Abstract
Although unspliced mRNA from influenza C virus RNA segment 6 (M gene) has a single open reading frame capable of encoding a 374-amino-acid protein (Mr, 42,000), the major polypeptide synthesized from this mRNA species is the CM2 protein, with an Mr of 18,000. The present study was performed to investigate the mechanism by which CM2 is generated from the unspliced mRNA. It was reported previously that the 374-amino-acid protein (P42) is an integral membrane protein having two internal hydrophobic domains, one of which (residues 241 to 252) is followed by two sequences (252 Ile-Thr-Ser and 257 Ala-Ser-Ala) favorable for cleavage by signal peptidase. To examine the possibility that P42 is cleaved by signal peptidase after Ser residue 254 or Ala residue 259 to yield CM2, we constructed three mutated M gene cDNAs in which either or both of the two sequences were eliminated and tested their ability to synthesize CM2 in the transfected COS cells. The results showed that CM2 synthesis was blocked completely when the second recognition motif for signal peptidase was removed. It was also found that when the mRNA transcript of the wild-type M gene was translated in vitro, P42, but not CM2, was synthesized in the absence of dog pancreas microsomal membranes, whereas CM2, in addition to a polypeptide (designated M1′) slightly larger than matrix protein (M1), was synthesized in the presence of microsomes. When the same experiment was done with the transcript of the mutated M gene in which the second recognition motif was removed, synthesis of CM2 could not be seen, even in the presence of microsomes. From these results, we conclude that cleavage of P42 by signal peptidase after Ala residue 259 produces CM2, composed of the C-terminal 115 amino acids, in addition to M1′, composed of the N-terminal 259 amino acids.
The influenza C virus genome consists of seven single-stranded RNA segments of negative polarity. RNA segment 6 (M gene) of C/Yamagata/1/88 is 1,181 nucleotides long and has a single open reading frame (positions 27 to 1148) capable of encoding a polypeptide of 374 amino acids, with a predicted Mr of 42,000 (2). However, the predominant mRNA transcript of this RNA segment lacks a region from nucleotides 755 to 982 and encodes a 242-amino-acid matrix (M1) protein with an Mr of 27,000; elimination of the intron results in the introduction of a termination codon (consisting of nucleotides 753, 754, and 983) after amino acid residue 242 (2, 14). Unspliced mRNA from RNA segment 6 is synthesized in infected cells, but its amount is very low (13% of spliced mRNA) (2). This mRNA species is capable of coding for a 374-amino-acid protein containing an additional 132 amino acids from the C terminus of M1. However, immunoprecipitation experiments with anti-GST/CM2 serum which was raised against the glutathione S-transferase fusion protein containing the extra C-terminal region identified a protein (CM2) with an Mr of 18,000 in infected cells (2). Recently, our laboratory (3) as well as Pekosz and Lamb (6) demonstrated that CM2 is an integral membrane protein that has many of the same biochemical properties (including NoutCin membrane orientation, the sizes of the ectodomain and cytoplasmic tail, and the ability to form disulfide-linked dimers and tetramers) as influenza A virus M2 and influenza B virus NB having an ion channel activity (1, 7, 10, 11). More recently, we succeeded in detecting the 374-amino-acid protein (designated P42) and its N-glycosylated form (designated P44) in influenza C virus-infected cells as well as in eukaryotic cells transfected with the M gene cDNA, although their amounts were extremely low compared with that of CM2 (4). P42/P44 is an integral membrane protein containing two regions (amino acid residues 241 to 252 and 287 to 318) sufficiently hydrophobic to interact with membranes (see Fig. 5 for its possible orientation in membranes) (4).
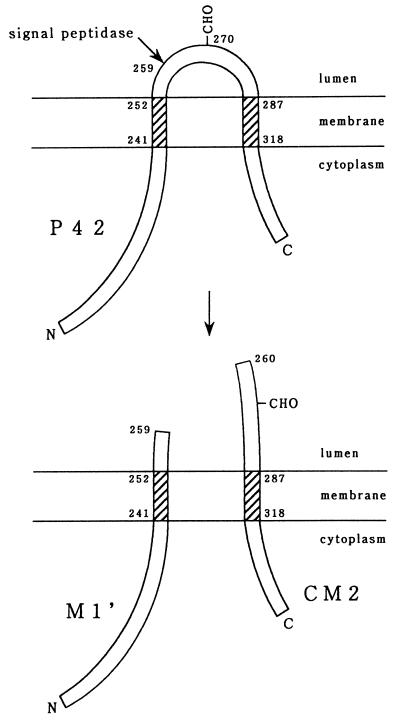
FIG. 5.
Proposed model for the biosynthesis of CM2. The 374-amino-acid protein (P42) has two hydrophobic domains capable of interacting with a lipid bilayer (shaded) and a N-glycosylation site (CHO). This protein is cleaved by signal peptidase after Ala residue 259 to produce the M1′ and CM2 proteins composed of the N-terminal 259 amino acids and C-terminal 115 amino acids, respectively.
The mechanism by which CM2 is produced from the unspliced mRNA is not known. However, it has previously been pointed out (2, 6) that three initiation codons lie in the same reading frame as that used for M1 at nucleotides 732 to 734, 741 to 743, and 747 to 749, one of which (residues 732 to 734) is in the context considered most favorable for initiation of protein synthesis (A/GNNAUGG) (5), suggesting that the unspliced mRNA may be translated from the initiation codon at nucleotides 732 to 734 to the termination codon at nucleotides 1,149 to 1,151, generating a 139-amino-acid CM2 protein. In this study, however, we present data showing that none of the three AUG codons located between nucleotides 732 and 749 are used for initiation of CM2 synthesis and provide evidence which indicates that a 374-amino-acid protein (P42/P44) is cleaved by signal peptidase to produce a 115-amino-acid CM2 protein, in addition to the previously unrecognized protein of 259 amino acids.
MATERIALS AND METHODS
Virus and cells.
The Yamagata/1/88 strain of influenza C virus was grown in the amniotic cavities of 9-day-old embryonated hen’s eggs (15). COS cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum.
Plasmid construction and site-directed mutagenesis.
To construct plasmid pME18S-CM2ORF used for expressing the putative CM2 coding region (nucleotides 732 to 1148), the DNA fragment corresponding to positions 728 to 1181 was obtained by PCR, with plasmid pCM5-3′F (2) (which contains nucleotides 3 to 1168 of the C/Yamagata/1/88 virus M gene and nucleotides 1169 to 1181 of the C/Ann Arbor/1/50 virus M gene) as a template and two primers (plus-sense primer composed of the EcoRI site followed by the sequence corresponding to positions 728 to 749 and minus-sense primer corresponding to positions 1181 to 1159). The PCR product was cut with EcoRI and EcoNI and inserted into the EcoRI and EcoNI sites of pCM5-3′F. The DNA fragment composed of residues 728 to 1181 was then excised by digestion with EcoRI and SalI and subcloned into the EcoRI and XhoI sites of a transient expression vector, pME18S (12) (a generous gift of Y. Takebe, National Institute of Infectious Diseases) to give pME18S-CM2ORF.
The mutated M gene cDNAs (except for DL705) were made by PCR with mutant primers. To create an ATG codon mutant IC-1 (see Fig. 1A), the 450-bp DNA fragment corresponding to positions 732 to 1181 was prepared by PCR with pCM5-3′F as a template and a plus-sense mutant primer (5′-dGCGGGACGAATGGCAATGAAATG [altered nucleotides are underlined]) corresponding to positions 732 to 754 and a minus-sense primer corresponding to positions 1181 to 1159. The 557-bp DNA fragment corresponding to positions 175 to 731 was also prepared with primers corresponding to positions 175 to 194 (plus-sense) and 731 to 711 (minus-sense). The 557- and 450-bp PCR products were cut with NcoI and EcoNI, respectively, and the resulting fragments (residues 237 to 731 and 732 to 1080) were phosphorylated with T4 polynucleotide kinase and then ligated into the NcoI and EcoNI sites of plasmid pCM5-5′3′F (4), which contains nucleotides 1 to 1168 of the C/Yamagata/1/88 virus M gene and nucleotides 1169 to 1181 of the C/Ann Arbor/1/50 virus M gene. The DNA molecules containing a full-length copy of the altered M gene were excised by digestion with EcoRI and SalI and then subcloned into the EcoRI and XhoI sites of pME18S. The remaining four mutated M genes shown in Fig. 1A were created according to the same procedures as those used for the creation of mutant IC-1, except that different mutant primers were used for preparation of the 450-bp DNA fragments (IC-1,2, IC-2,3, and IC-1,2,3) and that two DNA fragments corresponding to positions 175 to 704 and 706 to 1181 were first synthesized by PCR(DL705).
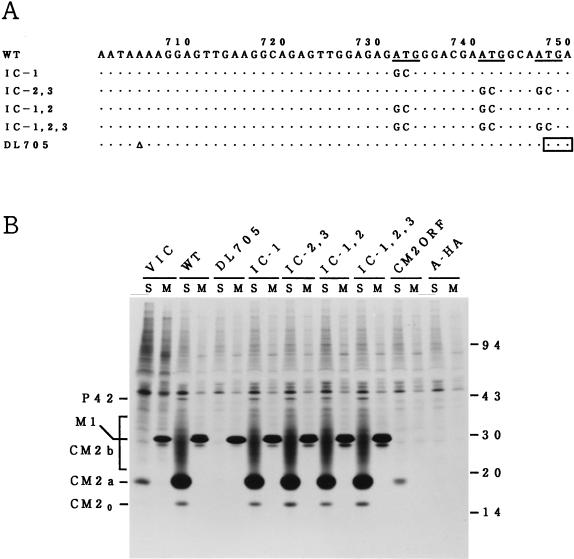
FIG. 1.
The three initiation codons present between nucleotides 732 and 749 are not used to initiate CM2 synthesis. (A) Nucleotide substitutions in the ATG codon mutants (IC-1; IC-2,3; IC-1,2; and IC-1,2,3) and the deletion mutant (DL705) are shown. The three initiation codons, which have previously been suggested to be used for initiating CM2 synthesis (2, 6), are underlined. The open triangle indicates deletion of a nucleotide residue. The position of the newly generated termination codon in the mutant DL705 is boxed. (B) COS cells were transfected with the recombinant plasmid constructed to contain the WT M gene (WT), the mutated M gene (DL705; IC-1; IC-2,3; IC-1,2; or IC-1,2,3), the putative CM2 open reading frame (CM2ORF), or the influenza A/Aichi/68 virus HA gene (A-HA) (a generous gift of E. Nobusawa, Nagoya City University). Cells were then labeled with [35S]methionine for 1 h at 48 h posttransfection and immunoprecipitated with either anti-GST/CM2 serum (lanes S) or anti-M1 monoclonal antibody (lanes M), and the resulting precipitates were analyzed by SDS-PAGE. COS cells infected with C/Yamagata/1/88 virus and labeled with [35S]methionine for 1 h at 26 h postinfection were also analyzed by immunoprecipitation followed by SDS-PAGE (VIC). In this experiment, P44 could not be unequivocally identified because of its comigration with a cellular protein. Numbers at the right show Mrs (in thousands).
To create mutant SP-1, having an amino acid sequence change of 252 Ile-Thr-Ser to 252 Met-Thr-Thr (see Fig. 3A), the 618-bp DNA fragment corresponding to nucleotide positions 175 to 792 was generated by PCR with a plus-sense primer (corresponding to positions 175 to 194) and a minus-sense mutant primer (5′-dGTTGAGTTGTCATAGAGAAATATATTATAACAAC [altered nucleotides are underlined]) corresponding to positions 792 to 759. In parallel, a 389-bp DNA fragment corresponding to positions 793 to 1181 was prepared by using primers corresponding to positions 793 to 815 (plus-sense) and 1181 to 1159 (minus-sense). For construction of mutant SP-2 with a change of 257 Ala-Ser-Ala to 257 Met-Ser-Met (see Fig. 3A), a plus-sense mutant primer (5′dCTATGTCTATGTGCAATCTAAAGACCTGTCTAAACC [altered nucleotides are underlined]) corresponding to 793 to 828 was used for synthesis of the 389-bp DNA fragment. In either case, the 618- and 389-bp PCR products were digested with NcoI and EcoNI, respectively, and the resulting fragments were phosphorylated and ligated into the NcoI and EcoNI sites of pCM5-5′3′F. The mutated M gene cDNA was then excised and subcloned into pME18S, as described above. The nucleotide sequences of all the mutant cDNAs in the pCM-5′3′F plasmid were confirmed by dideoxynucleotide chain-terminating sequencing.
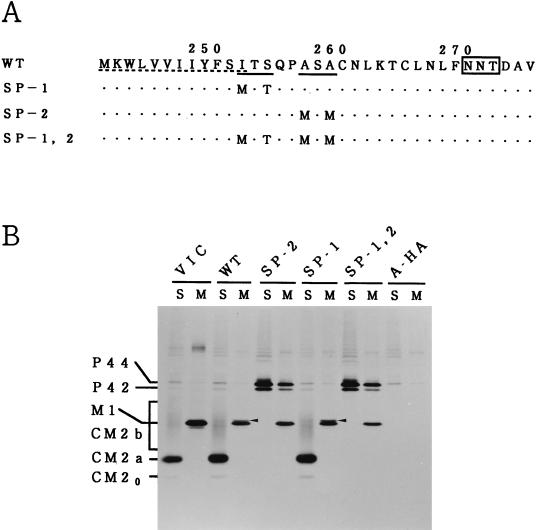
FIG. 3.
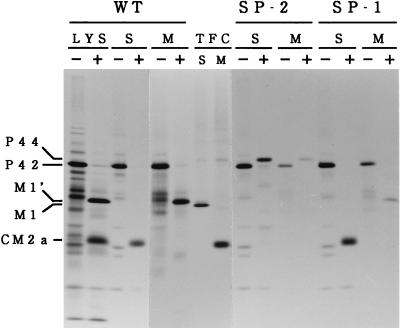
The existence of the second recognition motif for signal peptidase is essential for producing CM2. (A) Amino acid substitutions in mutants with alterations in the recognition motifs for signal peptidase are shown. Two sequences favorable for cleavage by signal peptidase are indicated by solid underlines. The hydrophobic domain that can interact with a lipid bilayer is indicated by a dotted underline. The N-glycosylation site is boxed. (B) COS cells transfected with the recombinant plasmid containing the WT M gene (WT), the mutated M gene (SP-2, SP-1, or SP-1,2) or influenza A/Aichi/68 virus HA gene (A-HA) were labeled with [35S]methionine for 1 h at 48 h posttransfection and immunoprecipitated with anti-GST/CM2 serum (lanes S) or anti-M1 monoclonal antibody (lanes M), and the resulting precipitates were analyzed by SDS-PAGE. C/Yamagata/1/88 virus-infected COS cells labeled with [35S]methionine for 1 h at 26 h postinfection were also analyzed by immunoprecipitation followed by SDS-PAGE (VIC). Arrowheads indicate the 259-amino-acid protein (M1′) that presumably contains an additional 17 amino acids from the C terminus of M1.
Transfection, metabolic labeling, and immunoprecipitation.
Subconfluent monolayers of COS cells in 3.5-cm-diameter petri dishes were transfected with the recombinant pME18S plasmid (1 μg/plate) containing the wild-type (WT) or mutated M gene by the lipofectamine procedure and incubated at 37°C. At 48 h posttransfection, cells were labeled with 40 μCi of [35S]methionine (ARC)/ml for 1 h in methionine-deficient Dulbecco’s modified Eagle’s medium. Cells were then disrupted in 0.01 M Tris-HCl (pH 7.4) containing 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.15 M NaCl, and a cocktail of protease inhibitors (3) and immunoprecipitated as described previously (8), utilizing anti-GST/CM2 serum or monoclonal antibody against the M1 protein (L2) (9). The immunoprecipitates obtained were analyzed by SDS-polyacrylamide gel electorophoresis (PAGE) on 17.5% gels containing 4 M urea under reducing conditions and processed for analysis by fluorography (15).
In vitro transcription and translation.
This procedure was carried out with the TnT T7 Quick Coupled Transcription/Translation system, which contains rabbit reticulocyte lysates, T7 RNA polymerase, ribonucleotides, and RNase inhibitor, according to the directions provided by the supplier (Promega). The recombinant Bluescript II KS(+) plasmid (1 μg), containing either the WT M gene (pCM5-5′3′F) or the mutated M gene, together with [35S]methionine (30 μCi), was added to a reaction mixture (50 μl), and reactions were allowed to proceed at 30°C for 70 min in the presence or absence of canine pancreas microsomal membranes (2.5 μl) (Promega). Translation products were analyzed by SDS-PAGE either directly or after immunoprecipitation with anti-GST/CM2 serum or anti-M1 monoclonal antibody.
RESULTS AND DISCUSSION
Three initiation codons present between nucleotides 732 and 749 are not used to initiate CM2 synthesis.
To examine the possibility that one of the three AUG codons located at nucleotide positions 732 to 734, 741 to 743, and 747 to 749 is used for initiation of CM2 synthesis, COS cells were transfected with the recombinant plasmid pME18S-CM2ORF constructed to contain nucleotides 728 to 1181 of the M gene, labeled with [35S]methionine, and analyzed by immunoprecipitation with either anti-GST/CM2 serum or anti-M1 monoclonal antibody. The results (Fig. 1B) show that CM2 (but not M1) was synthesized in the transfected cells, as has been observed by Pekosz and Lamb (6) with the vac-T7 expression system, a finding which supports the idea described above. To identify the initiation codon used for CM2 synthesis, therefore, we created four different ATG codon mutants (IC-1; IC-2,3; IC-1,2; and IC-1,2,3) shown in Fig. 1A and tested their ability to synthesize CM2 by expression in COS cells. When cells were transfected with plasmid pME18S-CM, containing the WT M gene (4), and immunoprecipitated with anti-GST/CM2 serum, it was found that three forms of CM2 (CM20, CM2a, and CM2b) were synthesized, in addition to a trace amount of P42 (Fig. 1B). We demonstrated previously that a mannose-rich oligosaccharide core is added to unglycosylated CM20 (Mr, 16,000) to form CM2a (Mr, 18,000) and that the maturation of oligosaccharide chain from the high-mannose type to the complex one converts CM2a into CM2b (Mr, 22,000 to 30,000) (3). Immunoprecipitation with anti-M1 monoclonal antibody also revealed the synthesis of M1 in the transfected cells.
Among the three AUG codons in question, the first one (nucleotides 732 to 734) is in the strongest context for ribosome initiation (A/GNNAUGG) (5) and has been thought to be the most likely initiation codon used for CM2 synthesis (2, 6). Unexpectedly, however, elimination of this initiation codon by converting residues 732 to 734 from ATG to GCG (mutant IC-1) did not affect the amounts of CM2, P42, and M1 synthesized in the transfected cells at all (Fig. 1B). Moreover, it became evident that CM2, in addition to M1 and P42, was synthesized in cells transfected with any of the double (IC-1,2 and IC-2,3) or triple (IC-1,2,3) ATG codon mutants. These results led us to conclude that none of the three initiation codons located between nucleotide positions 732 and 749 are used when CM2 is synthesized from the colinear mRNA transcript of the M gene, although one or more of them is used in the absence of nucleotides 1 to 727. To further confirm this conclusion, we introduced a deletion of nucleotide residue 705 into the M gene to give a mutant DL705 (Fig. 1A). In DL705, a deletion of residue 705 introduced a translation termination codon at positions 748 to 750, resulting in the loss of the ability to synthesize P42 and M1 as well as in the acquisition of the capacity to encode a 240-amino-acid protein, the N-terminal 227 residues of which are identical with those of M1. More importantly, mutant DL705 should retain the ability to synthesize CM2 if one of the three initiation codons described above is used for synthesis of the protein, since they are all preserved in the mutated M gene. Transfection of COS cells with mutant DL705 followed by immunoprecipitation with anti-GST/CM2 serum or anti-M1 monoclonal antibody showed that neither P42 nor CM2 was synthesized in the transfected cells, although synthesis of a protein with the electrophoretic mobility indistinguishable from that of M1 (which presumably represents a 240-amino-acid protein) could be detected (Fig. 1B). The finding that the failure of DL705 to synthesize P42 was accompanied by the failure to generate CM2 led us to postulate that synthesis of P42 may be a prerequisite for the production of CM2; P42 may be the primary product of unspliced mRNA from the M gene and may be cleaved co- or posttranslationally to yield CM2.
It should be noted in regard to Fig. 1B that the amount of CM2 (relative to the amount of M1) synthesized in cells transfected with the WT and mutant M gene cDNAs (except for DL705) was much larger than that in influenza C virus-infected cells, raising the possibility that virus-specific proteins other than the M gene products may regulate splicing of the colinear M gene transcript.
Kinetics of the production of P42 and CM2.
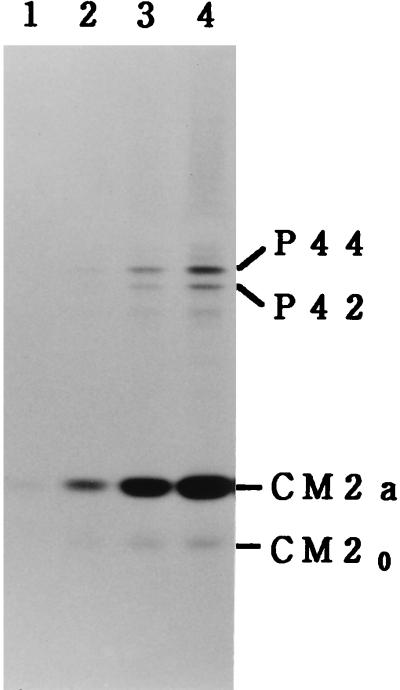
We reported previously that the precursor-product relationship between P42 and CM2 could not be detected in pulse-chase experiments in which a pulse-labeling period of 10 min was employed (4). To further examine the possibility that P42 is cleaved posttranslationally to yield CM2, COS cells transfected with pME18S-CM (containing the WT M gene) were pulse-labeled for an extremely short period (1, 2, 4, or 6 min) at 48 h posttransfection and then immunoprecipitated with anti-GST/CM2 serum. As shown in Fig. 2, the amounts of P42 and its N-glycosylated form (P44) were very low compared with those of CM2 (CM2a and CM20), even when cells were pulse-labeled for only 1 or 2 min, a result similar to that obtained with the transfected cells labeled for 1 h (Fig. 1B). This observation suggests strongly that if a cleavage event is involved in the biosynthesis of CM2, it must occur, cotranslationally or otherwise, immediately after the completion of translation.
FIG. 2.
[35S]methionine labeling of the M gene cDNA-transfected cells for a short period. COS cells transfected with the recombinant plasmid pME18S-CM (containing the WT M gene) were labeled with [35S]methionine for 1 min (lane 1), 2 min (lane 2), 4 min (lane 3), or 6 min (lane 4) and immunoprecipitated with anti-GST/CM2 serum, and the resulting precipitates were analyzed by SDS-PAGE.
Cleavage of P42/P44 by signal peptidase produces CM2.
The first hydrophobic domain (residues 241 to 252) of P42 is followed by two sequences (252 Ile-Thr-Ser and 257 Ala-Ser-Ala) favorable for cleavage by signal peptidase (13), raising the possibility that P42/P44, during or after penetration of the endoplasmic reticulum membrane, may be cleaved by signal peptidase after either Ser residue 254 or Ala residue 259, yielding CM2. To investigate this possibility, three different M gene mutants (SP-1, SP-2, and SP-1,2) shown in Fig. 3A, were constructed, and their ability to synthesize CM2 was tested. In mutant SP-1,2, the two recognition motifs for signal peptidase were both eliminated. Immunoprecipitation of SP-1,2-transfected COS cells with anti-GST/CM2 serum or anti-M1 monoclonal antibody followed by SDS-PAGE showed that this mutant lacks the ability to synthesize CM2, although it is capable of synthesizing M1 (Fig. 3B). It was also evident that the amounts of P42 and P44 synthesized in SP-1,2-transfected cells were much larger than those synthesized in the WT-transfected cells. These observations indicate that the existence of at least one of the two recognition motifs is essential for producing CM2 and that the failure of this mutant to produce CM2 is accompanied by the accumulation of P42 and P44. In mutants SP-1 and SP-2, the first and second consensus sequences for signal peptidase cleavage were removed, respectively. As clearly seen in Fig. 3B, synthesis of CM2 was detected in the SP-1-transfected cells but not in the SP-2-transfected cells, indicating the critical role of the second recognition motif in CM2 synthesis. If P42/P44 is certainly cleaved by signal peptidase after Ala residue 259, this cleavage event should yield a 259-amino-acid protein which contains 17 additional amino acids from the C terminus of M1 in addition to a 115-amino-acid protein, CM2. In the SDS-PAGE patterns of the immunoprecipitates obtained after the WT- or SP-1-transfected cell lysates were treated with anti-M1 monoclonal antibody, a thin band (indicated by an arrowhead) with slightly slower electrophoretic mobility than that of M1 could be seen (Fig. 3B). This band is likely to correspond to the 259-amino-acid protein described above, since it was undetectable in the cells transfected with SP-2 or SP-1,2 in which the cleavage of P42/P44 did not occur. This protein was designated M1′.
To obtain unequivocal evidence which indicates that P42/P44 is the primary product of unspliced mRNA from RNA segment 6 and is cleaved by signal peptidase to generate CM2, the WT and mutated M gene cDNAs (SP-1 and SP-2) were transcribed and then translated in vitro by using a kit described in Materials and Methods. The translation products obtained were analyzed by SDS-PAGE following immunoprecipitation with anti-GST/CM2 serum or anti-M1 monoclonal antibody (Fig. 4). We should mention here that because of the lack of splicing machinery, the M1 protein encoded by a spliced mRNA is not synthesized in this system. When the mRNA transcript of the WT M gene was translated in vitro in the absence of dog pancreas microsomal membranes, P42 was the only product detected. In the presence of microsomes, by contrast, the almost-complete disappearance of P42 was accompanied by the appearance of CM2a (detected by immunoprecipitation with anti-GST/CM2) and M1′ (detected by immunoprecipitation with anti-M1 monoclonal antibody). Essentially identical results were obtained with mutant SP-1. When mutant SP-2 was subjected to in vitro transcription and translation, by contrast, neither CM2 nor M1′ was synthesized, even in the presence of microsomal membranes, P44 being the only translation product detected.
FIG. 4.
In vitro transcription and translation of the mutant cDNAs with alteration in one of the two recognition motifs for signal peptidase. The WT M gene cDNA (WT) and the mutated M gene cDNAs (SP-2 and SP-1) were transcribed and then translated in vitro in the presence (+) or absence (−) of dog pancreas microsomal membranes, according to the procedures described in Materials and Methods. The translation products obtained were analyzed by SDS-PAGE either directly (LYS) or after immunoprecipitation with anti-GST/CM2 serum (S) or anti-M1 monoclonal antibody (M). The immunoprecipitates obtained from COS cells transfected with pME18S-CM (a plasmid containing the WT M gene) and then labeled with [35S]methionine for 1 h at 48 h posttransfection were also analyzed by SDS-PAGE (TFC).
Conclusions.
Based on the data presented here, we conclude that biosynthesis of CM2 proceeds in the following manner (Fig. 5). (i) Unspliced mRNA from RNA segment 6 is first translated into a 374-amino-acid protein, P42. (ii) Cotranslationally or immediately after the completion of translation, P42 begins to be inserted into the endoplasmic reticulum with the aid of the first hydrophobic domain, composed of amino acid residues 241 to 252. (iii) Translocation of P42 through the membrane is halted by the presence of the second hydrophobic domain, consisting of residues 287 to 318. (iv) P42 is then N-glycosylated at Asn residue 270 (6), generating P44. (v) Either before or after the addition of an oligosaccharide chain, P42/P44 is cleaved by signal peptidase at the C-terminal side of Ala residue 259, producing the M1′ and CM2 proteins, composed of the N-terminal 259 amino acids and the C-terminal 115 amino acids, respectively. It should be noted, however, that the orientation of M1′ in membranes, as well as the fate of the protein in infected cells and its role in virus replication, remains to be determined in future studies.
ACKNOWLEDGMENTS
We thank N. Iizuka (Nagoya City University) for helpful discussions and Y. Takebe (National Institute of Infectious Diseases) and E. Nobusawa (Nagoya City University) for providing the plasmids.
This work was supported by a grant-in-aid for scientific research from Ministry of Education, Science, and Culture, Japan.
REFERENCES
- 1.Ciampor F, Bayley P M, Nermut M V, Hirst E M A, Sugrue R J, Hay A J. Evidence that the amantadine-induced, M2-mediated conversion of influenza A virus hemagglutinin to the low pH conformation occurs in an acidic trans Golgi compartment. Virology. 1992;188:14–24. doi: 10.1016/0042-6822(92)90730-d. [DOI] [PubMed] [Google Scholar]
- 2.Hongo S, Sugawara K, Nishimura H, Muraki Y, Kitame F, Nakamura K. Identification of a second protein encoded by influenza C virus RNA segment 6. J Gen Virol. 1994;75:3503–3510. doi: 10.1099/0022-1317-75-12-3503. [DOI] [PubMed] [Google Scholar]
- 3.Hongo S, Sugawara K, Muraki Y, Kitame F, Nakamura K. Characterization of a second protein (CM2) encoded by RNA segment 6 of influenza C virus. J Virol. 1997;71:2786–2792. doi: 10.1128/jvi.71.4.2786-2792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hongo S, Gao P, Sugawara K, Muraki Y, Matsuzaki Y, Tada Y, Kitame F, Nakamura K. Identification of a 374 amino acid protein encoded by RNA segment 6 of influenza C virus. J Gen Virol. 1998;79:2207–2213. doi: 10.1099/0022-1317-79-9-2207. [DOI] [PubMed] [Google Scholar]
- 5.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 6.Pekosz A, Lamb R A. The CM2 protein of influenza C virus is an oligomeric integral membrane glycoprotein structurally analogous to influenza A virus M2 and influenza B virus NB proteins. Virology. 1997;237:439–451. doi: 10.1006/viro.1997.8788. [DOI] [PubMed] [Google Scholar]
- 7.Pinto L H, Holsinger L J, Lamb R A. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara K, Nishimura H, Kitame F, Nakamura K. Antigenic variation among human strains of influenza C virus detected with monoclonal antibodies to gp88 glycoprotein. Virus Res. 1986;6:27–32. doi: 10.1016/0168-1702(86)90054-7. [DOI] [PubMed] [Google Scholar]
- 9.Sugawara K, Nishimura H, Hongo S, Kitame F, Nakamura K. Antigenic characterization of the nucleoprotein and matrix protein of influenza C virus with monoclonal antibodies. J Gen Virol. 1991;72:103–109. doi: 10.1099/0022-1317-72-1-103. [DOI] [PubMed] [Google Scholar]
- 10.Sugrue R J, Hay A J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991;180:617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunstrom N A, Premkumar L S, Premkumar A, Ewart G, Cox G B, Gage P W. Ion channels formed by NB, an influenza B virus protein. J Membr Biol. 1996;150:127–132. doi: 10.1007/s002329900037. [DOI] [PubMed] [Google Scholar]
- 12.Takebe Y, Motoharu S, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita M, Krystal M, Palese P. Evidence that the matrix protein of influenza C virus is coded for by a spliced mRNA. J Virol. 1988;62:3348–3355. doi: 10.1128/jvi.62.9.3348-3355.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokota M, Nakamura K, Sugawara K, Homma M. The synthesis of polypeptides in influenza C virus-infected cells. Virology. 1983;130:105–117. doi: 10.1016/0042-6822(83)90121-6. [DOI] [PubMed] [Google Scholar]