Abstract
Candida auris is an emerging global public health threat and is resistant to most antifungal agents. Though fungi are significant pathogens for animals, the role of C. auris in animal health remains unexplored. Here, we analysed the microbial cultures of skin and ear swabs of 87 dogs in Delhi and performed fungal meta-barcode sequencing of ear and skin samples of 7 dogs with confirmed otitis externa (OE). Overall, 4.5% of dogs (4/87) with chronic skin infections contained evidence of C. auris in their ear canal (n = 3) and on their skin surface (n = 1). Of the three OE dogs with C. auris infection/colonisation, a diversity of fungi was observed, and their meta-barcode ITS sequence reads for C. auris ranged from 0.06% to 0.67%. Whole-genome sequencing of six C. auris strains obtained in culture from two dogs showed relatedness with Clade I clinical strains. The report highlights the isolation of C. auris from an animal source; however, the routes of transmission of this yeast to dogs and the clinical significance of transmission between dogs and humans remain to be investigated.
Keywords: otitis externa, meta-barcode sequencing, ITS, Candida auris, dogs, whole-genome sequencing
1. Introduction
Candida auris is a newly emerged human pathogenic yeast and is resistant to most antifungal agents. It has caused a diversity of infections and outbreaks in hospitals and presents a huge treatment challenge for clinicians and public health authorities. First reported in Japan in 2009, C. auris has spread rapidly worldwide [1,2,3] and caused the World Health Organisation to declare it as one of the four ‘critical priority’ fungal pathogens [4]. Outside hospital settings, C. auris has been isolated from the surface of stored apples, tidal marshes, hypersaline environments, and recently from wastewater, suggesting that this yeast can survive in harsh conditions [5,6,7,8]. Interestingly, trace sequences identical or highly similar to that of C. auris at the fungal barcode locus, i.e., the internal transcribed spacer (ITS) region, were found in metabarcoding data on an ear sample from a Spanish dog with otitis externa (OE) and from the skin of newts in the United Kingdom [9]. Though fungi are significant pathogens for animals, no live culture of C. auris has been isolated from animals, and its role in animal health remains to be explored [10]. We hereby report the isolation of C. auris from ear samples of two dogs. Additionally, culture-independent assessment of mycobiome analysis (DNA metabarcoding data) showed the presence of C. auris-specific ITS reads from the ear and skin of dogs with chronic skin infections.
2. Material and Methods
Swab collection and processing: In this study, we analysed the microbial communities of skin and ear swab samples in culture from a heterogeneous cohort of 87 dogs from a public referral hospital cum shelter in Delhi, India. The skin and ear swabs were collected and processed for bacterial and fungal cultures using the routine diagnostic protocol for suspected skin and ear infections. An ethics review was not requested by the Animal Care Centre because it was considered as a clinical observation study. The ear canal was sampled using sterile swabs (HIMEDIA, Mumbai, India) by rubbing the skin between the vertical–horizontal junctions. The skin swabs were obtained from the presenting lesions covering the dorsal–ventral skin, paws, and groin region. For microbiologic culture, the swabs were streaked directly on standard bacterial culture media, i.e., sheep blood agar (SBA) and MacConkey agar at 37 °C for 24 h. For fungal growth, Sabouraud dextrose agar (SDA) and CHROMagar (Becton, Dickinson, Baltimore, MD, USA) media were used and incubated at 37 °C for 24 h and 48 h, respectively. Additionally, for isolation of Candida auris, each swab was inoculated into yeast nitrogen base (YNB) broth with 10% NaCl at 37 °C for 4 days followed by streaking onto the SDA plates [7,11]. All microbial colonies, including bacteria and yeasts on blood agar, MacConkey agar, SDA, and CHROMagar, were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Biotyper OC version 3.1, Daltonics, Bremen, Germany) with a score ≥ 2. Candida auris colonies obtained in culture were further confirmed by sequencing of internal transcribed spacer region (ITS) within the ribosomal RNA gene cluster [12].
Fungal DNA extraction and ITS amplicon sequencing (Metagenomics): In addition to samples for microbiological cultures, ITS meta-barcode sequencing was conducted for swab samples (skin, n = 2; ear swabs, n = 7) from seven dogs to study the fungal communities in these dogs with OE. Swabs were collected and placed in 0.05 M phosphate buffer for 30 min with a continuous shaking at 200 rpm. Swabs were discarded, and the solution was stored at −20 °C until subsequent analyses [7]. Microbial genomic DNA was extracted from each sample using a column-based QIAamp DNA minikit (Qiagen, Hilden, Germany) and quantified by QUBIT 4 fluorometer (ThermoFisher, USA) using DNA HS assay kit (ThermoFisher). Primers ITS-1, 5′-TCCGTAGGTGAACCTTGCGG-3′, ITS2, 5′-GCTGCGTTCTTCATCGATGA-3′, ITS3, 5′-GCATCGATGAAGAACGCAGC-3′, and ITS4, 5′-TCCTCCGCTTATTGATATGC-3 were used for amplifying the ITS1 and ITS2 regions, respectively. Illumina sequencing platform (NOVASEQ 6000, PIPITS v2.7) was used to perform ITS fungal sequencing, and PIPITS v2.7 was used for analysis [13]. Magic-BLAST was used to map the raw metabarcoding sequences of each sample against the fungal ITS database containing 15,695 fungal species [14]. The reference ITS sequences can be downloaded at https://ftp.ncbi.nlm.nih.gov/refseq/TargetedLoci/Fungi/. To calculate the relative read abundance at the species level in each sample, the read counts of each species were divided by the total reads mapping to the fungal ITS database. Then, the read abundance was pooled into the genera level, and the average read abundance of the samples for each genus was used to select the taxa for the heatmap. A total of 28 genera with average read abundance over 0.11% were included, and the 28 genera covered an average of 98% reads that were mapped to the database. A heatmap was generated using matplotlib and seaborn [15,16]. Additionally, in each sample, we specifically searched for ITS sequences of C. auris. For samples that contained C. auris ITS sequences (>94% sequence identity over the cleaned and filtered ITS reads), the relative frequencies of C. auris ITS sequences in each sample were calculated. To better reveal the differences among samples, the read abundance for each genus was standardized. The standardization was carried out for each taxon by subtracting the minimum and dividing each by its maximum.
Antifungal susceptibility testing: The susceptibility testing of yeasts was performed using the CLSI broth microdilution method, following M27-A3 [17]. The antifungals tested were fluconazole (FLU, Sigma, St. Louis, MO, USA), itraconazole (ITC, Lee Pharma, Hyderabad, India), voriconazole (VRC, Pfizer, Groton, CT, USA), posaconazole (POS, Merck, Whitehouse Station, NJ, USA), isavuconazole (ISA, Basilea Pharmaceutical, Basel, Switzerland), 5-flucytosine (5-FC, Sigma, St. Louis, MO, USA), micafungin (MFG, Astellas, Toyama, Japan), anidulafungin (AFG, Pfizer, Groton, CT, USA), and amphotericin B (AMB, Sigma, Groton, CT, USA). The drugs were tested for 10 (two-fold) dilutions, and the drug concentration ranges were as follows: FLU, 0.25–>128 mg/L; ITC, VRC, and AMB, 0.03–16 mg/L; POS, ISA, AFG, MFG, 0.015–8 mg/L; 5-FC, 0.125–64 mg/L [18]. Candida krusei strain ATCC6258 and C. parapsilosis strain ATCC22019 were used as quality controls. All statistical parameters were calculated using Prism version 6.00 (GraphPad Software).
Antibiotic susceptibility testing was conducted for all bacterial isolates using disk diffusion method following CLSI M100, Ed-32. For Gram-positive bacteria, including Staphylococcus pseudointermedius, S. intermedius, S. simulans, and S. schleiferi, eight drugs were tested, namely penicillin, ampicillin, clindamycin, vancomycin, azithromycin, linezolid, cefoxitin, and cotrimoxazole. For Gram-negative bacteria, i.e., Acinetobacter baumanii, and Pseudomonas aeruginosa, ciprofloxacin, levofloxacin, gentamicin, amikacin, meropenem, cefepime, ceftazidime, ceftazidime-clavulanate, piperacillin, and piperacillin-tazobactam were tested. The inhibitory zone diameters obtained around the antibiotic discs were measured after incubation for 24 h at 37 °C and evaluated [19].
Whole Genome Sequencing (WGS): Genomic DNA of all C. auris isolates (n = 6 obtained from ear swabs of two dogs with OE) were extracted using a column-based QIAamp DNA minikit (Qiagen, Hilden, Germany) and quantified by a QUBIT 3 Fluorometer using dS DNA HS Dye. WGS libraries were prepared using NEBNext ultra II DNA FS kit (New England Biolabs, Ipswich, MA, USA), as described previously, and sequenced on Illumina HiSeq 4000 [5,11]. For phylogenetic analysis to identify the clade affinity of our strains, one reference genome from each of the four major clades was included for comparison, including strains B8441 (clade I), B11220 (clade II), B11221 (clade III), and B11245 (clade IV). All strains were subjected to NASP pipeline for genome sequencing analysis, as described previously [7]. The phylogenetic tree was constructed using RAxML v8.0.25 under ASC_GTRCAT nucleotide substitution model and 1000 bootstrap replicates [20]. A maximum-likelihood tree was constructed based on SNPs present in at least one sample. This analysis revealed that all of our strains belonged to clade I (see Results below). We then proceeded to conduct an additional phylogenetic analysis using B8441 as reference and including 569 previously reported clade I C. auris samples from different countries, including Indian clinical strains [21], strains recovered from apples [7], and strains from the marine environment of the Andaman Islands [5]. In this broad clade I-specific analysis, SNP sites with ambiguous calls in over 0.5% of the samples were removed, and the remaining SNPs were concatenated for each sample. Maximum-likelihood phylogeny was constructed using RAxML-HPC2 on XSEDE in the CIPRES Science Gateway [22]. The tree uses the ASC_GTRCAT nucleotide substitution model and 1000 bootstrap iterations and was visualized with iTOL [23].
3. Results
Population description: Among 87 dogs, 52 (60%) were stray dogs who were admitted in the intensive care unit (ICU) with severe skin lesions due to chronic skin diseases (group IA and IB). In group IA dogs with skin infections, eight had otitis externa with clinical signs of ear infection, i.e., erythema, oedema, erosion, and exudate in the affected ear (Table 1). In group IB all of the remaining 44 dogs exhibited skin infections. The group II composed of 35 pet dogs (40%) who were attending the outpatient services for minor ailments of the gastrointestinal and urinary tracts.
Table 1.
Distribution of yeasts and bacteria on the skin and in the ear of 87 dogs collected from North Delhi, India.
| Disease Categories in Dogs (Numbers) | Period of Sampling | Yeasts | Bacterial Isolation (Body Site, No. of Dogs Positive for the Given Species) |
|
|---|---|---|---|---|
| Culture (Body Site, No. of Dogs Positive for the Given Species) |
Amplicon Sequencing (ITS Reads for C. auris/Total Reads Matched to Fungal ITS; Percentage of ITS Reads) |
|||
| Group IA Chronic skin disease with otitis externa (n = 8), |
27-09-22 to 17-04-23 |
C. auris (E *, n = 1) (D1) @ C. rugosa (E, n = 2), C. krusei (E, n = 3) (D2) @ C. lusitaniae (E, n = 1) C. glabrata (S #, n = 1) (D3) @ |
C. auris from ear swab of D1 dog (34394/27920633; 0.12%) C. auris from ear swab of D2 dog (39577/59853350; 0.06%) C. auris from skin swab of D3 dog (3231/479708; 0.67%) |
A. baumanii (E, n = 1) S. pseudointermedius (S, n = 1) |
| Group IB Chronic skin disease (n = 44), |
14-08-22 to 17-04-23 |
C. auris (E, n = 1) (D4) @ M. pachydermatis (E, n = 11; S, n = 3) C. tropicalis (E, n = 8; S, n = 3), T. asahii (E, n = 7), C. glabrata (E, n = 6, S; n = 1), C. rugosa (E, n =1), C. albicans (E, n = 1), |
ND |
A. baumanii (E, n =15; S, n = 2) S. pseudointermedius (E, n = 9; S, n = 2) S. intermedius, (E, n = 1; S, n = 1) K. pneumoniae (E, n = 1) S. simulans (S, n = 1) S. schleiferi (S, n = 1) P. aeruginosa (E, n = 1) |
| Group II Negative for skin and ear infections (n = 35), |
11-05-22 to 02-09-22 |
C. tropicalis (E, n = 3; S, n = 3) M. pachydermatis (E, n = 3; S, n = 1) C. krusei (E, n = 2) C. lusitaniae (S, n = 2) C. parapsilosis (E, n = 1) |
ND |
A. baumanii (E, n = 1; S, n = 2), S. pseudointermedius (E, n = 6), P. aeruginosa (E, n = 3; S, n = 1) S. schleiferi (E, n = 1; S, n = 1) S. intermedius (S, n = 2), K. pneumoniae (S, n = 1), E. faecalis (S, n = 1), B. cereus (E, n = 1) |
* ear, @ dogs positive for C. auris either by culture or ITS amplicon sequencing, # skin.
Fungal and bacterial culture: Overall, the ear swabs of all 52 dogs (group IA and IB) yielded microbial growth. On the contrary, the skin samples of only 31% (n = 16) of the dogs had microbial growth, including the isolation of bacteria (n = 8) and yeast (n = 8) in eight dogs each. Furthermore, ear samples and skin samples of 14% (n = 7) and 4% (n = 2) of dogs contained both living yeasts and living bacteria. In the group IA and IB cohorts, Candida tropicalis (n = 3) and Malassezia pachydermatis (n = 3) were the predominant fungi on the skin (6% each), followed by Candida glabrata in a single dog. Also, in two (4%) dogs, Acinetobacter baumanii and Staphylococcus pseudointermedius were detected on the skin. In ear samples, M. pachydermatis was the predominant fungal species (n = 11, 21% dogs), followed by C. tropicalis (n = 8) in 15% cases (Table 1).
In group IA, all cases had confirmed ear infections, and the ear swabs yielded yeasts as the sole etiologic agent in four of eight cases. The ear swabs of the remaining four dogs had both yeasts and bacterial colonies in culture. Notably, a single dog with OE (D1 dog) showed colonisation with multiple yeasts in the ear skin, including C. auris (645-V-22), Candida lusitaniae, and Candida rugosa, along with Acinetobacter baumanii. The C. auris-positive dog (D1) was admitted with chronic skin infection and OE in the hospital for more than a month before our sampling, and the dog was on topical treatment with miconazole, mupirocin, and ofloxacin. A repeat sampling of the ear canal of this dog after four weeks again yielded C. auris (645-V-22_2). Furthermore, the ear swab obtained from another dog (D4) in group IB (with only skin infection) who had no symptoms of otitis was also positive for C. auris, yielding five colonies of C. auris (D_953_1 to D_953_5) in culture.
Interestingly, 63% (n = 22) of the group II cohort (n = 35) with no skin or ear infections showed microbial growth from ear and/or skin swabs, but none of the colonies were C. auris. Also, a high rate of skin colonisation (43%, n = 15) was noted in these otherwise healthy dogs, whereas ear samples of only seven dogs showed microbial growth. Of these seven dogs, both Candida tropicalis (n = 3) and M. pachydermatis (n = 3) were observed to be equally distributed in the ear samples, followed by Candida krusei (n = 2) and Candida parapsilosis (n = 1). The skin surface was predominately colonised by C. tropicalis (n = 3, 14%) and C. lusitaniae (n = 2, 9%), and bacterial colonisation was due to A. baumanii (n = 2, 5.7%) and S. intermedius (n = 2, 5.7%).
We investigated healthcare workers’ (HCW, n = 6) hand swabs for microbial growth. The ICU board of the Animal Facility guided the collection of hand swab cultures from healthcare workers (n = 6) handling the dogs and the environmental sampling of the ICU for microbial cultures. Also, the hand swab of a single HCW in the ICU was analysed for fungal diversity using metabarcoding. Hand colonisation by microbes was noted in five of the six HCWs. Overall, a varied spectrum of yeasts, including C. krusei, C. parapsilosis, C. tropicalis, and Trichosporan asahii, was observed on the skin surfaces of the hands of HCWs in culture. Additionally, the doors, benches, and floor of the ICU were found to be colonised by C. tropicalis and C. krusei.
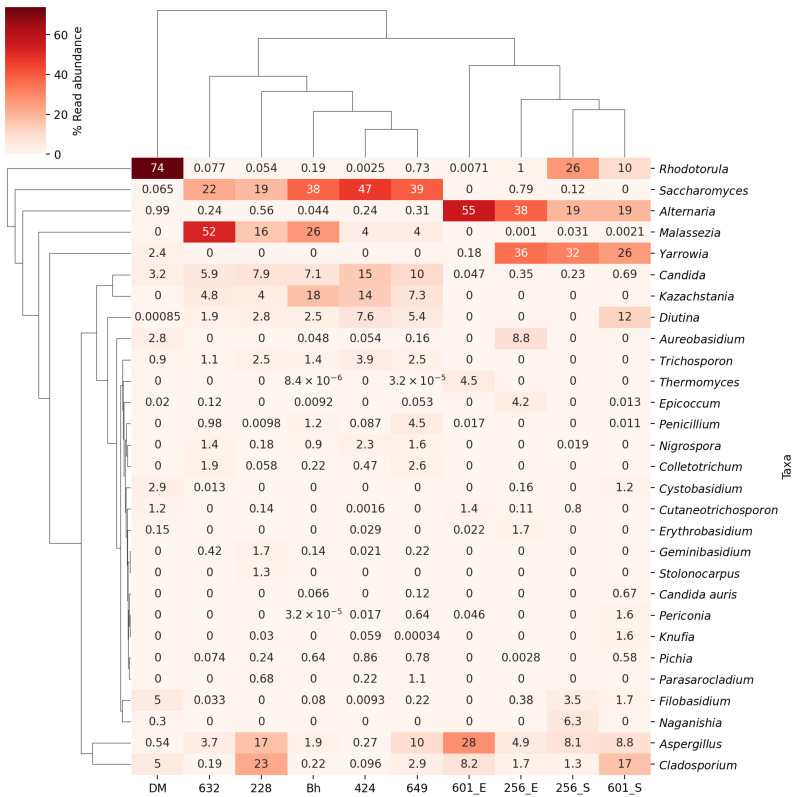
Metagenomics analysis: According to metabarcoding data, evidence of C. auris was found in the ear skin samples of two dogs (both with OE) and on the skin surface of a single dog with OE (in the group IA cohort). A total of 248,515,598 ITS reads were obtained from ten samples (nine samples from dogs and one HCW’s hands), with an average of 24,851,560 reads per sample. After sequence alignment, 151,552,302 ITS reads were matched to a fungal ITS database with an average of 15,155,230 reads per sample. The length of the high-quality reads was 152 base pairs. The meta-barcode data from the ear of the dog with a positive culture for C. auris (D1) were also found positive for 34,394 ITS reads (0.12%) for C. auris. In addition, two dogs with negative culture for C. auris also showed the presence of 39,577 (0.06%) and 3231 (0.6%) ITS reads for C. auris from the ear (D2) and skin (D3), respectively. The heatmap shows the percentage of ITS read abundance of fungi in the 10 samples (Figure 1). The percent ITS reads for all fungal species are given in Table S1.
Figure 1.
Heatmap showing the distribution of the 28 most common fungal genera in ear and skin samples from seven dogs and one healthcare worker. Different intensities of colours represent the percent read abundance of a genus in each sample. The dendrogram on the left is to indicate the similarity between different genera based on their read abundance profiles. Genera that are closer together on the dendrogram share more similar abundance patterns in our samples. The dendrogram on the top represents the clustering and relationships between the different samples based on their read abundance profiles of listed genera. Samples that are closer together on the dendrogram share more similar read abundance profiles.
Interestingly, among the three dogs containing ITS sequences for C. auris, there seemed to be a negative correlation between the ITS read abundance for Malassezia species and that of C. auris (Table S1). In addition, a single ear sample with the highest percentage (52.2%) of ITS reads for Malassezia did not have traces of C. auris. However, there was a big variation in the frequencies of Malassezia ITS sequences (0–52.2%) among C. auris-negative dogs. Aside from C. auris, other Candida species were frequently recovered. Of the seven dogs with OE whose ear swabs were subjected to ITS amplicon sequencing, there was an average of 15,155,230 ITS reads per samples for Candida species. In addition, the presence of C. tropicalis, C. krusei, C. parapsilosis and T. asahii, in the microbial cultures of the hand swab of an HCW showed correlation with the presence of ITS reads (3.2%) of Candida species.
Antifungal susceptibility testing (AFST): The MIC data of the antifungals tested are summarised in Table S2. The two C. auris strains (645-V-22, 645-V-22_2) from the D1 dog, were found to be resistant to fluconazole (FLU) (MIC > 128 mg/L), while all of the other five strains isolated from the D4 case showed 2–4-fold lower MICs for FLU (32–64 mg/L). All C. auris strains were susceptible to the other tested drugs (Table S2).
All of tested isolates of Staphylococcus, Pseudomonas, and Acinetobacter genera were susceptible to all drugs except S. pseudointermedius, which was found to be resistant to clindamycin and azithromycin.
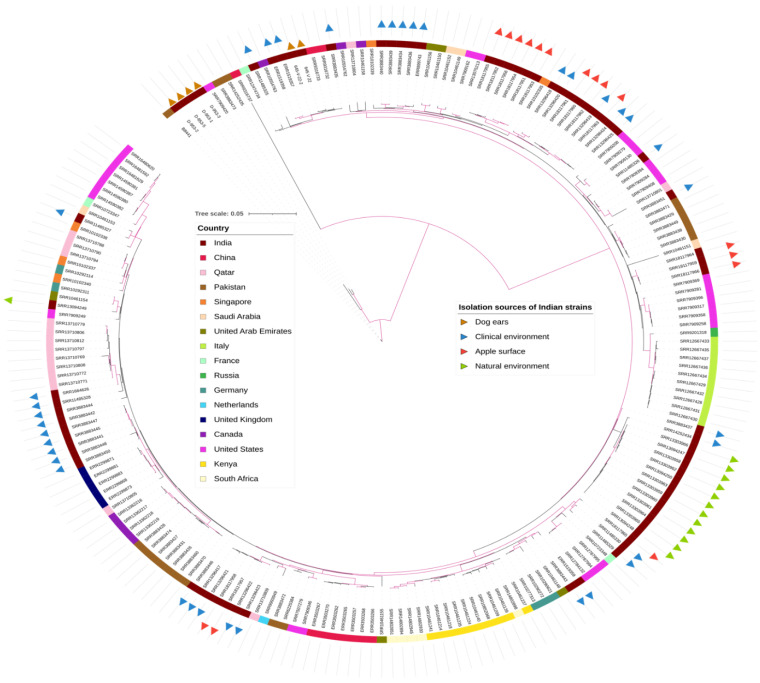
Whole genome sequencing: WGS analysis revealed that two C. auris strains (645-V-22, 645-V-22_2) obtained from the D1 dog four weeks apart differed by four SNPs from each other, suggesting that the same strain persisted during the four weeks in the colonised ear. In the D4 dog, four of the five isolates were sequenced, and clonal clustering of all four strains of C. auris was observed with a 2–6 SNPs difference among the strains. Notably, these four strains (D_953_1, D_953_2, D_953_3, and D_953_5) showed a difference of 955–965 SNPs from C. auris strains isolated from the D1 dog, suggesting the circulation of genetically distinct C. auris strains. All four C. auris strains from the D4 dog clustered with B8441, a clade I reference strain (Pakistan). Interestingly, the C. auris strains from the D4 dog showed close genetic relatedness (8–11 SNPs difference) with the Pakistan clade I reference strain. Phylogenetic analysis of 569 global clade I C. auris strains grouped the two D1 strains with two C. auris strains causing candidemia (7–14 SNPs) in Delhi, India (Figure 2) [3,21]. These two strains from the D1 dog showed K143R amino acid substitution in the ERG11 gene and A640V amino acid substitution in the TAC1B gene. Notably, four C. auris strains from the D4 dog showed the absence of mutation in azole-resistance-related genes, i.e., ERG11 and TAC1B.
Figure 2.
Genetic relationships of C. auris isolates from dog ears and clade I strains from other countries inferred based on concatenated genome-wide SNPs. Branches with bootstrap support over 0.95 are highlighted in purple. Indian strains were labelled with triangles in various colours to indicate their isolation sources. The inner colour strips specify the geographic location of the isolates.
4. Discussion
Our report documents for the first time the isolation of live C. auris culture from an animal source. Overall, 4 of the 87 dogs (4.5%) contained evidence of C. auris infection or colonisation in their ear and on the surface of their skin. Notably, three of the four C. auris-positive dogs, either by culture (n = 2) or by ITS meta-barcode sequencing (n = 2), harboured this yeast in the ear canal. Furthermore, among the eight dogs in the OE cohort, two had C. auris, suggesting an association of C. auris with external ear canal infections.
Candida auris has been previously isolated from ear infections in humans from Korea, Japan, and Iran [1,24,25], but those strains mainly belonged to Clade II and Clade V [3,25,26]. In a recent study from Spain investigating the bacterial and fungal microbiota in the ear canals of dogs with OE, 12 (0.01%) C. auris-specific ITS reads out of a total of 81,213 ITS reads were found in a single dog [9,27]. In contrast, in the present study, an ample number of C. auris reads accounting for 0.06% and 0.12% was observed in the ear samples, much higher than that in the ear sample of the Spanish dog. In addition, the skin of a single dog was also found to be colonised by C. auris, with 3231 (0.67%) ITS reads. The genomic data of C. auris strains from the ears of two dogs showed that genetically distinct strains of Clade I were infecting or colonising the dogs. A close association of C. auris strains from a single dog (D4) with the Pakistan clade I reference strain suggested a clonal transmission of this yeast in the Indian subcontinent. Interestingly, this strain with a MIC value of 32 mg/L for fluconazole had no known mutations in genes related to azole resistance, i.e., in the ERG11 and TAC1B genes. In contrast, C. auris strains from the D1 dog showed similarity with the Indian clinical strains and exhibited previously known K143R amino acid substitution in the ERG11 gene and A640V amino acid substitution in the TAC1B gene, likely contributing to the high MIC of fluconazole (MIC > 128 mg/L).
It is important to emphasise that in the present study, C. auris was not detected on the healthy intact skin of pet dogs using the culture method. However, the ITS amplicon sequencing was not performed on the skin and ear swabs of otherwise healthy dogs. C. auris colonised the breached skin of the ears of dogs with chronic skin conditions admitted to the ICU, but colonisation of the fomites samples in the ICU was not observed. This scenario is unlike the colonisation by C. auris in humans, where the close inanimate environment is readily contaminated with this yeast due to shedding of skin scales. The fact that, in the present study, the skin of the ears of dogs was found to be colonised by C. auris rather than the exposed (ventral and dorsal) skin probably contributed to reduced shedding in the environment. It is worth emphasising the circulation of closely related C. auris clade I strain in humans and dogs. However, the prevalence of C. auris transmissions between humans and domesticated animals warrants further investigations. Finally, the association of C. auris with ears in humans and dogs hints toward the ear canal possibly being an important reservoir of this yeast.
Acknowledgments
A.C. is a Fellow of the CIFAR program Fungal Kingdom: Threats & Opportunities. A.Y. and K.J. acknowledge support by the University Grants Commission (file no. 16-9/2019) and 596/(CSIR-UGC NET JUNE 2019) Government of India (GoI), New Delhi, and Council of Scientific & Industrial Research, GoI (file no. 08/728(0001)/2019-EMR-I), respectively, for their research fellowships. J.X. acknowledges a research grant from McMaster University’s Global Science Initiative (2020-03).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9070720/s1, Table S1: Distribution of the 28 most common fungal genera in the ten samples (9 samples from dogs and one HCW hands). Individual sheets list the percent ITS reads data for all fungal genera from ten samples. Table S2: In vitro antifungal susceptibility profile of 10 yeast species isolated from dogs (n = 87) against ten antifungal drugs using CLSI-BMD method.
Author Contributions
Conceptualization, A.C.; Data curation, Y.W.; Formal analysis, Y.W., J.X. and A.C.; Investigation, A.Y. and V.A.R.P.; Methodology, A.Y., K.J. and V.K.; Supervision, A.C.; Writing—original draft, A.Y., K.J. and A.C.; Writing—review and editing, V.A.R.P., H.K. and J.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
No ethical declarations are required as the study protocol was a part of routine diagnostic procedure, and no additional experiment was performed.
Informed Consent Statement
Not applicable.
Data Availability Statement
Genomic and meta-barcode data of the present study are submitted under bioproject number PRJNA917187.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Du H., Bing J., Hu T., Ennis C.L., Nobile C.J., Huang G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16:e1008921. doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Xu J. Population genomic analyses reveal evidence for limited recombination in the superbug Candida auris in nature. Comput. Struct. Biotechnol. J. 2022;20:3030–3040. doi: 10.1016/j.csbj.2022.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parums D.V. Editorial: The World Health Organization (WHO) fungal priority pathogens list in response to emerging fungal pathogens during the COVID-19 pandemic. Med. Sci. Monit. 2022;28:e939088. doi: 10.12659/MSM.939088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora P., Singh P., Wang Y., Yadav A., Pawar K., Singh A., Padmavati G., Xu J., Chowdhary A. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio. 2021;12:e03181-20. doi: 10.1128/mBio.03181-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escandón P. Novel environmental niches for Candida auris: Isolation from a coastal habitat in Colombia. J. Fungi. 2022;8:748. doi: 10.3390/jof8070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav A., Jain K., Wang Y., Pawar K., Kaur H., Sharma K.K., Tripathy V., Singh A., Xu J., Chowdhary A. Candida auris on apples: Diversity and clinical significance. mBio. 2022;13:e0051822. doi: 10.1128/mbio.00518-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez J., Iverson T., Hergert J., Oakeson K., LaCross N., Njoku C., Gorzalski A., Gerrity D. Candida auris discovery through community wastewater surveillance during healthcare outbreak, Nevada, USA, 2022. Emerg. Infect. Dis. 2023;29:422–425. doi: 10.3201/eid2902.221523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irinyi L., Roper M., Malik R., Meyer W. Finding a needle in a haystack—In silico search for environmental traces of Candida auris. Jpn. J. Infect. Dis. 2022;75:490–495. doi: 10.7883/yoken.JJID.2022.068. [DOI] [PubMed] [Google Scholar]
- 10.Seyedmousavi S., Bosco S.M.G., de Hoog S., Ebel F., Elad D., Gomes R.R., Jacobsen I.D., Jensen H.E., Martel A., Mignon B., et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018;56((Suppl. S1)):S165–S187. doi: 10.1093/mmy/myx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav A., Singh A., Wang Y., Haren M.H.V., Singh A., de Groot T., Meis J.F., Xu J., Chowdhary A. Colonisation and transmission dynamics of Candida auris among chronic respiratory diseases patients hospitalised in a chest hospital, Delhi, India: A comparative analysis of whole genome sequencing and microsatellite typing. J. Fungi. 2021;7:81. doi: 10.3390/jof7020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathuria S., Singh P.K., Sharma C., Prakash A., Masih A., Kumar A., Meis J.F., Chowdhary A. Multidrug-resistant Candida auris misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest Method. J. Clin. Microbiol. 2015;53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gweon H.S., Oliver A., Taylor J., Booth T., Gibbs M., Read D.S., Griffiths R.I., Schonrogge K. PIPITS: An automated pipeline for analyses of fungal internal transcribed spacer sequences from the Illumina sequencing platform. Methods Ecol. Evol. 2015;6:973–980. doi: 10.1111/2041-210X.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boratyn G.M., Thierry-Mieg J., Thierry-Mieg D., Busby B., Madden T.L. Magic-BLAST, an accurate RNA-seq aligner for long and short reads. BMC Bioinform. 2019;20:405. doi: 10.1186/s12859-019-2996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007;9:90–95. doi: 10.1109/MCSE.2007.55. [DOI] [Google Scholar]
- 16.Waskom M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021;6:3021. doi: 10.21105/joss.03021. [DOI] [Google Scholar]
- 17.Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. [Google Scholar]
- 18.Chowdhary A., Prakash A., Sharma C., Kordalewska M., Kumar A., Sarma S., Tarai B., Singh A., Upadhyaya G., Upadhyay S., et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–2017) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018;73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 19.Performance Standards for Antimicrobial Disk Susceptibility Testing. 32nd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2022. [Google Scholar]
- 20.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma C., Kumar N., Pandey R., Meis J.F., Chowdhary A. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect. 2016;13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees, in 2010 Gateway Computing Environments Workshop (GCE) IEEE; Piscataway, NJ, USA: 2010. pp. 1–8. [Google Scholar]
- 23.Letunic I., Bork P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abastabar M., Haghani I., Ahangarkani F., Rezai M.S., Taghizadeh Armaki M., Roodgari S., Kiakojuri K., Al-Hatmi A.M.S., Meis J.F., Badali H. Candida auris otomycosis in Iran and review of recent literature. Mycoses. 2019;62:101–105. doi: 10.1111/myc.12886. [DOI] [PubMed] [Google Scholar]
- 25.Chow N.A., de Groot T., Badali H., Abastabar M., Chiller T.M., Meis J.F. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019;25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spruijtenburg B., Badali H., Abastabar M., Mirhendi H., Khodavaisy S., Sharifisooraki J., Taghizadeh Armaki M., de Groot T., Meis J.F. Confirmation of fifth Candida auris clade by whole genome sequencing. Emerg. Microbes Infect. 2022;11:2405–2411. doi: 10.1080/22221751.2022.2125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puigdemont A., D’Andreano S., Ramió-Lluch L., Cuscó A., Francino O., Brazis P. Effect of an anti-inflammatory pomegranate otic treatment on the clinical evolution and microbiota profile of dogs with otitis externa. Vet. Dermatol. 2021;32:158-e37. doi: 10.1111/vde.12930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genomic and meta-barcode data of the present study are submitted under bioproject number PRJNA917187.