Abstract
Type 2 diabetes mellitus (T2DM) is a persistent metabolic condition that contributes to the development of cardiovascular diseases. Numerous studies have provided evidence that individuals with T2DM are at a greater risk of developing cardiovascular diseases, typically two to four times more likely than those without T2DM, mainly due to an increased risk of atherosclerosis. The rupture of an atherosclerotic plaque leading to pathological thrombosis is commonly recognized as a significant factor in advancing cardiovascular diseases caused by TD2M, with platelets inducing the impact of plaque rupture in established atherosclerosis and predisposing to the primary expansion of atherosclerosis. Studies suggest that individuals with T2DM have platelets that display higher baseline activation and reactivity than those without the condition. The expression enhancement of several platelet receptors is known to regulate platelet activation signaling, including platelet glycoprotein-Ib (GPIb). Furthermore, the high expression of platelet GP1b has been reported to increase the risk of platelet adhesion, platelet-leucocyte interaction, and thrombo-inflammatory pathology. However, the study exploring the role of GP1b in promoting platelet activation-induced cardiovascular diseases in T2DM patients is still limited. Therefore, we summarize the important findings regarding pathophysiological continuity between T2DM, platelet GPIb, and atherosclerosis and highlight the potential therapy targeting GPIb as a novel antiplatelet agent for preventing further cardiovascular incidents in TD2M patients.
Keywords: atherosclerosis, antiplatelet, glycoprotein-Ib (GP1b), platelet, type 2 diabetes mellitus (T2DM)
1. Introduction
For the past decades, type 2 diabetes mellitus (T2DM) cases have sharply increased in many countries of all income levels [1,2]. It is reported that T2DM is responsible for over 1 million deaths annually, ranking it as the ninth leading cause of mortality worldwide [1,2]. Projections indicate that the global prevalence of T2DM will continue to rise across all regions of the world, reaching 7079 individuals per 100,000 people by 2030 [1,2]. T2DM is a long-lasting medical condition characterized by insufficient insulin production and function, leading to chronically elevated glucose levels, one of its primary manifestations [3,4,5]. Individuals diagnosed with T2DM are at a high risk of developing cardiovascular diseases due to atherosclerosis, including stroke, acute coronary artery disease (CAD), and peripheral arterial disease (PAD) [3,4,5,6]. It is worth noting that T2DM not only promotes atherosclerosis but also influences its specific pattern [6,7,8]. For instance, metabolic dysregulation in T2DM increases the likelihood of arteries below the knee being affected by lower extremity PAD [6,7,8]. Additionally, T2DM contributes to excessive vascular stiffness, impacting disease progression [6,7,8]. Moreover, T2DM raises the risk of restenosis after interventions such as angioplasty with stent implantation, which are crucial for managing atherosclerosis through vascular therapy [6,7,8].
Atherosclerosis is a long-term inflammatory condition characterized by a disturbed interplay between inflammation and the hemostatic system, primarily involving the platelets, and can lead to serious cardiovascular events [9,10]. In the advanced stages of atherosclerosis, the plaque inside the arteries can become more vulnerable due to the generation of new blood vessels, causing the center of the plaque to become hypoxic [9,10,11]. Moreover, the involvement of inflammation in plaque vulnerability is significant [9,10,11]. Platelets are involved in multiple ways in the progression of atherosclerosis, including the production of the protein junctional adhesion molecule-A (JAM-A), activation of macrophages, and alteration of other immune cells’ functions [10,11]. Additionally, platelets can contribute to foam cell generation, which is the generation of immune cells that take up lipids and release inflammatory mediators and cytokines that promote atherosclerosis in a positive feedback loop [10,11].
Dysregulation of glucose metabolism in T2DM patients promotes an inflammatory environment, resulting in atherosclerosis development [3,4,5,9,10]. In individuals with T2DM, the metabolic condition increases oxidative stress, causing endothelial dysfunction and exacerbating the processes of inflammation, platelet activation, and thrombosis, resulting in atherosclerosis aggravation [3,4,5,9,10,12]. Moreover, inflammation in T2DM will lead to a prothrombotic state, stimulating the actions of platelets, endothelial–leukocyte adhesion molecules, and thromboxane to increase thrombosis [12,13]. Inflammation also generates clot persistence and formation, induces pro-coagulant agent production, and activates platelets [10,11,12,13]. The significance of platelets in vascular obstruction or occlusion is strongly linked with their adherence capacity to injured endothelial cells [10,13,14]. After an injury to the blood vessels, platelets rapidly adhere to the subendothelial cells through adhesion receptors to initiate the healing process [10,13,14]. Activated platelets then recruit more platelets and the expanding plug, resulting in increased thrombin generation [10,13,14]. Cumulatively, platelets play a crucial role in the development of atherosclerosis plaque and the formation of blood clots within the arteries, which can lead to cardiovascular events [9,10]. Considering that diabetic patients are at a higher risk of developing cardiovascular events, it is essential to block one or multiple pathways that regulate platelet activation and aggregation processes [4,5]. This blockade is crucial for reducing the risk of cardiovascular events, including ischemic events such as myocardial infarction, in diabetic patients [15,16,17]. Currently, there are multiple antiplatelet agents that are utilized to prevent and mitigate the risk of ischemic events in diabetic patients [15,16,17]. These include cyclooxygenase-1 (COX-1) inhibitors, ADP P2Y12 receptor antagonists, and platelet glycoprotein (GP) IIb/IIIa inhibitors [15,16,17]. These pharmacological treatments are primarily employed in the prevention and treatment of atherothrombotic disorders [15,16,17].
While these agents have been successful in reducing cardiovascular events in diabetic patients, there are reported limitations associated with the current treatment strategies [16,17]. These include issues such as resistance to aspirin and clopidogrel, as well as severe side effects of GP IIb/IIIa inhibitors [16,17,18,19,20]. As a result, ongoing efforts are being made to address these issues. These efforts involve exploring dose modifications, utilizing adjunctive therapies, and seeking out newer agents to improve treatment outcomes [16,17,18]. Establishing novel antiplatelet agents that can reliably and safely inhibit platelet activation and aggregation processes is considered the most encouraging approach for the future, where personalized antiplatelet drug regimens will be tailored to individual requirements [16,17,18]. This could involve utilizing drugs that specifically target dysfunctional pathways in particular patient groups, such as individuals with diabetes [16,17,18].
The platelet receptor glycoprotein (GP) Ib is a member of the family of leucine-rich repeat (LRR) protein kinases and has been recognized as transmembrane receptor type 1 [21,22]. It is also known as the Cluster of Differentiation 42 (CD42) protein [21,22]. GPIb, a major subunit of the GPIb-IX complex, is the second most prevalent adhesion receptor on platelets [22,23,24]. The GPIb-IX complex is also reported to be important for the hemostatic and prothrombotic functions of platelets [22,23]. It carries out its primary role by starting platelet adhesion when there is high force exerted by blood flow on the walls of blood vessels by binding to von Willebrand factor (vWF) in the subendothelial matrix [10,22,25]. Studies show that inhibiting the GPIbα–vWF binding site gives protective benefits, such as decreased microvascular occlusion, leading to better vascular conditions where blood vessels remain unobstructed [10,25,26]. The enhancement of platelet glycoprotein-Ib (GPIb) expression has been associated with the T2DM condition and has been reported to increase the risk of platelet aggregation, platelet-leucocyte interaction, and thrombo-inflammatory pathology [10,25,27,28,29]. Despite numerous studies demonstrating the link between T2DM and platelet hyperreactivity, research on the role of the platelet receptor GPIb in platelet activation-induced cardiovascular disease in T2DM patients remains limited. Therefore, this study comprehensively discusses GPIb’s role in inducing cardiovascular disease by promoting platelet activation, adhesion, and aggregation during T2DM disease progression. Understanding these pathophysiological mechanisms could lead to novel strategies targeting platelet activation to protect patients with T2DM from developing cardiovascular incidents.
2. The Physiology of Platelets
Platelets are non-nucleated blood components identified over 130 years ago [14,30]. It is known that platelets are the main cell that controls thrombosis and mediates myocardial infarction, stroke, and venous thromboembolism (VTE) [14,30]. Those make platelets important for blood vessel homeostasis. The human blood platelet diameter is about 2–4 μm, and its circulation number in healthy individuals approximately ranges from 150 to 350 × 109/L [14,30]. Platelets have a short lifespan, circulating in the blood only for one or two weeks following their elimination in the liver and spleen [14,30]. Platelet production primarily occurs in the bone marrow and is triggered by several transcription factors, including thrombopoietin hormone, to stimulate megakaryocyte development [30,31]. The process is initiated by forming polyploid megakaryocytes [30]. During maturation, megakaryocytes experience endomitotic cell cycles and become larger, resulting from increasing diameter [30,31]. After their differentiation in the bone marrow, megakaryocytes move toward the vascular cavity to be closer to the circulation [30,31]. Platelets have no genomic DNA; they only contain mRNA transcripts, enabling them to generate proteins such as cytokines and interleukins [30,31]. Furthermore, platelets can form microparticles with many bioactive compounds for the coagulation process [30,31].
The platelet’s cytoplasm contains three types of granules [32,33]. The first one is alpha granules, with an approximate number of 50 to 60 per platelet [32,33]. Alpha granules contain blood clotting factors such as vWF, growth factors, fibrinogen, coagulation factors V, XI, XIII, and chemokines [32,33]. The second one is platelet lysosomes, which comprise acid hydrolases and cathepsin D and E and play a role in the degradation of glycosaminoglycans, glycoproteins, and glycolipids [32,33]. These protective functions of lysosomes are fundamental for the extracellular matrix remodeling and regulation of the thrombus [32,33]. The third one is dense granules with an approximate number of 4 to 8 per platelet and consists of adhesion proteins, such as GPIIb/IIIa, GPIb, and P-selectin, as well as high concentrations of serotonin, adenine nucleotides, calcium, and phosphates, which are important in platelet aggregation and vascular contraction [32,33].
Hemostasis, thrombosis, and wound healing are regarded as the platelet’s fundamental functions and are achieved through a complicated activation process, resulting in plug development at the injury site, including vascular injury [34,35]. To avoid hemorrhage when blood vessels are damaged, the adhesive capabilities of platelets must be strictly controlled so the cells can rapidly activate accordingly [34,36]. Simultaneously, preventing unwanted platelet adhesion that can lead to thrombosis is crucial. Platelets have several adhesion molecules with specific individual functions that allow them to work distinctly in hemostatic and inflammatory conditions [34,35]. Furthermore, the platelet membrane encompasses diverse receptors, including glycoproteins, integrins, phospholipids, prostaglandin receptors, adenosine diphosphate receptors, immunoglobulin superfamily adhesion receptors, tyrosine kinase adhesive receptors, G-protein-coupled receptors, and leucine-rich adhesion receptors [34,35].
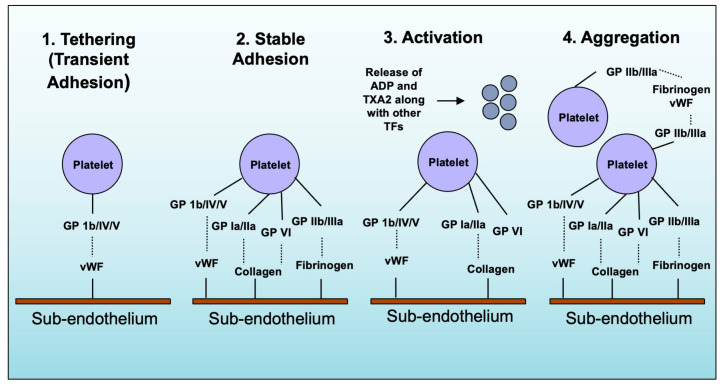
The hemostatic cascade signaling pathway starts with platelet receptors and some ligand interactions expressed on the surface of endothelial cells, in the subendothelial matrix, and as soluble proteins in the bloodstream [9,25,36]. This interaction triggers platelet adhesion and activation, developing thrombi [9,25,36]. GPIb/IV/V, GPVI, and GPIa/IIa receptors are particularly physiologically relevant to platelet adhesion [25,36]. The first step is tathering, the platelet interaction with the exposed endothelial cell matrix (ECM) [25,36]. Especially when blood flows through small arteries and arterioles at high rates, platelets first attach to the ECM by binding platelet GP1b and vWF [25,36]. However, the binding between vWF and GP1b has a quick off-rate and is consequently not enough to mediate firm adhesion [25,36]. Instead, it maintains a close connection between the platelet and the surface, facilitating the interaction between GPVI and collagen [25,36]. The next step is the rolling step. Due to the relatively low-affinity interactions between collagen and GPVI, the platelet GPIa/IIa receptor enhances the platelet interaction with collagen [25,36]. Following stable platelet adhesion, the next step is platelet activation, a paracrine- and autocrine-mediated signaling pathway [25,36]. The process begins with the discharge of thromboxane A2 (TXA2) and adenosine diphosphate (ADP) from platelets, along with the activation of thrombin by tissue factor present in the artery wall [25,36]. This integrates the integrins GPIIb/IIIa with fibrinogen and vWF, reinforcing the firm attachment of platelets and resulting in stable thrombus formation [25,36]. The action of thrombin causes fibrinogen to be changed into fibrin, promoting the growth of the thrombus [9,25]. The summary of platelet adhesion, activation, and aggregation is illustrated in Figure 1.
Figure 1.
Platelet adhesion, activation, aggregation. When blood vessels are damaged, platelets become activated and attach to the site of damage. This is facilitated by GPIbα and vWF, acting as “tether” and allowing other molecules such as GPVI to interact with collagen. This triggers a series of events that convert integrins on the surface of platelets to a high-affinity state and release ADP and TXA2, further activating platelets and promoting forming a stable blood clot. The tissue factor released from the damaged tissue activates thrombin, causing integrin GPIIb/IIIa to bind with fibrinogen and vWF, further strengthening the attachment of platelets and contributing to the formation of a stable blood clot.
3. Type 2 Diabetes Mellitus (T2DM) Promotes Atherosclerosis by Inducing Platelet Activation
Individuals with TD2M are at great risk of developing cardiovascular disease, which can lead to severe illness and death due to pathological thrombosis resulting from the rupture of atherosclerotic plaque [3,10,27]. Platelets play an important role in initiating and spreading thrombosis [9,10,11]. Recent findings suggest that patients with T2DM exhibit heightened platelet reactivity and basal activation compared to healthy individuals [27,28,29]. Studies have reported that individuals with diabetes have hyperactive platelets that metabolize more quickly, resulting in a faster turnover of platelets [37,38]. This further produces new hyperactive platelets prone to exaggerated responses to stimuli [37,38]. Additionally, platelet counts are higher in T2DM, particularly with an increased number of large platelets representing high reactivity [37,38,39]. The increased platelet hyperreactivity and baseline activation observed in T2DM are known multifactorial phenomena correlated with various biochemical factors, such as high lipid levels (hyperlipidemia), high blood sugar levels (hyperglycemia), insulin resistance, inflammation, and oxidative stress, thereby increasing the cardiovascular risk in T2DM [5,40,41].
Studies have shown that metabolic changes in T2DM can increase platelet activation, which is mostly associated with endothelial cell dysfunction [3,37,41,42,43]. Endothelial cells regulate various processes, including platelet activation and aggregation, vasodilation and vasoconstriction, thrombosis, and fibrinolysis [37,41,42,43]. Endothelial dysfunction, caused by increased blood glucose levels in T2DM, disrupts vascular homeostasis and initiates the atherosclerosis process [37,41,42,43]. Additionally, endothelial dysfunction results in the higher expression of adhesion molecules that promote platelet activation and aggregation, which is crucial in every stage of the atherosclerotic steps [37,41,42,43]. Under normal physiological conditions, endothelial cells produce and release vasodilator substances, such as prostacyclin (PGI2) and nitric oxide (NO), and vasoconstrictor substances, such as endothelin, to maintain vascular tone [37,42,44]. Insulin also affects platelet function by inhibiting P2Y12 signaling and increasing platelet responsiveness to the anti-aggregation effects of NO and PGI2 [17,27,37]. Therefore, a low insulin or insulin resistance level can increase platelet reactivity [5,17].
T2DM is associated with accelerated atherosclerosis due to reactive oxygen species (ROS) production, causing mitochondrial impairment, increased activation of protein kinase C (PKC), and advanced glycation end-products (AGEs) [45,46]. ROS can also stimulate nuclear poly (ADP-ribose) polymerase and switch early glycolytic intermediates into pathogenic pathways [45,46]. These processes lead to reduced mitochondrial biosynthesis, increased ROS production, and interference with the biorhythm of glucose and lipid metabolism [47,48]. The elevated glucose levels in T2DM increase ROS production, decrease NO and PGI2 synthesis through various mechanisms, such as activation of signaling pathways of NF-κB and protein kinase C (PKC), and decrease endothelial NO synthase (eNOS) activity [27,37,44]. These mechanisms result in altered adhesion molecules expression, impaired vasodilation, and advanced vascular inflammation [37,44]. Furthermore, in T2DM, inflammation cytokines are dysregulated and serve as a reciprocal correlation between inflammation and prothrombotic states [49].
Hyperglycemic conditions have also been associated with decreased antioxidant production, such as glutathione, associated with increased TXA2 production, leading to increased platelet activation [37,38]. Moreover, persistent hyperglycemia can increase the glycation of proteins on the platelet surface, causing changes in the activity and signaling of receptor proteins and reducing platelet membrane fluidity [37,50].
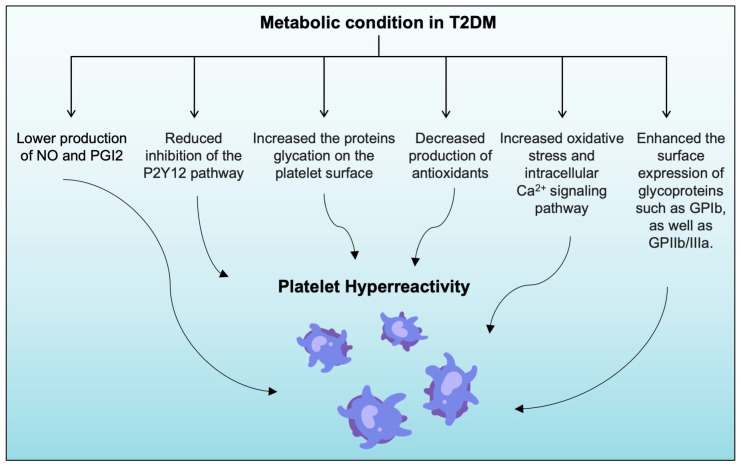
Thus, platelets become more responsive to thrombin and aggregate more, increasing platelet adhesion and sensitivity [37,50]. Some evidence also indicates that metabolic changes in patients with T2DM increase the surface expression of specific glycoproteins: GPIIb/IIIa and GPIb [27,28,37]. This increase in expression can lead to greater activation of GPIIb/IIIa, thereby increasing the binding of platelets to vWF and fibrinogen, ultimately leading to greater platelet aggregation. [27,28,37]. The summary of metabolic conditions in T2DM-induced platelet hyperreactivity is presented in Figure 2.
Figure 2.
Metabolic dysregulation in T2DM will contribute to greater platelet hyperreactivity. Dysregulated metabolic conditions in patients with T2DM will result in: (1) lower production of NO and PGI2; (2) reduced inhibition of the P2Y12 pathway; (3) increased oxidative stress and intracellular Ca2+ signaling activate the PKC pathway; (4) decreased production of antioxidants; (5) increased protein glycation on the platelet surface; (6) enhanced surface expression of glycoproteins, such as GPIb and GPIIb/IIIa. Together, these factors contribute to a prothrombotic environment, thus promoting the development of vascular occlusion and atherothrombosis in T2DM.
4. Platelet Glycoprotein Receptor-ib (GPIb)
During the pathological process, platelets play an important role in vascular interactions through their receptors [51,52]. Platelet membrane glycoproteins have an important role as receptors in two key processes: attachment to the subendothelial matrix and platelet aggregation [34,53,54]. As mentioned earlier, the atherosclerosis process is initiated by the adhesion step; whereas platelets adhere to intact endothelial cells, the process of transitioning from a passive circulating state to an active adhesive state in the extracellular matrix is regulated by platelet membrane receptors [9,25]. GPIb/IV/V, GPVI, and GPIa/IIa, along with other vital ligands, facilitate this adhesion [51,52]. When ligand-receptor engagement occurs, signaling mechanisms are activated, leading to changes in calcium oscillation, agonist molecule release, and platelet degranulation, which in turn induce events that lead to the activation of other platelets and the formation of a stable clot [51,52]. Among other platelet glycoprotein adhesion receptors, the GPIb/IX/V receptor complex represents the second-most expressed adhesion receptor found on platelets [22,23,24]. It comprises around 25,000 units of the GPIb-IX complex and 12,000 GPV units on the resting platelets [55].
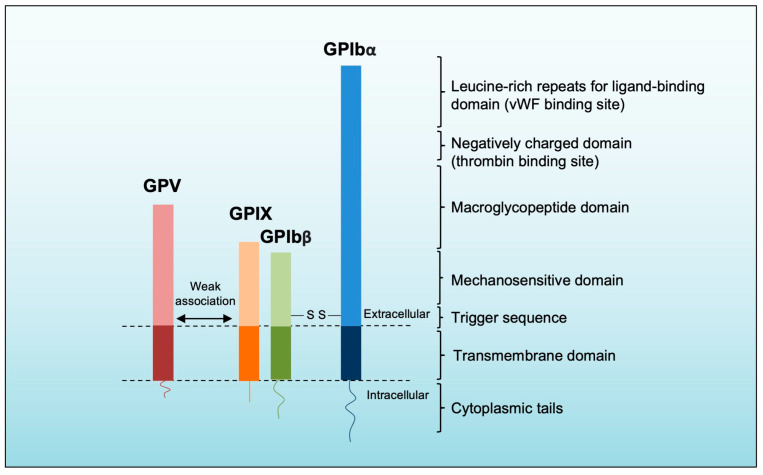
Platelet GPIb/IX/V’s structure has been well-reviewed elsewhere [24,56]. Briefly, GPIb/IX/V is a protein complex comprising three components: GPIbα-GPIbβ, GPIX, and GPV (Figure 3) [24]. The GPIbα, GPIbβ, and GPIX cluster into the GPIb/IX complex on the platelet membrane [24,57]. GPIbα is the largest one and mediates interactions with many known ligands [24]. It contains eight LRR that form the ligand-binding domain (LBD) and a negatively charged portion with three tyrosine residues with sulfate groups crucial for thrombin binding [24,56,58]. Additionally, GPIbα has a mucin-like macroglycopeptide region that aids in ligand-receptor complex formation [56]. The mechanosensitive domain (MSD), another identified domain in GPIbα, is located next to the macroglycopeptide region and can be cleaved by ADAM17, releasing soluble GPIbα fragments into the plasma [24,59]. Recently, a trigger sequence of 10 amino acid residues has been discovered near the transmembrane domain of GPIbα [56]. The discovery provides support for the GPIb-IX signaling model, proposing that when external forces act on the LBD of GPIbα, it induces the unfolding of the mechanosensitive domain (MSD) [56]. This unfolding exposes a trigger sequence, resulting in receptor activation and the subsequent initiation of signaling pathways within the platelet [56]. The other components of GPIbα-GPIbβ, the GPIbβ, make disulfide bonds with GPIbα and are connected to GPIX [56]. GPIbβ engages in intracellular interaction with calmodulin, whereas GPIX does not form associations with any intracellular molecules [24,57]. Moreover, the cytoplasmic tail of GPIbα possesses multiple binding sites for intracellular signaling molecules, facilitating the connection of the receptor complex to actin filaments within the cytoskeleton [24]. Interactions between the transmembrane domains of the components, including the engagement of GPIbβ and GPIX with the mechanosensitive domain (MSD) of GPIbα, stabilize the GPIb-IX complex [24]. The association of GPV with the complex is relatively weak, and its susceptibility to non-ionic detergents suggests that the GPIb/IX complex can potentially interact with other membrane receptors along with GPV [24].
Figure 3.
Platelet GPIb/IX/V’s structure. The largest subunit of the complex is GPIbα, comprising leucine-rich repeats in the ligand-binding domain, a negatively charged domain, a macroglycopeptide domain, a mechanosensitive domain, and the trigger sequence. GPIbα and GPIbβ are connected through disulfide bonds. The transmembrane domain facilitates close interaction with GPIbβ and GPIX, forming a stable parallel structure. GPV weakly interacts with GPIb-IX through polar interactions and can potentially be replaced by other receptors.
5. Platelet GPIb Hyperreactivity in T2DM and Its Role in the Pathogenesis of Atherosclerosis
The activation, adhesion, and aggregation of platelets are crucial processes significantly contributing to advanced atherosclerosis-induced cardiovascular disease, leading to serious clinical events of CAD such as myocardial infarction or heart attack [3,9,10]. These events occur due to the aggravation of atherosclerosis, which happens when endothelial plaque is disrupted, causing platelet adhesion, activation, and thrombus formation [3,9,10]. Patients with T2DM are at great risk of having cardiovascular diseases due to platelet hyperreactivity, which is common in T2DM patients with hyperglycemia [3,27,28]. Atherosclerosis is a condition where the walls of the arteries narrow due to lipid and cholesterol accumulation [41,60]. In patients with T2DM, changes in the coronary artery wall, platelet hyperreactivity, and fibrin deposition can cause progressive narrowing of the artery lumen [41,60]. These changes may lead to a sudden disruption of blood flow due to plaque rupture with thrombosis [41,60]. This can exacerbate the narrowing of the artery lumen, compromising blood flow and potentially leading to serious cardiovascular incidents [41,60].
T2DM has been marked as a chronic disease with prothrombotic status because it has shown platelet changes and coagulation characteristics [37,61,62]. Individuals with T2DM often have increased platelet adhesiveness, which is associated with higher levels of adhesive molecules such as the platelet receptors GPIb/CD42 and vWF [29,63,64,65,66,67,68]. The significance of platelets in vascular occlusion and thromboinflammation is strongly linked with their adherence capacity to injured endothelial cells and immune cells [34,69,70]. T2DM eventually precipitates endothelial dysfunction and magnifies inflammation, vasoconstriction, and thrombosis [62]. A study shows that in mice with streptozosocin-induced diabetes, platelets have more frequent interactions with endothelial vessels. This interaction is due to increased expression of vWF in endothelial cells and is mediated by platelet GPIb-IX-V [71].
The platelet GPIb/IX receptor complex is essential in regulating normal and pathological platelet processes [25]. For example, during the hemostasis process, the sequestration of platelets at the site of vascular injury is initiated through the interaction between platelet GPIb/IX and the vWF factor on the subendothelial matrix [25,72]. When there is damage to the endothelial layer of blood vessels, subendothelial collagen and other components are exposed, leading to platelet adhesion and activation [25,72]. The initial interaction is between the platelet GPIbα and vWF, and their interaction initiates platelet adhesion and triggers a signaling cascade that induces platelet integrin αIIbβ3 activation [25,72]. Activated αIIbβ3 then allows fibrinogen binding, leading to platelet aggregate formation [25]. Moreover, vWF can also be activated by high shear forces and becomes more adherent to the platelet GPIbα receptor, which further activates platelets and enhances thrombus formation [25,72].
In addition, the link between GP1b and vWF induces thromboxane A2 production, resulting in ADP secretion and fibrinogen receptor activation, the key steps in platelet activation [25,72]. The GP1b-IX-mediated platelet activation occurs through various signaling pathways, including the mitogen-activated protein kinase pathways, the phosphatidylinositol 3-kinase (PI3-kinase) protein kinase B (Akt) pathway, the FcRγ-Syk/PLCγ2 pathway, and the LIM kinase 1 (LIMK1) pathway [73,74]. After establishing adhesion, the platelets become more activated and generate more pro-inflammatory molecules and chemoattractants [31]. Platelets’ adhesion to the vascular endothelial cells delivers messages for leukocyte engagement and monocyte extravasation [31]. In atherosclerosis, the protective role of the vascular endothelium diminishes, resulting in an increased number of activated platelets [31]. Subsequently, the activated platelets produce more inflammatory molecules, generating chronic inflammation in the endothelium and resulting in vascular and cellular dysfunction [31].
The interaction between the platelet receptor GPIbα and the leukocyte integrin macrophage-1 antigen (Mac-1) also plays a role in thrombosis [75]. This interaction facilitates the mobilization, adherence, and migration of leukocytes to vascular lesion sites, promoting vascular inflammation [75]. Moreover, thrombus formation is entirely abolished in studies that use GPIb knockout mice, highlighting the crucial role of the GPIb receptor’s interaction with vWF in platelet adhesion and platelet interaction with immune cells. [25,75,76]. Additionally, apart from vWF, other ligands for the GPIb receptor might play a pivotal role in platelet aggregation and thrombosis, significantly contributing to platelet adhesion [25]. The GPV receptor interacts with additional ligands and counter-receptors within the bloodstream, participating in diverse aspects of platelet biology that are still under investigation [25]. Platelet GPIb receptors are important molecules during atherosclerosis’s early and later stages. Research indicates that inhibiting the interaction between GPIbα and vWF can have a protective effect by reducing the occurrence of microvascular blockages and improving the patency of blood vessels. [26]. As a result, several agents are currently being investigated to target the platelet adhesion signaling pathway by inhibiting GpIb for potential therapeutic applications.
6. Targeting GPIb as a Potential Therapy May Protect T2DM Patients from Developing Atherosclerosis-Induced Cardiovascular Diseases
Since patients with T2DM have demonstrated higher atherogenesis and atherothrombotic complications, antithrombotic drugs are needed as management therapy for these individuals [10,17,18,44,77]. Current treatment strategies for individuals with high risk of CAD, such as T2DM patients, primarily target enhancing vascular outcomes and revolve around restoring blood flow in obstructed arteries [10,17,18,78]. Therefore, antiplatelet agents are needed to prevent platelets from clumping and clotting from forming and growing in patients with T2DM [10,17,18,78]. Over the years, two primary targets for antithrombotic therapy have been extensively employed. These include using cyclooxygenase-1 (COX-1) inhibitors, aiming to diminish the production of TXA2 (e.g., low-dose aspirin), and utilizing platelet P2Y12 receptor antagonists (e.g., clopidogrel, prasugrel, ticagrelor, cangrelor, and elinogrel) [10,17,18,78]. However, despite their widespread use, the rates of recurrent atherothrombotic events remain high, particularly in diabetic patients, indicating inadequate protection against cardiovascular events [16,17,18,19,20]. The concept of antiplatelet drug resistance is relevant to aspirin and clopidogrel [16,17]. Furthermore, a randomized trial has suggested that aspirin may not effectively reduce the risk of cardiovascular events in primary prevention and could potentially increase the risk of gastrointestinal bleeding [16,79]. The variability in platelet response to clopidogrel can be attributed to genetic, cellular, and clinical factors [16,17,80,81]. Notably, diabetic patients, especially those requiring insulin therapy, are more likely to be nonresponsive to clopidogrel [16,17]. Additionally, dual antiplatelet therapy (DAPT), which combines aspirin with P2Y12 inhibitors (primarily clopidogrel), is commonly used in patients with CAD and those undergoing percutaneous coronary intervention (PCI), regardless of their T2DM status [82,83]. However, it is worth noting that reports of reduced responsiveness to DAPT and an increased risk of bleeding have been described [17,84,85]. Another class of antiplatelet agents used to reduce cardiovascular complications associated with PCI is GPIIb/IIIa antagonists, which inhibit platelet aggregation [10,17,25]. Currently, three intravenous GPIIb/IIIa antagonists are available: the monoclonal antibody abciximab, as well as the small molecules eptifibatide and tirofiban [10,17,25]. It is important to consider that the use of GP IIb/IIIa antagonists has been associated with an increased risk of thrombocytopenia and bleeding complications [4,17,20,86].
Collectively, it underscores the need for newer targeted antiplatelet treatment approaches in diabetic patients. This may involve utilizing more potent medications or combining different antiplatelet drugs to enhance effectiveness [4,16,17,18]. In the future, antiplatelet drug regimens could be utilized based on a “stage-specific” vascular management strategy and personalized needs [4,16,17,18]. The most promising strategy to accomplish this objective is to develop new antiplatelet agents that specifically target pathways commonly observed in a specific patient population, such as elevated GPIb levels in patients with T2DM [4,25,87,88].
Researchers have conducted several studies to develop drugs targeting the GPIb receptor, subsequently decreasing platelet adhesion and preventing thrombosis [10,25,87,89,90]. One study by David et al. utilized an inhibitory peptide called R9α557 that can penetrate the cell membrane and contains nine arginine amino acid residues to facilitate entry into the cell [25,91]. The peptide comprises a sequence of 11–13 amino acids from the cytoplasmic region of the GPIb receptor [25,91]. Further, the study demonstrated that the R9α557 peptide could effectively reduce vWF-mediated adhesion in human platelets [25,91]. Another experimental approach involved using a monoclonal antibody, 6B4, that targeted the GPIb receptor in nonhuman primates, as conducted by Cauwenberghs et al. [25,92]. The findings from this study showed that the monoclonal antibody could reduce thrombus formation without causing significant prolongation of bleeding time [25,92]. Additionally, Zahger et al. conducted a study in which they utilized VCL, a recombinant von Willebrand factor GP1b binding domain, as an antagonist to the GPIb receptor in rats [25,93]. Their investigation provided compelling evidence that using VCL, a platelet GP1b receptor antagonist, yielded notable reductions in platelet adhesion and the extent of intimal thickening following balloon injury to the femoral artery in rats [25,93].
Another interesting study involved compounds derived from snake venom called C-type lectins, which have generated mixed results in various experimental settings [25,87,94,95]. While some agents have led to platelet inhibition, others have resulted in platelet activation [25,87,94,95]. However, a group of researchers focused on one particular compound called anfibatide, a snake venom-derived GPIb antagonist [93,96,97,98]. They successfully synthesized it using recombinant technology and tested its ability to inhibit the GPIbα-vWF interaction in vitro and in vivo studies [96,99]. Their finding also indicates that the researchers developed a capable method of generating recombinant anfibatide in substantial quantities [96,99]. This approach aimed to overcome the challenges of purifying anfibatide from raw snake venom and the limited supply of this natural resource [96,99]. Next, after accumulating positive in vitro and in vivo results, the researchers evaluated the safety and efficacy of anfibatide in healthy human individuals [96,100,101,102,103]. Their investigations yielded compelling evidence demonstrating the specific inhibition of GPIbα-vWF interaction and associated platelet functions by anfibatide [96,103]. Notably, this inhibition was observed in various scenarios, such as ferric chloride- and laser-induced thrombus formation in mesenteric and cremaster muscle arterioles [96,103]. The positive outcomes prompted subsequent studies involving human subjects [96,103]. These results strongly suggest that anfibatide possesses selective antithrombotic properties targeting GPIb in humans [96,103]. This noteworthy finding was briefly mentioned during the American Society of Hematology’s 2013 annual meeting [96,103]. In managing and treating CAD characterized by elevated shear stress, anfibatide may emerge as a more favorable alternative to αIIbβ3 and vWF antagonists [96,103]. This results from the pivotal role of the GPIbα-vWF interaction in thrombosis under such conditions [96,103]. Consequently, utilizing anfibatide could potentially improve patients’ risk/benefit ratio in these scenarios [96,103]. Significantly, this marked the first instance of testing a GPIbα antagonist in humans, as registered in ClinicalTrials.gov. The outcomes from the Phase I study indicate that anfibatide may serve as a safe and effective therapeutic agent for antithrombotic therapy, specifically targeting platelet GPIbα [96,103].
Moreover, the study revealed a promising safety profile for anfibatide, providing a foundation for advancing to subsequent clinical trial phases: Phases Ib-IIa and Phase II [96]. Anfibatide holds potential as a therapeutic agent for treating CAD, with particular relevance for patients with T2DM who face an increased risk of cardiovascular complications accompanied by elevated levels of GPIb [93,96,100,101,102,103]. Further investigations are warranted to determine optimal dosing strategies and evaluate the safety profile for patients with a high intracoronary thrombus burden. Nonetheless, anfibatide exhibits promise as an anti-GPIb agent, offering prospects for enhanced treatment options.
7. Conclusions
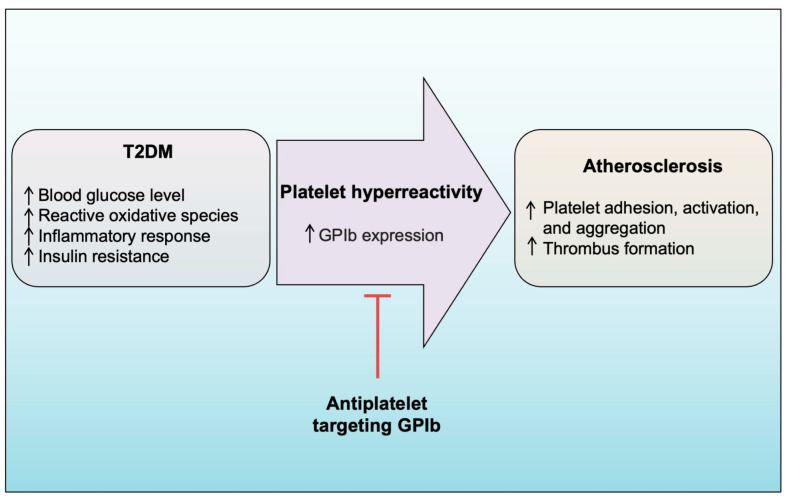
Platelet adhesion and aggregation are important in preventing excessive bleeding during tissue injury, including vascular injury. These processes are particularly significant in vascular thrombosis, such as in cerebral arteries or atherosclerotic coronary vessels, which can result in stroke, heart attack, and lower extremity PAD. Numerous studies have reported that individuals with T2DM face a high risk of atherosclerotic cardiovascular disease. To manage cardiovascular thrombotic disorders in patients with T2DM, antiplatelet agents such as aspirin, GPIIb/IIIa inhibitors, and clopidogrel are currently used as treatment options. However, recent studies have highlighted the limitations of these therapies, including resistance issues and severe side effects, indicating the need for newer antiplatelet agents. Studies have shown that individuals with T2DM often experience platelet hyperreactivity and a prothrombotic state linked to increased expression of various platelet glycoproteins, including GPIb. When exposed to strong shear forces, GPIb facilitates platelet adhesion by interacting with vWF. Subsequently, the adhered platelets become activated and aggregate, forming a hemostatic plug or an occlusive thrombus. Collectively, these findings highlight the crucial role of GPIb as a bridge connecting T2DM and atherosclerosis (Figure 4). Therefore, this review article emphasizes the discovery of the first antiplatelet agents targeting platelet GPIb, which have progressed to clinical trial studies, such as anfibatide. Anfibatide has demonstrated promising antiplatelet effects and a low bleeding tendency in clinical trials. Given that GPIb is an attractive target for attenuating thrombosis and its expression is heightened in patients with T2DM, anfibatide could be an effective antiplatelet agent to prevent cardiovascular disease complications in this specific population. Nevertheless, further studies and trials, encompassing experimental in vitro and in vivo investigations, including clinical trials, are necessary to thoroughly evaluate the effectiveness of anfibatide in achieving a higher antiplatelet effect, particularly in T2DM-specific populations.
Figure 4.
Platelet glycoprotein-Ib (GPIb) may act as a bridge between T2DM and atherosclerosis. In the context of T2DM, various risk factors such as high glucose levels, insulin resistance, oxidative stress, and inflammation can contribute to increased platelet hyperreactivity. One of the effects of these risk factors is the upregulation of GPIb expression, which promotes platelet adhesion, activation, and aggregation. These processes, in turn, contribute to the development and progression of atherosclerosis.
Author Contributions
Design and conceptualization, M.A., M.U.P. and F.C.S.; interpret the relevant literature, M.A., M.U.P., F.C.S., R.S. and B.W.; writing—original draft preparation, M.A. and M.U.P.; writing—review and editing, M.U.P. and F.C.S.; supervision and funding acquisition, F.C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research is funded by the Ministry of Education, Culture, Research, and Technology of Indonesia (Reference Number: NKB-786/UN2.RST/HKP.05.00/2022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Diabetes 2023. [(accessed on 13 January 2023)]; Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 2.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Rosa S., Arcidiacono B., Chiefari E., Brunetti A., Indolfi C., Foti D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front. Endocrinol. 2018;9:2. doi: 10.3389/fendo.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung J.H., Tantry U.S., Gurbel P.A., Jeong Y.H. Current antiplatelet treatment strategy in patients with diabetes mellitus. Diabetes Metab. J. 2015;39:95–113. doi: 10.4093/dmj.2015.39.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B., Ostolaza H., Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soyoye D.O., Abiodun O.O., Ikem R.T., Kolawole B.A., Akintomide A.O. Diabetes and peripheral artery disease: A review. World J. Diabetes. 2021;12:827–838. doi: 10.4239/wjd.v12.i6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiruvoipati T., Kielhorn C.E., Armstrong E.J. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J. Diabetes. 2015;6:961–969. doi: 10.4239/wjd.v6.i7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakubiak G.K., Pawlas N., Cieślar G., Stanek A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health. 2020;17:9339. doi: 10.3390/ijerph17249339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badimon L., Padró T., Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care. 2012;1:60–74. doi: 10.1177/2048872612441582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Tang C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020;21:9760. doi: 10.3390/ijms21249760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lordan R., Tsoupras A., Zabetakis I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2021;45:100694. doi: 10.1016/j.blre.2020.100694. [DOI] [PubMed] [Google Scholar]
- 12.Geovanini G.R., Libby P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018;132:1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 13.Patzelt J., Verschoor A., Langer H.F. Platelets and the complement cascade in atherosclerosis. Front. Physiol. 2015;6:49. doi: 10.3389/fphys.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018;122:337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capodanno D., Mehran R., Krucoff M.W., Baber U., Bhatt D.L., Capranzano P., Collet J.P., Cuisset T., De Luca G., De Luca L., et al. Defining Strategies of Modulation of Antiplatelet Therapy in Patients with Coronary Artery Disease: A Consensus Document from the Academic Research Consortium. Circulation. 2023;147:1933–1944. doi: 10.1161/CIRCULATIONAHA.123.064473. [DOI] [PubMed] [Google Scholar]
- 16.Russo I., Penna C., Musso T., Popara J., Alloatti G., Cavalot F., Pagliaro P. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc. Diabetol. 2017;16:71. doi: 10.1186/s12933-017-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angiolillo D.J. Antiplatelet therapy in diabetes: Efficacy and limitations of current treatment strategies and future directions. Diabetes Care. 2009;32:531–540. doi: 10.2337/dc08-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajjan R.A., Kietsiriroje N., Badimon L., Vilahur G., Gorog D.A., Angiolillo D.J., Russell D.A., Rocca B., Storey R.F. Antithrombotic therapy in diabetes: Which, when, and for how long? Eur. Heart J. 2021;42:2235–2259. doi: 10.1093/eurheartj/ehab128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald R., Pirmohamed M. Aspirin resistance: Effect of clinical, biochemical and genetic factors. Pharmacol. Ther. 2011;130:213–225. doi: 10.1016/j.pharmthera.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Labinaz M., Ho C., Banerjee S., Martin J., Chen S., Mensinkai S. Meta-analysis of clinical efficacy and bleeding risk with intravenous glycoprotein IIb/IIIa antagonists for percutaneous coronary intervention. Can. J. Cardiol. 2007;23:963–970. doi: 10.1016/S0828-282X(07)70858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Deng W., Zhou L., Xu Y., Yang W., Liang X., Wang Y., Kulman J.D., Zhang X.F., Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood. 2015;125:562–569. doi: 10.1182/blood-2014-07-589507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews R.K., Gardiner E.E., Shen Y., Whisstock J.C., Berndt M.C. Glycoprotein Ib-IX-V. Int. J. Biochem. Cell Biol. 2003;35:1170–1174. doi: 10.1016/S1357-2725(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 23.Li R., Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J. Thromb. Haemost. 2013;11:605–614. doi: 10.1111/jth.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendas G., Schlesinger M. The GPIb-IX complex on platelets: Insight into its novel physiological functions affecting immune surveillance, hepatic thrombopoietin generation, platelet clearance and its relevance for cancer development and metastasis. Exp. Hematol. Oncol. 2022;11:19. doi: 10.1186/s40164-022-00273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiefer T.L., Becker R.C. Inhibitors of platelet adhesion. Circulation. 2009;120:2488–2495. doi: 10.1161/CIRCULATIONAHA.109.886895. [DOI] [PubMed] [Google Scholar]
- 26.Maiocchi S., Alwis I., Wu M.C.L., Yuan Y., Jackson S.P. Thromboinflammatory Functions of Platelets in Ischemia-Reperfusion Injury and Its Dysregulation in Diabetes. Semin. Thromb. Hemost. 2018;44:102–113. doi: 10.1055/s-0037-1613694. [DOI] [PubMed] [Google Scholar]
- 27.Kakouros N., Rade J.J., Kourliouros A., Resar J.R. Platelet function in patients with diabetes mellitus: From a theoretical to a practical perspective. Int. J. Endocrinol. 2011;2011:742719. doi: 10.1155/2011/742719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschoepe D., Roesen P., Kaufmann L., Schauseil S., Kehrel B., Ostermann H., Gries F.A. Evidence for abnormal platelet glycoprotein expression in diabetes mellitus. Eur. J. Clin. Investig. 1990;20:166–170. doi: 10.1111/j.1365-2362.1990.tb02264.x. [DOI] [PubMed] [Google Scholar]
- 29.Soma P., Swanepoel A.C., du Plooy J.N., Mqoco T., Pretorius E. Flow cytometric analysis of platelets type 2 diabetes mellitus reveals ‘angry’ platelets. Cardiovasc. Diabetol. 2016;15:52. doi: 10.1186/s12933-016-0373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 31.Reinthaler M., Braune S., Lendlein A., Landmesser U., Jung F. Platelets and coronary artery disease: Interactions with the blood vessel wall and cardiovascular devices. Biointerphases. 2016;11:029702. doi: 10.1116/1.4953246. [DOI] [PubMed] [Google Scholar]
- 32.Hamilos M., Petousis S., Parthenakis F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc. Diagn. Ther. 2018;8:568–580. doi: 10.21037/cdt.2018.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharda A., Flaumenhaft R. The life cycle of platelet granules. F1000Research. 2018;7:236. doi: 10.12688/f1000research.13283.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gremmel T., Frelinger A.L., 3rd, Michelson A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016;42:191–204. doi: 10.1055/s-0035-1564835. [DOI] [PubMed] [Google Scholar]
- 35.Yun S.H., Sim E.H., Goh R.Y., Park J.I., Han J.Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed. Res. Int. 2016;2016:9060143. doi: 10.1155/2016/9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga-Szabo D., Pleines I., Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler. Thromb. Vasc. Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 37.Sobczak A.I.S., Stewart A.J. Coagulatory Defects in Type-1 and Type-2 Diabetes. Int. J. Mol. Sci. 2019;20:6345. doi: 10.3390/ijms20246345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morel O., Jesel L., Abbas M., Morel N. Prothrombotic changes in diabetes mellitus. Semin. Thromb. Hemost. 2013;39:477–488. doi: 10.1055/s-0033-1343888. [DOI] [PubMed] [Google Scholar]
- 39.Zaccardi F., Rocca B., Rizzi A., Ciminello A., Teofili L., Ghirlanda G., De Stefano V., Pitocco D. Platelet indices and glucose control in type 1 and type 2 diabetes mellitus: A case-control study. Nutr. Metab. Cardiovasc. Dis. 2017;27:902–909. doi: 10.1016/j.numecd.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler M., Wang X., Peter K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019;115:1178–1188. doi: 10.1093/cvr/cvz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poznyak A., Grechko A.V., Poggio P., Myasoedova V.A., Alfieri V., Orekhov A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020;21:1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Sala L., Prattichizzo F., Ceriello A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2019;26((Suppl. 2)):15–24. doi: 10.1177/2047487319878373. [DOI] [PubMed] [Google Scholar]
- 43.Ye J., Li L., Wang M., Ma Q., Tian Y., Zhang Q., Liu J., Li B., Zhang B., Liu H., et al. Diabetes Mellitus Promotes the Development of Atherosclerosis: The Role of NLRP3. Front. Immunol. 2022;13:900254. doi: 10.3389/fimmu.2022.900254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pechlivani N., Ajjan R.A. Thrombosis and Vascular Inflammation in Diabetes: Mechanisms and Potential Therapeutic Targets. Front. Cardiovasc. Med. 2018;5:1. doi: 10.3389/fcvm.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katakami N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018;25:27–39. doi: 10.5551/jat.RV17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan T., Yang T., Chen H., Fu D., Hu Y., Wang J., Yuan Q., Yu H., Xu W., Xie X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–260. doi: 10.1016/j.redox.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah M.S., Brownlee M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaneto H., Katakami N., Matsuhisa M., Matsuoka T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019;18:72. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aronson D., Rayfield E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002;1:1. doi: 10.1186/1475-2840-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X.R., Carrim N., Neves M.A., McKeown T., Stratton T.W., Coelho R.M., Lei X., Chen P., Xu J., Dai X., et al. Platelets and platelet adhesion molecules: Novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb. J. 2016;14((Suppl. 1)):29. doi: 10.1186/s12959-016-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibrahim H., Kleiman N.S. Platelet pathophysiology, pharmacology, and function in coronary artery disease. Coron. Artery Dis. 2017;28:614–623. doi: 10.1097/MCA.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 53.Kunicki T.J. Platelet membrane glycoproteins and their function: An overview. Blut. 1989;59:30–34. doi: 10.1007/BF00320245. [DOI] [PubMed] [Google Scholar]
- 54.Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36:195–198. doi: 10.1007/s10555-017-9677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen C.E., Qiu Y., McCarty O.J.T., Lam W.A. Platelet Mechanotransduction. Annu. Rev. Biomed. Eng. 2018;20:253–275. doi: 10.1146/annurev-bioeng-062117-121215. [DOI] [PubMed] [Google Scholar]
- 56.Quach M.E., Li R. Structure-function of platelet glycoprotein Ib-IX. J. Thromb. Haemost. 2020;18:3131–3141. doi: 10.1111/jth.15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo S.Z., Mo X., Afshar-Kharghan V., Srinivasan S., López J.A., Li R. Glycoprotein Ibalpha forms disulfide bonds with 2 glycoprotein Ibbeta subunits in the resting platelet. Blood. 2007;109:603–609. doi: 10.1182/blood-2006-05-024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong J., Ye P., Schade A.J., Gao S., Romo G.M., Turner N.T., McIntire L.V., López J.A. Tyrosine sulfation of glycoprotein I(b)alpha. Role of electrostatic interactions in von Willebrand factor binding. J. Biol. Chem. 2001;276:16690–16694. doi: 10.1074/jbc.M101035200. [DOI] [PubMed] [Google Scholar]
- 59.Gardiner E.E., Karunakaran D., Shen Y., Arthur J.F., Andrews R.K., Berndt M.C. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J. Thromb. Haemost. 2007;5:1530–1537. doi: 10.1111/j.1538-7836.2007.02590.x. [DOI] [PubMed] [Google Scholar]
- 60.Haas A.V., McDonnell M.E. Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol. Metab. Clin. North Am. 2018;47:51–63. doi: 10.1016/j.ecl.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Pretorius L., Thomson G.J.A., Adams R.C.M., Nell T.A., Laubscher W.A., Pretorius E. Platelet activity and hypercoagulation in type 2 diabetes. Cardiovasc. Diabetol. 2018;17:1–11. doi: 10.1186/s12933-018-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaur R., Kaur M., Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018;17:121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J.H., Bae H.Y., Kim S.Y. Clinical marker of platelet hyperreactivity in diabetes mellitus. Diabetes Metab. J. 2013;37:423–428. doi: 10.4093/dmj.2013.37.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Israels S.J., McNicol A., Dean H.J., Cognasse F., Sellers E.A. Markers of platelet activation are increased in adolescents with type 2 diabetes. Diabetes Care. 2014;37:2400–2403. doi: 10.2337/dc13-2718. [DOI] [PubMed] [Google Scholar]
- 65.Koga H., Sugiyama S., Kugiyama K., Fukushima H., Watanabe K., Sakamoto T., Yoshimura M., Jinnouchi H., Ogawa H. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur. Heart J. 2006;27:817–823. doi: 10.1093/eurheartj/ehi746. [DOI] [PubMed] [Google Scholar]
- 66.Vinik A.I., Erbas T., Park T.S., Nolan R., Pittenger G.L. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 67.Frankel D.S., Meigs J.B., Massaro J.M., Wilson P.W., O’Donnell C.J., D’Agostino R.B., Tofler G.H. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: The framingham offspring study. Circulation. 2008;118:2533–2539. doi: 10.1161/CIRCULATIONAHA.108.792986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaikina T., Minukhina D., Titova G., Rynchak P., Lantukhova N. Impact of percutaneous coronary intervention on prothrombogenic potential in patients with acute myocardial infarction and diabetes mellitus type 2. Wiad. Lek. 2022;75:339–343. doi: 10.36740/WLek202202102. [DOI] [PubMed] [Google Scholar]
- 69.Knobler H., Savion N., Shenkman B., Kotev-Emeth S., Varon D. Shear-induced platelet adhesion and aggregation on subendothelium are increased in diabetic patients. Thromb. Res. 1998;90:181–190. doi: 10.1016/S0049-3848(98)00050-4. [DOI] [PubMed] [Google Scholar]
- 70.Gauer J.S., Ajjan R.A., Ariëns R.A.S. Platelet-Neutrophil Interaction and Thromboinflammation in Diabetes: Considerations for Novel Therapeutic Approaches. J. Am. Heart Assoc. 2022;11:e027071. doi: 10.1161/JAHA.122.027071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Przygodzki T., Kassassir H., Talar M., Siewiera K., Watala C. Effects of three-month streptozotocin-induced diabetes in mice on blood platelet reactivity, COX-1 expression and adhesion potential. Int. J. Exp. Pathol. 2019;100:41–48. doi: 10.1111/iep.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spiel A.O., Gilbert J.C., Jilma B. von Willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation. 2008;117:1449–1459. doi: 10.1161/CIRCULATIONAHA.107.722827. [DOI] [PubMed] [Google Scholar]
- 73.Badolia R., Kostyak J.C., Dangelmaier C., Kunapuli S.P. Syk Activity Is Dispensable for Platelet GP1b-IX-V Signaling. Int. J. Mol. Sci. 2017;18:1238. doi: 10.3390/ijms18061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Estevez B., Du X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology. 2017;32:162–177. doi: 10.1152/physiol.00020.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y., Gao H., Shi C., Erhardt P.W., Pavlovsky A., D A.S., Bledzka K., Ustinov V., Zhu L., Qin J., et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nat. Commun. 2017;8:15559. doi: 10.1038/ncomms15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergmeier W., Chauhan A.K., Wagner D.D. Glycoprotein Ibalpha and von Willebrand factor in primary platelet adhesion and thrombus formation: Lessons from mutant mice. Thromb. Haemost. 2008;99:264–270. doi: 10.1160/TH07-10-0638. [DOI] [PubMed] [Google Scholar]
- 77.Picard F., Adjedj J., Varenne O. Diabetes Mellitus, a prothrombotic disease. Ann. Cardiol. Angeiol. 2017;66:385–392. doi: 10.1016/j.ancard.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Rivas Rios J.R., Franchi F., Rollini F., Angiolillo D.J. Diabetes and antiplatelet therapy: From bench to bedside. Cardiovasc. Diagn. Ther. 2018;8:594–609. doi: 10.21037/cdt.2018.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothwell P.M., Fowkes F.G., Belch J.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 80.Bonello L., Tantry U.S., Marcucci R., Blindt R., Angiolillo D.J., Becker R., Bhatt D.L., Cattaneo M., Collet J.P., Cuisset T., et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 81.Zaccardi F., Pitocco D., Willeit P., Laukkanen J.A. Efficacy and safety of P2Y12 inhibitors according to diabetes, age, gender, body mass index and body weight: Systematic review and meta-analyses of randomized clinical trials. Atherosclerosis. 2015;240:439–445. doi: 10.1016/j.atherosclerosis.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Collet J.P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 83.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 84.Chen J., Bhatt D.L., Dunn E.S., Shi C., Caro J.J., Mahoney E.M., Gabriel S., Jackson J.D., Topol E.J., Cohen D.J. Cost-effectiveness of clopidogrel plus aspirin versus aspirin alone for secondary prevention of cardiovascular events: Results from the CHARISMA trial. Value Health. 2009;12:872–879. doi: 10.1111/j.1524-4733.2009.00529.x. [DOI] [PubMed] [Google Scholar]
- 85.Dasgupta A., Steinhubl S.R., Bhatt D.L., Berger P.B., Shao M., Mak K.H., Fox K.A., Montalescot G., Weber M.A., Haffner S.M., et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial) Am. J. Cardiol. 2009;103:1359–1363. doi: 10.1016/j.amjcard.2009.01.342. [DOI] [PubMed] [Google Scholar]
- 86.Said S.M., Hahn J., Schleyer E., Müller M., Fiedler G.M., Buerke M., Prondzinsky R. Glycoprotein IIb/IIIa inhibitor-induced thrombocytopenia: Diagnosis and treatment. Clin. Res. Cardiol. 2007;96:61–69. doi: 10.1007/s00392-006-0459-7. [DOI] [PubMed] [Google Scholar]
- 87.Clemetson K.J., Clemetson J.M. Platelet GPIb complex as a target for anti-thrombotic drug development. Thromb. Haemost. 2008;99:473–479. doi: 10.1160/TH07-12-0718. [DOI] [PubMed] [Google Scholar]
- 88.López J.A. The platelet glycoprotein Ib-IX complex. Blood Coagul. Fibrinolysis. 1994;5:97–119. doi: 10.1097/00001721-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 89.Massberg S., Brand K., Grüner S., Page S., Müller E., Müller I., Bergmeier W., Richter T., Lorenz M., Konrad I., et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koltsova E.K., Sundd P., Zarpellon A., Ouyang H., Mikulski Z., Zampolli A., Ruggeri Z.M., Ley K. Genetic deletion of platelet glycoprotein Ib alpha but not its extracellular domain protects from atherosclerosis. Thromb. Haemost. 2014;112:1252–1263. doi: 10.1160/th14-02-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.David T., Ohlmann P., Eckly A., Moog S., Cazenave J.P., Gachet C., Lanza F. Inhibition of adhesive and signaling functions of the platelet GPIb-V-IX complex by a cell penetrating GPIbalpha peptide. J. Thromb. Haemost. 2006;4:2645–2655. doi: 10.1111/j.1538-7836.2006.02198.x. [DOI] [PubMed] [Google Scholar]
- 92.Cauwenberghs N., Meiring M., Vauterin S., van Wyk V., Lamprecht S., Roodt J.P., Novák L., Harsfalvi J., Deckmyn H., Kotzé H.F. Antithrombotic effect of platelet glycoprotein Ib-blocking monoclonal antibody Fab fragments in nonhuman primates. Arterioscler. Thromb. Vasc. Biol. 2000;20:1347–1353. doi: 10.1161/01.ATV.20.5.1347. [DOI] [PubMed] [Google Scholar]
- 93.Zahger D., Fishbein M.C., Garfinkel L.I., Shah P.K., Forrester J.S., Regnstrom J., Yano J., Cercek B. VCL, an antagonist of the platelet GP1b receptor, markedly inhibits platelet adhesion and intimal thickening after balloon injury in the rat. Circulation. 1995;92:1269–1273. doi: 10.1161/01.CIR.92.5.1269. [DOI] [PubMed] [Google Scholar]
- 94.Andrews R.K., Gardiner E.E., Shen Y., Berndt M.C. Structure-activity relationships of snake toxins targeting platelet receptors, glycoprotein Ib-IX-V and glycoprotein VI. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2003;1:143–149. doi: 10.2174/1568016033477559. [DOI] [PubMed] [Google Scholar]
- 95.Clemetson K.J. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon. 2010;56:1236–1246. doi: 10.1016/j.toxicon.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Li B.X., Dai X., Xu X.R., Adili R., Neves M.A.D., Lei X., Shen C., Zhu G., Wang Y., Zhou H., et al. In vitro assessment and phase I randomized clinical trial of anfibatide a snake venom derived anti-thrombotic agent targeting human platelet GPIbα. Sci. Rep. 2021;11:11663. doi: 10.1038/s41598-021-91165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lei X., Reheman A., Hou Y., Zhou H., Wang Y., Marshall A.H., Liang C., Dai X., Li B.X., Vanhoorelbeke K., et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb. Haemost. 2014;111:279–289. doi: 10.1160/TH13-06-0490. [DOI] [PubMed] [Google Scholar]
- 98.Gao Y., Ge H., Chen H., Li H., Liu Y., Chen L., Li X., Liu J., Niu L., Teng M. Crystal structure of agkisacucetin, a Gpib-binding snake C-type lectin that inhibits platelet adhesion and aggregation. Proteins. 2012;80:1707–1711. doi: 10.1002/prot.24060. [DOI] [PubMed] [Google Scholar]
- 99.Cheng X., Xu Z.Y., Liu Q.D., Li X.M., Li X.Y., Liu J. Purification and Characterization of a Platelet Agglutinating Inhibiting Protein (Agkisacutacin) from Agkistrodon acutus Venom. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao. 2000;32:653–656. [PubMed] [Google Scholar]
- 100.Gong P., Li R., Jia H.Y., Ma Z., Li X.Y., Dai X.R., Luo S.Y. Anfibatide Preserves Blood-Brain Barrier Integrity by Inhibiting TLR4/RhoA/ROCK Pathway After Cerebral Ischemia/Reperfusion Injury in Rat. J. Mol. Neurosci. 2020;70:71–83. doi: 10.1007/s12031-019-01402-z. [DOI] [PubMed] [Google Scholar]
- 101.Li T.T., Fan M.L., Hou S.X., Li X.Y., Barry D.M., Jin H., Luo S.Y., Kong F., Lau L.F., Dai X.R., et al. A novel snake venom-derived GPIb antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. Br. J. Pharmacol. 2015;172:3904–3916. doi: 10.1111/bph.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo S.Y., Li R., Le Z.Y., Li Q.L., Chen Z.W. Anfibatide protects against rat cerebral ischemia/reperfusion injury via TLR4/JNK/caspase-3 pathway. Eur. J. Pharmacol. 2017;807:127–137. doi: 10.1016/j.ejphar.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Hou Y., Lei X., Li B.X., Dai X., Yang Z., Qian F., Zhang G., Xu Z., Liu J., Liang C., et al. The First In Vitro and In Vivo Assessment of Anfibatide, a Novel Glycoprotein Ib Antagonist, in Mice and in a Phase I Human Clinical Trial. Blood. 2013;122:577. doi: 10.1182/blood.V122.21.577.577. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.