Abstract
The efficient packaging of genomic RNA into virions of human immunodeficiency virus type 1 (HIV-1) is directed by cis-acting encapsidation signals, which have been mapped to particular RNA stem-loop structures near the 5′ end of the genome. Earlier studies have shown that three such stem-loops, located adjacent to the major 5′ splice donor, are required for optimal packaging; more recent reports further suggest a requirement for the TAR and poly(A) hairpins of the 5′ R region. In the present study, we have compared the phenotypes that result from mutating these latter elements in the HIV-1 provirus. Using a single-round infectivity assay, we find that mutations which disrupt base pairing in either the TAR or poly(A) stems cause profound defects in both packaging and viral replication. Decreased genomic packaging in a given mutant was always accompanied by increased packaging of spliced viral RNAs. Compensatory mutations that restored base pairing also restored encapsidation, indicating that the secondary structures of the TAR and poly(A) stems, rather than their primary sequences, are important for packaging activity. Despite having normal RNA contents, however, viruses with compensatory mutations at the base of the TAR stem were severely replication defective, owing to a defect in proviral DNA synthesis. Our findings thus confirm that the HIV-1 TAR stem-loop is required for at least three essential viral functions (transcriptional activation, RNA packaging, and reverse transcription) and reveal that its packaging and reverse transcription activities can be dissociated genetically by mutations at the base of the TAR stem.
As a human immunodeficiency virus type 1 (HIV-1) capsid assembles on the inner surface of the host cell plasma membrane, two unspliced viral transcripts are packaged into each virion core. The capacity of HIV-1 to package its own viral RNAs specifically has been shown to require sequences within the nucleocapsid (NC) portion of Gag, as well as particular cis-acting RNA secondary structures located near the 5′ end of the genome, which are collectively termed the psi site or packaging signal (for a review, see reference 5). In particular, two zinc finger elements and flanking basic amino acids within HIV-1 NC have been shown to be critical for the fidelity of packaging (2, 5, 14, 17, 35, 41). When the HIV-1 NC domain is replaced by that of a different retrovirus, the resulting chimera preferentially packages the heterologous genome (7, 42). Although less well defined, the packaging signal of HIV-1 has been shown to consist of multiple functional hairpin structures located on both sides of the major splice donor (9, 31, 32). In particular, three stem-loops, which we have termed SL1, SL3, and SL4, each act as high-affinity binding sites for NC in vitro (8) and have been shown genetically to be critical for packaging specificity in vivo (9, 31, 32).
Accumulating lines of evidence indicate, however, that the autonomous packaging signal from HIV-1 is larger and more complex than originally thought. (i) Although one group reported that addition of SL3 to a heterologous RNA was sufficient to direct its encapsidation (20), others have not obtained similar results even when using much larger segments from this same region (6). On the other hand, several groups have shown that heterologous RNAs which begin with the first 350 to 400 nucleotides of the HIV-1 genome are readily incorporated into virion particles (24, 32, 34). (ii) Proviruses containing a deletion either of SL1 alone or of both SL1 and SL3 efficiently package spliced viral transcripts, even though these transcripts lack essentially all elements of the defined psi locus due to the removal of the gag-pol intron (9, 31, 32). This suggests the existence of additional psi elements in the spliced RNAs. (iii) Others have reported that more specific Gag binding sites are located at the extreme 5′ end of the genome (16). (iv) Mutational analysis recently has shown that another element, called the poly(A) stem-loop, located in the 5′ R (repeat) region, is necessary for efficient encapsidation (12).
Recent studies have also suggested a possible role of the TAR stem-loop of HIV-1 RNA in packaging and/or reverse transcription. In addition to its well-known involvement in mediating transcriptional regulation by the Tat protein (13, 15, 23, 36, 38), the TAR locus was recently reported to be necessary for efficient initiation of reverse transcription (19). However, other workers have observed that deleting TAR leads to a defect in encapsidation (32); this raises the question of whether the defects in reverse transcription were simply a consequence of deficient RNA packaging.
To address some of these issues, we have performed a mutational analysis of several of these RNA elements to evaluate their contributions to the specificity of RNA encapsidation, viral infectivity, and the efficiency of reverse transcription. We have found that mutations which disrupt base pairing at the bottom of the TAR stem cause severe defects in genomic RNA encapsidation. However, we have also identified a series of TAR mutants in which packaging is maintained at wild-type levels but which are severely defective both in infectivity and in the ability to initiate reverse transcription. This phenotype differs from that of the corresponding mutations in the poly(A) hairpin, whose defects in reverse transcription were attributable to defects in encapsidation. Our results therefore support the notion that the TAR element exerts effects both on RNA packaging and on the initiation of HIV-1 reverse transcription. These data may suggest novel strategies for interfering with the initiation of reverse transcription, a critical step of the viral life cycle.
MATERIALS AND METHODS
Cell culture.
Human osteosarcoma (HOS), 293T, and COS-7 cells were cultured in Dulbecco’s modified Eagle medium containing glucose (4.5 g/liter), penicillin G (100 U/ml), streptomycin sulfate (0.1 mg/ml), and 10% fetal calf serum at 37°C in 5% CO2.
Plasmid construction.
All mutations were introduced into the previously described HIV-gpt vector (27, 33) (gift of N. Landau and D. Littman). The amphotropic murine leukemia virus (A-MLV) Env expression vector has also been previously described (27, 33). Mutations in SL4, as well as the Δ214-243 deletion mutant, were created by oligonucleotide-directed mutagenesis (26) of the unique KasI-ClaI fragment of HIV-1 subcloned into pBluescript II KS+ (pBS/KS+; Stratagene) as described earlier (9). These KasI-ClaI fragments were then subcloned into the HIV-gpt vector cut with the same restriction enzymes. The 5′ TAR and poly(A) hairpin mutations were also created by oligonucleotide-directed mutagenesis within the BspEI-KasI (309 to 637) fragment of HIV-1 subcloned into pBS/KS+, after which DNAs were sequenced in order to confirm the mutations. This fragment was then subcloned back into the HIV-gpt vector, through a multistep subcloning process. Constructs for in vitro transcription of antisense riboprobes, used in the RNase protection assays, were made by subcloning the KpnI-ClaI fragment of wild-type or mutant HIV-gpt into pBS/KS+ cut with the same enzymes, as before (9). Prior to in vitro transcription with T7 RNA polymerase, plasmids were linearized with BspEI. Radiolabeled transcripts were prepared exactly as described previously (8, 10).
Virus production and infectivity assays.
All virions used in these studies consisted of HIV-1 core particles (strain HXB2) pseudotyped with the A-MLV Env protein (9). Viral stocks were prepared from transient calcium phosphate cotransfection of 293T cells exactly as before (9). Infectivity assays, using HOS cells, were performed in duplicate with serial dilutions of the viral supernatants as previously described (9). Infectivity assays were performed with different supernatants from at least three independent transfections, with similar results.
Virus quantitation and reverse transcriptase assays.
The concentration of viral antigen (p24) in the stocks was determined by using an enzyme immunoassay as recommended by the manufacturer (Coulter-Immunotech) and as previously described (9). Reverse transcriptase assays were performed in duplicate on virions pelleted from 0.5 ml of viral stocks at 25,000 × g for 1 h at 4°C as before (9).
RNase protection assays.
Viral stocks (10.5 ml) were layered onto a 1-ml 20% sucrose cushion (in phosphate-buffered saline [PBS]) and centrifuged at 150,000 × g in an SW41 rotor (Beckman) for 1.5 h at 4°C. Viral pellets were resuspended in 0.1 ml of PBS, and an aliquot was removed to determine the p24 concentration as described above. Virion and cytoplasmic RNAs were extracted exactly as described before (9). Viral and cytoplasmic RNA preparations were treated with 1.0 U of RQ1 RNase-free DNase (Promega) and 10 U of RNase inhibitor in 0.1 ml for 30 min at 37°C, followed by treatment with phenol-chloroform and ethanol precipitation to remove any plasmid DNA contamination. Amounts of viral RNAs were quantitated by using an RNase protection assay as recommended by the manufacturer (RPA II kit; Ambion). For virion-derived RNAs, the amount of RNA equivalent to 100 ng of pelleted p24 was annealed to an excess of 32P-labeled riboprobe (105 cpm, ≈200 pg). For cytoplasmic RNAs, approximately 1/20 of the RNA isolated from one T75 flask of 293T cells was used. The protected fragments were electrophoresed on denaturing 5% polyacrylamide–8 M urea sequencing gels and subjected to autoradiography. Radioactivity in the various bands was quantitated with a Molecular Dynamics PhosphorImager.
Semiquantitative PCR analysis.
Viral supernatants containing 500 ng of p24 were brought to a final volume of 4 ml with fresh medium. After addition of MgCl2 (5 mM, final concentration) and 100 U of RNase-free DNase, supernatants were incubated at 24°C for 30 min. After addition of 8 μg of Polybrene per ml, the DNase-treated supernatants were split into two samples. The reverse transcriptase inhibitor AZT (zidovudine) was added to one-half of the supernatants to a final concentration of 10 μM. COS-7 cell monolayers grown to about 50% confluence in 10-cm2 dishes were infected with 2 ml of DNase-treated viral supernatants. Those plates of cells infected with virus in the presence of 10 μM AZT had been pretreated with the same drug concentration for 3 h prior to infection. After a 90-min infection at 37°C, cell monolayers were extensively washed with PBS and fresh medium. An additional 10 ml of medium was added (with or without 10 μM AZT), and cells were cultured for about 20 h. After extensive washing with PBS, cells were briefly trypsinized and then pelleted. Total cell lysates were prepared by a previously published procedure (11). Briefly, cells were disrupted by the addition of lysis buffer (100 mM KCl, 20 mM Tris-HCl [pH 8.4], 0.2% Nonidet P-40, 500 μg of proteinase K per ml) and then incubated at 60°C for 2 h followed by 15 min at 95°C. Serial dilutions of the lysates were then assayed for the presence of the cellular CC chemokine receptor 5 (CCR5) gene, to ensure that approximately equal amounts of nucleic acids were present in all samples. A previously described “hot” PCR-based procedure was used (19, 40). Lysates were diluted in 10-fold increments, and 5 μl of each was used in the PCRs. The reaction contents were essentially as previously described (19) except that 50 ng of the unlabeled oligonucleotide (5′-ATGGATTATCAAGTGTCAAGT-3′ [sense]) and 25 ng of the 32P-labeled oligonucleotide (5′-GCAGGAGGCGGGCTGCAATTT-3′ [antisense]), which hybridized to the CCR5 gene, were added to each reaction. Thirty amplification cycles consisting of 93°C for 1 min and 65°C for 2 min were used, and reaction products were separated on 5% polyacrylamide gels. The CCR5 PCR product was 100 bp in length. Gels were visualized by autoradiography and quantitated with a Molecular Dynamics PhosphorImager. Identical reaction conditions were used for hot PCR of viral DNAs. The 104-fold dilutions of the cellular lysates were used in the PCRs because it was found that the viral DNA products fell within the linear range of the standard curves. Three oligonucleotide pairs, which hybridized to HIV-1 (HXB2), were used to amplify strong-stop (5′-ATCTGAGCCTGGGAGCTCTCT-3′ [sense] and 5′-ACTGCTAGAGATTTTCCACACTGA-3′ [antisense]), minus-strand jump (5′-CTTTCCGCTGGGGACTTTCCA-3′ [sense] and 5′-GAGAGCTCCCAGGCTCAGATCTGG-3′ [antisense]), and full-length (5′-TGTGCCCGTCTGTTGTGTGACTCT-3′ [sense] and 5′-TCCTGCGTCGAGAGAGCTCCTCTGG-3′ [antisense]) DNAs. Reaction products were visualized and quantitated as described above. The sizes of the PCR products were 162 bp for strong-stop, 141 bp for minus-strand jump, and 138 bp for full-length DNAs.
RESULTS
We introduced mutations into the parental vector HIV-gpt, which consists of a full-length provirus of HIV-1 (HXB2) into which a selectable marker gene (gpt) has been inserted in the place of env sequences (33). In the present study, all virions consisted of an HIV-1 core particle which was pseudotyped with the A-MLV Env protein. Specifically, we created a series of mutations in four regions at the 5′ end of the HIV-1 genome which previously have been implicated in the encapsidation process. Using oligonucleotide-directed mutagenesis, we mutated the SL4 stem-loop, created a deletion directly upstream of SL1, and introduced a series of disruption and compensatory mutations into the stems of the poly(A) and TAR hairpins (Fig. 1, 2, and 5). An RNase protection assay was used to quantify the efficiency with which various mutant RNAs were encapsidated into the pseudotyped virions. In addition, the particles are capable of undergoing one round of viral replication in which they transduce the marker gene to host cells; by counting the number of colonies which formed under selection, we obtained a quantitative measure of the infectivity of each mutant.
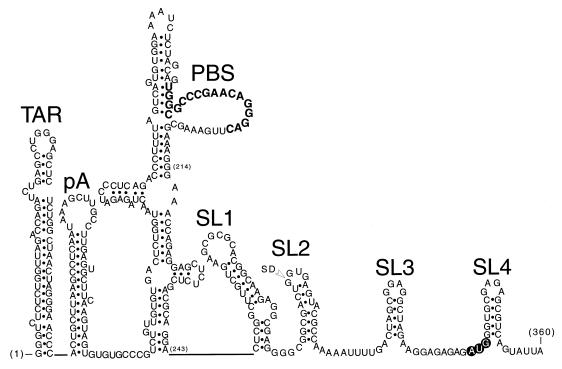
FIG. 1.
Diagrams of RNA secondary structures located at the 5′ end of the HIV-1 genome (nucleotides 1 to 360). Genetic evidence for the existence of the individual stem-loops has been published; however, the primer binding site (PBS) stem-loop is shown in a somewhat arbitrary fold, as in reference 4. The poly(A) hairpin is labeled pA, the Gag initiation codon is shown in open lettering, and the location of the major 5′ splice donor (SD) is indicated. The 18-nucleotide primer binding site is shown in boldface.
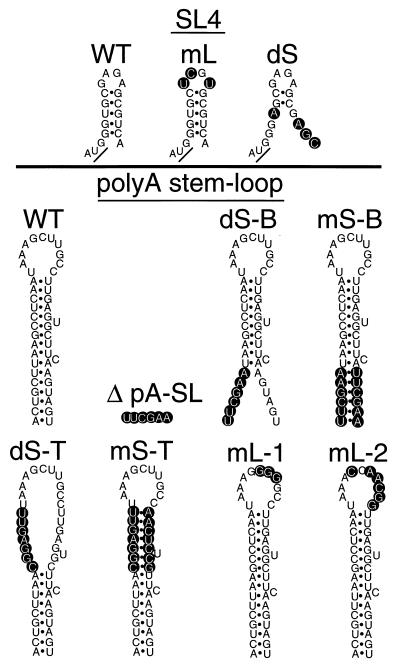
FIG. 2.
Diagram of the SL4 and poly(A) wild-type (WT) and mutant constructs used in this study. The nucleotide changes are shown in open lettering; the Gag start codon is underlined.
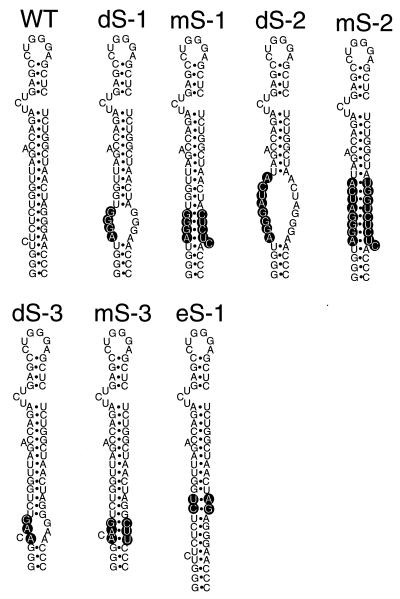
FIG. 5.
Diagrams of TAR wild-type (WT) and mutant constructs used in this study. Nucleotide changes are shown in open lettering.
Mutagenesis of the SL4 and poly(A) stem-loops.
Proviral constructs were transfected into 293T cells; 48 h later, supernatants were collected and assayed for the viral core antigen p24. As shown in Table 1, a 30-nucleotide deletion mutant (Δ214-243), both SL4 mutants, and the poly(A) stem-loop mutants (Fig. 2) each produced 130 to 400 ng of p24 per ml of supernatant. Approximately half of this p24 was judged associated with sucrose-pelletable virion particles (Table 1). These mutants also produced approximately equivalent amounts of reverse transcriptase activity per unit of p24, comparable to parental virions (Table 1).
TABLE 1.
Summary of mutant phenotypes
| Construct | Mean p24 concn (ng/ml)a ± SE | % Particle-associated p24b (mean ± SE) | RT/p24 ratioc | Relative genomic RNA contentd (mean ± SE)
|
Ratio of genomic/spliced RNAse (mean ± SE) in:
|
Infectivity (CFU/ng of p24 on HOS cells) | ||
|---|---|---|---|---|---|---|---|---|
| Virions | Cytoplasm | Virions | Cytoplasm | |||||
| HIV-gpt | 407 ± 86 | 53 ± 6 | 100 | 100 | 100 | 14.7 ± 2.5 | 1.0 ± 0.2 | 784 ± 21 |
| Δ214-243 | 129 ± 27 | 59 ± 1 | 108 | 20 | 51 | 0.7 | 0.8 | 34 ± 4 |
| SL4-mL | 256 ± 73 | 60 ± 4 | 95 | 72 ± 7 | 107 ± 1 | 1.4 ± 0.4 | 0.8 ± 0.2 | 278 ± 51 |
| SL4-dS | 356 ± 163 | 50 ± 9 | 100 | 61 ± 15 | 103 ± 7.5 | 1.8 ± 0.1 | 0.8 ± 0.2 | 359 ± 12 |
| Δ poly(A)-SL | 271 ± 106 | 71 ± 9 | 62 | 52 ± 9 | 205 ± 138 | 2.7 ± 0.3 | 1.1 ± 0.2 | 15 ± 0.5 |
| Poly(A) dS-B | 316 ± 46 | 56 ± 6 | 65 | 41 ± 8 | 73 ± 28 | 1.3 ± 0.1 | 0.6 ± 0.1 | 25 ± 2 |
| Poly(A) mS-B | 321 ± 85 | 57 ± 14 | ND | 139 ± 27 | 79 ± 29 | 17.1 ± 0.5 | 0.8 ± 0.1 | 251 ± 55 |
| Poly(A) dS-T | 259 ± 85 | 64 ± 15 | 74 | 49 ± 9 | 100 ± 49 | 3.0 ± 0.2 | 0.9 ± 0.1 | 48 ± 5 |
| Poly(A) mS-T | 336 ± 125 | 53 ± 1 | 83 | 95 ± 13 | 183 ± 104 | 12.6 ± 1.0 | 1.2 ± 0.2 | 200 ± 11 |
| Poly(A) mL-1 | 309 ± 16 | 61 ± 8 | ND | 108 ± 28 | 141 ± 47 | 9.5 ± 1.5 | 1.8 ± 0.2 | 662 ± 61 |
| Poly(A) mL-2 | 398 ± 52 | 59 ± 6 | ND | 126 ± 46 | 313 ± 45 | 10.2 ± 0.5 | 1.3 ± 0.2 | 607 ± 41 |
| HIV-gpt | 448 ± 33 | 62 ± 7 | 100 | 100 | 100 | 16.8 ± 2.2 | 0.6 ± 0.0 | 907 ± 143 |
| TAR dS-1 | 875 ± 111 | 56 ± 11 | 145 | 11 ± 2 | 207 ± 31 | 2.0 ± 0.0 | 0.7 ± 0.1 | 3 ± 0.1 |
| TAR mS-1 | 663 ± 289 | 66 ± 20 | 161 | 74 ± 24 | 35 ± 2 | 11.3 ± 1.5 | 0.6 ± 0.0 | 9 ± 0.3 |
| TAR dS-2 | 367 ± 22 | 58 ± 5 | 115 | 13 ± 1 | 52 ± 8 | 1.8 ± 0.1 | 0.4 ± 0.1 | 3 ± 0.3 |
| TAR mS-2 | 944 ± 80 | 76 ± 5 | 82 | 137 ± 11 | 224 ± 40 | 14.3 ± 2.8 | 0.8 ± 0.0 | 13 ± 2 |
| TAR dS-3 | 197 ± 32 | 71 ± 7 | 124 | 15 ± 5 | 101 ± 23 | 1.3 ± 0.3 | 0.7 ± 0.1 | 9 ± 0.3 |
| TAR mS-3 | 195 ± 42 | 65 ± 3 | 112 | 94 ± 38 | 59 ± 16 | 13.3 ± 5.3 | 0.8 ± 0.1 | 111 ± 7 |
| TAR eS-1 | 199 ± 36 | 35 ± 16 | 95 | 162 ± 49 | 92 ± 8 | 6.5 ± 0.5 | 0.5 ± 0.0 | 591 ± 15 |
Concentration in viral stocks from at least three independent transfections.
Amount recovered from viral stocks after pelleting through a 20% sucrose cushion.
Reverse transcriptase (RT) activity in viral stocks relative to the concentration of p24, using the ratio from HIV-gpt as 100; data from one representative experiment. ND, not determined.
Percentage of genomic RNA in virions relative to the parental wild type (HIV-gpt) or in the cytoplasm of producer cells relative to cells transfected with the parental wild type.
Ratio of genomic to spliced viral RNAs extracted from pelleted virions or from the cytoplasm of transfected cells (expressed as arbitrary PhosphorImager units in genomic/arbitrary PhosphorImager units in spliced band).
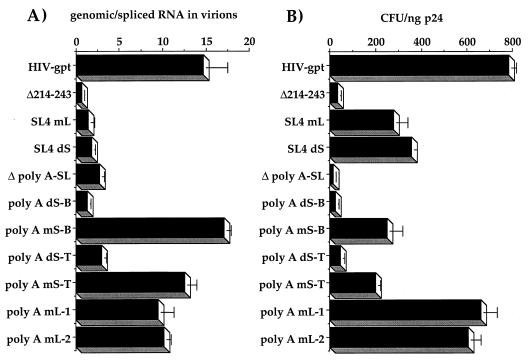
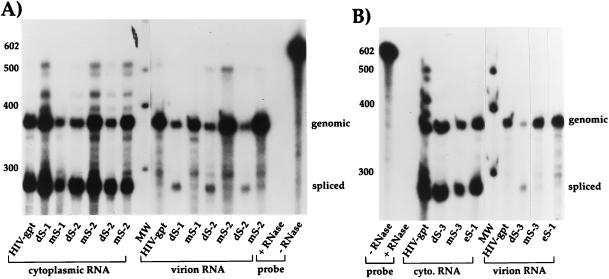
We used a previously described RNase protection assay (9) to quantitate the amounts and types of viral RNAs encapsidated by these mutants, as well as to evaluate cytoplasmic expression profiles (Fig. 3). Total RNA was extracted from sucrose-pelleted virions and from the cytoplasm of transfected 293T cells. RNAs from equivalent amounts of virion particles were then hybridized with a large excess of a labeled antisense riboprobe which spanned the major 5′ splice donor. After RNase digestion, protected fragments were analyzed by polyacrylamide gel electrophoresis. This technique allowed us to quantify not only genomic RNA but also spliced RNA, and possible contaminating proviral DNAs as well (9). For the SL4 and Δ214-243 mutants, as well as HIV-gpt, the RNase-treated riboprobe generated three protected fragments; the largest corresponded to genomic RNA, the second largest corresponded to spliced RNA, and the smallest corresponded to the 3′ long terminal repeat sequences. Little or no proviral DNA contamination was observed in any of our samples. Moreover, the overall amounts and ratios of genomic to spliced RNAs in the cytoplasm of transfected producer cells were similar in both the mutants and wild type (Table 1; Fig. 3A). However, the SL4-mL, SL4-dS, and Δ214-243 mutant virions all contained lower amounts of genomic RNA as well as higher levels of spliced RNAs than the parental virus (Table 1; Fig. 3A; Fig. 4A). These results indicate severe packaging defects for all of these mutants, with the phenotypes being very similar to those previously described for mutants in the SL1 and SL3 hairpins, each of which contains at least one Gag binding site (9). Because SL4 and an uncharacterized element immediately upstream of SL1 (partially deleted in Δ214-243) have also been found to contain at least one in vitro Gag interaction site (8), these findings are consistent with the idea that Gag binding to these sites is a major determinant of encapsidation.
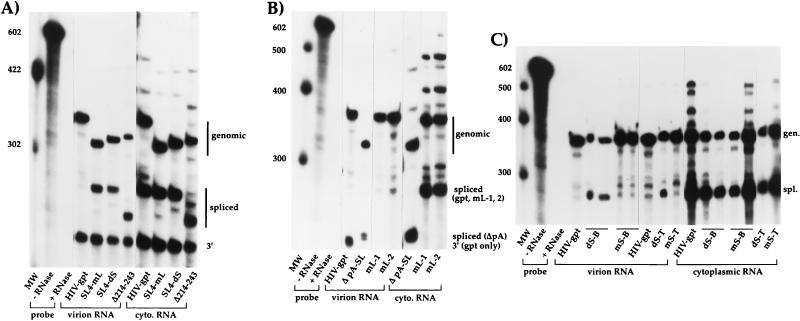
FIG. 3.
Representative RNase protection assays of Δ214-243 (A), SL4 (A), and poly(A) (B and C) mutants. Cytoplasmic (cyto.) or virion-derived RNAs were annealed to an excess of radiolabeled riboprobe and then exposed to single-strand-specific RNases; protected fragments were then separated on denaturing polyacrylamide gels. All RNAs containing mutations upstream of the major 5′ splice donor [including Δ214-243 and all poly(A) mutants] were annealed to mutant-specific riboprobes, whereas all SL4 mutants were annealed to the wild-type riboprobe. For all constructs, the top band corresponds to genomic (gen.), the second major band corresponds to spliced (spl.), and the bottom band, seen in panels A (lanes 4 to 11) and B (lane 4) only, corresponds to 3′ viral RNA sequences, as indicated at the right. The band corresponding to spliced RNA for the Δ pA-SL mutant (lanes 5 and 8) migrates at approximately the same position as the band representing 3′ viral RNA sequences in wild-type HIV-gpt (lane 4). All riboprobes were also mixed with 2 μg of Escherichia coli tRNA and subjected to the assay with or without RNase treatment. Represented in each panel is an aliquot (1/20) of the wild-type probe minus RNase. MW, molecular weight markers (indicated in nucleotides at the left).
FIG. 4.
Quantitation of RNA packaging specificity versus viral infectivities. (A) Ratio of genomic RNA to spliced RNA packaged in each mutant compared to that of the parental virus (HIV-gpt) after quantitation of the genomic and spliced bands derived from the RNase protection assays by PhosphorImager analysis. (B) Infectivities of the constructs expressed as the gpt+ CFU per nanogram of the viral antigen p24 on HOS cells. Assays of viral stocks from at least three independent transfection and infection assays yielded similar results.
We next tested a series of mutations located in the 5′ R region, in a structure termed the poly(A) stem-loop. This element has also recently been shown to be involved with encapsidation (12). While this hairpin is found in both the 5′ and 3′ ends of the genomic RNA, only the 5′ element was mutated in our proviral clones. Because the 3′ element is wild type, this creates a mismatch with our mutant riboprobes, allowing for its digestion, and thus only two fragments, corresponding to genomic and spliced RNAs, were protected by these mutants (Fig. 3B and C). Deletion of the entire hairpin [Δ poly(A)-SL] resulted in a virus which packaged about 50% of parental levels of genomic RNA with the same dramatically increased levels of spliced RNAs as found for the above-described packaging mutants (Table 1; Fig. 3B and 4A). Mutants with disruptions in base pairing either at the top [poly(A) dS-T] or at the bottom [poly(A) dS-B] of the stem had a packaging defect similar to that of the deletion (Table 1; Fig. 3C). Compensatory mutations which restored stem formation, albeit with different stem sequences [poly(A) mS-T and mS-B]), restored genomic RNA packaging while excluding the majority of spliced RNAs to a similar extent as the wild type (Table 1; Fig. 3C and 4A). Two loop mutants [poly(A) mL-1 and mL-2] appeared to have no effect on genomic packaging (Table 1; Fig. 3B and 4A). None of these mutations appeared to significantly alter the ratios of genomic and spliced RNAs in the cytoplasm of transfected producer cells (Table 1; Fig. 3B and C). There was some variability in the amounts of cytoplasmic genomic RNAs between the various mutants, which presumably resulted from variations in transfection efficiencies, but it was not correlated with packaging efficiencies (Table 1; Fig. 3B and C). These results indicate that the structure of the poly(A) stem-loop contributes to packaging, while its specific sequence does not. We previously made similar conclusions about the SL3 hairpin, which is located downstream of the major splice donor (9).
The infectivity of these mutants was assayed by their ability to stably transduce the marker (gpt) gene into cultured HOS cells (Table 1; Fig. 4B). All encapsidation-defective mutants were less infective than the wild-type virus (Fig. 4). However, the poly(A) hairpin mutants were even less infective than the SL4 mutants. For example, even the compensatory poly(A) stem mutants (mS-B and mS-T), which had normal encapsidation, had reduced infectivities compared to the parental virus (Fig. 4). Similarly sized mutations in the poly(A) loop (compare poly(A) mL-2 to mS-B), by contrast, had infectivities approaching the wild-type level (Table 1; Fig. 4). Therefore, there is a sequence-specific component in the poly(A) stem which is needed for maximal infectivity.
Mutagenesis of the TAR stem-loop.
Viral supernatants from cells transfected with the seven TAR constructs (Fig. 5) produced 200 to 950 ng of p24 per ml (Table 1). None of the TAR mutants produced significantly less p24 than the wild-type virus, showing that TAR-mediated transactivation was unaffected at the level of viral core protein expression. As before, about half of this p24 was judged associated with sucrose-pelletable virion particles (Table 1). The reverse transcriptase activity associated with these particles was about the same as for the wild type when normalized to the p24 level (Table 1).
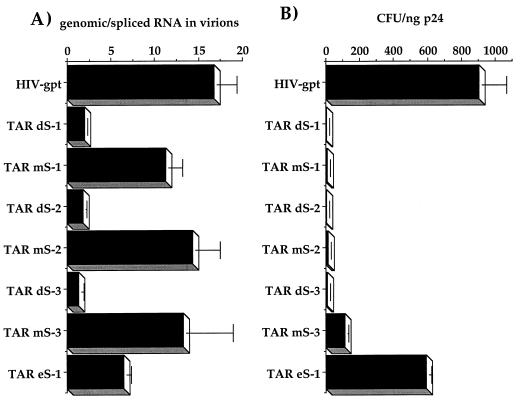
Using the RNase protection assay, we quantitated the amounts and types of RNAs associated with the mutant virions (Table 1; Fig. 6). Disruptions of the TAR stem (TAR dS-1, dS-2, and dS-3) caused severe reductions in the overall amount of genomic RNA packaged, to between 10 and 15% of wild-type levels (Table 1). As seen with all of our other packaging mutants, the reduced genomic content was associated with a corresponding increase in the amount of spliced RNAs in these virions (Table 1; Fig. 6). As with the poly(A) mutants, the overall ratios of genomic and spliced RNAs in the cytoplasm of transfected cells did not appear to be significantly affected by any of our TAR mutations (Table 1; Fig. 6). The overall amounts of cytoplasmic genomic RNAs tended to vary much more than the ratios of genomic to spliced RNAs, probably as a result of differences in transfection efficiencies (Table 1). However, these differences were not reproducibly correlated with packaging efficiencies. Again, this shows that viral gene expression, mediated through TAR, was not significantly affected by these mutations. Compensatory mutants (TAR mS-1, mS-2, and mS-3) restored genomic RNA packaging to approximately wild-type levels (Table 1; Fig. 6). These mutants also properly excluded spliced RNAs just as effectively as wild-type particles, as shown by the ratio of genomic to spliced RNAs in virions (Table 1; Fig. 6 and 7A). A mutant with a 2-bp stem extension (TAR eS-1) did not appear to be affected in its ability to properly encapsidate genomic RNA (Table 1; Fig. 6B).
FIG. 6.
Representative RNase protection assays of TAR mutants. Cytoplasmic or virion-derived RNAs were annealed to an excess of radiolabeled riboprobe and then exposed to single-strand-specific RNases; protected fragments were then separated on denaturing polyacrylamide gels. All RNAs containing TAR mutations were annealed to mutant-specific riboprobes. For all constructs, the top band corresponds to genomic and the second major band corresponds to spliced viral RNA sequences, as indicated at the right. All riboprobes were also mixed with 2 μg of E. coli tRNA and subjected to the assay with or without RNase treatment. Shown in each panel is an aliquot (1/20) of the wild-type probe minus RNase. MW, molecular weight markers (indicated in nucleotides at the left).
FIG. 7.
Quantitation of RNA packaging specificity versus viral infectivities. (A) Ratio of genomic RNA to spliced RNA packaged in each mutant compared to that of the parental virus (HIV-gpt) after quantitation of the genomic and spliced bands derived from the RNase protection assays by PhosphorImager analysis. (B) Infectivities of the constructs expressed as the gpt+ CFU per nanogram of the viral antigen p24 on HOS cells. Assays of viral stocks from at least three independent transfection and infection assays yielded similar results.
The infectivities of the majority of the TAR mutants were severely reduced (Table 1; Fig. 7B). Only the extended stem mutant, TAR eS-1, had an infectivity which approached the wild-type level (Table 1; Fig. 7B). Even infectivities of the compensatory mutants (TAR mS-1, mS-2, and mS-3), which had essentially wild-type packaging profiles, were reduced ≈10- to 100-fold (Table 1). These results imply that there is at least one other genetically separable function of the TAR hairpin, besides the transactivation and packaging functions, which contributes to full infectivity. Most probably, this other function involves the first-strand switch, in which the minus-strand strong-stop DNA is translocated from the 5′ to the 3′ R sequences during reverse transcription.
Effects of the poly(A) and TAR hairpin mutations on reverse transcription.
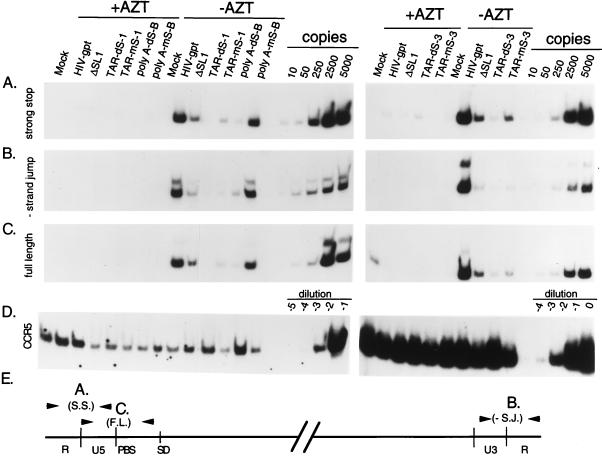
As our virions can undergo only one round of replication, we used a previously described semiquantitative PCR assay to examine the efficiency of the various steps of reverse transcription (19, 40). Three primer pairs which could distinguish between early (negative-strand strong-stop), middle (minus-strand jump), and late (full-length) reverse transcription products (Fig. 8E) were used. Equal amounts of DNase-treated virion-containing supernatants, as assayed by p24 antigen, were used to infect COS-7 cell monolayers which were either untreated or treated with 10 μM AZT. After 90 min, monolayers were extensively washed, refed with medium with or without AZT, and harvested about 20 h later. Appropriate dilutions of total cell lysates, containing approximately equivalent amounts of the nucleus-encoded cellular marker gene CCR5 (Fig. 8D), were assayed for the presence of the various reverse transcription products (Fig. 8).
FIG. 8.
Semiquantitative PCR analysis of the efficiency of reverse transcription by selected mutants. Equal amounts of DNase I-treated viral supernatants (containing equivalent amounts of p24) were used to infect cell monolayers as described in Materials and Methods. One-half of the cells were treated with 10 μM AZT. Total cell lysates, harvested 20 h postinfection, were assayed for the presence of strong-stop (A), minus (−)-strand jump (B), or full-length (C) viral DNA. The relative positions of the primer pairs used in the PCRs are shown schematically (E). PCR standards are shown for reaction mixtures that contained 10, 50, 250, 2,500, and 5,000 copies of proviral DNA in an HIV-gpt vector. To verify that approximately equal amounts of host cell-derived nucleic acids were present in the samples, PCR was performed with a primer pair that amplifies the cellular gene CCR5 (D). To amplify CCR5, cell lysates were used at dilutions of 100-fold (A) and 10-fold (right); for detection of viral DNAs, a 104 dilution was used (see Materials and Methods).
As shown in Fig. 8, this concentration of the drug AZT (10 μM) effectively suppressed viral DNA synthesis. The poly(A) stem disruption mutant (dS-B) produced substantially reduced (≈90%) amounts of the minus-strand strong-stop DNA products, as well as subsequent products of reverse transcription, compared to wild-type virions (Fig. 8). The defect appeared to be more severe than from a previously described packaging mutant containing a deletion of SL1 (ΔSL1), which produced about 70% less than the wild type (9). However, the compensatory mutant, mS-B, produced approximately wild-type levels of all products of reverse transcription (Fig. 8). These data agree with previously published results (12) and suggest that disruptions of the poly(A) stem cause reverse transcription defects primarily as a result of packaging defects. In contrast, two different disruption and compensatory mutant pairs in the TAR element showed phenotypes very different from those of these poly(A) mutants. As expected, both of the TAR stem disruptions, dS-1 and dS-3, produced very little viral DNA, about 95% reductions of all reverse transcription products, compared to the wild type (Fig. 8). However, the compensatory mutants, mS-1 and mS-3, which had wild-type packaging efficiencies, still produced about 90% less viral DNA than the wild type (Fig. 8). Both of these mutants appeared to be defective at the first step of reverse transcription; however, because of the mismatches between the mutant minus-strand strong-stop DNAs and the wild-type 3′ R sequences, a defect in the first-strand switch cannot be excluded.
These results show that the structure, but not the specific sequence, of the bottom of the TAR stem is critical for HIV-1 packaging, while a sequence(s) in this region is critical for the efficiency of HIV-1 reverse transcription.
DISCUSSION
In this study, we provide genetic evidence that the HIV-1 packaging signal includes secondary structures which extend from the extreme 5′ end of the genome to sequences located in the Gag open reading frame. These results support and extend two other recent studies which showed that both the TAR element (32), as well as the secondary structure of the poly(A) hairpin (12), are required for fully efficient HIV-1 genomic RNA packaging. Our results help explain why several groups have shown that heterologous RNAs which start with the first 350 to 400 nucleotides of HIV-1 are readily packaged into wild-type virions (24, 32, 34), whereas another study showed that a chimeric RNA containing HIV-1 RNA from nucleotides 19 to 505 was not packaged (6). The results presented here show that the secondary structure at the bottom of the TAR hairpin, from nucleotides +4 to +12, is critical for efficient packaging and leave unresolved the intriguing possibility that the 5′ cap structure may also function as part of the HIV-1 encapsidation signal. It has also been clearly shown that the first 1,018 nucleotides of HIV-1 can function as an autonomous packaging signal when either the HIV-1 Rev-responsive element or a viral constitutive-transport element is included in a heterologous transcript (32). Together with the work presented here, this finding suggests that the 5′ viral end forms a unified RNA element which is recognized as the packaging signal.
Although we did not identify any in vitro Gag binding sites in an RNA containing the TAR and poly(A) hairpins (8), another group reported that a matrix-deleted form of the Gag polyprotein did bind specifically to RNAs derived from the extreme 5′ end of the HIV-1 genome in a rabbit reticulocyte system (16). This difference may be explained by the fact that our in vitro-transcribed RNAs did not start with HIV-1 nucleotide 1 but instead contained extensive plasmid-derived sequences at their 5′ ends. It may be that in order to be specifically recognized and bound by Gag, the TAR and/or poly(A) hairpins must be presented very close to the 5′ end of an RNA molecule. This may explain why only the 5′ end of the HIV-1 genome is necessary for efficient RNA packaging even though the TAR and poly(A) hairpins are located at the 3′ ends of all viral transcripts as well. These 3′ elements may not be recognized as Gag binding sites because they have sequences 5′ to this TAR hairpin, as did our RNAs used in the in vitro Gag binding assays (8). Alternatively, it may be that the TAR and poly(A) hairpins do not function as Gag or NC binding sites at all, but instead serve to maintain the overall tertiary structure needed for specific NC binding which occurs further downstream at the SL1, SL3, and SL4 loci (8).
Our results agree with previous evidence which showed that the lower part of the TAR stem is critical for optimal HIV-1 replication (25). Our results also support and extend the recent observation that the TAR hairpin plays a critical role in mediating efficient proviral DNA synthesis (19). It has now been shown that there are at least three functions in TAR: mediating transcription through the Tat protein, allowing efficient encapsidation, and mediating efficient reverse transcription. HIV-1 proviral DNA synthesis has been intensively studied and is known to initiate from the 3′ end of a packaged host cell-derived tRNA3Lys which hybridizes through 18 nucleotides to the primer binding site on viral genomic RNA. There is evidence to suggest that the uridine-rich anticodon loop of the primer tRNA3Lys also interacts with an adenosine-rich stem-loop just upstream of the primer binding site (3, 21, 22) and that this interaction controls the switch from initiation to elongation by reverse transcriptase (22). There may be other, unknown primer-template interactions that control early events which occur during reverse transcription. Such an interaction might involve the lower part of the TAR stem with an unidentified region of the tRNA3Lys. This could explain the defects seen in our TAR mS-1, mS-2, and mS-3 mutants, which had normal packaging but showed severely reduced synthesis or stability of minus-strand strong-stop DNA. It is unlikely that these mutations would cause a reduction in the overall amount of primer tRNA3Lys incorporated into these virions since it has been shown that primer packaging occurs through interactions with the reverse transcriptase moiety of the Gag-Pol polyprotein, not through hybridization with the primer binding site (29). Alternatively, the TAR stem could function as a binding site for viral or cellular factors which are necessary for efficient proviral DNA synthesis. The same group which showed that the TAR hairpin was essential for efficient reverse transcription (19) has recently shown that the Tat protein plays a critical role in promoting efficient proviral DNA synthesis as well (18). How Tat affects reverse transcription is unclear. There is also published evidence that the virally encoded Nef (1, 37), integrase (28, 30), Vif (39), and NC (21) proteins each exert effects on reverse transcription. Therefore, the regulation of proviral DNA synthesis appears to be very complex. Because our viruses contain mutations in only the 5′ R elements, a mismatch would exist between the minus-strand strong-stop DNA and the 3′ R sequences; this may reduce the efficiency of the first-strand transfer and so indirectly promote the degradation of the initial products of reverse transcription. Given the prolonged incubation times postinfection (20 h), results of the semiquantitative PCR assay used in a similar study (19) and our own might lead to the conclusion that initiation of reverse transcription was itself defective. However, Harrich et al. (19) have shown that even at only 2 h postinfection, cells infected with certain TAR mutants contain significantly less strong-stop DNA than wild-type virus-infected cells. Although the mechanism remains to be determined, our results clearly show that a specific sequence in the lower part of the TAR hairpin is critical for efficient proviral DNA synthesis, starting with the synthesis and/or accumulation of minus-strand strong-stop DNA products.
ACKNOWLEDGMENTS
We thank Z. Mosquera for technical assistance.
This work was supported by a New Investigator award (grant K96-SF-006) from the Universitywide AIDS Research Program, University of California (to J.L.C.) and by NIH grants AI-29313, AI-36636, and AI-40317. D.A.E. was supported by Medical Scientist Training grant GM07618.
REFERENCES
- 1.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldovini A, Young R A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts E J, Stetor S R, Li X, Rausch J W, Howard K J, Ehresmann B, North T W, Wohrl B M, Goody R S, Wainberg M A, Grice S F. Initiation of (−) strand DNA synthesis from tRNA(3Lys) on lentiviral RNAs: implications of specific HIV-1 RNA-tRNA(3Lys) interactions inhibiting primer utilization by retroviral reverse transcriptases. Proc Natl Acad Sci USA. 1996;93:10063–10068. doi: 10.1073/pnas.93.19.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkhout B. Structure and function of the human immunodeficiency virus leader RNA. Prog Nucleic Acid Res Mol Biol. 1996;54:1–34. doi: 10.1016/s0079-6603(08)60359-1. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz R D, Hammarskjold M L, Helga-Maria C, Rekosh D, Goff S P. 5′ regions of HIV-1 RNAs are not sufficient for encapsidation: implications for the HIV-1 packaging signal. Virology. 1995;212:718–723. doi: 10.1006/viro.1995.1530. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz R D, Ohagen A, Hoglund S, Goff S P. Retroviral nucleocapsid domains mediate the specific recognition of genomic viral RNAs by chimeric Gag polyproteins during RNA packaging in vivo. J Virol. 1995;69:6445–6456. doi: 10.1128/jvi.69.10.6445-6456.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clever J, Sassetti C, Parslow T G. RNA secondary structure and binding sites for gag gene products in the 5′ packaging signal of human immunodeficiency virus type 1. J Virol. 1995;69:2101–2109. doi: 10.1128/jvi.69.4.2101-2109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clever J L, Parslow T G. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J Virol. 1997;71:3407–3414. doi: 10.1128/jvi.71.5.3407-3414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clever J L, Wong M L, Parslow T G. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J Virol. 1996;70:5902–5908. doi: 10.1128/jvi.70.9.5902-5908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collin M, Herbein G, Montaner L, Gordon S. PCR analysis of HIV1 infection of macrophages: virus entry is CD4-dependent. Res Virol. 1993;144:13–19. doi: 10.1016/s0923-2516(06)80006-3. [DOI] [PubMed] [Google Scholar]
- 12.Das A T, Klaver B, Klasens B I, van Wamel J L, Berkhout B. A conserved hairpin motif in the R-U5 region of the human immunodeficiency virus type 1 RNA genome is essential for replication. J Virol. 1997;71:2346–2356. doi: 10.1128/jvi.71.3.2346-2356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman T, Luban J, Goff S P, Haseltine W A, Gottlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher A G, Feinberg M B, Josephs S F, Harper M E, Marselle L M, Reyes G, Gonda M A, Aldovini A, Debouk C, Gallo R C, et al. The trans-activator gene of HTLV-III is essential for virus replication. Nature. 1986;320:367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- 16.Geigenmuller U, Linial M L. Specific binding of human immunodeficiency virus type 1 (HIV-1) Gag-derived proteins to a 5′ HIV-1 genomic RNA sequence. J Virol. 1996;70:667–671. doi: 10.1128/jvi.70.1.667-671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelick R J, Chabot D J, Rein A, Henderson L E, Arthur L O. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J Virol. 1993;67:4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrich D, Ulich C, Garcia-Martinez L F, Gaynor R B. Tat is required for efficient HIV-1 reverse transcription. EMBO J. 1997;16:1224–1235. doi: 10.1093/emboj/16.6.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrich D, Ulich C, Gaynor R B. A critical role for the TAR element in promoting efficient human immunodeficiency virus type 1 reverse transcription. J Virol. 1996;70:4017–4027. doi: 10.1128/jvi.70.6.4017-4027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi T, Shioda T, Iwakura Y, Shibuta H. RNA packaging signal of human immunodeficiency virus type 1. Virology. 1992;188:590–599. doi: 10.1016/0042-6822(92)90513-o. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Khorchid A, Gabor J, Wang J, Li X, Darlix J L, Wainberg M A, Kleiman L. The role of nucleocapsid and U5 stem/A-rich loop sequences in tRNA3Lys genomic placement and initiation of reverse transcription in human immunodeficiency virus type 1. J Virol. 1998;72:3907–3915. doi: 10.1128/jvi.72.5.3907-3915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isel C, Lanchy J M, Le Grice S F, Ehresmann C, Ehresmann B, Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the posttranscriptional modifications of primer tRNA3Lys. EMBO J. 1996;15:917–924. [PMC free article] [PubMed] [Google Scholar]
- 23.Kao S Y, Calman A F, Luciw P A, Peterlin B M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 24.Kaye J F, Richardson J H, Lever A M. cis-acting sequences involved in human immunodeficiency virus type 1 RNA packaging. J Virol. 1995;69:6588–6592. doi: 10.1128/jvi.69.10.6588-6592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaver B, Berkhout B. Evolution of a disrupted TAR RNA hairpin structure in the HIV-1 virus. EMBO J. 1994;13:2650–2659. doi: 10.1002/j.1460-2075.1994.tb06555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leavitt A D, Robles G, Alesandro N, Varmus H E. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J Virol. 1996;70:721–728. doi: 10.1128/jvi.70.2.721-728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak J, Jiang M, Wainberg M A, Hammarskjold M L, Rekosh D, Kleiman L. Role of Pr160gag-pol in mediating the selective incorporation of tRNALys into human immunodeficiency virus type 1 particles. J Virol. 1994;68:2065–2072. doi: 10.1128/jvi.68.4.2065-2072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda T, Planelles V, Krogstad P, Chen I S. Genetic analysis of human immunodeficiency virus type 1 integrase and the U3 att site: unusual phenotype of mutants in the zinc finger-like domain. J Virol. 1995;69:6687–6696. doi: 10.1128/jvi.69.11.6687-6696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride M S, Schwartz M D, Panganiban A T. Efficient encapsidation of human immunodeficiency virus type 1 vectors and further characterization of cis elements required for encapsidation. J Virol. 1997;71:4544–4554. doi: 10.1128/jvi.71.6.4544-4554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page K A, Landau N R, Littman D R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parolin C, Dorfman T, Palu G, Gottlinger H, Sodroski J. Analysis in human immunodeficiency virus type 1 vectors of cis-acting sequences that affect gene transfer into human lymphocytes. J Virol. 1994;68:3888–3895. doi: 10.1128/jvi.68.6.3888-3895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon D T K, Wu J, Aldovini A. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J Virol. 1996;70:6607–6616. doi: 10.1128/jvi.70.10.6607-6616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen C A, Sodroski J G, Haseltine W A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985;41:813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selby M J, Bain E S, Luciw P A, Peterlin B M. Structure, sequence, and position of the stem-loop in TAR determine transcriptional elongation by tat through the HIV-1 long terminal repeat. Genes Dev. 1989;3:547–558. doi: 10.1101/gad.3.4.547. [DOI] [PubMed] [Google Scholar]
- 39.von Schwedler U, Song J, Aiken C, Trono D. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Barklis E. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J Virol. 1997;71:6765–6776. doi: 10.1128/jvi.71.9.6765-6776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Barklis E. Nucleocapsid protein effects on the specificity of retrovirus RNA encapsidation. J Virol. 1995;69:5716–5722. doi: 10.1128/jvi.69.9.5716-5722.1995. . (Erratum, 71:5212, 1992) [DOI] [PMC free article] [PubMed] [Google Scholar]