Abstract
Objectives: The prevalence of chronic kidney disease (CKD) was reported to be higher in rheumatoid arthritis (RA) patients than in normal healthy individuals. Human leukocyte antigen (HLA) was associated with RA or CKD. Few studies on the association of HLA with CKD in RA have been reported. Here, we investigated the association of HLA polymorphisms with CKD in Japanese RA patients. Methods: HLA-DRB1 genotyping was conducted in 351 Japanese RA patients with CKD (estimated glomerular filtration rate [eGFR] lower than 60 [mL/min/1.73 m2]) and 959 without CKD (eGFR equal to or higher than 60 [mL/min/1.73 m2]). Associations of allele carrier frequencies of DRB1 with CKD were examined in the RA patients. Results: There was an association of DRB1*13:02 with CKD in RA, but this did not achieve statistical significance (p = 0.0265, odds ratio [OR] 1.70, pc = 0.7412, 95% confidence interval [CI] 1.09–2.64). The DR6 serological group was associated with CKD in RA (p = 0.0008, OR 1.65, 95% CI 1.24–2.20). A gene-dosage effect of DR6 was not detected. Logistic regression analysis showed that the association of DR6 with CKD in RA was independent of clinical characteristics. Conclusions: The present study first revealed the independent predisposing association of DR6 with CKD in Japanese RA patients, although DR6 is known to be protective against RA. Our data suggest direct or indirect roles of HLA for the development of CKD in RA, but the mechanisms are not clear.
Keywords: HLA, chronic kidney disease, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by the destruction of synovial joints. RA is commonly complicated with chronic kidney disease (CKD) [1,2,3,4], and the prevalence of CKD is higher in RA patients than that in the general population [5,6,7]. Several factors, including age and complications of hypertension, diabetes mellitus, secondary amyloidosis, rheumatoid vasculitis, drug-induced membranous nephropathy, and interstitial nephritis, are involved in the pathogenesis of CKD in RA [8,9]. Inflammation was reported to be a risk factor for the development of CKD in RA [10], and treatment with biological agents decreased the risk [11]. However, the cause of CKD is generally difficult to determine in most RA patients.
Human leukocyte antigen (HLA)-DRB1 is the most important genetic risk factor for RA. Many studies have reported the association of HLA polymorphisms with susceptibility to RA [12,13,14] or CKD [15,16]. Different DRB1 alleles associated with RA are dependent upon various ethnicities: DRB1*04:01 is associated with European RA [12], and DRB1*04:05 is associated with Japanese RA [13]. Amino acid residues at positions 70–74 in the DRβ chain were conserved as risk DRB1 alleles for RA, and these are called shared epitope (SE) alleles [12]. A gene-dosage effect was observed for the associations of DRB1 alleles with RA: homozygosity for risk alleles conferred a higher odds ratio (OR) than heterozygosity. Different HLA alleles have been associated with CKD in different studies [16], which might be explained by different underlying diseases or different ethnicities. However, few studies have examined the association of HLA with CKD in RA patients to date. Here, we evaluated the association of HLA polymorphisms with CKD in Japanese RA patients.
2. Materials and Methods
2.1. Patients and Controls
A total of 1310 RA patients were recruited at Sagamihara National Hospital and Tokyo National Hospital. All RA patients fulfilled the American College of Rheumatology Criteria for RA or Rheumatoid Arthritis Classification Criteria [17,18]. A total of 413 healthy individuals were recruited from Sagamihara National Hospital, Teikyo University, Kanazawa University, or by the Pharma SNP Consortium (Tokyo, Japan) [19,20]. Patients and healthy individuals were native Japanese living in Japan. The estimated glomerular filtration rate (eGFR) was calculated using the equation of the Japanese Society of Nephrology [21]: eGFR (mL/min/1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female). RA patients with an eGFR lower than 60 (mL/min/1.73 m2) were defined as CKD(+)RA. RA patients with an eGFR equal to or higher than 60 (mL/min/1.73 m2) were defined as CKD(−)RA.
The protocol of this study was reviewed and approved by the Research Ethics Committee of Tokyo National Hospital (190010) and Sagamihara National Hospital. Written informed consent was obtained from each participant. This study was conducted in accordance with the principles expressed in the Declaration of Helsinki.
2.2. Genotyping
The genotyping of HLA-DRB1 was performed by polymerase chain reaction with reverse sequence-specific oligonucleotide probes (WAKFlow HLA typing kits, Wakunaga, Akitakata, Japan), using the Bio-Plex system (Bio-Rad, Hercules, CA, USA). Genotyping results for some RA patients and healthy controls were reported in previous studies [13,22].
2.3. Statistical Analysis
Differences in the clinical characteristics between RA patients were analyzed by Fisher’s exact test using 2 × 2 contingency tables or the Student’s t-test. Associations of DRB1 allele carrier frequencies or genotype frequencies, or amino acid residue carrier frequencies were tested by Fisher’s exact test using 2 × 2 contingency tables. Deviation from Hardy–Weinberg equilibrium was detected by Genepop (http://genepop.curtin.edu.au/ (accessed on 13 January 2023)) [23]. Multiple logistic regression analysis under the additive model was used to examine whether DRB1 alleles were independently associated with CKD in RA patients. The corrected p (pc) values were generated by multiplying the p-values by the number of alleles or amino acid residues tested. Principal component analysis (PCA) was performed to discriminate between CKD(+)RA, CKD(−)RA, and healthy control groups based on the allele frequencies of DRB1.
3. Results
3.1. Clinical Characteristics of RA Patients
The clinical characteristics of RA patients are shown in Table 1. Of all RA patients, 351 are defined as CKD(+)RA and 959 as CKD(−)RA. CKD(+)RA patients are older than CKD(−)RA patients. The Steinbrocker class [24], body mass index, erythrocyte sedimentation rate, and disease activity score 28 (DAS28) are higher in CKD(+)RA patients than in CKD(−)RA patients.
Table 1.
Characteristics of RA patients.
| CKD(+)RA | CKD(−)RA | ||
|---|---|---|---|
| Number of patients | 351 | 959 | |
| Age, years (SD) | 73.5 (8.6) | 64.2 (12.4) | * 2.99 × 10−47 |
| Male, n (%) | 73 (20.8) | 161 (16.8) | 0.1033 |
| Disease duration, years (SD) | 17.7 (12.5) | 16.3 (11.3) | * 0.0632 |
| Steinbrocker stage III and IV, n (%) | 156 (45.3) | 418 (44.1) | 0.7043 |
| Steinbrocker class 3 and 4, n (%) | 61 (17.8) | 96 (10.1) | 0.0003 |
| Body mass index, kg/m2 (SD) | 22.3 (3.9) | 21.7 (3.5) | * 0.0171 |
| Rheumatoid factor positive, n (%) | 287 (82.0) | 766 (80.5) | 0.5782 |
| Anti-citrullinated peptide antibody positive, n (%) | 303 (86.6) | 823 (86.7) | 0.9269 |
| Serum creatinine, mg/dL (SD) | 0.96 (0.25) | 0.62 (0.12) | * 2.71 × 10−82 |
| CRP, mg/L (SD) | 4.6 (7.5) | 5.1 (11.8) | * 0.3548 |
| ESR, mm/h (SD) | 30.2 (21.0) | 26.2 (21.1) | * 0.0035 |
| DAS28 | 3.0 (1.0) | 2.8 (1.1) | * 0.0125 |
| DAS28-CRP | 2.2 (0.9) | 2.2 (0.9) | * 0.3548 |
Number or average values of each group are shown. Standard deviations or percentages are shown in parentheses. Differences were tested by Fisher’s exact test using 2 × 2 contingency tables or the Student’s t-test. * The Student’s t-test was used. RA: rheumatoid arthritis; CKD: chronic kidney disease; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; DAS28: disease activity score 28.
3.2. Association of HLA with CKD in RA Patients
DRB1 genotyping was conducted to compare allele carrier frequencies between RA patients with or without CKD (Table 2). Deviation from Hardy–Weinberg equilibrium is found in the CKD(−)RA group (p = 0.0140) but not in the CKD(+)RA group (p = 0.6059). There is an association of DRB1*13:02 with CKD in RA patients, but this does not achieve statistical significance (p = 0.0265, OR 1.70, pc = 0.7412, 95% confidence interval [CI] 1.09–2.64). The DR6 serological group is associated with CKD in RA patients (p = 0.0008, OR 1.65, 95% CI 1.24–2.20), indicating that DR6 is predisposing to CKD in RA. DRB1 genotype frequencies in the CKD(+)RA patients are compared with those of the CKD(−)RA patients to clarify the presence or absence of gene-dosage effects (Table 3). Homozygosity for DR6 does not have a higher risk for CKD compared to heterozygosity (DR6/not DR6: p = 0.0009, OR 1.66, 95%CI 1.24–2.23, DR6/DR6: p = 0.7558, OR 1.22, 95%CI 0.37–3.98), indicating the absence of the gene-dosage effect. The allele carrier frequency of DRB1 was also compared with those of healthy controls to confirm the protective role of DR6 against RA in CKD(+)RA group (Supplementary Table S1). The allele carrier frequency of DR6 is lower than that in healthy controls (p = 0.0481, OR 0.73, 95%CI 0.53–0.99), indicating that DR6 is still protective against RA in CKD(+)RA group. Thus, DR6 is associated with CKD in RA patients, although a gene-dosage effect is not observed.
Table 2.
DRB1 allele carrier frequency in RA patients with or without CKD.
| CKD(+)RA (n = 351) | CKD(−)RA (n = 959) | p | OR | pc | 95%CI | |
|---|---|---|---|---|---|---|
| DRB1*01:01 | 54 (15.4) | 136 (14.2) | 0.5955 | 1.10 | NS | (0.78–1.55) |
| DRB1*03:01 | 1 (0.3) | 0 (0.0) | 0.2679 | 8.21 | NS | (0.33–202.08) |
| DRB1*04:01 | 19 (5.4) | 75 (7.8) | 0.1478 | 0.67 | NS | (0.40–1.13) |
| DRB1*04:03 | 10 (2.8) | 28 (2.9) | 1.0000 | 0.98 | NS | (0.47–2.03) |
| DRB1*04:04 | 3 (0.9) | 5 (0.5) | 0.4483 | 1.64 | NS | (0.39–6.92) |
| DRB1*04:05 | 162 (46.2) | 487 (50.8) | 0.1513 | 0.83 | NS | (0.65–1.06) |
| DRB1*04:06 | 11 (3.1) | 45 (4.7) | 0.2799 | 0.66 | NS | (0.34–1.29) |
| DRB1*04:07 | 3 (0.9) | 4 (0.4) | 0.3934 | 2.06 | NS | (0.46–9.24) |
| DRB1*04:10 | 16 (4.6) | 39 (4.1) | 0.7557 | 1.13 | NS | (0.62–2.04) |
| DRB1*07:01 | 2 (0.6) | 5 (0.5) | 1.0000 | 1.09 | NS | (0.21–5.66) |
| DRB1*08:02 | 18 (5.1) | 29 (3.0) | 0.0917 | 1.73 | NS | (0.95–3.16) |
| DRB1*08:03 | 26 (7.4) | 81 (8.4) | 0.6487 | 0.87 | NS | (0.55–1.37) |
| DRB1*09:01 | 102 (29.1) | 252 (26.3) | 0.3258 | 1.15 | NS | (0.88–1.51) |
| DRB1*10:01 | 5 (1.4) | 18 (1.9) | 0.8123 | 0.76 | NS | (0.28–2.05) |
| DRB1*11:01 | 10 (2.8) | 30 (3.1) | 0.8584 | 0.91 | NS | (0.44–1.88) |
| DRB1*12:01 | 21 (6.0) | 70 (7.3) | 0.4625 | 0.81 | NS | (0.49–1.34) |
| DRB1*12:02 | 9 (2.6) | 27 (2.8) | 1.0000 | 0.91 | NS | (0.42–1.95) |
| DRB1*13:01 | 0 (0.0) | 2 (0.2) | 1.0000 | 0.54 | NS | (0.03–11.38) |
| DRB1*13:02 | 34 (9.7) | 57 (5.9) | 0.0265 | 1.70 | 0.7412 | (1.09–2.64) |
| DRB1*14:02 | 1 (0.3) | 2 (0.2) | 1.0000 | 1.37 | NS | (0.12–15.12) |
| DRB1*14:03 | 6 (1.7) | 21 (2.2) | 0.6672 | 0.78 | NS | (0.31–1.94) |
| DRB1*14:05 | 7 (2.0) | 18 (1.9) | 0.8237 | 1.06 | NS | (0.44–2.57) |
| DRB1*14:06 | 19 (5.4) | 30 (3.1) | 0.0691 | 1.77 | NS | (0.98–3.19) |
| DRB1*14:07 | 1 (0.3) | 1 (0.1) | 0.4642 | 2.74 | NS | (0.17–43.88) |
| DRB1*14:54 | 27 (7.7) | 46 (4.8) | 0.0559 | 1.65 | NS | (1.01–2.70) |
| DRB1*15:01 | 37 (10.5) | 116 (12.1) | 0.4967 | 0.86 | NS | (0.58–1.27) |
| DRB1*15:02 | 56 (16.0) | 163 (17.0) | 0.6769 | 0.93 | NS | (0.67–1.29) |
| DRB1*16:02 | 5 (1.4) | 14 (1.5) | 1.0000 | 0.98 | NS | (0.35–2.73) |
| DR6 (DRB1*13, *14) | 93 (26.5) | 172 (17.9) | 0.0008 | 1.65 | (1.24–2.20) |
Allele carrier frequencies are shown in parentheses (%). Associations were tested by Fisher’s exact test using 2 × 2 contingency tables. RA: rheumatoid arthritis; CKD: chronic kidney disease; OR: odds ratio; CI: confidence interval; pc: corrected p.
Table 3.
HLA-DRB1 genotype frequency in RA patients with or without CKD.
| CKD(+)RA (n = 351) | CKD(−)RA (n = 959) | p | OR | 95%CI | |
|---|---|---|---|---|---|
| DR6/not DR6 | 89 (25.4) | 163 (17.0) | 0.0009 | 1.66 | (1.24–2.23) |
| DR6/DR6 | 4 (1.1) | 9 (0.9) | 0.7558 | 1.22 | (0.37–3.98) |
Genotype frequencies are shown in parentheses (%). Associations were tested by Fisher’s exact test, using 2 × 2 contingency tables. RA: rheumatoid arthritis; CKD: chronic kidney disease; OR: odds ratio; CI: confidence interval.
3.3. PCA of the CKD(+)RA, CKD(−)RA, and Healthy Control Groups
PCA was performed to discriminate between the CKD(+)RA, CKD(−)RA, and healthy control groups based on the allele frequencies of DRB1 (Supplementary Figure S1). The results of PCA component 1 are related to the division of RA and healthy controls, although the results of PCA component 2 seem to explain the presence of CKD. Thus, CKD(+)RA group is different from CKD(−)RA or the healthy control groups.
3.4. Associations of Amino Acid Residues in the DRβ Chain of RA Patients with CKD
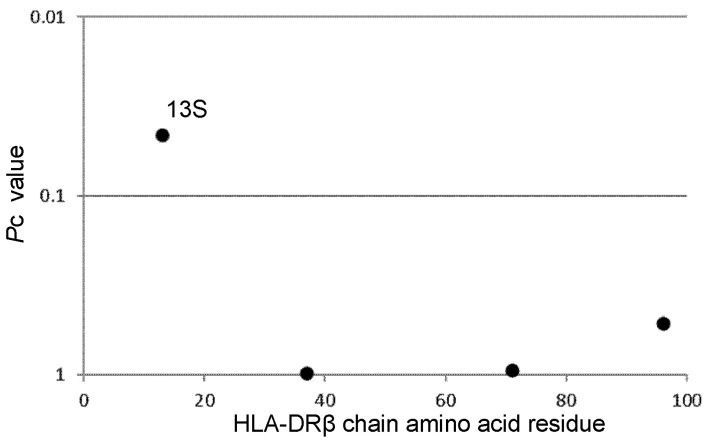
Associations of amino acid residues in the HLA-DRβ chain with CKD in RA patients were analyzed to reveal the effects of amino acid residues on the predisposition to or protection against CKD (Figure 1). Serine at position 13 (13S, p = 0.0013, OR 1.59, pc = 0.0456, 95% CI 1.21–2.11) is associated with CKD in RA patients. Thus, one amino acid residue is associated with CKD, probably through the presentation of antigens.
Figure 1.
Associations of amino acid residues in the DRβ chain with CKD in RA patients. The significance of the associations was established by Fisher’s exact test, using 2 × 2 contingency tables. Corrected p (pc) values were generated by multiplying p-values by the number of analyzed amino acid residues. Predisposing associations are indicated by filled circles. RA: rheumatoid arthritis; CKD: chronic kidney disease.
3.5. Logistic Regression Analysis of DR6 and Clinical Characteristics of CKD
Because older RA patients are prone to CKD, multiple logistic regression analyses of DR6 and the clinical characteristics of patients were performed to exclude the influence of clinical characteristics on the pathogenesis of CKD (Table 4). The association of DR6 remains significant (padjusted = 0.0196, ORadjusted 1.43, 95% CI 1.06–1.94) when conditioned on the clinical characteristics, suggesting the independent association of DR6 with CKD in RA patients. Age, Steinbrocker class, and body mass index remain associated with CKD when conditioned on other factors. Thus, DR6 is independently associated with CKD in RA patients.
Table 4.
Multiple logistic regression analysis of DR6 and clinical manifestations for CKD in RA patients.
| Unconditioned | Conditioned on Other Clinical Manifestations | |||||
|---|---|---|---|---|---|---|
| Clinical Manifestations | OR | 95%CI | p | ORadjusted | 95%CI | p adjusted |
| Age | 1.10 | (1.08–1.11) | 2.95 × 10−30 | 1.10 | (1.08–1.11) | 4.68 × 10−25 |
| Male | 1.30 | (0.96–1.77) | 0.0940 | 1.10 | (0.77–1.57) | 0.5978 |
| Disease duration | 1.01 | (1.00–1.02) | 0.0517 | 0.99 | (0.98–1.01) | 0.3772 |
| Steinbrocker class | 1.32 | (1.10–1.59) | 0.0024 | 1.24 | (1.00–1.52) | 0.0475 |
| Body mass index | 1.04 | (1.01–1.08) | 0.0126 | 1.05 | (1.01–1.09) | 0.0176 |
| ESR | 1.01 | (1.00–1.01) | 0.0040 | 1.00 | (0.99–1.01) | 0.7025 |
| DR6 | 1.55 | (1.19–2.03) | 0.0013 | 1.43 | (1.06–1.94) | 0.0196 |
p, OR, 95%CI, padjusted, and ORadjusted were calculated by logistic regression analysis of RA patients. RA: rheumatoid arthritis; CKD: chronic kidney disease; OR: odds ratio; CI: confidence interval.
4. Discussion
The present study revealed the independent association of DR6 with CKD in Japanese RA patients, although DR6 is protective against RA and other autoimmune diseases [13,22,25]. Our data suggest direct or indirect roles of HLA in the development of CKD in RA. Bucillamine, one of the disease-modifying anti-rheumatic drugs, occasionally causes drug-induced membranous nephropathy in RA patients. Bucillamine-induced membranous nephropathy in RA was associated with HLA [26]. Analogically, it is possible that DR6 is associated with drug-induced membranous nephropathy in RA patients. It could be suggested that other common genetic or environmental factors than HLA might contribute to the pathogenesis of both RA and CKD in RA patients with DR6. Single nucleotide polymorphisms, rare variants, structural variants outside of the HLA region, or smoking would contribute to the pathogenesis. Otherwise, drugs used in the treatment for RA could cause CKD in RA patients, and it would influence the results of this study.
The associations of HLA with RA have also been reported by many studies [12,13,14]. The association of SE with RA was reported and DRB1*01:01, DRB1*04:01, DRB1*04:04, DRB1*04:05, DRB1*04:10, DRB1*10:01, DRB1*14:02, and DRB1*14:06 were designated as SE alleles [12]. In this study, the allele carrier frequencies of SE alleles were not increased in CKD(+)RA patients compared to CKD(−)RA patients but were increased when compared with healthy controls. The protective association of DR6 with RA was reported previously [13], and DR6 includes the DRB1*13 and DRB1*14 alleles. The allele carrier frequencies of DR6 were increased in CKD(+)RA patients compared with CKD(−)RA patients but were decreased compared with healthy controls. These data suggested that DR6 alleles were still protective against RA, though they were predisposing to CKD. Thus, the DRB1 distribution pattern in CKD(+)RA patients is different from that in CKD(−)RA patients or healthy controls.
The current study detected an association of amino acid residue 13S in the DRβ chain with CKD in RA patients. The 13S residue is encoded by DRB1*13:02, DRB1*14:06, and DRB1*14:54; thus, the association of amino acid residue 13S reflects the predisposing effects of DRB1*13:02, DRB1*14:06, and DRB1*14:54 alleles for the development of CKD.
Conditioned logistic regression analyses suggested that DR6 independently contributed to the susceptibility of RA patients to CKD. Age, Steinbrocker class, and body mass index were also associated with CKD in RA patients. Age was an essential factor in the estimation of physiological renal function and is included in the equation for eGFR [21]. The increase in Steinbrocker’s class might have been a result of CKD in RA patients. Obesity found in CKD(+)RA patients might be explained by obesity-related chronic kidney disease associated with hypertension, diabetes mellitus, or the impaired production of adipokine or proinflammatory cytokines. However, other confounding factors could not be eliminated. Renal function in RA patients might also be affected by longstanding inflammation or the side effects of drugs [7,10,11]. DR6 was revealed to be a risk factor for CKD in RA patients and, therefore, might be useful for the prevention of CKD in RA patients with DR6: newly diagnosed RA patients should be genotyped, and RA patients with DR6 should be under the strict management of blood pressure or blood glucose levels. Because some drugs are excreted in the urine, the selection of therapeutic strategies for CKD(+)RA is tightly restricted. This prevention strategy for CKD in RA patients might help for better controls for RA.
The present study had some limitations. The sample size of this study was modest and only included those in the Japanese population. The distribution patterns of DRB1 alleles are different in other European or Asian ethnic populations. Although an association of DR6 was found in this study, other culprit genes in linkage disequilibrium with DRB1 loci might be involved in the pathogenesis of CKD. The associations of other HLA loci with CKD in RA patients should be investigated. Larger multiethnic studies of the total HLA region should be performed to confirm the associations of DR6 with CKD in RA patients. Data related to albuminuria were not available, and this might affect the diagnosis of CKD in RA patients and the results obtained in the present study. In conclusion, the present study revealed the independent association of DR6 with CKD in Japanese RA patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14071470/s1, Figure S1: Results of principal component analyses of CKD(+)RA, CKD(−)RA, and healthy control groups based on allele frequencies of DRB1; Table S1: DRB1 allele carrier frequency in RA patients with CKD and controls.
Author Contributions
H.F. and S.T. conceived and designed the experiments. T.H., S.O. and H.F. performed the experiments. T.H. and H.F. analyzed the data. H.F., K.S., A.H., A.K., T.M., N.F. and S.T. contributed reagents/materials/analysis tools, and T.H. and H.F. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The protocol of this study was reviewed and approved by the Research Ethics Committee of the Tokyo National Hospital (190010) and Sagamihara National Hospital.
Informed Consent Statement
Written informed consent was obtained from each participant. This study was conducted in accordance with the principles expressed in the Declaration of Helsinki.
Data Availability Statement
Data supporting the findings of this study are presented in the paper and the supplementary file. Other data are available from the authors upon reasonable request. However, the clinical information and genotype data of each participant are not available under the conditions of informed consent mandated by the Act on the Protection of Personal Information.
Conflicts of Interest
H.F. has the following conflicts, and the following funders are supported wholly or in part by the indicated pharmaceutical companies. The Japan Research Foundation for Clinical Pharmacology is run by Daiichi Sankyo, the Takeda Science Foundation is supported by an endowment from Takeda Pharmaceutical Company, and the Nakatomi Foundation was established by Hisamitsu Pharmaceutical Co., Inc. The Daiwa Securities Health Foundation was established by Daiwa Securities Group, Inc., and Mitsui Sumitomo Insurance Welfare Foundation was established by Mitsui Sumitomo Insurance Co., Ltd. H.F. was supported by research grants from Bristol-Myers Squibb Co. H.F. received honoraria from Ajinomoto Co., Inc., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Pfizer Japan, Inc., and Takeda Pharmaceutical Company, Luminex Japan Corporation, Ltd., and Ayumi Pharmaceutical Corporation. T.M. was supported by research grants from AbbVie Japan Co., Ltd., Asahi Kasei Pharma, Astellas Pharma, Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Pfizer, Inc., Mitsubishi-Tanabe Pharma Co., and Taisho Pharmaceutical Co., Ltd. T.M. received honoraria from AbbVie Japan Co., Ltd., Asahi Kasei Pharma, Astellas Pharma, Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Mitsubishi-Tanabe Pharma Co., Ono Pharmaceutical, Pfizer, Inc., Takeda Pharmaceutical Co., Ltd., and UCB Japan Co., Ltd. S.T. was supported by research grants from nine pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma, Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme, Inc., Pfizer Japan, Inc., Takeda Pharmaceutical Company Limited, and Teijin Pharma Limited. S.T. received honoraria from Asahi Kasei Pharma Corporation, Astellas Pharma, Inc., AbbVie GK, Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Pfizer Japan, Inc. The other authors declare no financial or commercial conflicts of interest.
Funding Statement
This work was supported by Grants-in-Aid for Scientific Research (B, C) (26293123, 22591090, 15K09543, 18K08402) and for Young Scientists (B) (24791018) from the Japan Society for the Promotion of Science, Health and Labour Science Research Grants from the Ministry of Health, Labour, and Welfare of Japan, Grants-in-Aid of the Practical Research Project for Allergic Diseases and Immunology (Research on Allergic Diseases and Immunology) from the Japan Agency for Medical Research and Development, Grants-in-Aid for Clinical Research from National Hospital Organization, Research Grants from Daiwa Securities Health Foundation, Research Grants from Japan Research Foundation for Clinical Pharmacology, Research Grants from The Nakatomi Foundation, Research Grants from Takeda Science Foundation, Research Grants from Mitsui Sumitomo Insurance Welfare Foundation, Bristol-Myers K.K., RA Clinical Investigation Grant from Bristol-Myers Squibb Co., and research grants from the following pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsuibishi Tanabe Pharma Corporation, Merck Sharp and Dohme Inc., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited, and Teijin Pharma Limited. The funders had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Boers M., Dijkmans B.A., Breedveld F.C., Camps J.A., Chang P.C., van Brummelen P., Pauwels E.K., Cats A. Subclinical renal dysfunction in rheumatoid arthritis. Arthritis Rheum. 1990;33:95–101. doi: 10.1002/art.1780330113. [DOI] [PubMed] [Google Scholar]
- 2.Koseki Y., Terai C., Moriguchi M., Uesato M., Kamatani N. A prospective study of renal disease in patients with early rheumatoid arthritis. Ann. Rheum. Dis. 2001;60:327–331. doi: 10.1136/ard.60.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karstila K., Korpela M., Sihvonen S., Mustonen J. Prognosis of clinical renal disease and incidence of new renal findings in patients with rheumatoid arthritis: Follow-up of a population-based study. Clin. Rheumatol. 2007;26:2089–2095. doi: 10.1007/s10067-007-0625-y. [DOI] [PubMed] [Google Scholar]
- 4.Karie S., Gandjbakhch F., Janus N., Launay-Vacher V., Rozenberg S., Mai Ba C.U., Bourgeois P., Deray G. Kidney disease in RA patients: Prevalence and implication on RA-related drugs management: The MATRIX study. Rheumatology. 2008;47:350–354. doi: 10.1093/rheumatology/kem370. [DOI] [PubMed] [Google Scholar]
- 5.Hill A.J., Thomson R.J., Hunter J.A., Traynor J.P. The prevalence of chronic kidney disease in rheumatology outpatients. Scott. Med. J. 2009;54:9–12. doi: 10.1258/rsmsmj.54.2.9. [DOI] [PubMed] [Google Scholar]
- 6.Hickson L.J., Crowson C.S., Gabriel S.E., McCarthy J.T., Matteson E.L. Development of reduced kidney function in rheumatoid arthritis. Am. J. Kidney Dis. 2014;63:206–213. doi: 10.1053/j.ajkd.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saisho K., Yoshikawa N., Sugata K., Hamada H., Tohma S. Prevalence of chronic kidney disease and administration of RA-related drugs in patients with RA: The NinJa 2012 study in Japan. Mod. Rheumatol. 2016;26:331–335. doi: 10.3109/14397595.2015.1088620. [DOI] [PubMed] [Google Scholar]
- 8.Helin H.J., Korpela M.M., Mustonen J.T., Pasternack A.I. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. 1995;38:242–247. doi: 10.1002/art.1780380213. [DOI] [PubMed] [Google Scholar]
- 9.Nakano M., Ueno M., Nishi S., Shimada H., Hasegawa H., Watanabe T., Kuroda T., Sato T., Maruyama Y., Arakawa M. Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin. Nephrol. 1998;50:154–160. [PubMed] [Google Scholar]
- 10.Kochi M., Kohagura K., Shiohira Y., Iseki K., Ohya Y. Inflammation as a Risk of Developing Chronic Kidney Disease in Rheumatoid Arthritis. PLoS ONE. 2016;11:e0160225. doi: 10.1371/journal.pone.0160225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumida K., Molnar M.Z., Potukuchi P.K., Hassan F., Thomas F., Yamagata K., Kalantar-Zadeh K., Kovesdy C.P. Treatment of rheumatoid arthritis with biologic agents lowers the risk of incident chronic kidney disease. Kidney Int. 2018;93:1207–1216. doi: 10.1016/j.kint.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reveille J.D. The genetic contribution to the pathogenesis of rheumatoid arthritis. Curr. Opin. Rheumatol. 1998;10:187–200. doi: 10.1097/00002281-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Oka S., Furukawa H., Kawasaki A., Shimada K., Sugii S., Hashimoto A., Komiya A., Fukui N., Ito S., Nakamura T., et al. Protective effect of the HLA-DRB1*13:02 allele in Japanese rheumatoid arthritis patients. PLoS ONE. 2014;9:e99453. doi: 10.1371/journal.pone.0099453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa H., Oka S., Shimada K., Hashimoto A., Tohma S. Human leukocyte antigen polymorphisms and personalized medicine for rheumatoid arthritis. J. Hum. Genet. 2015;60:691–696. doi: 10.1038/jhg.2015.36. [DOI] [PubMed] [Google Scholar]
- 15.Le Pham N.M., Ong T.P., Vuong N.L., Van Tran B., Nguyen T.T.H. HLA types and their association with end-stage renal disease in Vietnamese patients: A cross-sectional study. Medicine. 2022;101:e31856. doi: 10.1097/MD.0000000000031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe M., Jervis S., Payton A., Poulton K., Worthington J., Gemmell I., Verma A. Systematic review of associations between HLA and renal function. Int. J. Immunogenet. 2022;49:46–62. doi: 10.1111/iji.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S., Healey L.A., Kaplan S.R., Liang M.H., Luthra H.S., et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd, Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 19.Kamatani N., Kawamoto M., Kitamura Y., Harigai M., Okumoto T., Sumino Y. Establishment of B-cell lines derived from 996 Japanese individuals. Tissue Cult. Res. Commun. 2004;23:71–80. [Google Scholar]
- 20.Kamitsuji S., Matsuda T., Nishimura K., Endo S., Wada C., Watanabe K., Hasegawa K., Hishigaki H., Masuda M., Kuwahara Y., et al. Japan PGx Data Science Consortium Database: SNPs and HLA genotype data from 2994 Japanese healthy individuals for pharmacogenomics studies. J. Hum. Genet. 2015;60:319–326. doi: 10.1038/jhg.2015.23. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Oka S., Furukawa H., Yasunami M., Kawasaki A., Nakamura H., Nakamura M., Komori A., Abiru S., Nagaoka S., Hashimoto S., et al. HLA-DRB1 and DQB1 alleles in Japanese type 1 autoimmune hepatitis: The predisposing role of the DR4/DR8 heterozygous genotype. PLoS ONE. 2017;12:e0187325. doi: 10.1371/journal.pone.0187325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousset F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 24.Steinbrocker O., Traeger C.H., Batterman R.C. Therapeutic criteria in rheumatoid arthritis. J. Am. Med. Assoc. 1949;140:659–662. doi: 10.1001/jama.1949.02900430001001. [DOI] [PubMed] [Google Scholar]
- 25.Oka S., Higuchi T., Furukawa H., Shimada K., Hashimoto A., Komiya A., Matsui T., Fukui N., Suematsu E., Ohno S., et al. Predisposition of HLA-DRB1*04:01/*15 heterozygous genotypes to Japanese mixed connective tissue disease. Sci. Rep. 2022;12:9916. doi: 10.1038/s41598-022-14116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furukawa H., Oka S., Shimada K., Sugii S., Hashimoto A., Komiya A., Fukui N., Miyashita T., Migita K., Suda A., et al. HLA-DRB1*08:02 is associated with bucillamine-induced proteinuria in Japanese rheumatoid arthritis patients. Biomark. Insights. 2014;9:23–28. doi: 10.4137/BMI.S13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are presented in the paper and the supplementary file. Other data are available from the authors upon reasonable request. However, the clinical information and genotype data of each participant are not available under the conditions of informed consent mandated by the Act on the Protection of Personal Information.