Abstract
In recent years, concerns about a good-quality diet have increased. Food supplements such as prebiotics have great nutritional and health benefits. Within the diverse range of prebiotics, xylooligosaccharides (XOs) show high potential, presenting exceptional properties for the prevention of systemic disorders. XOs can be found in different natural sources; however, their production is limited. Lignocellulosic biomasses present a high potential as a source of raw material for the production of XOs, making the agro-industrial by-products the perfect candidates for production on an industrial scale. However, these biomasses require the application of physicochemical pretreatments to obtain XOs. Different pretreatment methodologies are discussed in terms of increasing the production of XOs and limiting the coproduction of toxic compounds. The advance in new technologies for XOs production could decrease their real cost (USD 25–50/kg) on an industrial scale and would increase the volume of market transactions in the prebiotic sector (USD 4.5 billion). In this sense, new patents and innovations are being strategically developed to expand the use of XOs as daily prebiotics.
Keywords: xylooligosaccharides, prebiotics, lignocellulosic biomass, agro-industrial by-products
1. Introduction
In recent years, the food industry has developed different types of new food products in order to obtain functional foods with greater benefits for health and nutrition. In this sense, the functional food market is proliferating to increase the value of ingredients added to food, particularly food supplements that prevent the occurrence of serious diseases that can cause public health problems [1]. These new products have been widely accepted by consumers, who demand products with lower fat, salt, and sugar content, supplemented with functional food ingredients (prebiotics and probiotics), which help in health care.
Prebiotics are widely used as nondigestible food supplements mainly due to their different health benefits for various systemic disorders such as gastrointestinal, cardiovascular, neurological, inflammatory, oncological, and endocrine systems [2]. There are different types of prebiotics, which are mostly indigestible fibers, among which low-molecular-weight oligosaccharides stand out.
Xylooligosaccharides (XOs) are nondigestible oligosaccharides composed mainly of xylose units with high prebiotic potential. Due to their physicochemical characteristics, XOs are highly resistant to gastrointestinal enzymes and gastric acids, which allows them to pass through the upper gastrointestinal tract without being digested, until they reach the lower intestine and are metabolized by probiotic bacteria, mainly from the Bifidobacterium and Lactobacillus genera. An XOs-supplemented diet promotes host health in multifaceted ways due to XOs’ immunomodulatory, anticancer, antimicrobial, growth-regulating, antioxidant, and other bioactive properties [2,3]. That is why XOs present a high demand in the food market. However, the natural extraction source of these prebiotics leads to low concentrations of them, which limits their industrial use [4]. For this reason, new sources for the extraction and production of XOs have been developed and studied, focusing on the high potential of lignocellulosic biomass as a new source of raw material.
Lignocellulosic biomass is composed of carbohydrates and lignin polymers, where hemicellulose represents the second most abundant polysaccharide and is mainly composed of xylan [5]. In this way, lignocellulosic biomass is emerging as a high potential source for the production of XOs. Within the vast diversity of lignocellulosic biomass, agro-industrial by-products are of strong interest to the industry, due to their high production volume, worldwide distribution, and low market price. However, due to its recalcitrant nature, it is necessary to expand physicochemical pretreatments [6]. Different types of pretreatments have been used in order to obtain greater efficiency in the production of XOs from agro-industrial by-products. Traditional pretreatment methods using acid, alkaline, and high temperatures allow the release of XOs with different polymerization degrees and toxic chemical derivatives [7]. New pretreatment methods using recyclable and/or biodegradable catalysts, which allow high production efficiency without generating toxic compounds, are being developed by different study groups.
In this review, the high potential of XOs as a food supplement is shown, describing their different health benefits. Alternative sources of extraction and production of XOs are discussed, highlighting the use of agro-industrial by-products due to their high hemicellulose content, their renewable capacity, and their low price, which would enable a higher competitiveness of XOs in the market. The different pretreatment methods applied in the production of XOs and the demand of these prebiotics in the global market scenario are also shown. Finally, a discussion about the different patents developed for the production and commercialization of XOs is presented, including how innovation is facing the new challenges in technological development to consolidate XOs as a food supplement for the daily diet.
2. The Importance of Xylooligosaccharides as Prebiotics
2.1. Prebiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”. This definition is applicable to both humans and animals [8]. In this sense, prebiotics are indigestible food supplements that play an important role in stimulating the growth of beneficial bacteria for health. These beneficial bacteria, also known as probiotics, are part of the intestinal microbiota, with the genera Lactobacillus (some former Lactobacillus probiotics are now classified in genera Ligilactobacillus, Lactiplantibacillus, and Limosilactobacillus) and Bifidobacterium being the main beneficial probiotics [9,10,11].
In addition to increasing the levels of healthy microbiota in the gut, a diet supplemented with prebiotics increases the metabolism of probiotic bacteria, producing beneficial chemical compounds such as propionate, primary butyrate, and secondary butyrate. On the other hand, pathogenic bacteria of the Enterobacteriaceae family (enteric bacteria) and of the Clostridium genus do not have the capacity to metabolize prebiotics, avoiding their proliferation and indirectly stimulating the colonization of the intestinal epithelium by probiotics [1].
Nondigestible oligosaccharides are low-molecular-weight prebiotics derived from carbohydrates like polysaccharides found in plants. These prebiotics have high potential due to their great abundance and are classified according to their chemical nature. Thus, there are a wide variety of prebiotics defined by the most abundant type of monosaccharide in the main chain, such as fructooligosaccharides, galactooligosaccharides, maltooligosaccharides, isomaltooligosaccharides, and XOs [1,12,13].
2.2. Xylooligosaccharides as Prebiotics
XOs are nondigestible oligosaccharides that present in their main chain xylose monosaccharides of 2–10 units in size linked by β-1,4-xylosidic bonds. This type of link allows XOs to resist the attack of gastric enzymes, so they pass through the upper gastrointestinal tract without being digested, until they reach the lower intestine and are metabolized by the probiotic microbiota. The prebiotic effectiveness of XOs depends on the polymerization degree of its main chain. Smaller chains with a polymerization degree of 2–4 are particularly effective in promoting the growth of specific probiotics, especially those belonging to the Bifidobacterium genus. Additionally, XOs exhibit improved prebiotic properties when there is a limited presence or an absence of monosaccharides, such as glucose and xylose [5].
A characteristic of XOs is their high stability at acidic pH, with ranges of 2.5–8, which gives them the capacity to resist gastric juices. They present a high stability at temperatures above 100 °C. On the other hand, XOs have organoleptic properties of a moderate sweetness level and do not have an unpleasant taste, which allows their supplementation in any type of food, and the xylobiose has 30% of the sweetness of sucrose [14].
Arabinoxylans (AX) are xylans with arabinose substitution at the C-2 and/or C-3 positions of the xylan backbone, and are most present in cereals like wheat, barley, maize, and rice [15]. Hydrolysis of AX leads to a mixture of unsubstituted and arabinose-substituted XOs, the so-called arabinoxylan-oligosaccharides (AXOs) [16]. Each species of probiotic has specific preferences between XOs and AXOs assimilation. When in the colon, AXO and XO fragments are further degraded to xylose and arabinose by extracellular and/or intracellular arabinofuranosidases and xylosidases produced by specialized bacteria, including Bifidobacterium species [16]. AXO, with its arabinose side chains, has been shown to selectively stimulate the growth of bifidobacteria, whereas the effects of xylan-oligosaccharides may vary depending on their specific composition.
For example, in the human microbiota, 12 different phyla are predominant, among them Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes [17,18]. The most well-known probiotic strains, such as Bifidobacterium (Actinobacteria) and Lactobacillus (Firmicutes), have distinct systems for utilizing (A)XOs. Cross-feeding plays a crucial role in the breakdown of intricate substrates in the gut. Strains that generate arabinofuranosidases can utilize the arabinose attachments found in AXOs. In contrast, other strains can consume the unsubstituted XOs produced. Bifidobacterium adolescentis, for example, can consume both AXOs and undecorated XOs, while Lactobacillus brevis utilizes only XOs [18]. Similarly, Weissella confusa (Firmicutes), a potential probiotic, has been found to use XOs but not AXOs [16,18].
XOs prebiotics can be consumed in many forms in food and feed (Table 1). For animals, it is normally incorporated at a given percentage in the daily diet. In the case of human consumption, it can be ingested directly as a pure supplement, as in the case of capsule intake [19], or it can be incorporated in beverages (like juices), biscuits, and breakfast cereals, among other things [20,21,22,23]. The addition of XOs in those products has great acceptance according to the literature since they contribute to sensorial properties by increasing sweetness and taste intensity. Further, the heat stability of XOs makes them suitable for baked products [24].
Table 1.
Recent examples of effects of xylooligosaccharides prebiotics in different organisms.
| Organism | Prebiotic Formulation |
Main Components and Manufacturer |
Effects | Reference |
|---|---|---|---|---|
| Humans | Capsule supplement containing 2 g XOs (2.8 g of 70% XOs) | Not provided (purchased from Shandong Longlive Bio-Technology Co., Ltd., Yucheng, Shandong, China) | Modifications on gut microbiota in both healthy and pre-diabetes mellitus subjects (shifts of 4 bacterial taxa associated with the condition) | [19] |
| Humans | 2.2 or 4.8 g/d arabino-xylan-oligosaccharides (AXOs) | Not provided (purchased from Kellogg Company, Battle Creek, MI, USA) | Selectively increased fecal bifidobacterial and postprandial ferulic acid concentrations | [23] |
| Humans | Prebiotic berry juice (50 mL, XO quantity not provided) |
Not provided (purchased from AKK FormulaTM) | Improved levels of skin brightness, moisture, elasticity, spots, and brown spots—mechanisms not detailed | [20] |
| Megalobrama amblycephala (Fish) | 1.0% of XOs supplemented in the daily diet | Not provided (purchased from Yuanye Biotechnology Co., Ltd., Shanghai, China) | Improved growth performance and glycolipid metabolism (upregulating glucose transport, glycolysis, glycogenesis, pentose phosphate pathway, and fatty acids β-oxidation; downregulating gluconeogenesis and fatty acid biosynthesis) | [25] |
| Oreochromis niloticus (Fish) | 5.0% of XOs supplemented in the daily diet | Not provided (purchased from Cargill, Wayzata, MI, USA) | Enhanced production of glutathione-related proteins—antioxidation and detoxification effects | [26] |
| Broiler Chickens | 0.005% or 0.01% of XOs supplemented in the daily diet | Not provided | Enhanced production of SCFA through increased cecal fermentation; improvements in body weight |
[27] |
| Broiler Chickens | Dietary supplementation with 150 mg/kg XOs | Xylobiose, xylotriose, and xylotetraose (purchased from Zhengzhou Yicong Biotechnology Co., Ltd., Zhengzhou, China) | Increased villus height of duodenum, jejunum, and ileum, and VH/CD (villus height/crypt depth) ratio of jejunum; enhanced nitrogen metabolism and reduced fecal ammonia release | [28] |
| Pigs | Dietary supplementation with 100, 250, and 500 g/t XOs | Xylobiose, xylotriose, and xylotetraose (purchased from Shandong Longlive Biotechnology Co., Ltd., China) | Reduced pathogenic bacteria (Proteobacteria and Citrobacter) and enhanced beneficial bacteria (Firmicutes and Lactobacillus); decreased level of 1,7-heptane diamine and increased concentrations of acetic acid, straight-chain fatty acids, and total SCFAs in the intestine |
[29] |
2.3. Beneficial Properties of Xylooligosaccharides
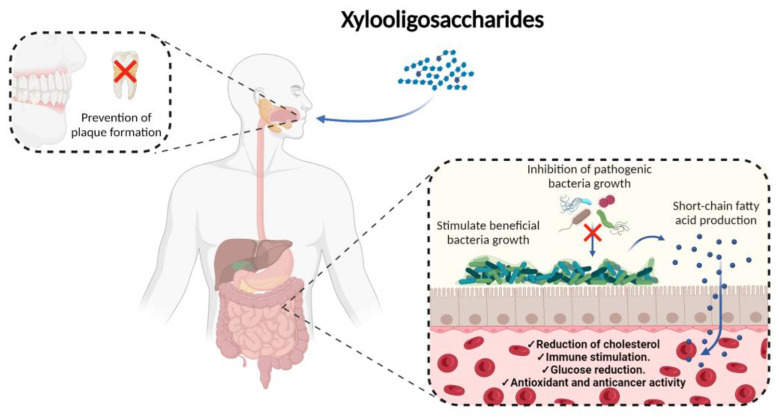
In general, XOs increase digestion and absorption of nutrients, but also present health benefits by preventing the growth of pathogenic bacteria. The inhibition of pathogenic bacteria due to the action of XOs is mediated by two mechanisms: (i) increasing the proliferation and colonization of probiotic bacteria in the intestinal epithelium, and (ii) decreasing the pH by inducing the production of organic acids, such as lactic acid and acetic acid. Thanks to these mechanisms, XOs indirectly prevent gastrointestinal infections, maintaining fecal water levels and preventing diarrhea. In addition to suppressing the activity of enteric bacteria, they decrease the production of toxic compounds such as amines [14]. Other favorable effects of XOs are to improve the proliferation of cecal epithelial cells and prevent the formation of dental plaque (Figure 1) [30,31].
Figure 1.
Xylooligosaccharides beneficial characteristics as prebiotic in humans.
On the other hand, XOs present specific indirect actions within the intestinal tract. These prebiotics are used and metabolized by probiotic bacteria, generating an increase in the production levels of small-chain fatty acids (SCFAs), with butyrate and propionate SCFAs being the most produced. High levels of SCFAs maintain the integrity of the gastrointestinal barrier by regulating cecal cell proliferation and apoptosis, and by encouraging goblet cell differentiation via Notch and Wnt/β-catenin signaling pathways [31]. It has also been reported that dietary supplementation with XOs can increase the expression of molecular chaperones and improve the ubiquitination of proteases, which would also demonstrate that XOs have a healthy protective activity in the mammalian intestine. Finally, the SCFAs induced by the metabolization of XOs induce the activation of expression genes and the synthesis of proteins in cecal cells, and are able to suppress the expression of proinflammatory cytokines through activating host GPR109a or inhibiting histone deacetylases [31,32].
These characteristics give XOs greater potential compared to other established prebiotics such as fructooligosaccharides, galactooligosaccharides, and inulin [9,33], hence making them interesting supplementary food ingredients. Further, probiotics of the Bifidobacterium genus present a greater predisposition for the consumption of pentoses compared to prebiotics composed of hexoses (fructooligosaccharides and galactooligosaccharides). In addition, it has been reported that due to the high induction of responses beneficial to health, smaller daily doses of XOs are necessary in food supplementation compared to other prebiotics, with XO dietary supplementation requiring only 1.4–2.8 g per day [34,35]. This makes XOs more economically competitive than other prebiotics, which has high potential for consumer preference as a healthy food product.
However, the natural sources for obtaining XOs such as vegetables, fruits, honey, milk, and bamboo shoots are scarce and limited [4]. In this sense, industrial-scale production seeks new sources of cheap and renewable raw materials to deal with the high demand for existing prebiotics. In recent years, lignocellulose biomasses have emerged as a source of raw material in the production of high-value-added biomolecules, including XOs prebiotics [36]. The great distribution worldwide, its great abundance, and its renewable nature make lignocellulosic biomass a natural source that is easily accessible and cheap compared to other raw materials. Within the huge set of lignocellulosic biomasses, agro-industrial residues or by-products obtain greater interest due to their high production volumes [5].
3. Agro-Industrial by-Products as Substrates for Xylooligosaccharides Production
3.1. Agro-Industrial by-Products Employed for Xylooligosaccharides Production
The cost of the production of XOs is a limiting factor, so their obtention from agro-industrial waste, including many grain by-products, has been considered a potential cost-reduction strategy as this is a low-cost, renewable, and abundant raw material [35]. Xylan, the second most abundant biopolymer in nature, constitutes most of the hemicellulose that can be degraded into XOs, using combinations of pretreatments and enzymatic hydrolysis [37]. Despite the benefits of using agro-industrial residues, their varying composition is a challenge, because depending on the chemical composition, the residues may be more or less suitable for XOs production. Residues with higher amounts of xylan and low amounts of lignin are better options. Corn cob, wheat straw, rice straw, corn stover, switchgrass, and sugarcane bagasse are some of the relevant residues for XOs production, due to their high levels of hemicellulose [35,38]. The high xylan content of corn cob is considered attractive for XOs production [39]. Xylans from agro-industrial residues are classified depending on the type of side groups and degree of substitutions. For example, arabinoxylans are generally present in sugarcane and cereals, such as wheat, rye, barley, oats, rice, corn, and sorghum, and in grasses there is a higher presence of L-arabinose [40].
3.2. Lignocellulosic Biomass Pretreatment
Agro-industrial by-products are mostly composed of cellulose, hemicellulose, and lignin structures. Cellulose and hemicellulose can be enzymatically hydrolyzed into glucose and xylose; however, cellulose is strongly associated with hemicelluloses and lignin, preventing the access of hydrolytic agents, and its crystalline structure is also an extra obstacle to hydrolysis [41]. To increase the efficiency of the use of lignocellulosic residues, it is necessary to perform a step before the enzymatic hydrolysis, called pretreatment, to extract xylan from the plant cell wall. Several pretreatment techniques have been reported in the literature, such as steam explosion, solvent extraction, and thermal pretreatment using acids or bases, organosolv, deep eutectic solvents, and hydrothermal pretreatment [6,42,43]. During pretreatment, hemicellulose can be depolymerized to produce various xylosugars, among them short-chain polysaccharides with different degrees of polymerization, which are considered attractive because of their potential use to promote the growth of intestinal bacteria and improve immunity [41]. Alkaline pretreatment has the disadvantage of partially degrading carbohydrates and causing equipment corrosion and environmental contamination. Acid pretreatment generates undesirable sugar monomers and many toxic by-products, such as furfural, hydroxymethylfurfural, and formic acid, and excessive degradation of xylan to xylose results in lower purity of the XOs. Hydrolysis with acetic acid has been used to prepare XOs because of its fast reaction and high yield [44].
Lignin and polysaccharides, such as hemicellulose and cellulose, are primarily bound together by noncovalent bonds, specifically hydrogen bonds and van der Waals forces. These bonds play a significant role in the structural integrity of plant cell walls, where lignin acts as a matrix that binds and strengthens the polysaccharide components. While noncovalent interactions are predominant, there can also be some covalent linkages between lignin and polysaccharides, such as ester bonds, which are susceptible to hydrolysis. Alkaline extraction is performed using salts at high temperatures, which leads to deacetylation of the compounds. Xylan, a type of hemicellulose, naturally presents O-acetyl groups at the hydroxyl ends of its structure, improving its solubility in water. However, during the alkaline extraction process, the acetyl groups are removed. On the other hand, extraction with water can isolate water-soluble hemicelluloses, which have high molar mass and less substitution of arabinose. In addition, this form of extraction helps in the preservation of the hemicellulose structure [1].
Acid or alkaline pretreatments can cause environmental pollution because reagents need to be discarded after the process. Alternatively, hot water pretreatment has been used with the advantage of not requiring reagents. It is based on the autoionization of water at high temperatures, producing protons that break the glycosidic bonds and generate organic acids, which can act as catalysts for the degradation of the hemicellulose and lignin fractions of the biomass [45]. Pretreatment using imidazolium brings positive results in biomass delignification, allowing the high recovery of cellulose-rich and hemicellulose-rich fractions separately. Imidazole has the advantage of low vapor pressure, high boiling point, and low toxicity, and it is recyclable and reusable [46].
Ionic liquids are solvents that are integrally composed of ions, which present organic properties to reduce the intractability of the lignin/carbohydrate complex, decreasing the recalcitrance of lignocellulosic biomass. On the other hand, deep eutectic solvents have great potential as a method to be applied in the extraction of lignocellulosic fractions, such as hemicellulose. Deep eutectic solvents generally have a component that acts as a hydrogen-bond acceptor and another component that acts as a hydrogen-bond donor, and are renewable, biodegradable, and profitable [47]. Ionic liquids and deep eutectic solvents are included within green solvents [48]. Wang et al. (2017) [49] evaluated imidazole-based ionic liquid with an anionic component (HSO4). It generated greater hydrolysis of xylan in the pretreatment of xylan-rich hardwood, hydrolyzing more than half of the xylan in the products as xylose or XOs. Morais et al. (2018) [50] used deep eutectic solvents (choline chloride and urea) to extract xylan from hardwood, demonstrating the successful extraction of xylan from Eucalyptus globulus wood.
Comparative to conventional approaches, microwave pretreatment techniques are a promising approach to increase the efficiency and speed of XOs production, providing a faster reaction rate and lower energy consumption [51]. Recently, the use of microwaves has been considered a promising heating method for the selective and controlled hydrothermal depolymerization of biomass. The energy from microwaves is absorbed by the water present in the reaction medium, directly generating heat in the substrate. This results in more efficient and faster heating compared to conventional methods. The application of microwaves during the enzymatic hydrolysis of xylan can increase the reaction rate, accelerating the breakdown of glycosidic bonds through rapid and selective heating [52]. Controlled microwave application can lead to more precise and specific enzymatic hydrolysis, preventing excessive degradation of XOs into monosaccharides. Microwave treatment causes fragmentation and swelling, leading to the degradation of lignin and hemicellulose, thereby improving pentose yield. Results have shown that microwave pretreatment is a promising method for lignocellulosic degradation of rice husks [53]. Microwave-assisted reactions significantly decrease xylose content in hydrolysates and greatly increase the yield of XOs compared to conventional heating methods [54]. Microwave irradiation treatment involves exposing lignocellulosic biomass to electromagnetic waves with wavelengths between 1 mm and 1 m and frequencies ranging from 300 to 300,000 MHz [42]. The principle of microwave heating involves rapid rotation of polar molecules, resulting in faster and more intense activation of polar species. Therefore, the use of microwave-assisted heating for reactions under hydrothermal conditions is considered a highly favorable strategy to obtain selective, nontoxic, and high-purity production of XOs from lignocellulosic biomass [55].
Microwave-assisted pretreatment generally avoids the use of solvents, auxiliary chemicals, or separating agents. Additionally, the lower chance of waste generation after the pretreatment process is recognized as an advantage of microwave-based approaches. Compared to other conventional heating approaches, microwaves reduce the process time by tenfold, resulting in lower energy consumption [56]. In an investigation of microwave-assisted acid hydrolysis of sugarcane bagasse for XOs production, response surface analysis indicated that microwave-assisted acid hydrolysis using H2SO4 at a concentration of 0.24 M for 31 min resulted in a maximum XOs yield of 290.2 mg/g. It was observed that the xylose yield increased with increasing acid concentration and residence time, and no degraded sugar products were formed during the process [57]. In a study on microwave-assisted hydrothermal depolymerization of beechwood hemicellulose aimed at producing high-purity XOs, a good balance was achieved between the net yield (81%) and the purity of XOs (96% by carbon mass) [55]. Regarding the impact of microwave-assisted deep eutectic solvent pretreatment on the production of XOs from wheat straw, the results demonstrated that this pretreatment is an efficient, fast, and promising technique for xylan extraction. However, further research is needed to develop economically viable methods for the separation and purification of the XOs extracted from the mixture [58].
In Table 2, examples of XOs produced from agro-industrial residues using different pretreatment methods are shown.
Table 2.
Composition, types of pretreatments, and yields derived from agro-industrial residues used in xylooligosaccharides production.
| Residue | Composition | Pretreatment | Yield | Reference |
|---|---|---|---|---|
| Sugarcane bagasse | Glucan 37.61% | Hydrothermal | XOs: 67.12% | [59] |
| Xylan 21.87% | ||||
| Lignin 20.60% | ||||
| Sugarcane bagasse | Cellulose 38.9% | Hydrothermal and organosolv | Conversion of 90% into XOs | [60] |
| Hemicellulose 28.2% | ||||
| Lignin 19.7% | ||||
| Sugarcane bagasse | Glucan 43.49% | Hydrothermal | XOs: 50.53% | [61] |
| Xylan 22.68% | ||||
| Lignin 20.75% | ||||
| Corn cob | Glucan 37.5% | Hydrogen peroxide-acetic acid | XOs: 58.3 g of XOs from 1 kg of corn cob | [44] |
| Xylan 32.9% | ||||
| Lignin 13.7% | ||||
| Corn cob | Glucan 30.3 ± 0.9 | Alkali and hydrothermal | Alkali: 59 mg of XOs; hydrothermal: 115 mg of XOs per gram of initial biomass | [62] |
| Xylan 25.8 ± 0.4 | ||||
| Lignin 20 ± 1 | ||||
| Corn cob | Glucan 33.1% | Gluconic acid | 180 g of XOs per 1 kg of corn cob | [63] |
| Xylan 32.8% | ||||
| Lignin 19.0% | ||||
| Wheat straw | Glucose 41.17 ± 2.04 | Hydrothermal | N.C. | [64] |
| Xylose 25.73 ± 1.28 | ||||
| Lignin 20.13 ± 1.01 | ||||
| Poplar | Glucan 44.0% | Sodium chlorite and acetic acid | 4.0 g of XOs and 6.6 g of xylose per 100 g of poplar | [65] |
| Xylan 18.4% | ||||
| Lignin 26.3% | ||||
| Bamboo shoot shell | Glucan 38.2 ± 1.0 | Hydrothermal | 6.6 g of XOs per 100 g of bamboo shoot shell | [66] |
| Xylan 25.7 ± 0.1 | ||||
| Lignin 25.2 ± 0.2 | ||||
| Cassava peel-based | Cellulose 26.07 ± 2.46 | Sodium hydroxide | 24.21 mg of XOs per 1 g of pretreated biomass | [67] |
| Hemicellulose 13.09 ± 0.62 | ||||
| Lignin 38.30 ± 2.24 | ||||
| Poplar sawdust | Glucan 46.9 ± 0.3 | Acetic acid | 7.2 g XOs per 100 g of poplar sawdust | [68] |
| Xylan 18.6 ± 0.3 | ||||
| Lignin 28.3 ± 0.6 | ||||
| Peach palm—inner sheath | Cellulose 34.2 ± 4% | Sodium hydroxide | XOs: 50.1% | [69] |
| Hemicellulose 19 ± 2% | ||||
| Lignin 23 ± 2% | ||||
| Peach palm—peel | Cellulose 36.0 ± 4% | Sodium hydroxide | XOs: 48.8% | [69] |
| Hemicellulose 20 ± 4% | ||||
| Lignin 20 ± 3% | ||||
| Pineapple peel waste | Cellulose 20.9 ± 0.6 | Hydrothermal | XOs: 25.7 ± 0.4 g/100 g of xylan; xylose: ~91.3% |
[70] |
| Hemicellulose 31.8 ± 1.9 | ||||
| Lignin 10.4 ± 1 | ||||
| Birch sawdust | Glucan 46.6 | Hydrothermal | XOs: 46.1% | [71] |
| Xylan 22.4 | ||||
| Lignin 23.7 | ||||
| Rice husk | Cellulose 28.6% | Microwave | Arabinoxylan: 9.01 g/100 |
[53] |
| Hemicellulose 28.6% | ||||
| Lignin 24.4% | ||||
| Sugarcane bagasse | Cellulose 43.6% | Microwave | 290.2 mg/g | [57] |
| Hemicellulose 33.5% | ||||
| Lignin 18.1% | ||||
| Wheat straw | Glucan 33.2% ± 0.4 | Microwave-assisted deep eutectic solvent | 72.7 g/kg wheat straw | [58] |
| Xylan 19.7% ± 0.1% | ||||
| Lignin 21.1% ± 0.1 |
N.C. not calculated.
3.3. Enzymatic Hydrolysis for Xylooligosaccharides Production
Enzymatic methods are commonly utilized to obtain XOs, especially due to their high yields and specificity in generating products. The advantages also include the use of milder operating conditions, resulting in less generation of polluting waste. The enzymatic method can be used either with enzymes in biological treatments or applied after physicochemical pretreatment to increase XOs production from lignocellulosic biomass (Table 3) [72,73,74,75].
The group of enzymes involved in this method is called hemicellulases, which can degrade hemicellulose into smaller polymers and sugars. Xylanases (endo-1,4-β-xylanases, EC 3.2.1.8) are one of the most representative enzymes within this group, classified as endo enzymes that catalyze the hydrolysis of the β-1,4 linked chains of xylose to produce small XOs [40,73,76,77]. Xylanases are grouped as glycoside hydrolases (GH) in the families of glycosidases 10 (GH10) and 11 (GH11) [72]. GH10 enzymes have a preference for groups at the terminal of xylan bonds, particularly favoring the reducing end, and can degrade dorsal branches with many substitutions because they have low substrate specificity. On the other hand, GH11 enzymes show a preference for internal xylan bonds and unsubstituted xylan chains, acting primarily on the xylose unit in the center of the oligosaccharide chain, hydrolyzing only xylan [78].
Some other enzymes of the hemicellulases group can be used in the production of XOs in conjunction with xylanases, aiming to increase the yield and specificity of production [79,80]. Arabinofuranosidases (EC 3.2.1.55) can act on polysaccharides containing arabinose, catalyzing the hydrolysis of the glycosidic bond of the nonreducing terminal, generating arabinoglucoxylans, arabinoxylan, and arabinans [81,82]. Feruloyl esterases hydrolyze the ester bond between the arabinose and ferulic acid substituents in xylose, while acetyl esterases hydrolyze acetyl substituents in xylose [40]. The β-xylosidases (EC 3.2.1.37) have the ability to hydrolyze xylobiose at the nonreducing end, as well as soluble XOs, releasing xylose. However, xylose is a monosaccharide that decreases the prebiotic effect of XOs, which is why β-xylosidases are not usually used in the production of XOs [83,84,85].
Table 3.
Enzymes used in xylooligosaccharides production from different agro-industrial residues.
| Residue | Enzyme | Hydrolysis Condition | Yield | Reference |
|---|---|---|---|---|
| Corn cob | Endoxylanase | 50 °C, pH 5.4, 14 h | 10.2 mg/mL | [86] |
| Nervosum grass | Endoxylanase from Trichoderma viridae | 40 °C, pH 5.11, 16.5 h | 0.11 g/g | [87] |
| Corn cob | Endoxylanase | 40 °C, 140 rpm, 7 d | 150 mg/g | [88] |
| Bean culm | 105 mg/g | |||
| Bagasse | 133 mg/g | |||
| Mahogany wood | Xylanase | 50 °C, pH 5.0, 12 h | 572.00 mg/g | [89] |
| Mango wood | 504.00 mg/g | |||
| Wheat straw | Endo-β-1,4-xylanase | 50 °C, pH 4.8, 150 rpm, 48 h | 7.10 mg/mL | [90] |
| Arecanut husk | Endo-β-1,4-xylanase | 50 °C, pH 5.0, 24 h | 350.00 mg/g | [91] |
| Rice husk | Endo-β-1,4-xylanase | 60 °C, pH 6.0, 9 h | 17.35 mg/mL | [75] |
| Maize straw | Xylanase | 50 °C, pH 5.0, 200 rpm, 7 h | 670.00 mg/g | [92] |
| Poplar | Xylanase and celullase from Aspergillus orizae | 50 °C, pH 5.0, 200 rpm | 16.9 g/kg | [93] |
| Sugarcane bagasse | α-l-arabinofuranosidase, endo-1,4-xylanase, and feruloyl esterase | 50 °C, pH 5.0, 48 h | 10.23 mg/mL | [73] |
| Coffee husk | 8.45 mg/mL | |||
| Coffee peel | Endo-β-1,4-D-xylanase | 40 °C, 24 h | 407.5 mg/g | [94] |
| Beech wood | Crude xylanase | 40 °C, pH 6, 24 h, 180 rpm | 10.1 mg/mL | [95] |
| Sugarcane bagasse | Endoxylanase (rHlxyn11 A) | 40 °C, pH 5.5, 180 rpm, 96 h | 587.30 mg/g | [96] |
| Corn cob powder | Immobilized xylanase | 40 °C, pH 5, 2 h | 126.7 mg/g | [97] |
| Milled rice straw | Immobilized xylanase | 50 °C, pH 7, 5 h | 143 mg/g | [98] |
| Milled corn cob | 152 mg/g | |||
| Birch wood | Recombinant xylanase (reBlxA) | 40 °C, pH 6.0, 24 h | 3.02 mg/mL | [99] |
| Pineapple bagasse | Recombinant xylanase | 50 °C, pH 5.2, 180 min | 2.70 mg/mL | [100] |
| 2.31 mg/mL | ||||
| Kenaf stem | Recombinant xylanase and arabinofuranosidase (Xyn2:AnabfA) | 40 °C, pH 4.0, 48 h | 351.46 mg/g | [101] |
| Corn cob | Recombinant xylanase LC9 | 40 °C, 200 rpm, 72 h | 6.91 mg/mL | [74] |
| Beech | Xylanase (XynB) and α-glucuronidase (AguA) | 80 °C, pH 7.0, 6 h | 17.29 mg/mL | [102] |
| Soybean fiber | Recombinant endo-1,4-xylanase (xynC) and α-l-arabinofuranosidase (abfB) | 50 °C, pH 4.7, 10 h | 37.25 mg/X2 | [80] |
| 288.6 mg/g X3 | ||||
| 143 mg/g X4 | ||||
| Sugarcane bagasse | Recombinant endoxylanase modified from GH10 xylanase of alkaliphilic Bacillus Halodurans | 60 °C, pH 7.0, 24 h | 4.66 g/L | [103] |
| Sugarcane bagasse | Recombinant xylanase (XynA) | 60 °C, pH 5, 180 rpm, 6 h | 33.32 g/L | [104] |
| Wheat bran | Recombinant endo-β-1,4-xylanase (Baxyl11) | 44.3 °C, pH 7.98, 12 h | 5.3 mg/mL | [105] |
| Bamboo hemicellulose (BCH) | Recombinant xylanase (HoXyn10), α-glucuronidase (AnGus67), and α-L-arabinofuranosidase (AnAxh62A) | 50 °C, 24 h | 7.08 g/L | [106] |
| Corn cob | Recombinant endoxylanases and exoxylanases (GH10 and GH11) | 50 °C, pH 6.0, 48 h. | 115 mg/g | [62] |
| Sorghum stalk | Immobilized recombinant endo-1, 4-β-D-xylanase (XynC) | 50 °C, 72 h | 40.81 mg/g | [107] |
| Sugarcane bagasse | 38.43 mg/g | |||
| Hydrothermal liquor of Eucalyptus wood chips | Immobilized recombinant xylanase (MpXyn10) | 50 °C, pH 5.0, 3 h | 0.9 mg/mL | [108] |
Several studies have demonstrated the ability of xylanases to obtain XOs from agro-industrial residues. Brienzo et al. [109] and Khat-udomkiri et al. [75] demonstrated that reaction time and enzyme concentration influenced XOs yields, regardless of whether unpurified enzyme extract or commercial enzyme was used. Decreasing the enzyme concentration resulted in decreased hydrolysis, while increasing the reaction time led to higher concentrations of xylobiose and xylose and decreased xylotriose concentration. The production of XOs can be achieved through simultaneous enzymatic production and enzymatic hydrolysis [88]. Li et al. [110] successfully produced xylotetrose (X4) and xylopentose (X5) from various substrates using the hydrolytic capacity of xylanase produced by Streptomyces rameus L2001 in submerged fermentation.
Some techniques have also been studied to increase the enzymatic efficiency of XOs production, such as immobilization and recombinant production. Li et al. [111] (2014) immobilized a recombinant xylanase from Trichoderma reesei in ethylene glycol methyl ether 5000 (MPEG5000) in an aqueous biphasic MPEG5000/sodium citrate system and observed a higher yield of XOs production from birch wood compared to the free enzyme. Additionally, Rajagopalan et al. [112] demonstrated that immobilizing endoxylanase in calcium alginate resulted in a more specific production of xylobiose and xylotriose. Furthermore, Purohit et al. [98] developed a magnetic cross-linked xylanase immobilized by using Acinetobacter pitti MASK 25 and achieved specific production of XOs (xylopentose and xylohexose) using pretreated rice straw and corn cob. The magnetic immobilized xylanase promoted over 60% of xylan conversion into XOs.
3.4. Biorefinery Concept Applied to Xylooligosaccharides Production
XOs are a high-value material, and their production from agricultural by-products is a biorefinery concept, as it aims to maximize the utility and value of biomass by its conversion into various products [9]. It is a promising strategy in the production of high-value-added compounds from the hemicellulosic fraction of biomass, promoting the sustainability of the biorefinery model in terms of circular bioeconomy [42]. Integrated lignocellulosic biomass biorefineries can develop bioprocesses to generate bioproducts such as biofuels, bioenergy, enzymes, and chemicals of commercial interest [113]. Cellulose, hemicellulose, and lignin, which are the main components of lignocellulosic biomass, can be used as feedstocks for biorefinery products. Together with XOs, it is possible to produce ethanol, butanol, acetic acid, and citric acid [114].
Some studies have reported XOs production in combination with other products. In a study by Zhang et al. [115], maleic acid was applied to the pretreatment of corn cob for the production of XOs, increasing the efficiency of enzymatic hydrolysis and resulting in a glucose yield of 87.5%. The production of xylanases and other enzymes and the application of enzyme complexes in the production of XOs through the enzymatic hydrolysis of sugarcane bagasse xylan was performed in another study [5]. It was also reported that the integrative biorefinery process for the coproduction of value-added products (gluconic acid and XOs) of a pretreatment method is a good alternative for the integral utilization of sugarcane bagasse [41].
Biorefineries help to reduce the emission of greenhouse gases and generate by-products with added value in a closed production cycle, contributing to the development of sustainability and bioeconomy. However, studies are still needed to advance new technologies in the deployment of biorefineries on a large scale [116].
4. The Market of Xylooligosaccharides Prebiotics
4.1. Current Market of Xylooligosaccharides
The global scenario and market potential of prebiotics have shown that the oligosaccharide market is increasing promptly, reaching a market size of USD 3.3 billion in 2016 and an expectancy of growth by 8.7% (compound annual growth rate—CAGR) by 2026, with profits up to USD 9.4 billion, twice the estimated market in 2020 (USD 4.5 billion) [117]. The food industry is the first consumer of the prebiotic market due to the addition in formulation, representing 82% of the market [118]. According to the lower requirement to achieve the prebiotic effect, XOs (dose given of 1.4–2.8 g/day) are considered the most competitively priced prebiotic, with a final cost of less than USD 0.1 per dose [34].
The XOs market has increased in the last two decades, since it seems more efficient in boosting and improving the gut microbiota balance and reducing proinflammatory responses than fructooligosaccharides in diet supplementations, potential features for immunity enhancement and cardiovascular and inflammatory diseases [38]. According to the Market Study Reports, the use of XOs is not exclusively in the food industry; nowadays, XOs can also be applied as an immune-stimulating agent in medicine and as an antioxidant agent, nutraceutical, and cosmetic in the pharmaceutical industry [40]. However, XOs application in food products is suitable due to their high stability, acid pH resistance, absorption, digestion, and fermentation of single-cell fatty acids [119].
The market price for XOs prebiotics can vary from USD 25 to 50/kg according to their purity, and the minimum selling price is USD 3430–7500, 4030–8970, and 4840–10,640 per metric ton for 80, 90, and 95% purity [120]. The purity of XOs depends on the presence of remaining compounds after processing, such as glucose and xylose, which affect the calorific value and sweetness. Thus, the purity may affect the prebiotic effect [24]. The processes for XOs extraction include autohydrolysis, chemical hydrolysis, enzymatic processes, and combinations among them. A simple extraction process can reach yields of 15.4 to 66% of XOs from raw materials before the purification steps [121]. The minimum daily dose of this prebiotic is about 1.4–2.8 g, making this prebiotic competitive among others due to the price per dose [34,122]. Although there is no XOs daily intake restriction, 5 g per day is the maximum recommended; an excess is not harmful but can cause gases and discomfort [123].
Since the market is still expanding, a lucrative value of USD 7.3 billion is estimated by 2023, with an increment projection of USD 130 million by 2025. In 2018, the growth rate was circa 4.1%, and by 2023, it is expected to be 5.3% [38,124]. This prebiotic alternative is still an attractive market and an added-value product since it can be easily produced from hemicellulose residues such as rice straw, wheat straw, and sugarcane [9,55].
Currently, the Asiatic market is the highest consumer, especially Japan, the leading country [125]. Meanwhile, China produces 224.9 million tons of corn annually, mainly used for XOs production. Using XOs in animal nutrition, on the other hand, is another alluring market. The benefits of XOs for improving gastrointestinal health can be found in livestock formulations for animal nutrition, including those for cattle, poultry, horses, and swine. Given this, Future Market Insights (FMI) predicted that the market would grow by 5.6% in 2022, with sales of about 6 MT (volume) [126].
4.2. Challenges of Xylooligosaccharides for the Food Industry
There are several crucial challenges for the successful integration of XOs as a functional food supplement in the food industry. These challenges are currently being addressed to enable the incorporation of XOs into innovative and healthier food products [1,127,128]. Some of the common challenges are as follows:
-
(a)
Consolidation of scientific studies: While XOs show potential health benefits as prebiotics, further research is needed to establish the specific mechanisms of action, optimal dosage, and potential interactions with other food components. It is essential to have a solid scientific foundation regarding the effects of XOs as prebiotics.
-
(b)
Production costs and availability: The cost and availability of XOs can pose a significant challenge for the food industry. The production of XOs involves enzymatic processes and may require specific raw materials, such as lignocellulosic biomass. Developing cost-effective and sustainable production methods for XOs is necessary to make them economically viable for widespread use in the food industry.
-
(c)
Organoleptic characteristics and stability: XOs, like other prebiotic ingredients, may have unique flavor and texture characteristics that could impact the sensory profile of food products. Ensuring that XOs do not negatively affect the taste, texture, or overall quality of food products is crucial for consumer acceptance. Moreover, XOs can undergo changes during processing and storage, which may affect stability and shelf life.
-
(d)
Quality standards: The food industry must comply with regulatory standards and requirements related to the use of XOs as a food ingredient. Regulatory bodies may have specific guidelines and limits on the concentration and labeling of XOs in food products. Adhering to these standards is necessary to ensure the safe and appropriate use of XOs in food applications.
-
(e)
Consumer acceptance: XOs may be relatively new to the market, and consumers may have limited knowledge about their benefits and potential uses. Educating consumers and raising awareness about XOs as a functional food ingredient is crucial for promoting acceptance and adoption. Creating the right conditions based on solid scientific evidence to support the benefits of XOs is important for building consumer confidence, obtaining regulatory approval, and facilitating the seamless integration of XOs into the food market.
5. Patents and Innovation in Technical Development
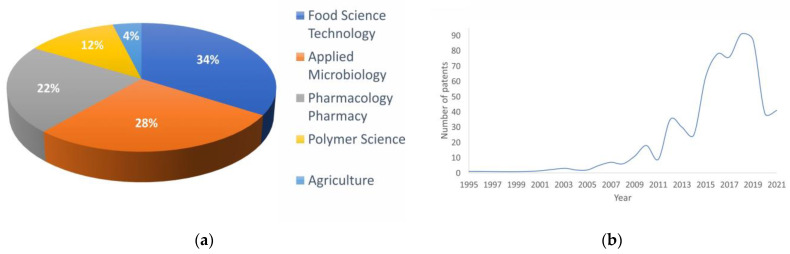
The production of XOs has been recorded since 1995; according to the Derwent database, there are currently 659 registered patents in different fields (Figure 2). Oji Paper cO, Shandong Longlive Bio Technology Co, Ltd., Yucheng, Shandong, China, and the University of Nanjing are the top three assignees. The most significant XOs producer is Shandong Longlive Biotechnology, located in China, who had the generally recognized as safe (GRAS) certification granted in 2013, as well as Prenexus Health from the USA.
Figure 2.
(a) Fields of innovation of registered patents in Derwent database; (b) number of papers registered annually from 1995 until today.
The patent granted to Towa Kasei Kogyo KK was mainly about the methods for producing this prebiotic [129]. The application and innovations only started in 2000, when Suntory Holdings Ltd. (Osaka, Japan) patented agents, among them xylobiose, to lower ammonia in blood and hyperammonemia. The top 10 patents, according to citation and relevance of the database, are from 2012 to 2018. In 2015, Beijing Dongfang Xingqi Food Ind and Tech (Beijing, China) patented a nutritional formulation helpful in improving gastrointestinal tract function; the composition of this formulation contained microcrystalline cellulose, probiotics, XOs, whey protein, and plant extract [130]. Shaoxing Shangyu Hongsheng Technology company (Shaoxing, China) patented another food formulation for enhancing immunity by adding mushroom extracts [131].
Two products were also patented in 2015; the first was by Anhui Yunjiuchang Group Co., Ltd. (Anhui, China), who released a sesame white wine with hepatoprotective properties using XOs as a prebiotic, rice husks, sesame, and plant extracts [132]. The second was a fertilizer for tobacco that included XOs and growth promoters such as potassium tripolyphosphate, citric acid, abscisic acid, and fermented extracts, which was used as a base reference to develop a complete nutrition foliar fertilizer for companies such as Liaoning Agric Sci Acad [133].
In 2014, the University of Jiangnan had a patent granted for preparing XOs and lignin using microcrystalline cellulose to harness agricultural crop straws [134]. In 2013, products and methods for biscuit preparation were also developed, patented, and well cited. The Institute of Agricultural Products, Processing, and Nuclear Agriculture of Hubei [135] developed this product for dietary supplement innovations such as gluten-free and digestive products [136]. In 2012 and 2011, XOs were considered a potential ingredient for addition to animal feed [137] and in alcohol production [138].
Recent innovations and research on XOs have focused beyond the prebiotic qualities that have attracted attention to other potential uses and characteristics of XOs. Recent research on the possible antitumoral action of XOs shows a decrease in the activity of colorectal cancer cell lines [20] and acute lymphocytic leukemia cell lines [139], as well as antiaging effects [140].
In parallel, the incorporation of XOs in biomaterials was also successfully demonstrated. According to Neves et al. [141], XOs can be used as carriers of bioactive compounds. They obtained promising results when XOs nanoparticles were formed to carry a natural blue colorant, genipin. The application as a coating agent was also successfully demonstrated in fruits such as blueberries [142].
Due to their effectiveness as a sugar replacement, moisture retention, heat resistance, and many other characteristics, XOs are still a trend in food composition. However, there are still a few desirable characteristics being studied to create the ideal XOs prebiotic, including the absence of undesirable effects like the gas produced for the metabolism of helpful bacteria, high molecular weight for persistence through the colon without being absorbed, viscosity, stability and storage, and sweetness. Those improvements are desired since the food industry accounted for approximately 49.61% of the XOs market demand in 2017, along with the pharmaceutical sector (25.39%) and food and beverage formulation (23.18%).
Another critical matter to optimize is the industrial scale to produce from an economic point of view, as well as to validate all new properties and characteristics through animal models or human clinical trials since studies of new antitumoral or cytotoxic effects are increasing.
6. Conclusions
Prebiotics have become a necessary food supplement in recent years, improving health and nutrition. XOs have a high prebiotic potential compared to other nondigestible oligosaccharides, showing various nutritional and preventive benefits against various systemic disorders. The use of agro-industrial by-products could boost the industrial production of XOs and make it economically viable. New pretreatment methodologies in the production of XOs are more friendly to the environment, reducing the production of toxic compounds. Due to their beneficial properties and characteristics for health, XOs are quickly positioning themselves in the world market, which may approach USD 10 billion in the prebiotics sector by 2026. Different patents and innovations are being developed for the sustainable increase and production of XOs that can consolidate them as a fixed food supplement in the world market of prebiotics.
Acknowledgments
This work was supported by CAPES-PROEX process number 23038.019661/2022-09, and CNPq provided financial support.
Author Contributions
K.K.V.-D.: investigation, formal analysis, visualization, writing—review and editing. L.P.d.S.V.: visualization, review and editing, funding acquisition. S.V.: visualization, writing. L.D.G.-M.: visualization, writing. M.C.M.: visualization, writing. P.B.G.d.M.: visualization, writing. V.T.S.: writing—review. C.R.S.: resources, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was partially funded by the National Council for Scientific and Technological Development (CNPq) of Brazil via grant No. 151455/2022–8.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Palaniappan A., Antony U., Naushad M. Trends in Food Science & Technology Current Status of Xylooligosaccharides: Production, Characterization, Health Benefits and Food Application. Trends Food Sci. Technol. 2021;111:506–519. doi: 10.1016/j.tifs.2021.02.047. [DOI] [Google Scholar]
- 2.Oniszczuk A., Oniszczuk T., Gancarz M., Szymańska J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules. 2021;26:1172. doi: 10.3390/molecules26041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta M., Bangotra R., Sharma S., Vaid S., Kapoor N., Dutt H.C., Bajaj B.K. Bioprocess Development for Production of Xylooligosaccharides Prebiotics from Sugarcane Bagasse with High Bioactivity Potential. Ind. Crops Prod. 2022;178:114591. doi: 10.1016/j.indcrop.2022.114591. [DOI] [Google Scholar]
- 4.Samanta A.K., Jayapal N., Jayaram C., Roy S., Kolte A.P., Senani S., Sridhar M. Xylooligosaccharides as Prebiotics from Agricultural By-Products: Production and Applications. Bioact. Carbohydr. Diet. Fibre. 2015;5:62–71. doi: 10.1016/j.bcdf.2014.12.003. [DOI] [Google Scholar]
- 5.Valladares-Diestra K.K., Porto de Souza Vandenberghe L., Soccol C.R. A Biorefinery Approach for Enzymatic Complex Production for the Synthesis of Xylooligosaccharides from Sugarcane Bagasse. Bioresour. Technol. 2021;333:125174. doi: 10.1016/j.biortech.2021.125174. [DOI] [PubMed] [Google Scholar]
- 6.Valladares-Diestra K.K., Porto de Souza Vandenberghe L., Zevallos Torres L.A., Zandoná Filho A., Lorenci Woiciechowski A., Ricardo Soccol C. Citric Acid Assisted Hydrothermal Pretreatment for the Extraction of Pectin and Xylooligosaccharides Production from Cocoa Pod Husks. Bioresour. Technol. 2022;343:126074. doi: 10.1016/j.biortech.2021.126074. [DOI] [PubMed] [Google Scholar]
- 7.Lorenci Woiciechowski A., Dalmas Neto C.J., Porto de Souza Vandenberghe L., de Carvalho Neto D.P., Novak Sydney A.C., Letti L.A.J., Karp S.G., Zevallos Torres L.A., Soccol C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020;304:122848. doi: 10.1016/j.biortech.2020.122848. [DOI] [PubMed] [Google Scholar]
- 8.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 9.Mhetras N., Mapre V., Gokhale D. Xylooligosaccharides (XOS) as Emerging Prebiotics: Its Production from Lignocellulosic Material. Adv. Microbiol. 2019;9:14–20. doi: 10.4236/aim.2019.91002. [DOI] [Google Scholar]
- 10.Holscher H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng J., Wittouck S., Salvetti E., Franz C.M.A.P., Harris H.M.B., Mattarelli P., O’Toole P.W., Pot B., Vandamme P., Walter J., et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 12.Sangwan V., Tomar S.K., Singh R.R.B., Singh A.K., Ali B. Galactooligosaccharides: Novel Components of Designer Foods. J. Food Sci. 2011;76:R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- 13.Meyer T.S.M., Miguel A.S.M., Fernández D.E.R., Ortiz G.M.D. Food Production and Industry. InTech Open; London, UK: 2015. Biotechnological Production of Oligosaccharides—Applications in the Food Industry. [Google Scholar]
- 14.Vázquez M.J., Alonso J.L., Domínguez H., Parajó J.C. Xylooligosaccharides: Manufacture and Applications. Trends Food Sci. Technol. 2000;11:387–393. doi: 10.1016/S0924-2244(01)00031-0. [DOI] [Google Scholar]
- 15.Mendis M., Martens E.C., Simsek S. How Fine Structural Differences of Xylooligosaccharides and Arabinoxylooligosaccharides Regulate Differential Growth of Bacteroides Species. J. Agric. Food Chem. 2018;66:8398–8405. doi: 10.1021/acs.jafc.8b01263. [DOI] [PubMed] [Google Scholar]
- 16.Patel A., Falck P., Shah N., Immerzeel P., Adlercreutz P., Stålbrand H., Prajapati J.B., Holst O., Nordberg Karlsson E. Evidence for Xylooligosaccharide Utilization in Weissella Strains Isolated from Indian Fermented Foods and Vegetables. FEMS Microbiol. Lett. 2013;346:20–28. doi: 10.1111/1574-6968.12191. [DOI] [PubMed] [Google Scholar]
- 17.Flint H.J., Scott K.P., Louis P., Duncan S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 18.Nordberg Karlsson E., Schmitz E., Linares-Pastén J.A., Adlercreutz P. Endo-Xylanases as Tools for Production of Substituted Xylooligosaccharides with Prebiotic Properties. Appl. Microbiol. Biotechnol. 2018;102:9081–9088. doi: 10.1007/s00253-018-9343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Summanen P., Henning S., Hsu M., Lam H.M., Huang J., Tseng C.-H., Dowd S., Finegold S., Heber D., et al. Xylooligosaccharide Supplementation Alters Gut Bacteria in Both Healthy and Prediabetic Adults: A Pilot Study. Front. Physiol. 2015;6:216. doi: 10.3389/fphys.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang H.-C., Liang C.-H., Lin Y.-J., Lin Y.-H., Lin Y.-H., Kuan C.-M. Investigation of the Synergistic Effect of Berry Juice and Xylooligosaccharides on Skin Health: A Clinical Evaluation. J. Food Nutr. Res. 2020;8:268–272. doi: 10.12691/jfnr-8-6-4. [DOI] [Google Scholar]
- 21.Silva E.K., Arruda H.S., Pastore G.M., Meireles M.A.A., Saldaña M.D.A. Xylooligosaccharides Chemical Stability after High-Intensity Ultrasound Processing of Prebiotic Orange Juice. Ultrason. Sonochem. 2020;63:104942. doi: 10.1016/j.ultsonch.2019.104942. [DOI] [PubMed] [Google Scholar]
- 22.Juhász R., Penksza P., Sipos L. Effect of Xylo-oligosaccharides (XOS) Addition on Technological and Sensory Attributes of Cookies. Food Sci. Nutr. 2020;8:5452–5460. doi: 10.1002/fsn3.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki K.C., Gibson G.R., Dickmann R.S., Kendall C.W.C., Chen C.-Y.O., Costabile A., Comelli E.M., McKay D.L., Almeida N.G., Jenkins D., et al. Digestive and Physiologic Effects of a Wheat Bran Extract, Arabino-Xylan-Oligosaccharide, in Breakfast Cereal. Nutrition. 2012;28:1115–1121. doi: 10.1016/j.nut.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Yan F., Tian S., Du K., Xue X., Gao P., Chen Z. Preparation and Nutritional Properties of Xylooligosaccharide from Agricultural and Forestry Byproducts: A Comprehensive Review. Front. Nutr. 2022;9:977548. doi: 10.3389/fnut.2022.977548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W.-L., Ge Y.-P., Sun M., He C.-F., Zhang L., Liu W.-B., Li H.-X., Li X.-F. Insights into the Correlations between Prebiotics and Carbohydrate Metabolism in Fish: Administration of Xylooligosaccharides in Megalobrama Amblycephala Offered a Carbohydrate-Enriched Diet. Aquaculture. 2022;561:738684. doi: 10.1016/j.aquaculture.2022.738684. [DOI] [Google Scholar]
- 26.Xu W., Lutz C.G., Taylor C.M., Ortega M.C. Improvement of Fish Growth and Metabolism by Oligosaccharide Prebiotic Supplement. Aquac. Nutr. 2022;2022:5715649. doi: 10.1155/2022/5715649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A.K., Mishra B., Bedford M.R., Jha R. Effects of Supplemental Xylanase and Xylooligosaccharides on Production Performance and Gut Health Variables of Broiler Chickens. J. Anim. Sci. Biotechnol. 2021;12:98. doi: 10.1186/s40104-021-00617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Wu X., Ma W., Chen W., Zhao F. Effects of Dietary Xylooligosaccharides Supplementation on the Intestinal Morphology, Nitrogen Metabolism, Faecal Ammonia Release, Antioxidant Capacity, and Immune Organ Indices of Broilers. Ital. J. Anim. Sci. 2022;21:1352–1361. doi: 10.1080/1828051X.2022.2113747. [DOI] [Google Scholar]
- 29.Pan J., Yin J., Zhang K., Xie P., Ding H., Huang X., Blachier F., Kong X. Dietary Xylo-Oligosaccharide Supplementation Alters Gut Microbial Composition and Activity in Pigs According to Age and Dose. AMB Express. 2019;9:134. doi: 10.1186/s13568-019-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valladares-Diestra K.K., de Souza Vandenberghe L.P., de Carvalho Neto D.P., Goyzueta-Mamani L.D., Soccol C.R. Microbial Enzymes in Production of Functional Foods and Nutraceuticals. CRC Press; Boca Raton, FL, USA: 2022. Microbial Enzymes for Production of Fructooligosaccharides; pp. 153–172. [Google Scholar]
- 31.Tang S., Chen Y., Deng F., Yan X., Zhong R., Meng Q., Liu L., Zhao Y., Zhang S., Chen L., et al. Xylooligosaccharide-Mediated Gut Microbiota Enhances Gut Barrier and Modulates Gut Immunity Associated with Alterations of Biological Processes in a Pig Model. Carbohydr. Polym. 2022;294:119776. doi: 10.1016/j.carbpol.2022.119776. [DOI] [PubMed] [Google Scholar]
- 32.Taggart A.K.P., Kero J., Gan X., Cai T.-Q., Cheng K., Ippolito M., Ren N., Kaplan R., Wu K., Wu T.-J., et al. (D)-β-Hydroxybutyrate Inhibits Adipocyte Lipolysis via the Nicotinic Acid Receptor PUMA-G. J. Biol. Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 33.Mano M.C.R., Neri-Numa I.A., Da Silva J.B., Paulino B.N., Pessoa M.G., Pastore G.M. Oligosaccharide Biotechnology: An Approach of Prebiotic Revolution on the Industry. Appl. Microbiol. Biotechnol. 2018;102:17–37. doi: 10.1007/s00253-017-8564-2. [DOI] [PubMed] [Google Scholar]
- 34.Finegold S.M., Li Z., Summanen P.H., Downes J., Thames G., Corbett K., Dowd S., Krak M., Heber D. Xylooligosaccharide Increases Bifidobacteria but Not Lactobacilli in Human Gut Microbiota. Food Funct. 2014;5:436–445. doi: 10.1039/c3fo60348b. [DOI] [PubMed] [Google Scholar]
- 35.Amorim C., Silvério S.C., Prather K.L.J., Rodrigues L.R. From Lignocellulosic Residues to Market: Production and Commercial Potential of Xylooligosaccharides. Biotechnol. Adv. 2019;37:107397. doi: 10.1016/j.biotechadv.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Vandenberghe L.P.S., Valladares-Diestra K.K., Bittencourt G.A., Zevallos Torres L.A., Vieira S., Karp S.G., Sydney E.B., de Carvalho J.C., Thomaz Soccol V., Soccol C.R. Beyond Sugar and Ethanol: The Future of Sugarcane Biorefineries in Brazil. Renew. Sustain. Energy Rev. 2022;167:112721. doi: 10.1016/j.rser.2022.112721. [DOI] [Google Scholar]
- 37.de Mello Capetti C.C., Vacilotto M.M., Dabul A.N.G., Sepulchro A.G.V., Pellegrini V.O.A., Polikarpov I. Recent Advances in the Enzymatic Production and Applications of Xylooligosaccharides. World J. Microbiol. Biotechnol. 2021;37:169. doi: 10.1007/s11274-021-03139-7. [DOI] [PubMed] [Google Scholar]
- 38.Santibáñez L., Henríquez C., Corro-Tejeda R., Bernal S., Armijo B., Salazar O. Xylooligosaccharides from Lignocellulosic Biomass: A Comprehensive Review. Carbohydr. Polym. 2021;251:117118. doi: 10.1016/j.carbpol.2020.117118. [DOI] [PubMed] [Google Scholar]
- 39.Wu B., Yu Q., Chang S., Monteiro M., Gao Z., He B., Schenk G. Expansin assisted bio-affinity immobilization of endoxylanase from Bacillus subtilis onto corncob residue: Characterization and efficient production of xylooligosaccharides. Food Chem. 2019;282:101–108. doi: 10.1016/j.foodchem.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Marim A.V.C., Gabardo S. Xylooligosaccharides: Prebiotic Potential from Agro-Industrial Residue, Production Strategies and Prospects. Biocatal. Agric. Biotechnol. 2021;37:102190. doi: 10.1016/j.bcab.2021.102190. [DOI] [Google Scholar]
- 41.Zhou X., Xu Y. Integrative Process for Sugarcane Bagasse Biorefinery to Co-Produce Xylooligosaccharides and Gluconic Acid. Bioresour. Technol. 2019;282:81–87. doi: 10.1016/j.biortech.2019.02.129. [DOI] [PubMed] [Google Scholar]
- 42.Pinales-Márquez C.D., Rodríguez-Jasso R.M., Araújo R.G., Loredo-Treviño A., Nabarlatz D., Gullón B., Ruiz H.A. Circular Bioeconomy and Integrated Biorefinery in the Production of Xylooligosaccharides from Lignocellulosic Biomass: A Review. Ind. Crops Prod. 2021;162:113274. doi: 10.1016/j.indcrop.2021.113274. [DOI] [Google Scholar]
- 43.Gong W., Zhang C., He J., Gao Y., Li Y., Zhu M., Wen J. A Synergistic Hydrothermal-Deep Eutectic Solvents (DES) Pretreatment for Acquiring Xylooligosaccharides and Lignin Nanoparticles from Eucommia Ulmoides Wood. Int. J. Biol. Macromol. 2022;209:188–197. doi: 10.1016/j.ijbiomac.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Hao X., Xu F., Zhang J. Effect of Pretreatments on Production of Xylooligosaccharides and Monosaccharides from Corncob by a Two-Step Hydrolysis. Carbohydr. Polym. 2022;285:119217. doi: 10.1016/j.carbpol.2022.119217. [DOI] [PubMed] [Google Scholar]
- 45.Fang L., Su Y., Wang P., Lai C., Huang C., Ling Z. Bioresource Technology Co-Production of Xylooligosaccharides and Glucose from Birch Sawdust by Hot Water Pretreatment and Enzymatic Hydrolysis. Bioresour. Technol. 2022;348:126795. doi: 10.1016/j.biortech.2022.126795. [DOI] [PubMed] [Google Scholar]
- 46.Valladares-Diestra K.K., Porto de Souza Vandenberghe L., Zevallos Torres L.A., Nishida V.S., Zandoná Filho A., Woiciechowski A.L., Soccol C.R. Imidazole Green Solvent Pre-Treatment as a Strategy for Second-Generation Bioethanol Production from Sugarcane Bagasse. Chem. Eng. J. 2021;420:127708. doi: 10.1016/j.cej.2020.127708. [DOI] [Google Scholar]
- 47.Loow Y.L., Wu T.Y., Yang G.H., Ang L.Y., New E.K., Siow L.F., Jahim J.M., Mohammad A.W., Teoh W.H. Deep Eutectic Solvent and Inorganic Salt Pretreatment of Lignocellulosic Biomass for Improving Xylose Recovery. Bioresour. Technol. 2018;249:818–825. doi: 10.1016/j.biortech.2017.07.165. [DOI] [PubMed] [Google Scholar]
- 48.Fu G.-Q., Hu Y.-J., Bian J., Li M.-F., Peng F., Sun R.-C. Isolation, Purification, and Potential Applications of Xylan. In: Fang Z., Smith R.L. Jr., Tian X.-F., editors. Production of Materials from Sustainable Biomass Resources. Springer Nature; Singapore: 2019. pp. 3–35. [Google Scholar]
- 49.Wang Z., Gräsvik J., Jönsson L.J., Winestrand S. Comparison of [HSO4]−, [Cl]− and [MeCO2]− as Anions in Pretreatment of Aspen and Spruce with Imidazolium-Based Ionic Liquids. BMC Biotechnol. 2017;17:82. doi: 10.1186/s12896-017-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morais E.S., Mendonça P.V., Coelho J.F.J., Freire M.G., Freire C.S.R., Coutinho J.A.P., Silvestre A.J.D. Deep Eutectic Solvent Aqueous Solutions as Efficient Media for the Solubilization of Hardwood Xylans. ChemSusChem. 2018;11:753–762. doi: 10.1002/cssc.201702007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Y., Tao Y., Meng Q., Qu J., Ma S., Han S., Zhang Y. Microwave-Combined Advanced Oxidation for Organic Pollutants in the Environmental Remediation: An Overview of Influence, Mechanism, and Prospective. Chem. Eng. J. 2022;441:135924. doi: 10.1016/j.cej.2022.135924. [DOI] [Google Scholar]
- 52.Aguilar-Reynosa A., Romaní A., Rodríguez-Jasso R.M., Aguilar C.N., Garrote G., Ruiz H.A. Comparison of Microwave and Conduction-Convection Heating Autohydrolysis Pretreatment for Bioethanol Production. Bioresour. Technol. 2017;243:273–283. doi: 10.1016/j.biortech.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 53.Klangpetch W., Pattarapisitporn A., Phongthai S., Utama-ang N., Laokuldilok T., Tangjaidee P., Wirjantoro T.I., Jaichakan P. Microwave-Assisted Enzymatic Hydrolysis to Produce Xylooligosaccharides from Rice Husk Alkali-Soluble Arabinoxylan. Sci. Rep. 2022;12:11. doi: 10.1038/s41598-021-03360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mobarec H., Villagomez R., Nordberg Karlsson E., Linares-Pastén J.A. Microwave-Assisted Xylanase Reaction: Impact in the Production of Prebiotic Xylooligosaccharides. RSC Adv. 2021;11:11882–11888. doi: 10.1039/D1RA00449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remón J., Li T., Chuck C.J., Matharu A.S., Clark J.H. Toward Renewable-Based, Food-Applicable Prebiotics from Biomass: A One-Step, Additive-Free, Microwave-Assisted Hydrothermal Process for the Production of High Purity Xylo-Oligosaccharides from Beech Wood Hemicellulose. ACS Sustain. Chem. Eng. 2019;7:16160–16172. doi: 10.1021/acssuschemeng.9b03096. [DOI] [Google Scholar]
- 56.Anoopkumar A.N., Reshmy R., Aneesh E.M., Madhavan A., Kuriakose L.L., Awasthi M.K., Pandey A., Binod P., Sindhu R. Progress and Challenges of Microwave-Assisted Pretreatment of Lignocellulosic Biomass from Circular Bioeconomy Perspectives. Bioresour. Technol. 2023;369:128459. doi: 10.1016/j.biortech.2022.128459. [DOI] [PubMed] [Google Scholar]
- 57.Bian J., Peng P., Peng F., Xiao X., Xu F., Sun R.C. Microwave-Assisted Acid Hydrolysis to Produce Xylooligosaccharides from Sugarcane Bagasse Hemicelluloses. Food Chem. 2014;156:7–13. doi: 10.1016/j.foodchem.2014.01.112. [DOI] [PubMed] [Google Scholar]
- 58.Isci A., Thieme N., Lamp A., Zverlov V., Kaltschmitt M. Production of Xylo-Oligosaccharides from Wheat Straw Using Microwave Assisted Deep Eutectic Solvent Pretreatment. Ind. Crops Prod. 2021;164:113393. doi: 10.1016/j.indcrop.2021.113393. [DOI] [Google Scholar]
- 59.Zhang X., Zhang W., Lei F., Yang S., Jiang J. Coproduction of Xylooligosaccharides and Fermentable Sugars from Sugarcane Bagasse by Seawater Hydrothermal Pretreatment. Bioresour. Technol. 2020;309:123385. doi: 10.1016/j.biortech.2020.123385. [DOI] [PubMed] [Google Scholar]
- 60.Milessi T.S., Corradini F.A.S., Kopp W., Giordano R.C., Giordano R.L.C. Xylooligosaccharides Production Chain in Sugarcane Biorefineries: From the Selection of Pretreatment Conditions to the Evaluation of Nutritional Properties. Ind. Crops Prod. 2021;172:114056. doi: 10.1016/j.indcrop.2021.114056. [DOI] [Google Scholar]
- 61.Zhang W., Zhang X., Lei F., Jiang J. Co-Production Bioethanol and Xylooligosaccharides from Sugarcane Bagasse via Autohydrolysis Pretreatment. Renew. Energy. 2020;162:2297–2305. doi: 10.1016/j.renene.2020.10.034. [DOI] [Google Scholar]
- 62.Cesar C., Capetti D.M., Oliveira V., Pellegrini A., Cristina M., Santo E., Abreu A., Falvo M., Aprigio A., Curvelo S., et al. Enzymatic Production of Xylooligosaccharides from Corn Cobs: Assessment of Two Different Pretreatment Strategies. Carbohydr. Polym. 2023;299:120174. doi: 10.1016/j.carbpol.2022.120174. [DOI] [PubMed] [Google Scholar]
- 63.Han J., Cao R., Zhou X., Xu Y. Bioresource Technology An Integrated Biorefinery Process for Adding Values to Corncob in Co- Production of Xylooligosaccharides and Glucose Starting from Pretreatment with Gluconic Acid. Bioresour. Technol. 2020;307:123200. doi: 10.1016/j.biortech.2020.123200. [DOI] [PubMed] [Google Scholar]
- 64.Xu J., Liu B., Wu L., Hu J., Hou H., Yang J. A Waste-Minimized Biorefinery Scenario for the Hierarchical Conversion of Agricultural Straw into Prebiotic Xylooligosaccharides, Fermentable Sugars and Lithium-Sulfur Batteries. Ind. Crops Prod. 2019;129:269–280. doi: 10.1016/j.indcrop.2018.12.002. [DOI] [Google Scholar]
- 65.Wen P., Zhang T., Xu Y., Zhang J. Industrial Crops & Products Co-Production of Xylooligosaccharides and Monosaccharides from Poplar by a Two-Step Acetic Acid and Sodium Chlorite Pretreatment. Ind. Crops Prod. 2020;152:112500. doi: 10.1016/j.indcrop.2020.112500. [DOI] [Google Scholar]
- 66.Wang Q., Su Y., Gu Y., Lai C., Ling Z., Yong Q. Valorization of Bamboo Shoot Shell Waste for the Coproduction of Fermentable Sugars and Xylooligosaccharides. Front. Bioeng. Biotechnol. 2022;10:1006925. doi: 10.3389/fbioe.2022.1006925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogoski W., Nayana G., Karina P., Moisés C., Eduardo S., Stambuk B.U., Ávila P.F., Goldbeck R., De Oliveira D., José C. Production of Cassava Peel-Based Xylooligosaccharides Using Endo-1,4-β-Xylanase from Trichoderma longibrachiatum: The Effect of Alkaline Pretreatment. Biomass Convers. Biorefinery. 2022 doi: 10.1007/s13399-022-03287-2. [DOI] [Google Scholar]
- 68.Su Y., Fang L., Wang P., Lai C., Huang C., Ling Z., Yong Q. Coproduction of Xylooligosaccharides and Monosaccharides from Hardwood by a Combination of Acetic Acid Pretreatment, Mechanical Refining and Enzymatic Hydrolysis. Bioresour. Technol. 2022;358:127365. doi: 10.1016/j.biortech.2022.127365. [DOI] [PubMed] [Google Scholar]
- 69.Vieira T.F., Corrêa R.C.G., de Fatima Peralta Muniz Moreira R., Peralta R.A., de Lima E.A., Helm C.V., Garcia J.A.A., Bracht A., Peralta R.M. Valorization of Peach Palm (Bactris Gasipaes Kunth) Waste: Production of Antioxidant Xylooligosaccharides. Waste Biomass Valorizat. 2021;12:6727–6740. doi: 10.1007/s12649-021-01457-3. [DOI] [Google Scholar]
- 70.Banerjee S., Patti A.F., Ranganathan V., Arora A. Hemicellulose Based Biorefinery from Pineapple Peel Waste: Xylan Extraction and Its Conversion into Xylooligosaccharides. Food Bioprod. Process. 2019;117:38–50. doi: 10.1016/j.fbp.2019.06.012. [DOI] [Google Scholar]
- 71.Yan F., Tian S., Chen H., Gao S., Dong X., Du K. Advances in Xylooligosaccharides from Grain Byproducts: Extraction and Prebiotic Effects. Grain Oil Sci. Technol. 2022;5:98–106. doi: 10.1016/j.gaost.2022.02.002. [DOI] [Google Scholar]
- 72.Morgan N.K., Wallace A., Bedford M.R., Choct M. Efficiency of Xylanases from Families 10 and 11 in Production of Xylo-Oligosaccharides from Wheat Arabinoxylans. Carbohydr. Polym. 2017;167:290–296. doi: 10.1016/j.carbpol.2017.03.063. [DOI] [PubMed] [Google Scholar]
- 73.Ávila P.F., Martins M., de Almeida Costa F.A., Goldbeck R. Xylooligosaccharides Production by Commercial Enzyme Mixture from Agricultural Wastes and Their Prebiotic and Antioxidant Potential. Bioact. Carbohydr. Diet. Fibre. 2020;24:100234. doi: 10.1016/j.bcdf.2020.100234. [DOI] [Google Scholar]
- 74.Chang S., Guo Y., Wu B., He B. Extracellular Expression of Alkali Tolerant Xylanase from Bacillus Subtilis Lucky9 in E. Coli and Application for Xylooligosaccharides Production from Agro-Industrial Waste. Int. J. Biol. Macromol. 2017;96:249–256. doi: 10.1016/j.ijbiomac.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 75.Khat-udomkiri N., Sivamaruthi B.S., Sirilun S., Lailerd N., Peerajan S., Chaiyasut C. Optimization of Alkaline Pretreatment and Enzymatic Hydrolysis for the Extraction of Xylooligosaccharide from Rice Husk. AMB Express. 2018;8:115. doi: 10.1186/s13568-018-0645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verma D., Satyanarayana T. Molecular Approaches for Ameliorating Microbial Xylanases. Bioresour. Technol. 2012;117:360–367. doi: 10.1016/j.biortech.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 77.Porto de Souza Vandenberghe L., Karp S.G., Binder Pagnoncelli M.G., von Linsingen Tavares M., Libardi Junior N., Valladares Diestra K., Viesser J.A., Soccol C.R. Biomass, Biofuels, Biochemicals. Elsevier; Amsterdam, The Netherlands: 2020. Classification of Enzymes and Catalytic Properties; pp. 11–30. [DOI] [Google Scholar]
- 78.Törrönen A., Rouvinen J. Structural and Functional Properties of Low Molecular Weight Endo-1,4-p-Xylanases. J. Biotechnol. 1997;57:137–149. doi: 10.1016/S0168-1656(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 79.Akpinar O., Erdogan K., Bostanci S. Production of Xylooligosaccharides by Controlled Acid Hydrolysis of Lignocellulosic Materials. Carbohydr. Res. 2009;344:660–666. doi: 10.1016/j.carres.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 80.Pereira G.F., de Bastiani D., Gabardo S., Squina F., Ayub M.A.Z. Solid-State Cultivation of Recombinant Aspergillus Nidulans to Co-Produce Xylanase, Arabinofuranosidase, and Xylooligosaccharides from Soybean Fibre. Biocatal. Agric. Biotechnol. 2018;15:78–85. doi: 10.1016/j.bcab.2018.05.012. [DOI] [Google Scholar]
- 81.Saha B.C. α-L-Arabinofuranosidases: Biochemistry, Molecular Biology and Application in Biotechnology. Biotechnol. Adv. 2000;18:403–423. doi: 10.1016/S0734-9750(00)00044-6. [DOI] [PubMed] [Google Scholar]
- 82.Song L., Siguier B., Dumon C., Bozonnet S., O’Donohue M.J. Engineering Better Biomass-Degrading Ability into a GH11 Xylanase Using a Directed Evolution Strategy. Biotechnol. Biofuels. 2012;5:3. doi: 10.1186/1754-6834-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beg Q.K., Kapoor M., Mahajan L., Hoondal G.S. Microbial Xylanases and Their Industrial Applications: A Review. Appl. Microbiol. Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- 84.Qing Q., Li H., Kumar R., Wyman C.E. Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals. John Wiley & Sons; Hoboken, NJ, USA: 2013. Xylooligosaccharides Production, Quantification, and Characterization in Context of Lignocellulosic Biomass Pretreatment. [Google Scholar]
- 85.Rezende M.I., De Melo Barbosa A., Vasconcelos A.F.D., Endo A.S. Xylanase Production by Trichoderma harzianum Rifai by Solid State Fermentation on Sugarcane Bagasse. Braz. J. Microbiol. 2002;33:67–72. doi: 10.1590/S1517-83822002000100014. [DOI] [Google Scholar]
- 86.Aachary A.A., Prapulla S.G. Value Addition to Corncob: Production and Characterization of Xylooligosaccharides from Alkali Pretreated Lignin-Saccharide Complex Using Aspergillus Oryzae MTCC 5154. Bioresour. Technol. 2009;100:991–995. doi: 10.1016/j.biortech.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 87.Samanta A.K., Jayapal N., Kolte A.P., Senani S., Sridhar M., Suresh K.P., Sampath K.T. Enzymatic Production of Xylooligosaccharides from Alkali Solubilized Xylan of Natural Grass (Sehima Nervosum) Bioresour. Technol. 2012;112:199–205. doi: 10.1016/j.biortech.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 88.Li X., Li E., Zhu Y., Teng C., Sun B., Song H., Yang R. A Typical Endo-Xylanase from Streptomyces Rameus L2001 and Its Unique Characteristics in Xylooligosaccharide Production. Carbohydr. Res. 2012;359:30–36. doi: 10.1016/j.carres.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Rajagopalan G., Shanmugavelu K., Yang K.L. Production of Prebiotic-Xylooligosaccharides from Alkali Pretreated Mahogany and Mango Wood Sawdust by Using Purified Xylanase of Clostridium Strain BOH3. Carbohydr. Polym. 2017;167:158–166. doi: 10.1016/j.carbpol.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Huang C., Lai C., Wu X., Huang Y., He J., Huang C., Li X., Yong Q. An Integrated Process to Produce Bio-Ethanol and Xylooligosaccharides Rich in Xylobiose and Xylotriose from High Ash Content Waste Wheat Straw. Bioresour. Technol. 2017;241:228–235. doi: 10.1016/j.biortech.2017.05.109. [DOI] [PubMed] [Google Scholar]
- 91.Singh R.D., Banerjee J., Sasmal S., Muir J., Arora A. High Xylan Recovery Using Two Stage Alkali Pre-Treatment Process from High Lignin Biomass and Its Valorisation to Xylooligosaccharides of Low Degree of Polymerisation. Bioresour. Technol. 2018;256:110–117. doi: 10.1016/j.biortech.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Qian S., Zhou J., Chen X., Ji W., Zhang L., Hu W., Lu Z. Evaluation of an Efficient Fed-Batch Enzymatic Hydrolysis Strategy to Improve Production of Functional Xylooligosaccharides from Maize Straws. Ind. Crops Prod. 2020;157:112920. doi: 10.1016/j.indcrop.2020.112920. [DOI] [Google Scholar]
- 93.Hao X., Wen P., Wang J., Wang J., You J., Zhang J. Bioresource Technology Production of Xylooligosaccharides and Monosaccharides from Hydrogen Peroxide-Acetic Acid-Pretreated Poplar by Two-Step Enzymatic Hydrolysis. Bioresour. Technol. 2020;297:122349. doi: 10.1016/j.biortech.2019.122349. [DOI] [PubMed] [Google Scholar]
- 94.Ratnadewi A.A.I., Amaliyah Zain M.H., Nara Kusuma A.A.N., Handayani W., Nugraha A.S., Siswoyo T.A. Lactobacillus Casei Fermentation towards Xylooligosaccharide (XOS) Obtained from Coffee Peel Enzymatic Hydrolysate. Biocatal. Agric. Biotechnol. 2020;23:101446. doi: 10.1016/j.bcab.2019.101446. [DOI] [Google Scholar]
- 95.Gautério G.V., Hübner T., da Rosa Ribeiro T., Ziotti A.P.M., Kalil S.J. Xylooligosaccharide Production with Low Xylose Release Using Crude Xylanase from Aureobasidium Pullulans: Effect of the Enzymatic Hydrolysis Parameters. Appl. Biochem. Biotechnol. 2022;194:862–881. doi: 10.1007/s12010-021-03658-x. [DOI] [PubMed] [Google Scholar]
- 96.Zhao S., Zhang G.L., Chen C., Yang Q., Luo X.M., Wang Z.B., Wu A.M., Feng J.X. A Combination of Mild Chemical Pre-Treatment and Enzymatic Hydrolysis Efficiently Produces Xylooligosaccharides from Sugarcane Bagasse. J. Clean. Prod. 2021;291:125972. doi: 10.1016/j.jclepro.2021.125972. [DOI] [Google Scholar]
- 97.Rajagopalan G., Shanmugavelu K., Yang K.L. Production of Xylooligosaccharides from Hardwood Xylan by Using Immobilized Endoxylanase of Clostridium Strain BOH3. RSC Adv. 2016;6:81818–81825. doi: 10.1039/C6RA17085D. [DOI] [Google Scholar]
- 98.Purohit A., Rai S.K., Chownk M., Sangwan R.S., Yadav S.K. Xylanase from Acinetobacter Pittii MASK 25 and Developed Magnetic Cross-Linked Xylanase Aggregate Produce Predominantly Xylopentose and Xylohexose from Agro Biomass. Bioresour. Technol. 2017;244:793–799. doi: 10.1016/j.biortech.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 99.Liu M.Q., Liu G.F. Expression of Recombinant Bacillus Licheniformis Xylanase A in Pichia Pastoris and Xylooligosaccharides Released from Xylans by It. Protein Expr. Purif. 2008;57:101–107. doi: 10.1016/j.pep.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 100.Zhao L.C., Wang Y., Lin J.F., Guo L.Q. Adsorption and Kinetic Behavior of Recombinant Multifunctional Xylanase in Hydrolysis of Pineapple Stem and Bagasse and Their Hemicellulose for Xylo-Oligosaccharide Production. Bioresour. Technol. 2012;110:343–348. doi: 10.1016/j.biortech.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 101.Izyan N., Azelee W., Jahim J., Fauzi A., Fatimah S., Mohamad Z., Rahman R.A., Illias R. High Xylooligosaccharides (XOS) Production from Pretreated Kenaf Stem by Enzyme Mixture Hydrolysis. Ind. Crops Prod. 2016;81:11–19. [Google Scholar]
- 102.Zhou T., Xue Y., Ren F., Dong Y. Antioxidant Activity of Xylooligosaccharides Prepared from Thermotoga Maritima Using Recombinant Enzyme Cocktail of β-Xylanase and α-Glucuronidase. J. Carbohydr. Chem. 2018;37:210–224. doi: 10.1080/07328303.2018.1455843. [DOI] [Google Scholar]
- 103.Tseng Y.H., Lee W.C., Krisomdee K., Natesuntorn W., Chatsurachai S., Sriroth K. Xylooligosaccharide Production from Sugarcane Bagasse Using Recombinant Endoxylanase of Bacillus Halodurans. Sugar Tech. 2022;24:1029–1036. doi: 10.1007/s12355-021-01096-x. [DOI] [Google Scholar]
- 104.de Oliveira Nascimento C.E., de Oliveira Simões L.C., de Cassia Pereira J., da Silva R.R., de Lima E.A., de Almeida G.C., Penna A.L.B., Boscolo M., Gomes E., da Silva R. Application of a Recombinant GH10 Endoxylanase from Thermoascus Aurantiacus for Xylooligosaccharide Production from Sugarcane Bagasse and Probiotic Bacterial Growth. J. Biotechnol. 2022;347:1–8. doi: 10.1016/j.jbiotec.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 105.Liu J., Liu C., Qiao S., Dong Z., Sun D., Zhu J., Liu W. One-Step Fermentation for Producing Xylo-Oligosaccharides from Wheat Bran by Recombinant Escherichia coli Containing an Alkaline Xylanase. BMC Biotechnol. 2022;22:6. doi: 10.1186/s12896-022-00736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xue Y., Song Y., Wu J., Gan L., Long M., Liu J. Characterization of the Recombinant GH10 Xylanase from Trichoderma Orientalis EU7-22 and Its Synergistic Hydrolysis of Bamboo Hemicellulose with α-Glucuronidase and α-L-Arabinofuranosidase. Ind. Crops Prod. 2023;194:116330. doi: 10.1016/j.indcrop.2023.116330. [DOI] [Google Scholar]
- 107.Shivudu G., Khan S., Chandraraj K., Selvam P. Immobilization of Recombinant Endo-1,4-β-Xylanase on Ordered Mesoporous Matrices for Xylooligosaccharides Production. ChemistrySelect. 2019;4:11214–11221. doi: 10.1002/slct.201901593. [DOI] [Google Scholar]
- 108.Alnoch R.C., Alves G.S., Salgado J.C.S., de Andrades D., Freitas E.N.d., Nogueira K.M.V., Vici A.C., Oliveira D.P., Carvalho V.P., Jr., Silva R.N., et al. Immobilization and Application of the Recombinant Xylanase GH10 of Malbranchea Pulchella in the Production of Xylooligosaccharides from Hydrothermal Liquor of the Eucalyptus (Eucalyptus Grandis) Wood Chips. Int. J. Mol. Sci. 2022;23:13329. doi: 10.3390/ijms232113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brienzo M., Carvalho W., Milagres A.M.F. Xylooligosaccharides Production from Alkali-Pretreated Sugarcane Bagasse Using Xylanases from Thermoascus Aurantiacus. Appl. Biochem. Biotechnol. 2010;162:1195–1205. doi: 10.1007/s12010-009-8892-5. [DOI] [PubMed] [Google Scholar]
- 110.Li X., Mupondwa E., Panigrahi S., Tabil L., Sokhansanj S., Stumborge M. A review of agricultural crop residue supply in Canada for cellulosic ethanol production. Renew. Sustain. Energy Rev. 2012;16:2954–2965. doi: 10.1016/j.rser.2012.02.013. [DOI] [Google Scholar]
- 111.Li X., Shan Z., Song X., Ouyang J., Xu Y., Yong Q., Yu S. Immobilization of Xylanase on Poly (Ethylene Glycol) Methyl Ether 5000 and Its Self-Extractive Bioconversion for the Production of Xylo-Oligosaccharides. Appl. Biochem. Biotechnol. 2014;172:2022–2029. doi: 10.1007/s12010-013-0666-4. [DOI] [PubMed] [Google Scholar]
- 112.Rajagopalan G., He J., Yang K.-L. One-pot fermentation of agricultural residues to produce butanol and hydrogen by Clostridium strain BOH3. Renew. Energy. 2016;85:1127–1134. doi: 10.1016/j.renene.2015.07.051. [DOI] [Google Scholar]
- 113.Carvalho A.F.A., de Figueiredo F.C., Campioni T.S., Pastore G.M., de Oliva Neto P. Improvement of Some Chemical and Biological Methods for the Efficient Production of Xylanases, Xylooligosaccharides and Lignocellulose from Sugar Cane Bagasse. Biomass Bioenergy. 2020;143:105851. doi: 10.1016/j.biombioe.2020.105851. [DOI] [Google Scholar]
- 114.Kumar V., Bahuguna A., Ramalingam S., Kim M. Developing a Sustainable Bioprocess for the Cleaner Production of Xylooligosaccharides: An Approach towards Lignocellulosic Waste Management. J. Clean. Prod. 2021;316:128332. doi: 10.1016/j.jclepro.2021.128332. [DOI] [Google Scholar]
- 115.Zhang F., Lan W., Zhang A., Liu C. Green approach to produce xylo-oligosaccharides and glucose by mechanical-hydrothermal pretreatment. Biores. Technol. 2022;344:126298. doi: 10.1016/j.biortech.2021.126298. [DOI] [PubMed] [Google Scholar]
- 116.Valladares-Diestra K.K., Porto de Souza Vandenberghe L., Soccol C.R. Integrated Xylooligosaccharides Production from Imidazole-Treated Sugarcane Bagasse with Application of in House Produced Enzymes. Bioresour. Technol. 2022;362:127800. doi: 10.1016/j.biortech.2022.127800. [DOI] [PubMed] [Google Scholar]
- 117.Vivek N., Parhi P., Mohan B., Hazeena S.H., Kumar A.N., Gullón B., Srivastava A., Nair L.M., Alphy M.P., Sindhu R. Valorization of Renewable Resources to Functional Oligosaccharides: Recent Trends and Future Prospective. Bioresour. Technol. 2021;346:126590. doi: 10.1016/j.biortech.2021.126590. [DOI] [PubMed] [Google Scholar]
- 118.Grand View Research . Prebiotics Market Size, Share & Trends Analysis by Ingredients (FOS, Inulin, GOS, MOS), by Application (Food and Beverages, Dietary Supplements, Animal Feed), by Region, and Segment Forecasts, 2014–2024. Grand View Research; San Francisco, CA, USA: 2020. [Google Scholar]
- 119.Huang C., Wang X., Liang C., Jiang X., Yang G., Xu J., Yong Q. A Sustainable Process for Procuring Biologically Active Fractions of High-Purity Xylooligosaccharides and Water-Soluble Lignin from Moso Bamboo Prehydrolyzate. Biotechnol. Biofuels. 2019;12:189. doi: 10.1186/s13068-019-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lan K., Xu Y., Kim H., Ham C., Kelley S.S., Park S. Techno-Economic Analysis of Producing Xylo-Oligosaccharides and Cellulose Microfibers from Lignocellulosic Biomass. Bioresour. Technol. 2021;340:125726. doi: 10.1016/j.biortech.2021.125726. [DOI] [PubMed] [Google Scholar]
- 121.Ho A.L., Carvalheiro F., Duarte L.C., Roseiro L.B., Charalampopoulos D., Rastall R.A. Production and Purification of Xylooligosaccharides from Oil Palm Empty Fruit Bunch Fibre by a Non-Isothermal Process. Bioresour. Technol. 2014;152:526–529. doi: 10.1016/j.biortech.2013.10.114. [DOI] [PubMed] [Google Scholar]
- 122.Alfa M.J., Strang D., Tappia P.S., Graham M., Van Domselaar G., Forbes J.D., Laminman V., Olson N., DeGagne P., Bray D. A Randomized Trial to Determine the Impact of a Digestion Resistant Starch Composition on the Gut Microbiome in Older and Mid-Age Adults. Clin. Nutr. 2018;37:797–807. doi: 10.1016/j.clnu.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 123.Valdemiro Carlos S. The Importance of Prebiotics in Functional Foods and Clinical Practice. Food Nutr. Sci. 2011;2:133–144. [Google Scholar]
- 124.Jana U.K., Kango N., Pletschke B. Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation. Front. Nutr. 2021;8:670817. doi: 10.3389/fnut.2021.670817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Freitas C., Carmona E., Brienzo M. Xylooligosaccharides Production Process from Lignocellulosic Biomass and Bioactive Effects. Bioact. Carbohydr. Diet. Fibre. 2019;18:100184. doi: 10.1016/j.bcdf.2019.100184. [DOI] [Google Scholar]
- 126.Future Market Insights . Xylooligosaccharides Market Outlook—2022–2032. Future Market Insights; Newark, DE, USA: 2022. [Google Scholar]
- 127.Pimentel T.C., Torres de Assis B.B., dos Santos Rocha C., Marcolino V.A., Rosset M., Magnani M. Prebiotics in Non-Dairy Products: Technological and Physiological Functionality, Challenges, and Perspectives. Food Biosci. 2022;46:101585. doi: 10.1016/j.fbio.2022.101585. [DOI] [Google Scholar]
- 128.Hussein L.A. Chapter 30—Novel Prebiotics and next-Generation Probiotics: Opportunities and Challenges. In: Singh R.B., Watanabe S., Isaza A.A., editors. Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases. Academic Press; Cambridge, MA, USA: 2022. pp. 431–457. [Google Scholar]
- 129.Horitsu H. Xylooligosaccharide and method of producing its reduced substances. JP1994343486(A) Granted Patent. 1995 May 1;
- 130.Fang K., Jian F., Zhao J. Nutritional Healthcare Food Useful for e.g. Improving Gastrointestinal Tract Function, Contains Microcrystalline Cellulose, Probiotic Powder, Xylooligosaccharide, Isomaltitol, Concentrated Whey Protein, and Saussurea Extract. CN104509864-A. Granted Patent. 2015 January 20;
- 131.Shao S. Healthcare Food Product used for e.g. Enhancing Immunity, Contains D-Mannitol, Maltodextrin, Microcrystalline Cellulose, Probiotic Powder, Modified Dietary Fiber, Xylooligosaccharide, Isomaltitol and Mushroom Extract. CN104543679-A. Granted Patent. 2015 April 29;
- 132.Wang Z. Sesame White Wine Useful for Protecting Liver, Prepared Using Rice Wine Lees, Rice, Rice Husk, Enteromorpha, Sesame, Fleeceflower Root, Cuscuta Chinensis, Pilose Antler, Medlar, Koji, Tea Polyphenol and Xylooligosaccharide. CN105062748-A. Granted Patent. 2015 November 18;
- 133.Wang Z., Zhou L., Yu M., Pan B., Wu T., Cui Y., Zhao Z. Full-Nutrition Foliar Fertilizer Includes Photosynthetic Bacteria, Glacial Acetic Acid, Potassium Humate, Sorbitol, Xylitol, Mannitol, Potassium Hydroxide, Sodium Hydroxide, Magnesium Nitrate, Urea, Potassium Hydroxide and Sodium Glutamate. CN108147903-A. Granted Patent. 2018 June 12;
- 134.Yang R., Zhao W., Hu J. Preparing Xylooligosaccharide and Lignin using Microcrystalline Cellulose Straw Involves Crushing the Agricultural Crop Straw, Water Leaching, Straw Flash Explosion Depolymerization Process, and Xylanase Enzymolysis Processing. CN103981237-A. Granted Patent. 2014 August 13;
- 135.Che M., Gao H., Guo P., Li L., Shi D., Wang C., Xue S., Yang D., Zhou M. Method for Manufacturing Sweet Potato Dregs Biscuit, Involves Rinsing Sweet Potato Dregs, Filtering and Drying by Filter Screen, and Mixing Eggs, White Granulated Sugar and Xylooligosaccharide Followed by Baking Biscuits in Electric Oven. CN103210985-A. Granted Patent. 2013 July 24;
- 136.Liu X., Chen J. Manufacture of Gluten-Free Baked Pancake Involves Dissolving Food Glue in Water, Dissolving Yeast in Water, Activating, and Pouring Sweet Potato Crude Dietary Fiber Coarse Powder, Protein, Sugar, Salt, and Yeast in Noodle Machine. CN104431882-A. Granted Patent. 2015 March 25;
- 137.Du J., Hu Z., Zhao L. Additive Premix for Laying Type Chickens Includes Bean Pulp, Calcium Hydrophosphate, Composite vitamin, Niacin, D-Calcium Pantothenate, Biotin, Choline Chloride, Xylooligosaccharide, Microbial Ecological Agent, and Rice Husk Bran. CN102293339-A. Granted Patent. 2012 January 1;
- 138.Bao S., Long M., Long Y. Grade Separation of Forestry Cellulose Biomass Component for Preparing Fuel Alcohol and Xylooligosaccharide Involves Crushing Agriculture and Forestry Biomass Solid Phase Material, Filtering Adding Oxidant, and Extracting. CN102261007-A. Granted Patent. 2011 November 30;
- 139.Batsalova T., Georgiev Y., Moten D., Teneva I., Dzhambazov B. Natural Xylooligosaccharides Exert Antitumor Activity via Modulation of Cellular Antioxidant State and TLR4. Int. J. Mol. Sci. 2022;23:10430. doi: 10.3390/ijms231810430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ashwini A., Ramya H.N., Ramkumar C., Reddy K.R., Kulkarni R.V., Abinaya V., Naveen S., Raghu A.V. Reactive Mechanism and the Applications of Bioactive Prebiotics for Human Health. J. Microbiol. Methods. 2019;159:128–137. doi: 10.1016/j.mimet.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 141.Neves M.I.L., Strieder M.M., Prata A.S., Silva E.K., Meireles M.A.A. Xylooligosaccharides as an Innovative Carrier Matrix of Spray-Dried Natural Blue Colorant. Food Hydrocoll. 2021;121:107017. doi: 10.1016/j.foodhyd.2021.107017. [DOI] [Google Scholar]
- 142.Bambace M.F., Alvarez M.V., del Rosario Moreira M. Novel Functional Blueberries: Fructo-Oligosaccharides and Probiotic Lactobacilli Incorporated into Alginate Edible Coatings. Food Res. Int. 2019;122:653–660. doi: 10.1016/j.foodres.2019.01.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.