Abstract
The effects of inactivation of the genes encoding penicillin-binding protein 1a (PBP1a), PBP1b, and PBP2a in Streptococcus pneumoniae were examined. Insertional mutants did not exhibit detectable changes in growth rate or morphology, although a pbp1a pbp1b double-disruption mutant grew more slowly than its parent did. Attempts to generate a pbp1a pbp2a double-disruption mutant failed. The pbp2a mutants, but not the other mutants, were more sensitive to moenomycin, a transglycosylase inhibitor. These observations suggest that individually the pbp1a, pbp1b, and pbp2a genes are dispensable but that either pbp1a or pbp2a is required for growth in vitro. These results also suggest that PBP2a is a functional transglycosylase in S. pneumoniae.
High-molecular-mass penicillin-binding proteins (PBPs) are membrane-bound enzymes that possess essential transpeptidase and transglycosylase activities responsible for bacterial cell wall peptidoglycan cross-linking and elongation, respectively (10). The transpeptidase activity of PBPs is inhibited by β-lactam antibiotics (10). In Streptococcus pneumoniae, five high-molecular-mass PBPs have been detected by penicillin-binding assays (12), and the genes encoding them have been identified and sequenced (7, 13, 20, 23, 24). The role of these proteins in resistance to β-lactam antibiotics has been studied extensively (6, 13, 21, 24).
The high-molecular-mass PBPs have been subdivided into class A and class B, which differ in part by the sequences of the N-terminal regions (9). In S. pneumoniae, class A PBPs are represented by PBP1a, PBP1b, and PBP2a and class B PBPs are represented by PBP2x and PBP2b. The C-terminal domains of both classes appear to possess transpeptidase activity (10, 11). The functions of the N-terminal domains are less established, but for the class A high-molecular-mass PBPs evidence suggests that they possess transglycosylase activity. The N-terminal domains of the class A PBPs contain four conserved motifs that are also present in monofunctional transglycosylases (2, 9, 32), and several of the class A proteins have been shown to catalyze the polymerization of the polysaccharide backbone of peptidoglycan in vitro (16, 25, 28, 33, 34, 38).
To examine the essential nature of the class A PBPs of S. pneumoniae, we generated insertion mutations in the pbp1a, pbp1b, and pbp2a genes.
Identification and sequence analysis of the pbp1b and pbp2a genes.
Searches of our collection of random sequences of the S. pneumoniae (hex) R6 genome (1) for genes that encode the conserved motifs in the N-terminal domains of class A PBPs uncovered the genes that encode PBP1b and PBP2a (13). A comparison of the sequences with recently published sequences for these genes (13) revealed that the pbp1b genes were identical, while the pbp2a genes differed by three nucleotides (C1887T, A2110G, and A2192G), resulting in two amino acid changes (N704D and H731R).
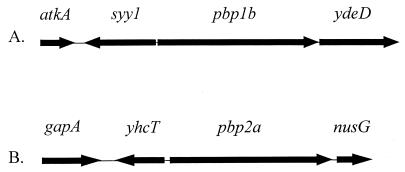
We sequenced the genomic regions surrounding pbp1b and pbp2a (Fig. 1). None of the identifiable neighboring genes are known to encode proteins related to cell wall biosynthesis. However, the genes at the 3′ end of pbp1b and the 5′ end of pbp2a have unknown function. The locations of the promoters for transcription of pbp1b and pbp2a are not known. The genes at the 5′ ends of both genes are transcribed in the opposite direction, but the genes at the 3′ ends are transcribed in the same direction. It is not known whether either gene is part of a transcription unit with these downstream genes.
FIG. 1.
Genomic organizations of the S. pneumoniae pbp1b (A) and pbp2a (B) genes. The arrows show the direction of transcription and relative sizes of the genes that surround the pbp1b and pbp2a genes on the 6- and 5.4-kb regions of the chromosome, respectively. atkA, gene encoding cation-transporting ATPase; syy1, gene encoding tyrosyl-tRNA synthetase; gapA, gene encoding glyceraldehyde-3-phosphate dehydrogenase; nusG, gene encoding a transcription antitermination protein; ydeD and yhcT, genes encoding hypothetical proteins in Bacillus subtilis (19).
Construction of insertion mutations in the pbp1a, pbp1b, and pbp2a genes of S. pneumoniae.
Gene disruptions of nanA, pbp1a, pbp1b, and pbp2a in S. pneumoniae were generated by conjugation from Escherichia coli S17-1 to insert an Eryr plasmid between the regions of the gene of interest encoding the third and fourth conserved motifs (9). The nanA gene, which encodes a surface-expressed neuraminadase (4), was used as a control. We have previously shown that nanA disruption in R6 affords no apparent phenotypic change other than loss of the enzymatic function it encodes (30). For single-crossover integrations, a fragment of approximately 500 bp from near the 5′ end of each gene was amplified by PCR and cloned into Eryr plasmid pCZA342 (26, 27), which can replicate in E. coli but not in S. pneumoniae and which can be transferred from an appropriate E. coli host into S. pneumoniae by conjugation. The specific fragments used for each gene included bp 50 to 615 of the coding region for nanA (4), bp 18 to 519 of the coding region for pbp1a (23), bp 217 to 721 of the coding region for pbp1b (13), and bp 27 to 526 of the coding region for pbp2a (13).
Conjugation into S. pneumoniae (hex) R6 and selection for erythromycin resistance (0.3 mg/ml) gave strains (with genotypes nanA::Eryr, pbp1a::Eryr, pbp1b::Eryr, and pbp2a::Eryr) in which the plasmid became integrated into the chromosome by a single homologous crossover event. In some cases, two or more plasmids became integrated in tandem. Insertion of the plasmid into the pbp1a gene was confirmed by Southern blotting analyses and by a penicillin-binding assay, which demonstrated that the PBP1a protein was missing in the pbp1a::Eryr mutants (data not shown). PCR analyses confirmed that the pbp1b::Eryr and pbp2a::Eryr mutants contained the 5.4-kb plasmid insertion because amplification of the wild-type pbp1b and pbp2a genes gave predicted fragments with sizes of 0.57 and 0.60 kb, respectively, but amplification of these genes in the insertion mutants gave predicted sizes of 5.97 and 6.0 kb, respectively. Western blotting analysis also confirmed the absence of the PBP2a protein in the pbp2a::Eryr mutant (37).
These results established that insertion mutations in the pbp1a, pbp1b, and pbp2a genes could be obtained and that none of the three genes individually was essential for the growth of S. pneumoniae in vitro. These results confirm a previous report that the pbp1a gene is dispensable for S. pneumoniae (18).
Construction of double-disruption insertion mutations.
Studies of class A high-molecular-mass PBPs of E. coli demonstrated that neither pbp1a nor pbp1b alone was essential for growth in vitro, but disruption of both genes could not be achieved in the same strain (17, 36). These observations indicated that the pbp1a and pbp1b genes encode proteins that can perform similar essential functions. To determine whether either the pbp1a or pbp1b gene of S. pneumoniae is also required for viability, we attempted to generate a mutant strain in which both genes were inactivated. Our approach was to disrupt the pbp1a gene by insertion of the Spcr gene, then to transform the resulting mutant with chromosomal DNA from mutants in which the second gene was disrupted by insertion of DNA containing the Eryr gene.
The protocol used for transformation was as follows. S. pneumoniae (hex) R6 was grown overnight in brain heart infusion (BHI) broth at 37°C. The culture was diluted 1:10,000 in BHI with 10 mM glucose and 10% heat-inactivated horse serum (22), competence-stimulating peptide I (14) was added at a final concentration of 25 ng/ml, and the mixture was incubated at 37°C for 30 min before the transforming DNA was added. The mixture was incubated for 4 to 6 h at 37°C, and dilutions were plated on chocolate II agar containing the appropriate antibiotic. Plates were incubated at 37°C, and transformants were scored after 24 h.
For disruption of the pbp1a gene by integration of the Spcr gene via a double-crossover event, we constructed a plasmid, pJT011, in which the Spcr gene in pGEM7Zf(−)Spcr (3) was flanked by internal fragments of the pbp1a gene (bp 264 to 1068 and bp 1151 to 2055) (23). This Spcr vector did not contain any regions homologous to the Eryr plasmid that had been used to disrupt pbp1a, pbp1b, and pbp2a. The bla gene in pGEM7Zf(−)Spcr was inactivated by deleting an internal AvaII fragment. We transformed S. pneumoniae (hex) R6 with the plasmid pJT011 to generate a strain in which the pbp1a gene was disrupted by the Spcr gene. Selection for spectinomycin resistance (250 μg/ml) produced a strain in which the Spcr gene replaced the region of the pbp1a gene between nucleotides 1068 and 1151 by a double-crossover homologous recombination event that was confirmed by PCR analysis (data not shown).
We then introduced the pbp1b::Eryr mutation into the pbp1a::Spcr strain by transformation with genomic DNA and selection for resistance to erythromycin and spectinomycin. Control experiments were done with the S. pneumoniae (hex) R6 parent as a recipient and with the nanA::Eryr strain as a donor. Transformants were readily obtained when the pbp1a::Spcr mutant or the S. pneumoniae (hex) R6 parent strain was used as a recipient and either the nanA::Eryr or the pbp1b::Eryr strain was used as a donor, yielding 400 to 1,200 colonies per transformation. PCR analyses confirmed that these pbp1a pbp1b mutants contained insertions in both genes (data not shown). These results demonstrated that the pbp1a and pbp1b genes could be inactivated in the same strain and therefore that they are not essential for the viability of S. pneumoniae in vitro.
We also attempted to generate a pbp1a pbp2a double-disruption mutant by using the pbp1a::Spcr strain as a recipient and the pbp2a::Eryr strain as a donor. However, only five transformants were obtained. A similar number of transformants were obtained in several repeats of this experiment. PCR analyses demonstrated that none of the five putative mutants contained an insertion in the pbp2a gene, although they still contained an insertion in the pbp1a gene. These results demonstrated that either the pbp1a or the pbp2a gene is required for the viability of S. pneumoniae in vitro. Thus, both E. coli and S. pneumoniae appear to require the presence of at least one class A PBP for viability, suggesting that perhaps the class A PBPs are functionally redundant. Furthermore, the results suggest that the PBP1a and PBP2a proteins of S. pneumoniae may be the functional homologues of the E. coli PBP1a and PBP1b enzymes.
Each of the PBP mutations described here resulted from insertion of a plasmid into the N-terminal domain, which would inactivate the C-terminal domain as well. Thus, it is not clear whether the apparent essential nature of the pbp1a or the pbp2a gene is related to the putative N-terminal transglycosylase domain, the C-terminal transpeptidase domain, or perhaps both.
The plasmid used for the insertions in pbp1a and pbp2a has been found to have polar effects on downstream genes when inserted into a multigene transcription unit (data not shown). However, we do not know whether either pbp1a or pbp2a is part of a multigene transcription unit, and therefore whether the insertions have a polar effect on downstream genes. The ability to isolate insertions in both genes suggest that the insertions are not polar on any absolutely essential genes. However, we cannot rule out the formal possibility that the essential genes implied by our observation are actually downstream genes rather than pbp1a or pbp2a.
Characterization of the pbp1a, pbp1b, pbp2a, and pbp1a pbp1b insertion mutants of S. pneumoniae.
The pbp1a, pbp1b, and pbp2a mutants obtained were characterized with respect to their growth, morphology, and sensitivity to antibiotics. Morphology was evaluated by light microscopy with cells grown in Todd-Hewitt broth and BHI blood agar. The mutants did not exhibit any detectable changes in their growth rates (Table 1) or morphology compared with their parent strain, except that the pbp1a pbp1b double mutant grew slightly more slowly than the parent strain (Table 1). We also found that the pbp2a mutants were 8- to 16-fold more sensitive to moenomycin, a transglycosylase inhibitor, although susceptibilities to other agents tested remained unchanged (for example, MICs of vancomycin and penicillin for all strains were 0.25 and 0.016 μg/ml, respectively). In contrast to the pbp2a mutants, the pbp1a, pbp1b, and pbp1a pbp1b mutants had the same sensitivity to moenomycin as the parent.
TABLE 1.
Antibiotic susceptibilities and growth rates of S. pneumoniae pbp insertional mutants
| Isolate | Moenomycin MIC (μg/ml)a | Doubling time (min)b |
|---|---|---|
| R6c | 2 | 27.0 ± 0.5 |
| R6 nanA::Eryr | 2 | 27.5 ± 0.5 |
| R6 pbp2a::Eryr-1d | 0.25 | 26.0 ± 0.5 |
| R6 pbp2a::Eryr-2d | 0.13 | 27.5 ± 0.5 |
| R6 pbp1a::Eryr | 4 | 28.0 ± 0.5 |
| R6 pbp1b::Eryr | 2 | 27.5 ± 0.5 |
| R6 pbp1a::Spcrpbp1b::Eryr | 2 | 34.0 ± 0.5 |
| R6 pbp1a::SpcrnanA::Eryr | 2 | 28.0 ± 0.5 |
MICs were determined by the broth microdilution method. Antibiotic susceptibilities were determined by broth microdilution as recommended by the National Committee for Clinical Laboratory Standards (29) by using Mueller-Hinton II medium supplemented with 5% lysed horse blood. Other agents tested included vancomycin, penicillin G, cefazolin, and cefaclor (Eli Lilly and Co.), imipenem (Merck Sharp & Dohme), ramoplanin and teicoplanin (Gruppo Lepetit S.P.A., Milan, Italy), trimethoprim-sulfamethoxazole (Hoffmann-La Roche), and enduracidin, bacitracin, fosfomycin, chloramphenicol, tetracycline, rifampin, and ciprofloxacin (Sigma Chemical Co.). Moenomycin was prepared by fermentation and isolated at Lilly Research Laboratories. Optochin susceptibility was determined by disk diffusion.
Growth rates in Todd-Hewitt broth supplemented with 0.125% yeast extract and 1% IsoVitaleX. Cultures were set up by adding 10 μl of frozen stock to 210 μl of medium in microtiter trays. Then, 100 μl of mineral oil was layered in each well, and plates were incubated at 37°C in a Molecular Devices Thermomax plate reader programmed to take optical density (absorbance at 650 nm) measurements at 15-min intervals for 12 h. Values are means of calculated minimum doubling time ± standard errors.
R6, S. pneumoniae (hex) R6.
S. pneumoniae (hex) R6 pbp2a::Eryr-1 appears to have a single copy of the Eryr plasmid integrated into pbp2a, while S. pneumoniae (hex) R6 pbp2a::Eryr-2 has two or more.
The finding that the pbp2a mutants were hypersensitive to moenomycin is consistent with the hypothesis that the PBP2a protein functions as a transglycosylase for cell wall biosynthesis. Recent studies of PBP1a of S. pneumoniae demonstrated that PBP1a may interact with moenomycin (5). Moenomycin inhibits the transglycosylase activity of high-molecular-mass PBPs (8, 15), probably by direct interaction with the enzyme (35). If the PBP2a protein performs this function and has a lower affinity for moenomycin than other transglycosylase enzymes in the cell, then the inactivation of the PBP2a protein would result in the enhanced sensitivity of the mutants to moenomycin. These results also suggest that PBP2a may be a major source of transglycosylase activity for S. pneumoniae since we would not expect the moenomycin sensitivity to change so significantly if PBP2a played a minor role.
In summary, we found that the insertion mutations in the pbp1a, pbp1b, and pbp2a genes could be readily obtained, indicating that none of these genes alone was essential for the growth of S. pneumoniae. We also found that either the pbp1a or the pbp2a gene appears to be essential for viability, suggesting that either alone is sufficient for the growth of S. pneumoniae. Insertions into the pbp2a gene increased the sensitivity of the strains to moenomycin, which suggests that PBP2a functions as a transglycosylase in S. pneumoniae. These studies are consistent with those of Paik et al. (31), who recently reported a similar mutational analysis of the class A high-molecular-mass PBPs of S. pneumoniae.
Nucleotide sequence accession numbers.
The nucleotide sequences of pbp1b and pbp2a have been submitted to GenBank under the accession no. AF10178 and AF101780, respectively.
Acknowledgments
We thank D. Morrison (University of Illinois, Chicago) for the gift of CSP-1, A. Tomasz (Rockefeller University, New York, N.Y.) for providing the S. pneumoniae (hex) R6 strain, P. Rockey and P. Rosteck for DNA sequencing, and F. H. Norris for database searches. We also thank H. L. Watson and J. I. Glass for critical review of the manuscript.
REFERENCES
- 1.Baltz R H, Norris F H, Matsushima P, DeHoff B S, Rockey P, Porter G, Burgett S, Peery R, Hoskins J, Braverman L, Jenkins I, Solenberg P, Young M, McHenney M A, Skatrud P L, Rosteck P R., Jr DNA sequence sampling of the Streptococcus pneumoniae genome to identify novel targets for antibiotic development. Microbial Drug Resistance. 1998;4:1–9. doi: 10.1089/mdr.1998.4.1. [DOI] [PubMed] [Google Scholar]
- 2.Berardino M D, Dijkstra A, Stuber D, Keck W, Gubler M. The monofunctional glycosyltransferase of Escherichia coli is a member of a new class of peptidoglycan-synthesising enzymes. FEBS Lett. 1996;392:184–188. doi: 10.1016/0014-5793(96)00809-5. [DOI] [PubMed] [Google Scholar]
- 3.Buckley N D, Lee L, LeBlanc D J. Use of a novel mobilizable vector to inactivate the scrA gene of Streptococcus sobrinus by allelic replacement. J Bacteriol. 1995;177:5028–5034. doi: 10.1128/jb.177.17.5028-5034.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camara M, Boulnois G J, Andrew P W, Mitchell T J. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect Immun. 1994;62:3688–3695. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.di Guilmi A M, Mouz N, Andrieu J P, Hoskins J, Jaskunas S R, Gagnon J, Dideberg O, Vernet T. Identification, purification, and characterization of transpeptidase and glycosyltransferase domains of Streptococcus pneumoniae penicillin-binding protein 1a. J Bacteriol. 1998;180:5652–5659. doi: 10.1128/jb.180.21.5652-5659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowson C G, Hutchison A, Brannigan J A, George R C, Hansman D, Linares J, Tomasz A, Smith J H, Spratt G B. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1989;86:8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowson C G, Hutchison A, Spratt B G. Nucleotide sequence of the penicillin-binding protein 2B gene of Streptococcus pneumoniae strain R6. Nucleic Acids Res. 1989;17:7518. doi: 10.1093/nar/17.18.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale E F, Cundliffe E, Reynolds P E, Richmond M H, Waring M J, editors. The molecular basis of antibiotic action. 2nd ed. New York, N.Y: John Wiley; 1981. [Google Scholar]
- 9.Ghuysen J-M. Molecular structures of penicillin-binding proteins and beta-lactamases. Trends Microbiol. 1994;2:372–380. doi: 10.1016/0966-842x(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 10.Ghuysen J M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 11.Ghuysen J-M, Dive G. Biochemistry of the penicilloyl serine transferases. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 103–129. [Google Scholar]
- 12.Hakenbeck R, Ellerbrok H, Briese T. Antibodies against the benzylpenicillin moiety as a probe for penicillin-binding proteins. Eur J Biochem. 1986;157:101–106. doi: 10.1111/j.1432-1033.1986.tb09644.x. [DOI] [PubMed] [Google Scholar]
- 13.Hakenbeck R, Konig A, Kern I, van der Linden M, Keck W, Billot-Klein D, Legrand R, Schoot B, Gutmann L. Acquisition of five high-Mr penicillin-binding protein variants during transfer of high-level β-lactam resistance from Streptococcus mitis to Streptococcus pneumoniae. J Bacteriol. 1998;180:1831–1840. doi: 10.1128/jb.180.7.1831-1840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber F, Nesemann F. Moenomycin: an inhibitor of cell wall synthesis. Biochem Biophys Res Commun. 1968;30:7–13. doi: 10.1016/0006-291x(68)90704-3. [DOI] [PubMed] [Google Scholar]
- 16.Ishino F, Mitsui K, Tamaki S, Matsuhashi M. Dual enzyme activities of cell wall peptidoglycan synthesis: peptidoglycan transglycosylase and penicillin-sensitive transpeptidase in purified preparations of Escherichia coli penicillin-binding protein 1A. Biochem Biophys Res Commun. 1980;97:287–293. doi: 10.1016/s0006-291x(80)80166-5. [DOI] [PubMed] [Google Scholar]
- 17.Kato J, Suzuki H, Hirota Y. Dispensability of either penicillin-binding protein-1a or -1b involved in the essential process for cell elongation in Escherichia coli. Mol Gen Genet. 1985;200:272–277. doi: 10.1007/BF00425435. [DOI] [PubMed] [Google Scholar]
- 18.Kell C M, Sharma U K, Dowson D G, Town C, Balganesh T S, Spratt B G. Deletion analysis of the essentiality of penicillin-binding proteins 1A, 2B and 2X of Streptococcus pneumoniae. FEMS Microbiol Lett. 1993;106:171–176. doi: 10.1111/j.1574-6968.1993.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 19.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 20.Laible G, Hakenbeck R, Sicard M A, Joris B, Ghuysen J-M. Nucleotide sequences of the pbpX genes encoding the penicillin-binding proteins 2x from Streptococcus pneumoniae R6 and a cefotaxime-resistant mutant, C506. Mol Microbiol. 1989;3:1337–1348. doi: 10.1111/j.1365-2958.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 21.Laible G, Spratt B G, Hakenbeck R. Inter-species recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991;5:1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc D J, Lee L N, Abu-Al-Jaibat A. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1a plasmid of oral streptococcal origin. Plasmid. 1992;28:130–145. doi: 10.1016/0147-619x(92)90044-b. [DOI] [PubMed] [Google Scholar]
- 23.Martin C, Briese T, Hakenbeck R. Nucleotide sequences of genes encoding penicillin-binding proteins from Streptococcus pneumoniae and Streptococcus oralis with high homology to Escherichia coli penicillin-binding proteins 1A and 1B. J Bacteriol. 1992;174:4517–4523. doi: 10.1128/jb.174.13.4517-4523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin C, Sibold C, Hakenbeck R. Relatedness of penicillin-binding protein 1a genes from different clones of penicillin-resistant Streptococcus pneumoniae isolated in South Africa and Spain. EMBO J. 1992;11:3831–3836. doi: 10.1002/j.1460-2075.1992.tb05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuhashi M. Utilization of lipid-linked precursors and the formation of peptidoglycan in the process of cell growth and division. Membrane enzymes involved in the final steps of peptidoglycan synthesis and the mechanism of their regulation. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Sciences; 1994. pp. 55–72. [Google Scholar]
- 26.Matsushima, P. Unpublished data.
- 27.Matsushima P, Broughton M C, Turner J R, Baltz R H. Conjugal transfer of cosmid DNA from Escherichia coli to Saccharopolyspora spinosa: effects of chromosomal insertions on macrolide A83543 production. Gene. 1994;146:39–45. doi: 10.1016/0378-1119(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa J, Tamaki S, Tomioka S, Matsuhashi M. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. J Biol Chem. 1984;259:13937–13946. [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. Approved standard M7-A2. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically. 2nd ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 30.Nicas, T. I. Unpublished data.
- 31.Paik J, Kern I, Lurz R, Hakenbeck R. Mutational analysis of the Streptococcus pneumoniae bimodular class A penicillin-binding proteins. J Bacteriol. 1999;181:3852–3856. doi: 10.1128/jb.181.12.3852-3856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spratt B G, Zhou J, Taylor M, Merrick M J. Monofunctional biosynthetic peptidoglycan transglycosylases. Mol Microbiol. 1996;19:639–647. doi: 10.1046/j.1365-2958.1996.442924.x. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, van Heijenoort Y, Tamura T, Mizoguchi J, Hirota Y, van Heijenoort J. In vitro peptidoglycan polymerization catalysed by penicillin binding protein 1b of Escherichia coli K-12. FEMS Microbiol Lett. 1980;110:245–249. doi: 10.1016/0014-5793(80)80083-4. [DOI] [PubMed] [Google Scholar]
- 34.Tamaki S, Nakajima S, Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci USA. 1977;74:5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Heijenoort Y, Leduc M, Singer H, van Heijenoort J. Effects of moenomycin on Escherichia coli. J Gen Microbiol. 1987;133:667–674. doi: 10.1099/00221287-133-3-667. [DOI] [PubMed] [Google Scholar]
- 36.Yousif S Y, Broome-Smith J K, Spratt B G. Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985;131:2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]
- 37.Zhao, G. Unpublished data.
- 38.Zijderveld C A L, Waisfisz Q, Aarsman M E G, Nanninga N. Hybrid proteins of the transglycosylase and the transpeptidase domains of PBP1b and PBP3 of Escherichia coli. J Bacteriol. 1995;177:6290–6293. doi: 10.1128/jb.177.21.6290-6293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]