Abstract
Skewing of the T-cell receptor repertoire of CD8+ T cells has been shown in some persistent infections with viruses, such as human immunodeficiency virus, simian immunodeficiency virus, and Epstein-Barr virus. We have demonstrated that similar distortions also occur in nonpersistent measles virus infection. In addition, two of four children immunized with live, attenuated measles virus showed larger and more persistent CD8+ T-cell expansions than their naturally infected counterparts. The expanded lymphocyte populations were monoclonal or oligoclonal and lysed target cells infected with recombinant vaccinia virus expressing measles virus protein. These results demonstrate that the expansions of CD8+ T lymphocytes are antigen driven.
Measles virus (MV) is a negative-strand RNA virus. The clinical symptoms caused by MV infection appear between 2 and 3 weeks after infection. The appearance of the rash is a sign of the peaking immune response and is associated with clearance of the virus (17). Although now well controlled by vaccination programs in developed countries, measles is still a major problem in sub-Saharan Africa (14, 15). Children below the age of 1 year are particularly at risk and are hard to protect with the current vaccine (30). In young children, MV causes around one million deaths per annum and there is also a large morbidity. Secondary infection by other agents is common (2, 20), while malnutrition may increase the risk of this complication (1, 32). Such secondary infections are believed to largely result from immunosuppression by MV infection which can persist for several weeks (33). The effect was first described as a delayed type hypersensitivity defect in MV-infected patients (37). Proliferation of lymphocytes in response to mitogen in vitro is also reduced after MV infection (16, 33, 39, 42).
While the humoral immune response is important in protecting against reinfection, the cellular response, especially the CD8+ T-cell response, is important in the clearance of established MV infection (3, 18, 36). Some primary infections with persistent viruses, such as human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), and Epstein-Barr virus (EBV), stimulate a very strong CD8+ T-cell response, with development of large clonal and oligoclonal expansions of these cells (5, 7, 25, 40). Such expansions may comprise up to 40% of CD8+ T cells and thus cause distortions of the T-cell receptor (TCR) repertoire. Clonally expanded populations can persist for many months after primary infection with these persistent viruses. While indirect evidence suggests that these T-cell expansions are antigen driven, little is known about their functional capacity, such as cytolysis.
In this study, we wished to determine whether similar distortions in the T-cell repertoire occur in MV infection. We therefore studied the TCR repertoire in peripheral blood lymphocytes of children with acute MV infection and of healthy Gambian infants given the standard live, attenuated MV vaccine. We found marked distortions of the T-cell repertoires in the majority of infected patients and in two of the four vaccinees. These expanded T lymphocytes have cytotoxic activity and are likely therefore to play a major role in the clearance of infected cells.
MATERIALS AND METHODS
Patients and controls.
Nineteen patients with acute MV infection from The Gambia were studied. The patients were aged from 6 months to 9 years, with a mean of 2.8 years. Categorization of disease severity was based on previously described criteria (21). Five showed severe and 10 showed moderate symptoms; the rest had mild disease. The study also included four children who were vaccinated with the attenuated Edmonston strain of MV at the age of 9 months. Patients were bled within a week of the onset of rash, and vaccinees were sampled 2 to 4 weeks after vaccination. Ten Gambian children, with a mean age of 8.1 years, recovering from malaria infection at least 4 months, were included as controls. Approval for this study was given by the Gambian Government/MRC Ethics Committee.
Isolation and fractionation of lymphocyte preparations.
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation. The cells were cryopreserved until tested. In the clonality expansion study, CD4+ cells were depleted from PBMC by anti-CD4-conjugated Dynabeads (Dynal UK Ltd.).
Flow cytometry analysis for T-cell repertoire.
Two-color staining was carried out as described previously (28). PBMC were stained with a panel of monoclonal antibodies (MAb) specific for the β-chain variable region of the TCR (Vβ) by using a second-layer rabbit anti-mouse antibody directly conjugated to fluorescein isothiocyanate (Dako Ltd.). The anti-CD8 MAb was directly conjugated to phycoerythrin (Dako Ltd.). The panel of anti-human TCR Vβ-region MAb is as follows: B237.2 (BV1), E2.2E7.2 (BV2), LE89 (BV3), 30/3D6 (BV5S2/S3), OT145 (BV6S7), 3G5D5 (BV7S1), JR2 (BV8), MKB1P.2-10 (BV9), S511 (BV12), H131 (BV13S1), H132 (BV13S2), C1 (BV17), ELL1.4 (BV20), 1G125 (BV21S3), and HUT78#7 (BV23). These antibodies were part of the human TCR MAb workshop. Cells were analyzed on a Becton-Dickinson FACScan using CELLQUEST software. CD8+ T lymphocytes within an individual Vβ family were considered to have attained significant expansion when the percent staining was higher than three standard deviations above the mean staining of that Vβ family in the control population.
Molecular cloning and sequencing of TCR beta chains.
RNA was extracted from CD4-depleted T cells by using TRIreagent (Sigma Chemical Co.) and used in first-strand cDNA synthesis with CB14 primer, CTCAGCTCCACGTG as described previously (40). The cDNA was used as a template in PCRs using 3′ CB-R primer (ATACTGGAGTCGACCTTCTGATGGCTCAAACAC) and 5′ BV primer. The 5′ primers were BV7, ATAAGAATGCGGCCGCGTTTGTCTACAGCTATGAGAAACTCT; BV8, TTCTAGAAGCGGCCGCACGTTCCGATAGATGATTCAG; and BV17, ATAAGAATGCGGCCGCACAGCGTCTCTCGGGAGA. The PCR conditions were as previously described (5). The PCR products were gel purified using the Wizard PCR purification kit (Promega). The purified product was cloned in a pMOSBLUE T-vector (Amersham Life Sciences). Plasmid DNA was extracted by the DNA purification system (Promega). Double-stranded DNA sequencing was performed with T7 DNA polymerase (Pharmacia) and M13 −20 primer (GTAAAACGACGGCCAGT).
Depletion of expanded T cells.
PBMC were cultured overnight at 37°C in the presence of 10 U of interleukin-2/ml of RPMI 1640 plus 10% fetal calf serum (R10). Cells were counted for live cells and split into two groups. One group was kept as untreated cells. The other was incubated with antibodies against the expanded Vβ TCR for 1 h at 4°C. After washing, goat anti-mouse immunoglobulin G-coated Dynabeads (Dynal) were added to the cells and rotated for 1 h at 4°C and then removed using a magnet. Unbound cells were tested for cytotoxic T-lymphocyte (CTL) activity.
CTL activity.
CTL activity was assayed as described previously (18). Briefly, autologous EBV-transformed B cells, target cells, were infected with vaccinia virus recombinants expressing the measles virus fusion protein (F), hemagglutinin (H), or nucleoprotein (NP) at a multiplicity of infection of 5 for 2 h at 37°C. Cells were then washed and left at 37°C in R10 for 16 h before labelling them with 51chromium. An effector-to-target ratio of 20 to 1 was used in a 4-h 51chromium release assay.
RESULTS
The CD8+ T-cell repertoire is distorted during acute MV infection.
By staining PBMC with a panel of 15 MAbs specific for the TCR Vβ chain, we found expanded populations of CD8+ T cells expressing particular Vβ chains (Vβ expansions) in 16 of 19 children with acute MV infection and 2 of 4 vaccinees (Table 1). Up to four different Vβ expansions were found in any one individual. The expansions were not confined to particular TCR Vβ families, and there was no association with HLA class I (Table 1). There was no correlation between severity of the disease and the number or size of the expansions (data not shown). Follow-up samples were examined 4 to 9 months after acute infection and 8 to 10.5 months after vaccination. By this time, most of the expansions were reduced in size or were no longer detectable, with only a few Vβ expansions persisting.
TABLE 1.
The T-cell repertoire in MV-infected patients and vaccinees
| Sample source | HLA A and B type | Sample timea | V beta (BV) gene segment usage among CD8+ T lymphocytes
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV1 | BV2 | BV3 | BV5S2/S3 | BV6S7 | BV7S1 | BV8 | BV9 | BV12 | BV13S1 | BV13S2 | BV17 | BV20 | BV21S3 | BV23 | |||
| Vaccinee | |||||||||||||||||
| CBL108 | NDe | 1st (2) | 2.36 | 3.55 | 2.08 | 3.80 | 0.51 | 7.48b | 1.54 | 0.50 | 0.63 | 1.37 | 0.97 | 21.76 | 10.53 | 0.74 | 15.53 |
| 2nd (42) | 3.93 | 3.52 | 3.85 | 3.56 | 0.86 | 7.98 | 2.21 | 0.51 | 0.64 | 2.14 | 1.07 | 9.60 | 8.39 | 1.63 | 12.34 | ||
| CBL121 | ND | 1st (6) | 3.39 | 2.34 | 0.49 | 4.18 | 0.50 | 1.00 | 2.70 | 7.81 | 0.99 | 2.16 | 2.68 | 31.13 | 1.08 | 0.33 | 1.33 |
| 2nd (32) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 30.80 | ND | ND | ND | ||
| CBL134 (twin 1)c | ND | 1st (4) | 2.71 | 4.65 | 1.15 | 6.13 | 3.30 | 3.95 | 2.66 | 1.34 | 1.43 | 2.50 | 1.11 | 3.29 | 2.71 | 2.51 | 0.98 |
| CBL134 (twin 2)c | ND | 1st (4) | 5.90 | 5.65 | 0.93 | 3.85 | 2.91 | 1.43 | 5.72 | 1.30 | 2.15 | 4.32 | 0.96 | 4.97 | 2.57 | 1.92 | 0.81 |
| Patient | |||||||||||||||||
| M34 | ND | A | 12.16 | 4.62 | 0.27 | 1.00 | 0.70 | 3.20 | 9.15 | 1.35 | 3.80 | 2.63 | 0.41 | 4.49 | 0.36 | 1.12 | 0.86 |
| M35 | A2,3; B12,17 | A | 4.40 | 4.06 | 0.66 | 7.51 | 1.90 | 3.35 | 3.01 | 4.64 | 2.50 | 2.81 | 2.40 | 3.53 | 3.95 | 3.21 | 0.82 |
| M36 | A19,28; B22,41 | A | 2.15 | 3.49 | 0.00 | 7.38 | 0.89 | 3.70 | 0.00 | 0.00 | 3.61 | 3.45 | 0.00 | 1.49 | 1.03 | 0.00 | 0.78 |
| M37 | A9,28; B12,53 | A | 1.26 | 0.66 | 1.98 | 1.46 | 0.40 | 1.85 | 3.64 | 1.29 | 2.12 | 4.25 | 0.78 | 2.67 | 0.36 | 6.25 | 4.07 |
| F (18) | 4.36 | 5.09 | 1.59 | 2.63 | 1.89 | 1.49 | 4.29 | 0.59 | 1.10 | 6.04 | 2.09 | 4.21 | 1.03 | 4.60 | 1.89 | ||
| M39 | A2,28; B5,35 | A | 1.57 | 7.72 | 6.81 | 2.89 | 3.00 | 2.50 | 1.17 | 0.45 | 0.69 | 1.80 | 8.01 | 1.02 | 0.70 | 1.78 | 21.30 |
| F (24) | 5.54 | 10.37 | 3.10 | 2.44 | 2.72 | 0.86 | 3.34 | 0.48 | 1.85 | 2.73 | 4.58 | 2.42 | 0.83 | 1.20 | 12.38 | ||
| M40 | A19; B5,35 | A | 1.39 | 2.45 | 10.42 | 2.61 | 1.14 | 3.53 | 3.67 | 0.67 | 1.36 | 8.08 | 0.99 | 3.79 | 0.37 | 2.01 | 0.58 |
| M41 | A28; B15 | A | 3.79 | 3.23 | ND | 3.56 | 0.27 | 2.91 | 3.22 | 0.44 | 0.24 | 2.52 | 1.67 | 6.07 | 2.01 | 2.83 | 0.89 |
| M42 | A19,28; B5,35 | A | 5.00 | 3.58 | 2.41 | 5.94 | 0.83 | 0.59 | 16.04 | 0.18 | 1.28 | 3.23 | 0.57 | 1.98 | 4.55 | 2.00 | 0.32 |
| F (20) | ND | ND | ND | ND | ND | ND | 4.60 | ND | ND | ND | ND | ND | ND | ND | ND | ||
| M43 | A1,9; B16 | A | 8.24 | 11.45 | 0.15 | 2.52 | 2.20 | 10.07 | 4.01 | 2.27 | 1.03 | 2.05 | 2.21 | 2.60 | 1.67 | 2.05 | 0.52 |
| F (16) | ND | ND | ND | ND | ND | 5.60 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| M44 | A1,10; B8,49 | A | 6.54 | 2.58 | 0.85 | 0.62 | 1.20 | 1.48 | 3.43 | 1.69 | 7.22 | 2.25 | 4.09 | 3.03 | 0.78 | 2.01 | 2.92 |
| M45 | A2,3; B7 | A | 2.17 | 3.66 | 1.14 | 2.35 | 1.08 | 2.70 | 2.15 | 0.00 | 0.00 | 1.32 | 5.10 | 3.57 | 4.17 | 1.06 | 4.26 |
| F (23) | 5.16 | 3.65 | 0.89 | 3.89 | 2.11 | 1.22 | 4.58 | 0.31 | 1.25 | 3.40 | 2.65 | 3.09 | 1.52 | 1.05 | 1.66 | ||
| M47 | A30,32; B35,53 | A | 2.38 | 5.62 | 8.60 | 6.01 | 1.57 | 0.45 | 13.51 | 1.58 | 1.68 | 2.07 | 1.45 | 4.83 | 1.16 | 0.82 | 0.61 |
| F (8) | 3.58 | 3.87 | 3.95 | 6.70 | 1.99 | 2.31 | 7.62 | ND | ND | ND | ND | ND | 1.42 | 1.99 | 0.56 | ||
| M49 | A19; B35,22 | A | 10.36 | 16.92 | 8.02 | 3.54 | 0.27 | 1.44 | 2.73 | 0.48 | 6.71 | 2.76 | 5.29 | 4.96 | 1.35 | 1.49 | 0.42 |
| F (15) | 7.57 | 10.19 | 1.58 | 4.41 | 2.16 | 5.22 | 3.65 | 0.99 | 3.83 | 2.19 | 2.82 | 4.43 | 1.88 | 3.33 | 1.46 | ||
| M51 | A19; B14,35 | A | 0.98 | 2.02 | 1.61 | 4.71 | 0.23 | 0.64 | 1.19 | 0.28 | 12.32 | 0.69 | 0.25 | 0.49 | 17.71 | 0.20 | 11.56 |
| M53 | A26,19; B8 | A | 3.39 | 5.77 | 0.63 | 3.17 | 0.55 | 6.11 | 6.87 | 1.13 | 1.56 | 4.99 | 1.69 | 5.52 | 4.17 | 1.93 | 1.40 |
| F (17) | 2.39 | 6.23 | 1.36 | 4.76 | 1.38 | 3.12 | 4.73 | 1.31 | 1.51 | 3.57 | 0.85 | 7.27 | 3.29 | 1.11 | 1.05 | ||
| M111 | A2,33; B15,50 | A | 1.92 | 4.09 | 1.22 | 2.08 | 0.55 | 0.72 | 4.34 | 0.07 | 1.24 | 7.80 | 0.65 | 23.05 | 12.34 | 7.00 | 5.68 |
| M152 | A31,68; B35,53 | A | 4.21 | 5.32 | 6.39 | 4.39 | 4.34 | 3.96 | 3.40 | 3.27 | 0.95 | 9.28 | 0.65 | 3.90 | 3.03 | 3.15 | 3.74 |
| M342 | A2,23; B7,53 | A | 2.73 | 4.53 | 5.54 | 2.75 | 1.68 | 3.96 | 3.02 | 1.95 | 1.33 | 8.06 | 1.62 | 4.57 | 2.64 | 2.49 | 10.89 |
| M132 | A | 7.17 | 4.67 | 3.67 | 3.70 | 1.18 | 2.67 | 5.84 | 0.44 | 1.81 | 0.81 | 0.96 | 5.25 | 2.31 | 1.24 | 0.55 | |
| 10 Gambian controls | |||||||||||||||||
| Mean | 3.73 | 4.69 | 2.20 | 3.55 | 2.11 | 1.77 | 4.34 | 0.74 | 1.51 | 3.35 | 1.99 | 3.80 | 3.01 | 1.69 | 1.03 | ||
| Standard deviation | 1.22 | 2.13 | 1.73 | 2.67 | 1.78 | 0.81 | 2.29 | 0.47 | 0.54 | 0.96 | 1.44 | 1.72 | 2.08 | 0.97 | 0.50 | ||
| Cutoff pointd | 7.40 | 11.06 | 7.38 | 11.57 | 7.45 | 4.20 | 11.22 | 2.15 | 3.13 | 6.22 | 6.31 | 8.96 | 9.24 | 4.60 | 2.54 | ||
| 4 Caucasian controls | |||||||||||||||||
| Mean | 6.46 | 4.62 | 3.75 | 3.88 | 2.33 | 2.04 | 4.51 | 0.89 | 1.59 | 3.54 | 3.59 | 3.63 | 2.42 | 1.94 | 1.53 | ||
| Standard deviation | 3.48 | 1.71 | 0.90 | 0.86 | 1.12 | 1.26 | 1.29 | 0.35 | 0.63 | 1.08 | 1.98 | 1.70 | 0.97 | 0.36 | 0.75 | ||
| Cutoff pointd | 16.90 | 9.75 | 6.45 | 6.46 | 5.69 | 5.82 | 8.38 | 1.94 | 3.48 | 6.78 | 9.53 | 8.73 | 5.33 | 3.02 | 3.78 | ||
1st, 2nd, A, and F represent first, second, acute, and follow-up samples, respectively. The number in parentheses indicates weeks after vaccination or infection.
Percentage of a particular TCR Vβ shown in bold was considered to be expanded.
These children are twins.
The cutoff point for a particular TCR Vβ family was calculated as the mean plus 3 standard deviations among controls (10 Gambian children recovering from malaria infection or 4 healthy Caucasian controls).
ND, not done.
In contrast, the CD8+ TCR repertoires of the children recovering from malaria infection were very similar to each other and to those previously recorded in control Caucasian populations (Table 1). They showed no expansions.
CD8+ TCR Vβ expansions are clonal or oligoclonal.
To determine whether the expansions observed were derived from one (clonal) or a few (oligoclonal) T-cell clones, we examined the predicted amino acid sequences of the Vβ chain CDR3 regions (between Vβ and Jβ) of the expanded CD8+ T cells in three children. In patient M42, 64% of the expanded BV8+ CD8+ T-cell transcripts were identical, showing that a single clone of T cells accounted for most of this expansion of 16% of CD8+ T cells (Table 2). In the follow-up sample taken from this child, the repertoire, as assessed using TCR Vβ-specific MAbs, had returned to normal. However, sequence analysis of the BV8 CDR3 region showed that the clonotype that dominated the primary response was still detectable, albeit at a lower frequency (44%).
TABLE 2.
Analysis of BV8TCR sequences in acute and follow-up samples in patient M42
| Sample time | End sequence of BV8 | CDR3 | BJ | Frequency |
|---|---|---|---|---|
| Acute infectiona | CAS | SPGGIGAF | FG 1S1 | 7/11 |
| CAS | SLGGREQY | FG 2S7 | 1/11 | |
| CAS | SSYSEAF | FG 1S1 | 1/11 | |
| CAS | SPDBRGEPTDTQY | FG 2S3 | 1/11 | |
| CAS | SLAGPNEKLF | FG 1S4 | 1/11 | |
| Follow-upb | CAS | SPGGIGAF | FG 1S1 | 4/9 |
| CAS | SFWGLGETQY | FG 2S5 | 1/9 | |
| CAS | TPPRDRPISPQH | FG 1S5 | 1/9 | |
| CAS | SSWEPGIVTEAF | FG 1S1 | 1/9 | |
| CAS | SSTAENSPLH | FG 1S6 | 1/9 | |
| CAS | SPTLGRGDSNAGELF | FG 2S2 | 1/9 |
At this time, 16.0% of CD8+ T cells were expressing BV8.
At the 20-week follow-up, 4.6% of CD8+ T cells were expressing BV8.
Patient M43 showed an oligoclonal expansion in BV7S1+ CD8+ T cells (Table 3). The TCRs of these clones showed restricted CDR3 length (9 to 11 amino acids) and joining chain (J) use which was biased toward use of the BJ2S7 gene, 78% compared to 15 to 25% in healthy individuals (29, 30, 38). Extensive analysis of CDR3 sequences associated with BV7S1 in healthy persons has shown a wide range of CDR3 lengths and Jβ gene usage (4a). This restricted CDR3 pattern is consistent with an antigen-driven CD8+ T-cell response (23). In the M43 follow-up sample, BV7S1+ CD8+ T cells showed a polyclonal response with no clonotype using BJ2S7.
TABLE 3.
Analysis of BV7S1 TCR sequences in acute and follow-up samples in patient M43
| Sample time | End sequence of BV7S1 | CDR3c | BJ | Frequency |
|---|---|---|---|---|
| Acute infectiona | CAS | SQKMVPTPYT | FG 1S2 | 4/18 |
| CAS | SQGDRVNEQF | FG 2S1 | 3/18 | |
| CAS | SQVGGQETQY | FG 2S5 | 2/18 | |
| CAS | SQERRQKQY | FG 2S7 | 1/18 | |
| CAS | SQEGWYEQY | FG 2S7 | 1/18 | |
| CAS | SPQRDRAYEQY | FG 2S7 | 1/18 | |
| CAS | SQGQANYEQY | FG 2S7 | 1/18 | |
| CAS | SPGTGRPEQY | FG 2S7 | 1/18 | |
| CAS | SPGDRVYEQY | FG 2S7 | 1/18 | |
| CAS | RKDRGTYEQY | FG 2S7 | 1/18 | |
| CAS | SQVWTGDQPQH | FG 1S5 | 1/18 | |
| CAS | SQEGAGGDSTNEKLF | FG 1S4 | 1/18 | |
| Follow-upb | ||||
| CAS | SQGQGADISRGYT | FG 1S2 | 2/9 | |
| CAS | SQKMVPTPYT | FG 1S2 | 1/9 | |
| CAS | SPTQGGGEKLF | FG 1S4 | 1/9 | |
| CAS | SQTITSTYSPLH | FG 1S6 | 1/9 | |
| CAS | SQDMVGLAVDEQF | FG 2S1 | 1/9 | |
| CAS | SQWTPSEAF | FG 1S1 | 1/9 | |
| CAS | SQVQDTEAF | FG 1S1 | 1/9 | |
| CAS | SNNWGVTEAF | FG 1S1 | 1/9 |
At this time, 10.1% of CD8+ T cells were expressing BV7S1.
At the 16-week follow-up, 5.6% of CD8+ T cells were expressing BV7S1.
Glutamate-glutamine motif is highlighted.
In vaccinee CBL121, the BV17+ CD8+ T-cell expansion, which consisted of 31.13% CD8+ T cells, was predominantly clonal, with 82% of sequences shown to be identical (Table 4). In this individual the expansion persisted, with the same clonotype continuing to dominate (92%) 32 weeks later.
TABLE 4.
Analysis of BV17 TCR sequences in acute and follow-up samples in vaccinee CBL121
| Sample | End sequence of BV17 | CDR3 | BJ | Frequency |
|---|---|---|---|---|
| Firsta | CAS | SHDGYTEAF | FG 1S1 | 9/11 |
| CAS | GPAEAMNTEAF | FG 1S1 | 1/11 | |
| CAS | SPDNQPQH | FG 1S5 | 1/11 | |
| Secondb | CAS | SHDGYTEAF | FG 1S1 | 11/12 |
| CAS | NPGYGYT | FG 1S2 | 1/12 |
At 6 weeks after vaccination, 31.1% of CD8+ T cells were expressing BV17.
At the 32-week follow-up, 30.1% of CD8+ T cells were expressing BV17.
Expanded T lymphocytes show virus-specific cytotoxic function.
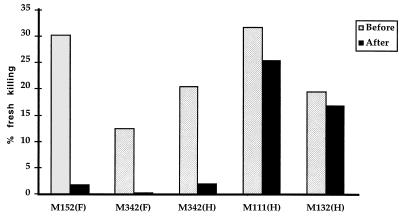
To determine whether the expanded T cells were cytotoxic, the patients’ PBMC were tested with autologous B cells infected with vaccinia virus expressing MV protein, i.e., fusion protein (F), hemagglutinin (H), or nucleoprotein (NP), at an effector/target ratio of 20:1. Patient M152 had a CD8+ T-cell expansion of T cells expressing BV13S1 (9.28% compared to 3.35% ± 0.96 in Gambian controls) (Table 1) at the time of acute MV infection. PBMC from this patient after overnight culture in the absence of antigen lysed F-expressing vaccinia virus-infected cells. This killing activity was reduced from 30 to 1% after removing the BV13S1+ cells from PBMC (Fig. 1).
FIG. 1.
Cytotoxic activity from patients with acute MV infection was tested before and after depleting Vβ expansions from PBMC. The percent specific lysis of target cells expressing vaccinia virus recombinants expressing fusion protein (F) and hemagglutinin (H) was shown. In patients M152 and M342, all the expanded T cells were removed. In patient M111, only 2 of 4 Vβ expansions were depleted. Patient M132, who had no particular TCR expansion, was used as negative control. PBMC of these patients were also tested for cytotoxic activity against β-galactosidase-expressing vaccinia virus, on a different occasion, as a control. All samples had less than 5% killing against this recombinant.
In another patient, M132, the TCR repertoire, as determined using TCR Vβ-specific MAbs, was within normal limits. PBMC from this patient lysed H-expressing vaccinia virus-infected target cells. Depletion of BV13S1+ T lymphocytes from this patient did not significantly reduce this lytic activity, showing that the antibody and depletion procedure did not nonspecifically reduce cytotoxicity.
Patient M342 had CD8+ T-cell expansions of cells expressing BV13S1 (8.06% versus 3.35 ± 0.96 in Gambian controls) and BV23 (10.89% versus 1.03% ± 0.50). CTL in this patient showed 12.5 and 20.8% specific cytotoxicity, ex vivo, against target cells infected with vaccinia virus expressing F and H, respectively. The activity was decreased to 0 and 2.1% after removing just the expanded BV13S1+ and BV23+ populations.
Patient M111 had CD8+ T-cell expansions of cells expressing BV17 (23% versus 3.8% ± 1.72), BV13S1 (7.8% versus 3.35% ± 0.50), BV20 (12% versus 3.01% ± 2.08), and BV21S3 (7% versus 1.69% ± 0.97). PBMC from this patient showed killing activity against H-expressing vaccinia virus-infected cells but not against target cells infected with vaccinia virus expressing F and NP. The percent lysis against H of this patient was reduced from 32 to 26% after depletion of BV17+ and BV21S3+ cells.
DISCUSSION
Distortion of the human T-cell repertoire during acute and chronic infection with HIV, SIV, and EBV has been demonstrated (5, 7, 25, 40). Those reports described expansions in particular Vβ T-cell subsets during the acute phase of infection. Most of the expansions declined to baseline levels in follow-up samples several weeks later, but in some cases Vβ T-cell expansions could persist, as detected by PCR, for up to 2 years (40, 41). In order to determine whether skewing of the T-cell repertoire is also characteristic of nonpersistent virus infections, we examined the TCR repertoire of CD8+ T cells in patients with acute MV infection. Expansions similar to those detected during primary HIV or EBV infection were found. Recipients of attenuated MV vaccine showed similar results; in two of four vaccinees the T-cell responses were greater than those in naturally infected patients. This may reflect the reduced immunosuppressive effects of the vaccine strain of virus. It is noteworthy that no TCR expansions were found in control children recovering from malaria infection. Although there is good evidence that CD8+ T cells respond to liver-stage antigens (27), the response may be very weak compared to that in an acute virus infection.
The TCR β-chain CDR3 region highly variable. It is important in interacting with antigenic peptides presented by HLA molecules (9, 12, 13). Sequence analysis of this highly variable region of the TCR indicates whether the T cells are clonal or oligoclonal, suggesting selection by an antigen, or polyclonal. In patient M42 and vaccinee CBL121, the Vβ expansions were dominated by a single clone, whereas in patient M43 the expanded BV7S1+ CD8+ T cells showed oligoclonality, with selection for CDR3 length and heavy bias toward the use of joining chain BJ2S7. This joining chain is one of two Jβ families containing a glutamate-glutamine motif (highlighted in Table 3) (35) which has been shown to be involved in antigen recognition by the TCR (19). The clonal and oligoclonal nature of the expansions found during primary MV infection strongly suggests that they are driven by antigen specificity.
Following recovery from MV infection, the T-cell repertoire, as determined using TCR Vβ-specific MAbs, usually returned to normal. However, a few expansions sometimes remained. Half the vaccinees showed results similar to those infected naturally, with some persistent, oligoclonal T-cell expansions. Chronic clonal expansion in a persistent infection probably results from continuous activation by antigens; however, in MV infection the virus disappears from the blood and tissue within 1 month (17), yet the expansions can persist much longer. Why these clones persist and escape from activation-induced cell death is not known. Not all clones persist, however, for example, the BV7S1 population in M43 was lost.
Until now it has been difficult to show that the expanded CD8+ T cells found in acute virus infections can mediate antigen-specific cytotoxicity (5, 25). Recently, staining of expanded T cells with tetrameric HLA-peptide complexes has confirmed that they are antigen specific (6, 11, 41), and when sorted by flow cytometry, these T cells secrete gamma interferon in response to antigen challenge (10). Pantaleo et al. (25) showed cytotoxic capacity in sorted CD8+ T cells expressing BV17, an expanded cell population in an HIV patient. However, the sorted cells had to be stimulated in culture with an anti-CD3 MAb for 10 days, and a surprisingly high effector-to-target (E:T) cell ratio was needed to demonstrate significant killing. Here, we tested the virus-specific cytotoxic activity of fresh unstimulated PBMC before and after depleting the expanded population. When the expanded T cells were removed from PBMC of patients with acute MV infection, cytotoxic activity against target cells infected with MV-expressing vaccinia virus was completely lost. The antibody only removed the T cells carrying that receptor, and the BV13S1 antibody only impaired the lytic response when there was a BV13S1 expansion, excluding nonspecific effects. This study therefore provides direct evidence that the expanded CD8+ T cells are specific for MV antigens and function as cytotoxic effector cells.
The magnitude of the clonal expansions which develop in response to acute virus infection is very variable. Massive clonal expansions could lead to exhaustion and anergy (22, 26). Gallimore et al. (11) showed reduced function in some expanded CD8+ T-cell populations in mice after acute infection with an aggressive variant of lymphocytic choriomenigitis virus. In patients M152 and M342, expansions comprised 8 to 11% CD8+ T lymphocytes. Cytotoxicity was abolished after depleting these particular populations. In contrast, in patient M111, no significant cytotoxicity was found in the expanded T cells. This patient had four expansions, the largest against BV17, consisting of 23% of the CD8+ T cells. PBMC from this patient killed target cells expressing H but not those expressing F and NP. The killing activity was only slightly decreased after depleting cells expressing BV17 and BV21S3 which together comprised 30% of CD8+ T cells. Thus, the specificity for H probably resided in the smaller expansions. The depleted T-cell clones might react to other MV proteins which were not tested, although Jaye et al. (18) have shown that F, H, and NP are normally the major targets for CTL. Alternatively, the large, expanded BV17 and BV21S3 T-cell population in this patient might comprise cells at a late stage of differentiation with poor cytotoxic function (8). Thus, although the expanded CD8+ T cells can be shown in some cases to have very specific cytotoxic function, it is possible that further expansion could lead to a decrease in function. This issue needs further examination. A third possibility is that these cells are bystander expansions in response to cytokines; although bystander effects have been described previously (34), such expansions are not oligoclonal and their magnitude has been questioned (4, 24).
In conclusion, we have demonstrated large expansions of virus-specific CD8+ T-cell clones that have cytolytic activity in acute MV infection. Similar expansions were found after vaccination with live, attenuated MV. In many cases, the expansions persisted for several months. Such expansions comprise a few clones and are remarkable for their magnitude. It is likely that they contribute to the control and clearance of MV infection.
ACKNOWLEDGMENTS
We thank Siriraj Hospital Faculty of Medicine, Mahidol University Thailand, a Training Research Fellowship from Rockefeller Foundation, and the Medical Research Council for support of personnel and expenses.
We thank P. Marrack (Howard Huges Medical Institute Research Laboratories) for BV13S1 and BV13S2 antibodies, F. Wild (Institute Pasteur de Lyon) for the vaccinia virus recombinants, and L. Tan for unpublished data. We also acknowledge T. Corrah, A. Sadiq, M. Ngong, and C. Vimteh for excellent field work, and finally, we are very grateful to all the subjects and controls.
REFERENCES
- 1.Axton J H. Measles and the state of nutrition. S Afr Med J. 1979;55:125–126. [PubMed] [Google Scholar]
- 2.Beckford A P, Kaschula R O, Stephen C. Factors associated with fatal cases of measles. A retrospective autopsy study. S Afr Med J. 1985;68:858–863. [PubMed] [Google Scholar]
- 3.Burnet F M. Measles as an index of immunological function. Lancet. 1968;ii:610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- 4.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Callan, M., and L. Tan. Unpublished data.
- 5.Callan M F, Steven N, Krausa P, Wilson J D, Moss P A, Gillespie G M, Bell J I, Rickinson A B, McMichael A J. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 6.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O’Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualization of antigen-specific CD8(+) T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z W, Kou Z C, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin N L. T cell receptor vβ repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian-human immunodeficiency virus. J Exp Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d’Angeac A D, Monier S, Pilling D, Travaglio Encinoza A, Reme T, Salmon M. CD57+ T lymphocytes are derived from CD57− precursors by differentiation occurring in late immune responses. Eur J Immunol. 1994;24:1503–1511. doi: 10.1002/eji.1830240707. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y H, Smith K J, Garboczi D N, Utz U, Biddison W E, Wiley D C. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar P R, Ogg G S, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 11.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garboczi D N, Ghosh P, Utz U, Fan Q R, Biddison W E, Wiley D C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 13.Garcia K C, Degano M, Stanfield R L, Brunmark A, Jackson M R, Peterson P A, Teyton L, Wilson I A. An alphabeta T cell receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 14.Gellin B G, Katz S L. Putting a stop to a serial killer: measles. J Infect Dis. 1994;170:S1–S2. doi: 10.1093/infdis/170.supplement_1.s1. [DOI] [PubMed] [Google Scholar]
- 15.Gellin B G, Katz S L. Measles: state of the art and future directions. J Infect Dis. 1994;170:S3–S14. doi: 10.1093/infdis/170.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 16.Greenstein J I, McFarland H F. Response of human lymphocytes to measles virus after natural infection. Infect Immun. 1983;40:198–204. doi: 10.1128/iai.40.1.198-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin D E, Ward B J, Esolen L M. Pathogenesis of measles virus infection: an hypothesis for altered immune responses. J Infect Dis. 1994;170:S24–S31. doi: 10.1093/infdis/170.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- 18.Jaye A, Magnusen A F, Whittle H C. Human leukocyte antigen class I- and class II-restricted cytotoxic T lymphocyte responses to measles antigens in immune adults. J Infect Dis. 1998;177:1282–1289. doi: 10.1086/515271. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen J L, Reay P A, Ehrich E W, Davis M M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- 20.Miller D. The frequency of complications of measles. Br Med J. 1963;2:75–78. doi: 10.1136/bmj.2.5401.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morley D. Severe measles in Africa. London, United Kingdom: Butterworths; 1973. pp. 207–230. [Google Scholar]
- 22.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 23.Moss P A, Moots R J, Rosenberg W M, Rowland-Jones S J, Bodmer H C, McMichael A J, Bell J I. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci USA. 1991;88:8987–8990. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, Fauci A S. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. . Comments. [DOI] [PubMed] [Google Scholar]
- 26.Pantaleo G, Soudeyns H, Demarest J F, Vaccarezza M, Graziosi C, Paolucci S, Daucher M, Cohen O J, Denis F, Biddison W E, Sekaly R P, Fauci A S. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–9853. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plebanski M, Aidoo M, Whittle H C, Hill A V. Precursor frequency analysis of cytotoxic T lymphocytes to pre-erythrocytic antigens of Plasmodium falciparum in West Africa. J Immunol. 1997;158:2849–2855. [PubMed] [Google Scholar]
- 28.Posnett D N, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy.”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiros Roldan E, Sottini A, Bettinardi A, Albertini A, Imberti L, Primi D. Different TCRBV genes generate biased patterns of V-D-J diversity in human T cells. Immunogenetics. 1995;41:91–100. doi: 10.1007/BF00182318. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg W M, Moss P A, Bell J I. Variation in human T cell receptor V beta and J beta repertoire: analysis using anchor polymerase chain reaction. Eur J Immunol. 1992;22:541–549. doi: 10.1002/eji.1830220237. [DOI] [PubMed] [Google Scholar]
- 31.Samb B, Aaby P, Whittle H, Seck A M, Simondon F. Decline in measles case fatality ratio after the introduction of measles immunization in rural Senegal. Am J Epidemiol. 1997;145:51–57. doi: 10.1093/oxfordjournals.aje.a009031. [DOI] [PubMed] [Google Scholar]
- 32.Samsi T K, Ruspandji T, Susanto I, Gunawan K. Risk factors for severe measles. Southeast Asian J Trop Med Public Health. 1992;23:497–503. [PubMed] [Google Scholar]
- 33.Tamashiro V G, Perez H H, Griffin D E. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Tough F D, Borrow P, Sprent J. Induction of bystander T cell proliferation by virus and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 35.Toyonaga B, Yoshikai Y, Vadasz V, Chin B, Mak T W. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci USA. 1985;82:8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Binnendijk R S, Poelen M C, Kuijpers K C, Osterhaus A D, Uytdehaag F G. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. Clonal analyses of human CD8+ class I MHC-restricted CTL. J Immunol. 1990;144:2394–2399. [PubMed] [Google Scholar]
- 37.von Pirqute C. Das verhalten der kutanen Tuberkulin-Reaction wahrend der Masern. Deutsch Med Wochenschr. 1908;343:1297–1300. [Google Scholar]
- 38.Walser Kuntz D R, Weyand C M, Weaver A J, O’Fallon W M, Goronzy J J. Mechanisms underlying the formation of the T cell receptor repertoire in rheumatoid arthritis. Immunity. 1995;2:597–605. doi: 10.1016/1074-7613(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 39.Ward B J, Johnson R T, Vaisberg A, Jauregui E, Griffin D E. Spontaneous proliferation of peripheral mononuclear cells in natural measles virus infection: identification of dividing cells and correlation with mitogen responsiveness. Clin Immunol Immunopathol. 1990;55:315–326. doi: 10.1016/0090-1229(90)90107-2. [DOI] [PubMed] [Google Scholar]
- 40.Wilson J D K, Cranage M, Cook N, Leech S, McMichael A J, Callan M F C. Evidence for the persistence of monoclonal expansions of CD8+ T cells following primary simian immunodeficiency virua infection. Eur J Immunol. 1998;28:1172–1180. doi: 10.1002/(SICI)1521-4141(199804)28:04<1172::AID-IMMU1172>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Wilson J D K, Ogg G, Allen R L, Goulder P J, Kelleher T, Sewell A, O’Callaghan C A E, Rowland-Jones S L, McMichael A J. Oligoclonal expansions of CD8+ T cells in chronica HIV infection are antigen specific. J Exp Med. 1998;188:785–790. doi: 10.1084/jem.188.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanagi Y, Cubitt B A, Oldstone M B. Measles virus inhibits mitogen-induced T cell proliferation but does not directly perturb the T cell activation process inside the cell. Virology. 1992;187:280–289. doi: 10.1016/0042-6822(92)90316-h. [DOI] [PubMed] [Google Scholar]