Abstract
Lipid bilayer experiments indicated that the cell wall of Mycobacterium tuberculosis contains at least two different porins: (i) a cation-selective, heat-sensitive 0.7-nS channel which has a short-lived open state and is probably composed of 15-kDa subunits and (ii) a 3-nS, >60-kDa channel with a long-lived open state, resembling porins from fast-growing mycobacteria.
The mycobacterial cell wall differs from that of most other bacteria (1) and forms a diffusion barrier which is 100- to 1,000-fold less permeable to hydrophilic molecules than that of Escherichia coli (4). The permeation properties of this barrier are determined by porins, which were observed in the cell walls of fast-growing mycobacteria such as Mycobacterium chelonae (18) and Mycobacterium smegmatis (17) and allow the diffusion of small and hydrophilic molecules into the periplasm. MspA is the major porin from M. smegmatis. It is a water-filled channel with a conductance of 4.6 nS in 1 M KCl and has a high channel-forming activity in lipid bilayer experiments (9). Porins with similar properties have not yet been described for slow-growing mycobacteria, including the pathogen Mycobacterium tuberculosis. OmpATb from M. tuberculosis produces channels with low activity in liposome swelling experiments and a conductance of 0.7 nS in 1 M KCl (15). However, the crystal structure of the transmembrane domain of OmpA from E. coli and lipid bilayer experiments indicated that OmpA has no channel-forming activity (10). It was suggested that the observed channel-forming activities may have been caused by a fraction of molecules with a nonnative conformation (10). This may be true also for other members of the OmpA family, indicating that channel-forming proteins other than OmpATb might exist in M. tuberculosis. In this study, we present the analysis of channel-forming activities in the cell wall of M. tuberculosis.
Porins of M. tuberculosis can be extracted with organic solvents.
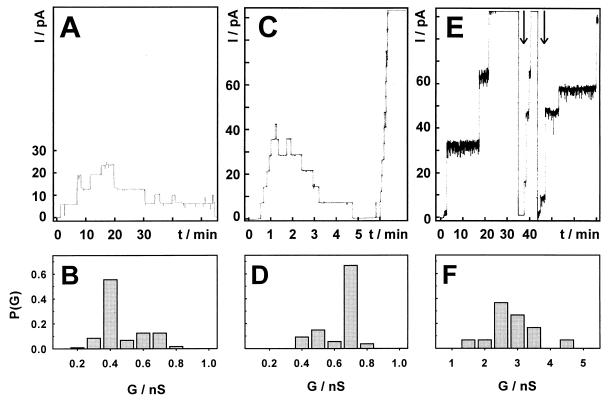
Inactivated cells of M. tuberculosis H37Rv were obtained from J. Belisle (Colorado State University, Fort Collins, Colo.). A 1.8-g portion of cells (wet weight) was extracted with 30 ml of a mixture of methylenechloride and methanol (1:2) as described for the isolation of porins from other bacteria of the Corynebacterium-Mycobacterium-Nocardia group (6, 9, 12). After sedimentation of the cells, 100 μl of the supernatant was mixed with 300 μl of PG05 buffer (0.5% of the detergent isotridecyl-polyethylene glycol ether [Genapol], 100 mM Na phosphate [pH 6.5], 150 mM NaCl). Ten microliters was added to each side of a planar membrane made from diphytanoyl phosphatidylcholine and diphytanoyl phosphatidylserine (4:1) and analyzed for the presence of channel-forming proteins as previously described (9). Channels with a low conductance (about 0.4 nS) were observed with these extracts (Fig. 1A and B). These channels did not show the typical staircase increase of current after reconstitution of porins in a lipid membrane (Fig. 1A). For porins from gram-negative bacteria, such a channel characteristic can be caused by a loss of the porin from the membrane but is usually interpreted as closing of the channel, which can be modulated by ligands in vivo (13) and by the applied voltage, the type of membrane, and the porin preparation in vitro (7). Such closing events were very rarely observed with MspA from M. smegmatis under the same experimental conditions (9). Only a total of 10 to 20 channels was detected in each lipid bilayer experiment with the methylenechloride-methanol extract of M. tuberculosis. These findings are in contrast to the high activity of 2.3-nS porins in similar extracts of M. smegmatis (9) and could be caused either by a smaller number of porins in extracts from M. tuberculosis or by unfavorable reconstitution conditions. Gel analysis of the methylenechloride-methanol extract did not reveal any protein (data not shown), in contrast to results for similar extracts of M. smegmatis (9), supporting the assumption that the extract contained only minor amounts of protein.
FIG. 1.
Single-channel recordings of a planar lipid bilayer in the presence of different preparations of cell wall proteins from M. tuberculosis. The aqueous phase contained 1 M KCl and was buffered with 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) at pH 6.0. Protein solutions were added on both sides of membranes formed from diphytanoyl phosphatidylcholine and diphytanoyl phosphatidylserine (molar ratio, 4:1). Membrane current was measured after application of a potential of 10 mV as described previously (9). Data were collected from at least three different membranes. Graphs A, C, and E show traces of the current (I), which started at zero. Graphs B, D, and F show the probability (P) of conductance steps (G) of the corresponding current traces. (A and B) Extract of M. tuberculosis cells with a mixture of methylenechloride and methanol (1:2). The protein concentration was too low to be measurable by standard methods. The record shows the reconstitution of three 0.4-nS pores and one 0.2-nS pore which were all closed at the end of this trace. A total of 119 single-channel events were analyzed. (C and D) Extract of M. tuberculosis cells with 0.5% Genapol. The protein concentration was about 100 ng/ml in each compartment of the bilayer cuvette. The record shows the reconstitution and the subsequent closure of seven pores of 0.7 nS, which is followed by a rapid reconstitution of pores of 0.7 nS in a staircase manner until the disruption of the membrane. A total of 108 single-channel events were analyzed. (E and F) Proteins from M. tuberculosis in fraction 9 after anion-exchange chromatography. The protein concentration was too low to be measurable by standard methods. The record shows the reconstitution of five 3-nS pores, two 0.7-nS pores, and three 1.5- to 3-nS pores. The pores which were reconstituted after current suppression as indicated by an arrow are only partially recorded. A total of 30 single-channel events were analyzed.
Detergent extracts of M. tuberculosis exhibit a low activity of porins with conductances of 0.7 and 3 nS.
Since the preparation of cell extracts with a high channel activity is an important step for the purification of porins from M. tuberculosis, we tested different extraction and reconstitution conditions for increased channel-forming activity. Therefore, 100 mg (wet weight) of M. tuberculosis H37Rv cells was suspended in 1 ml of PG05E buffer (PG05 buffer containing 40 mM EDTA) and extracted by shaking at room temperature for 1 h. Rapidly closing channels with a main conductance of 0.7 nS (Fig. 1C and D) and also few pores with a conductance of 2.4 to 3.7 nS (data not shown) were observed in lipid bilayer experiments using 10 μl of the cell extract. The dominance of a low-conductance porin in extracts from M. tuberculosis is in contrast to observations made with fast-growing mycobacteria, which produce mainly channels with single-channel conductances above 2 nS (17, 18). No pores could be detected after boiling of the Genapol extract for 30 min. This indicated that these channels were not resistant to denaturation by heat, in contrast to MspA, which is an extremely stable porin (9). Macroscopic conductance measurements showed the reconstitution of up to 660 ± 80 channels into diphytanoyl phosphatidylcholine-diphytanoyl phosphatidylserine (4:1) membranes in the presence of Genapol extracts (about 100 ng of protein/ml). Thus, the concentration of channel-forming proteins appeared to be higher than that in extracts with methylenechloride-methanol, but the activity was still orders of magnitude lower than that obtained with similar extracts from M. smegmatis, from which up to 105 channels reconstituted in planar lipid membranes. However, neither variation of the detergent (sodium dodecyl sulfate [SDS], cholate, cetyltrimethylammonium bromide, or N,N-dimethyldodecylamine-N-oxide) nor variation of the extraction conditions such as temperature (20, 28, or 37°C) or time (up to 17 h) with Genapol as a detergent significantly improved the channel-forming activity of cell extracts from M. tuberculosis compared to that obtained with the Genapol extract described above. Also, with the Genapol extract, variations of reconstitution conditions such as the temperature between 11 and 37°C or the pH between 4 and 9 or use of negatively charged membranes did not change the frequency of pore reconstitution in lipid bilayer experiments. These results suggested that the quantity of porins in cell extracts of M. tuberculosis might be significantly lower than that in extracts from M. smegmatis.
The 0.7- and the 3-nS porins of M. tuberculosis are localized in the cell wall.
Cell wall from M. tuberculosis H37Rv was prepared by using a sucrose gradient as previously described (3) and provided by J. Belisle. The extract of 50 mg of cell wall (wet weight) with 500 μl of PG05E buffer was done as described above for whole cells of M. tuberculosis. Five microliters produced channels with the same conductances as extracts of whole cells of M. tuberculosis H37Rv, although the frequency of reconstitution into lipid membranes was lower (data not shown). This demonstrated that the observed channels are located in the cell wall.
The 0.7-nS porin is probably an oligomeric protein composed of 15-kDa subunits.
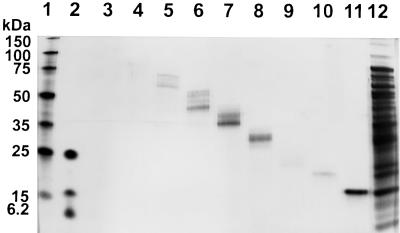
We used preparative gel electrophoresis, as described for the isolation of MspA (9), to purify a porin from M. tuberculosis. Proteins were extracted from 2 g (wet weight) of M. tuberculosis H37Rv cells with 20 ml of PG05E buffer, precipitated with acetone, and separated on a denaturing polyacrylamide gel. The gel of each lane was cut into 14 polyacrylamide strips, which were eluted overnight with PG05E buffer. Eluted proteins were detected in fractions 1 to 9 with apparent molecular masses from above 150 kDa to 15 kDa (Fig. 2). Channels with conductances of approximately 3 nS were reconstituted from proteins in fractions 1 (proteins larger than 75 kDa), 2 (proteins approximately equal to 75 kDa), and 3 (proteins from 60 to 75 kDa). Fast-closing channels with a conductance of 0.7 nS were detected in fractions with proteins larger than 60 kDa and in fraction 9 with proteins of approximately 15 kDa. We did not observe any channel in fractions 10 to 14 containing proteins of less than 15 kDa. This indicated that the 0.7-nS channel was formed by partially SDS-resistant oligomers of 15-kDa monomers and that both the monomeric and the oligomeric forms of this protein are reconstituted as 0.7-nS channels in lipid bilayer experiments. The oligomeric structure is in agreement with results obtained for other porins from bacteria of the Corynebacterium-Mycobacterium-Nocardia group, which are composed of subunits of molecular masses between 5 and 23 kDa (6, 9, 12), and suggested further that the 3-nS channel which was observed in addition to the 0.7-nS channel in fractions 1 to 3 containing proteins larger than 60 kDa is probably formed by a different protein. In addition, the 0.7-nS channel has a lifetime in the open state in the range of only seconds to minutes, whereas the 3-nS channel was open for hours under the same experimental conditions. This result supports the assumption that the 0.7- and the 3-nS channel are formed by different proteins, an observation reminiscent of the fact that even the closely related OmpF and OmpC porins from E. coli can be distinguished unequivocally by their closing characteristics (2).
FIG. 2.
Mass-fractionated cell wall proteins from M. tuberculosis H37Rv. Proteins were separated on an SDS–10% polyacrylamide gel with a buffer containing Tricine (14). The gel was stained with silver (8). Lanes: 1, molecular mass marker 1 (150, 100, 75, 50, 35, 25, and 15 kDa; Novagen, Madison, Wis.); 2, molecular mass marker 2 (26.6, 17, 14.6, 6.5, 3.5, and 1 kDa; Sigma); 3 to 11, fractions 1 to 9 of gel-eluted proteins after preparative gel electrophoresis of an extract of cells of M. tuberculosis H37Rv with Genapol (lane 12). Protein concentrations in this experiment were low and, therefore, could not be determined by standard methods. In particular, proteins with a molecular mass larger than 75 kDa in lanes 3 and 4 were only faintly visible. All samples were incubated at 37°C for 30 min before the gel was loaded.
The 0.7-nS porin is not identical to OmpATb.
We did not observe any channel in fractions 4 to 8, which include the fractions expected to contain OmpATb (33.5 kDa) (15). Our finding that porins other than OmpATb, which does not allow the diffusion of disaccharides (15), exist in M. tuberculosis is supported by the transport properties of its cell wall, which is permeable to large and hydrophilic antibiotics such as the aminoglycoside streptomycin and the cyclic peptide capreomycin (11).
The 0.7-nS porin is a minor protein in Genapol extracts of M. tuberculosis.
Proteins of fraction 9 produced exclusively 0.7-nS channels and migrated as a single band at 15 kDa in a denaturing polyacrylamide gel (Fig. 2). Therefore, the band at 15 kDa was transferred to a polyvinylidene difluoride membrane which was stained with Coomassie blue and subjected to Edman degradation (Toplab, Munich, Germany). The N-terminal sequence was determined to be A-T-T-L-P-V-Q-R-H-P-(R)-S-L-F-(P)-E-F (amino acids which were not clearly identified are in parentheses). This sequence is identical with the N-terminus of the α-crystallin-like 16-kDa antigen from M. tuberculosis, which belongs to the family of small heat shock proteins (19) and appears to be associated with the cell wall (5). However, the 16-kDa antigen was found exclusively in the aqueous phase in Triton X-114 phase-partitioning experiments (19), which showed that it is a water-soluble protein. This property excludes the 16-kDa antigen as a porin, and therefore we assume that the porin activity in fraction 9 was caused by a minor protein of M. tuberculosis H37Rv with a molecular mass of about 15 kDa.
The 0.7-nS porin is cation selective.
The single channel conductance of Genapol extracts of M. tuberculosis was influenced considerably by salt composition of the solution bathing the lipid bilayer in reconstitution experiments (Table 1). The mobility of cations through the 0.7-nS porin decreased with the radii of the hydrated ions. This suggested that the 0.7-nS porin forms a water-filled channel. Exchange of K+ for the less mobile Tris+ reduced the single channel conductance by a factor of about 10, whereas replacement of Cl− by the less mobile acetate anion had almost no effect. This indicated a selectivity of the 0.7-nS porin for cations as found for all previously described mycobacterial porins (9, 16, 17).
TABLE 1.
Average single-channel conductances of the 0.7-nS porin from M. tuberculosis in 1 M solutions of different saltsa
| Salt | Radius of hydrated cation (nm)b | Conductance (nS) |
|---|---|---|
| Tris Cl | 0.321 | 0.08 |
| LiCl | 0.216 | 0.3c |
| NaCl | 0.163 | 0.5 |
| KCl | 0.110 | 0.7 |
| RbCl | 0.105 | 0.8 |
| CsCl | 0.106 | 0.7 |
| KOAc | 0.110 | 0.5 |
M. tuberculosis H37Rv cells (100 mg, wet weight) were extracted with 1 ml of PG05E buffer. Then, 10 μl of these extracts was added to 1 M salt solutions on both sides of the membranes. The protein concentration was too low to be measurable by standard methods. Salt solutions were buffered with 10 mM MES at pH 6.0. The pH values of solutions of Tris Cl and potassium acetate (KOAc) were 8.0 and 7.3, respectively. Data were collected from at least three different membranes. Average single-channel conductances were calculated from at least 80 events.
Data from reference 17.
Average conductance from five channels.
Enrichment of the 3-nS porin after anion-exchange chromatography.
M. tuberculosis H37Rv cells (500 mg) were extracted with 5 ml of a buffer containing 0.5% Genapol, 25 mM NaCl, and 200 mM Tris HCl (pH 8.0) as described above. The supernatant was diluted with water to a final Genapol concentration of 0.2%. The channel-forming activity of this extract was similar to that of extracts with PG05E buffer (data not shown). This sample was applied to an anion-exchange column (HQ/M Poros, 20 μm, Biocad workstation) and eluted with a linear gradient from 0 to 1.5 M NaCl. Nineteen fractions of 10 ml each were collected and analyzed by lipid bilayer experiments for channel-forming activity (data not shown). An enrichment of pores with a main channel conductance of about 3 nS and a long lifetime in the open state was observed in fraction 9 (Fig. 1E and F). These channel properties resembled those of the porins from the fast-growing M. smegmatis (17) and M. chelonae (16). However, the increased activity of the 3-nS porin could not be correlated with a protein in silver-stained SDS-polyacrylamide gels (not shown), suggesting that it was a minor protein in this fraction.
Conclusions.
The prevalence of a 0.7-nS porin in cell extracts appears to be special for M. tuberculosis, which might reflect the fact that its genome does not contain genes with a significant similarity to the mspA gene of M. smegmatis (9). The porin with a conductance of 3 nS has properties similar to those of the porins from fast-growing mycobacteria. However, none of these porins could be identified by preparative gel electrophoresis or by anion-exchange chromatography, which were both successfully used to purify porins from M. smegmatis (2a, 9). The low channel-forming activity of extracts of M. tuberculosis indicated that either its porins are not as easy to solubilize from the cell wall as those from M. smegmatis or the number of porins is much lower.
Acknowledgments
We are indebted to John Belisle for providing cell wall and cells of M. tuberculosis through the NIH program “Tuberculosis Research Materials and Vaccine Testing” funded by NIAID, contract NO1-AI-75320. We thank Wolfgang Hillen for continuous support, Kristin Birkness, Harald Engelhardt, and Sabine Ehrt for critically reading the manuscript, Roland Benz for discussions, and the reviewers for valuable suggestions.
This work was partly supported by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Brennan P J, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 2.Buehler L K, Kusumoto S, Zhang H, Rosenbusch J P. Plasticity of Escherichia coli porin channels. Dependence of their conductance on strain and lipid environment. J Biol Chem. 1991;266:24446–24450. [PubMed] [Google Scholar]
- 2a.Heinz, C., and M. Niederweis. Unpublished results.
- 3.Hirschfield G R, McNeil M, Brennan P J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990;172:1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarlier V, Nikaido H. Permeability barrier to hydrophilic solutes in Mycobacterium chelonei. J Bacteriol. 1990;172:1418–1423. doi: 10.1128/jb.172.3.1418-1423.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee B Y, Hefta S A, Brennan P J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtinger T, Burkovski A, Niederweis M, Kramer R, Benz R. Biochemical and biophysical characterization of the cell wall porin of Corynebacterium glutamicum: the channel is formed by a low molecular mass polypeptide. Biochemistry. 1998;37:15024–15032. doi: 10.1021/bi980961e. [DOI] [PubMed] [Google Scholar]
- 7.Mathes A, Engelhardt H. Voltage-dependent closing of porin channels: analysis of relaxation kinetics. J Membr Biol. 1998;165:11–18. doi: 10.1007/s002329900416. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey J H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 9.Niederweis M, Ehrt S, Heinz C, Klöcker U, Karosi S, Swiderek K M, Riley L W, Benz R. Cloning of the mspA gene encoding a porin from Mycobacterium smegmatis. Mol Microbiol. 1999;33:933–945. doi: 10.1046/j.1365-2958.1999.01472.x. [DOI] [PubMed] [Google Scholar]
- 10.Pautsch A, Schulz G E. Structure of the outer membrane protein A transmembrane domain. Nat Struct Biol. 1998;5:1013–1017. doi: 10.1038/2983. [DOI] [PubMed] [Google Scholar]
- 11.Rastogi N, Labrousse V, Goh K S. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol. 1996;33:167–175. doi: 10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 12.Riess F G, Lichtinger T, Cseh R, Yassin A F, Schaal K P, Benz R. The cell wall porin of Nocardia farcinica: biochemical identification of the channel-forming protein and biophysical characterization of the channel properties. Mol Microbiol. 1998;29:139–150. doi: 10.1046/j.1365-2958.1998.00914.x. [DOI] [PubMed] [Google Scholar]
- 13.Samartzidou H, Delcour A H. Excretion of endogenous cadaverine leads to a decrease in porin-mediated outer membrane permeability. J Bacteriol. 1999;181:791–798. doi: 10.1128/jb.181.3.791-798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 15.Senaratne R H, Mobasheri H, Papavinasasundaram K G, Jenner P, Lea E J, Draper P. Expression of a gene for a porin-like protein of the OmpA family from Mycobacterium tuberculosis H37Rv. J Bacteriol. 1998;180:3541–3547. doi: 10.1128/jb.180.14.3541-3547.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trias J, Benz R. Characterization of the channel formed by the mycobacterial porin in lipid bilayer membranes. Demonstration of voltage gating and of negative point charges at the channel mouth. J Biol Chem. 1993;268:6234–6240. [PubMed] [Google Scholar]
- 17.Trias J, Benz R. Permeability of the cell wall of Mycobacterium smegmatis. Mol Microbiol. 1994;14:283–290. doi: 10.1111/j.1365-2958.1994.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 18.Trias J, Jarlier V, Benz R. Porins in the cell wall of mycobacteria. Science. 1992;258:1479–1481. doi: 10.1126/science.1279810. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Crane D D, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]