Abstract
Thromboembolic (TE) risk scores used for atrial fibrillation (AF) patients do not include mitral annular calcification (MAC) as a potential indicator of vascular disease. This research evaluated the correlation between MAC and TE risk scores (CHADS2 and CHA2DS2-VASc). We compared TE risk score values and clinical and echocardiographic data in patients with and without MAC. We included, prospectively, 103 patients: 40.8% with AF, 83.5% with hypertension, 30.1% with type II diabetes mellitus, 79.6% with chronic heart failure, and 7.8% with a history of stroke. We identified MAC in 50.5% of patients. The mean CHADS2 and CHA2DS2-VASc scores were 2.56 ± 1.135 and 4.57 ± 1.61, respectively. In MAC patients, both scores tended to increase significantly compared with the control (2.88 ± 1.114 versus 2.24 ± 1.06, p = 0.005, and 5.21 ± 1.51 versus 3.92 ± 1.46, p < 0.001, respectively). The left ventricular ejection fraction negatively correlated with the presence of MAC (r = −0.254, p = 0.01). The presence of MAC was a risk factor for vascular disease (OR = 2.47, χ2 = 34.32, p < 0001). Conclusions: The presence of MAC is associated with greater TE risk scores and a higher risk of vascular disease. It appears that adding MAC as a vascular disease parameter to TE risk scores may have benefits for patients by improving their predictive value.
Keywords: mitral annulus calcification, thromboembolic risk, stroke, CHA2-DS2-VASc score, atherosclerosis
1. Introduction
Before becoming symptomatic and affecting patient survival, atherothrombosis and valvular calcifications involve a silent process. The early identification of the clinical significance of these calcifications is essential for the appropriate classification of patients into TE risk groups in order to produce practical prevention methods for adverse cardiac events.
Mitral annular calcification (MAC) is the result of a chronic, degenerative, and progressive process of calcification of the fibrous mitral annulus [1]. Although MAC was first thought to be an age-associated process, current research shows that it is an active mechanism that involves inflammation, hemodynamic stress, lipid deposition, and the production of new bone [2]. In many cases, it is discovered alongside other atherosclerotic risk factors such as tobacco use, arterial hypertension (HTN), obesity, dyslipidemia, and type II diabetes mellitus (DM) [3].
Despite being an incidental finding and frequently asymptomatic, MAC has clinical relevance because of its correlation with a greater number of cardiovascular diseases, which independently affect cardiovascular and all-cause mortality. It is also related to mitral valve disease and conduction abnormalities [2,4,5]. Recent research has found a link between MAC and the risk of stroke. In patients without clinical cardiovascular disease, MAC serves as an independent predictor of stroke [6,7].
The CHA2DS2-VASc score and its predecessor, CHADS2, are used to predict TE risk in patients with AF [8]. An elevated risk of TE events is attributed to vascular involvement (history of myocardial infarction—MI, aortic atherosclerosis plaques, peripheral arterial disease—PAD), but further research is needed to determine the role of MAC. The current data attest to a correlation between MAC and TE risk to some level. To the best of our knowledge, the clinical importance of MAC in a patient group has not yet been fully characterized; however, data from the literature reveals relationships that are statistically significant and may have therapeutic implications.
The aim of this study was to evaluate the postulated usefulness of MAC as a possible marker of vascular disease for TE risk calculation, in addition to other parameters of the CHA2DS2-VASc and CHADS2 scores.
2. Materials and Methods
2.1. Patient Enrolment, Inclusion, and Exclusion Criteria
This study is a pilot, retrospective study carried out in the Internal Medicine Clinic of the “Sfântul Spiridon” Emergency Hospital, Iași, Romania.
Patients with or without calcification of the mitral annulus who were over the age of 18 with the ability to comprehend and accept an informed consent form were included in this research. The other inclusion criteria were the diagnosis of HTN, dyslipidemia, type II DM, ischemic heart disease (IHD), angina pectoralis, or chronic MI.
Patients under the age of 18 years; patients without signed informed consent; patients with chronic kidney disease with creatinine clearance below 30 mL/min/1.73 m2, hemodynamically significant valv diseases (more than mild severity), mechanical prostheses, or aortic or mitral valve reconstruction; and patients with surgical myocardial revascularization were excluded from the study.
2.2. Clinical Investigation and Data Collection
For the patients who were enrolled, we gathered clinical, biochemical, electrocardiographic (ECG), and echocardiographic data. Thromboembolic risk was determined using the CHADS2 and CHA2DS2-VASc scores. These variables were compared in patients with MAC (study group) and those without MAC (control group).
Sex, age, body surface area (BSA), and body mass index (BMI) were included in the demographic characteristics. The patients’ smoking or non-smoking status was also assessed. We also noted patient-associated comorbidities such as a history of HTN, heart failure (HF), DM, aortic atherosclerosis plaques, dyslipidemia, peripheral arterial disease (PAD), IHD, myocardial infarction (MI), transient ischemic attack (TIA), and stroke. Chronic treatment with statins, oral anticoagulants, or antiplatelet agents in all patients was noted.
For the patients’ biological profiles, we gathered information on their total cholesterol and triglyceride levels and conducted liver function tests, including alanine aspartate transferase (AST), alanine aminotransferase (ALT), gamma-glutamyl-transferase (GGT), and fasting blood sugar levels.
The CHADS2 score assigns 1 point each for congestive HF or a left ventricular ejection fraction (LVEF) below 40% (C), HTN (H), age over 75 years (A), and DM (D) and 2 points each for a previous stroke and TIA or systemic thromboembolism, totaling a maximum of 6 points. The CHA2DS2-VASc score differs from the first by adding other parameters—vascular disease (history of MI, PAD, or the presence of atherosclerotic plaques on the aortic cross) for which 1 point is given; female sex, 1 point; age between 64 and 75 years, 1 point; and over 75 years, 2 points, with a maximum value of 9 points [9]. In addition, recently, hypertrophic cardiomyopathy was added to the letter C in the acronym.
All patients underwent an ECG to determine the presence of sinus rhythm or AF upon admission, before transthoracic echocardiography.
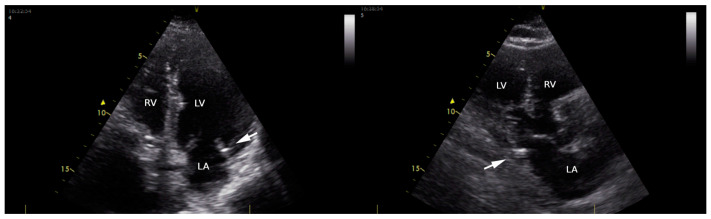
A cardiac structural and functional assessment, including the presence of MAC, was conducted via transthoracic echocardiography using a Vivid T8 Pro, GE Healthcare (according to the newest recommendations of the European Association of Cardiovascular Imaging) [10]. MAC (Figure 1) was defined as an echo-dense structure, located at the junction of the atrioventricular groove and the posterior or anterior mitral leaflet on the parasternal long-axis view; apical 4- or 2-chamber view; and parasternal short-axis view [11]. The parameters quantified during the transthoracic echocardiographic examination were as follows: left ventricular (LV) dimensions (interventricular septum—IVS, left ventricular posterior wall—LVPW, left ventricular end-diastolic diameter—LVEDD); LV systolic function (LV ejection fraction via Simpson method—LVEF), and LV diastolic function (E/A; E/e’; left atrium volume). E/A ratio was assessed in patients in sinus rhythm in apical 4- or 2-chamber view; E represents the maximum velocity of early diastolic filling of the mitral flow, and A represents the maximum velocity of the late diastolic filling of mitral flow. The diastolic index or E/e’ ratio (the ratio of early mitral in-flow to tissue velocity of the mitral annulus) was assessed in apical 4- or 2-chamber view in patients with AF (A wave being absent in these patients). We used the e’ velocity obtained via Tissue Doppler Imaging from the septal and lateral mitral annulus. For patients in AF during the echocardiographic examination, we used average velocity measurements taken during 10 consecutive cycles.
Figure 1.
Transthoracic echocardiography in apical four-chamber view (left) and long parasternal view (right), showing mitral annular calcification (arrow) in an enrolled patient. LA: left atrial; LV: left ventricle; RV: right ventricle.
2.3. Statistical Analysis
Statistical analysis was performed using the SPSS version 29.0 software package (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as frequencies and percentages and continuous variables were presented as mean ± standard deviation. Categorical and ordinal variables were compared between groups using the χ2 test, and continuous variables were compared between groups using Student’s t-test and the Mann–Whitney test (when the precondition of normal repartition of values was not verified). Pearson and Spearman correlation coefficients were used to assess the degree of association between variables (Pearson for continuous variables and Spearman for nominal or ordinal variables), and binary logistic regression was used for multivariate analysis. The association between continuous variables, if any, was analyzed through linear regression. Statistical significance was assessed at a value of p < 0.05, and the confidence interval (CI) was 95%.
3. Results
A total of 103 patients satisfied the requirements of the study, and they were divided into two groups: those with MAC present at the time of the echocardiogram examination (52 patients), and those without MAC present at the time of the echocardiography assessment (51 patients). To fulfill the outlined goals, a variety of comparisons and statistical assessments were performed between the two groups.
3.1. Study Group Description
In this retrospective study, we enrolled 103 patients, of whom 57 were women (55.3%) and 46 were males (44.7%). The mean age was 72.59 years ± 9.9 years. The general characteristics of the patients included are summarized in Table 1. The data are presented in the table both for all patients and grouped according to the presence or absence of MAC.
Table 1.
Demographic, clinical, laboratory, and echocardiographic characteristics of all patients included in the study (with and without mitral annular calcification).
| Parameter | Overall (n = 103) |
with MAC (n = 52) |
without MAC (n = 51) |
p-Value |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age (years) | 72.59 ± 9.914 | 74.46 ± 10.019 | 70.69 ± 9.528 | 0.042 * |
| BMI (kg/m2) | 28.65 ± 5.939 | 29.20 ± 6.463 | 28.10 ± 5.378 | 0.669 |
| Female gender (%) | 57 (55.3%) | 30 (57.7%) | 27 (52.9%) | 0.628 |
| Smoking (%) | 45 (43.7%) | 24 (46.2%) | 21 (41.2%) | 0.611 |
| Comorbidities | ||||
| Arterial hypertension (%) | 86 (83.5%) | 46 (88.5%) | 40 (78,4%) | 0.170 |
| Diabetes mellitus (%) | 31 (30.1%) | 22 (42.3%) | 9 (17.6%) | 0.006 * |
| Obesity (%) | 35 (34.0%) | 20 (38.5%) | 15 (29.4%) | 0.332 |
| Heart failure (%) | 82 (79.6%) | 41 (78.8%) | 41 (80.4%) | 0.846 |
| Myocardial infarction (%) | 7 (6.8%) | 5 (9.6%) | 2 (3.9%) | 0.437 |
| Prior stroke (%) | 8 (7.8%) | 6 (11.5%) | 2 (3.9%) | 0.269 |
| Atrial fibrillation (%) | 42 (40.8%) | 24 (46.2%) | 18 (35.3%) | 0.262 |
| Aortic atherosclerosis plaques (%) | 68 (66.0%) | 47 (90.4%) | 21 (41.2%) | <0.001 * |
| Vascular disease (%) | 67 (65.0%) | 48 (92.3%) | 19 (37.3%) | <0.001 * |
| Peripheral artery disease (%) | 22 (21.4%) | 12 (23.1%) | 10 (19.6%) | 0.668 |
| Biological parameters | ||||
| Total cholesterol (mg/dL) | 191.86 ± 54.270 | 189.00 ± 51.406 | 194.78 ± 57.407 | 0.591 |
| Triglycerides (mg/dL) | 117.75 ± 53.757 | 114.94 ± 59.352 | 120.61 ± 47.808 | 0.288 |
| Fasting Glycemia (mg/dL) | 120.44 ± 47.576 | 126.85 ± 61.046 | 113.78 ± 26.418 | 0.870 |
| AST (U/L) | 29.31 ± 39.397 | 32.33 ± 48.947 | 26.24 ± 26.522 | 0.484 |
| ALT (U/L) | 30.54 ± 35.991 | 33.13 ± 41.888 | 27.90 ± 28.959 | 0.702 |
| GGT (U/L) | 66.85 ± 122.845 | 94.72 ± 173.207 | 42.39 ± 32.441 | 0.633 |
| Echocardiographic parameters | ||||
| LVEF (%) | 49.50 ± 13.085 | 46.48 ± 13.126 | 52.59 ± 12.423 | 0.010 * |
| IVS (mm) | 12.14 ± 1.754 | 12.27 ± 1.868 | 12.00 ± 1.637 | 0.263 |
| LVPW (mm) | 12.16 ± 1.685 | 12.25 ± 1.823 | 12.08 ± 1.545 | 0.583 |
| LVEDD (mm) | 50.82 ± 9.045 | 50.52 ± 8.012 | 51.14 ± 10.062 | 0.653 |
| TE risk scores | ||||
| CHADS2 | 2.56 ± 1.135 | 2.88 ± 1.114 | 2.24 ± 1.069 | 0.005 * |
| CHA2-DS2-VASc | 4.57 ± 1.619 | 5.21 ± 1.513 | 3.92 ± 1.468 | <0.001 * |
| Treatment | ||||
| Statins (%) | 70 | 71 | 69 | 0.623 |
| Antiplatelets (%) | 57 | 58 | 57 | 0.779 |
| Oral anticoagulants (%) | 39 | 50 | 27 | 0.018 * |
* Statistical significance (p < 0.05); AST: alanine aspartate transferase; ALT: alanine amino transferase; BMI: body mass index; CHADS2 and CHA2DS2VASc: thromboembolic risk scores; GGT: gamma-glutamyl transpeptidase; IVS: interventricular septum; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVPW: left ventricular posterior wall; MAC: mitral annular calcification.
An echocardiographic assessment of the LV systolic function showed a mean LVEF of 49.50%, with a standard deviation of 13.085%, a minimum value of 10%, and a maximum value of 74%. Other mean values calculated based on echocardiographic data were as follows:
Interventricular septum (IVS): 12.14 ± 1.754 mm;
LV posterior wall: 12.16 ± 1.685 mm;
LV end-diastolic diameter (LVEDD): 50.82 ± 9.045 mm.
3.2. Relationship between MAC and Clinical, Biological, and Echocardiographic Parameters
Patients with MAC had a significantly greater prevalence of DM, aortic atherosclerosis plaques, and vascular disease. When compared with individuals without MAC, they were also significantly older.
According to the laboratory tests, individuals with MAC had non-significantly smaller triglyceride levels than those without MAC (114.94 ± 59.352 vs. 120.61 ± 47.808 mg/dL); in the case of hepatic function, non-significant variations were registered; patients with MAC presented non-significantly increased fasting glycemia compared with the patients without MAC (126.85 ± 61.046 vs. 113.78 ± 26.418 mg/dL).
When compared with the control group, the study group’s LV ejection percent was considerably lower (46.48 ± 13.126% vs. 52.59 ± 12.423%). In addition, sinus rhythm patients (62.83%) with MAC showed a significantly decreased LVEF as compared with those without MAC—55.73 ± 12.3% vs. 46.96 ± 14.5%, p = 0.013. The difference in LVEFs between MAC patients and the control was not significant in the AF patients (45.92 ± 11.594% vs. 46.83 ± 10.601%, p = 0.794).
3.3. Relationship between MAC and Other Comorbidities
We also emphasized the association between MAC and other diseases in this investigation. When compared with the control group, the patients from our research with MAC often had a greater prevalence of DM (p = 0.006). Among the 52 patients who had MAC, 22 of them (42.3%) also had DM, and among the 51 patients without MAC, only 9 patients (17.6%) had DM, values which indicate DM as a risk factor for MAC (OR = 3.422).
Only 19 patients (37.3%) of the 51 patients without MAC had vascular disease, compared with 48 (92.3%) of the 52 patients with MAC who also had vascular involvement. Therefore, the presence of vascular disease was a risk factor for MAC in our research (OR = 20.211). The presence of aortic atherosclerosis plaques is also significantly associated with MAC: 47 patients with MAC had this diagnosis (90.4%), compared with only 21 patients without MAC (41.2%), leading to a calculated risk of OR = 13.429.
Regarding the other investigated comorbidities, no significant associations with MAC were identified, even though among the patients with MAC we found increased incidences of HTN (88.5%), obesity (38.5%), prior stroke (11.5%), AF (46.2%), and PAD (23.1%).
3.4. Multivariate Analysis of the Relationship between MAC and Clinical, Biological, and Echocardiographic Parameters
We introduced parameters statistically significantly associated with MAC in a binary logistic regression model in order to investigate their combined action as risk factors for MAC. The model was achieved in order to evaluate the effects of age, diabetes, aortic atherosclerosis plaques, vascular disease, and LVEF on the probability of MAC in the investigated patients. The built model was statistically significant (p < 0.001) and explains 48.4% of the variation in MAC occurrence; the model’s sensibility is 88.5%, and its specificity is 64.7%. Among the five predictor variables used, two were identified as statistically significant: the presence of diabetes mellitus and the presence of vascular disease. The patients with diabetes mellitus had a 4.226 times higher risk than the others of developing MAC, and those with vascular disease had a 18.027 times higher risk than the others of developing MAC (Table 2).
Table 2.
ORs for the significant risk factors (univariate and multivariate analyses).
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| p | OR (95% CI) | p | OR (95% CI) | B Coef. | |
| Age (years) | 0.042 * | - | 0.200 | 1.037 (0.981 ÷ 1.097) | 0.036 |
| Diabetes mellitus (%) | 0.006 * | 3.422 (1.383 ÷ 8.469) | 0.017 * | 4.226 (1.293 ÷ 13.814) | 1.441 |
| Aortic atherosclerosis plaques (%) | <0.001 * | 13.429 (4.572 ÷ 39.444) | 0.955 | 1.071 (0.100 ÷ 11.430) | 0.068 |
| Vascular disease (%) | <0.001 * | 20.211 (6.290 ÷ 64.944) | 0.019 * | 18.027 (1.618 ÷ 200.800) | 2892 |
| LVEF (%) | 0.010 * | - | 0.461 | 0.986 (0.949 ÷ 1.024) | −0.014 |
| Constant | −4.420 | ||||
* Statistical significance where p < 0.05; LVEF: left ventricular ejection fraction.
3.5. Relationship between MAC and Thromboembolic Risk Scores
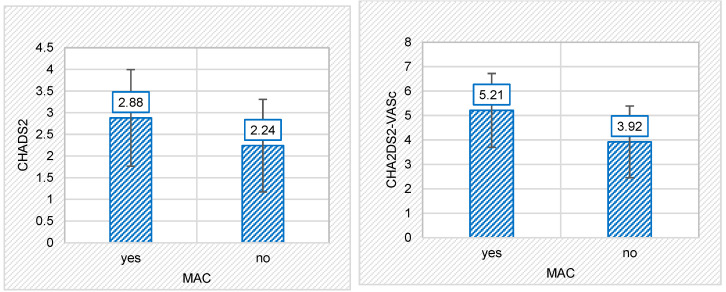
TE risk scores were calculated for the entire patient population. Overall, the mean of the CHADS2 score was 2.56 ± 1.135, and for the CHA2DS2-VASc score, it was 4.57 ± 1.619. For the patients with MAC, the mean CHADS2 score was 2.88 ± 1.114, but in those without MAC, the mean score was 2.24 ± 1.069—a statistically significant difference (p = 0.005). Patients with MAC had a mean CHA2DS2-VASc score of 5.21 ± 1.513, whereas those without MAC had a mean of 3.92 ± 1.468 (again, a statistically significant difference: p < 0.001). The values of both TE scores were, thus, considerably higher in the MAC group as compared with the control group. The relationship between MAC and the TE risk score is shown graphically in Figure 2.
Figure 2.
The relationship between the presence or absence of mitral annular calcification (MAC) and thromboembolic risk scores: MAC and CHADS2 risk score (left); MAC and CHA2DS2-VASc risk score (right).
Regarding the correlations between the TE risk scores and clinical, biological, and echocardiographic parameters, it was found that the CHADS2 score was strongly positively correlated with age (r = 0.456, p < 0.001) and blood glucose values (r = 0.294, p = 0.003). The other biological parameters under investigation did not statistically substantially correlate with TE risk scores (Table 3).
Table 3.
Correlation coefficients (Pearson linear) and the linear regression equation between the TE risk scores and the clinical, biological, and echocardiographic parameters.
| CHADS2 | CHA2DS2-VASC | |||||
|---|---|---|---|---|---|---|
| r | p | Regression Line | r | p | Regression Line | |
| Age (years) | 0.456 | 0.000 * | y = 0.052 ∗ x − 1.229 | 0.612 | 0.000 * | y = 0.100 ∗ x − 2.683 |
| BMI (kg/m2) | 0.138 | 0.230 | 0.032 | 0.782 | ||
| Total cholesterol (mg/dL) | −0.142 | 0.151 | −0.006 | 0.952 | ||
| Triglycerides (mg/dL) | 0.000 | 0.997 | 0.023 | 0.821 | ||
| Fasting Glycemia (mg/dL) | 0.294 | 0.003 * | y = 0.007 ∗ x + 1.762 | 0.257 | 0.009 * | y = 0.009 ∗ x + 3.533 |
| AST (U/L) | 0.122 | 0.221 | 0.076 | 0.443 | ||
| ALT (U/L) | 0.126 | 0.204 | 0.035 | 0.727 | ||
| GGT (U/L) | 0.057 | 0.591 | −0.051 | 0.626 | ||
| LVEF (%) | −0.161 | 0.104 | −0.139 | 0.161 | ||
| IVS (mm) | 0.146 | 0.141 | 0.090 | 0.367 | ||
| LVPW (mm) | 0.106 | 0.285 | 0.042 | 0.676 | ||
| LVEDD (mm) | 0.031 | 0.755 | −0.050 | 0.619 | ||
* Statistical significance where p < 0.05; AST: alanine aspartate transferase; ALT: alanine amino transferase; BMI: body mass index; GGT: gamma-glutamyl transpeptidase; IVS: interventricular septum; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVPW: left ventricular posterior wall.
Regarding the CHA2DS2-VASc score, it also positively and better correlated with age (r = 0.612, p < 0.001). Among the biological parameters, it had a weak positive correlation with blood glucose levels (r = 0.257, p= 0.009). No additional statistical relationships between the evaluated parameters were found (Table 3).
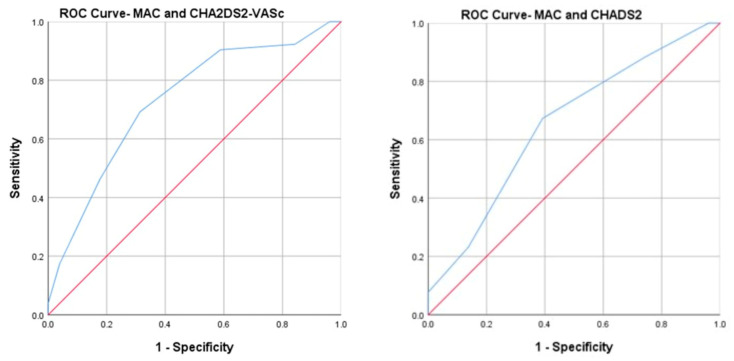
Furthermore, ROC curve analyses (Figure 3) showed that the areas under the curve (AUCs) for CHA2DS2-VASC with MAC and CHADS2 with MAC were 0.73 (95% CI, 0.63–0.82) and 0.65 (95% CI, 0.54–0.75), respectively.
Figure 3.
Area under the curve (ROC curve) analysis for mitral annular calcification (MAC) with CHA2DS2-VASC risk score (left) and with CHADS2 risk score (right).
3.6. Relationship between MAC and Thromboembolic Risk Depending on Gender
Additionally, a difference in the CHADS2 score based on gender can be seen for the group with MAC. The mean for the female gender was found to be greater than for the male gender (3 ± 1.050 vs. 2.73 ± 1.203). Also, the CHA2DS2-VASc score was higher in women than in men (5.87 ± 1.167 vs. 4.32 ± 1.492). A ROC curve analysis showed that both scores were higher in women: AUC in women: 0.795 (95%CI, 0.677–0.913) for CHA2DS2-VASC and 0.683 (95% CI, 0.543–0.824) for CHADS2 (Figure 4).
Figure 4.
Area under the curve (ROC curve) analysis for mitral annular calcification (MAC) with CHA2DS2-VASc risk score (left) and CHADS2 risk score (right) in women.
4. Discussion
Mitral valve abnormalities are relatively common in the general population, and their incidence increases in patients with cardiovascular diseases, diabetes, and metabolic syndrome. We chose MAC to determine if it influences patients’ TE risk and other clinical and biological parameters, such as the association between MAC and related diseases. There are currently limited data available in the literature.
Considering that the link between MAC and TE risk is already emphasized in the most recent literature, we believe it is worthwhile to investigate the potential of including this variable in the current risk scores in order to increase their predictive power. We draw attention to the fact that, in the current AF guidelines, in the assessment of the CHA2DS2-VASc risk score, vascular disease (the letter V) is a parameter, but it strictly refers to peripheral arterial disease, myocardial infarction, and aortic atherosclerosis. Therefore, we believe that verifying the association between MAC and the TE risk score is not redundant but, on the contrary, could improve its predictive power.
This study examined the relationship between MAC and the two TE risk scores, CHADS2 and CHA2DS2-VASc, biochemical and echocardiographic differences in the studied groups, and the prevalence of other diseases.
4.1. Clinical Data
Old age, obesity, and a history of diabetes were the factors that mainly characterized the studied population. Congestive heart failure, prior stroke, and vascular diseases were also present in our patients. Our results were consistent with the information already available in the literature. Kanjanauthai et al. in the MESA study observed an increased incidence of MAC in elderly patients [12]. This association can be explained by the fact that MAC is considered a manifestation of atherosclerosis. Furthermore, in our study, MAC was diagnosed in a higher proportion in women than in men. Similar investigations have demonstrated the relationship between MAC and the feminine gender. This suggests a unique pathophysiological process involving calcium metabolism [13]. MAC in elderly women can be explained by postmenopausal osteoporosis and ectopic calcium deposition [14]. In cases such as diabetes, chronic kidney disease, and atherosclerosis or because of advanced age, the balance between the factors that inhibit and those that promote calcification becomes unstable, and thus, calcifications can appear at any level [15]. MAC is considered a manifestation of atherosclerotic disease since the risk factors and pathophysiological mechanisms are common [5,16].
Additionally, MAC was identified in our study as a risk factor for vascular disease. The association between MAC and carotid and peripheral arterial disease has been demonstrated before. In multiple studies, the presence of MAC was associated with a higher incidence of PAD [17,18]. For instance, in a trial that aimed to establish a connection between MAC and PAD, the mean ankle/brachial systolic pressure index was considerably lower in the MAC group than in the control group [19].
Similarly, in a study that included 17,735 patients under the age of 65 years, patients with MAC had a higher prevalence of severe IHD (including left main coronary artery and triple vessel disease) than patients without MAC. Thus, MAC represents a predictive factor for IHD, and the idea of MAC being a risk factor for vascular disease is further strengthened [20].
Our research shows that the MAC patients had a higher incidence of DM than the control group. It should be noted that, like vascular disease, this pathology is a component of the CHA2DS2-VASc risk score. A recent study on 138 diabetic individuals found a higher frequency of MAC in the population under consideration [21]. In patients with DM, the occurrence of MAC, as a form of atherosclerotic disease, can be explained by the generation of oxidative stress influenced by high blood sugar. Endothelial dysfunction occurs as a result of this process, and along with hypercholesterolemia, atherosclerosis will later develop [22]. More than that, to support this theory, blood tests of our patients with MAC showed higher blood glucose levels when compared with the control group. There is evidence that oxidative stress influences stages of certain thrombotic processes, including the activation of platelets by decreasing the bioavailability of nitric oxide [23]. Therefore, in individuals with MAC, increased oxidative stress might increase morbidity and mortality rates through TE episodes.
4.2. Echocardiographic Data
In our study, echocardiographic examinations revealed 52 patients with MAC. Furthermore, sinus rhythm patients with MAC showed significantly decreased LVEF values compared with those without MAC. These findings are in agreement with those of previous studies. Rao et al. demonstrated that patients with chronic kidney disease and severe MAC are associated with a decrease in LV systolic function [24]. In addition, reduced LV systolic function has been observed in diabetic patients with MAC [25]. A speckle-tracking analysis performed on 91 patients with MAC and a control group of 48 patients demonstrated that the presence of MAC is related to a decrease in LV systolic and diastolic function. Also, these changes are correlated with the severity of calcification [26]. Various mechanisms can be used to explain this observation. First of all, it is assumed that myocardial ischemia present in the small coronary vessels is involved [27]. Another argument highlighted in the literature involves both the metabolism of vitamin D and parathormone or the metabolism of calcium and phosphorus. Imbalances at this level promote the calcification of the mitral valve and, thus, LV dysfunction [27]. Lastly, systemic inflammation is associated with the presence of MAC and systolic LV dysfunction [26,28]. This link between MAC and LV systolic dysfunction may be an explanation of the cardiovascular events associated with MAC. Early diagnosis of LV systolic dysfunction is of major importance, and the unhesitant initiation of treatment can delay or prevent the onset of heart failure. Thus, in our study, the difference in LVEF values between MAC patients and the control was not significant in AF patients.
4.3. Thromboembolic Risk Scores
Cerebrovascular events have been associated with MAC since 1946, when Rytand et al. described the case of a patient with MAC and stroke [29]. Subsequently, Benjamin et al. demonstrated that the risk of stroke is doubled by the presence of MAC, after adjusting for other risk factors [30]. Important studies, such as the Framingham Heart Study [30], the LIFE study [6], and the Strong Heart Study [7], found similar results, headlining the increased risk of stroke in populations with MAC. Patients diagnosed with MAC have an increased risk of developing arrhythmias, for example, AF, and, of course, an increased risk of TE [31,32,33]. Now, new data from the literature reveal this increased risk even among patients without AF [5].
MAC is an independent risk factor for TE events and stroke, beyond AF or other common cardiovascular risk factors. A recent study highlighted the link between MAC and the increased risk of cerebral embolism. This study was conducted for a period of 15 years and included 6814 patients (of which 644 had MAC), and of the total number of strokes reported, 79% were ischemic strokes. Calicchio et al. observed that MAC was associated with an increased risk of all strokes after adjusting for all parameters assessed in their study [34].
Several theories have tried to explain the association between TE risk and MAC. On the one hand, some authors blame cerebral embolic events on the relationship between MAC and carotid stenosis. On the other hand, autopsies performed have described calcium emboli in the cerebral arteries of MAC patients [16]. Another theory is the relationship between MAC and atrial dysfunction or atrial fibrosis, which can cause the formation of thrombi in the left atrium [32,35]. However, some authors state that, for the time being, it cannot be established with accuracy whether TE risk is caused by calcification itself or by its association with other cerebrovascular risk factors [16].
The association between MAC and TE risk scores, like CHADS2 and CHA2DS2-VASc, is not well established in the literature. In our study, the average TE risk scores (CHADS2 and CHA2DS2-VASc) were higher in patients in the MAC group than in the control group. This finding is consistent with other recent studies that discussed this association [5,21]. The difference that we noticed between the two categories allows us to say that MAC influences both TE risk scores. Regarding the correlations between TE risk scores and clinical, biological, and echocardiographic parameters, it was observed that the CHADS2 score was positively correlated with blood glucose values, but with the other parameters, it had no statistical significance. The CHA2DS2-VASc score correlated positively with age, and there was an association with blood sugar levels.
This study brings to the foreground the importance of a routine clinical and paraclinical examination of patients at risk of cardiovascular events and that MAC discovered incidentally in these patients raises a red flag regarding their increased risk of TE, even in the absence of AF or other risk factors. Simple TE risk scores that are easy to use and available at any time, such as CHADS2 and CHA2DS2-VASc, can indicate to the clinician the need for TE prevention, increasing patient survival and life expectancy. Although its predecessor, the CHADS2 score, has maintained its predictive usefulness over time, it is important to remember that the CHA2DS2-VASc score is the current guideline recommendation since the greatest amount of evidence suggests that it can predict thromboembolic risk [8].
More than that, this study brings, as a novelty, the possibility of including MAC in the calculation of the CHA2DS2-VASc TE risk score, together with the other parameters of vascular disease, in order to increase its predictive value.
Since the data in the literature are limited at the moment, the current study shows that MAC seems to have multiple associations with patient mortality. Moreover, the findings of our study provide clues regarding the potential for further investigation.
4.4. Study Limitations
Our clinical small study was a pilot study, which provides the opportunity for future research on a larger scale with more patients and more monitored parameters over a longer period. For a more detailed description of MAC regarding its extent and location, computer tomography is the preferred method. However, the availability of this imaging method is limited and exposes the patient to irradiation. Therefore, we chose to use a less expensive, more accessible technique for early MAC detection. MAC is classified in the literature according to the extent of focal calcifications. However, this classification is not commonly applied in current practice, in which the examination is performed using echocardiography. In this study, MAC was not graded by severity. The data interpretation and results can only be applied to patients referred to a hospital for a first evaluation; they cannot be extended to the MAC population in general.
5. Conclusions
MAC is a progressive, chronic, age-related process, usually asymptomatic, that evolves toward important cardiovascular complications, influencing the risk of cardiovascular events and mortality. Our study reinforces the fact that simple and widely available tools such as blood tests, risk scores, and echocardiography are important in the clinical approach to patients with a high risk of MAC and TE events. We demonstrated that the CHA2DA2-VASc and CHADS2 scores were significantly higher in patients with MAC. Both risk scores correlated very well with the presence of MAC, but the current recommended score (CHA2DS2-VASc) had a better degree of correlation and higher statistical significance. This research highlights the possibility of incorporating MAC in the CHA2DS2-VASc risk score calculation, together with the other vascular disease indicators, in terms of enhancing its predictive value.
Acknowledgments
The authors would like to thank the “Grigore T. Popa” University of Medicine and Pharmacy and the “Sf. Spiridon” Emergency Clinical Hospital for providing institutional access and assistance in developing this study.
Author Contributions
Conceptualization, M.F., P.C.M. and D.M.T.; methodology, M.F. and L.Ş.; software, A.F.O., C.G.M. and C.G.D.; validation, I.-D.M., G.L.B. and D.M.T.; formal analysis, C.G.M. and P.C.M.; investigation, C.G.M., P.C.M. and C.P.C.; resources, D.M.T. and A.F.O.; data curation, C.P.C. and O.S.; writing—original draft preparation, P.C.M., M.F., D.M.T. and C.G.D.; writing—review and editing, P.C.M., M.F., O.S. and C.G.D.; visualization, O.S., G.L.B. and D.E.I.; supervision, M.F., L.Ş., I.-D.M., A.F.O. and D.E.I.; project administration, M.F. and L.Ş. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the “St. Spiridon” Emergency Hospital, Iași (no. 28/9 March 2023), and by the Ethics Committee of the “Grigore T. Popa” University of Medicine and Pharmacy, Iași (no 303/16 May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Abramowitz Y., Jilaihawi H., Chakravarty T., Mack M.J., Makkar R.R. Mitral Annulus Calcification. J. Am. Coll. Cardiol. 2015;66:1934–1941. doi: 10.1016/j.jacc.2015.08.872. [DOI] [PubMed] [Google Scholar]
- 2.Massera D., Kizer J.R., Dweck M.R. Mechanisms of Mitral Annular Calcification. Trends Cardiovasc. Med. 2020;30:289–295. doi: 10.1016/j.tcm.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcanti L.R.P., Sá M.P.B.O., Perazzo Á.M., Escorel Neto A.C., Gomes R.A.F., Weymann A., Zhigalov K., Ruhparwar A., Lima R.C. Mitral Annular Calcification: Association with Atherosclerosis and Clinical Implications. Curr. Atheroscler. Rep. 2020;22:9. doi: 10.1007/s11883-020-0825-3. [DOI] [PubMed] [Google Scholar]
- 4.Eberhard M., Schönenberger A.L.N., Hinzpeter R., Euler A., Sokolska J., Weber L., Kuzo N., Manka R., Kasel A.M., Tanner F.C., et al. Mitral Annular Calcification in the Elderly—Quantitative Assessment. J. Cardiovasc. Comput. Tomogr. 2021;15:161–166. doi: 10.1016/j.jcct.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Aksoy F., Guler S., Kahraman F., Kuyumcu M.S., Bagcı A., Bas H.A., Uysal D., Varol E. The Relationship between Mitral Annular Calcification, Metabolic Syndrome and Thromboembolic Risk. Braz. J. Cardiovasc. Surg. 2019;34:535–541. doi: 10.21470/1678-9741-2019-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Marco M., Gerdts E., Casalnuovo G., Migliore T., Wachtell K., Boman K., Dahlöf B., Olsen M.H., Kizer J.R., Devereux R.B., et al. Mitral Annular Calcification and Incident Ischemic Stroke in Treated Hypertensive Patients: The LIFE Study. Am. J. Hypertens. 2013;26:567–573. doi: 10.1093/ajh/hps082. [DOI] [PubMed] [Google Scholar]
- 7.Kizer J.R., Wiebers D.O., Whisnant J.P., Galloway J.M., Welty T.K., Lee E.T., Best L.G., Resnick H.E., Roman M.J., Devereux R.B. Mitral Annular Calcification, Aortic Valve Sclerosis, and Incident Stroke in Adults Free of Clinical Cardiovascular Disease: The Strong Heart Study. Stroke. 2005;36:2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., Bax J.J., Boriani G., Dan G.A., Fauchier L., Kalman J.M., Lane D.A., Lettino M., et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 9.Chen J.Y., Zhang A.D., Lu H.Y., Guo J., Wang F.F., Li Z.C. CHADS2 versus CHA2DS2-VASc Score in Assessing the Stroke and Thromboembolism Risk Stratification in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Geriatr. Cardiol. 2013;10:258–266. doi: 10.3969/j.issn.1671-5411.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang R.M., Badano L.P., Victor M.A., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Kohsaka S., Jin Z., Rundek T., Boden-Albala B., Homma S., Sacco R.L., Di Tullio M.R. Impact of Mitral Annular Calcification on Cardiovascular Events in a Multiethnic Community. The Northern Manhattan Study. JACC Cardiovasc. Imaging. 2008;1:617–623. doi: 10.1016/j.jcmg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanjanauthai S., Nasir K., Katz R., Rivera J.J., Takasu J., Blumenthal R.S., Eng J., Budoff M.J. Relationships of Mitral Annular Calcification to Cardiovascular Risk Factors: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;213:558–562. doi: 10.1016/j.atherosclerosis.2010.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmariah S., Budoff M.J., Delaney J.A.C., Hamirani Y., Eng J., Fuster V., Kronmal R.A., Halperin J.L., O’Brien K.D. Risk Factors Associated with the Incidence and Progression of Mitral Annulus Calcification: The Multi-Ethnic Study of Atherosclerosis. Am. Heart J. 2013;166:904–912. doi: 10.1016/j.ahj.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugihara N., Matsuzaki M. The Influence of Severe Bone Loss on Mitral Annular Calcification in Postmenopausal Osteoporosis of Elderly Japanese Women. Jpn. Circ. J. 1993;57:14–26. doi: 10.1253/jcj.57.14. [DOI] [PubMed] [Google Scholar]
- 15.Johnson R.C., Leopold J.A., Loscalzo J. Vascular Calcification: Pathobiological Mechanisms and Clinical Implications. Circ. Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 16.Adler Y., Fink N., Spector D., Wiser I., Sagie A. Mitral Annulus Calcification—A Window to Diffuse Atherosclerosis of the Vascular System. Atherosclerosis. 2001;155:1–8. doi: 10.1016/S0021-9150(00)00737-1. [DOI] [PubMed] [Google Scholar]
- 17.Garg P.K., Buzkova P., Meyghani Z., Budoff M.J., Lima J., Criqui M., Cushman M., Allison M. Valvular Calcification and Risk of Peripheral Artery Disease: TheMulti-Ethnic Study of Atherosclerosis (MESA) Eur. Heart J. Cardiovasc. Imaging. 2020;21:1152–1159. doi: 10.1093/ehjci/jez284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barasch E., Gottdiener J.S., Marino Larsen E.K., Chaves P.H.M., Newman A.B., Manolio T.A. Clinical Significance of Calcification of the Fibrous Skeleton of the Heart and Aortosclerosis in Community Dwelling Elderly. The Cardiovascular Health Study (CHS) Am. Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 19.Adler Y., Levinger U., Koren A., Gabbay R., Shapira Y., Vaturi M., Fink N., Herz I., Zelikovski A., Sagie A. Association between Mitral Annulus Calcification and Peripheral Arterial Atherosclerotic Disease. Angiology. 2000;51:639–646. [PubMed] [Google Scholar]
- 20.Atar S., Jeon D.S., Luo H., Siegel R.J. Mitral Annular Calcification: A Marker of Severe Coronary Artery Disease in Patients under 65 Years Old. Heart. 2003;89:161–164. doi: 10.1136/heart.89.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grigorescu E.-D., Lăcătușu C.-M., Floria M., Cazac G.-D., Onofriescu A., Ceasovschih A., Crețu I., Mihai B.-M., Șorodoc L. Association of Inflammatory and Metabolic Biomarkers with Mitral Annular Calcification in Type 2 Diabetes Patients. J. Pers. Med. 2022;12:1484. doi: 10.3390/jpm12091484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asmat U., Abad K., Ismail K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuentes E., Palomo I. Role of Oxidative Stress on Platelet Hyperreactivity during Aging. Life Sci. 2016;148:17–23. doi: 10.1016/j.lfs.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Rao A.K., Djamali A., Korcarz C.E., Aeschlimann S.E., Wolff M.R., Stein J.H. Mitral Annular Calcification Is Associated with Reduced Left Ventricular Function and Inflammation in Patients with Chronic Kidney Disease. J. Am. Soc. Echocardiogr. 2008;21:747–750. doi: 10.1016/j.echo.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Cioffi G., Faganello G., De Feo S., Berlinghieri N., Tarantini L., Di Lenarda A., Pinamonti B., Candido R., Faggiano P. Combined Circumferential and Longitudinal Left Ventricular Systolic Dysfunction in Patients with Type 2 Diabetes Mellitus without Myocardial Ischemia. Exp. Clin. Cardiol. 2013;18:e26–e31. [PMC free article] [PubMed] [Google Scholar]
- 26.Gökdeniz T., Boyaci F., Hatem E., Bektaş H., Kalaycioʇlu E., Gürsoy M.O., Aykan A.Ç., Yildiz B.Ş., Altintaş B. Association of Mitral Annular Calcification with Left Ventricular Mechanics: A Speckle Tracking Study. Echocardiography. 2015;32:1374–1383. doi: 10.1111/echo.12861. [DOI] [PubMed] [Google Scholar]
- 27.Faganello G., Faggiano P., Candido R., Tarantini L., Di Lenarda A., De Feo S., Cioffi G. The Worrisome Liaison between Left Ventricular Systolic Dysfunction and Mitral Annulus Calcification in Type 2 Diabetes without Coronary Artery Disease: Data from the SHORTWAVE Study. Nutr. Metab. Cardiovasc. Dis. 2013;23:1188–1194. doi: 10.1016/j.numecd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Kurtoǧlu E., Korkmaz H., Aktürk E., Ylmaz M., Altaş Y., Uçkan A. Association of Mitral Annulus Calcification with High-Sensitivity C-Reactive Protein, Which Is a Marker of Inflammation. Mediat. Inflamm. 2012;2012:606207. doi: 10.1155/2012/606207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rytand D.A. Clinical aspects of calcification of the mitral annulus fibrosus. Arch. Intern. Med. 1946;78:544–564. doi: 10.1001/archinte.1946.00220050049003. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin E.J., Plehn J.F., D’Agostino R.B., Belanger A.J., Comai K., Fuller D.L., Wolf P.A., Levy D. Mitral Annular Calcification and the Risk of Stroke in an Elderly Cohort. N. Engl. J. Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 31.Qasim A.N., Rafeek H., Rasania S.P., Churchill T.W., Yang W., Ferrari V.A., Jha S., Master S.M., Mulvey C.K., Terembula K., et al. Cardiovascular Risk Factors and Mitral Annular Calcification in Type 2 Diabetes. Atherosclerosis. 2013;226:419–424. doi: 10.1016/j.atherosclerosis.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neal W.T., Efird J.T., Nazarian S., Alonso A., Michos E.D., Szklo M., Heckbert S.R., Soliman E.Z. Mitral Annular Calcification Progression and the Risk of Atrial Fibrillation: Results from MESA. Eur. Heart J. Cardiovasc. Imaging. 2018;19:279–284. doi: 10.1093/ehjci/jex093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T.K.M., Griffin B.P., Xu B., Rodriguez L.L., Popovic Z.B., Gillinov M.A., Pettersson G.B., Desai M.Y. Relationships between Mitral Annular Calcification and Cardiovascular Events: A Meta-Analysis. Echocardiography. 2020;37:1723–1731. doi: 10.1111/echo.14861. [DOI] [PubMed] [Google Scholar]
- 34.Calicchio F., Onuegbu A., Kinninger A., Nakanishi R., Carr J.J., Nasir K., Gottesman R., Budoff M.J. Mitral annular calcification as a predictor of stroke in the multi ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2022;79:1199. doi: 10.1016/S0735-1097(22)02190-8. [DOI] [PubMed] [Google Scholar]
- 35.Fashanu O.E., Bizanti A., Al-Abdouh A., Zhao D., Budoff M.J., Thomas I.C., Longstreth W.T., Michos E.D. Progression of Valvular Calcification and Risk of Incident Stroke: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2020;307:32–38. doi: 10.1016/j.atherosclerosis.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.