Abstract
Azoles are the main antifungal agents employed in clinical practice to treat invasive candidiasis. Nonetheless, their efficacy is limited by fungal resistance mechanisms, mainly the overexpression of efflux pumps. Consequently, candidiasis has a worrisome death rate of 75%. One potential strategy to overcome efflux-mediated resistance is to inhibit this process. Ailanthus altissima is a Chinese tree that produces several active substances, including altissimacoumarin D. Due to the low yield of its extraction and the need to search for new drugs to treat candidiasis, this study aimed to synthesize altissimacoumarin D and its analogues, as well as evaluating their ability to reverse the resistance phenotype of Candida albicans. Coumarin isofraxidin was prepared via total synthesis through a solvent-free Knoevenagel condensation as the key step. Isofraxidin and other commercially available coumarins were alkylated with prenyl or geranyl groups to yield the natural product altissimacoumarin D and seven analogues. The antifungal activity of the coumarins and their ability to reverse the fungal resistance phenotype were assessed using microbroth methodologies. Toxicity was evaluated using erythrocytes and an in silico prediction. All compounds improved the antifungal activity of fluconazole by inhibiting efflux pumps, and ACS47 and ACS50 were the most active. None of the coumarins were toxic to erythrocytes. In silico predictions indicate that ACS47 and ACS50 may be safe for human use. ACS47 and ACS50 are promising candidates when used as adjuvants in the antifungal therapy against C. albicans-resistant strains.
Keywords: altissimacoumarin D, Candida spp., coumarins, efflux transporters, isofraxidin, multidrug resistance
1. Introduction
Fungi belonging to the Candida genus can be found within the human microbiome, at sites such as skin and urinary tract [1]. Nonetheless, these microorganisms may invade inner organs and cause life-threatening infections, mainly in immunocompromised patients. In this context, Candida spp. is the most significant fungal pathogen in humans, and its infection has mortality rates of 46–75% [2].
Currently, treating invasive fungal infections relies on a limited number of antifungal agents—azoles, polyenes and echinocandins—and the increasing incidence of resistance to them jeopardizes the success of therapy [3]. Azole drugs, such as fluconazole, itraconazole and ketoconazole, are first-line treatments and are subject to several mechanisms of resistance by Candida spp. [4].
The most important treatment involves the overexpression of efflux pumps within the plasma membrane [5]. These transporters may belong to the ATP-Binding Cassette (ABC) superfamily, which uses ATP hydrolysis as energy source to extrude drugs from the cytoplasm to the extracellular milieu [6], or to the Major Facilitator Superfamily (MFS), which uses proton gradient to transport the drugs [7] and confers a multidrug resistance (MDR) phenotype. Overcoming efflux-pump-mediated resistance is thereby an important strategy for combating fungal resistance to current drugs [8].
Ailanthus altissima is a Chinese native tree with a history of use in traditional herbal medicines for a variety of ailments that range from vaginal infections to epilepsy. Several compounds can be extracted and isolated from the plant, including approximately one hundred coumarins. These substances mostly show antitumor and deacetylating activity. Nonetheless, they are not the most studied group of compounds found in A. altissima and require further investigation [9]. Coumarins, and especially prenylated coumarins, have already been studied and have antibacterial [10,11], antifungal [12], antileishmanial [13], anticancer [14], anti-inflammatory and antiviral [15] activities.
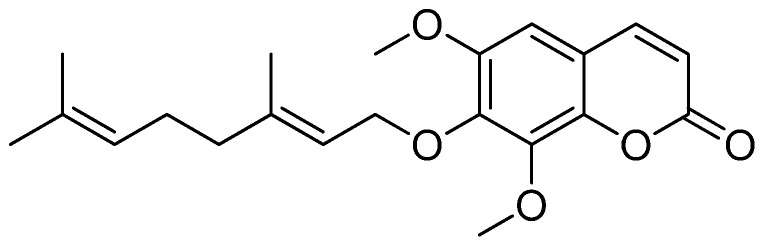
In 2012, a group of coumarins was isolated for the first time from A. altissima, and their deacetylating activity was demonstrated. However, no other studies have been performed since then, which was probably a consequence of the difficulty of obtaining such compounds, considering that 3 kg of the tree bark provides 6.5 to 22 mg of the coumarins [16]. In a previous study by our group, we were able to perform the first total synthesis of altissimacoumarin D (Figure 1), the most active compound isolated from A. altissima by Dao et al. [17].
Figure 1.
Structure of altissimacoumarin D (ACS51).
In this study, we aimed to improve the total synthesis of altissimacoumarin D by decreasing the number of steps and toxic reagents used, avoiding high temperatures (e.g., ethylene glycol reflux) and the use of anhydrous solvents. Analogues were also synthesized, and we demonstrated that these compounds have the ability to reverse the MDR phenotype in Candida albicans.
2. Experimental Section
2.1. General Experiment Procedure
All reagents were obtained commercially (Sigma-Aldrich Co., St. Louis, MO, USA) and used without further purification, except fluconazole which was obtained commercially from the university pharmacy (UFJF, Juiz de Fora—MG, Brazil). Analytical thin-layer chromatography was carried out on 0.25 mm silica gel 60 F254 plates, and compounds were visualized using UV irradiation or staining with 2,4-dinitrophenylhydrazine. Nuclear magnetic resonance (NMR) spectra were obtained on a Bruker Avance-III spectrometer (Bruker Corporation, Billerica, MA, USA) (Figures S1–S16). The splitting patterns are reported as s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Fluconazole stock solutions were prepared in distilled water, sterilized by filtration (0.22 μm), and maintained at −20 °C.
2.2. Synthetic Procedures
2.2.1. 3,5-Dibromo-2,4-dihydroxybenzaldehyde
This compound was prepared as previously described [17]. ¹H NMR (400 MHz, DMSO-d6) δ 7.96 (s, 1H), 9.78 (s, 1H); ¹³C NMR (100 MHz, DMSO-d6) δ 100.1, 101.9, 116.3, 135.7, 158.1, 158.7, 193.5.
2.2.2. 2,4-Dihydroxy-3,5-dimethoxybenzaldehyde
This compound was prepared as previously described [17]. ¹H NMR (400 MHz, CDCl3) δ 3.98 (s, 6H), 7.28 (s, 1H), 8.0 (s, 1H); ¹³C NMR (100 MHz, CDCl3) δ 56.6, 60.9, 109.0, 112.9, 134.6, 141.3, 147.1, 151.7, 194.6.
2.2.3. 7-Hydroxy-6,8-dimethoxy-2H-chromen-2-one (isofraxidin)
2,4-dihydroxy-3,5-dimethoxybenzaldehyde (1 eq), Meldrum’s acid (1.2 eq) and ammonium carbonate (0.2 eq) were placed in a sealed reaction tube and stirred at 75 °C for 2 h. The reaction was monitored using thin-layer chromatography with a solution of iron (III) chloride to determine the consumption of the starting aldehyde. Then, the temperature was increased to 140 °C for decarboxylation. This step was also monitored using thin-layer chromatography. After completion, a small amount of acetone was added to solubilize the reaction mixture, which was then transferred into a saturated solution of sodium bicarbonate. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent evaporated was in vacuo [18]. ¹H NMR (400 MHz, CDCl3) δ 3.86 (s, 3H), 4.01 (s, 3H), 6.21 (d, J 9.0 Hz, 1H), 6.59 (s, 1H), 7.54 (d, J 9.0 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 56.2, 60.8, 105.9, 109.5, 112.8, 134.2, 144.5, 145.9, 146.9, 149.6, 164.2. Yield: 60%.
2.2.4. €-7-((3,7-Dimethylocta-2,6-dien-1-yl)oxy)-6,8-dimethoxy-2H-chromen-2-one (ACS51)
K2CO3 (1.2 eq) and geranyl bromide (1.0 eq) were added to a solution of isofraxidin (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.59 (s, 3H), 1.66 (s, 3H), 1.69 (s, 3H), 2.05 (m, 4H), 3.89 (s, 3H), 4.03 (s, 3H), 4.68 (d, J 7 Hz, 2H), 5.06 (t, J 8 Hz, 1H), 5.55 (t, J 8 Hz, 1H), 6.35 (d, J 8 Hz, 1H), 6.66 (s, 1H), 7.60 (d, J 12 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 16.4, 17.7, 25.7, 26.4, 39.6, 56.3, 61.7, 70.3, 103.5, 114.4, 115.1, 119.6, 123.8, 131.7, 141.8, 142.5, 143.0, 143.5, 144.9, 150.7, 160.6. Yield: 70%.
2.2.5. 6,8-Dimethoxy-7-((3-methylbut-2-en-1-yl)oxy)-2H-chromen-2-one (ACS50)
K2CO3 (1.2 eq) and prenyl bromide (1.0 eq) were added to a solution of isofraxidin (1.0 eq) in acetone (5 mL) and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.71 (s, 3H), 1.77 (s, 3H), 3,89 (s, 3H), 4.03 (s, 3H), 4.65 (d, J 8 Hz, 2H), 5.56 (t, J 8 Hz, 1H), 6.35 (d, J 8 Hz, 1H), 6.67 (s, 1H), 7.63 (d, J 8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 18.2, 26.0, 56.5, 62.0, 70.5, 103.8, 114.6, 115.3, 120.1, 139.5, 142.1, 143.2, 143.7, 150.9, 160.8. Yield: 77%.
2.2.6. (E)-7-((3,7-Dimethylocta-2,6-dien-1-yl)oxy)-2H-chromen-2-one (ACS48)
K2CO3 (1.2 eq) and geranyl bromide (1.0 eq) were added to a solution of umbelliferone (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.62 (s, 3H), 1.68 (s, 3H), 1.77 (s, 3H), 2.11 (m, 4H), 4.61 (d, J 6 Hz, 2H), 5.01 (m, 1H), 5.48 (m, 1H), 6.26 (d, J 8 Hz, 1H), 6.85 (m, 2H), 7.36 (d, J 8 Hz, 1H), 7.65 (d, J 9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 17.0, 17.9, 25.9, 26.5, 39.7, 65.7, 101.8, 112.6, 113.2, 113.5, 118.6, 123.8, 128.9, 132.2, 142.6, 143.7, 156.1, 161.5, 162.4. Yield: 82%.
2.2.7. 7-((3-Methylbut-2-en-1-yl)oxy)-2H-chromen-2-one (ACS47)
K2CO3 (1.2 eq) and prenyl bromide (1.0 eq) were added to a solution of umbelliferone (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.77 (s, 3H), 1.82 (s, 3H), 4.59 (d, J 7 Hz, 2H), 5.48 (t, J 6 Hz, 1H), 6.25 (d, J 9 Hz, 1H), 6.86 (m, 2H), 7.36 (d, J 8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 18.3, 25.8, 65.4, 101.6, 112.4, 113.0, 113.2, 118.6, 128.7, 139.3, 143.5, 155.9, 161.3, 162.1. Yield: 79%.
2.2.8. (E)-7-((3,7-Dimethylocta-2,6-dien-1-yl)oxy)-4-methyl-2H-chromen-2-one (ACS52)
K2CO3 (1.2 eq) and geranyl bromide (1.0 eq) were added to a solution of 4-methylumbelliferone (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.62 (s, 3H), 1.68 (s, 3H), 1.77 (s, 3H), 2.11 (m, 4H), 2.41 (s, 3H), 4.61 (d, J 6 Hz, 2H), 5.01 (m, 1H), 5.48 (m, 1H), 6.14 (s, 1H), 6.83 (s, 1H), 6.87 (d, J 9 Hz,1H), 7.50 (d, J 9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 16.8, 17.7, 18.7, 25.7, 26.3, 39.5, 65.5, 101.6, 111.9, 112.9, 113.5, 118.5, 123.6, 125.4, 132.0, 142.3, 152.6, 155.3, 161.4, 162.0. Yield: 91%.
2.2.9. 4-Methyl-7-((3-methylbut-2-en-1-yl)oxy)-2H-chromen-2-one (ACS54)
K2CO3 (1.2 eq) and prenyl bromide (1.0 eq) were added to a solution of 4-methylumbelliferone (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.78 (s, 3H), 1.82 (s, 3H), 2.40 (s, 3H), 4.59 (d, J 7 Hz, 2H), 5.48 (t, J 6 Hz, 1H), 6.13 (s, 1H), 6.83 (s, 1H), 6.86 (d, J 7 Hz, 1H), 7.48 (d, J 9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 18.3, 18.7, 25.8, 65.4, 101.6, 111.8, 112.9, 113.5, 118.7, 125.5, 139.2, 152.6, 155.2, 161.4, 161.9. Yield: 86%.
2.2.10. (E)-7-((3,7-Dimethylocta-2,6-dien-1-yl)oxy)-3,4,8-trimethyl-2H-chromen-2-one (ACS55)
K2CO3 (1.2 eq) and prenyl bromide (1.0 eq) were added to a solution of 7-hydroxy-3,4,8-trimethylcoumarin (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.59 (s, 3H), 1.65 (s, 3H), 1.73 (s, 3H), 2.08 (m, 4H), 2.16 (s, 3H), 2.29 (s, 3H), 2.34 (s, 3H), 4.60 (d, J 6 Hz, 2H), 5.07 (m, 1H), 5.46 (m, 1H), 6.79 (d, J 9 Hz,1H), 7.36 (d, J 9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 8.5, 13.4, 15.3, 17.0, 17.9, 25.9, 26.5, 39.7, 65.9, 108.2, 114.3, 114.5, 118.8, 119.7, 122.1, 123.9, 132.1, 141.4, 146.6, 151.4, 158.8, 162.9. Yield: 85%.
2.2.11. 3,4,8-Trimethyl-7-((3-methylbut-2-en-1-yl)oxy)-2H-chromen-2-one (ACS56)
K2CO3 (1.2 eq) and geranyl bromide (1.0 eq) were added to a solution of 7-hydroxy-3,4,8-trimethylcoumarin (1.0 eq) in acetone (5 mL), and the mixture was stirred under reflux until completion. The product was extracted using ethyl acetate (5 × 20 mL). The organic layer was washed with brine, dried with Na2SO4, filtered, and the solvent was evaporated in vacuo. ¹H NMR (400 MHz, CDCl3) δ 1.71 (s, 3H), 1.77 (s, 3H), 2.18 (s, 3H), 2,31 (s, 3H), 2.36 (s, 3H), 4.60 (d, J 7 Hz, 2H), 5.50 (t, J 6 Hz, 1H), 6.83 (d, J 9 Hz, 1H), 7.40 (d, J 9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 8.3, 13.2, 15.1, 18.3, 25.8, 65.6, 108.0, 114.1, 114.3, 118.6, 119.6, 121.9, 138.0, 146.4, 151.2, 158.6, 162.7. Yield: 83%.
2.3. Biological Assays
2.3.1. Strains
In this study, four mutant strains of S. cerevisiae were used. These strains were kindly provided by Dr. Richard Cannon and Dr. Brian Monk (University of Otago, Dunedin, New Zealand). Firstly, a null mutant was constructed by deleting genes related to ABC transporters (Pdr5p, Yor1p, Snq2p, Pdr10p, Pdr11p, and Ycf1p). Then, three fluconazole-resistant strains were created by heterologous expression of transporters originally found in Candida spp., as summarized in Table 1 [19]. Also, two fluconazole-resistant C. albicans clinical isolates were used. While 95-142 overexpresses the ABC transporters, CaCdr1p and CaCdr2p [5], the PRI strain overexpresses the MFS transporter CaMdr1p [20]. The 95-142 strain was kindly provided by Dr. Theodore White (University of Missouri, Kansas City, MO, USA). Additionally, one fluconazole-sensitive C. albicans clinical isolate was used (ATCC 10231; American Type Culture CollectionTM, Manassas, VA, USA). The strains were grown in YPD medium (2% glucose, 1% yeast extract, 2% peptone) at 30 °C (S. cerevisiae) or 37 °C (C. albicans) via agitation and were harvested in the exponential phase of growth whenever experiments were about to be performed.
Table 1.
Strains used in this study.
| Strain | Efflux Transporter | Family | Species |
|---|---|---|---|
| Null mutant | None | - | S. cerevisiae |
| CaCdr1p+ | CaCdr1p | ABC | S. cerevisiae |
| CaCdr2p+ | CaCdr2p | ABC | S. cerevisiae |
| CaMdr1p+ | CaMdr1p | MFS | S. cerevisiae |
| 95-142 | CaCdr1p and CaCdr2p | ABC | C. albicans |
| PRI | CaMdr1p | MFS | C. albicans |
| ATCC 10231 | None | - | C. albicans |
2.3.2. Antifungal Activity of Coumarins against C. albicans and S. cerevisiae Strains
Briefly, 5 × 103 cells/mL of C. albicans strains (95-142, PRI and ATCC 10231) and 2 × 104 cells/mL of the S. cerevisiae null mutant were incubated in RPMI-1640 (Sigma-Aldrich Co., St. Louis, MO, USA) at 37 °C or YPD medium at 30 °C, respectively, for 48 h via agitation (75 rpm) in the presence of 100 μg/mL of the compounds [21]. Cell growth was measured at 600 nm (Fluostar Optima, BMG Labtech, Offenburg, Germany).
2.3.3. Antifungal Activity of Coumarins Combined with Fluconazole against S. cerevisiae Strains
Briefly, 2 × 104 cells/mL of S. cerevisiae mutant strains (CaCdr1p+, CaCdr2p+, and CaMdr1p+) were incubated in YPD medium at 30 °C for 48 h using agitation (75 rpm), in the presence of 100 μg/mL of the coumarins with or without fluconazole at sub-inhibitory concentrations (125 μg/mL, 15.6 μg/mL and 15.6 μg/mL, respectively) [21]. Cell growth was measured at 600 nm (Fluostar Optima, BMG Labtech, Offenburg, Germany).
2.3.4. Synergism Evaluation through Checkerboard Assay
This assay was conducted with the compounds active against S. cerevisiae in the presence of fluconazole. Briefly, 2 × 104 cells/mL of S. cerevisiae mutant strains (CaCdr1p+, CaCdr2p+, and CaMdr1p+) were incubated in YPD medium at 30 °C for 48 h with agitation (75 rpm), in the presence of serial concentrations of the coumarins (100–6.25 μg/mL) and fluconazole (250–3.91 μg/mL). Cell growth was measured at 600 nm (Fluostar Optima, BMG Labtech, Offenburg, Germany). Serial dilutions of the coumarins and fluconazole alone were made to obtain the minimum inhibitory concentration (MIC) of each compound. The interaction between the coumarins and fluconazole was assessed using the fractional inhibitory concentration index (FICI). The FICI is calculated by summing the fractional inhibitory concentration (FIC) of each drug, and the FIC is the ratio of the MIC of a drug combined with a second drug and the MIC of a drug alone. Synergistic, additive, indifferent, and antagonistic interactions correspond to FICI ≤ 0.5, >0.5–1.0, 1.0–4.0, and >4.0, respectively [21].
After evaluating the association between coumarins and fluconazole against S. cerevisiae strains, the combinations that presented either additive or synergistic interactions were tested against the growth of C. albicans. The compounds active against CaCdr1p+ or CaCdr2p+ were tested against the 95-142 strain, while the compounds active against CaMdr1p+ were tested against the PRI strain. Briefly, 5 × 103 cells/mL of C. albicans resistant strains was incubated in RPMI-1640 medium at 37 °C for 48 h with agitation (75 rpm) in the presence of serial concentrations of the coumarins (100–6.25 μg/mL) and fluconazole (1000–15.6 μg/mL). Cell growth was measured at 600 nm (Fluostar Optima, BMG Labtech, Offenburg, Germany), and the FICI values were calculated using the aforementioned method.
2.3.5. Erythrocyte Viability
The effect of the compounds on erythrocyte viability was assessed as described elsewhere [22]. Cells were washed three times and resuspended in phosphate-buffered saline (PBS) to a final of concentration of 2% v/v. Then, cells were incubated in the presence of the compounds at 100 μg/mL for 60 min at 37 °C. Afterward, cells were harvested by centrifugation at 3000× g for 5 min and 1 mL of supernatant was transferred to glass cuvettes. The absorbance of hemoglobin released from erythrocytes was measured at 540 nm (SP220, Biospectro, Curitiba, Brazil). Controls of 100% and 0% hemolysis were performed by incubating the cells in PBS in the presence or absence of 1% Triton X-100, respectively. Also, controls with DMSO and acetonitrile were performed.
2.3.6. In Silico Toxicity Prediction
The toxicity of the coumarins was predicted in silico using OSIRIS Property Explorer [23], which provides information regarding mutagenic, tumorigenic, irritant, and reproductive effects, and GUSAR (General Unrestricted Structure–Activity Relationships), which provides a LD50 (lethal dose to 50% of a given population) of the substances in rats, considering four routes of administration (intraperitoneal, intravenous, oral, and subcutaneous) [24].
2.3.7. Statistical Analysis
All the experiments were performed at least three times, and the results were expressed as mean ± standard deviation. Data were analyzed using Student’s t test, and p values lower than 0.05 were considered significant.
3. Results
3.1. Synthesis
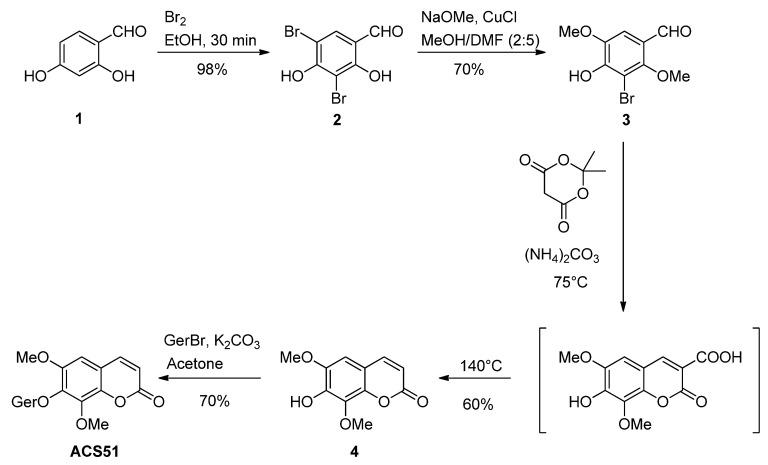
The synthesis of altissimacoumarin D is summarized in Figure 2. This coumarin was first isolated by Dao et al. in 2012 [16], and the first total synthesis performed by our group [17] involved the alkylation of isofraxidin, another natural occurring coumarin. In this study, our goal was to improve that first synthesis via the most crucial step, the formation of the coumarin ring. The first two steps of the synthesis, which lead to the formation of the methoxylated intermediate, were kept the same, since the yields were already improved, and the purification process was simple.
Figure 2.
Total synthesis of altissimacoumarin D in four steps.
Attempts were made to form the coumarin ring in a way that would be more efficient, greener and easier to purify. Then, the reaction was performed as described by Schijndel et al. [18], with slight modifications. Instead of forming the coumarin ring using malonic acid, 2-hydroxybenzadehyde and ammonium bicarbonate, we used ammonium carbonate, 2,4-dihydroxy-3,5-dimethoxybenzaldehyde and Meldrum’s acid with no solvent in this study. Isofraxidin was obtained with a yield of 60%.
After obtaining isofraxidin, the next step was to obtain altissimacoumarin D via an alkylation. In our previous study, this step was performed via a Mitsunobu reaction that, although efficient, required the use of a dry solvent, a 24 h reaction, and made the purification of the product challenging. Thus, in this study, we performed the alkylation via a simpler methodology using acetone, potassium carbonate and geranyl bromide; altissimacoumarin D was obtained with a yield of 70%.
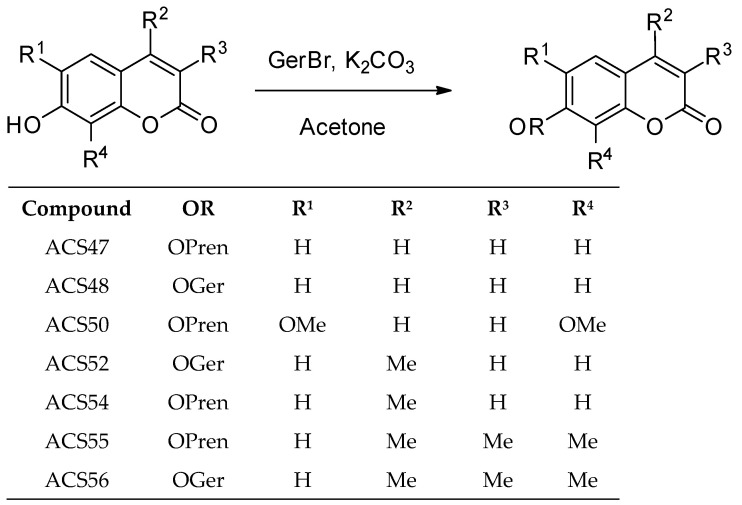
Since only one biological test was performed with altissimacoumarin D [16], and the compound was shown to be promising due to its prenylated coumarin nature, seven other coumarins with different substitution patterns were synthesized, varying the alkyl chain between geranyl and prenyl. Figure 3 shows the structures of the analogues of altissimacoumarin D that were synthesized in this study.
Figure 3.
Analogues of altissimacoumarin D obtained through the alkylation of commercially available compounds. OMe—methoxy group; Me—methyl group; OGer—geranyl group; OPren—prenyl group.
3.2. Antifungal Activity of Coumarins against C. albicans and S. cerevisiae Strains
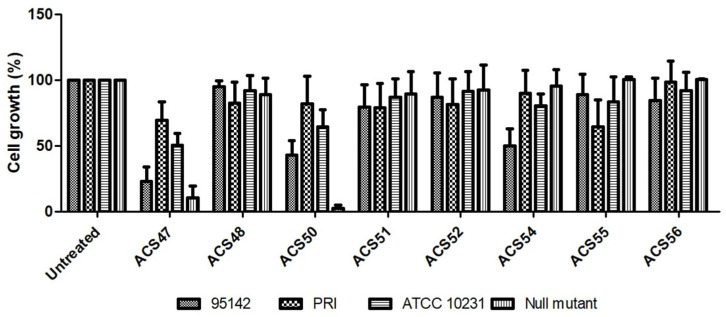
Altissimacoumarin D and the coumarin analogues were screened for antifungal activity against C. albicans and S. cerevisiae strains (Figure 4).
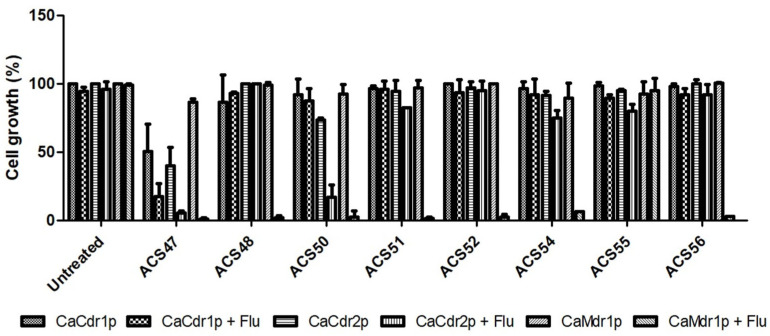
Figure 4.
Antifungal activity screening against C. albicans and S. cerevisiae strains. Cells (95-142, PRI, ATCC 10231 and Null mutant) were incubated with 100 μg/mL of coumarins for 48 h. Cell growth was measured at 600 nm. Data are expressed as mean ± standard deviation of three independent experiments.
While the natural product was inactive at the tested concentration, ACS47, ACS50, and ACS54 at 100 μg/mL presented antifungal activity. ACS47 inhibited the growth of 95-142, PRI, ATCC 10231, and Null mutant strains by 77.2%, 30.4%, 49.5%, and 89.8%, respectively. ACS50 inhibited the growth of 95-142, ATCC 10231 and Null mutant strains by 57%, 35.5%, and 97.6%, respectively. ACS54 inhibited the growth of the 95-142 strain by 50.2%.
3.3. Antifungal Activity of Coumarins Combined with Fluconazole against S. cerevisiae Strains
To assess if the compounds inhibit C. albicans efflux pumps, their antifungal activity alone and combined with fluconazole were evaluated against S. cerevisiae mutant strains that overexpress these transporters. Compounds ACS48, ACS51, ACS52, ACS54, ACS55 and ACS56 did not exhibit antifungal activity alone. On the other hand, ACS47 inhibited the growth of CaCdr1p+ and CaCdr2p+ by 49.6% and 50.3%, respectively, and ACS50 inhibited the growth of CaCdr2p+ by 26.5%. However, in combination with fluconazole, all compounds, except for ACS55, inhibited the growth of CaMdr1p+ by 93.6–99.1%. Moreover, the combination of ACS47 with fluconazole inhibited the growth of CaCdr1p+ and CaCdr2p+ by 82.5% and 94.7%, respectively, while the combination of ACS50 and fluconazole inhibited the growth of CaCdr2p+ by 83.2% (Figure 5).
Figure 5.
Antifungal activity of coumarins combined with fluconazole against S. cerevisiae strains. Cells (CaCdr1p+, CaCdr2p+, and CaMdr1p+) were incubated with 100 μg/mL of coumarins and sub-inhibitory concentrations of fluconazole for 48 h. Cell growth was measured at 600 nm. Data are expressed as the mean ± standard deviation of three independent experiments.
3.4. Synergism Evaluation through Checkerboard Assay
Since the coumarins presented antifungal activity when combined with fluconazole, a checkerboard assay was performed to determine the type of interaction between the compounds. Firstly, the S. cerevisiae mutant strains were used. As shown in Table 2, ACS47 at 50 μg/mL was synergistic with fluconazole against CaCdr1p+ and CaCdr2p+, reducing the fluconazole MIC by 4–8 fold. The interaction between ACS50 (at 25 μg/mL) and fluconazole was classified as additive, and the coumarin halved fluconazole MIC against CaCdr2p+. All the coumarins were able to diminish fluconazole MIC against CaMdr1p+. Compounds ACS47–54 displayed synergistic activity with fluconazole, with FICI values ranging from 0.188 to 0.500. ACS55 and ACS56 presented an additive interaction with fluconazole.
Table 2.
Checkerboard assays of coumarins against S. cerevisiae mutant strains.
| Strain and Compound | Compound (µg/mL) | Fluconazole (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| MICa | MICb | FIC | MICa | MICb | FIC | FICI | Outcome | |
| CaCdr1p+ | ||||||||
| ACS47 | >100 | 50 | 0.250 | >250 | 62.5 | 0.125 | 0.375 | S |
| CaCdr2p+ | ||||||||
| ACS47 | >100 | 50 | 0.250 | 62.5 | 15.6 | 0.250 | 0.500 | S |
| ACS50 | >100 | 25 | 0.125 | 62.5 | 31.3 | 0.500 | 0.625 | A |
| CaMdr1p+ | ||||||||
| ACS47 | >100 | 25 | 0.125 | 62.5 | 3.91 | 0.063 | 0.188 | S |
| ACS48 | >100 | 50 | 0.250 | 62.5 | 7.81 | 0.125 | 0.375 | S |
| ACS50 | >100 | 25 | 0.125 | 62.5 | 15.6 | 0.250 | 0.375 | S |
| ACS51 | >100 | 50 | 0.250 | 62.5 | 15.6 | 0.250 | 0.500 | S |
| ACS52 | >100 | 12.5 | 0.063 | 62.5 | 15.6 | 0.250 | 0.313 | S |
| ACS54 | >100 | 12.5 | 0.063 | 62.5 | 7.81 | 0.125 | 0.188 | S |
| ACS55 | >100 | 100 | 0.500 | 62.5 | 31.3 | 0.500 | 1.000 | A |
| ACS56 | >100 | 100 | 0.500 | 62.5 | 15.6 | 0.250 | 0.750 | A |
MICa, MIC of compound alone; MICb, MIC of combined compound; FIC, fractional inhibitory concentration; FICI, fractional inhibitory concentration index; S, synergism; A, additive.
Due to these positive results, the checkerboard assay was also performed using C. albicans strains. All the compounds were tested against PRI because this strain overexpresses CaMdr1p. Furthermore, ACS47 and ACS50 were tested against 95-142, a C. albicans strain that overexpresses both CaCdr1p and CaCdr2p. At 50 μg/mL, ACS 47 and ACS50 reduced fluconazole MIC against 95-142 by 4–8 fold, with FICI values of 0.375 and 0.500, respectively. Against the PRI strain, only ACS47 and ACS50 showed a combined activity with fluconazole. Both compounds reduced the fluconazole MIC from 1000 μg/mL to 15.6 μg/mL (Table 3).
Table 3.
Checkerboard assays of coumarins against fluconazole-resistant clinical strains of Candida albicans.
| Strain and Compound | Compound (µg/mL) | Fluconazole (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| MICa | MICb | FIC | MICa | MICb | FIC | FICI | Outcome | |
| 95-142 | ||||||||
| ACS47 | >100 | 50 | 0.250 | 500 | 62.5 | 0.125 | 0.375 | S |
| ACS50 | >100 | 50 | 0.250 | 500 | 125 | 0.250 | 0.500 | S |
| PRI | ||||||||
| ACS47 | >100 | 100 | 0.500 | 1000 | 15.6 | 0.016 | 0.516 | A |
| ACS48 | >100 | >100 | 1 | 1000 | 1000 | 1 | 2 | I |
| ACS50 | >100 | 100 | 0.500 | 1000 | 15.6 | 0.016 | 0.516 | A |
| ACS51 | >100 | >100 | 1 | 1000 | 1000 | 1 | 2 | I |
| ACS52 | >100 | >100 | 1 | 1000 | 1000 | 1 | 2 | I |
| ACS54 | >100 | >100 | 1 | 1000 | 1000 | 1 | 2 | I |
| ACS55 | >100 | >100 | 1 | 1000 | 1000 | 1 | 2 | I |
| ACS56 | >100 | >100 | 1 | 1000 | 1000 | 1 | 2 | I |
MICa, MIC of compound alone; MICb, MIC of combined compound; FIC, fractional inhibitory concentration; FICI, fractional inhibitory concentration index; I, indifferent; A, additive.
3.5. Erythrocyte Viability
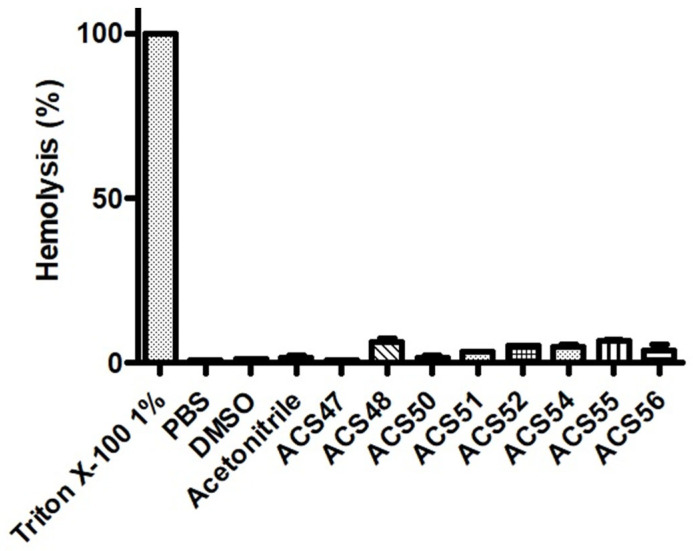
At 100 μg/mL, the compounds exerted 0.8–6.6% of hemolysis. The lowest hemolytic compounds were ACS47 and ACS50, which presented results comparable to PBS and DMSO controls (Figure 6).
Figure 6.
Hemolysis assay. Erythrocytes were incubated in the presence of coumarins, and the absorbance of released hemoglobin was measured at 540 nm. Triton X-100 and PBS were used as controls of 100% and 0% hemolysis, respectively. DMSO and acetonitrile were also used as controls. Data are expressed as mean ± standard deviation of three independent experiments. PBS: phosphate-buffered saline. DMSO: dimethyl sulfoxide.
3.6. In Silico Toxicity Prediction
The toxicity of the prenylated coumarins was predicted in silico using two free software programs. According to OSIRIS Property Explorer, ACS47 and ACS50 were predicted not to be mutagenic, tumorigenic and irritant; they do not have any reproductive effects on humans, a toxicity profile similar to fluconazole. ACS48 and ACS51 may be irritants, while ACS54 may induce reproductive effects. Also, ACS52, ACS55, and ACS56 may be irritant and exert reproductive effects. None of the compounds are predicted to be mutagenic or tumorigenic (Table 4).
Table 4.
Toxicity risk prediction by Osiris Property Explorer.
| Compound | Mutagenic | Tumorigenic | Irritant | Reproductive Effect |
|---|---|---|---|---|
| ACS47 | No | No | No | No |
| ACS48 | No | No | Yes | No |
| ACS50 | No | No | No | No |
| ACS51 | No | No | Yes | No |
| ACS52 | No | No | Yes | Yes |
| ACS54 | No | No | No | Yes |
| ACS55 | No | No | Yes | Yes |
| ACS56 | No | No | Yes | Yes |
Data obtained by the GUSAR software is summarized in Table 5. Coumarins intraperitoneal, intravenous, oral, and subcutaneous LD50 ranged from 333.1 to 581 mg/kg, 53.41 to 121.6 mg/kg, 1057 to 2690 mg/kg, and 845.7 to 2462 mg/kg, respectively. Fluconazole LD50 ranged from 200.4 mg/kg to 708 mg/kg.
Table 5.
Rat acute toxicity prediction by GUSAR (mg/kg).
| Compound | Rat IP LD50 | Rat IV LD50 | Rat Oral LD50 | Rat SC LD50 |
|---|---|---|---|---|
| ACS47 | 476.4 | 65.99 | 1551 | 2462 |
| ACS48 | 456.5 | 90.71 | 2157 | 1234 |
| ACS50 | 426.8 | 93.38 | 1057 | 1461 |
| ACS51 | 525 | 121.6 | 1401 | 845.7 |
| ACS52 | 401.5 | 83.72 | 1979 | 918.6 |
| ACS54 | 581.3 | 66.26 | 1470 | 2452 |
| ACS55 | 333.1 | 53.41 | 2445 | 1116 |
| ACS56 | 351.9 | 74.89 | 2690 | 948.3 |
| Fluconazole | 708 | 200.4 | 584.4 | 511.3 |
4. Discussion
4.1. Synthesis of Prenylated Coumarins
Natural products have been extensively explored as potential sources of new drugs, due to their wide variety of pharmacological activities. The Chinese tree A. altissima, for example, possesses compounds with anti-inflammatory [25], anticancer [26], and neuroprotective [27] properties. Also, Li et al. (2021) isolated an alkaloid with antifungal activity against Fusarium oxysporum [28], and Dao et al. (2012) purified terpenylated coumarins with potential anticancer activity from A. altissima [16]. Coumarins are considered privileged scaffolds for the synthesis of potential drugs with higher yields when compared to isolation from natural sources [29]. The presence of terpenylated coumarins in A. altissima, the feasibility of their synthesis, their potential pharmacological activity, and the need to discover substances that reverse antifungal resistance in Candida spp. encouraged the synthesis and evaluation of antifungal activity of altissimacoumarin D, the main compound of the class found in A. altissima and its analogues. In the present study, isofraxidin was efficiently prepared in a one-pot solvent-free reaction. Also, the Mitsunobu reaction, which was a different approach from the previous synthesis of altissimacoumarin D that we reported, was not used in this study [17]. These modifications led to a lower use of organic solvent to obtain the desired coumarins, without worsening the yields of the reactions.
4.2. Antifungal Activity of Coumarins against C. albicans and S. cerevisiae Strains
After preparing the coumarins, their ability to inhibit the growth of fluconazole-sensitive and fluconazole-resistant strains was evaluated. Only three compounds (ACS47, ACS50, and ACS54) presented antifungal activity, and all of them are substituted with a prenyl group. Their geranyl-substituted counterparts (ACS48, ACS51, and ACS52, respectively) did not display antifungal activity. ACS47 was active against the four strains tested, while ACS50 and ACS54 inhibited the growth of three strains and one strain, respectively. It may be observed that the insertion of methyl groups into the benzopyran moiety decreased the antifungal activity of the compounds, which may explain why ACS55 was not active against the yeasts, since it bears three methyl groups. ACS47 and ACS50 differ due to the presence of two methoxy substitutions in the latter. Results show that this substitution pattern does not influence the antifungal activity of the compound. Kurdelas et al. (2010) evaluated the antifungal activity of three prenylated coumarins with geranyl groups (auraptene, 5′-hydroxy auraptene, and 5′-oxo-auraptene) against yeasts and filamentous fungi and observed that none of the substances inhibited the growth of C. albicans, C. tropicalis, and S. cerevisiae [30], such as in this study. Jia et al. (2019) observed that coumarin (1,2-benzopyrone) exerts anti-C. albicans activity at 0.5 mg/mL–2 mg/mL by inducing apoptosis [31]. In this study, coumarins inhibited C. albicans and S. cerevisiae growth at 0.1 mg/mL. This action may be related to apoptosis induction, as seen in the unsubstituted coumarin, and the prenyl substitution could ease the interaction between the coumarins and plasma membrane, explaining the antifungal activity observed at a lower concentration [32].
4.3. Antifungal Activity of Coumarins Combined with Fluconazole against S. cerevisiae Strains
To evaluate whether the compounds inhibit C. albicans efflux pumps, hence precluding multidrug resistance and sensitizing the fungi to fluconazole, a screening assay using S. cerevisiae mutant strains was performed. Each strain heterologously expresses a specific MDR transporter, making it possible to comprehend exactly which efflux pumps the coumarins may affect. All coumarins, except for ACS55, improved the fluconazole antifungal activity against the S. cerevisiae strain that overexpresses CaMdr1p, an MFS transporter. This finding indicates that the compounds are able to inhibit this specific efflux pump, regardless of whether the coumarin possesses a prenyl or a geranyl substitution. ACS55, the only coumarin that did not affect CaMdr1p in this assay, bears three methyl groups that may impair its binding to the transporter. Moreover, data show that ACS47 and ACS50 also inhibit ABC transporters. These two coumarins carry a prenyl-substitution and are unsubstituted in the lactone ring. The presence of one methyl group is the only difference between ACS47, which inhibits CaCdr1p and CaCdr2p, as well as ACS54, which did not affect ABC transporters. In a previous study, it was observed that beta-lapachone impaired CgCdr2p ATPase activity, while beta-nor-lapachone did not have an inhibitory activity, and the difference between these two naphthoquinones is a methylene group [21]. Esposito et al. (2017) also observed that minor modifications in the structures of jatrophanes modified their inhibitory activity against CaCdr1p and CaMdr1p [33]. These data suggest that small increases in substitution may impact the inhibition of Candida spp. efflux transporters, which is important to optimize the design of new MDR inhibitors. The results of the screening assay were confirmed using a checkerboard. Eight out of the eleven tested combinations displayed synergism, and compounds ACS47 and ACS54 were the most active substances. Also, when comparing FICI values, it was not possible to determine a link between the presence of prenyl or geranyl substitutions and the inhibitory activity of the coumarins on the MFS transporter. To our knowledge, there are no reports on the ability of prenylated coumarins to inhibit Candida efflux pumps. However, the effects of geraniol have already been described. Singh et al. (2018) assessed the influence of the monoterpene on CaCdr1p and CaMdr1p, and observed that it affects only the ABC transporter. In silico analysis revealed that geraniol binds to CaCdr1p via hydrophobic interactions and H-bonds, while the control compound farnesol binds to the efflux pump only via hydrophobic interactions. Also, geraniol presented a lower binding energy than farnesol, indicating a higher affinity to the active site of CaCdr1p [34]. ACS47 and ACS50 are prenyl-substituted, and it may be hypothesized that the reduced size of the prenyl side chain, in comparison to a geranyl or a farnesyl chain, would increase the affinity of the coumarins to CaCdr1p, explaining the ability of these specific two compounds to inhibit ABC transporters. Aside from geraniol, the inhibitory activity of coumarin scopoletin on efflux pumps was also evaluated [35]. This substance is an unprenylated coumarin, presenting one hydroxyl and one methoxy substitution. Scopoletin exerted an antifungal effect on C. tropicalis, and at a sub-inhibitory concentration, promoted a four-fold decrease in fluconazole MIC, an effect related to the inhibition of efflux pumps. Although geraniol and scopoletin separately modulate Candida spp. efflux pumps, the geranylated coumarins tested in this study were unable to inhibit ABC transporters. One of the substances tested, ACS48 (also known as auraptene), induced the expression of P-glycoprotein in human intestinal cells [36]. Since this efflux pump is analogous to CaCdr1p and CaCdr2p, ACS48 and the other coumarins could induce the expression of CDR1 and CDR2 instead of inhibiting the transporters, which may explain the lack of activity in these substances combined with fluconazole against S. cerevisiae mutant strains that overexpress ABC transporters.
4.4. Antifungal Activity of Coumarins Combined with Fluconazole against Strains of C. albicans
The S. cerevisiae mutant strains used in this study are important tools to assess the action of substances in individual efflux pumps. Nonetheless, to endorse a possible application of these compounds combined with fluconazole, it is essential to test them against pathogenic strains. Thus, fluconazole-resistant C. albicans clinical strains were also employed in this study. Data show that none of the compounds were synergistic with fluconazole against PRI strain. It is important to consider that FICI is a parameter that may not be strictly followed. In this study, FICI of 0.516 were obtained using ACS47 and ACS50 combined with fluconazole and, according to these results, the interactions are additive. However, the fact that no synergism was observed does not disqualify these substances as promising candidates to be used in clinics. Both coumarins reduced fluconazole MIC by 64 times, reinforcing their ability to inhibit efflux pumps. Although the other six coumarins can also inhibit CaMdr1p, indifferent interactions were detected against PRI strain. These compounds present geranyl groups and/or substitutions in the lactone ring, increasing their hydrophobicity. Since the plasma membranes from S. cerevisiae and C. albicans differ in composition, the diffusion of more hydrophobic substances across the membrane may be hindered in C. albicans [37]. Therefore, the impossibility of concentrating in the cytoplasm may justify the lack of activity of some compounds, even when they are able to inhibit efflux pumps. This could explain why ACS47 and ACS50, two prenyl-substituted coumarins, performed better than the other substances. ACS47 and ACS50 also improved the antifungal activity of fluconazole against 95-142. This C. albicans strain overexpresses two ABC transporters, CaCdr1p and CaCdr2p, and ACS47 is able to block both of them. Interestingly, ACS50 inhibits only CaCdr2p, but synergized with fluconazole against 95-142. This could be explained by a possible lower expression of CDR1 in comparison to CDR2. In this scenario, the rate of fluconazole extrusion by CaCdr1p would not be sufficient, and the inhibition of CaCdr2p would promote fluconazole intracellular accumulation, allowing the antifungal agent to exert its activity. Moraes et al. (2020) observed that the naphthoquinone beta-lapachone exhibits the same inhibitory pattern of ACS50, i.e., the compound inhibits only CaCdr2p but displays synergism with fluconazole against the 95-142 strain [38].
4.5. Toxicity
Aside from being effective in treating candidiasis, alone and/or combined with fluconazole, the prenylated coumarins tested in this study must be safe for human use to be good candidates as antifungal agents. In this study, in vitro and in silico assays were conducted to verify the toxicity of the coumarins. The substances had a low hemolytic effect, which is essential to ensure the safety of a substance, since drugs reach the bloodstream after administration to the infection site. The lack of in vitro toxicity corroborates the findings of Maleki et al. (2019), who assessed the in vitro toxicity of O-prenylated coumarins against an HDF cell line and observed that ACS47, ACS48 and umbelliprenin did not affect its viability [39].
Although the absence of hemolysis is an important feature of any drug, it may be considered an initial test to assess toxicity [40]. Other cells constitute the human body, and a non-hemolytic drug may not be exempt from toxic effects. To better understand the toxicity profile of the coumarins, an in silico analysis was performed. None of the compounds showed mutagenic or tumorigenic effects. However, eight compounds were predicted to be irritant, and four compounds may have reproductive effects. Comparing the compounds, it is possible to observe that the presence of three methyl groups in the coumarin moiety induces irritant effects, regardless of the presence of a prenyl or a geranyl substitution. Among the coumarins with less substitutions, the prenylated coumarins do not have irritant effects, while their geranylated counterparts are toxic. On the other hand, all the compounds that bear a methyl group in the lactone ring cause reproductive effects, aside from the presence of a prenyl or geranyl substitution. The toxicity profiles predicted for ACS47 and ACS50 are similar to that observed for fluconazole, reinforcing a possible role of these two coumarins as new anti-Candida agents. The in silico analysis also provided LD50 for different routes of administration. The intraperitoneal and intravenous LD50 values for all coumarins were lower than those obtained for fluconazole. Contrarily, the coumarins presented higher oral and subcutaneous LD50 values than fluconazole. These results corroborate the lack of toxicity and encourage future studies with animals to test the in vivo activity of the coumarins against C. albicans.
5. Conclusions
In this study, we were able to improve the synthesis of altissimacoumarin D. Using a smaller amount of organic solvent, altissimacoumarin D and analogues were obtained in good yields and purity. ACS47, ACS50 and ACS54 presented antifungal activity alone, while all the coumarins (except for ACS55) inhibited at least one efflux pump. ACS48, ACS51, ACS52, ACS54 and ACS56 inhibited only CaMdr1p. No classic CaMdr1p inhibitors are available to be used as positive controls in experiments, and these coumarins could be used to fill this gap. ACS47 and ACS50 have synergistic activity when combined with fluconazole against the growth of Candida albicans. This effect is related to the inhibition of the efflux pumps CaCdr1p, CaCdr2p, and/or CaMdr1p. ACS47 inhibits all three transporters, which is important considering that the goal is to develop a new antifungal treatment since the substance is able to enhance fluconazole activity regardless of the efflux pump related to the resistance. Also, these compounds did not present toxicity in vitro and in silico. Therefore, ACS47 and ACS50 emerge as potential candidates for use as adjuvants in candidiasis therapy caused by resistant strains.
Acknowledgments
We thank UFRJ for the facilities used in the elaboration of this work, and CNPq and FAPERJ for financial assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9070758/s1. Figure S1. 1H NMR spectra of ACS51; Figure S2. 13C NMR spectra of ACS51; Figure S3. 1H NMR spectra of ACS50; Figure S4. 13C NMR spectra of ACS50; Figure S5. 1H NMR spectra of ACS48; Figure S6. 13C NMR spectra of ACS48; Figure S7. 1H NMR spectra of ACS47; Figure S8. 13C NMR spectra of ACS47; Figure S9. 1H NMR spectra of ACS52; Figure S10. 13C NMR spectra of ACS52; Figure S11. 1H NMR spectra of ACS54; Figure S12. 13C NMR spectra of ACS54; Figure S13. 1H NMR spectra of ACS56; Figure S14. 13C NMR spectra of ACS56; Figure S15. 1H NMR spectra of ACS55; Figure S16. 13C NMR spectra of ACS55.
Author Contributions
Conceptualization, A.G. and C.C.L.; methodology, A.C.S., D.C.d.M., D.C.d.C. and G.C.C.G.; data curation, A.C.S. and D.C.d.M.; writing—original draft preparation, A.C.S. and D.C.d.M.; writing—review and editing, A.F-P. and C.C.L.; visualization, A.G.; supervision, R.S.C.L., A.F.-P. and C.C.L.; project administration, C.C.L.; funding acquisition, R.S.C.L. and C.C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)–Finance Code 001.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Soll D.R., Galask R., Schmid J., Hanna C., Mac K., Morrow B. Genetic Dissimilarity of Commensal Strains of Candida Spp. Carried in Different Anatomical Locations of the Same Healthy Women. J. Clin. Microbiol. 1991;29:1702–1710. doi: 10.1128/jcm.29.8.1702-1710.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown G.D., Denning D.W., Gow N.A.R., Levitz S.M., Netea M.G., White T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Lewis R.E. Current Concepts in Antifungal Pharmacology. Mayo Clin. Proc. 2011;86:805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz R., Kelly S.L., Lamb D.C., Kelly D.E., Ruhnke M., Morschhäuser J. Multiple Molecular Mechanisms Contribute to a Stepwise Development of Fluconazole Resistance in Clinical Candida Albicans Strains. Antimicrob. Agents Chemother. 1998;42:3065–3072. doi: 10.1128/AAC.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White T.C., Holleman S., Dy F., Mirels L.F., Stevens D.A. Resistance Mechanisms in Clinical Isolates of Candida Albicans. Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ter Beek J., Guskov A., Slotboom D.J. Structural Diversity of ABC Transporters. J. Gen. Physiol. 2014;143:419–435. doi: 10.1085/jgp.201411164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumari S., Kumar M., Khandelwal N.K., Kumari P., Varma M., Vishwakarma P., Shahi G., Sharma S., Lynn A.M., Prasad R., et al. ABC Transportome Inventory of Human Pathogenic Yeast Candida Glabrata: Phylogenetic and Expression Analysis. PLoS ONE. 2018;13:e0202993. doi: 10.1371/journal.pone.0202993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon R.D., Lamping E., Holmes A.R., Niimi K., Baret P.V., Keniya M.V., Tanabe K., Niimi M., Goffeau A., Monk B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Li Y., Ma S., Zhao Q., Wu J., Duan L., Xie Y., Wang S. Traditional Uses, Phytochemistry, and Pharmacology of Ailanthus Altissima (Mill.) Swingle Bark: A Comprehensive Review. J. Ethnopharmacol. 2021;275:114121. doi: 10.1016/j.jep.2021.114121. [DOI] [PubMed] [Google Scholar]
- 10.Qin H.L., Zhang Z.W., Ravindar L., Rakesh K.P. Antibacterial Activities with the Structure-Activity Relationship of Coumarin Derivatives. Eur. J. Med. Chem. 2020;207:112832. doi: 10.1016/j.ejmech.2020.112832. [DOI] [PubMed] [Google Scholar]
- 11.Tan N., Yazıcı-Tütüniş S., Bilgin M., Tan E., Miski M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos Hulusii. Molecules. 2017;22:1098. doi: 10.3390/molecules22071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fotso G.W., Ngameni B., Storr T.E., Ngadjui B.T., Mafu S., Stephenson G.R. Synthesis of Novel Stilbene–Coumarin Derivatives and Antifungal Screening of Monotes Kerstingii-Specialized Metabolites against Fusarium Oxysporum. Antibiotics. 2020;9:537. doi: 10.3390/antibiotics9090537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iranshahi M., Arfa P., Ramezani M., Jaafari M.R., Sadeghian H., Bassarello C., Piacente S., Pizza C. Sesquiterpene Coumarins from Ferula Szowitsiana and in Vitro Antileishmanial Activity of 7-Prenyloxycoumarins against Promastigotes. Phytochemistry. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Tayarani-Najaran Z., Tayarani-Najaran N., Eghbali S. A Review of Auraptene as an Anticancer Agent. Front. Pharmacol. 2021;12:698352. doi: 10.3389/fphar.2021.698352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y.P., Yan G., Xie Y.T., Lin T.C., Zhang W., Li J., Wu Y.J., Zhou J.Y., Fu Y.H. Bioactive Prenylated Coumarins as Potential Anti-Inflammatory and Anti-HIV Agents from Clausena Lenis. Bioorg. Chem. 2020;97:103699. doi: 10.1016/j.bioorg.2020.103699. [DOI] [PubMed] [Google Scholar]
- 16.Dao T.T., Tran T.L., Kim J., Nguyen P.H., Lee E.H., Park J., Jang I.S., Oh W.K. Terpenylated Coumarins as SIRT1 Activators Isolated from Ailanthus Altissima. J. Nat. Prod. 2012;75:1332–1338. doi: 10.1021/np300258u. [DOI] [PubMed] [Google Scholar]
- 17.Silva A.C., Benelkebir H., Lopes R.S.C., Lopes C.C., Ganesan A. Total Synthesis of Altissimacoumarin D, a Small Molecule Sirtuin 1 Activator. J. Braz. Chem. Soc. 2018;29:1157–1161. [Google Scholar]
- 18.Van Schijndel J., Molendijk D., Canalle L.A., Rump E.T., Meuldijk J. Temperature Dependent Green Synthesis of 3-Carboxycoumarins and 3,4-Unsubstituted Coumarins. Curr. Org. Synth. 2018;16:130–135. doi: 10.2174/1570179415666180924124134. [DOI] [PubMed] [Google Scholar]
- 19.Lamping E., Monk B.C., Niimi K., Holmes A.R., Tsao S., Tanabe K., Niimi M., Uehara Y., Cannon R.D. Characterization of Three Classes of Membrane Proteins Involved in Fungal Azole Resistance by Functional Hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:1150–1165. doi: 10.1128/EC.00091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto A.C.C., Rocha D.A.S., Moraes D.C.D., Junqueira M.L., Ferreira-Pereira A. Candida Albicans Clinical Isolates from a Southwest Brazilian Tertiary Hospital Exhibit MFS-Mediated Azole Resistance Profile. An. Acad. Bras. Cienc. 2019;91:e20180654. doi: 10.1590/0001-3765201920180654. [DOI] [PubMed] [Google Scholar]
- 21.Clemente de Moraes D., do Carmo Freire Ribeiro Pinto M., Tenório Sousa Domingos L., do Valle Pereira Midlej V., Ferreira-Pereira A. Effects of β-Lapachone and β-nor-Lapachone on Multidrug Efflux Transporters and Biofilms of Candida Glabrata. Bioorg. Med. Chem. 2022;63:116749. doi: 10.1016/j.bmc.2022.116749. [DOI] [PubMed] [Google Scholar]
- 22.Niimi K., Harding D.R.K., Parshot R., King A., Lun D.J., Decottignies A., Niimi M., Lin S., Cannon R.D., Goffeau A., et al. Chemosensitization of Fluconazole Resistance in Saccharomyces Cerevisiae and Pathogenic Fungi by a D -Octapeptide Derivative. Antimicrob. Agents Chemother. 2004;48:1256–1271. doi: 10.1128/AAC.48.4.1256-1271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Moraes D.C., Cardoso K.M., Domingos L.T.S., do Carmo Freire Ribeiro Pinto M., Monteiro R.Q., Ferreira-Pereira A. β-Lapachone Enhances the Antifungal Activity of Fluconazole against a Pdr5p-Mediated Resistant Saccharomyces Cerevisiae Strain. Braz. J. Microbiol. 2020;51:1051–1060. doi: 10.1007/s42770-020-00254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagunin A., Zakharov A., Filimonov D., Poroikov V. QSAR Modelling of Rat Acute Toxicity on the Basis of PASS Prediction. Mol. Inform. 2011;30:241–250. doi: 10.1002/minf.201000151. [DOI] [PubMed] [Google Scholar]
- 25.Cho S.K., Jeong M., Jang D.S., Choi J.H. Anti-Inflammatory Effects of Canthin-6-One Alkaloids from Ailanthus Altissima. Planta Med. 2018;84:527–535. doi: 10.1055/s-0043-123349. [DOI] [PubMed] [Google Scholar]
- 26.Jeong M., Kim H.M., Ahn J.H., Lee K.T., Jang D.S., Choi J.H. 9-Hydroxycanthin-6-One Isolated from Stem Bark of Ailanthus Altissima Induces Ovarian Cancer Cell Apoptosis and Inhibits the Activation of Tumor-Associated Macrophages. Chem. Biol. Interact. 2018;280:99–108. doi: 10.1016/j.cbi.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Shao Q., Li T.Y., Quan W., Wang H., Yan K.Y., Qiu P.C., Tang H.F., Lu Y.Y. The Alkaloids with Neuroprotective Effect from the Root Bark of Ailanthus Altissima. Nat. Prod. Res. 2022:1–7. doi: 10.1080/14786419.2022.2149518. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Zhao M., Zhang Z. Quantitative Proteomics Reveals the Antifungal Effect of Canthin-6-One Isolated from Ailanthus Altissima against Fusarium Oxysporum f. Sp. Cucumerinum in Vitro. PLoS ONE. 2021;16:e0250712. doi: 10.1371/journal.pone.0250712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annunziata F., Pinna C., Dallavalle S., Tamborini L., Pinto A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020;21:4618. doi: 10.3390/ijms21134618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurdelas R.R., Lima B., Tapia A., Feresin G.E., Sierra M.G., Rodríguez M.V., Zacchino S., Enriz R.D., Freile M.L. Antifungal Activity of Extracts and Prenylated Coumarins Isolated from Baccharis Darwinii Hook & Arn. (Asteraceae) Molecules. 2010;15:4898–4907. doi: 10.3390/molecules15074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia C., Zhang J., Yu L., Wang C., Yang Y., Rong X., Xu K., Chu M. Antifungal Activity of Coumarin against Candida Albicans Is Related to Apoptosis. Front. Cell. Infect. Microbiol. 2019;9:445. doi: 10.3389/fcimb.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X., Mukwaya E., Wong M.S., Zhang Y. A Systematic Review on Biological Activities of Prenylated Flavonoids. Pharm. Biol. 2014;52:655–660. doi: 10.3109/13880209.2013.853809. [DOI] [PubMed] [Google Scholar]
- 33.Esposito M., Nim S., Nothias L.F., Gallard J.F., Rawal M.K., Costa J., Roussi F., Prasad R., Di Pietro A., Paolini J., et al. Evaluation of Jatrophane Esters from Euphorbia Spp. as Modulators of Candida Albicans Multidrug Transporters. J. Nat. Prod. 2017;80:479–487. doi: 10.1021/acs.jnatprod.6b00990. [DOI] [PubMed] [Google Scholar]
- 34.Singh S., Fatima Z., Ahmad K., Hameed S. Fungicidal Action of Geraniol against Candida Albicans Is Potentiated by Abrogated CaCdr1p Drug Efflux and Fluconazole Synergism. PLoS ONE. 2018;13:e0203079. doi: 10.1371/journal.pone.0203079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemos A.S.O., Florêncio J.R., Pinto N.C.C., Campos L.M., Silva T.P., Grazul R.M., Pinto P.F., Tavares G.D., Scio E., Apolônio A.C.M., et al. Antifungal Activity of the Natural Coumarin Scopoletin Against Planktonic Cells and Biofilms from a Multidrug-Resistant Candida Tropicalis Strain. Front. Microbiol. 2020;11:1525. doi: 10.3389/fmicb.2020.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabekura T., Kawasaki T., Kato Y., Kawai K., Fiorito S., Epifano F., Genovese S., Uwai Y. Citrus Auraptene Induces Drug Efflux Transporter P-Glycoprotein Expression in Human Intestinal Cells. Food Funct. 2020;11:5017–5023. doi: 10.1039/D0FO00315H. [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay K., Kohli A., Prasad R. Drug Susceptibilities of Yeast Cells Are Affected by Membrane Lipid Composition. Antimicrob. Agents Chemother. 2002;46:3695–3705. doi: 10.1128/AAC.46.12.3695-3705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moraes D.C., Reis de Sá L.F., Domingos L.T.S., Pinto M.d.C.F.R., Soares R.M.d.A., Ferreira-Pereira A. Synergistic Interactions between β-Lapachone and Fluconazole in the Inhibition of CaCdr2p and CaMdr1p in Candida Albicans. Rev. Iberoam. Micol. 2020;37:104–106. doi: 10.1016/j.riam.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Maleki E.H., Bahrami A.R., Sadeghian H., Matin M.M. Discovering the Structure–Activity Relationships of Different O-Prenylated Coumarin Derivatives as Effective Anticancer Agents in Human Cervical Cancer Cells. Toxicol. Vitr. 2020;63:104745. doi: 10.1016/j.tiv.2019.104745. [DOI] [PubMed] [Google Scholar]
- 40.Greco I., Molchanova N., Holmedal E., Jenssen H., Hummel B.D., Watts J.L., Håkansson J., Hansen P.R., Svenson J. Correlation between Hemolytic Activity, Cytotoxicity and Systemic in Vivo Toxicity of Synthetic Antimicrobial Peptides. Sci. Rep. 2020;10:13206. doi: 10.1038/s41598-020-69995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.