Abstract
Aim of the study
Portal vein congestion index has shown promise in detecting early portal venous hemodynamic changes in chronic liver disease. The aim of this study was to compare the portal vein congestion index of adult patients with chronic liver disease to that of healthy controls, and to evaluate the differences in portal vein congestion index, if any, between the common etiologies of chronic liver disease (chronic viral hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease).
Method and materials
Eighty participants with chronic liver disease and 80 healthy controls had their sociodemographic variables, anthropometric indices, liver size/echotexture, spleen size, presence of ascites, and portal vein parameters (diameter, cross-sectional area, velocity, and congestion index) evaluated. P ≤0.05 was considered statistically significant.
Results
There were 48 (60%) males and 32 (40%) females in the control group, while 56 (70%) males and 24 (30%) females were included in the chronic liver disease group (p = 0.185). Of the eighty people with chronic liver disease, 57 (71.2%) were diagnosed with alcoholic liver disease, while 23 (28.8%) were diagnosed with chronic viral hepatitis. There were no cases of non-alcoholic fatty liver disease during the study period. The mean liver spans of the control and chronic liver disease groups were 13.45 ± 0.85 cm and 16.50 ± 4.96 cm, respectively. All the controls had normal hepatic parenchymal echogenicity, while 45 (56.3%) subjects with chronic liver disease (36 alcoholic liver disease and 9 chronic viral hepatitis) had increased hepatic echogenicity. The mean values of the portal vein congestion index for the control and chronic liver disease groups were 0.0775 ± 0.02 cm/sec and 0.1037 ± 0.03 cm/sec, respectively (p <0.0001).
Conclusion
The chronic liver disease group showed a significantly higher mean portal vein congestion index than the control group.
Keywords: chronic liver disease, congestion index, portal vein, portal hypertension
Introduction
Chronic liver disease (CLD) is a progressive deterioration of liver functions (synthesis of clotting factors/proteins, detoxification of harmful products of metabolism, and excretion of bile) for more than six months. It is a continuous process of inflammation, destruction, and regeneration of liver parenchyma, which leads to fibrosis and cirrhosis. Cirrhosis is the final stage of CLD that results in disruption of liver architecture, the formation of widespread nodules, vascular reorganization, neo-angiogenesis, and deposition of the extracellular matrix(1). Progressive hepatic fibrosis leads to increased intrahepatic resistance and portal hypertension(2). Liver cirrhosis is the most common cause of portal hypertension(3). In addition, portal venous pressure correlates significantly with the degree of disease chronicity and fibrosis in liver disease(4,5).
CLD has a wide variety of causes, including long-term alcoholism, chronic viral infection (hepatitis B and C viruses), autoimmune diseases, metabolic diseases (e.g., non-alcoholic fatty liver disease, NAFLD), and hereditary disorders(1,2).
As of 2021, there were 1.5 billion people living with CLD (including any stage of disease severity) worldwide(6). Currently, the pre-eminent causes of liver cirrhosis globally are NAFLD, hepatitis B virus (HBV), hepatitis C virus (HCV), and alcoholic liver disease, in that order(6). In sub-Saharan Africa, CLD accounted for an estimated 157,558.69 deaths (equivalent to 2.11% of all deaths) in 2017(7). The prevalence rates of HBV and HCV in our locality are 4.3–23.3% and 0.5–15%, respectively(8).
Liver biopsy has been the traditional gold standard for diagnosing liver disease, while hepatic venous pressure gradient (HPVG) is the standard for diagnosing portal hypertension(9–11). However, these procedures are highly invasive. Furthermore, liver biopsy is prone to significant sampling errors, as it samples only 1/50,000 part of the liver(12).
Ultrasonography is a key non-invasive method for diagnosing liver diseases using B-mode/grayscale imaging, elastography, and Doppler parameters. The diameter of the portal vein is variable, and various studies carried out to determine the sonographic value for their respective populations reveal a range of about 7–15 mm(13–15).
On Doppler interrogation, the normal flow through the portal vein is hepatopetal (towards the liver), a finding which may be absent or reversed in portal hypertension. The normal blood flow velocity in the portal vein is 13–55 cm/s. The portal waveform is monophasic with an undulating appearance due to its variations with cardiac activity and respiration(15–17).
The portal vein congestion index (PVCI) was first introduced and analyzed by Moriyasu et al.(18) as a duplex Doppler tool for detecting portal venous hemodynamic changes in certain cases of liver disease, and is defined as the ratio of the portal vein cross-sectional area (cm2) and the mean blood flow velocity (cm/sec)(3,18,19). By relating two quantifiable factors affected by portal hypertension, it tends to magnify the differences in measured parameters between study groups, and is therefore said to be the most sensitive and specific triplex Doppler parameter for detecting portal hypertension(20).
The aim of this study was to compare the PVCI of adult patients with CLD to that of healthy controls, and to evaluate the differences in PVCI, if any, between the common etiologies of CLD (CVH, ALD, and NAFLD).
Materials and methods
Study design
This was a prospective descriptive comparative sonographic study done at the Radiology Department of the Delta State University Teaching Hospital from January 2020 to January 2021. A total of 160 participants (80 subjects and 80 controls) were enrolled. The Research and Ethics Committee of the institution approved the study protocol (HREC/PAN/2019/072/0324). Subjects were recruited into the study after an informed written consent had been granted following a thorough explanation of the aims and objectives of the study, methods of examination, and benefits.
Study population
The participants were adult patients (>18 years) diagnosed with CLD or liver cirrhosis at the gastroenterology clinic based on clinical (stigmata of CLD)(21) and laboratory parameters(1). The subjects were enrolled consecutively until the sample size was complete. The exclusion criteria included portal hypertension from other causes (e.g., portal vein thrombosis, right heart failure), prior therapies for portal hypertension, tachypnea, pregnancy, recent upper abdominal surgery, and medications (vasoactive drugs, diuretics or anti-inflammatory medications).
The control group included healthy volunteers, without any history or laboratory evidence of liver-related diseases (normal liver function test, LFT), recruited among patients’ relatives, staff and students of the hospital and affiliated university. The exclusion criteria for controls included obesity (BMI >30 kg/m2), hepatobiliary diseases, abnormal LFT, splenomegaly, cardiac diseases, recent abdominal surgery, tachypnea, pregnancy, and anatomical variants of the portal vein (e.g., double portal vein).
Study technique
The relevant demographic data and medical history were recorded in structured questionnaires, including the participants’ age, sex, height, weight, and body mass index [BMI = weight/height2 (kg/m2)]. BMI of <18.5 kg/m2, 18.5–24.9 kg/m2, 25.0–29.9 kg/m2, 30– 39.9 kg/m2, and >40 kg/m2 were classified as underweight, normal, overweight, obese, and morbidly obese, respectively(22). The last menstrual period (LMP) of female participants was documented and a pelvic ultrasound scan was done to exclude cyesis. Blood samples were collected from the participants under aseptic conditions and sent to the laboratory for liver function tests.
Abdominal ultrasound was done using the curvilinear transducer (2.8–5.2 MHz frequency) of a Siemens ultrasound machine with Doppler facility (Sonoline G50, Siemens Medical Solutions Inc., USA) or a Mindray DC-30 ultrasound scanner (Shenzhen Mindray Bio-Medical Electronic Co. Ltd. China). The participants were scanned in the supine position following a minimum of 4 hours fast (to minimize bowel gas and reduce the likelihood of portal venous hemodynamic alterations by nutrient load)(15,23). The liver was scanned to determine echogenicity, presence of nodules, and craniocaudal span at the midclavicular line(14). Liver spans <10 cm and >15 cm were defined as shrunken liver and hepatomegaly, respectively(24). The splenic span was also measured. Splenomegaly was defined as craniocaudal splenic span >12 cm(25).
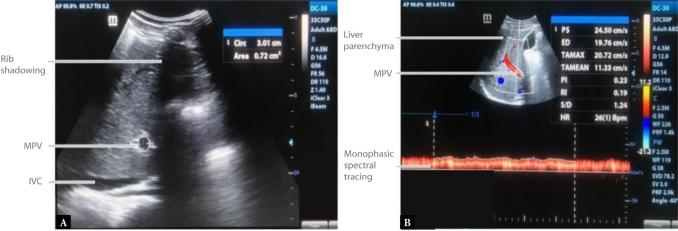
A subcostal approach (with the transducer pointing posterocephalad) or a right intercostal approach (transducer pointing medially) was employed to evaluate the portal vein for thrombus(23). The transducer was placed at the epigastrium in both the transverse and longitudinal planes to assess the main portal vein during intermittent breath holds or quiet inspiration(23). Erect right anterior oblique or left posterior oblique views were employed to adequately visualize the distal extrahepatic portal vein whenever it was obscured by significant duodenal gas. The portal vein’s cross-sectional area and the mean flow velocity were measured at a point just distal to the union of the splenic and superior mesenteric veins(20). On grayscale imaging, a frozen transverse cut-section of a well demonstrated portal vein was obtained and the cross-sectional area was measured using the ellipse mode of the ultrasound machine. The ellipse was placed to approximate the inner margins of the echogenic wall of the portal vein (Fig. 1).
Fig. 1.
B-mode transverse view ( A ) of the main portal vein (MPV) taken at the epigastrium, showing the cross-sectional area of the MPV = 0.72 cm 2 ; and triplex sonogram of the MPV showing its typical forward (hepatopetal) flow and characteristic monophasic spectral pattern with gentle undulations caused by adjacent cardiac and respiratory motion
Portal vein Doppler waveforms were obtained using an insonation angle <60°, following which the mean flow velocity [also known as the Time Averaged Mean Velocity (TAmean)](26–28) was calculated automatically by the ultrasound machine. All measurements were taken three times by one investigator, and the average was calculated to reinforce the reliability of the outcome and minimize intra-observer variability. The portal vein congestion index (cm/sec) was computed using the formula: Cross-Sectional Area (cm2)/Mean Flow Velocity (cm/sec).
Data analysis
The study data collected were entered and subsequently analyzed using the IBM SPSS Statistics for Windows version 24 (IBM Corp., Armonk, New York, USA). Data normality was tested using the Kolmogorov-Smirnov test. Categorical data were expressed in proportion or percentages, while continuous data was expressed as mean (± standard deviation). For categorical data, the test of association between proportions was done using chi-square or odds ratio, as appropriate, while for continuous variables, Student’s t-test or analysis of variance (ANOVA) was used to test for difference. Confounding variables were controlled for by stratification or regression analysis. P values ≤0.05 were considered statistically significant at 95% confidence interval.
Results
Socio-demographic characteristics
One hundred and sixty (160) participants, comprising eighty (80) subjects with chronic liver disease (CLD) and eighty (80) healthy controls, were recruited. There were 56 (70%) males and 24 (30%) females in the CLD group, while the control group had 48 (60%) male and 32 (40%) female participants. The mean age for the study population was 40.28 ± 12.09 years. The other socio-demographic details are presented in Tab. 1.
Tab. 1.
Socio-demographic characteristics of the study population
| N | Controls | CLD | Total | Test statistics | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| (%) | n | (%) | n | (%) | |||||
| Age (years) | <20 | 7 | 8.8 | 0 | 0.0 | 7 | 4.4 | χ2 = 17.948 | 0.006 |
| 21–30 | 21 | 26.3 | 12 | 15.0 | 33 | 20.6 | |||
| 31–40 | 17 | 21.3 | 23 | 28.7 | 40 | 25.0 | |||
| 41–50 | 23 | 28.7 | 22 | 27.5 | 45 | 28.1 | |||
| 51–60 | 12 | 15.0 | 16 | 20.0 | 28 | 17.5 | |||
| 61–70 | 0 | 0.0 | 5 | 6.3 | 5 | 3.1 | |||
| >71 | 0 | 0.0 | 2 | 2.5 | 2 | 1.3 | |||
| Mean | 36.81 ±11.60 | 43.74 ± 11.63 | 40.28 ±12.09 | t = -3.770 | <0.0001 | ||||
| Sex | Male | 48 | 60.0 | 56 | 70.0 | 104 | 65.0 | χ2 = 1.758 | 0.185 |
| Female | 32 | 40.0 | 24 | 30.0 | 56 | 35.0 | |||
| Occupation | Unemployed | 4 | 5.0 | 7 | 8.8 | 11 | 6.9 | χ2 = 17.140 | 0.002 |
| Self-employed | 15 | 18.8 | 28 | 35.0 | 43 | 26.9 | |||
| Student | 26 | 32.5 | 9 | 11.3 | 35 | 21.9 | |||
| Civil servant | 35 | 43.8 | 32 | 40.0 | 67 | 41.9 | |||
| Retired | 0 | 0.0 | 4 | 5.0 | 4 | 2.5 | |||
| Ethnic group | Urhobo | 45 | 56.3 | 38 | 47.5 | 83 | 51.9 | χ2 = 3.143 | 0.871 |
| Ijaw | 8 | 10.0 | 10 | 12.5 | 18 | 11.3 | |||
| Itsekiri | 12 | 15.0 | 9 | 11.3 | 21 | 13.1 | |||
| Igbo | 5 | 6.3 | 8 | 10.0 | 13 | 8.1 | |||
| Yoruba | 2 | 2.5 | 4 | 5.0 | 6 | 3.8 | |||
| Bini | 3 | 3.8 | 4 | 5.0 | 7 | 4.4 | |||
| Isoko | 4 | 5.0 | 6 | 7.5 | 10 | 6.3 | |||
| Hausa | 1 | 1.3 | 1 | 1.3 | 2 | 1.3 | |||
P-value – probability value; χ2 – Pearson’s chi-squared test; t – Student’s t-test; control = range (18–55 years); median (37.5 years); CLD – chronic liver disease = range (24–74 years), median (43.5 years)
Anthropometric and clinical parameters
The mean weight of subjects in the CLD group was 72.63 ± 4.87 kg, while in the control group it was 73.02 ± 5.13 kg (p = 0.040). The CLD group had a mean height of 1.74 ± 0.09 m, whereas the control group had a mean height of 1.71 ± 0.09 m (p = 0.12). The mean BMI was 25.02 ± 2.71 kgm2 and 24.32 ± 3.05 kg/m2 for the cases and controls, respectively (p = 0.12). Of the eighty people with CLD, 57 (71.2%) were diagnosed with alcoholic liver disease (ALD), while 23 (28.8%) were diagnosed with chronic viral hepatitis (CVH). There were no cases of NAFLD in the study population.
Ultrasonographic B-mode findings
Increased liver echogenicity was observed in 45/80 (56.3%) of the CLD group, comprising 36/57 (63.2%) of the ALD subgroup and in 9/23 (39.1%) of the CVH subgroup (p = 0.05) Tab. 2. The craniocaudal liver span was 8.5–26.4 cm (mean = 16.50 ± 4.96 cm), 9.3–26.4 cm (mean = 16.63 ± 5.02 cm), 8.5–25.8 cm (mean = 16.17 ± 4.88 cm), 12.1–14.8 cm (mean = 13.45 ± 0.85 cm) in the CLD group, ALD subgroup, CVH subgroup, and the controls, respectively. The splenic span was 7.2–16.4 cm (mean = 11.10 ± 2.51 cm), 7.2–16.4 cm (mean = 11.25 ± 2.64 cm), 7.7–15.8 (mean = 10.74 ± 2.19 cm), 6.5–10.7 cm (mean = 8.53 ± 1.15 cm) in the CLD group, ALD subgroup, CVH subgroup, and the controls, respectively.
Tab. 2.
B-mode liver findings in the CLD subgroups
| Alcoholic liver disease | Chronic viral hepatitis | Total | χ2 | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||||
| Abnormal echogenicity | 36 | 63.2 | 9 | 39.1 | 45 | 56.3 | χ2 = 3.844 | 0.051 | |
| Hepatic nodules | 17 | 29.8 | 5 | 21.7 | 22 | 27.5 | χ2 = 11537 | 0.464 | |
| Liver span | Normal | 19 | 33.3 | 6 | 26.1 | 25 | 31.3 | χ2 = 0.402 | 0.818 |
| Hepatomegaly | 27 | 47.4 | 12 | 52.2 | 39 | 48.8 | |||
| Shrunken | 11 | 19.3 | 5 | 21.7 | 16 | 20.0 | |||
Twenty-four (30%) patients with CLD had ascites, out of which 19 (33.3%) were in the ALD subgroup and 5 (21.7%) in the CVH subgroup. The total number of study participants that had splenomegaly was 20 (25%), with 16 (28.1%) in the ALD subgroup and 4 (17.4%) in the CVH subgroup.
The mean congestion index in the CLD study subjects was 1.1037 ± 0.03 cm/sec, and this showed a significant weak positive correlation with the presence of ascites (r = 0.24, p = 0.035) and splenomegaly (r = 0.26, p = 0.020).
Portal vein parameters
Tab. 3 and Tab. 4 show the differences in portal vein diameter (PVD), portal vein cross-sectional area (PVSA), portal vein mean velocity (PVMV), and portal vein congestion index (PVCI) between the study groups and subgroups. Table 5 is a post-hoc analysis which shows that all the intergroup differences were statistically significant.
Tab. 3.
Portal vein parameters of the control and CLD groups
| Controls (n = 80) | Chronic liver disease (n = 80) | t | P-value | |
|---|---|---|---|---|
| PV diameter (cm) | 1.16 ± 0.14 | 1.34 ± 0.15 | t = -7.604 | <0.0001 |
| PV cross-sectional area (cm2) | 1.07 ± 0.26 | 1.42 ± 0.32 | t = -7.500 | <0.0001 |
| PV mean velocity (cm/s) | 14.01 ± 1.60 | 13.90 ± 1.47 | t = 0.462 | 0.645 |
| PV congestion index (cm/sec) | 0.0775 ± 0.02 | 0.1037 ± 0.03 | t = -6.735 | <0.0001 |
PV – portal vein; P-value – probability value; t – Student’s t-test
Tab. 4.
Portal vein parameters of the ALD and CVH subgroups
| Alcoholic liver disease (n = 57) | Chronic viral hepatitis (n = 23) | t | P-value | |
|---|---|---|---|---|
| PV diameter (cm) | 1.36 ± 0.16 | 1.28 ± 0.10 | t = 2.234 | 0.028 |
| PV cross-sectional area (cm2) | 1.47 ± 0.35 | 1.29 ± 0.20 | t = 2.330 | 0.022 |
| PV mean velocity (cm/s) | 13.90 ± 1.50 | 13.90 ± 1.42 | t = 0.008 | 0.993 |
| PV congestion index (cm/sec) | 0.1077 ± 0.03 | 0.0939 ± 0.02 | t = 2.006 | 0.048 |
PV – portal vein; P-value – probability value; t – Student’s t-test
Tab. 5.
Post-hoc analysis of intergroup differences in portal vein congestion index
| Total | t | P-value | |
|---|---|---|---|
| Normal (n = 80) | 0.0775 ± 0.02 | 6.735 | <0.0001 |
| Chronic liver disease (n = 80) | 0.1037 ± 0.03 | ||
| Alcoholic liver disease (n = 57) | 0.1077 ± 0.03 | 2.006 | 0.048 |
| Chronic viral hepatitis (n = 23) | 0.0939 ± 0.02 | ||
| Normal (n = 80) | 0.0775 ± 0.02 | 47.562 | <0.0001 |
| Alcoholic liver disease (n = 57) | 0.1077 ± 0.03 | ||
| Normal (n = 80) | 0.0775 ± 0.02 | 47.562 | 0.0001 |
| Chronic viral hepatitis (n = 23) | 0.0939 ± 0.02 |
P-value – probability value; t – Student’s t-test; range of congestion index for control = 0.0398–0.1129; chronic liver disease = 0.0632–0.1818
The highest mean PVCI (0.0817 ± 0.022 cm/sec) in the control group was found in subjects in the third decade, while the lowest mean PVCI (0.0731 ± 0.021 cm/sec) in the same group was found in the fourth decade. By contrast, in the CLD group, the lowest mean PVCI (0.0980 ± 0.040 cm/sec) and the highest mean PVCI (0.1287 ± 0.010 cm/sec) were found in the seventh and eighth decades, respectively. The differences in mean PVCI among the different age groups in both the control and CLD study groups were not statistically significant (p = 0.143) (Tab. 6).
Tab. 6.
Effect of age, sex, and BMI on PVCI in the ALD and CVH subgroups
| Mean ± SD | Alcoholic liver disease | Chronic viral hepatitis | χ2 | F | P-value | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean ± SD | n (%) | ||||||
| Age (years) | 21–30 | 0.1196 ± 0.041 | 7 (12.3) | 0.0863 ± 0.010 | 5(21.7) | 3.675 | 0.597 | |
| 31–40 | 0.0997 ± 0.027 | 19 (33.3) | 0.0848 ± 0.008 | 4(17.4) | 1.359 | 0.250 | ||
| 41–50 | 0.1143 ± 0.030 | 16 (28.1) | 0.0994 ± 0.020 | 6 (26.1) | ||||
| 51–60 | 0.1101 ± 0.035 | 10 (17.5) | 0.0986 ± 0.020 | 6 (26.1) | ||||
| 61–70 | 0.0852 ± 0.019 | 4 (7.0) | 0.0773 | 1 (4.3) | ||||
| >70 | 0.1356 | 1 (1.8) | 0.1217 | 1 (4.3) | ||||
| Sex | Male | 0.1072 ± 0.033 | 41 (71.9) | 0.0911 ± 0.018 | 15 (65.2) | 0.352 | 0.553 | |
| Female | 0.1090 ± 0.028 | 16 (28.1) | 0.0992 ± 0.016 | 8 (34.8) | 0.173 | 0.678 | ||
| BMI | Underweight | 0.1006 ± 0.027 | 4 (7.0) | – | – | 1.822 | 0.402 | |
| Normal | 0.1022 ± 0.030 | 30 (52.6) | 0.0919 ± 0.016 | 14 (60.9) | 1.652 | 0.198 | ||
| Overweight | 0.1161 ± 0.033 | 23 (40.4) | 0.0970 ± 0.020 | 9 (39.1) | ||||
ALD – alcoholic liver disease, CVH – chronic viral hepatitis, BMI – body mass index, PVCI – portal vein congestion index
The mean PVCI was higher in females than males in both study groups, with males in the control group having a mean PVCI value of 0.0742 ± 0.020 cm/sec, and females having a mean PVCI of 0.0825 ± 0.019 cm/sec. In the CLD group, males and females had mean PVCI values of 0.1029 ± 0.030 cm/sec and 0.1058 ± 0.024 cm/ sec, respectively. The gender differences in PVCI were not statistically significant (p = 0.536). There was no statistical significance in the mean PVCI of the different BMI categories between the two groups (p = 0.379).
Discussion
Portal hypertension is a common finding in decompensated chronic liver disease/liver cirrhosis, and several researchers have validated the suitability of Doppler sonography for evaluating portosystemic changes within the liver(14). In this study, the hepatic and portal venous changes in 80 participants with CLD were compared to healthy controls.
The most common cause of CLD was alcoholic liver disease (ALD), which was seen in 57 (71.3%) subjects. The number of CLD patients with ALD was significantly higher than those diagnosed with CVH. This is at variance with studies which found chronic viral hepatitis B to be the most common cause of CLD(29–31). The higher prevalence of ALD in this study was probably due to the high rate of alcohol consumption (locally brewed gin called ‘ogogoro’ or ‘kai-kai’) in our region(32).
A greater number of subjects in the ALD group showed more features of hepatic decompensation (ascites, splenomegaly, hepatic nodularity, shrunken liver with irregular outline) than the CVH group. This can probably be explained by the fact that subjects with alcoholic liver disease were more inclined to show signs of more advanced signs of liver disease due to late presentation, poor level of compliance and higher rates of relapse. Some of the hepatic and extrahepatic sonographic findings in the CLD group of this study were similar to those documented by Maàji et al.(33): hepatomegaly (31%), shrunken liver (25%), abnormal liver echotexture (95%), liver edge irregularities (93%), splenomegaly (9%), and ascites (74%). These values suggest a more advanced decompensated CLD in comparison with the index study.
The portal vein diameter (PVD) was significantly higher in the CLD group than the controls. This is in agreement with the findings from the study by Achim et al.(13) and Ayele et al.(34) who observed a significantly higher PVD in the CLD groups than the controls. The reason for this significant difference can be adduced to the presence of portal hypertensive changes in patients with CLD, resulting in a number of hemodynamic portal venous alterations, one of which is increased venous diameter. Furthermore, subjects with ALD had a significantly higher PVD than those with CVH. The difference in PVD between these two groups is probably due to the aforementioned reason of generally more advanced disease seen in ALD than the CVH subgroup. However, the PVD should not be used in isolation to diagnose portal hypertension because of its poor sensitivity to the presence of collateral channels that decompress the portovenous system(14).
The mean PVCSA of the CLD group was significantly higher than that of controls. This was similar to the findings by Moriyasu et al.(18) where a similar mean portal vein cross-sectional area value for liver cirrhosis patients was obtained (1.49 ± 0.49 cm2), which was significantly higher than that of controls (p = 0.001). This similarity is most probably attributable to portal hypertensive changes which result in slightly increased portal vein caliber in both study groups. The ALD subgroup had a significantly higher mean PVCSA than the CVH subgroup. The variable disease severity between the two subgroups might be responsible for this disparity. There were no significant differences in mean PVCSA and portal venous velocity between males and females in both groups (controls and CLD patients). The mean portal vein cross-sectional area (PVCSA) for controls was 1.07 ± 0.26 cm2. This is similar to the mean PVCSA obtained by Moriyasu et al.(18) (0.99 ± 0.28 cm2) and Aiyekomogbon et al.(20) (1.097 ± 0.20 cm2), and also close to the value reported by Ibinaiye et al.(35) (1.10 ± 0.20 cm2). The similar PVCSA values from these studies suggest that there might be no significant ethnic differences in PVCSA.
The CLD group had a mean PVCI of 0.104 ± 0.03 cm/sec, which was significantly higher than the value for controls. Of the two CLD subgroups, the ALD subgroup showed a significantly higher mean PVCI than the CVH subgroup. The reason ALD patients had a higher mean PVCI than CVH patients may be due to the poor health seeking behavior of this category of patients, leading to more advanced liver disease states at presentation. This is probably worsened by the higher number of males than females in this subcategory, as men in this society have been shown to have worse health seeking habits than women(36). Furthermore, non-compliance may also play a significant role, as alcoholics tend to show poor compliance with health intervention strategies, with higher rates of relapse to the detriment of their liver health.
Other studies also found a significant difference in PVCI between CLD cases and controls(19,26,37–39). Ehtisham et al.(38) showed a more significant statistical difference between the mean PVCI for controls and CLD subjects (0.05 ± 0.00 cm/sec vs. 0.14 ± 0.02 cm/sec respectively; p = 0.0001). However, the authors did not subcategorize the CLD participants into etiological subgroups to observe for PVCI differences therein.
Chakravarthy and co-researchers(19), on the other hand, subdivided the CLD participants into three subcategories of ALD, CVH, and non-alcoholic fatty liver disease (NAFLD), and measured the PVCI in each of these subgroups. The PVCI values recorded for each of these conditions were significantly higher than the value obtained in their controls, i.e., medians of 0.027, 0.050 and 0.060 for NAFLD, CVH, and ALD, respectively. The absolute values in their study differed significantly from those of the index research possibly due to interobserver variability, differing Doppler angles, and racial differences.
Similarly, Iliopoulos et al.(26) recorded a mean PVCI of 0.06 ± 0.03 cm/sec for subjects in the CVH group. Unlike the index study, however, this was not significantly higher than the PVCI of the control group (0.05 ± 0.02 cm/sec) (p = 0.251). This discordance with the index study may be explained by the fact that Iliopoulos et al. further subdivided subjects with CVH into five subgroups (F1–F5) on the basis of histologic activity index (necroinflammatory score). Those in subgroups F1–F4 had lower necroinflammatory scores than those in the F5 group, who showed a more advanced stage of fibrosis; this was reflected in the PVCI, which was significantly higher in F5 than the F1–F4 stages (p = 0.041). This significant difference was, however, not reflected upon averaging of the subgroups into the composite CVH group due to a higher number of participants in the F1–F4 subgroups than the F5 subgroup.
Haag et al.(37) found the PVCI to be highly sensitive and specific in diagnosing elevated portal pressure. Similarly to this study, they reported a significant difference in PVCI between control and CLD groups. However, there was no attempt to correlate the PVCI with Class-Pugh classification in this study, as was done by Haag and colleagues(37). On the other hand, the authors did not investigate possible variations in PVCI based on etiology, as was done in the index study.
Bolognesi et al.(39) in Italy investigated the relationship between the etiology of cirrhosis and splanchnic hemodynamics in patients with CLD scheduled for orthotopic liver transplant. Only patients with ALD or CVH were included. Most of the splanchnic hemodynamic parameters evaluated, including the PVCI, did not show any significant difference between the two disease categories. This is in discordance with our study in which there was a statistically significant PVCI difference between the two groups. Furthermore, the mean PVCI recorded in their research was significantly higher than that recorded in the present study, most probably because the patients recruited by the authors had very advanced stages of liver disease requiring a transplant. This same reason could also account for why the PVCI values between the ALD and CVH groups in their study did not show a significant difference.
We encountered some limitations in this study. Firstly, due to the location of the portal vein, a large body habitus or significant bowel gas usually made scanning difficult. Oblique views were employed to overcome this limitation. The subjects whose portal veins could still not be assessed were excluded from the study. Secondly, we could not correlate the PVCI with the Child-Pugh classification.
Conclusion
In conclusion, the patients with CLD had a significantly higher PVCI than healthy controls. Similarly, the PVCI of the ALD subgroup was significantly higher than that of the CVH subgroup. The significant difference in PVCI between the control and CLD groups suggests that PVCI can be an adjunct parameter for detecting chronic liver diseases.
Footnotes
Conflict of interest: The authors do not report any financial or personal connections with other persons or organizations which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Author contributions: Original concept of study: ODO, AOZ. Writing of manuscript: ODO, AOZ, BMI. Analysis and interpretation of data: ODO, AOZ, BMI. Final approval of manuscript: ODO, AOZ, JEI, NNN, BMI, GIO. Collection, recording and/or compilation of data: AOZ. Critical review of manuscript: ODO, JEI, NNN, BMI, GIO.
References
- 1.Sharma A, Nagalli S . StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Chronic liver disease. : . , . Available from: http://www.ncbi.nlm.nih.gov/books/NBK554597/ (date of access: 18.02.2022). [PubMed] [Google Scholar]
- 2.Muir AJ Understanding the complexities of cirrhosis Clin Ther. 2015;37(8):1822. doi: 10.1016/j.clinthera.2015.05.507. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 3.Robinson KA, Middleton WD, Al-Sukaiti R, Teefey SA, Dahiya N Doppler sonography of portal hypertension Ultrasound Q. 2009;25(1):3. doi: 10.1097/ruq.0b013e31819c8685. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 4.Ljubicić N, Duvnjak M, Rotkvić I, Kopjar B Influence of the degree of liver failure on portal blood flow in patients with liver cirrhosis Scand J Gastroenterol. 1990;25(4):395. doi: 10.3109/00365529009095505. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 5.Procopet B, Berzigotti A Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy Gastroenterol Rep. 2017;5(2):79. doi: 10.1093/gastro/gox012. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheemerla S, Balakrishnan M Global epidemiology of chronic liverdisease Clin Liver Dis. 2021;17(5):365. doi: 10.1002/cld.1061. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terefe Tesfaye B, Gudina EK, Bosho DD, Mega TA Short-term clinical outcomes of patients admitted with chronic liver disease to selected teaching hospitals in Ethiopia PloS One. 2019;14(8):e0221806. doi: 10.1371/journal.pone.0221806. : . ; ( ): . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agada SA, Odama RI, Kenechukwu CO, Okang SO, Ezeh CO Epidemiology of chronic liver disease in Nigeria: a review Asian J Adv Med Sci. 2020;2(3):1. : . ; ( ): –. . [Google Scholar]
- 9.Cadranel JF, Rufat P, Degos F Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatol Baltim Md. 2000;32(3):477. doi: 10.1053/jhep.2000.16602. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 10.Spycher C, Zimmermann A, Reichen J The diagnostic value of liver biopsy BMC Gastroenterol. 2001;1:12. doi: 10.1186/1471-230x-1-12. : . ; : . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG Measurement of portal pressure and its role in the management of chronic liver disease Semin Liver Dis. 2006;26(4):348. doi: 10.1055/s-2006-951603. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 12.Smith A, Baumgartner K, Bositis C Cirrhosis: diagnosis and management Am Fam Physician. 2019;100(12):759. : . ; ( ): –. . [PubMed] [Google Scholar]
- 13.Achim CA, Bordei P, Dumitru E The role of ultrasonography in the evaluation of portal hemodynamics in healthy adults and pathologic conditions ARS Medica Tomitana. 2016;22(2):128. doi: 10.1515/arsm-2016-0022. : . ; ( ): –. . DOI: . [DOI] [Google Scholar]
- 14.Gerstenmaier JF, Gibson RN Ultrasound in chronic liver disease Insights Imaging. 2014;5(4):441. doi: 10.1007/s13244-014-0336-2. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Nakeep S, Ziska SK . StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Doppler liver assessment, protocols, and interpretation of results. : . , . Available from: http://www.ncbi.nlm.nih.gov/books/NBK567725/ (date of access: 22.10.2022). [PubMed] [Google Scholar]
- 16.Kruskal JB, Newman PA, Sammons LG, Kane RA Optimizing Doppler and color flow US: application to hepatic sonography Radiographics. 2004;24(3):657. doi: 10.1148/rg.243035139. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 17.McNaughton DA, Abu-Yousef MM Doppler US of the liver made simple Radiographics. 2011;31(1):161. doi: 10.1148/rg.311105093. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 18.Moriyasu F, Nishida O, Ban N, Nakamura T, Sakai M, Miyake T, et al. ‘Congestion index’ of the portal vein Am J Roentgenol. 1986;146(4):735. doi: 10.2214/ajr.146.4.735. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 19.Chakravarthy A, Thomas S, Mohanan K, Puthussery PV, Resmi S, Raini KP Congestion index of portal vein in the evaluation of liver disease J Med Sci Clin Res. 2017;5(5):22666. : . ; ( ): –. . [Google Scholar]
- 20.Aiyekomogbon JO, Ibinaiye PO, Tabari AM, Chom ND, Yusuf R, Aiyebelehin AO, et al. Determination of normal portal vein congestive index on ultrasound scan among adults in Zaria, Nigeria Arch Int Surg. 2014;4(3):146. : . ; ( ): –. . [Google Scholar]
- 21.Karnath B. Stigmata of chronic liver disease Hosp Physician. 2003;39:14. . ; : –. . [Google Scholar]
- 22.Weir CB, Jan A . StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. BMI classification percentile and cut off points. : . , . Available from: http://www.ncbi.nlm.nih.gov/books/NBK541070/ (date of access: 22.10.2022). [PubMed] [Google Scholar]
- 23.Pozniak MA, Allan PL . Clinical Doppler Ultrasound. 3rd ed. Churchill Livingstone; 2013. : . [Google Scholar]
- 24.Afzal S, Masroor I, Beg M Evaluation of chronic liver disease: does ultrasound scoring criteria help? Int J Chronic Dis. 2013;2013:1. doi: 10.1155/2013/326231. : ; : –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niknejad MT Splenomegaly. : . Available from: http://radiopaedia.org/articles/6003 (date of access: 22.10.2022).
- 26.Iliopoulos P, Vlychou M, Karatza C, Yarmenitis SD, Repanti M, Tsamis I, et al. Ultrasonography in differentiation between chronic viral hepatitis and compensated early stage cirrhosis World J Gastroenterol. 2008;14(13):2072. doi: 10.3748/wjg.14.2072. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idowu BM, Ibitoye BO, Adetiloye VA Uterine artery doppler velocimetry of uterine leiomyomas in Nigerian women Rev Bras Ginecol E Obstet. 2017;39(9):464. doi: 10.1055/s-0037-1604489. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idowu BM, Ibitoye BO Doppler sonography of perifibroid and intrafibroid arteries of uterine leiomyomas Obstet Gynecol Sci. 2018;61(3):395. doi: 10.5468/ogs.2018.61.3.395. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asrani SK, Devarbhavi H, Eaton J, Kamath PS Burden of liver diseases in the world J Hepatol. 2019;70(1):151. doi: 10.1016/j.jhep.2018.09.014. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 30.Kooffreh-Ada M, Okpara H, Oku A, Okonkwo U, Ihekwaba A Risk factors of chronic liver disease amongst patients receiving care in a Gastroenterology practice in Calabar IOSR J Dent Med Sci. 2015;14(12):6. : . ; ( ): –. . [Google Scholar]
- 31.Aidoo M, Sulemana Mohammed B Distribution and determinants of etiologies and complications of chronic liver diseases among patients at a tertiary hospital in a lower economic region of Ghana Cent Afr J Public Health. 2020;6(5):280. : . ; ( ): –. . [Google Scholar]
- 32.Iwegbue CMA, Ojelum AL, Bassey FI A survey of metal profiles in some traditional alcoholic beverages in Nigeria Food Sci Nutr. 2014;2(6):724. doi: 10.1002/fsn3.163. : . ; ( ): –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Màaji SM, Yakubu A, Odunko DD Pattern of abnormal ultrasonographic findings in patients with clinical suspicion of chronic liver disease in Sokoto and its environs Asian Pac J Trop Dis. 2013;3(3):202. : . ; ( ): –. . [Google Scholar]
- 34.Ayele T, Gebremickael A, Alemu Gebremichael M, George M, Wondmagegn H, Esubalew H, et al. Ultrasonographic determination of portal vein diameter among adults with and without chronic liver disease at selected referral hospitals in Southern Ethiopia Int J Gen Med. 2022;15:45. doi: 10.2147/ijgm.s342087. : . ; : –. . DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibinaiye PO, Aiyekomogbon JO, Tabari MA, Chom ND, Hamidu AU, Yusuf R Determination of normal portal vein parameters on triplex ultrasound scan among adults in Zaria, Nigeria Sub-Sahar Afr J Med. 2015;2(1):33. : . ; ( ): –. . [Google Scholar]
- 36.Olanrewaju FO, Ajayi LA, Loromeke E, Olanrewaju A, Allo T, Nwannebuife O Masculinity and men’s health-seeking behaviour in Nigerian academia Cogent Soc Sci. 2019;5(1):1682111. : . ; ( ): . [Google Scholar]
- 37.Haag K, Rössle M, Ochs A, Huber M, Siegerstetter V, Olschewski M, et al. Correlation of duplex sonography findings and portal pressure in 375 patients with portal hypertension AJR Am J Roentgenol. 1999;172(3):631. doi: 10.2214/ajr.172.3.10063849. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]
- 38.Ehtisham-Ul-Haq H, Shami AS, Noreen A, Malik MA, Ul-Hasan Z, Bacha R, et al. Sonographic comparison of congestive index of portal vein with and without chronic liver parenchymal disease J Health Med Sci. 2019;2(1):80. : . ; ( ): –. . [Google Scholar]
- 39.Bolognesi M, Sacerdoti D, Mescoli C, Bombonato G, Cillo U, Merenda R, et al. Different hemodynamic patterns of alcoholic and viral endstage cirrhosis: analysis of explanted liver weight, degree of fibrosis and splanchnic Doppler parameters Scand J Gastroenterol. 2007;42(2):256. doi: 10.1080/00365520600880914. : . ; ( ): –. . DOI: . [DOI] [PubMed] [Google Scholar]