Abstract
Several literatures have examined the risk of chronic respiratory diseases in association with short-term ambient PM2.5 exposure in China. However, little evidence has examined the chronic impacts of PM2.5 exposure on morbidity of chronic respiratory diseases in cohorts from high pollution countries. Our study aims to investigate the associations. Based on a retrospective cohort among adults in northern China, a Cox regression model with time-varying PM2.5 exposure and a concentration-response (C-R) curve model were performed to access the relationships between incidence of chronic respiratory diseases and long-term PM2.5 exposure during a mean follow-up time of 9.8 years. Individual annual average PM2.5 estimates were obtained from a satellite-based model with high resolution. The incident date of a chronic respiratory disease was identified according to self-reported physician diagnosis time and/or intake of medication for treatment. Among 38,047 urban subjects analyzed in all-cause chronic respiratory disease cohort, 482 developed new cases. In CB (38,369), asthma (38,783), and COPD (38,921) cohorts, the onsets were 276, 89, and 14, respectively. After multivariable adjustment, hazard ratio and 95% confidence interval for morbidity of all-cause chronic respiratory disease, CB, asthma, and COPD were 1.15 (1.01, 1.31), 1.20 (1.00, 1.42), 0.76 (0.55, 1.04), and 0.66 (0.29, 1.47) with each 10 μg/m3 increment in PM2.5, respectively. Stronger effect estimates were suggested in alcohol drinkers across stratified analyses. Additionally, the shape of C-R curve showed an increasing linear relationship before 75.00 μg/m3 concentrations of PM2.5 for new-onset all-cause chronic respiratory disease, and leveled off at higher levels. These findings indicated that long-term exposure to high-level PM2.5 increased the risks of incident chronic respiratory diseases in China. Further evidence of C-R curves is warranted to clarify the associations of adverse chronic respiratory outcomes involving air pollution.
Keywords: Satellite-based model, PM2.5, Cohort study, Chronic respiratory diseases, Incidence, China
1. Introduction
Over the past few decades, the global burden of chronic respiratory diseases remains a sustainable global concern. During 1990 and 2017, disability-adjusted life-years (DALYs) counts of chronic respiratory diseases significantly increased (GBD Chronic Respiratory Disease Collaborators, 2020). As the Global Burden of Disease Study reported, in 2017, chronic respiratory diseases caused almost 3.91 million deaths, accounting for approximately 7% of total deaths globally (GBD, 2017 Causes of Death Collaborators., 2018; Li et al., 2020b). In 2016, 9% of the all-cause non-communication disease mortality was attributed to chronic respiratory diseases with an estimated number of 0.87 million in China (Guan et al., 2016; Li et al., 2020b; Mokoena et al., 2019).
The development of urbanization and industrialization brings severe air pollution and poor air quality problems, causing over 7 million premature deaths each year worldwide (World Health Organization, 2019), especially ambient PM2.5. PM2.5 is widely deposited in the lung and constitutes about 96% of particles existing in human pulmonary parenchyma (Churg and Brauer, 1997), contributing to 4.8 million deaths in 2015 (Cohen et al., 2017). Ambient PM2.5 pollution is still a major global health issue.
Nowadays, several epidemiological studies have demonstrated that being exposed to daily PM2.5 was positively related to the acute risk of hospitalizations or emergency room visits of chronic respiratory diseases such as chronic bronchitis (CB), asthma, and chronic obstructive pulmonary disease (COPD) (Chi et al., 2019; Dominici et al., 2006; Lin et al., 2020; Wang et al., 2019; Yao et al., 2020; Zuo et al., 2019). However, the evidence on morbidity of chronic respiratory diseases related to long-term exposure to PM2.5 was mostly obtained from North American and European settings, and the annual concentrations of PM2.5 pollution in these countries were commonly under 35 μg/m3 (Atkinson et al., 2015; Carey et al., 2016; Doiron et al., 2021; Fisher et al., 2016; Gan et al., 2013; Guo et al., 2018; Hooper et al., 2018; Weichenthal et al., 2017; Young et al., 2014). Yet, the studies derived from longitudinal cohorts with relatively high pollution exposure such as in China was scarce. It was mainly related to the fact that China did not have available PM2.5 ground-based monitoring data until 2013. Currently, combining the historical PM2.5 estimates assessed by a satellite-based spatiotemporal model, some nationwide studies have reported the harmful impacts of lung cancer incidence attributable to long-term PM2.5 exposure among Chinese (Guo et al., 2020; Li et al., 2020a), but we still lacked the accurate evidence on prolonged PM2.5 exposure related to the risks of other chronic respiratory diseases.
To our best knowledge, due to large emissions of traffic engines, fugitive dusts, smokes or soot associated with coal and/or biofuel combustion, the accumulated concentrations of PM2.5 in China remain much higher compared with that in other western countries (Cao et al., 2018; Ebisu and Bell, 2012; Pandey et al., 2013; Wang et al., 2017; Wu et al., 2013). Additionally, dominate elements on the surface of PM2.5 are various in different regions, which may stimulate different toxic effects (Cao et al., 2005; Kioumourtzoglou et al., 2015; Zhang et al., 2013). Therefore, the estimated effects of PM2.5 on chronic respiratory outcomes obtained from developed regions with relatively low levels may not directly extended to those in China.
Herein, based on a cohort with urban adults in northern China, this study is intended to investigate the impacts of PM2.5 pollution on chronic respiratory diseases by using evaluated PM2.5 data from satellite-inversion model during 2000–2009.
2. Methods
2.1. Study area and population
39, 054 adults in this retrospective study were recruited from four cities in northern China: Rizhao, Taiyuan, Shenyang, and Tianjin. A published article has provided the complete information of geography and location of environment monitoring sites in these cities (Yan et al., 2020; Zhang et al., 2014). Briefly, Tianjin, as one of the earliest industrial cities in China, comprises a population of 12.3 million in 2009 and located southeast of Beijing. Shenyang is the capital city of Liaoning Province and has major industries including steel manufacturing, nonferrous metals, machinery, coke-related industries, and electric power generation. Taiyuan, known for the largest coal-producing area in China, lies on the eastern edge of the Loess Plateau. As an emerging coastal city, Rizhao is located on the west coast of the Yellow China Sea with a 2.7 million population in 2009. The PM2.5 pollution in these four cities can partially cover the wide range of PM2.5 pollution levels in northern urban China. The study participants based-on community were recruited from above four cities in 2009. Firstly, according to geographical and population sizes of each city, one, two, five, and seven environmental monitoring stations were identified in Rizhao, Taiyuan, Shenyang, and Tianjin, respectively. Secondly, all apartment buildings or street blocks as small neighborhoods within a radius of 1 km around the local environmental monitoring stations of each city were selected and those small neighborhoods including 500–700 urban residents were numbered to form a sampling frame. The samples of each neighborhood were randomly drawn until a desired number of approximately 10,000 participants in each city were recruited. The eligible participants included that (1) candidates were born before January 1, 1975; (2) they had already resided in the defined sites since January 1, 1998; and (3) they were able to be contacted for the information of age, sex, years of residence details from local neighborhood offices and survey questionnaire. After obtaining the information on age, sex, and residential addresses from local neighborhood offices, the trained interviewers contacted the participants to retrospectively collect their basic socio-demographic, lifestyle and medical information in 1998, and the disease and/or medical conditions during the period from 1998 to 2009 with a standardized questionnaire, establishing a retrospective cohort in northern China. The baseline information was basic information in 1998, and the cohort was followed once in 2009. Initially, a total of 39,054 participants completed the cohort investigation. Because the data of satellite-derived PM2.5 exposure were available since 2000, we excluded 113 deceased individuals before 2000. Besides, participants suffering from chronic respiratory diseases before January 1st, 2000 and those missing information of diagnosis were further excluded at the baseline, with a sample size of 38,047, 38,369, 38,783 and 38,921 participants were eligible for the analysis on the occurrence of all-cause chronic respiratory disease, CB, asthma, and COPD related to PM2.5, respectively.
The ethical committee of the coordinating center of Tianjin Medical University approved this study. All participants signed informed consents before the investigation started.
2.2. Health data acquirement and Assessment of chronic respiratory diseases
In the current study, the data on characteristics of demography, status of diseases, drug usage, and lifestyle habits during the follow-up period from baseline to 2009 were obtained by local neighborhood offices and/or trained investigators through a uniformed questionnaire. For decedents, we collected information from their family members recalling. Smoking status was divided into yes (current and former smokers) or no (never smoke) by asking subjects (1) whether he/she smoked ≥ 1 cigarette a day for one year; (2) whether he/she was a former or current smoker at the baseline survey. Alcohol intake was classified into “yes” or “no” according to self-reported intake of alcohol once or more times per week in the last year or not. Passive smoke status was divided into “yes” or “no” according to whether a resident was exposed to second-hand smoke at least 6 months or not. Physical activity was assigned as “yes” or “no” based on whether participants had one or more leisure physical activities one week or not. Disease related-data were collected by asking individuals whether he/she or their families suffered chronic respiratory diseases. If yes, the information on (1) physician diagnosed time from at least a three-class hospital; (2) and/or whether they took treatment measures were also obtained through self-reporting.
According to the International Classification of Diseases-tenth revision, the defined all-cause chronic respiratory disease (J40-J47) was coded, including CB (J40-J42), COPD (J44), asthma (J45-J46), and other chronic respiratory diseases (J43 and J47). The definition of incident date was based on self-reported physician diagnosed time from at least a three-class hospital, and/or intake of medication for treating these respiratory diseases since diagnosis, which was usually used previously (Camargo et al., 1999; Fisher et al., 2016).
PM2.5 exposure data were available after 2000. Considering the time consistency of PM2.5 exposure and population survey, the follow-up started at January 1st, 2000 (baseline date), and the basic data in 1998 were used as the baseline information of all participants in this study. The end time was the date of chronic respiratory diseases occurrence, the date of death or the end of follow-up, when an ending event appeared firstly. Follow-up time was calculated from baseline time to end time. For the morbidity of all-cause chronic respiratory disease, CB, asthma and COPD, the follow-up time was calculated respectively.
2.3. Assessment of ambient pollution exposure
In the present study, because the monitoring stations did not measure PM2.5 levels in mainland China before 2013, the ambient PM2.5 concentrations were evaluated by the spatiotemporal model based on satellite in this study, with high spatial resolution of 1 km × 1 km. The details of the model, and assessments of personal PM2.5 exposure have been reported previously (Liang et al., 2020; Xiao et al., 2018).
In brief, the Aerosol Optical Depth (AOD) product obtained from NASA was retrieved by the Multi-Angle Implementation of Atmospheric Correction (MAIAC) algorithm, which was employed to calculate PM2.5 exposure estimates at 1 km × 1 km spatial resolution. To minimize the bias induced by cloud cover, we filled the missing AOD values by a multiple imputation method and then we use random forest and extreme gradient boosting models to combine with land use information, meteorology, road information, air pollution emissions indicators, and population density data for prediction of PM2.5 levels. What’s more, the accuracy of PM2.5 pollution estimates were assessed from two aspects. For data available from ground PM2.5 measurements from the China Environmental Monitoring Center (i.e., data after 2013), a random 10-fold cross-validation R2 was calculated (R2 =0.93 at monthly level and R2 = 0.95 at annual level). For those that couldn’t be obtained from national ground observations in mainland China (i.e., data during 2000–2012), the predictions of PM2.5 were compared with the available PM2.5 ground measurements from Hong Kong, Taiwan, the US Embassy, with R2 of 0.80 and a root mean squared error of 8.90 μg/m3 at the annual level.
As a published article reported (Yang et al., 2021), individual pollution exposure was calculated via combining with the latitude and longitude coded by each resident’s address and satellite-based PM2.5 data. Accounting for the temporal variations of PM2.5 during 2000–2009, the annual mean PM2.5 levels were calculated as a time-varying exposure, which is time-varying PM2.5 exposure on 1-calendar year scale, and they were assigned to each participant who was alive or not suffering chronic respiratory diseases in that year during the follow-up period based on the individual coded address.
Till now, satellite-driven techniques are preferred methods to predict long-term PM2.5 concentrations in China given their high accuracy and extensive temporal coverage. In this study, high-resolution PM2.5 concentration estimates with 1 km × 1 km would provide more accurate exposure gradients within population clusters. This model has been widely applied in the published studies on historical PM2.5 exposure levels with human health (Li et al., 2020a; Liang et al., 2020; Lin et al., 2021; Yang et al., 2021).
Annual average gaseous pollutants levels in each city were acquired from the database in the China National Environmental Monitoring Center (CNEMC), the methods of ambient pollutants measurement and individual exposure estimates were described in detail in previous literature (Zhang et al., 2014; Yan et al., 2020). Briefly, the data were derived from the monitoring stations (seven in Tianjin, five in Shenyang, two in Taiyuan, and one in Rizhao) in CNEMC, which were collected by the local government agency. The daily average levels of NO2 (μg/m3) and SO2 (μg/m3) were measured using chemiluminescence, and ultraviolet fluorescence, respectively. Because CNEMC didn’t have ground monitoring data of O3 before 2013 in mainland of China, O3 was not considered in this study. Based on daily monitoring data of the nearest monitoring stations, we calculated annual (2000–2009) average concentrations, from which the concentration of NO2 and SO2 exposure to individuals was estimated during the follow-up time.
2.4. Statistical analyses
Characteristics of study subjects at baseline were displayed as numbers (percentages) for categorical variables and mean ± standard derivations for continuous variables. Given that the annual mean PM2.5 levels changed over the follow-up period, we used a Cox proportional hazards model with time-varying PM2.5 exposure on 1-year scale to assess the relationships between long-term PM2.5 pollution and onset of each chronic respiratory disease. PM2.5 levels were introduced in analyses as continuous variable. As well, PM2.5 were categorized into four groups according to quartiles, and reported hazard ratio (HR) for top 3 quartile groups compared with Q1 (Quartile 1) group as well. All analyses were performed by introducing certain risk factors gradually in adjustment models. The basic model is a crude model without introducing other covariates. Age, gender, and BMI were adjusted for Model 1; Model 2 was additionally adjusted for individual monthly income, and education level. In Model 3, smoking status, passive smoking, physical activity, drink status, and family history of related chronic respiratory diseases (All-cause respiratory disease: all chronic respiratory diseases; CB: CB; Asthma: asthma; COPD: COPD) were also taken into account. Finally, city was added as a covariate for adjustment in Model 4. Apart from single-pollutant models, considering that gas pollutants and PM2.5 might have co-effects on incidence of chronic respiratory disease, two-pollutant models were further performed to evaluate the influences of multiple air pollution exposures on chronic respiratory diseases morbidity. The combinations of the double-pollutant model were PM2.5-NO2 and PM2.5-SO2 models.
Stratified analyses were performed to assess the effect modifications of each potential covariate on PM2.5-related chronic respiratory diseases. We also introduced a cross-product term to examine the statistical interactions between time-varying PM2.5 exposure and baseline confounders in the Cox proportional hazards model.
We further performed concentration-response (C-R) curves using restricted cubic spline functions with 4 degrees of freedom (df) after adjusting for potential confounders in Model 4 for morbidity of all-cause chronic respiratory disease linked-to prolonged exposure to PM2.5.
To test robustness, several sensitivity analyses were carried out. Firstly, due to recall bias, the diagnosed time of respiratory diseases might not be precise, a logistic regression model was performed to examine the impacts of the mean annual concentrations of PM2.5 on the chronic respiratory diseases during the follow-up from 2000 to 2009. Secondly, occupational particulate matter (PM) may influence the main effect estimates potentially, so we restricted the subjects who were free of occupational PM exposure at baseline. Thirdly, considering that the confounding effects were derived from burning of household biofuels, study participants cooking with biofuels in the baseline were excluded.
All the statistical analyses were conducted via SAS (version 9.4) and R (version 4.1.1) software. The statistic significant test was two sided and P-value was below 0.05 across all analyses.
3. Results
3.1. Descriptive characteristics of the study populations
During the follow-up, there were 482 incident cases of all-cause chronic respiratory disease (372,509 person-years of follow-up), 276 onset of CB (376,507 person-years of follow-up), 89 new cases of asthma (381,129 person-years of follow-up), and 14 (382,936 person-years of follow-up) new COPD incidents. Table 1 showed the characteristics at the baseline of the study populations investigated in four cities for the cohort of all-cause chronic respiratory disease. The average age of participants was 43.87 ± 13.62 years old and the mean BMI was 22.63 ± 2.95 kg/m3 at baseline. The proportion of gender is approximately equal. More than half of the overall subjects had a personal monthly income ≥ 500 CNY, more than one-quarter were smokers, and about one-fifth were alcohol drinkers. Furthermore, the baseline demographic characteristics of cohort population for other chronic respiratory diseases (CB, asthma, and COPD) were similar to those of all-cause respiratory disease, which were showed in Table S1-S3 in Supplementary Materials.
Table 1.
Baseline characteristics of all-cause chronic respiratory disease cohort by cities.
| Characteristic | Rizhao | Shenyang | Taiyuan | Tianjin | Total |
|---|---|---|---|---|---|
| Total numbers (%) | 9277 | 9542 | 9821 | 9407 | 38047 |
| Gender (%) | |||||
| Male | 4879 (52.59) | 4613 (48.34) | 4883 (49.72) | 4530 (48.16) | 18905 (49.69) |
| Female | 4398 (47.41) | 4929 (51.66) | 4938 (50.28) | 4877 (51.84) | 19142 (50.31) |
| Age (years) | 38.38 ± 12.39 | 46.85 ± 14.26 | 44.33 ± 13.79 | 45.76 ± 12.33 | 43.87 ± 13.62 |
| BMI (kg/m2) | 22.34 ± 2.51 | 22.38 ± 3.36 | 22.86 ± 2.65 | 22.93 ± 3.16 | 22.63 ± 2.95 |
| Monthly income (%) | |||||
| < 500 CNY | 4482 (48.31) | 4703 (49.29) | 3609 (36.75) | 4448 (47.28) | 17242 (45.32) |
| ≥ 500 CNY | 4795 (51.69) | 4839 (50.61) | 6212 (63.25) | 4959 (52.72) | 20805 (54.68) |
| Education (%) | |||||
| < High school | 6557 (70.68) | 5693 (59.66) | 4754 (48.41) | 4487 (47.70) | 21491 (56.49) |
| ≥ High school | 2506 (27.01) | 3849 (40.34) | 5067 (51.59) | 4860 (51.66) | 16282 (42.79) |
| Smoking status (%) | |||||
| No | 7412 (79.90) | 6608 (69.25) | 7123 (72.53) | 6387 (67.90) | 27530 (72.36) |
| Yes | 1865 (20.10) | 2934 (30.75) | 2698 (27.47) | 3020 (32.10) | 10517 (27.64) |
| Alcohol intake (%) | |||||
| No | 7295 (78.64) | 7615 (79.81) | 8055 (82.02) | 7411 (78.78) | 30376 (79.84) |
| Yes | 1982 (21.36) | 1927 (20.19) | 1766 (17.98) | 1996 (21.22) | 7671 (20.16) |
| Exercise status (%) | |||||
| No | 4437 (47.83) | 5500 (57.64) | 4979 (50.70) | 4128 (43.88) | 19044 (50.05) |
| Yes | 4839 (52.16) | 4041 (42.35) | 4835 (49.20) | 5268 (56.12) | 18983 (49.89) |
| Passive smoking status (%) | |||||
| No | 7376 (79.51) | 5745 (60.21) | 5302 (53.99) | 4740 (50.39) | 23163 (60.88) |
| Yes | 1892 (20.39) | 3539 (37.09) | 4411 (44.91) | 4482 (47.65) | 14324 (37.65) |
| Family history of all-cause chronic respiratory disease (%) | |||||
| No | 9202 (99.19) | 9439 (98.92) | 9704 (98.81) | 9220 (98.01) | 37154 (97.65) |
| Yes | 27 (0.29) | 112 (1.17) | 3 (0.03) | 187 (1.99) | 79 (0.21) |
| New cases of all-cause chronic respiratory disease (%) | 75 (0.81) | 103 (1.08) | 117 (1.19) | 187 (1.99) | 482 (1.27) |
The values are presented as percentages (%) or means ± standard.
3.2. PM2.5 exposure in association with incidence of chronic respiratory diseases
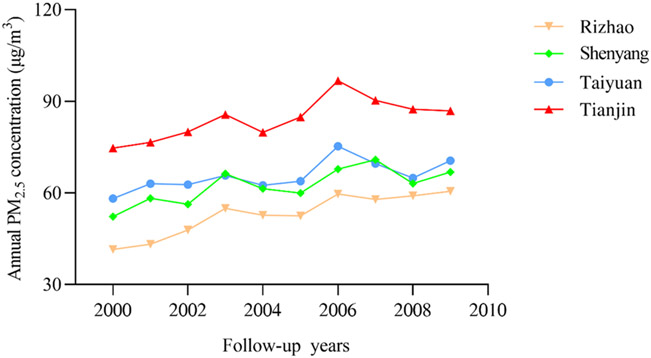
Among the original cohort of 39,054 participants without exclusion of any chronic respiratory disease at baseline, the time-varying trends of annual PM2.5 concentrations in four cities were displayed in Fig. 1. For the entire cohort, the average concentration of annual-mean PM2.5 exposure from 2000 to 2009 was 66.51 μg/m3. The lowest level appeared in Rizhao and it was 41.44 μg/m3. By contrast, Tianjin was the highest with 96.75 μg/m3. Furthermore, we calculated the mean annual PM2.5 level in the cohort of each chronic respiratory disease (approximately as same as 66.51 μg/m3), which was revealed in Fig. S1. Besides, the variations of PM2.5 concentrations by city were displayed in Fig. S2.
Fig. 1.
The temporal trends for average annual concentrations of PM2.5 (μg/m3) during the period from 2000 to 2009 across the four cities in northern China.
As illustrated in Table 2, PM2.5 attributed increased risks of all-cause respiratory disease, and CB incidence in both crude and multivariable-adjusted models. The hazard ratio (HR) for occurrence of all-cause respiratory disease and CB were 1.15 (95% confidence interval [CI]: 1.01, 1.31) and 1.20 (95% CI: 1.00, 1.42) per 10 μg/m3 increment of PM2.5 after multiple covariates were adjusted for. Additionally, after the PM2.5 concentrations were categorized based on quartiles, higher risks of all-cause chronic respiratory disease morbidity were observed with HR of 2.11 (95% CI: 1.50, 2.97), 2.03 (95% CI: 1.40, 2.94), and 3.13 (95% CI: 1.90, 5.16) for the Q2, Q3, and Q4 group, respectively, compared with the Q1 as reference (Table S4). The estimate results in CB analyses also revealed consistent significant trends. By contrast, no significant associations of PM2.5 with the onset of asthma and COPD were reported in any analyses.
Table 2.
Adjusted HR (95% CI) for the incidence of chronic respiratory diseases in relation to each 10 μg/m3 increase in PM2.5.
| Baseline variable adjusted for | HR (95% CI) for incidence of chronic respiratory diseases |
|||
|---|---|---|---|---|
| All-cause respiratory disease | CB | Asthma | COPD | |
| Crude model | 1.30 (1.21, 1.39) | 1.15 (1.05, 1.26) | 1.48 (1.27, 1.73) | 0.95 (0.63, 1.44) |
| Model 1 | 1.27 (1.18, 1.36) | 1.12 (1.02, 1.23) | 1.45 (1.24, 1.70) | 0.83 (0.53, 1.31) |
| Model 2 | 1.28 (1.19, 1.37) | 1.14 (1.03, 1.25) | 1.45 (1.23, 1.70) | 0.82 (0.51, 1.32) |
| Model 3 | 1.20 (1.12, 1.29) | 1.09 (1.00, 1.20) | 1.35 (1.15, 1.60) | 0.86 (0.54, 1.38) |
| Model 4 | 1.15 (1.01, 1.31) | 1.20 (1.00, 1.42) | 0.76 (0.55, 1.04) | 0.66 (0.29, 1.47) |
Abbreviations: HR, hazard ratio; CI, confidence interval; CB, chronic bronchitis; COPD, chronic obstructive pulmonary disease;
Crude model: adjusted no factors associated with respiratory disease and PM2.5. Model 1: adjusted for gender (male and female), age (continuous variable), and BMI (continuous variable). Model 2: adjusted for Model 1 plus education level (below high school or high school or above), personal income (< 500 CNY or ≥ 500 CNY per month). Model 3: adjusted for Model 2 plus smoking status (yes or no), passive smoking status (yes or no), alcohol consumption (yes or no), physical activity (yes or no) and family history of related chronic respiratory diseases (yes or no). Model 4: adjusted for Model 3 + city (Rizhao, Shenyang, Taiyuan, and Tianjin).
In double-pollutant models (Table S5), the effect estimates of PM2.5 levels on all-cause chronic respiratory disease were enhanced after adjustment for NO2 or SO2 exposure levels. Similar increasing risk relationships were also observed for the PM2.5 levels linked to CB after NO2 or SO2 levels were controlled. Overall, the results of these two-pollutant analyses were consistent with that in the single-PM2.5 models.
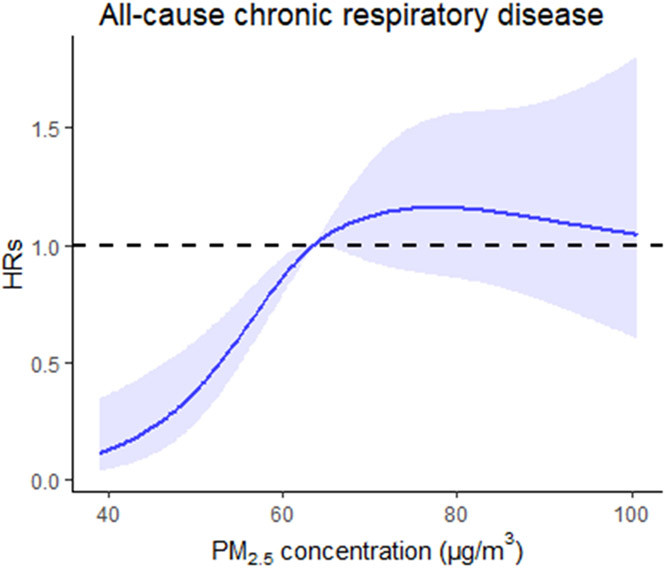
Fig. 2 illustrated the shape of C-R curve of PM2.5 and the onset of all-cause chronic respiratory disease after adjustment for multi-confounders. The curve of all-cause chronic respiratory disease showed no evidence of a threshold, with a steeply increasing linear pattern at the concentration range from approximately 40.00 to 75.00 μg/m3, and no increase in risks at the levels over 80.00 μg/m3.
Fig. 2.
The concentration-response curve for the association between long-term PM2·5 exposure and morbidity of all-cause chronic respiratory disease. The X-axis is the average concentration of PM2.5 in the cohort study. The Y-axis is the log relative risk. The solid line represents the mean risk estimate, and the dotted lines represent the 95% confidence intervals.
3.3. Stratified subgroups
In this study, a pooled stratified Cox proportional hazards analysis was carried out to detect the possible modification for the risks of incident chronic respiratory diseases associated with prolonged PM2.5 exposure by the selected characteristics in Model 4. Stronger effect estimates were observed in residents younger than 60 years old, males, the participants with lower education levels, smokers, and alcohol drinkers. However, only significant interaction of subgroups with PM2.5 exposure was reported in alcohol drinking status among the cohorts of all-cause chronic respiratory disease and cohort. For instance, the HR was 1.52 (95% CI: 1.21, 1.91) and 1.79 (95% CI: 1.31, 2.44) per 10 μg/m3 increase in PM2.5 among drinkers in all-cause chronic respiratory disease cohort and CB cohort, respectively. But no appreciable differences were revealed in the subgroups classified by other characteristics for all diseases. These results were illustrated in Table 3. Besides, the results of two-pollutant stratified models were summarized in Table S6-S7, and the results of all chronic respiratory diseases with ambient PM2.5 exposure were similar to those from each single-PM2.5 model.
Table 3.
Estimated HR for the incidence of chronic respiratory diseases per 10 μg/m3 increase of PM2.5 by stratified Cox proportional hazards models.
| Characteristics | All-cause chronic respiratory disease |
CB |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of onset | HRs | 95% CI | Pinteraction value | No. of onset | HRs | 95% CI | Pinteraction value | |
| Age | 482 | 0.35 | 276 | 0.19 | ||||
| < 60 | 303 | 1.31 | 1.11–1.54 | 178 | 1.32 | 1.06–1.64 | ||
| ≥ 60 | 179 | 1.04 | 0.83–1.29 | 98 | 1.08 | 0.80–1.45 | ||
| Gender | 0.77 | 0.92 | ||||||
| Male | 300 | 1.27 | 1.08–1.50 | 166 | 1.32 | 1.06–1.65 | ||
| Female | 182 | 1.09 | 0.88–1.35 | 110 | 1.03 | 0.78–1.36 | ||
| Education level | 0.43 | 0.76 | ||||||
| < High school | 287 | 1.23 | 1.04–1.46 | 171 | 1.37 | 1.09–1.71 | ||
| ≥ High school | 194 | 1.02 | 0.83–1.26 | 104 | 0.95 | 0.72–1.26 | ||
| Monthly income | 0.22 | 0.68 | ||||||
| < 500 CNY | 188 | 1.15 | 0.93–1.43 | 108 | 1.28 | 0.96–1.70 | ||
| ≥ 500 CNY | 294 | 1.14 | 0.96–1.34 | 168 | 1.15 | 0.92–1.43 | ||
| Smoking status | 0.28 | 0.16 | ||||||
| No | 246 | 1.08 | 0.90–1.30 | 144 | 1.07 | 0.84–1.36 | ||
| Yes | 236 | 1.23 | 1.02–1.50 | 132 | 1.37 | 1.06–1.76 | ||
| Passive smoking status | 0.44 | 0.38 | ||||||
| No | 234 | 1.09 | 0.90–1.31 | 147 | 1.16 | 0.91–1.47 | ||
| Yes | 242 | 1.22 | 1.01–1.46 | 127 | 1.02 | 1.00–1.03 | ||
| Alcohol intake | < 0.001 | < 0.001 | ||||||
| No | 310 | 0.99 | 0.84–1.17 | 181 | 0.98 | 0.79–1.22 | ||
| Yes | 172 | 1.52 | 1.21–1.91 | 95 | 1.79 | 1.31–2.44 | ||
| Physical activity | 0.34 | 0.60 | ||||||
| No | 310 | 1.13 | 0.91–1.40 | 106 | 1.11 | 0.83–1.49 | ||
| Yes | 172 | 1.14 | 0.97–1.35 | 170 | 1.23 | 0.99–1.52 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; CB, chronic bronchitis; COPD, chronic obstructive pulmonary disease.
Covariates: adjusted for age, sex, BMI, smoking status (yes or no), education level (below high school or high school or higher), personal income ( < 500 CNY and ≥ 500 CNY per month), smoking status (yes or no), passive smoking status (yes or no), alcohol intake (yes or no), physical activity (yes or no), family history of related chronic respiratory diseases (yes or no), and city (Rizhao, Shenyang, Taiyuan, and Tianjin).
Pinteraction values are the interaction results between PM2.5 and the variables.
3.4. Sensitivity analyses
In the sensitivity analyses, the results of the logistic regression analysis resembled to those displayed from the main analyses, the corresponding odds ratio (OR) were 1.15 (95% CI: 1.01, 1.31), 1.19 (95% CI: 1.00, 1.42), 0.76 (95% CI: 0.55, 1.04) and 0.65 (95% CI: 0.29, 1.46) for the incidence of all-cause respiratory disease, CB, asthma, and COPD, respectively (Table S8). In addition, after excluding participants who are exposed to occupational PM or use biofuels for cooking at the baseline, we found similar effect estimates of PM2.5 on occurrence of studied chronic respiratory diseases, and the corresponding HR were outlined in Table S9-S10. Overall, in most sensitivity analyses, no substantial changes were revealed in estimations of the relationship between long-term PM2.5 exposure and the occurrence of chronic respiratory diseases.
4. Discussion
This cohort study conducted in northern China reported significant associations between the risk of incident all-cause chronic respiratory disease, and CB linked to long-term ambient PM2.5 exposure with high levels. The corresponding HR were up to 1.15 (95% CI: 1.01, 1.31), and 1.20 (95% CI: 1.00, 1.42), respectively. These results suggested the significance of exploring the adverse respiratory outcomes affected by air particles and jointly with certain covariates effects in the following directions.
4.1. Chronic respiratory diseases
Prior literatures have demonstrated the deleterious impacts on chronic respiratory diseases when people were exposed to ambient PM2.5 for a short time. For instance, two meta-analyses reported that short-term PM2.5 exposure elevated 1.4% (95% CI: 0.7%, 2.1%) or 0.33% (95% CI: 0.21%, 0.46%) risk of COPD acute exacerbation or hospitalizations in China (Li et al., 2016; Wang et al., 2019). Furthermore, similar findings were obtained for the associations between PM2.5 exposure and hospital visits of CB, asthma, and COPD in China, with the strongest effects of 1.104 (95% CI: 1.032, 1.176) on CB at lag4, 1.072 (95% CI: 1.024, 1.119) on asthma at lag5 and 1.091 (95% CI: 1.047, 1.135) on COPD at lag 3 (Chi et al., 2019; Lin et al., 2020; Tian et al., 2018), separately. There was no analysis for multiple diseases classified by northern and southern regions in China in the same research, while the levels of PM2.5 pollution in northern areas were the most severe (He et al., 2017). The biological mechanism underlying PM2.5-caused respiratory morbidity remains evolving. It’s well known that the common ones involve the oxidative stress pathway and free radical reaction, triggering an inflammation cascade such as chronic airway inflammation, with the inflammatory cytokine receptors increasing. (Danesh Yazdi et al., 2019; Gowers et al., 2012; Grievink et al., 2000; Li et al., 2018). Another proposed mechanism reported that PM particles could interact with airway wall, resulting in the structural changes and remodeling (Churg et al., 2003; Gowers et al., 2012). These changes subsequently cause chronic airflow obstruction. Given that chronic airflow limitation is a major pathogeneses factor of chronic respiratory diseases (Kirkham and Barnes, 2013; Li et al., 2018), it can be assumed that PM2.5 may result in chronic respiratory diseases occurrence and development (Kirkham and Barnes, 2013). However, according to previous epidemiologic literature exploring the risks of long-term exposure to PM2.5 in association with chronic respiratory diseases, most of them focused on the prevalent outcome (Cai et al., 2014; Doiron et al., 2021; Hooper et al., 2018). Whether PM2.5 contributes to the incidence of chronic respiratory diseases remains scarce and controversial (Schikowski et al., 2014). This is the first longitudinal study that provide evidence for the impacts of prolonged exposure to air PM2.5 on incident chronic respiratory diseases in northern China. To diminish the influence of confounding as much as possible, demographics, BMI, lifestyle habits, family history of related disease, and city were adjusted in final model, and the analyses were conducted after the exclusion of subjects exposed to occupational PM or biofuels. The stable and significant positive association from each analysis confirmed the hypothesis mentioned above.
4.2. CB
In this study, a positive association was obtained between incident CB and chronic exposure to ambient PM2.5. The HR was 1.20 (95% CI: 1.00, 1.42) in the final adjusted model, which was similar to the results presented from some prior literatures. For example, a combined analysis of cross-sectional data in two large Europeans (654,835) from ESCAPE study revealed the adverse impact of prolonged PM2.5 exposure on wheezing with OR up to 1.16 (95% CI: 1.11, 1.21) per 5 μg/m3 (Doiron et al., 2017). What’s more, another investigation conducted in 39,844 American women found that CB increased by 1.18 (95% CI: 1.04, 1.34) per 4.4 μg/m3 increment in PM2.5 concentrations among never smokers (Hooper et al., 2018). However, the PM2.5 levels were only assessed in the period of 1 or 2 years from these two studies, which may ignore and mask the temporal impacts of long-term PM2.5 concentrations on CB morbidity. Additionally, all of them were performed in western countries, and the annual average concentrations of PM2.5 pollution in these places were often lower than 35 μg/m3. Apart from these positive results, a cohort study from Netherland also reported no significant link between PM2.5 and incident CB. The possible reason is that in this study, pollution data were evaluated in 2010 monitoring data. By contrast, CB incident information was collected from 2014 to 2017. The bias may be attributed to different accumulated concentrations of PM2.5 caused by long time interval (4–7 years). In special, the annual mean PM2.5 concentrations generally decreased during the follow-up years. As such, almost 10-year long-term moving average satellite-inversion data of time-varying PM2.5 level were distributed to subjects in a Cox regression model in the present article, which modified above limitations and provided more robust association results (Doiron et al., 2021).
4.3. Asthma
When it comes up to asthma, data on the impact of ambient PM2.5 were limited. In an analysis of 1057,722 participants from the Canadian cohort, an increment of 10 μg/m3 in PM2.5 was linked to an elevated risk of asthma onset with the HR of 1.03 (95% CI: 1.00, 1.10) (Weichenthal et al., 2017). The longitudinal data from ESCAPE cohorts across six European districts indicated positive but nonsignificant associations of PM exposure with incident asthma, with the effect estimate up to 1.08 (95% CI: 0.77, 1.51) for PM2.5 per 10 μg/m3 increment (Jacquemin et al., 2015) and the consistent result was reported among 23,704 American females (HR=1.66, 95% CI: 0.97, 2.86) per 10 μg/m3 increment (Young et al., 2014). In our study, when “city” was additionally adjusted for in model 4, the effect estimate was null-significant in asthma analysis with 0.76 (95% CI: 0.55, 1.04) per 10 μg/m3 increase of PM2.5. The heterogeneity in the associations between PM2.5 and asthma morbidity in distinct settings might be partially attributable to the differences in regional PM2.5 compositions (Ge et al., 2020; Li et al., 2019; Rosa et al., 2016; Siddique et al., 2020; Wang et al., 2014a, 2014b; Xu et al., 2016). To our best knowledge, PM exists in nature as a complex with chemicals, heavy metals, and biological substances attached (Pandey et al., 2013). As we know, over 20% of global total PAHs emissions in 2014 were contributed to China (Wang et al., 2014a). Another investigation observed that 16 PM2.5-associated polycyclic aromatic hydrocarbons (PAHs) in Zhengzhou were higher than those in certain cities of Korea and USA, which appeared to cause different asthma symptoms (Liu et al., 2016; Wang et al., 2014b). What’s more, due to the inconsistent development of economics and medical conditions in different cities, the incident rates of asthma distributed slightly differently among four cities in our study, which may explain the difference of the results between Model 4 and other models. These above results suggested that it’s necessary to carry out studies on the associations of ambient PM2.5 components, socioeconomics and medical conditions with incidence of asthma in the future direction.
4.4. COPD
Our study indicated null relationship between PM2.5 exposure and onset of COPD, and the HR was 0.66 (95% CI: 0.29, 1.47) with a 10 μg/m3 increment in PM2.5. After the potential risks such as occupational PM exposure, the burning of biomass and fossil fuels were eliminated in sensitive analyses, the main results seemed no changes in direction of associations. These findings only indicated possible trend of inverse associations, but not real inverse associations, which agreed with those from certain prior literatures. An English (Atkinson et al., 2015) and a Canadian cohort study (Gan et al., 2013) found that the HR linked to incidence of COPD were 1.05 (95% CI: 0.98, 1.13) for PM2.5 with each 10 μg/m3 increment and 1.02 (95% CI: 0.98, 1.07) with a 1.58 μg/m3 increase in PM2.5, separately. But the average levels of PM2.5 pollution (range from 4.1 to 12.9 μg/m3) in these two works was much lower than that (66.51 μg/m3) in our research. A US nurses’ Health Study containing 121,701 nurses revealed the HR of incident COPD was 0.93 (95% CI: 0.66, 1.31) per 10 μg/m3 increment of PM2.5, however, the study was only recruited females for investigation (Fisher et al., 2016).
Despite the above irrelevant results, whether chronic exposure to air pollution is linked to increased morbidity of COPD is inconclusive. Several articles have documented the evidence for potential adverse impacts of PM2.5 on COPD. A meta-analysis analyzed a positive association of long-term exposure to PM2.5 with the COPD incidence by combining six prospective cohort studies (Park et al., 2021). Among European subjects, chronic PM2.5 exposure was related to the elevated emergency room visits (ERVs), hospital admissions or outpatient visits of COPD with the HR up to 1.17 (95% CI: 1.06, 1.29) (Liu et al., 2021). In a Canadian cohort with Toronto residents, PM2.5 affected COPD morbidity and the HR was 1.06 (95% CI: 1.04, 1.08) per 3.2 μg/m3 increase in PM2.5 (Weichenthal et al., 2017). Furthermore, there were two Asian cohort articles containing Korean elders and Taiwan individuals exposed to relatively high-settings revealed significant positive relationships of COPD development with PM2.5 levels in the adjusted HR (Guo et al., 2018; Han et al., 2020). The possible explanations for the inconsistent results compared with ours is that most of the above articles identified new-onset COPD by using records of hospital admission, which may capture more COPD incidence than the reals because hospital admissions may incorporate acute exacerbation of previously diagnosed as COPD cases, leading to the overestimated effect values. To provide more comparable evaluations, standard for defining incident of COPD in further literatures should be uniformed. What’s more, the subjects from Korean and European works were the elderly with more sensitivity to air pollution than other residents (Ferreira et al., 2016), causing bias of overestimated effect values. These differed results also may be attributed to the adjustment of confounding factors (whether adjusted for other air pollutants, smoking habits or not), the difference of racial distinction, the evaluation methods of pollutants and the identification of COPD morbidity. In addition, considering short-term air pollution exposure was significantly associated with acute exacerbation of COPD, and high correlation was observed in short- and long-term exposure (Schikowski et al., 2014), it’s difficult to explore the single chronic effect of air pollution on COPD. Last, it’s worth noting that long-term follow-up time interval (9.8 years) might contribute to survival bias. As a result, the number of new COPD cases in the study period may be less than real incidents. For instance, in this study, only 14 individuals were new cases of COPD, and the incidence of COPD was 0.04%. The limited sample size may lead to underestimated effect estimates in this study. Herein, it implies that more studies should be conducted to assess the COPD morbidity attributable to ambient air pollution. In addition, future research directions can target to the effects of air pollution on COPD symptom classifications.
4.5. C-R curves
Although the C-R curve in this study showed no evidence of a threshold for all-cause respiratory disease, and disagreed with the shape in previous literature studied on respiratory admissions for short-term exposure to PM2.5 (Yao et al., 2020), generally, the incident risk of all-cause respiratory disease was observed under high PM2.5 exposure ranges in both articles. In this study, the risk revealed when PM2.5 was over 63.00 μg/m3 level, and it exceeded the annual average threshold for the air PM2.5 exposure levels set by WHO (5 μg/m3) (World Health Organization, 2021). We also found that after PM2.5 level was more than 80 μg/m3, the curve showed a slightly downward trend, which suggested that the following researches should provide more comprehensive and credible evidence on the shape of curve among residents who are exposed to high PM2.5 pollution levels. Notably, the annual average concentrations of PM2.5 among four cities in northern China was 66.51 μg/m3, which was about 2–16 times higher than those of western countries (4.10 μg/m3-26.91 μg/m3) (Atkinson et al., 2015; Carey et al., 2016; Fisher et al., 2016; Gan et al., 2013; Weichenthal et al., 2017). Due to the heavier PM2.5 pollution in northern China compared with western countries, we were unable to determine the C-R curve in the relatively lower PM2.5 level ranges. These findings suggested that more comprehensive and valid data of cohorts are needed to explore the influence pattern of air pollution on chronic respiratory diseases morbidity, and hence provide the basis and directions in evaluation of the respiratory damages induced by air pollutions, ascertainment of threshold level, and prevention for chronic respiratory damages.
4.6. Effect modification
As to the effect modification, no significant difference was obtained from the relationships between PM2.5 exposure and incident chronic respiratory diseases by most characteristics (e.g., age, gender, education level, and personal behavior habits). However, interestingly, we found alcohol drinkers were more sensitive to the ambient PM2.5 in all-cause respiratory disease cohort and CB cohort than non-alcoholic drinkers. The similar trend was also observed by Li et al. and colleges based on China-PAR project, though null interactions were observed of PM2.5 exposure with drinking status (Li et al., 2020a). Yet, the role of alcohol drinking status in this process remains unclear, which is a topic that requires further research and verification.
4.7. Strengths and Limitations
This study has several strengths. It is a large cohort study based on Chinese adults with a long period of almost 10-year follow-up to access the relationship and the C-R curves between prolonged ambient PM2.5 exposure and onset of chronic respiratory diseases. To our best knowledge, the information from the C-R plot plays an important role in public health assessment for the prevention of diseases. Besides, almost 40 thousand residents’ epidemiological information were collected in four cities, where data of PM2.5 pollution levels represented the broad range of northern China. What’s more, compared with the exposure assessment such us monitoring station or land-use regression model in part of the prior analysis, the estimate of individual PM2.5 exposure in our study was derived from high resolution (1 km × 1 km) through an ensemble machine-learning spatiotemporal. In addition, among diverse risk factors, smoking is the most important one for respiratory diseases development but little articles eliminate the effects of second-hand smokers. This is the first study that considered passive smoking status as an adjustment variable. An increasing number of epidemiological and biological evidence showed that second-hand smoke exists as a major risk factor of respiratory diseases in never smokers. It would irritate the airways, cause or worsen asthmatic diseases and allergies (Braun et al., 2020; Kim et al., 2018). As such, it’s recommended that more researches should attach on the modification of second-hand smoke. Finally, air pollutants exist as complex mixture in nature, some literatures also indicated that gas pollutants such as NO2, O3, and SO2 may have adverse impact on chronic respiratory diseases (Doiron et al., 2019; Park et al., 2021; Saygın et al., 2017; Zhang et al., 2018), and the impacts of ambient PM2.5 exposure on chronic respiratory diseases might be affected by gas pollutants exposure. Thus, we conducted bi-pollutant models such as PM2.5-NO2 and PM2.5-SO2 to evaluate the impacts of multi-pollutant exposure on chronic respiratory outcomes. Analysis of the above data provided rare but valuable evidence of unfavorable impacts on all-cause respiratory diseases, CB, and asthma morbidity influenced by long-term PM2.5 exposure in China.
Despite these strengths, some limitations should be noticed. Firstly, the design of a retrospective cohort study caused the bias of recall for certain self-reported contents, inducing exercise, lifestyle habits, etc. Further study directions could target to the risk influences of prolonged air pollution exposure on incident chronic respiratory diseases collected from hospital admissions. Secondly, the effects induced by PM2.5 may differ in different districts of China because of the various attachments of PM2.5. Nevertheless, we are unable to carry out the related analyses due to the lack of data on mixture compositions of PM2.5 (Wang et al., 2014a, 2014b; Zhu et al., 2018). More specific respiratory damages involved to particulate components should be assessed in the future. Thirdly, due to the lack data of O3 levels before 2013 in mainland China, PM2.5-O3 model was not conducted in this study. Further studies are needed to explore the impacts of the mixed effects of PM2.5 and O3 exposure on chronic respiratory outcomes. Fourthly, lifestyles such as smoking and drinking status may be potential risk factors for chronic respiratory diseases, but we lack time scale data of these covariates, and they were not analyzed as time-varying covariates, which is one of the limitations in our study. More researches needed to evaluate the confounding effects of time-varying lifestyles on the incidence of chronic respiratory diseases linked to prolonged PM2.5 exposure in the future. Additionally, an increasing number of researches have indicated that certain common biomarker known as C-reactive protein (CRP) was associated with respiratory diseases development, but it was not available in the current study (Lock-Johansson et al., 2014). Therefore, to provide more comprehensive and powerful results, the information of common and possible biomarkers at the baseline survey should be provided in the following studies. Finally, cautions should be treated to the results. Indoor fuel contamination might be the potential risk factor of adverse respiratory outcomes (Chan et al., 2019). Yet, it was not considered as a confounder because the lack of indoor pollutants’ data in our study. Future directions target to independent but comprehensive multi-center studies with uniformed participants’ characteristics, complete disease-related information, and more accurate methods of air pollution estimation.
5. Conclusions
In conclusion, we found that prolonged exposure to PM2.5 pollution at high levels was a risk factor contributing to the increased morbidity of chronic respiratory diseases among adults in northern China. The evidence provided the local governments a better prospect to regulate and formulate the strategies on PM2.5 pollution emissions for prevention from unfavorable outcomes of chronic respiratory diseases in China.
Supplementary Material
Acknowledgment
This work was supported by the Special Environmental Research Fund for Public Welfare from Ministry of Environmental Protection of China (No. 200709048), and the National Key Research and Development Program of China (2017YFC0211600 and 2017YFC0211605 and 2017YFC0211704). The work of Yang Liu is supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (Grant No. 1R01ES032140). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ecoenv.2022.114025.
Data Availability
The data that has been used is confidential.
References
- Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG, 2015. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup. Environ. Med 72, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Klingelhöfer D, Oremek GM, Quarcoo D, Groneberg DA, 2020. Influence of second-hand smoke and prenatal tobacco smoke exposure on biomarkers, genetics and physiological processes in children-an overview in research insights of the last few years. Int J. Environ. Res Public Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Schikowski T, Adam M, Buschka A, Carsin AE, Jacquemin B, Marcon A, Sanchez M, Vierkötter A, Al-Kanaani Z, Beelen R, Birk M, Brunekreef B, Cirach M, Clavel-Chapelon F, Declercq C, de Hoogh K, de Nazelle A, Ducret-Stich RE, Valeria Ferretti V, Forsberg B, Gerbase MW, Hardy R, Heinrich J, Hoek G, Jarvis D, Keidel D, Kuh D, Nieuwenhuijsen MJ, Ragettli MS, Ranzi A, Rochat T, Schindler C, Sugiri D, Temam S, Tsai MY, Varraso R, Kauffmann F, Krämer U, Sunyer J, Künzli N, Probst-Hensch N, Hansell AL, 2014. Cross-sectional associations between air pollution and chronic bronchitis: an ESCAPE meta-analysis across five cohorts. Thorax 69, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Camargo CA Jr., Weiss ST, Zhang S, Willett WC, Speizer FE, 1999. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch. Intern Med 159, 2582–2588. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Wu F, Chow JC, Lee SC, Li Y, Chen SW, An ZS, Fung KK, Watson JG, Zhu CS, Liu SX, 2005. Characterization and source apportionment of atmospheric organic and elemental carbon during fall and winter of 2003 in Xi’an, China. Atmos. Chem. Phys 5, 3127–3137. [Google Scholar]
- Cao Q, Rui G, Liang Y, 2018. Study on PM2.5 pollution and the mortality due to lung cancer in China based on geographic weighted regression model. BMC Public Health 18, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM, Anderson HR, Atkinson RW, Beevers S, Cook DG, Dajnak D, Gulliver J, Kelly FJ, 2016. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup. Environ. Med 73, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Kurmi OP, Bennett DA, Yang L, Chen Y, Tan Y, Pei P, Zhong X, Chen J, Zhang J, Kan H, Peto R, Lam K, Chen Z, China Kadoorie Biobank Collaborative Group, 2019. Solid fuel use and risks of respiratory diseases. A cohort study of 280,000 Chinese never-smokers. Am. J. Respir. Crit. Care Med 199, 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi R, Li H, Wang Q, Zhai Q, Wang D, Wu M, Liu Q, Wu S, Ma Q, Deng F, Guo X, 2019. Association of emergency room visits for respiratory diseases with sources of ambient PM(2.5). J. Environ. Sci. (China) 86, 154–163. [DOI] [PubMed] [Google Scholar]
- Churg A, Brauer M, 1997. Human lung parenchyma retains PM2.5. Am. J. Respir. Crit. Care Med 155, 2109–2111. [DOI] [PubMed] [Google Scholar]
- Churg A, Brauer M, del Carmen Avila-Casado M, Fortoul TI, Wright JL, 2003. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect 111, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH, 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J, 2019. Long-term exposure to PM(2.5) and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ. Int 130, 104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doiron D, de Hoogh K, Probst-Hensch N, Mbatchou S, Eeftens M, Cai Y, Schindler C, Fortier I, Hodgson S, Gaye A, Stolk R, Hansell A, 2017. Residential air pollution and associations with wheeze and shortness of breath in adults: a combined analysis of cross-sectional data from two large European cohorts. Environ. Health Perspect 125, 097025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, Hansell AL, 2019. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur. Respir. J 54, 1802140. [DOI] [PubMed] [Google Scholar]
- Doiron D, Bourbeau J, de Hoogh K, Hansell AL, 2021. Ambient air pollution exposure and chronic bronchitis in the Lifelines cohort. Thorax 76, 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM, 2006. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama 295, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu K, Bell ML, 2012. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ. Health Perspect 120, 1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira TM, Forti MC, de Freitas CU, Nascimento FP, Junger WL, Gouveia N, 2016. Effects of particulate matter and its chemical constituents on elderly hospital admissions due to circulatory and respiratory diseases. Int J. Environ. Res Public Health 13, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JA, Puett RC, Hart JE, Camargo CA Jr., Varraso R, Yanosky JD, Laden F, 2016. Particulate matter exposures and adult-onset asthma and COPD in the nurses’ health study. Eur. Respir. J 48, 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M, 2013. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am. J. Respir. Crit. Care Med 187, 721–727. [DOI] [PubMed] [Google Scholar]
- GBD, 2017. Causes of Death Collaborators., 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Chronic Respiratory Disease Collaborators, 2020. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med 8, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Yang H, Lu X, Wang S, Zhao Y, Huang J, Xi Z, Zhang L, Li R, 2020. Combined exposure to formaldehyde and PM(2.5): Hematopoietic toxicity and molecular mechanism in mice. Environ. Int 144, 106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers AM, Cullinan P, Ayres JG, Anderson HR, Strachan DP, Holgate ST, Mills IC, Maynard RL, 2012. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology 17, 887–898. [DOI] [PubMed] [Google Scholar]
- Grievink L, Smit HA, Brunekreef B, 2000. Anti-oxidants and air pollution in relation to indicators of asthma and COPD: a review of the current evidence. Clin. Exp. Allergy 30, 1344–1354. [DOI] [PubMed] [Google Scholar]
- Guan WJ, Zheng XY, Chung KF, Zhong NS, 2016. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet 388, 1939–1951. [DOI] [PubMed] [Google Scholar]
- Guo C, Zhang Z, Lau AKH, Lin CQ, Chuang YC, Chan J, Jiang WK, Tam T, Yeoh EK, Chan TC, Chang LY, Lao XQ, 2018. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health 2, e114–e125. [DOI] [PubMed] [Google Scholar]
- Guo H, Li W, Wu J, 2020. Ambient PM2.5 and annual lung cancer incidence: a nationwide study in 295 Chinese counties. Int J. Environ. Res Public Health 17, 1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Oh J, Lim YH, Kim S, Hong YC, 2020. Long-term exposure to fine particulate matter and development of chronic obstructive pulmonary disease in the elderly. Environ. Int 143, 105895. [DOI] [PubMed] [Google Scholar]
- He MZ, Zeng X, Zhang K, Kinney PL, 2017. Fine particulate matter concentrations in Urban Chinese Cities, 2005-2016: a systematic review. Int J. Environ. Res Public Health 14, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LG, Young MT, Keller JP, Szpiro AA, O’Brien KM, Sandler DP, Vedal S, Kaufman JD, London SJ, 2018. Ambient Air Pollution and Chronic Bronchitis in a Cohort of U.S. Women. Environ. Health Perspect 126, 027005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, Bellisario V, Buschka A, Bono R, Brunekreef B, Cai Y, Cirach M, Clavel-Chapelon F, Declercq C, de Marco R, de Nazelle A, Ducret-Stich RE, Ferretti VV, Gerbase MW, Hardy R, Heinrich J, Janson C, Jarvis D, Al Kanaani Z, Keidel D, Kuh D, Le Moual N, Nieuwenhuijsen MJ, Marcon A, Modig L, Pin I, Rochat T, Schindler C, Sugiri D, Stempfelet M, Temam S, Tsai MY, Varraso R, Vienneau D, Vierkötter A, Hansell AL, Krämer U, Probst-Hensch NM, Sunyer J, Künzli N, Kauffmann F, 2015. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE). Environ. Health Perspect 123, 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Ko HJ, Kwon JH, Lee JM, 2018. Exposure to secondhand smoke and risk of cancer in never smokers: a meta-analysis of epidemiologic studies. Int J. Environ. Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Austin E, Koutrakis P, Dominici F, Schwartz J, Zanobetti A, 2015. PM2.5 and survival among older adults: effect modification by particulate composition. Epidemiology 26, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham PA, Barnes PJ, 2013. Oxidative stress in COPD. Chest 144, 266–273. [DOI] [PubMed] [Google Scholar]
- Li J, Sun S, Tang R, Qiu H, Huang Q, Mason TG, Tian L, 2016. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J. Chron. Obstruct Pulmon Dis 11, 3079–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhu LL, Wang N, Liu M, Wang S, Qu J, 2019. Characteristics and source apportionment of PAHs in atmospheric particles PM2.5 in Shenyang. Adm. Tech. Environ. Monit 31, 24–28. [Google Scholar]
- Li JX, Lu XF, Liu FC, Liang FC, Huang KY, Yang XL, Xiao QY, Chen JC, Liu XQ, Cao J, Chen SF, Shen C, Yu L, Lu FH, Wu XP, Zhao LC, Wu XG, Li Y, Hu DS, Huang JF, Zhu M, Liu Y, Shen HB, Gu DF, 2020a. Chronic Effects of High Fine Particulate Matter Exposure on Lung Cancer in China. Am. J. Respir. Crit. Care Med 202, 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhou R, Zhang J, 2018. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol. Lett 15, 7506–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cao X, Guo M, Xie M, Liu X, 2020b. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. Bmj 368, m234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FC, Xiao Q, Huang K, Yang X, Liu F, Li J, Lu X, Liu Y, Gu D, 2020. The 17-y spatiotemporal trend of PM(2.5) and its mortality burden in China. Proc. Natl. Acad. Sci. USA 117, 25601–25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Li D, Lu JM, Yu ZB, Zhu Y, Shen P, Tang ML, Jin MJ, Lin HB, Shui LM, Chen K, Wang JB, 2020. Short-term associations between ambient fine particulate matter pollution and hospital visits for chronic obstructive pulmonary disease in Yinzhou District, China. Environ. Sci. Pollut. Res Int 27, 21647–21653. [DOI] [PubMed] [Google Scholar]
- Lin Y, Yan XL, Lian FC, Huang KY, Liu FC, L JX, Xiao QY, Chen JC, Liu XQ, Cao J, Chen SF, Shen C, Yu L, Lu FH, Wu XP, Zha LC, Wu XG, Li Y, Hu DS, Huang JF, Lu XF, Liu Y, Gu DF, 2021. Benefits of active commuting on cardiovascular health modified by ambient fine particulate matter in China: a prospective cohort study. Ecotoxicol. Environ. Saf 224, 112641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu C, Jiang ZY, Gu A, 2016. Association of polycyclic aromatic hydrocarbons and asthma among children 6-19 years: NHANES 2001-2008 and NHANES 2011-2012. Respir. Med 110, 20–27. [DOI] [PubMed] [Google Scholar]
- Liu S, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, Magnusson PKE, Rizzuto D, Hvidtfeldt UA, Raaschou-Nielsen O, Wolf K, Hoffmann B, Brunekreef B, Strak M, Chen J, Mehta A, Atkinson RW, Bauwelinck M, Varraso R, Boutron-Ruault MC, Brandt J, Cesaroni G, Forastiere F, Fecht D, Gulliver J, Hertel O, de Hoogh K, Janssen NAH, Katsouyanni K, Ketzel M, Klompmaker JO, Nagel G, Oftedal B, Peters A, Tjønneland A, Rodopoulou SP, Samoli E, Bekkevold T, Sigsgaard T, Stafoggia M, Vienneau D, Weinmayr G, Hoek G, Andersen ZJ, 2021. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ. Int 146, 106267. [DOI] [PubMed] [Google Scholar]
- Lock-Johansson S, Vestbo J, Sorensen GL, 2014. Surfactant protein D, Club cell protein 16, Pulmonary and activation-regulated chemokine, C-reactive protein, and Fibrinogen biomarker variation in chronic obstructive lung disease. Respir. Res 15, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena KK, Ethan CJ, Yu Y, Shale K, Liu F, 2019. Ambient air pollution and respiratory mortality in Xi’an, China: a time-series analysis. Respir. Res 20, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Patel DK, Khan AH, Barman SC, Murthy RC, Kisku GC, 2013. Temporal distribution of fine particulates (PM2.5:PM10), potentially toxic metals, PAHs and Metal-bound carcinogenic risk in the population of Lucknow City, India. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng 48, 730–745. [DOI] [PubMed] [Google Scholar]
- Park J, Kim HJ, Lee CH, Lee CH, Lee HW, 2021. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ. Res 194, 110703. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, Benedetti C, Peli M, Donna F, Nazzaro M, Fedrighi C, Zoni S, Marcon A, Zimmerman N, Wright R, Lucchini R, 2016. Association between personal exposure to ambient metals and respiratory disease in Italian adolescents: a cross-sectional study. BMC Pulm. Med 16, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygın M, Gonca T, Öztürk Ö, Has M, Çalışkan S, Has ZG, Akkaya A, 2017. To investigate the effects of air pollution (PM10 and SO(2)) on the respiratory diseases asthma and chronic obstructive pulmonary disease. Turk. Thorac. J 18, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T, Mills IC, Anderson HR, Cohen A, Hansell A, Kauffmann F, Krämer U, Marcon A, Perez L, Sunyer J, Probst-Hensch N, Künzli N, 2014. Ambient air pollution: a cause of COPD? Eur. Respir. J 43, 250–263. [DOI] [PubMed] [Google Scholar]
- Siddique AE, Rahman M, Hossain MI, Karim Y, Hasibuzzaman MM, Biswas S, Islam MS, Rahman A, Hossen F, Mondal V, Banna HU, Huda N, Hossain M, Sultana P, Nikkon F, Saud ZA, Haque A, Nohara K, Xin L, Himeno S, Hossain K, 2020. Association between chronic arsenic exposure and the characteristic features of asthma. Chemosphere 246, 125790. [DOI] [PubMed] [Google Scholar]
- Tian Y, Xiang X, Juan J, Song J, Cao Y, Huang C, Li M, Hu Y, 2018. Short-term effects of ambient fine particulate matter pollution on hospital visits for chronic obstructive pulmonary disease in Beijing, China. Environ. Health 17, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Geng NB, Xu YF, Zhang WD, Tang XY, Zhang RQ, 2014a. PAHs in PM2.5 in Zhengzhou: concentration, carcinogenic risk analysis, and source apportionment. Environ. Monit. Assess 186, 7461–7473. [DOI] [PubMed] [Google Scholar]
- Wang K, Hao Y, Au W, Christiani DC, Xia ZL, 2019. A systematic review and meta-analysis on short-term particulate matter exposure and chronic obstructive pulmonary disease hospitalizations in China. J. Occup. Environ. Med 61, e112–e124. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ying Q, Hu J, Zhang H, 2014b. Spatial and temporal variations of six criteria air pollutants in 31 provincial capital cities in China during 2013-2014. Environ. Int 73, 413–422. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi L, Lee M, Liu P, Di Q, Zanobetti A, Schwartz JD, 2017. Long-term exposure to PM2.5 and mortality among older adults in the Southeastern US. Epidemiology 28, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Bai L, Hatzopoulou M, Van Ryswyk K, Kwong JC, Jerrett M, van Donkelaar A, Martin RV, Burnett RT, Lu H, Chen H, 2017. Long-term exposure to ambient ultrafine particles and respiratory disease incidence in in Toronto, Canada: a cohort study. Environ. Health 16, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2019. Ten threats to Global Health in 2019. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019, Accessed date: 5 April 2019.
- World Health Organization, 2021. WHO global air quality guidelines. https://www.who.int/news-room/q-a-detail/who-global-air-quality-guidelines, Accessed date:22 September 2021.
- Wu S, Deng F, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Wei H, Hao Y, Lv H, Lu X, Guo X, 2013. Blood pressure changes and chemical constituents of particulate air pollution: results from the healthy volunteer natural relocation (HVNR) study. Environ. Health Perspect 121, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Chang HH, Geng G, Liu Y, 2018. An ensemble machine-learning model to predict historical PM(2.5) concentrations in china from satellite data. Environ. Sci. Technol 52, 13260–13269. [DOI] [PubMed] [Google Scholar]
- Xu H, Cao J, Chow JC, Huang RJ, Shen Z, Chen LW, Ho KF, Watson JG, 2016. Inter-annual variability of wintertime PM2.5 chemical composition in Xi’an, China: Evidences of changing source emissions. Sci. Total Environ 546–555. [DOI] [PubMed] [Google Scholar]
- Yan MF, Li CK, Zhang LW, Chen X, Yang XL, Shan AQ, Li XJ, Wu H, Ma Z, Zhang Y, Guo PY, Dong GH, Liu YM, Chen J, Wang T, Zhao BX, Tang NJ, 2020. Association between long-term exposure to Sulfur dioxide pollution and hypertension incidence in northern China: a 12-year cohort study. Environ. Sci. Pollut. Res Int 27, 21826–21835. [DOI] [PubMed] [Google Scholar]
- Yang XL, Zhang LW, Chen X, Liu FC, Shan AQ, Liang FC, Li XJ, Wu H, Yan MF, Ma Z, Dong GH, Liu YM, Chen J, Wang T, Zhao BX, Liu Y, Gu DF, Tang NJ, 2021. Long-term exposure to ambient PM 2.5 and stroke mortality among urban residents in northern China. Ecotoxicol. Environ. Saf 213, 112063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Wang Y, Williams C, Xu C, Kartsonaki C, Lin Y, Zhang P, Yin P, Lam KBH, 2020. The association between high particulate matter pollution and daily cause-specific hospital admissions: a time-series study in Yichang, China. Environ. Sci. Pollut. Res Int 27, 5240–5250. [DOI] [PubMed] [Google Scholar]
- Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ, 2014. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am. J. Respir. Crit. Care Med 190, 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LW, Chen X, Xue XD, Sun M, Han B, Li CP, Ma J, Yu H, Sun ZR, Zhao LJ, Zhao BX, Liu YM, Chen J, Wang PP, Bai ZP, Tang NJ, 2014. Long-term exposure to high particulate matter pollution and cardiovascular mortality: a 12-year cohort study in four cities in northern China. Environ. Int 62, 41–47. [DOI] [PubMed] [Google Scholar]
- Zhang R, Jing J, Tao J, Hsu SC, Wang G, Cao J, Lee CSL, Zhu L, Chen Z, Zhao Y, Shen Z, 2013. Chemical characterization and source apportionment of PM2.5 in Beijing: seasonal perspective. Atmos. Chem. Phys 13, 7053–7074. [Google Scholar]
- Zhang Z, Wang J, Lu W, 2018. Exposure to nitrogen dioxide and chronic obstructive pulmonary disease (COPD) in adults: a systematic review and meta-analysis. Environ. Sci. Pollut. Res Int 25, 15133–15145. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Huang L, Li J, Ying Q, Zhang H, Liu X, Liao H, Li N, Liu Z, Mao Y, Fang H, Hu J, 2018. Sources of particulate matter in China: Insights from source apportionment studies published in 1987-2017. Environ. Int 115, 343–357. [DOI] [PubMed] [Google Scholar]
- Zuo B, Liu C, Chen R, Kan H, Sun J, Zhao J, Wang C, Sun Q, Bai H, 2019. Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere 237, 124497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.