Abstract
Evidence from clinical and experimental studies of human and chimpanzees suggests that hepatitis C virus (HCV) envelope glycoprotein E2 is a key antigen for developing a vaccine against HCV infection. To identify B-cell epitopes in HCV E2, six murine monoclonal antibodies (MAbs), CET-1 to -6, specific for HCV E2 protein were generated by using recombinant proteins containing E2t (a C-terminally truncated domain of HCV E2 [amino acids 386 to 693] fused to human growth hormone and glycoprotein D). We tested whether HCV-infected sera were able to inhibit the binding of CET MAbs to the former fusion protein. Inhibitory activity was observed in most sera tested, which indicated that CET-1 to -6 were similar to anti-E2 antibodies in human sera with respect to the epitope specificity. The spacial relationship of epitopes on E2 recognized by CET MAbs was determined by surface plasmon resonance analysis and competitive enzyme-linked immunosorbent assay. The data indicated that three overlapping epitopes were recognized by CET-1 to -6. For mapping the epitopes recognized by CET MAbs, we analyzed the reactivities of CET MAbs to six truncated forms and two chimeric forms of recombinant E2 proteins. The data suggest that the epitopes recognized by CET-1 to -6 are located in a small domain of E2 spanning amino acid residues 528 to 546.
Most individuals who contact hepatitis C virus (HCV), responsible for most cases of posttransfusion and non-A, non-B hepatitis (4), develop a chronic infection which is a major cause of liver cirrhosis and hepatocellular carcinoma and more rarely leads to liver cancer (1, 33). Despite the recognition of HCV as an important cause of morbidity throughout the world and the advances in epidemiology and molecular virology, the pathogenesis of this disease and the molecular mechanism of viral persistence with high rates are not fully understood (7).
HCV, a positive-stranded RNA virus with a genomic size of about 9.5 kb, has one large open reading frame that encodes a polypeptide of 3,011 amino acids (aa). The single polypeptide precursor processed by cellular and viral proteases results in a core protein (C), two glycosylated envelope proteins (E1 and E2/NS1), and nonstructural proteins (NS2 to NS5) (5, 16, 39).
Comparative genome alignments suggest that the HCV E2 protein corresponds to the flavivirus NS1 glycoprotein and the major pestivirus envelope protein gp53/gp55 (gp53 in bovine viral diarrhea virus and gp55 in hog cholera virus) (26). Both flaviviral NS1 and pestiviral gp53/55 are known to elicit protective antibodies in hosts vaccinated with these proteins (32, 44).
In a chimpanzee model study of HCV, in vivo protection was achieved by vaccination with recombinant HCV E1/E2 proteins, and the anti-E2 antibody titers were shown to correlate with the protection (3). In another model study of chimpanzee, antibodies present in patient sera could prevent infection when incubated in vitro with virus prior to infection (8). In addition, HCV E2 protein expressed in Chinese hamster ovary (CHO) cells bound to human cells with high affinity, and sera from protected chimpanzees contained antibodies which neutralized the binding of E2 protein to target cells (31). Thus, several pieces of evidence suggest that the envelope glycoprotein E2 is a key antigen for vaccine development against HCV infection (21, 24, 30, 38).
Several observations suggest that hypervariable region 1 (HVR-1), which is located at the N terminus of E2 (12, 18, 42) and contains cytotoxic T-lymphocyte epitopes and several B-cell linear epitopes (35, 43, 46), may be involved in the neutralization of HCV, and antibodies directed at this region are shown to prevent binding of viruses (9, 19, 20, 37). However, the higher genetic variability of this region may allow virus to escape immune surveillance, and the variability of the HCV genome has posed serious problems in development of a broadly reactive vaccine against HCV infection (11, 17, 29, 41). In addition, some studies have reported the existence of B-cell epitopes within the HCV E2 protein downstream of HVR-1. However, detailed mapping of those regions has not been done (27, 28, 40).
In this study, to identify epitopes of HCV E2 glycoprotein, we generated six monoclonal antibodies (MAbs), CET-1 to -6, against HCV E2 antigen by using recombinant fusion proteins. To characterize the MAbs, we evaluated the competitive reactivity to E2 protein with HCV-immune sera and performed surface plasmon resonance (SPR) analyses. Finally, from the relative reactivities of MAbs to chimeric and truncated forms of E2 protein, we could identify a domain containing the epitopes of CET MAbs in the E2 region.
MATERIALS AND METHODS
Mice and MAbs.
Five- to six-week-old BALB/c female mice (Charles River Laboratory, Osaka, Japan) were immunized with 10 μg of a truncated form of HCV E2 protein (amino acid residues 386 to 693) lacking the C-terminal hydrophobic region (E2t) fused to human growth hormone (hGH) or herpes simplex virus type 1 (HSV-1) glycoprotein D (gD) (hGHE2t or gDE2t) three times by intraperitoneal injection. The first immunization was done with complete Freund’s adjuvant (Sigma, St. Louis, Mo.); the second and the third immunizations were done with incomplete Freund’s adjuvant (Sigma). Three days after the third injection, splenocytes obtained from the immunized mice were fused with SP2/0 cells as described by Galfre et al. (10). Hybridoma cell lines were tested by enzyme-linked immunosorbent assay (ELISA). Hybridomas producing MAbs with high affinity to E2t or gD were selected and subcloned twice. MAbs against E2t protein were designated CET MAbs and a MAb against gD was named 3H9. MAbs were purified from ascites fluid by protein A-Sepharose CL-4B (Pharmacia Biotech, Uppsala, Sweden) affinity column chromatography. Biotinylation of CET MAbs was performed with EZ-Link Sulfo-NHS (N-hydroxysuccinimide)-LC-Biotin (Pierce, Rockford, Ill.) according to the manufacturer’s instructions.
Fusion proteins.
To establish the recombinant CHO cell lines expressing gDE2t, we constructed the expression vector pMT3-gDE2t as follows. The cDNA encoding a C-terminally truncated gD (aa 1 to 326) was amplified by PCR from HSV-1 (KOS strain) and then inserted into pMT3 to produce pMT3-gD. Then the cDNA of HCV-N, genotype 1b, encoding the E2t region spanning amino acid residues 386 to 693 was obtained by PCR amplification and fused in frame to the cDNA encoding amino acid residue 326 of gD in pMT3-gD (23). The gDE2t protein in culture media of the recombinant CHO cells was purified by affinity column chromatography using CNBr-activated Sepharose-4B linked with anti-gD MAb. hGHE2t was also expressed in CHO cells and purified as described previously (23).
Human HCV sera.
Human sera were obtained from patients with chronic hepatitis C who visited Korea Cancer Center Hospital and College of Medicine, Seoul National University, Seoul, Korea. A total of eight samples were heat inactivated at 56.5°C for 30 min prior to experiments.
ELISA.
To detect murine MAbs specifically binding to E2t or gD, wells of a Maxisorp Immunoplate (Nunc InterMed, Roskilde, Denmark) were coated with hGHE2t, gDE2t, hGH, or gD at a concentration of 1.5 μg/ml at 4°C overnight. After blocking with 1% bovine serum albumin solution for 30 min, hybridoma culture media were added to the wells. After incubation for 2 h at room temperature, alkaline phosphatase (AP)-conjugated goat anti-mouse immunoglobulin (Ig) at a concentration of 100 ng/ml was added to each well, and the immunoplate was incubated for 2 h. p-Nitrophenyl phosphate was added to the wells, and optical density at 405 nm was measured.
The binding affinity of antibodies in human HCV sera to recombinant hGHE2t was assessed by using an immunoplate coated with hGHE2t protein at a concentration of 1.5 μg/ml. Human HCV sera at a dilution of 1/100 were added to the wells. Subsequent steps were the same as for the method used to detect murine MAbs specifically binding to E2t protein, but using AP-conjugated goat anti-human Ig instead of AP-conjugated goat anti-mouse Ig.
The competitive reactivity of CET MAbs to E2t protein with antibodies in HCV in human sera was assayed. Wells of an immunoplate were coated with hGHE2t, and human HCV sera at a dilution of 1/20 were added. Ten minutes later, biotinylated CET MAbs were added to each well to a final concentration of 400 ng/ml, and then the plate was incubated for 2 h. After an additional 2-h incubation with AP-conjugated extravidin at a concentration of 100 ng/ml, development of the reaction was performed as described above.
To investigate epitope specificities of CET MAbs by competitive ELISA, wells of an immunoplate were coated with hGHE2t at a concentration of 2 μg/ml. The unlabeled CET MAbs were added to the wells at serial fivefold dilutions beginning at 100 μg/ml. After incubation for 10 min, biotinylated CET MAbs were added to each well to a final concentration of 1 μg/ml, and the plate was incubated for an additional 2 h. Addition of secondary antibody and development of the reaction were performed as described above.
For mapping of the epitopes, the culture supernatant samples of transfected CHO cells or COS-7 cells expressing a chimeric, truncated, or wild-type form of E2t were assayed for binding to six CET MAbs. Primarily, the culture supernatant samples were tested for the presence of various types of E2t. The culture supernatant samples of transfectant CHO cells or COS-7 cells were added to the wells of an immunoplate coated with anti-gD MAb 3H9 at a concentration of 2 μg/ml. After incubation for 4 h, human HCV sera at a dilution of 1/500 were added to the wells. Subsequent steps were the same as for the methods used to assay the binding affinity of antibodies in human HCV sera to hGHE2t. Then the culture supernatant samples were assayed for binding to each MAb. The samples were added to an immunoplate coated with 3H9. After incubation for 24 h, each of biotinylated CET MAbs was added for an additional incubation for 4 h. Addition of secondary antibody and development of the reaction were performed as described above.
SPR analysis.
Experiments were run on a BIAcore X instrument (Biosensor, Uppsala, Sweden) at 25°C, using HEPES-buffered saline (10 mM HEPES [pH 7.4], 150 mM NaCl, 3.4 mM EDTA, 0.005% surfactant P20) as flow buffer. We activated a research-grade CM-5 sensor chip (Biosensor) by a standard amine coupling method with a 7-min pulse of 0.05 M NHS–0.2 M EDC [N-ethyl-N′-(dimethylaminopropyl) carbodiimide]. Immobilization of gDE2t protein on the activated sensor chip was performed by incubation of 1 μg of gDE2t in 10 mM acetate buffer (pH 4.8) with the sensor chip. Excessive NHS groups were inactivated with ethanolamine. Immobilization of 4,000 resonance units (RU) was achieved. For epitope mapping, series of successive addition of MAbs (1 μM in flow buffer) were performed. For kinetic assay of each MAb, gDE2t immobilization of 800 RU was achieved on another sensor chip by using standard methods described above with injection of each MAb at a concentration of 100 nM in flow buffer at a flow rate of 20 μl/min for 4 min. Apparent association and dissociation rates were determined by curve fitting using BIAevaluation 2.1 (Biosensor).
Construction of expression plasmids.
HCV cDNA covering the E2 region was obtained by reverse transcription-PCR from the serum sample of a Korean patient. The complete nucleotide sequence of the clone was determined; the clone was classified as genotype 1b and named HCV-N (23).
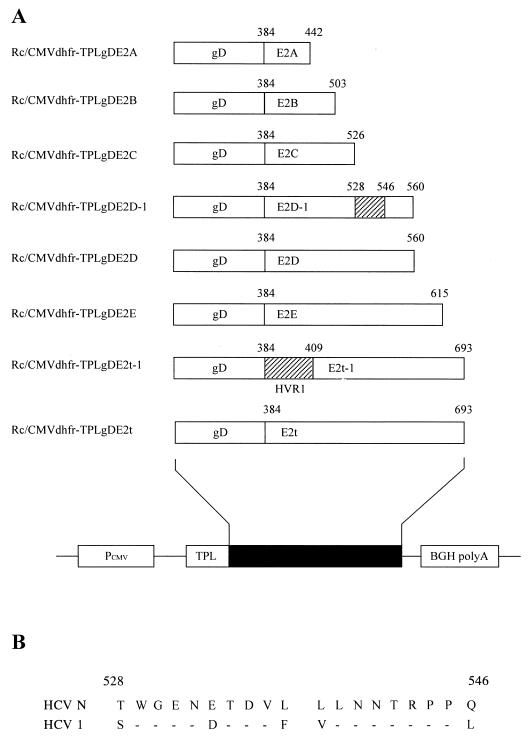
To construct an expression vector encoding a truncated form of E2 protein spanning amino acid residues 384 to 693, the TPLgDE2t region of pSK-TPLgDE2t was amplified by PCR. The PCR product digested with ClaI and XbaI was inserted into the Rc/CMVdhfr expression vector to generate Rc/CMVdhfr-TPLgDE2t (Fig. 2A) (23).
FIG. 2.
Schematic diagrams of expression plasmids. (A) Constructs expressing C-terminally truncated forms of HCV E2 differing in length and form. All constructs were based on Rc/CMVdhfr-TPLgDE2t. The E2t cDNA was modified by replacing the AgeI-XbaI fragment near the full-length E2t gene with PCR products of partially E2t-derived sequences except E2t-1, in which the NdeI-AluI segment encoding HVR-1 of E2t cDNA was replaced by a newly synthesized NdeI-AluI DNA fragment (described in Materials and Methods). Names of recombinant expression plasmids are shown at the left. The striped boxes in E2t-1 and E2D-1 represent the regions replaced with the corresponding sequences of HCV-1. Positions of amino acids at both ends of derivatives of E2t cDNA are shown. PCMV, human cytomegalovirus promoter; BGH polyA, bovine growth hormone polyadenylation signal; TPL, adenovirus tripartite leader. (B) Alignment of amino acid sequences of the region replaced in E2D-1 obtained from HCV-1 (group 1a) and HCV-N (group 1b). Amino acids are indicated in single-letter code. Positions of amino acids at both ends of the region are shown. Horizontal bars designate common sequences. Amino acid numbering is according to Choo et al. (5).
For the construction of expression vectors encoding truncated forms of E2t protein, five partial E2t-derived sequences (E2A, E2B, E2C, E2D, and E2E) were amplified by PCR from the Rc/CMVdhfr-TPLgDE2t expression vector by using an upstream primer (5′-GCC GCA CGA CCA ACC GGT TCG TGA-3′) and the following downstream primers: 5′-GCT CTA GAA CAG TGC GGC AAT AAA-3′ for E2A, 5′-GTC TAG ACC TGC GAT GCG GGT ACG-3′ for E2B, 5′-GCT CTA GAC TAC GTA GGG GCA CCG GAA-3′ for E2C, 5′-GCT CTA GAA CCC AGT GCT ATT CAT-3′ for E2D, and 5′-GCT CTA GAG CCT GTA TGG GTA GTC-3′ for E2E. We positioned the stop codon of each truncated E2t open reading frame not to be located at antigenic domains deduced from the hydrophilicity plot of HCV E2. The PCR products were digested with AgeI and XbaI. The DNA fragment encoding amino acid residues 397 to 693 of the E2t region in plasmid Rc/CMVdhfr-TPLgDE2t was replaced by five PCR products, using AgeI and XbaI sites in the E2t region, resulting in expression vectors Rc/CMVdhfr-TPLgDE2A to -E.
For the construction of an expression vector encoding HVR-1-replaced E2t protein, a DNA fragment encoding HVR-1 of E2t (amino acid residues 384 to 411) in Rc/CMVdhfr-TPLgDE2t was replaced by a DNA fragment encoding amino acid residues of E2 HVR-1 from another strain, HCV-1 (subtype 1a). A 96-bp DNA fragment for replacement HVR-1 was constructed as follows. Two oligonucleotides, 5′-GGA ATT CCA TAT GGA AAC CCA CGT CAC CGG GGG AAG TGC CGG CCA CAC TGT GTC TGG ATT TGT T-3′ and 5′-TTT ACA AGC TGG ACG TTC TGC TTG GCG CCT GGT GCG AGG AGG CTA ACA AAT CCA GAC ACA GTG T-3′, were partially annealed by a 3′ stretch of complementary nucleotides, and 5′ single strands were filled by the Klenow fragment of DNA polymerase I (Promega, Madison, Wis.). The 108-mer DNA constructed was digested with NdeI and AluI and replaced the NdeI-AluI DNA fragment of the original E2t HVR-1, resulting in the expression vector Rc/CMVdhfr-TPLgDE2t-1.
For the construction of an expression vector encoding a chimeric derivative of E2D, E2D sequences in Rc/CMVdhfr-TPLgDE2D were amplified by two rounds of PCR with three primers. The first-round PCR used the downstream primer for E2D (described above) and an upstream primer, 5′-AGC TGG GGT GAA AAT GAT ACG GAC GTC TTC GTC CTT AAC AAT ACC AGG CCA CCG CTG GGC-3′, containing the sequences encoding amino acid residues 528 to 546 of HCV-1. The first-round PCR product (105 bp) was used as the downstream primer for the second-round PCR in conjunction with the upstream primer used for the construction of E2t derivatives described above. The final PCR product was designated E2D-1, encoding E2D in which the domain spanning amino acid residues 528 to 546 was replaced by that of HCV-1. Subsequent steps were the same as for the methods used to construct expression vectors encoding variant forms of E2t, resulting in the expression vector Rc/CMVdhfr-TPLgDE2D-1.
All nucleotide sequences of the PCR products were determined by the dideoxynucleotide chain termination method (34) using a Li-COR (Lincoln, Neb.) sequencer. All oligonucleotide primers used in this study were purchased from Bioneer Inc., Cheongwon, Korea.
Transfection and cell lines.
CHO cells deficient in the dihydrofolate reductase gene (CHO DHFR− cells) were maintained in RPMI 1640 medium (GIBCO, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (GIBCO), 10 mM l-glutamine, hypoxanthine, and thymidine plus antibiotics. All DNA constructs expressing different forms of gDE2t protein were linearized by AhdI and introduced into CHO DHFR− cells by electroporation as follows. CHO DHFR− cells were resuspended in phosphate-buffered saline at a concentration of 107 cells/ml. Linearized plasmid was added to the cell suspension at a concentration of 20 μg/ml. An electric pulse was applied at 250 V and 500 μF with a Gene Pulser (Bio-Rad, Hercules, Calif.). Two days after transfection, G418 (GIBCO) was added to media at a concentration of 500 μg/ml to select for neomycin resistance. After 14 days of selection, G418 was replaced by 10 nM methorexate (Sigma). The concentration of MTX in the media was increased to a maximum of 1 μM. When monolayers reached confluence, the expression of different forms of gDE2t protein was assayed by ELISA.
COS-7 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 10 mM l-glutamine plus antibiotics. The DNA construct Rc/CMVdhfr-TPLgDE2D-1 was introduced into COS-7 cells by liposome-mediated transfection using Lipofectin reagent (GIBCO). We used 2 μg of DNA in 10 μl of Lipofectin per 2 × 105 cells, and performed the transfection according to the manufacturer’s instructions. Three days after transfection, the expression of gDE2D-1 protein was assayed by ELISA.
RESULTS
Generation of anti-E2 MAbs.
To produce MAbs specific for HCV E2 protein, recombinant fusion proteins hGHE2t and gDE2t were used as immunogens. They were expressed in CHO cells in secreted form (23). Splenocytes from immunized BALB/c mice were used to generate hybridomas secreting anti-E2 MAbs. The MAbs produced were expected to bind to the E2t portion or the hGH or gD portion of the recombinant E2 protein. Therefore, we performed ELISA to select MAbs binding to E2t only, not to hGH or gD protein. MAbs which bound to hGHE2t and gDE2t with considerable affinity but not to hGH or gD were selected and named CET-1 to -6 (Table 1). The binding activities of CET-1 to -5 to gDE2t were lower than those to hGHE2t, and reactivity of CET-6 to gDE2t was higher than its reactivity to hGHE2t. This observation suggested that differences in the binding of CET MAbs might be attributed to slightly different epitope structures of the two E2 fusion proteins. At the same time, 3H9 (Table 1), a MAb with specific affinity to gD, was developed from a fusion by using a mouse immunized with gDE2t. All six CET MAbs and 3H9 were identified as IgG1 isotype and κ light chain (data not shown). SPR analyses were performed to obtain association and dissociation rate constants for all MAbs. Calculated Kd values for CET MAbs were evenly distributed and ranged from 6.3 × 10−7 to 4.8 × 10−8 M (data not shown).
TABLE 1.
Binding of CET MAbs to recombinant E2 proteins
| MAba (1 μg/ml) | Binding activityb (A405)
|
|||
|---|---|---|---|---|
| gDE2t | gD | hGHE2t | hGH | |
| CET-1 | 0.753 | 0.087 | 1.738 | 0.042 |
| CET-2 | 0.972 | 0.032 | 1.728 | 0.029 |
| CET-3 | 0.733 | 0.033 | 1.706 | 0.033 |
| CET-4 | 0.836 | 0.033 | 1.660 | 0.034 |
| CET-5 | 0.869 | 0.034 | 1.732 | 0.031 |
| CET-6 | 2.224 | 0.028 | 1.183 | 0.058 |
| 3H9 | 2.045 | 1.469 | 0.053 | 0.061 |
| MAG 49c | 0.049 | 0.032 | 0.027 | 0.034 |
MAbs CET-1 and 3H9 were generated from the fusion of gDE2t-immune splenocytes, and CET-2 to -6 were generated from the fusion of hGHE2t-immune splenocytes.
Analyzed by ELISA as described in Materials and Methods.
Murine IgG, MAb specific for HIV gp 120 (15); used as a negative control.
Comparison of epitope specificities of CET MAbs with those of Abs in HCV-infected individuals.
To compare the epitope specificities of CET MAbs with those of Abs in HCV-immune sera, we investigated whether the serum samples from eight individuals were able to inhibit the binding of CET MAbs to hGHE2t by ELISA. Prior to the inhibition assay, HCV serotyping was performed by ELISA. We tested the binding activities of serum samples against HVR-1 peptides derived from the E2 sequence of subtype 1a or 1b. Eight samples were all reactive preferentially to the HVR-1 peptide of subtype 1b sequence, homologous to that of E2t fusion proteins used for generating CET MAbs (data not shown). The sera were also tested for binding to hGHE2t; seven of the eight sera showed binding to hGHE2t (Table 2).
TABLE 2.
Inhibition of CET MAb binding to hGHE2t by sera of HCV-infected individuals
| Serum | Binding to hGHE2ta | Inhibitory activityb
|
|||||
|---|---|---|---|---|---|---|---|
| CET-1 | CET-2 | CET-3 | CET-4 | CET-5 | CET-6 | ||
| 1 | − | − | − | − | − | − | − |
| 2 | ++ | − | − | − | − | − | − |
| 3 | +++ | ++ | + | + | + | ++ | + |
| 4 | ++ | ++ | + | + | + | + | + |
| 5 | + | + | + | + | + | + | + |
| 6 | +++ | ++ | − | ++ | + | ++ | + |
| 7 | ++ | ++ | − | − | − | − | − |
| 8 | ++ | + | − | − | − | − | − |
Analyzed by ELISA as described in Materials and Methods and presented as A405of <twofold (−), two- to fivefold (+), five- to eightfold (++), or >eightfold (+++) that of control the MAb (MAG 49, specific for HIV gp120).
Analyzed by ELISA as described in Materials and Methods and presented as <30% (−), 30 to 60% (+), or >60% (++) inhibition. Percent inhibition = 100 × (A405 of CET Ab − A405 of CET Ab bound to hGHE2t in presence of HCV sera)/A405 of CET Ab.
Inhibitory activity was observed in six of the seven sera. In case of CET-1, most sera showed inhibitory activities. In CET-3 and CET-5, four of seven sera showed modest or weak inhibitory activities. In CET-2, -4, and -6, four of seven sera showed weak inhibitory activities. These data suggested that antibodies similar in epitope specificity to CET-1 prevalently existed in HCV-infected individuals. Antibodies similar to CET-3 and CET-5 less frequently existed, and those similar to CET-2, -4, and -6 were barely present in HCV-infected individuals.
Epitope specificities of CET MAbs determined by SPR analysis and ELISA.
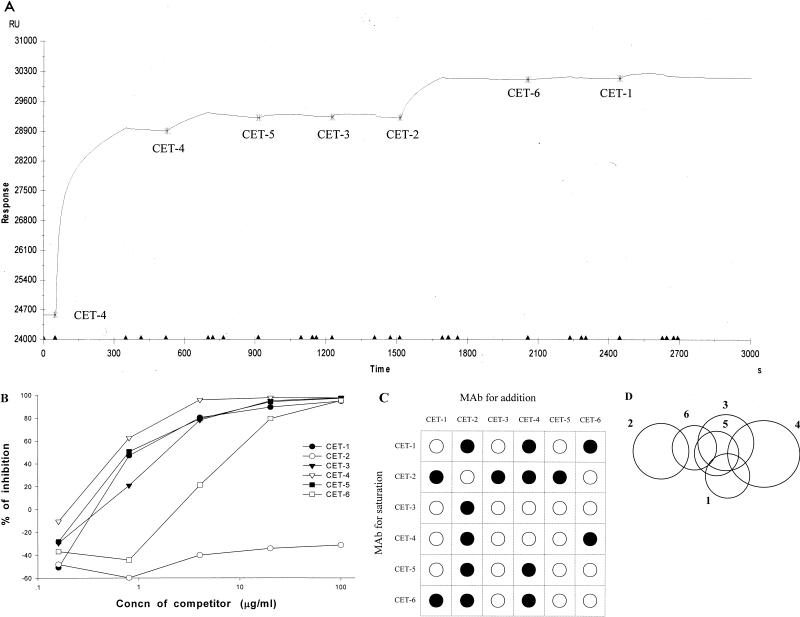
To analyze the epitopes on HCV E2 protein recognized by six CET MAbs, we tested simultaneous binding of CET MAbs by a series of SPR analyses (13, 14). We designed an experiment of sequential addition of five MAbs to gDE2t of which the surface binding sites were previously saturated with one of the CET MAbs. The saturation of binding sites on gDE2t coated on a sensor chip was accomplished by double injections of a CET MAb and followed by the sequential addition of the other five CET MAbs. We performed this experiment repeatedly with all of the six CET MAbs, changing the order of addition. The increase in RU level upon the addition of each MAb in the sensorgram allowed us to map the epitope specificity of the six CET MAbs. The competitive ELISA of CET MAbs confirmed the results of SPR analyses and permitted a more comprehensive interpretation of the sensorgram data.
Figure 1A shows an example of a sensorgram obtained from SPR analyses described above. The early stage of the sensorgram shows the saturation step by two consecutive injections of CET-4. For the sensor chip-immobilized gDE2t to be completely bound to CET-4, we used an excessive amount of CET-4 for the first injection. To avoid a false-positive response, unoccupied CET-4 sites on gDE2t must be blocked before subsequent injections of samples. This was accomplished by an additional injection of CET-4. The initial increase in RU from the baseline level and the next small increase confirm that the available sites for CET-4 are almost occupied by initial two injections of CET-4. In the next five injections, CET-5, -3, -2, -6, and -1 were sequentially added to the gDE2t protein saturated with CET-4. A significant increase in RU level is shown only after the injection of CET-2, indicating that the binding of CET-2 is not hindered by the previous binding of CET-4. CET-4 and CET-2 MAbs showing simultaneous binding in the sensorgram are therefore judged to bind to independent epitopes. The competitive ELISA performed in a pairwise manner showed results equivalent to those for the SPR analysis. CET-2 could not inhibit the binding of CET-4, whereas CET-1, -3, and -5 could completely inhibit the binding of CET-4 (Fig. 1B). Inhibitory activity was also shown in CET-6, but to a lesser extent, indicating that CET-4 and CET-6 bound to independent epitopes which were adjacently located.
FIG. 1.
SPR analysis for determining the epitope specificities of CET MAbs. (A) Sensorgram showing sequential addition of CET MAbs. Experimental conditions are described in Materials and Methods. SPR response was measured in RU. Buffer flow was maintained at 5μl/min throughout the analysis. The saturation step was performed with two injections of CET-4. CET-4 was bound to gDE2t by the first injection, and the unoccupied binding sites on gDE2t were blocked by the second. CET-5, -3, -2, -6, and -1 were added in turn. Each injection start point and the name of corresponding MAb are indicated. The injection volumes were 25 μl for the first injection of CET-4 and 15 μl in all other cases. (B) Competitive ELISA for binding to hGHE2t. Various amounts of unlabeled CET MAbs were used as competitors. hGHE2t-coated wells were incubated with competitors for 10 min. After incubation, biotin-labeled CET-4 was added to the wells. Percent inhibition = 100 × (A405 of CET-4 bound to hGHE2t − A405 of CET-4 bound to hGHE2t in presence of competitor)/A405 of CET-4 bound to hGHE2t. (C) Reactive pattern matrix showing the binding ability of pairs of CET MAbs to E2t protein. •, pairs of CET MAbs that bind concurrently. ○, pairs of CET MAbs that interfere with binding. (D) Two-dimensional surface-like map of the epitopes on E2t recognized to six CET MAbs. Numbers 1 to 6 correspond to the CET-1 to CET-6; overlapping circles represent MAb groups within which pairs of MAb cannot bind simultaneously.
From the interpretation of a series of sensorgram and ELISA data, we could display the reactivity patterns for six CET MAbs as a 6 × 6 matrix (Fig. 1C). We identified the existence of six different epitopes for six CET MAbs. Accordingly, we could construct a two-dimensional surface-like map of epitopes on E2t protein (Fig. 1D). The diagram demonstrated a cluster of epitopes composed of three major epitopes: the first recognized by CET-2, the second recognized by CET-1, -3, -5, and -6, and the third recognized by CET-4. The second epitope consisted of four minor overlapping epitopes. The first epitope recognized by CET-2 and the third recognized by CET-4 did not overlap at all.
Identification of an antigenic domain on E2 protein.
For mapping antigenic determinants on HCV E2 protein recognized by CET MAbs, we screened E2 region by examining the reactivities of CET MAbs to a series of different recombinant E2 proteins stably expressed in CHO cell lines. These recombinant CHO cell lines were established by the construction of seven recombinant expression vectors. The primary construct was the expression plasmid Rc/CMVdhfr-gDE2t (23), encoding E2 protein truncated to remove a C-terminal hydrophobic domain that appeared to anchor the protein in the endoplasmic reticulum (25, 36). For efficient expression and purification in cell culture system, the signal sequence of E2 was replaced by the coding region of HSV-1 gD because HCV E2 protein was shown to be secreted, depending on the signal peptide or the secretory protein fused to the E2 protein (23).
Six derivatives of this plasmid were constructed to express five truncated forms of E2t with different lengths and a chimeric form of E2t (Fig. 2A). It was determined whether the six derivatives of E2t as well as the original form of E2t were present in the culture media by ELISA with HCV type 1b-immune sera. For all seven constructs, the culture supernatant of CHO cells showed positive reactivity to HCV-immune sera (Table 3).
TABLE 3.
Reactivities of various forms of recombinant E2 protein to HCV-immune sera and different CET MAbsa
| Superna-tantb | Binding (A405) to:
|
||||||
|---|---|---|---|---|---|---|---|
| HCV (+) serum | CET-1 | CET-2 | CET-3 | CET-4 | CET-5 | CET-6 | |
| gDE2t | 1.062 | 0.559 | 0.596 | 0.589 | 0.584 | 0.638 | 0.585 |
| gDE2t-1 | 1.278 | 0.507 | 0.516 | 0.547 | 0.527 | 0.508 | 0.579 |
| gDE2E | 1.073 | 1.423 | 1.220 | 1.472 | 1.237 | 1.032 | 1.133 |
| gDE2D | 1.404 | 0.072 | 0.668 | 0.754 | 0.734 | 0.683 | 0.552 |
| gDE2D-1 | 1.439 | 0.051 | 0.020 | 0.019 | 0.042 | 0.019 | 0.031 |
| gDE2C | 1.113 | 0.078 | 0.047 | 0.048 | 0.058 | 0.095 | 0.065 |
| gDE2B | 0.984 | 0.096 | 0.048 | 0.053 | 0.057 | 0.044 | 0.070 |
| gDE2A | 1.702 | 0.093 | 0.055 | 0.045 | 0.058 | 0.046 | 0.080 |
| Negative control | 0.123c | 0.075 | 0.046 | 0.043 | 0.060 | 0.070 | 0.066 |
| 0.175d | 0.030 | 0.018 | 0.017 | 0.038 | 0.017 | 0.022 | |
Analyzed by ELISA as described in Materials and Methods.
The name of culture supernatant corresponds to the kind of recombinant protein expressed in CHO cells except for gDE2D-1, expressed in COS-7 cells as shown in Fig. 2A.
Culture supernatant of CHO cells transfected by expression plasmid Rc/CMVdhfr.
Culture supernatant of COS-7 cells transfected by expression plasmid Rc/CMVdhfr.
To identify the epitopes on E2, we tested six CET MAbs for reactivities with a panel of culture supernatant samples containing different forms of E2t. The plasmid expressing gDE2t-1 was designed to determine whether the epitopes recognized by CET MAbs were present within HVR-1 of E2 protein, considered as the major neutralization epitope (6). To minimize the conformational change of the whole E2 molecule, HVR-1 was replaced with the corresponding region of another strain HCV-1, subtype 1a. The culture supernatant samples of gDE2t and gDE2t-1 bound to all CET MAbs; affinities were not significantly different among the MAbs. The result showed that HVR-1 was not involved as an epitope recognized by CET MAbs (Table 3). Whereas the culture supernatant samples of gDE2A, gDE2B, and gDE2C did not show binding activity with any of CET MAbs, the culture supernatant samples of gDE2D and gDE2E showed binding activities with all CET MAbs (Table 3). This finding indicated that the epitopes recognized by CET-1 to -6 may exist in a region spanning amino acid residues 527 to 560.
For precise mapping of the domain recognized by CET MAbs, we designed an additional plasmid construct, Rc/CMVdhfr-TPLgDE2D-1, expressing a chimeric form of gDE2D with 19 amino acid residues (aa 528 to 546) replaced by the corresponding region of HCV-1 of another subtype 1a (Fig. 2B). The culture supernatant of recombinant COS-7 cells expressing gDE2D-1 showed positive reactivity to HCV-immune serum (Table 3). However, the binding reactivity was not shown with any of CET MAbs. This analysis suggested that the antigenic determinants of CET-1 to -6 were located in a small domain within E2 spanning amino acid residues 528 to 546.
DISCUSSION
It is clear that the development of a broadly reactive vaccine is the most effective method for preventing hepatitis C. The viral envelope E2 glycoprotein has been suggested to be responsible for binding of the virus to target cells, and antibodies to this region have been proposed to neutralize the virus and to drive immune selection. Despite the strong variability of the HCV E2 sequences, certain domains of biological importance (e.g., ligands for viral attachment to target cells) must be preserved. Thus, determining which region of E2 is critical in binding to host cell receptors and identifying the genotype-conserved determinants are very important for the development of an HCV vaccine (2, 22, 27, 28, 40, 45). Recently, it was also reported that the neutralizing epitope(s) in HCV E2 protein may be downstream of HVR-1 (31).
The purpose of our study was to identify the B-cell epitopes in HCV E2 protein by using MAbs specific for the E2 protein. We generated six murine MAbs specific for HCV E2 protein by using recombinant E2 proteins expressed in CHO cells. It was previously reported that the full-length E2 protein remained membrane anchored due to the C-terminal hydrophobic region and that the signal peptide of E2 was not appropriate for the efficient expression in CHO cells. Therefore, we designed two fusion constructs, hGHE2t and gDE2t, for the efficient expression of E2 in CHO cells, and they were detected in the culture media of the recombinant CHO cells (23).
Since CET MAbs were generated by using nonnatural forms of the HCV E2 protein, we compare the epitope specificities of CET MAbs with those of anti-E2 antibodies in HCV-immune sera by competitive ELISA. The serum samples all showed the same HCV serotype, 1b. Therefore, the inhibitory activity of sera may not be influenced by the serotype of HCV infection. The data showed that the HCV-immune sera had the anti-E2 antibodies which could bind to the same epitopes that were recognized by CET MAbs. We could classify the epitopes into three groups: epitopes recognized by CET-1 (most prevalent), those recognized by CET-3 and -5 (less frequent), and those recognized by CET-2, -4, and -6 (barely detected).
The epitope specificities of CET MAbs were determined by SPR analysis. We tested the abilities of the MAb to bind simultaneously to the gDE2t covalently linked to a sensor chip, using the sequential multideterminant binding method. In addition, we performed the competitive ELISA in a pairwise manner. The ELISA data strongly supported the conclusions from the SPR analysis. Since the conformational changes in the antigen due to the previous binding of the MAb(s) and the electrostatic interactions between the MAbs may distort the binding patterns of CET MAbs, the diagram does not correspond to the actual physical map of the binding sites on E2. These cases are seen as asymmetry in the reactivity pattern matrix; e.g., CET-1 could not bind to the antigen previously bound with CET-4. However, the binding of CET-1 did not influence that of CET-4 (Fig. 1C). We depict this as the overlap of two circles of different sizes in Fig. 1D. We interpreted the proposed surface-like map of epitopes shown in the diagram in accordance with the results of a previous epitope specificity assay as follows. The major epitope is recognized by CET-1. The minor epitope recognized by CET-6 neighbors the major one. These two epitopes do not overlap and are surrounded by the other two minor epitopes recognized by CET-2 and -4. The other minor epitopes recognized by CET-3 and CET-5 are depicted as conformational ones and extremely overlapped. HVR-1 of HCV E2 protein, which is the most variable region of the HCV genome, has been known to contain linear epitopes recognized by patient antibodies, although it is disputed whether the antibodies against HVR-1 can actually neutralize HCV infection (17, 20, 43). In our experiment, HVR-1 was not an epitope recognized by CET MAbs.
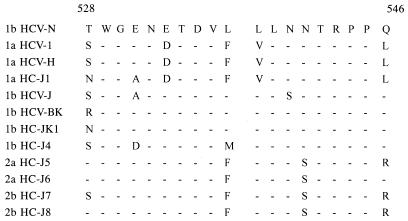
We used a series of E2 proteins with C-terminal truncations to determine the locations of the B-cell epitopes recognized by CET MAbs. The HCV-immune sera were reactive to each truncated type and to wild-type E2 equivalently, indicating that the overall structure integrity of each protein is not significantly compromised as a consequence of the truncations. The binding reactivities of CET MAbs to the truncated E2 proteins showed an all-or-none binding pattern: all CET MAbs showed binding activities only with E2t, E2E, and E2D. This result indicates that the epitopes recognized by CET MAbs all exist in a region spanning amino acid residues 527 to 560. However, it was also possible that the epitopes were present in a region upstream of amino acid residue 527 and that the C-terminal deletion for the construction of E2C distorted the conformational epitopes. To determine which explanation was appropriate, we constructed E2D-1, in which the domain spanning amino acid residues 528 to 546 was replaced with the corresponding domain of HCV-1, subtype 1a, and found that E2D-1 was reactive to HCV-immune sera. If the latter explanation were the case, CET MAbs could bind to E2D-1. However, E2D-1 did not show binding activity with any of CET MAbs, indicating that the epitopes are present in the region spanning amino acid residues 528 to 546. We compared the amino acid sequences of this region with those of other HCV strains and identified an increased divergence in the alignment (Fig. 3). This observation suggests that the B-cell epitopes in this region may be involved in the genetic drift by immunoselection. Therefore, it will be important to determine whether the epitopes in this domain are involved in HCV infection.
FIG. 3.
Alignment of 19 amino acids (in single-letter code) corresponding to the putative B-cell antigenic domain spanning map positions 528 to 546. The sequence of HCV-N is compared with that from 11 different isolates. Names of strains and subtypes are indicated at the left. Horizontal bars designate common sequences. Amino acid numbering is according to Choo et al.(5). GenBank database accession numbers of the sequences: HCV-1, M62321; HCV-H, M67463; HC-J1, D10749; HCV-J, D90208; HCV-BK, M58335; HC-JK1, X61596; HC-J4, D00832; HC-J5, D10076; HC-J6, D00944; HC-J7, D10076; HC-J8, D01221.
To date, evidence suggestive of the neutralizing role of antibodies directed by HCV E2 protein has accumulated. However, few data are available regarding mapping of the B-cell epitopes in E2 protein other than HVR-1 (27, 28, 40). We report here the identification of B-cell epitopes in a newly described domain on E2 protein by measuring the reactivities of truncated or chimeric E2 proteins to anti-E2 MAbs. Additional studies will be needed for the fine mapping of the individual epitopes in the putative B-cell immunogenic domain that we identified. Also, defining the neutralization potential of this domain will be important for future vaccine development.
ACKNOWLEDGMENTS
We thank R. Ward, U.S. Environmental Protection Agency, for a review of the manuscript.
This work was supported by the Korea Science and Engineering Foundation through the Research Center for New Drug Development at Seoul National University.
REFERENCES
- 1.Alter H J. The chronic consequences of non-A, non-B hepatitis. In: Seeff L B, Lewis J H, editors. Current perspectives in hepatology. New York, N.Y: Plenum; 1989. pp. 83–97. [Google Scholar]
- 2.Chan S-W, Bye J M, Jackson P, Allain J-P. Human recombinant antibodies specific for hepatitis C virus core and envelope E2 peptides from an immune phage display library. J Gen Virol. 1996;77:2531–2539. doi: 10.1099/0022-1317-77-10-2531. [DOI] [PubMed] [Google Scholar]
- 3.Choo Q-L, Kuo G, Ralston R, Weiner A, Chien D, Nest G V, Han J, Berger K, Thudium K, Kuo C, Kansopon J, Mcfarland J, Tabrizi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 5.Choo Q-L, Richman K H, Han J H, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby A, Barr P J, Weiner A J, Bradley D W, Kuo G, Houghton M. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choo S H, So H-S, Cho J M, Ryu W-S. Association of hepatitis C virus particles with immunoglobulin: a mechanism for persistent infection. J Gen Virol. 1995;76:2337–2341. doi: 10.1099/0022-1317-76-9-2337. [DOI] [PubMed] [Google Scholar]
- 7.Farci P, Alter H J, Govindarajan S, Wong D C, Engel R, Lesniewski R R, Mushawar I K, Desai S M, Miller R H, Ogata N, Purcell R H. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 8.Farci P, Alter H J, Wong D C, Millers R H, Govindarajan S, Engle R, Shapiro M, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farci P, Shimoda A, Wong D, Cabezon T, Gioannis D D, Strazzera A, Shimizu Y, Shapiro M, Alter H J, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galfre G, Howe S C, Milstein C, Butcher G W, Howard J C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977;266:550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- 11.Higashi Y, Kakumu S, Yoshioka K, Wakita T, Mizokami M, Ohba K, Ito Y, Ishikawa T, Takayanagi M, Nagai Y. Dynamics of genome change in the E2/NS1 region of hepatitis C virus in vivo. Virology. 1993;197:659–668. doi: 10.1006/viro.1993.1641. [DOI] [PubMed] [Google Scholar]
- 12.Hijikawa M, Kato N, Ootsuyama Y, Nakagawa M, Ohkoshi S, Shimotohno K. Hypervariable regions in the putative glycoprotein of hepatitis C virus. Biochem Biophys Res Commun. 1991;175:220–228. doi: 10.1016/s0006-291x(05)81223-9. [DOI] [PubMed] [Google Scholar]
- 13.Johne B, Gadnell M, Hansen K. Epitope mapping and binding kinetics of monoclonal antibodies studied by real time biospecific interaction analysis using surface plasmon resonance. J Immunol Methods. 1993;160:191–198. doi: 10.1016/0022-1759(93)90177-9. [DOI] [PubMed] [Google Scholar]
- 14.Johne B, Hansen K, Mork E, Holtlund J. Colloidal gold conjugated monoclonal antibodies, studied in the BIAcore biosensor and in the Nycocard immunoassay format. J Immunol Methods. 1995;183:167–174. doi: 10.1016/0022-1759(95)00047-e. [DOI] [PubMed] [Google Scholar]
- 15.Kang C-Y, Hariharan K, Nara P L, Sodroski J, Moore J P. Immunization with a soluble CD4-gp120 complex preferentially induces neutralizing anti-human immunodeficiency virus type 1 antibodies directed to conformational-dependent epitopes of gp120. J Virol. 1994;68:5854–5862. doi: 10.1128/jvi.68.9.5854-5862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato N, Hijikata M, Ootsuyama Y, Nakagawa M, Ohkoshi S, Sugimura T, Shimotono K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA. 1990;87:9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato N, Ootsuyama Y, Sekiya H, Ohkoshi S, Nakazawa T, Hijikata M, Shimotohno K. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68:4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato N, Ootsuyama Y, Tanaka T, Nakagawa M, Nakazawa T, Muraiso K, Ohkoshi S, Hijikata M, Shimotohno K. Marked sequence diversity in the putative envelope proteins of hepatitis C viruses. Virus Res. 1992;22:107–123. doi: 10.1016/0168-1702(92)90038-b. [DOI] [PubMed] [Google Scholar]
- 19.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimotohno K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima M, Osuga T, Tsuda F, Tanaka T, Okamoto H. Influence of antibodies to the hypervariable region of E2/NS1 glycoprotein on the selective replication of hepatitis C virus in chimpanzees. Virology. 1994;204:665–672. doi: 10.1006/viro.1994.1582. [DOI] [PubMed] [Google Scholar]
- 21.Lanford R E, Notvall L, Chavez D, White R, Frenzel G, Simonsen C, Kim J. Analysis of hepatitis C virus capsid, E1, and E2/NS1 proteins expressed in insect cells. Virology. 1993;197:225–235. doi: 10.1006/viro.1993.1583. [DOI] [PubMed] [Google Scholar]
- 22.Lee I-H, Kim C-H, Ryu W-S. Presentation of the hydrophilic domains of hepatitis C viral E2 envelope glycoprotein on hepatitis B surface antigen particles. J Med Virol. 1996;59:145–151. doi: 10.1002/(SICI)1096-9071(199610)50:2<145::AID-JMV7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Lee K J, Suh Y-A, Cho Y G, Cho Y S, Ha G W, Chung K-H, Hwang J H, Yun Y D, Lee D S, Kim C M, Sung Y-C. Hepatitis C virus E2 protein purified from mammalian cells is frequently recognized by E2-specific antibodies in patient sera. J Biol Chem. 1997;272:30040–30046. doi: 10.1074/jbc.272.48.30040. [DOI] [PubMed] [Google Scholar]
- 24.Matsuura Y, Harada S, Suzuki R, Watanabe Y, Inoue Y, Saito I, Miyamura T. Expression of processed envelope protein of hepatitis C virus in mammalian and insect cells. J Virol. 1992;66:1425–1431. doi: 10.1128/jvi.66.3.1425-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuura Y, Suzuki T, Suzuki R, Sato M, Aizaki H, Saito I, Miyamura T. Processing of E1 and E2 glycoproteins of hepatitis C virus expressed in mammalian and insect cells. Virology. 1994;205:141–150. doi: 10.1006/viro.1994.1629. [DOI] [PubMed] [Google Scholar]
- 26.Miller R H, Purcell R H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci USA. 1990;87:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mink M A, Benichou S, Madaule P, Tiollais P, Prince A M, Inchauspe G. Characterization and mapping of a B-cell immunogenic domain in hepatitis C virus glycoprotein using a yeast peptide library. Virology. 1994;200:246–255. doi: 10.1006/viro.1994.1182. [DOI] [PubMed] [Google Scholar]
- 28.Nakano I, Maertens G, Major M E, Vitvitski L, Dubuisson J, Fournillier A, Martynoff G D, Trepo C, Inchauspe G. Immunization with plasmid DNA encoding hepatitis C virus envelope E2 antigenic domains induces antibodies whose immune reactivity is linked to the injection mode. J Virol. 1997;71:7101–7109. doi: 10.1128/jvi.71.9.7101-7109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamato H, Kojima M, Okada S-I, Yoshizawa H, Lizuka H, Tanaka T, Muchmore E E, Peterson D A, Ito Y, Mishiro S. Genetic drift of hepatitis C virus during an 8.2-year infection in a chimpanzee: variability and stability. Virology. 1992;190:894–899. doi: 10.1016/0042-6822(92)90933-g. [DOI] [PubMed] [Google Scholar]
- 30.Ralston R, Thudium K, Berger K, Kuo C, Gervase B, Hall J, Selby M, Kuo G, Houghton M, Choo Q-L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J Virol. 1993;67:6753–6761. doi: 10.1128/jvi.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner A J, Lau J Y N, Choo Q-L, Chien D, Pileri P, Houghton M, Abrignani S. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rumenapf T, Stark R, Meyers G, Thiel H-J. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Gen Virol. 1991;65:589–597. doi: 10.1128/jvi.65.2.589-597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, Choo Q-L, Houghton M, Kuo G. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 35.Scarselli E, Cerino A, Esposito G, Silini E, Mondelli M U, Traboni C. Occurrence of antibodies reactive with more than one variant of the putative envelope glycoprotein (gp70) hypervariable region 1 in viremic hepatitis C virus-infected patients. J Virol. 1995;69:4407–4412. doi: 10.1128/jvi.69.7.4407-4412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selby M J, Glazer E, Masiara F, Houghton M. Complex processing and protein: protein interactions in the E2:NS2 region of HCV. Virology. 1994;204:114–112. doi: 10.1006/viro.1994.1515. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu Y K, Hijikata M, Iwamoto A, Alter H J, Pursell R H, Yoshikura H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spaete R R, Alexander D, Rugroden M E, Choo Q-L, Berger K, Crawford K, Kuo C, Leng S, Lee C, Ralston R, Thudium K, Tung J W, Kuo G, Houghton M. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992;188:819–830. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]
- 39.Takamizawa A, Mori C, Fuke I, Manabe S, Murakami S, Fujita J, Onishi E, Andoh T, Yoshida I, Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991;65:1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedeschi V, Akatsuka T, Shih J W-K, Bttegay M, Feinstone S M. A specific antibody response to HCV E2 elicited in mice by intramuscular inoculation of plasmid DNA containing coding sequences for E2. Hepatology. 1997;25:459–462. doi: 10.1002/hep.510250234. [DOI] [PubMed] [Google Scholar]
- 41.van Doorn L J, Capriles I, Maertens G, DeLeys R, Murray K, Kos T, Schellekens H, Quint W. Sequence evolution of the hypervariable region in the putative envelope region E2/NS1 of hepatitis C virus is correlated with specific humoral immune responses. J Virol. 1995;69:773–778. doi: 10.1128/jvi.69.2.773-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiner A J, Brauer M J, Rosenblatt J, Richman K H, Tung J, Crawford K, Bonino F, Saracco G, Choo Q-L, Houghton M, Han J H. Variable and hypervariable domains are in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991;180:842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- 43.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino F, Crawford K, Marion C D, Crawford K A, Brunetto M, Barr P J, Miyamura T, Mchutchison J, Houghton M. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y-M, Hayes E P, McCarty T C, Dubois D R, Summers P L, Eckels K E, Chanock R M, Lai C-J. Immunization of mice with dengue structural proteins and nonstructural protein NS1 expressed by baculovirus recombinant induced resistance to dengue virus encephalitis. J Virol. 1988;62:3027–3031. doi: 10.1128/jvi.62.8.3027-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zibert A, Kraas W, Meisel H, Jung G, Roggendorf M. Epitope mapping of antibodies directed against hypervariable region 1 in acute self-limiting and chronic infections due to hepatitis C virus. J Virol. 1997;71:4123–4127. doi: 10.1128/jvi.71.5.4123-4127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zibert A, Schreier E, Roggendorf M. Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology. 1995;208:653–661. doi: 10.1006/viro.1995.1196. [DOI] [PubMed] [Google Scholar]