Abstract
In diazotrophic organisms, nitrogenase synthesis and activity are tightly regulated. Two genes, nifL and nifA, are implicated as playing a major role in this regulation. NifA is a transcriptional activator, and its activity is inhibited by NifL in response to availability of excess fixed nitrogen and high O2 tension. It was postulated that NifL binds to NifA to inhibit NifA-mediated transcriptional activation of nif genes. Mutational analysis combined with transcriptional activation studies clearly is in agreement with the proposal that NifL interacts with NifA. However, several attempts to identify NifA-NifL interactions by using methods such as coimmunoprecipitations and chemical cross-linking experiments failed to detect direct interactions between these proteins. Here we have taken a genetic approach, the use of a yeast two-hybrid protein-protein interaction assay system, to investigate NifL interaction with NifA. A DNA fragment corresponding to the kinase-like domain of nifL was PCR amplified and was used to generate translation fusions with the DNA binding domain and the DNA activation domain of the yeast transcriptional activator GAL4 in yeast two-hybrid vectors. Similarly, a DNA fragment corresponding to the catalytic domain of nifA was PCR amplified and used to generate translation fusions with the DNA-binding domain and the DNA-activation domain of GAL4 in yeast two-hybrid vectors. After introducing appropriate plasmid combinations in yeast cells, the existance of direct interaction between NifA and NifL was analyzed with the MATCHMAKER yeast two-hybrid system by testing for the expression of lacZ and his3 genes. These analyses showed that the kinase-like domain of NifL directly interacts with the catalytic domain of NifA.
The biological nitrogen fixation reaction is catalyzed by a complex metalloenzyme called nitrogenase (6, 14). Nitrogenase is composed of two separately purified proteins, both of which are extremely oxygen sensitive. The larger of the two proteins, designated the MoFe protein, has a molecular mass of 230,000 Da (6, 14, 16, 17, 24). The MoFe protein is a tetramer in its biologically active form and is composed of two identical halves, each containing an α-subunit and a β-subunit encoded by the nifD and nifK genes, respectively. The smaller of the two proteins, designated the Fe protein, has a molecular mass of about 60,000 Da and is a dimer of identical subunits encoded by the nifH gene (11, 12, 14, 23). Besides the structural genes of nitrogenase, there are a number of nif-specific genes (20 identified to date) that comprise the nif regulon (15). These genes are generally clustered and arranged in multiple cistrons. Coordinated expression of the genes and interaction of gene products are required for synthesis and assembly of an active nitrogenase.
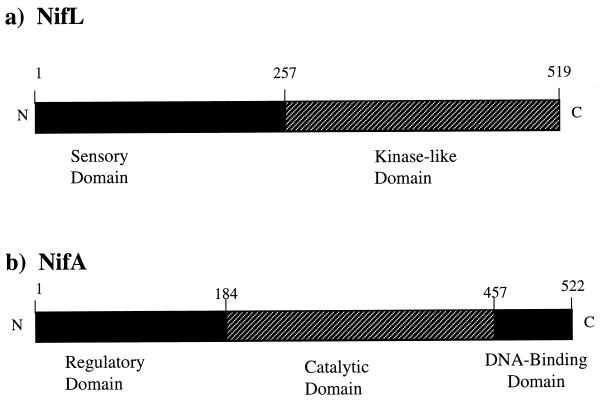
Expression of the nif genes is regulated at the transcriptional level by the products of nifA and nifL in response to molecular oxygen or ammonia (8). NifA is a specific transcriptional activator of the nif genes and acts in conjunction with RNA polymerase holoenzyme containing the alternative sigma factor, sigma 54. NifA binds to a characteristic palindromic motif, TGT-N10-ACA, also known as upstream activation sequence (UAS), that is located more than 100 bp upstream of nif promoters. The UAS-bound NifA makes contact with promoter-bound sigma 54 with the help of a DNA loop which is induced by the DNA-binding protein, the integration host factor (5, 13, 18). The integration host factor binds to a site between the NifA-binding site and the promoter to facilitate DNA loop formation and productive interaction between NifA and sigma 54. The NifA protein has three arbitrarily designated domains (20): an amino-terminal domain which is implicated in regulatory function, a catalytic domain that interacts with the sigma-RNA polymerase holoenzyme, and a C-terminal helix-turn-helix motif which recognizes the UAS on the nif promoters (Fig. 1).
FIG. 1.
Physical and genetic map of the (a) NifL- and the (b) NifA-coding regions from A. vinelandii. NifL is divided into a sensory domain and a kinase-like domain. NifA is divided into a regulatory domain, a catalytic domain, and a DNA-binding domain. The hatched regions in NifL and NifA correspond to regions used to construct fusion proteins with GAL4 domains. Numbers correspond to amino acid residue numbering. N and C represent the amino and carboxyl termini, respectively.
On the other hand, the NifL protein consists of two distinct domains separated by a glutamine-rich flexible linker sequence (3, 4, 8, 26). The amino-terminal region is designated as the sensory domain, and it has been proposed that this region may be involved in O2 sensing. The C-terminal region of the NifL shows some homology to the histidine protein kinase domain of two-component regulatory sensor proteins (Fig. 1). This domain has five highly conserved characteristic regions and a histidine residue which are present in other histidine autokinase transmitter domains. Furthermore, it was shown that the C-terminal domain of NifL is sufficient to inhibit transcriptional activity by the catalytic domain of NifA (22). Site-directed mutational analysis combined with biochemical experiments with purified proteins has thus far failed to detect either phosphotransfer to NifA or autophosphorylation of NifL (1, 19, 25). Hence, NifL and NifA make an atypical two-component regulatory system in which the communication does not involve phosphotransfer between these proteins. Therefore, in this case, the protein-protein communication is not through the covalent modification which is generally found in the members of two-component response regulator family proteins. Furthermore, evidence suggests that the inhibition of NifA activity by NifL requires stoichiometric levels of both proteins (9). When the NifA is overexpressed, even in the presence of wild-type levels of NifL, the transcriptional activity of the NifA is not inhibited. In the same way, the overproduction of NifL always causes inhibition of NifA activity, even in the absence of oxygen or combined nitrogen (1, 2). Taken together, these results suggest that NifL may directly interact with NifA and interfere with the contact between NifA and sigma 54 holoenzyme. Even though genetic analysis clearly suggests the formation of the NifL-NifA complex, coimmunoprecipitations and chemical cross-linking experiments on these proteins failed to detect NifL-NifA complex formation (22). In this communication, we report a molecular genetic approach, the use of the MATCHMAKER yeast two-hybrid system (10), to identify NifL-NifA protein-protein interactions in vivo.
The MATCHMAKER yeast two-hybrid system utilizes two plasmids, pAS2-1 and pGAD424 (7). The plasmid pAS2-1 contains the DNA-binding domain of the transcriptional activator GAL4 (GAL4 BD) and appropriate restriction sites for constructing a translational fusion with either NifL or NifA. The plasmid pGAD424 contains the DNA-activation domain of the transcriptional activator GAL4 (GAL4 AD) and appropriate restriction sites for constructing a translational fusion with either NifL or NifA. If the NifA and the NifL fusion peptides physically interacted in yeast cells, the GAL4 AD would be brought in close proximity to the GAL4 BD and result in the reassembly of the transcriptional activator and the activation of gene transcription from the gal1 promoter. Since the expression host, Saccharomyces cerevisiae CG1945, has the two genes, lacZ and his3, under the control of the gal1 promoter the interaction of the target proteins is easily determined phenotypically by growing the yeast on His-deficient media and measuring β-galactosidase activity.
Initially, an 815-bp DNA fragment encoding the catalytic domain of NifA was obtained by PCR amplification (21) with specific primers and using Azotobacter vinelandii OP chromosome as template. This PCR-amplified product was cloned into the pCR 2.1 plasmid to generate pBG500 (Table 1). The DNA fragment encoding the open reading frame was released by digesting pBG500 with EcoRI and was ligated with the EcoRI-digested pAS2-1 and pGAD424. This ligation resulted in the creation of an in-frame transitional fusion of the GAL4 BD-NifA peptide in plasmid pBG502 and GAL4 AD-NifA peptide in plasmid pBG504, respectively (Table 1 and Fig. 2). To construct fusion proteins in which the kinase-like domain of NifL is fused to either GAL4 BD or GAL4 AD, the two hybrid cloning vectors, pAS2-1 and pGAD424, were used, respectively. The strategy used for constructing these fusions was similar to that described to generate the NifA fusions. A 789-bp DNA fragment that encodes the kinase-like domain of NifL (Fig. 1) was generated by PCR amplification (25) using specific primers and the A. vinelandii OP chromosome as template. The PCR product was cloned into the pCR 2.1 plasmid to generate pBG501 (Table 1). The DNA fragment specifying the NifL kinase-like domain open reading frame was released by digesting pBG501 with EcoRI and was ligated into pAS2-1 and pGAD424 which were previously digested with EcoRI. These ligations resulted in the creation of an in-frame transitional fusion of the GAL4 BD-NifL peptide in plasmid pBG503 and GAL4 AD-NifL peptide in plasmid pBG505, respectively (Fig. 2). In all these plasmids, the fusion junctions were verified by nucleotide sequence analyses, and the deduced peptide sequence is shown in Fig. 2. These constructs were then cotransformed into the expression host S. cerevisiae CG1945, and transformants were selected for growth on SD medium lacking Leu and Trp. Since in this host system the gal1 promoter on the chromosome is transcriptionally fused to the his3 gene, the transformants would grow on plates lacking His only if the his3 gene was transcribed from a gal1 promoter that was activated by a GAL4 two-hybrid complex. The strains carrying various plasmid combinations were tested for their ability to grow on SD plates lacking His, Leu, and Trp, and the results of these experiments are presented in Table 2. It was observed that yeast colonies showed positive growth only when both NifL and NifA hybrid proteins were expressed, suggesting that direct protein-protein interaction exists between NifA and NifL.
TABLE 1.
Bacterial strains, yeast strains, and plasmids used in this study
| Strain or plasmid | Relevant characteristics and description | Source or reference |
|---|---|---|
| A. vinelandii OP | Wild-type, nitrogen-fixing, soil bacterium | Laboratory stock |
| Escherichia coli TG1 | K-12 Δ(lac-pro) supE thi hsd-5/F′ traD36 proA+B+ lacIq lacZΔM15 | Amersham Life Sciences, Inc. |
| E. coli INVαF′ | F′ endA1 recA1 hsdR17(rK− mK+) Δ(lac)U169 φ80lacΔM15 Δ(lacZYA-argF)U169 λ− supE44 thi-1 relA1 gyrA96 | Invitrogen Corp. |
| S. cerevisiae CG1945 | MATa ura3-52 his3-200 lys2-801 ade2-101 leu2-3 trp1-901 112 gal4-542 gal180-538 cyhr2 LYS2::GAL1UAS-GAL1TATA-HIS3 URA3::GAL417-mers(×3)-CYC1TATA-lacZ | CLONTECH Laboratories, Inc. |
| pVA3-1 | Murine p53(72–390) in pAS2-1, TRP1, Ampr (9.4 kb) | CLONTECH Laboratories, Inc. |
| pTD1-1 | SV40 large T antigen (84–708) in pACT2, LEU2, Ampr (9.9 kb) | CLONTECH Laboratories, Inc. |
| pCR 2.1 | Ampr Kanr (3.9 kb); used for direct cloning of PCR products | Invitrogen Corp. |
| pBG500 | Derivative of pCR 2.1 in which 815-bp DNA fragment that encodes the central domain (catalytic domain, Fig. 1b) of NifA was cloned; this fragment was generated by PCR amplification using the primers described in the text; this fragment could be released by digesting with EcoRI and could be cloned into the EcoRI site of pAS2-1 or pGAD424 to generate in-frame fusions with GAL4 DNA BD or AD, respectively | This work |
| pBG501 | Derivative of pCR 2.1 in which 789-bp DNA fragment that encodes the central domain (catalytic domain, Fig. 1a) of the NifL was cloned; this fragment was generated by PCR amplification using the primers described in the text; this fragment could be released by digesting with EcoRI and could be cloned into the EcoRI site of pAS2-1 or pGAD424 to generate in-frame fusions with GAL4 DNA BD or AD, respectively | This work |
| pAS2-1 | GAL4(1–147) DNA-BD TRP1 AmprCYHs2 (8.5 kb); unique cloning sites include NdeI, NcoI, SfiI, EcoRI, XmaI/SmaI, BamHI, SalI, and PstI | CLONTECH Laboratories, Inc. |
| pGAD424 | GAL4(768–881) AD LEU2 Ampr hemagglutinin epitope tag (8.1 kb); unique cloning sites include SfiI, NcoI, XmaI/SmaI, BamHI, EcoRI, SacI, and XhoI | CLONTECH Laboratories, Inc. |
| pBG502 | pAS2-1 derivative in which the EcoRI fragment that encodes the catalytic domain of the NifA (obtained by EcoRI digestion of pBG500) was cloned to generate the in-frame GAL4 BD-NifA translation fusion | This work |
| pBG503 | pAS2-1 derivative in which the EcoRI fragment that encodes the kinase-like domain of the NifL (obtained by EcoRI digestion of pBG501) was cloned to generate the in-frame GAL4 BD-NifL translation fusion | This work |
| pBG504 | pGAD424 derivative in which the EcoRI fragment that encodes the catalytic domain of the NifA (obtained by EcoRI digestion of pBG500) was cloned to generate the in-frame GAL4 AD-NifA translation fusion | This work |
| pBG505 | pGAD424 derivative in which the EcoRI fragment that encodes the kinase-like domain of the NifL (obtained by EcoRI digestion of pBG501) was cloned to generate the in-frame GAL4 AD-NifL translation fusion | This work |
FIG. 2.
The nucleotide sequence and predicted amino acid sequence (represented in single letter code) of fusion protein junctions in pBG502, pBG503, pBG504, and pBG505 are shown. The nucleotide sequences corresponding to NifA and NifL are shown in bold. (A) Fusion junction of the carboxyl-terminal sequence of the GAL4 DNA-binding domain and the catalytic domain of NifA present in pBG502. (B) Fusion junction of the carboxyl-terminal sequence of the GAL4 DNA-binding domain and the kinase-like domain of NifL present in pBG503. (C) Fusion junction of the carboxyl-terminal sequence of the GAL4-activation domain and the catalytic domain of NifA present in pBG504. (D) Fusion junction of the carboxyl-terminal sequence of GAL4-activation domain and the kinase-like domain of NifL present in pBG505.
TABLE 2.
Results of the liquid β-galactosidase assay with ONPG as substrate to demonstrate NifL-NifA interactions
| Plasmid to which GAL4 BD was translationally fused | Plasmid to which GAL4 AD was translationally fused | β-Galactosidase activity (Miller units)a | Growth on SD plates lacking Leu, Trp, and His/3ATb | Interacting peptides |
|---|---|---|---|---|
| pBG502 (GAL4 BD:NifA) | pBG505 (GAL4 AD:NifL) | 5.60 ± 0.22 | ++ | NifA and NifL |
| pBG503 (GAL4 BD:NifL) | pBG504 (GAL4 AD:NifA) | 5.71 ± 0.37 | ++ | NifA and NifL |
| pAS2-1 (GAL4 BD) | pBG504 (GAL4 AD:NifA) | 0.63 | −− | None |
| pAS2-1 (GAL4 BD) | pBG505 (GAL4 AD:NifL) | 0.53 | −− | None |
| pBG502 (GAL4 BD:NifA) | pGAD424 (GAL4 AD) | 0.50 | −− | None |
| pBG503 (GAL4 BD:NifL) | pGAD424 (GAL4 AD) | 0.71 | −− | None |
| pVA3-1 (GAL4 BD:p53) | pTD1-1(GAL4 AD:SV40 large T antigen) | 35.9 ± 2.61 | ++ | p53 and large T antigen |
| pAS2-1 (GAL4 BD) | pABCT2 (GAL4 AD) | 1.13 | −− | None |
The β-galactosidase activity assay was performed as described in the MATCHMAKER two-hybrid system (20). Briefly, cells from 1.5-ml samples of exponential culture were collected by centrifugation and resuspended in 300 μl of Z-buffer (20). A 100-μl aliquot of the resuspended cells was lysed by quick freeze-thaw (treatment with liquid nitrogen followed by thawing at 37°C). To measure the β-galactosidase activity in the cell lysate, a 0.7-ml sample of the Z-buffer–β-mercaptoethanol solution was added to each tube followed by 0.16 ml of Z-buffer–ONPG (4 mg of ONPG per 1 ml of Z-buffer). The time of ONPG addition was recorded, and the tubes were incubated at 30°C with shaking. When yellow color was visible, 400 μl of 1 M NaCO3 was added to each tube to terminate the reaction, and the time was recorded. The tubes were then centrifuged for 10 min at 10,000 × g to remove cellular debris, and the optical density at 420 nm was recorded. β-Galactosidase units were defined as the amount of enzyme which hydrolyzes 1 μmol of ONPG to o-nitrophenol and d-galactose per min.
S. cerevisiae CG1945 was transformed with plasmid DNA by utilizing the YEASTMAKER yeast transformation system (22) (CLONTECH Laboratories, Inc.). Transformants were selected on appropriate nutrition-deficient SD medium selection plates by incubating at 30°C for 2 to 3 days. Single colonies from each transformation experiment were purified and were used to determine growth characteristics on SD media lacking His.
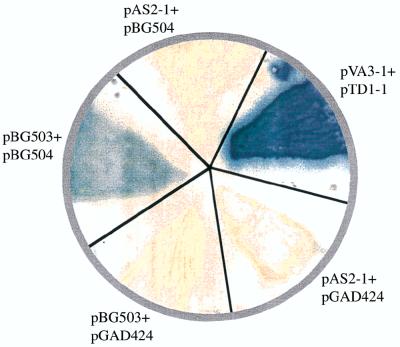
Further confirmation of the interaction between NifA and NifL was obtained by measuring the expression of the second marker gene, lacZ, in yeast strains that carried different combinations of yeast two-hybrid plasmids expressing translation fusions of NifA or NifL to different domains of GAL4. This was carried out by filter-lift assays with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as substrate and by liquid β-galactosidase activity assays using ONPG (o-nitrophenyl-β-d-galactopyranoside) as substrate (7). Figure 3 shows the expression of β-galactosidase in a yeast strain containing the combination of pBG503 (GAL4 BD:NifL) and pBG504 (GAL4 AD:NifA) by filter-lift assay. Yeast strains containing plasmid combinations that expressed only the NifA or NifL translational fusions with the different domains of GAL4 did not express any β-galactosidase. The positive control was S. cerevisiae CG1945 carrying the plasmids pVA3-1 and pTD1-1, the DNA BD and AD fusion plasmids, respectively. pVA3-1 encoded a fusion protein in which murine p53 had been fused to the GAL4 BD and pTD1-1 encoded a fusion protein in which simian virus 40 (SV40) large T antigen was fused to GAL4 AD. This strain was used as a standard positive control in the MATCHMAKER yeast two-hybrid protein-protein interaction assay. The negative control was S. cerevisiae CG1945 carrying the cloning vectors, pAS2-1 and pGAD424. Table 2 shows the results of liquid β-galactosidase activity assays with ONPG as the substrate. These results also support that the GAL4 BD and GAL4 AD domains were brought together and that the lacZ gene was expressed only when the yeast strains contained the plasmid combinations that would express translation fusions of GAL4 BD-NifA plus GAL4 AD-NifL or GAL BD-NifL plus GAL4 AD-NifA. The negative control was S. cerevisiae CG1945 carrying the cloning vectors, pAS2-1 and pGAD424. To confirm that the results we were seeing were not due to any type of false positives, we used different experimental controls (Table 2). Strains of S. cerevisiae CG1945 carrying pAS2-1 plus pBG504, pAS2-1 plus pBG505, pBG502 plus pGAD424, or pBG503 plus pGAD424 did not show any β-galactosidase activity. These strains also failed to grow on SD plates lacking His, Leu, and Trp (Table 2). Since the interaction between the NifA and NifL domains remained the same irrespective of the sequences surrounding them (as seen in yeast strains carrying GAL4 BD-NifA plus GAL4 AD-NifL or GAL BD-NifL plus GAL4 AD-NifA), we conclude that direct interaction exists between these two domains.
FIG. 3.
Colony filter-lift assay to identify the specificity of interaction between NifL and NifA. In these experiments, the strains of S. cerevisiae CG1945 carrying different combinations of plasmids were grown on SD plates lacking Trp and Leu and were transferred to a Whatman no. 5 paper filter. Cells were permeabilized by freeze-thaw treatment of the filters (freezing in liquid nitrogen and allowing them to thaw at room temperature). The filters carrying the cells were then placed over filters presoaked with Z-buffer–X-Gal solution according to the protocols of the MATCHMAKER yeast two-hybrid assay. β-Galactosidase activity was detected only when S. cerevisiae CG1945 contained pBG503 (GAL4 BD:NifL) and pBG504 (GAL4 AD:NifA).
Previous experimental analyses suggested that NifA is a transcriptional activator and that its activity is inhibited by NifL associating with NifA in response to the availability of excess fixed nitrogen or high O2 tension. As mentioned before, experiments to demonstrate direct interaction between NifA and NifL using coimmunoprecipitations and cross-linking experiments were unable to detect such an interaction. Here we show direct interaction between NifA and NifL by using a genetic assay, which may provide us with a method to probe the specificity of this interaction at the molecular level.
Acknowledgments
We thank the members of the Gavini and Pulakat laboratories at Bowling Green State University for their helpful discussions.
This work was supported by National Institutes of Health grant GM57636 to N. Gavini.
REFERENCES
- 1.Austin S, Buck M, Cannon W, Eydmann T, Dixon R. Purification and in vitro activities of the native nitrogen fixation control proteins NifA and NifL. J Bacteriol. 1994;176:3460–3465. doi: 10.1128/jb.176.12.3460-3465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger D K, Narberhaus F, Kustu S. The isolated catalytic domain of NIFA, a bacterial enhancer-binding protein, activates transcription in vitro: activation is inhibited by NIFL. Proc Natl Acad Sci USA. 1994;91:103–107. doi: 10.1073/pnas.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco G, Drummond M, Woodley P, Kennedy C. Sequence and molecular analysis of the nifL gene of Azotobacter vinelandii. Mol Microbiol. 1993;9:869–879. doi: 10.1111/j.1365-2958.1993.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 4.Blanco G, Woodley P, Drummond M, Bali A, Kennedy C. The nifL gene of Azotobacter vinelandii: novel features of sequence, expression and mutant phenotypes. In: Palacios R, Mora J, Newton W E, editors. New horizons in nitrogen fixation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 429–434. [Google Scholar]
- 5.Buck M, Cannon W. Activator-independent formation of a closed complex between sigma 54- holoenzyme and nifH and nifU promoters of Klebsiella pneumoniae. Mol Microbiol. 1992;6:1625–1630. doi: 10.1111/j.1365-2958.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 6.Burgess B K, Lowe D J. Mechanism of molybdenum nitrogenase. Chem Rev. 1996;96:2983–3011. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 7.CLONTECH Laboratories, Inc. User manual for MATCHMAKER yeast two-hybrid system. Palo Alto, Calif: CLONTECH Laboratories, Inc.; 1997. [Google Scholar]
- 8.Dixon R. The oxygen-responsive NIFL-NIFA complex: a novel two-component regulatory system controlling nitrogenase synthesis in gamma-proteobacteria. Arch Microbiol. 1998;169:371–380. doi: 10.1007/s002030050585. [DOI] [PubMed] [Google Scholar]
- 9.Eydmann T, Soderback E, Jones T, Hill S, Austin S, Dixon R. Transcriptional activation of the nitrogenase promoter in vitro: adenosine nucleotides are required for inhibition of NIFA activity by NIFL. J Bacteriol. 1995;177:1186–1195. doi: 10.1128/jb.177.5.1186-1195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields S. The two-hybrid system to detect protein-protein interactions. Methods (Orlando) 1993;5:116–124. [Google Scholar]
- 11.Gavini N, Burgess B K. FeMo cofactor synthesis by a nifH mutant with altered MgATP reactivity. J Biol Chem. 1992;267:21179–21186. [PubMed] [Google Scholar]
- 12.Georgiadis M M, Komiya H, Chakrabarti P, Woo D, Kornuc J J, Rees D C. Crystallographic structure of the nitrogenase iron protein from Azotobacter vinelandii [see comments] Science. 1992;257:1653–1659. doi: 10.1126/science.1529353. [DOI] [PubMed] [Google Scholar]
- 13.Hoover T R, Santero E, Porter S, Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990;63:11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- 14.Howard J B, Rees D C. Structural basis of biological nitrogen fixation. Chem Rev. 1996;96:2965–2982. doi: 10.1021/cr9500545. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson M R, Brigle K E, Bennett L T, Setterquist R A, Wilson M S, Cash V L, Beynon J, Newton W E, Dean D R. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989;171:1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Rees D C. Crystallographic structure and functional implications of the nitrogenase molybdenum-iron protein from Azotobacter vinelandii. Nature. 1992;360:553–560. doi: 10.1038/360553a0. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Rees D C. Nitrogenase and biological nitrogen fixation. Biochemistry. 1994;33:389–397. doi: 10.1021/bi00168a001. [DOI] [PubMed] [Google Scholar]
- 18.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of ς54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H S, Narberhaus F, Kustu S. In vitro activity of NifL, a signal transduction protein for biological nitrogen fixation. J Bacteriol. 1993;175:7683–7688. doi: 10.1128/jb.175.23.7683-7688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morett E, Segovia L. The ς54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993;175:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 22.Narberhaus F, Lee H S, Schmitz R A, He L, Kustu S. The C-terminal domain of NifL is sufficient to inhibit NifA activity. J Bacteriol. 1995;177:5078–5087. doi: 10.1128/jb.177.17.5078-5087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulakat L, Hausman B S, Lei S, Gavini N. Nif− phenotype of Azotobacter vinelandii UW97. Characterization and mutational analysis. J Biol Chem. 1996;271:1884–1889. doi: 10.1074/jbc.271.4.1884. [DOI] [PubMed] [Google Scholar]
- 24.Schindelin H, Kisker C, Schlessman J L, Howard J B, Rees D C. Structure of ADP × AIF4(−)-stabilized nitrogenase complex and its implications for signal transduction. Nature. 1997;387:370–376. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]
- 25.Woodley P, Drummond M. Redundancy of the conserved His residue in Azotobacter vinelandii NifL, a histidine autokinase homologue which regulates transcription of nitrogen fixation genes. Mol Microbiol. 1994;13:619–626. doi: 10.1111/j.1365-2958.1994.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhulin I B, Taylor B L, Dixon R. PAS domain S-boxes in Archea, Bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/s0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]