Abstract
Vanderbylia robiniophila (Huaier in Chinese) has been used as a traditional herbal medicine in China for over 1600 years. However, the secondary metabolites of V. robiniophila have not been systematically examined. Corresponding chemical investigation in this study led to the discovery of two new compounds, (22E, 24R)-6β, 7α-dimethoxyergosta-8(14), 22-diene-3β, 5α-diol (1) and vanderbyliolide A (8), along with eight known ones (2–7, 9–10). Their structures were determined by extensive spectroscopic analyses and electronic circular dichroism (ECD) calculations. The tyrosinase inhibitory activity of all isolated compounds was evaluated, and compound 10 showed a potential tyrosinase inhibitory effect with an IC50 value of 60.47 ± 2.63 μM. Kinetic studies of the inhibition reactions suggested that 10 provides the inhibitory ability on tyrosinase in an uncompetitive way.
Keywords: Vanderbylia robiniophila (Huaier), secondary metabolites, tyrosinase inhibitory activity, inhibition mechanism
1. Introduction
Melanin is the primary pigment responsible for skin color and also protects human skin against harmful effects by absorbing ultraviolet (UV) rays and mitigating oxidative stress [1]. The excessive production of melanin can lead to hyperpigmentation-related disorders and even melanoma in severe cases [2]. Tyrosinase is a reaction rate-limiting enzyme in the process of melanogenesis [3,4]. The skin-lightening products have been investigated as the melanogenesis inhibitors in the medical and cosmetic industry. They are in large demand with a worldwide market of USD 23 billion in 2020 [5]. However, most of the commercially available tyrosinase inhibitors have some drawbacks. For instance, vitamin C is susceptibly degradable and sensitive to temperature and air [6]. Kojic acid and arbutin are reported to cause safety issues such as skin irritation [7], while arbutin is chemically instable and might result in leukemia due to the metabolized products of benzene analogues [8]. Therefore, safe, stable, and effective tyrosinase inhibitors are still in great need for the treatment of dyspigmentation disease and cosmetic applications.
Mushrooms have been traditionally used as food ingredients and folk medicine since antiquity, and recent studies have elucidated their functional benefits, such as anticancer, anti-inflammatory, antiviral, antibacterial and hepatoprotective properties, which have been attributed to various mushroom components [9,10]. Medicinal mushrooms are known for producing specialized molecules with great chemo-diversity that have relevant impacts on human health and diseases [11]. These compounds have proved immensely valuable in the pharmaceutical industry as they provide the structural template and pharmacophores of commercially successful drugs with excellent clinical efficacies and acceptable side-effects [12]. These include the antibiotic retapamulin from Pleurotus multilus [13] and the first-in-class sphingosine-1-phosphate (S1P) receptor modulator named fingolimod from Isaria sinclairii [14]. Vanderbylia robiniophila (Murrill) B.K. Cui & Y.C. Dai (Huaier) is a medicinal mushroom mainly parasitized on the trunk of Robinia pseudoacacia L. It has been widely used for more than 1600 years in traditional Chinese medicine (TCM) [15]. Nowadays, “Huaier granule” is recognized by Chinese State Food and Drug Administration as a complementary medicine for the treatment of multiple malignancies, including liver cancer, lung cancer, digestive system cancers, and breast carcinoma [16,17].
The mechanisms behind the significant antitumor effect exerted by Vanderbylia robiniophila are fascinating and intrigue many researchers [18]. It has been revealed that V. robiniophila directly inhibits the growth and proliferation of cancer cells [19], arrests the cell cycle [20], restrains invasion and metastasis [21], interferes with angiogenesis [22], induces cell apoptosis [23] and regulates immune responses [24]. In contrast to the pharmacological activities, there is limited information about the chemical constituents of V. robiniophila. Recent studies have shown that polysaccharides, proteoglycan, and amino acids are the primary ingredients in V. robiniophila extract [25,26]. A metabolomic comparison between the naturally and the artificially cultured Huaier extract using LC-MS analysis showed that the former contains more amino acids, alkaloids, and terpenoids [27]. However, few researchers have been able to conduct any systematic research into the isolation and exact structure elucidation of the secondary metabolites of V. robiniophila [28].
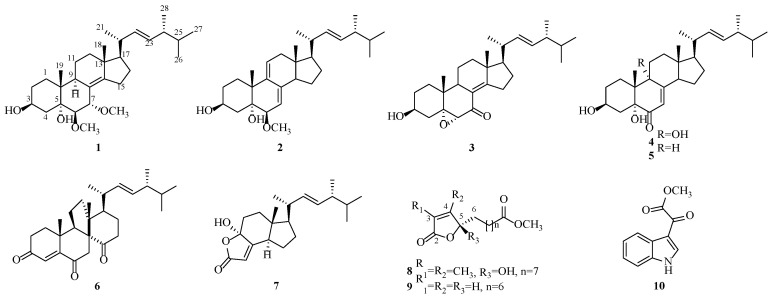
During our investigations on macrofungal resources, a wild-derived strain was isolated from the fruit body of Vanderbylia robiniophila, and the EtOAc fraction of the strain showed significant tyrosinase inhibitory activity. To discover potential tyrosinase inhibitors in V. robiniophila, a detailed chemical investigation of the large-scale fermentation in rice was carried out, which resulted in the purification of two novel secondary metabolites, along with eight known ones, including seven steroids (1–7), two 2(5H)-furanone derivatives (8–9), and a monoindole alkaloid (10) (Figure 1). The tyrosinase inhibitory activities of the isolated compounds were evaluated. Herein, the details of the isolation, structure elucidation, and bioactivities of compounds 1–10 are described.
Figure 1.
Chemical structures of compounds 1–10.
2. Materials and Methods
2.1. General Experimental Procedures
UV spectra were recorded on an ultraviolet-visible spectrometer (UV-1500PC, Macy Instruments, Inc., Shanghai, China) and IR spectra were measured on a Thermo Fisher Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Madison, WI, USA). Optical rotation values were obtained using a JASCO DIP-370 digital polarimeter (JASCO, Tokyo, Japan) at 20 °C. The ECD spectrum was measured by a BioLogic Science MOS-450 spectrometer (Bio-Logic Science Instruments, Seyssinet-Pariset, France). HRESIMS data were obtained in the positive-ion mode on a Waters Xevo G2-S QTOF mass spectrometer (Waters Corporation, Milford, MA, USA). 1D and 2D NMR were tested using a Bruker AVANCE NEO 600 MHz NMR spectrometer (Bruker Corporation, Bremen, Germany), where chemical shifts (δ) are given in parts per million (ppm) units with tetramethylsilane (TMS) as an internal standard. Chromatographic silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China), DIAION HP-20 macroporous adsorption resin (250–850 μm, Mitsubishi Chemical Corporation, Tokyo, Japan), and reversed-phase C18 (RP-C18) silica gel (50 μm, YMC Co., Ltd., Kyoto, Japan) were used for column chromatography. Thin-layer chromatography (TLC) (GF254, 10–40 μm, Qingdao Bangkai Hi-Tech Materials Co., Ltd., Qingdao, China) was employed to monitor the fractions. TLC spots were visualized by UV light (254 and 365 nm) and further reacted with 20% sulfuric acid spray reagent solution under the condition of heat. HPLC was conducted with an LC-20AT liquid chromatography system equipped with a Shimadzu SPD-20A UV/VIS detector and a Shimadzu RID-20A RI detector (Shimadzu, Kyoto, Japan) using a YMC Pack ODS-A column (250 × 10 mm, 5 μm, YMC Company, Kyoto, Japan). The OD value was determined using a BioTek PowerWave XS2 microplate reader (BioTek, Winooski, VT, USA).
2.2. Reagents and Chemicals
The HPLC-grade solvents, such as methanol and acetonitrile, and the analytical reagent solvents, such as petroleum ether, dichloromethane, ethyl acetate, and ethanol, were purchased from Tianjin Yongda Chemical Reagent Co., Ltd. (Tianjin, China). Tyrosinase (1100 U/mg) from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China) was used as the enzyme. L-tyrosine (98%, Biotopped, Beijing, China) served as the substrate and arbutin (98%, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) was used as a positive control.
2.3. Fungal Material
Vanderbylia robiniophila was collected from Liaoning Province, China, in August 2020 and authenticated based on the morphology analysis and ITS gene sequencing (GenBank Accession number OR116090) (Figure S33) [2]. The fungus was preserved at the Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang.
2.4. Fermentation and Extraction
Vanderbylia robiniophila was cultured on malt extract agar (MEA) plates at 28 °C for 7 days. Then, 5 pieces of mycelia were inoculated in a 250 mL conical flask containing 150 mL liquid medium (3% malt extract) and shaking cultured at 28 °C for 7 days at 140 rpm. Afterward, the seed liquid culture (8 mL) was transferred into rice medium (100 g rice and 100 mL water) and fermented at 28 °C statically for 3 months. Subsequently, the cultures were extracted by 90% EtOH 5 times at room temperature. The residue was suspended in water and partitioned with EtOAc.
2.5. Purification
The EtOAc layer (214 g) was subjected to silica gel CC (200–300 mesh) and stepped gradient elution with petroleum ether-EtOAc (v/v, 50:1, 20:1, 10:1, 5:1, 1:1) and CH2Cl2-CH3OH (v/v, 100:1, 50:1, 30:1, 20:1, 10:1, 5:1, 1:1) to yield fractions A–D. Fr. B (9.5 g) was chromatographed using RP-C18 silica gel (EtOH-H2O, 10–90%) to obtain 5 fractions (Fr.B1–Fr.B5). Fr. B1 (2.5 g) was submitted to silica gel CC (petroleum ether-EtOAc, 50:1-1:1) and separated by HPLC (90.0% CH3CN-H2O) to afford 3 (5.5 mg, tR = 33.8 min). Fr. B2 (1.3 g) was purified by Sephadex LH-20 CC (CH3OH) and separated by HPLC (78.0% CH3CN-H2O) to afford 6 (3.0 mg, tR = 33.8 min) and 7 (2.1 mg, tR = 25.4 min). Fr. B5 (1.2 g) was divided into 4 subfractions (Fr. B5-1~Fr. B5-4) by silica gel with the mobile phase CH2Cl2-CH3OH (from 50:1 to 1:1). Fr. B5-1, B5-3, B5-4 were isolated by HPLC (CH3CN-H2O: 38.0%, 38.0%, 30.0%) to give compounds 8 (3.3 mg, tR = 57.6 min), 9 (1.0 mg, tR = 43.2 min), and 10 (2.8 mg, tR = 27.3 min). Fr.D (40 g) was separated on a glass column packed with HP-20 macroporous resin and eluted with increasing concentrations of EtOH in water to afford 3 subfractions (Fr.D1~Fr.D3). Fr. D1 (4.5 g), which was eluted by petroleum ether-EtOAc (v/v, 50:1, 30:1, 20:1, 10:1, 5:1, 1:1), was further purified using silica gel CC to obtain 3 subfractions (Fr. D1-1~Fr. D1-3). Compounds 1 (3.0 mg, tR = 70.0 min) and 2 (4.9 mg, tR = 44.2 min) were isolated from Fr. D1-1 (320.5 mg) via semipreparative HPLC (90.0% CH3CN-H2O). Compounds 4 (5.3 mg, tR = 34.1 min) and 5 (2.8 mg, tR = 51.7 min) were separated from Fr. D1-3 (200.7 mg) by semipreparative HPLC (78.0% CH3CN-H2O).
2.6. Spectroscopic Data
2.6.1. Novel Compounds
(22E, 24R)-6β, 7α-dimethoxyergosta-8(14), 22-diene-3β, 5α-diol (1): White amorphous solid powder, [α −227.78 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 238 (+1.09); IR (MeOH) υmax: 3680, 2935, 2869, 1455, 1369, 1033, 1013, 971 cm−1; 1H and 13C NMR data, Table 1; HRESIMS m/z 497.3616 [M + Na]+ (calcd for C30H50O4Na, 497.3607). (Figures S1–S9).
Table 1.
The 1H (600 MHz) and 13C NMR (150 MHz) Data a of 1 and 8 in CDCl3.
| 1 (δ in ppm, Multi, J in Hz) | 8 (δ in ppm, Multi, J in Hz) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | δ H | δ C | No. | δ H | δ C | No. | δ H | δ C |
| 1 | 1.75 (m) 1.29 (m) |
31.7 | 16 | 1.43 (o) 1.73 (m) |
27.6 | 2 | - | 172.0 |
| 2 | 1.43 (o) 1.86 (m) |
31.1 | 17 | 1.19 (m) | 57.3 | 3 | - | 125.5 |
| 3 | 4.11 (tt, 11.0, 5.2) | 67.5 | 18 | 0.91 (s) | 17.7 | 4 | - | 157.7 |
| 4 | 1.69 (m) 1.95 (m) |
40.6 | 19 | 0.91 (s) | 17.8 | 5 | - | 106.9 |
| 5 | - | 76.8 | 20 | 2.14 (m) | 39.4 | 6 | 1.75 (m) 1.97 (m) |
36.2 |
| 6 | 3.12 (d, 2.3) | 85.7 | 21 | 1.05 (d, 6.7) | 21.5 | 7 | 1.17 (m) 1.27 (o) |
23.0 |
| 7 | 4.22 (d, 2.3) | 76.4 | 22 | 5.19 (dd, 15.3, 8.2) | 135.4 | 8 | 1.27 (o) | 29.4 |
| 8 | - | 122.2 | 23 | 5.25 (dd, 15.3, 7.3) | 132.5 | 9 | 1.27 (o) | 29.3 |
| 9 | 2.33 (m) | 36.7 | 24 | 1.86 (m) | 43.0 | 10 | 1.27 (o) | 29.2 |
| 10 | - | 41.1 | 25 | 1.48 (m) | 33.2 | 11 | 1.27 (o) | 29.2 |
| 11 | 1.53 (m) 1.57 (m) |
19.3 | 26 | 0.84 (d, 6.8) | 20.1 | 12 | 1.60 (m) | 25.0 |
| 12 | 1.16 (m) 2.00 (m) |
37.1 | 27 | 0.83 (d, 6.7) | 19.8 | 13 | 2.29 (t, 7.5) | 34.2 |
| 13 | - | 43.9 | 28 | 0.93 (d, 6.8) | 17.4 | 14 | - | 174.5 |
| 14 | - | 153.6 | 5-OH | 4.40 (s) | - | 15 | 1.82 (d, 1.2) | 8.6 |
| 15 | 2.32 (m) 2.40 (m) |
25.8 | 6-OCH3 | 3.36 (s) | 59.5 | 16 | 1.94 (d, 1.2) | 10.8 |
| 7-OCH3 | 3.21 (s) | 54.7 | 14-OCH3 | 3.66 (s) | 51.6 | |||
a Overlapping signals are expressed as o.
Vanderbyliolide A (8): Colorless transparent oil, [α −744.44 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 235 (+1.28); IR (MeOH) υmax: 3680, 2923, 2858, 1737, 1436, 1381, 1261, 1033, 960, 899, 765 cm−1; 1H and 13C NMR data, Table 1; HRESIMS m/z 321.1697 [M + Na]+ (calcd for C16H26O5Na, 321.1678). (Figures S22–S28).
2.6.2. Known Compounds
(22E, 24R)-6β-methoxyergosta-7, 9(11), 22-triene-3β, 5α-diol (2): 1H NMR (600 MHz, CDCl3) δH 5.71 (1H, d, J = 6.9, 2.1 Hz), 5.52 (1H, d), 5.24 (1H, dd, J = 15.3, 7.6 Hz), 5.17 (1H, dd, J = 15.3, 8.3 Hz), 4.10 (1H, m), 3.42 (3H, s), 3.34 (1H, dd, J = 5.6, 2.0 Hz), 2.35 (1H, m), 2.27 (1H, m), 2.15 (1H, m), 2.05 (1H, o), 2.05 (1H, o), 1.95 (1H, m), 1.86 (1H, m), 1.80 (1H, o), 1.80 (1H, o), 1.80 (1H, o), 1.80 (1H, o), 1.74 (1H, m), 1.56 (1H, m), 1.48 (1H, o), 1.48 (1H, o), 1.34 (1H, o), 1.34 (1H, o), 1.21 (3H, s), 1.02 (3H, d, J = 6.6 Hz), 0.92 (3H, d, J = 6.8 Hz), 0.84 (3H, d, J = 6.8 Hz), 0.83 (3H, d, J = 6.9 Hz), 0.60 (3H, s) ppm; 13C NMR (150 MHz, CDCl3) δC 140.6, 138.9, 135.5, 132.4, 126.0, 116.3, 82.9, 75.7, 67.9, 58.5, 56.2, 51.7, 43.0, 42.7, 42.5, 41.0, 40.5, 38.4, 33.2, 31.3, 31.1, 28.9, 26.0. 23.3, 20.8, 20.1, 19.8, 17.8, 11.6 ppm. (Figures S10 and S11).
5α, 6α-epoxy-3β-hydroxy-(22E)-ergosta-8(14), 22-dien-7-one (3): 1H NMR (600 MHz, CDCl3) δH 5.24 (1H, m), 5.19 (1H, dd, J = 15.3, 7.9 Hz), 3.92 (1H, tt, J = 11.3, 4.6 Hz), 3.16 (1H, s), 2.72 (2H, o), 2.72 (2H, o), 2.24 (1H, dd, J = 13.4, 11.6 Hz), 2.14 (1H, m), 2.01 (1H, o), 2.01 (1H, o), 1.87 (1H, m), 1.80 (1H, m), 1.75 (1H, m), 1.59 (1H, o), 1.59 (1H, o), 1.48 (1H, o), 1.48 (1H, o), 1.48 (1H, o), 1.48 (1H, o), 1.48 (2H, o), 1.24 (1H, m), 1.04 (3H, d, J = 6.7 Hz), 0.92 (3H, m), 0.92 (3H, m), 0.84 (3H, m), 0.82 (3H, m) ppm; 13C NMR (150 MHz, CDCl3) δC 195.6, 171.4, 134.9, 132.8, 124.0, 68.9, 68.9, 63.0, 54.7, 46.2, 43.0, 41.4, 39.2, 39.0, 36.4, 35.7, 33.2, 32.9, 31.2, 30.7, 27.9, 21.5, 20.1, 19.8, 19.6, 19.2, 17.8, 16.3 ppm. (Figures S12 and S13).
3β, 5α, 9α-trihydroxy- (22E, 24R)-ergosta-7, 22-dien-6-one (4): 1H NMR (600 MHz, CDCl3) δH 5.62 (1H, s), 5.23 (1H, dd, J = 15.2, 7.6 Hz), 5.16 (1H, dd, J = 15.2, 8.4 Hz), 4.06 (1H, m), 2.76 (1H, m), 2.34 (1H, m), 2.10 (1H, m), 1.94 (1H, m), 1.94 (1H, m), 1.87 (1H, o), 1.87 (1H, o), 1.80 (1H, m), 1.74 (1H, o), 1.74 (1H, o), 1.74 (1H, o), 1.58 (1H, m), 1.47 (1H, o), 1.47 (1H, o), 1.47 (1H, o), 1.47 (1H, o), 1.35 (1H, m), 1.03 (1H, d, J = 6.6 Hz), 0.99 (3H, s), 0.92 (3H, d, J = 6.8 Hz), 0.84 (3H, d, J = 6.8 Hz), 0.82 (3H, d, J = 6.8 Hz), 0.61 (3H, s) ppm; 13C NMR (150 MHz, CDCl3) δC 198.5, 165.1, 135.2, 132.6, 119.9, 79.7, 74.8, 67.4, 56.1, 51.9, 45.5, 43.0, 41.9, 40.4, 37.0, 35.1, 33.2, 30.1, 28.8, 28.0, 25.7, 22.6, 21.2, 20.5, 20.1, 19.8, 17.8, 12.4 ppm. (Figures S14 and S15).
(22E, 24R)-ergost-7, 22-dien-3β, 5α-diol-6-one (5): 1H NMR (600 MHz, CDCl3) δH 5.65 (1H, s), 5.24 (1H, dd, J = 15.3, 7.7 Hz), 5.16 (1H, dd, J = 15.3, 8.5 Hz), 4.04 (1H, m), 2.52 (1H, ddd, J = 12.1, 6.9, 2.5 Hz), 2.12 (1H, o), 2.12 (1H, o), 2.12 (1H, o), 2.04 (1H, o), 2.04 (1H, o), 1.87 (1H, m), 1.78 (1H, o), 1.78 (1H, o), 1.73 (1H, o), 1.73 (1H, o), 1.62 (1H, o), 1.62 (1H, o), 1.62 (1H, o), 1.62 (1H, o), 1.47 (1H, o), 1.47 (1H, o), 1.47 (1H, o), 1.36 (1H, m), 1.25 (1H, m), 1.03 (3H, d, J = 6.6 Hz), 0.95 (3H, s), 0.92 (3H, d, J = 6.8 Hz), 0.84 (3H, d, J = 6.8 Hz), 0.82 (3H, d, J = 6.8 Hz), 0.60 (3H, s) ppm; 13C NMR (150 MHz, CDCl3) δC 198.4, 165.4, 135.2, 132.7, 119.9, 78.0, 67.6, 56.2, 56.0, 44.9, 44.0, 43.0, 40.6, 40.4, 39.0, 36.7, 33.2, 30.5, 30.4, 28.0, 22.6, 22.1, 21.3, 20.1, 19.8, 17.7, 16.6, 12.8 ppm. (Figures S16 and S17).
Dankasterone (6): 1H NMR (600 MHz, CDCl3) δH 6.36 (1H, s), 5.27 (1H, m), 5.26 (1H, m), 2.81 (1H, t, J = 9.1 Hz), 2.66 (1H, d, J = 16.9, 1.5 Hz), 2.51 (1H, o), 2.51 (1H, o), 2.47 (2H, o), 2.47 (1H, o), 2.42 (1H, m), 2.03 (2H, o), 2.03 (1H, o), 1.87 (1H, o), 1.87 (1H, o), 1.87 (1H, o), 1.77 (1H, m), 1.71 (1H, o), 1.71 (1H, o), 1.48 (1H, o), 1.48 (1H, o), 1.26 (3H, s), 1.09 (3H, d, J = 7.0 Hz), 0.98 (3H, s), 0.91 (3H, d, J = 6.8 Hz), 0.83 (3H, d, J = 6.7 Hz), 0.81 (3H, d, J = 6.8 Hz) ppm; 13C NMR (150 MHz, CDCl3) δC 214.9, 200.2, 199.3, 156.2, 135.3, 132.5, 126.7, 62.3, 54.1, 49.5, 49.5, 43.4, 41.0, 39.1, 38.5, 38.1, 37.4, 36.2, 34.5, 33.2, 25.3, 24.2, 23.8, 23.3, 20.2, 19.8, 17.8, 17.2 ppm. (Figures S18 and S19).
4-hydroxy-17R-methylincisterol (7): 1H NMR (600 MHz, CDCl3) δH 5.64 (1H, d, J = 1.8 Hz), 5.26 (1H, d, J = 15.3, 8.5 Hz), 5.17 (1H, d, J = 15.3, 7.8 Hz), 2.64 (1H, m), 2.28 (1H, ddd, J = 14.2, 4.2, 2.4 Hz), 2.06 (1H, m), 1.98 (1H, ddd, J = 13.4, 4.8, 2.5 Hz), 1.90 (1H, m), 1.88 (1H, m), 1.85 (1H, m), 1.73 (1H, m), 1.63 (1H, m), 1.49 (1H, o), 1.49 (1H, o), 1.49 (1H, o), 1.49 (1H, o), 1.04 (3H, d, J = 6.7 Hz), 0.92 (3H, d, J = 6.8 Hz), 0.84 (3H, d, J = 6.8 Hz), 0.83 (3H, d, J = 6.8 Hz), 0.61 (3H, s) ppm; 13C NMR (150 MHz, CDCl3) δC 170.9, 170.6, 134.8, 133.0, 112.5, 104.9, 55.5, 50.5, 49.0, 43.0, 40.3, 35.4, 35.2, 33.2, 29.0, 21.5, 21.2, 20.1, 19.8, 17.7, 11.9 ppm. (Figures S20 and S21).
Cornilkone C (9): [α -723.55 (c 0.1, MeOH); 1H NMR (600 MHz, CDCl3) δH 7.44 (1H, dd, J = 5.7, 1.5 Hz), 6.11 (1H, dd, J = 5.7, 2.0 Hz), 5.03 (1H, ddd, J = 7.2, 5.3, 2.7 Hz), 3.67 (3H, s), 2.30 (2H, t, J = 7.5 Hz), 1.76 (1H, m), 1.64 (2H, o), 1.64 (2H, o), 1.31 (2H, o), 1.31 (2H, o), 1.31 (2H, o), 1.31 (2H, o) ppm; 13C NMR (150 MHz, CDCl3) δC 174.4, 173.3, 156.4, 121.7, 83.5, 51.6, 34.2, 33.3, 29.2, 29.1, 29.1, 25.0, 25.0 ppm. (Figures S29 and S30).
(1H-indol-3-yl) oxoacetic acid methyl ester (10): 1H NMR (600 MHz, CDCl3) δH 8.51 (1H, d, J = 3.2 Hz), 8.46 (1H, m), 7.45 (1H, m), 7.36 (1H, m), 7.34 (1H, m), 3.96 (3H, s) ppm; 13C NMR (150 MHz, CDCl3) δC 177.8, 163.3, 136.6, 136.1, 126.2, 124.7, 123.8, 122.8, 114.6, 111.7, 52.9 ppm. (Figures S31 and S32).
2.7. ECD Calculations
The conformational searches were carried out employing SPARTAN 14 in the MMFF force field. The conformers under the 3.0 kJ/mol energy window were further optimized at the B3LYP/6-31G(d) level by DFT using the Gaussian 09 package [29]. The optimized stable conformers were calculated using TDDFT methods at the B3LYP/6-311+g(d,p) level with an implicit solvent model for CDCl3. Then, the calculated ECD curve weighted by the Boltzmann distribution was produced by SpecDis 1.71 [30].
2.8. Bioassays
2.8.1. Anti-Tyrosinase Activities
The inhibitory effects of the isolated compounds on the tyrosinase were determined according to the literature procedure with some modifications [31]. L-tyrosine solution was used as substrate in concentration of 2 mM (40 uL). Compounds 1–10 (40 μL) with increased concentrations (5, 10, 50, 100, 200 μM), tyrosinase (40 μL, 100 U/mL), and 80 µL of phosphate-buffered saline (PBS) solution (25 mM, pH 6.8) were added to a 96-well microplate. A quantity of 40 μL 20% MeOH solution took the place of the sample as a blank solution. The mixtures were incubated at 37 °C for 30 min. After incubation, the produced dopachrome was measured at 492 nm by a microplate reader. The inhibition ratio was calculated by [1 − (C − D)/(A − B) × 100%], where A is the absorbance of blank solution after incubation, B is the absorbance of blank solution before incubation, C is the absorbance of sample solution after incubation, and D is the absorbance of sample solution before incubation. The IC50 values and statistical analyses were performed using GraphPad Prism 7 software, and the results were expressed as means ± SD of triplicate determination.
2.8.2. Kinetic Analysis
The inhibition type was measured by the reaction rate–substrate concentration curve and the Lineweaver–Burk plot. L-tyrosine solutions were diluted to different concentrations (5 mM, 7.5 mM, 9 mM, 12 mM, 15 mM) as the substate. The inhibition constant was determined by the second plot of the y-intercept versus the concentration of the inhibitor. The values of Kis were calculated from the following formula:
3. Results and Discussion
3.1. Structure Elucidation
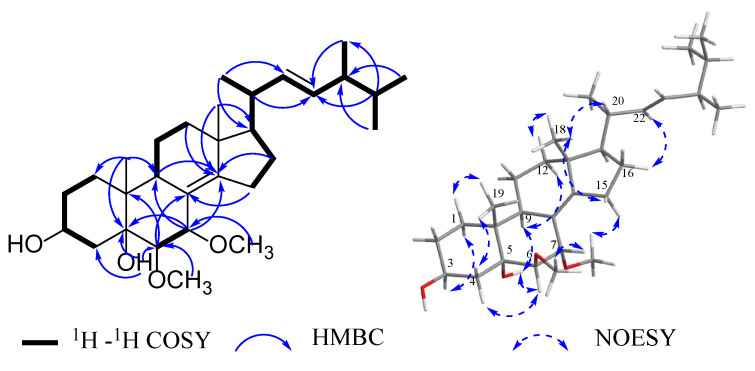
Compound 1, a white amorphous solid powder, was assigned the molecular formula C30H50O4 by analyses of HRESIMS m/z 497.3616 [M + Na]+ (calcd for C30H50O4Na, 497.3607) and NMR data (Table 1). 1H NMR spectrum of 1 revealed characteristic signals for two methyl singlets at δH 0.91 (H-18 and H-19 with the specific data δH 0.911 and 0.907), four methyl doublets at δH 1.05 (3H, J = 6.7 Hz, H-21), 0.84 (3H, J = 6.8 Hz, H-26), 0.83 (3H, J = 6.7 Hz, H-27) and 0.93 (3H, J = 6.8 Hz, H-28), two disubstituted double bonds at δH 5.19 (1H, dd, J = 15.3, 8.2 Hz, H-22), 5.25 (1H, dd, J = 15.3, 7.3 Hz, H-23), a sp3 oxygenated methine group at δH 4.11 (1H, tt, J = 11.0, 5.2 Hz, H-3), and two methoxy groups at δH 3.36 (3H, s, 6-OCH3), 3.21 (3H, s, 7-OCH3). The 13C NMR data (Table 1) showed resonances for four olefinic carbons [δC 122.2 (C-8), 132.5 (C-14), 135.4 (C-22), 153.6 (C-23)], four oxygenated carbons [δC 67.5 (C-3), 76.4 (C-7), 76.8 (C-5), 85.7 (C-6)], two methoxy groups (δC 54.7, 59.5) and the other 22 signals for aliphatic carbons. The above results indicated that the planar structure of 1 was most likely an ergostane-type steroid. The 1H-1H COSY correlations of H-1/H-2/H-3/H-4, H-6/H-7, H-9/H-11/H-12, together with the HMBC correlations of H-6/C-8, 10, H-7/C-5, 9, 14, H-9, 12, 16, 18/C-14, H-19/C-1, 5, 9, and methoxyl protons/C-6, 7 (Figure 2) further verified the steroidal tetracyclic skeleton of 1 with two hydroxy groups located at C-3 and C-5, two methoxyl groups linked to C-6 and C-7, and a Δ8(14) double bond. A long spin-coupling system from H-15 to H-28 recognized in the 1H-1H COSY spectrum, combined with the HMBC correlations of H-26,27/C-24, H-25/C-23,28, H-20,28/C-23, H-21/C-17,22, established the alkyl side chain at C-17 with a trans double bond between C-22 and C-23.
Figure 2.
Key 1H-1H COSY, HMBC and NOESY correlations of compound 1.
The relative configuration of 1 was elucidated as shown on the basis of a NOESY experiment (Figure 2). The α-orientation of H-3 and H-6 was ascertained by the cross-peaks in sequences of H-19/H-1α, 4α, H-3/H-1β, and H-4α/H-6/5-OH/H-10, while the β-orientation of H-7 was evidenced by the correlation of H-7/6-OCH3. The observation of NOE signals between H-18 and H-12β, 15β, 20, H-22 and H-16, 7-OCH3, and H-15α was consistent with the α-oriented H-17 and the 20R* stereoisomer. The 24R configuration was determined by comparing the 13C NMR resonance peaks of C-28 (δC 17.4) in CDCl3, which should be about 0.4 ppm downfield shifted for C-28 in the 24S epimer [32,33]. The above data showed that 1 was a 6,7-OCH3 substituted ergostane-type steroid compared to the known compound [34]. Therefore, the structure of 1 was identified as (22E, 24R)-6β, 7α-dimethoxyergosta-8(14), 22-diene-3β, 5α-diol.
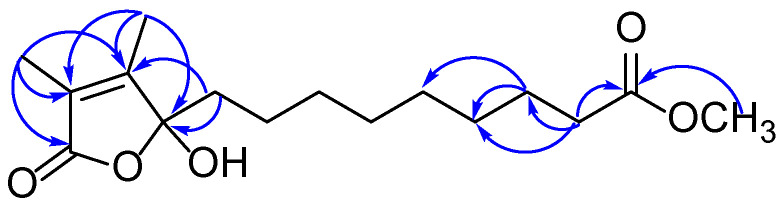
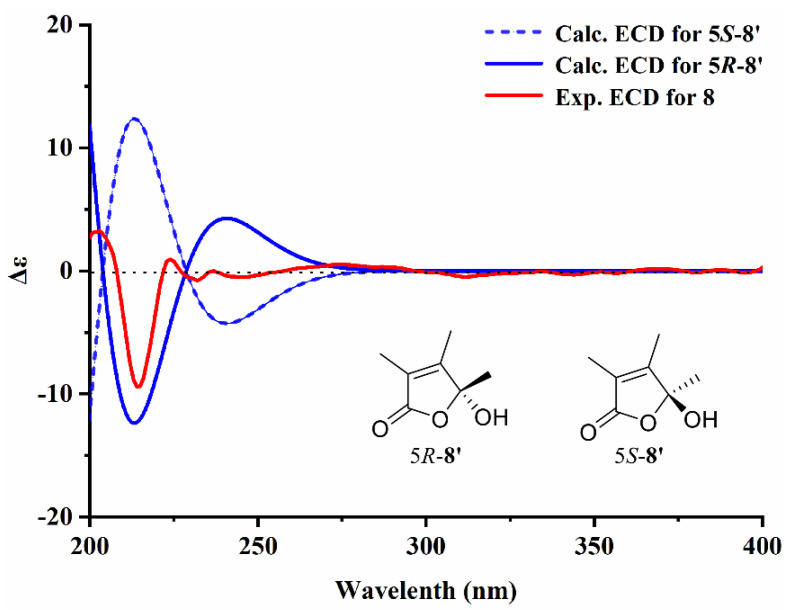
Compound 8 was isolated as a transparent oil with the chemical formula of C16H26O5 deduced from HRESIMS m/z 321.1697 [M + Na]+ (calcd for C16H26O5Na, 321.1678). Analysis of the 1D and 2D NMR spectra of 8 revealed that a sets of long-range coupling methyls [δH 1.82 (3H, d, J = 1.2 Hz, H-15), 1.94 (3H, d, J = 1.2 Hz, H-16) and δC 8.6 (C-15), 10.8 (C-16)], two olefinic carbons [δC 157.7 (C-3) and 125.5 (C-4)], an ester carbonyl [δC 172.0 (C-2)] and an oxygenated quaternary carbon [δC 106.9 (C-5)] formed a α, β-unsaturated 2,3-dimethyl-γ-lactone moiety by key HMBC correlations from H-15 to C-2, 4 and from H-16 to C-3, 5 (Figure 3). The monocyclic system was further confirmed by the four degrees of unsaturation. Moreover, a methyl nonanoate side chain was established by the signals of methylene groups at δH 2.29 (2H, t, J = 7.5 Hz, H-13), 1.97 (1H, m, H-6a), 1.75 (1H, m, H-6b), 1.60 (1H, m, H-12), 1.17–1.27 (10H, overlap, H-1~11) and terminal COOCH3 unit at δH 3.66 (3H, s, 14-OCH3) and δC 174.5 (C-14), as well as the HMBC correlations from 14-OCH3 and H-12, 13 to C-14. It was directly connected to C-5 in the lactonic ring deciphered by the key interactions from H-6 to C-5 in the HMBC spectrum. Given the flexible aliphatic chain could produce excess conformations, the calculated ECD method was applied on a simplified model compound to determine the absolute configuration of 8 at B3LYP/6-31G (d, p) level in MeOH (Figure 4). As a result, the experimental Cotton effects of 8 were in agreement with the calculated Cotton effects for 5S-8′. These data were similar to the known compound caulerpalide A [35]; the only difference between them is that an ethoxy group at position C-14 in caulerpalide A was substituted by a methoxy group in 8. Thus, compound 8 was established as shown and named vanderbyliolide A.
Figure 3.
Key HMBC correlations of compound 8.
Figure 4.
Calculated and experimental ECD spectra for 8.
Eight known compounds were identified as (22E, 24R)-6β-methoxyergosta-7, 9(11), 22-triene-3β, 5α-diol (2) [36], 5α,6α-epoxy-3β-hydroxy-(22E)-ergosta-8(14),22-dien-7-one (3) [37], 3β, 5α, 9α-trihydroxy-(22E, 24R)-ergosta-7, 22-dien-6-one (4) [38], (22E, 24R)-ergost-7, 22-dien-3β, 5α-diol-6-one (5) [39], dankasterone (6) [40], 4-hydroxy-17R-methylincisterol (7) [41], cornilkone C (9) [42], and (1H-indol-3-yl) oxoacetic acid methyl ester (10) [43] by comparing their spectroscopic data with the literature. All compounds were isolated from the species of the genus Vanderbylia for the first time. Among them, cornilkone C (9) was first isolated from fungi. It was originally discovered and purified from corn silk as a pair of enantiomeric compounds with weak anti-Aβ1–42 aggregation activity [42]. Notably, the type of 2(5H)-furanone derivatives (8–9) in our study exhibited significant specific rotations, which are significantly different from the minor magnitudes in the literature. It was indicated that 8 and 9 derived from fungi were likely to be optical pure compounds or racemic mixtures in different proportions. Compound 10 had been obtained from marine-derived fungi with weak cytotoxic activity against HeLa cells [44,45,46]. It was detected from macrofungi for the first time in this study.
3.2. Anti-Tyrosinase Activities
Tyrosinase inhibitors have been clinically used for diseases associated with melanin hyperpigmentation [47]. In this study, the inhibitory effects of 1–10 on tyrosinase were determined spectrophotometrically compared to the positive control arbutin (Table 2). Among them, compound 10 showed the highest tyrosinase inhibitory activity with IC50 values of 60.47 ± 2.63 μM, which were comparable to those of arbutin (IC50 = 58.17 ± 6.09 μM). Compounds 2, 4, 5, and 8 exhibited weak inhibitory activities with IC50 values ranging from 94.16 to 148.38 μM. The remaining compounds did not show any tyrosinase inhibitory activity at the texted concentration. These observations demonstrated that the indole alkaloid exhibited much stronger inhibitory potency than the other structures. It has been reported that the amino groups such as an indole ring could improve the tyrosinase inhibitory activity, which was consistent with our results [48,49]. Among the isolated steroids, compound 6 has a structural feature of C-ring migration and compound 7 is a highly degraded sterol belonging to the class incisterols. They were inactive even at 200 μM, indicating the importance of the tetracyclic ergostane-type scaffold. Detailed inspection of the structures of compounds 2, 4, and 5 showed the common features of a double bond at Δ7(8) within these molecules, which indicated that the position of olefinic bond is crucial for the activity.
Table 2.
The inhibitory effects of 1–10 on tyrosinase.
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | >200 | 6 | >200 |
| 2 | 148.38 ± 23.67 | 7 | >200 |
| 3 | >200 | 8 | 102.53 ± 4.05 |
| 4 | 116.36 ± 13.45 | 9 | >200 |
| 5 | 94.16 ± 13.69 | 10 | 60.47 ± 2.63 |
| Arbutin | 58.17 ± 6.09 |
3.3. Enzyme Kinetic Analysis
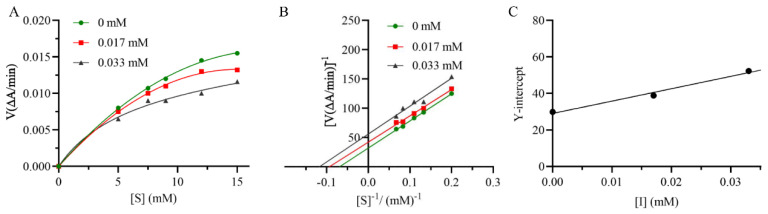
To confirm the inhibition mechanism of the most potent compound (10) on the inhibitory activity of tyrosinase, the V-S and Lineweaver–Burk plots were constructed. As shown in Figure 5A,B, both Km and Vm values of 10 decreased with the increase in concentration, but the ratio of Km/Vm remained unchanged. Thus, 10 belongs to an uncompetitive inhibitor, demonstrating that it inhibits the enzyme by combining with the enzyme–substrate complex. The inhibition constant Kis was obtained from the plot of the y-intercept versus the concentration of 10, which was calculated to be 0.04 mM (Figure 5C).
Figure 5.
Inhibition mechanism on tyrosinase of compound 10: (A) reaction rate–substrate concentration curve; (B) Lineweaver–Burk plots; (C) the secondary replots of y-intercept vs. [inhibitor].
4. Conclusions
In summary, two new compounds, (22E, 24R)-6β, 7α-dimethoxyergosta-8(14), 22-diene-3β, 5α-diol (1) and vanderbyliolide A (8), together with eight known ones (2–7, 9–10), were isolated from the cultures of Vanderbylia robiniophila. All compounds were discovered from the genus for the first time. Compound 10 showed potential tyrosinase inhibitory activity comparable to that of arbutin. The kinetics of the enzymatic reaction indicated that 10 was an uncompetitive inhibitor on tyrosinase. This study provides evidence for the development and utilization of V. robiniophila in skin disorders associated with melanin hyperpigmentation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9070702/s1, Figures S1–S32: The spectrum of compounds 1–10; Figure S33: Basidiomes of Vanderbylia robiniophila.
Author Contributions
Conceptualization, H.Y. and Y.W. (Yuxi Wang); methodology, Y.W. (Yuxi Wang) and J.J.; data analysis and bioassays, Q.W. and J.J.; writing-original draft preparation, Y.W. (Yuxi Wang); writing—review and editing, H.Y. and Y.W. (Yulian Wei). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are included in manuscript and Supplementary Materials files.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was financed by the China Postdoctoral Science Foundation (2022M713296), the National Natural Science Foundation of China (Project No. 32100013 and 32270017) and the Shenyang Science and Technology Plan (22-322-3-07).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pavic A., Ilic-Tomic T., Glamočlija J. Unravelling anti-melanogenic potency of edible mushrooms Laetiporus sulphureus and Agaricus silvaticus in vivo using the zebrafish model. J. Fungi. 2021;7:834. doi: 10.3390/jof7100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y.M., Huang J.H., Lu J.Y., Ding Y.F., Jiang L., Hu S.H., Chen J., Zeng Q.H. The role and mechanism of Asian medicinal plants in treating skin pigmentary disorders. J. Ethnopharmacol. 2019;245:112173–112186. doi: 10.1016/j.jep.2019.112173. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.Y., Baek N., Nam T. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016;31:1–13. doi: 10.3109/14756366.2015.1004058. [DOI] [PubMed] [Google Scholar]
- 4.Kishore N., Twilley D., Blom van Staden A., Verma P., Singh B., Cardinali G., Kovacs D., Picardo M., Kumar V., Lall N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018;81:49–56. doi: 10.1021/acs.jnatprod.7b00564. [DOI] [PubMed] [Google Scholar]
- 5.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caritá A.C., Fonseca-Santos B., Shultz J.D., Michniak-Kohn B., Chorilli M., Leonardi G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine. 2020;24:102117–102132. doi: 10.1016/j.nano.2019.102117. [DOI] [PubMed] [Google Scholar]
- 7.Shim J.H. Inhibitory effects of cycloheterophyllin on melanin synthesis. Molecules. 2021;26:2526. doi: 10.3390/molecules26092526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald T.A., Holland N.T., Skibola C., Duramad P., Smith M.T. Hypothesis: Phenol and hydroquinone derived mainly from diet and gastrointestinal flora activity are causal factors in leukemia. Leukemia. 2001;15:10–20. doi: 10.1038/sj.leu.2401981. [DOI] [PubMed] [Google Scholar]
- 9.Fogarasi M., Socaci S.A., Dulf F.V., Diaconeasa Z.M., Fărcaș A.C., Tofană M., Semeniuc C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules. 2018;23:3272. doi: 10.3390/molecules23123272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogarasi M., Diaconeasa Z.M., Pop C.R., Fogarasi S., Semeniuc C.A., Fărcaş A.C., Țibulcă D., Sălăgean C.-D., Tofană M., Socaci S.A. Elemental composition, antioxidant and antibacterial properties of some wild edible mushrooms from Romania. Agronomy. 2020;10:1972. doi: 10.3390/agronomy10121972. [DOI] [Google Scholar]
- 11.Schueffler A., Anke T. Fungal natural products in research and development. Nat. Prod. Rep. 2014;31:1425–1448. doi: 10.1039/C4NP00060A. [DOI] [PubMed] [Google Scholar]
- 12.Gressler M., Lohr N.A., Schafer T., Lawrinowitz S., Seibold P.S., Hoffmeister D. Mind the mushroom: Natural product biosynthetic genes and enzymes of Basidiomycota. Nat. Prod. Rep. 2021;38:702–722. doi: 10.1039/D0NP00077A. [DOI] [PubMed] [Google Scholar]
- 13.Rittenhouse S., Biswas S., Broskey J., McCloskey L., Moore T., Vasey S., West J., Zalacain M., Zonis R., Payne D. Selection of retapamulin, a novel pleuromutilin for topical use. Antimicrob. Agents Chemother. 2006;50:3882–3885. doi: 10.1128/AAC.00178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiba K. Discovery of fingolimod based on the chemical modification of a natural product from the fungus, Isaria sinclairii. J. Antibiot. 2020;73:666–678. doi: 10.1038/s41429-020-0351-0. [DOI] [PubMed] [Google Scholar]
- 15.Pan J., Yang C.H., Jiang Z., Huang J. Trametes robiniophila Murr: A traditional Chinese medicine with potent anti-tumor effects. Cancer Manag. Res. 2019;11:1541–1549. doi: 10.2147/CMAR.S193174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q., Shu C., Laurence A.D., Chen Y., Peng B.G., Zhen Z.J., Cai J.Q., Ding Y.T., Li L.Q., Zhang Y.B., et al. Effect of Huaier granule on recurrence after curative resection of HCC: A multicentre, randomised clinical trial. Gut. 2018;67:2006–2016. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Wang X., Chen T., Wang W., Yang Q. Trametes robiniophila Murr in the treatment of breast cancer. Biomed. Pharm. 2020;128:110254–110264. doi: 10.1016/j.biopha.2020.110254. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y.X., Yuan H.S. Research progress on chemical constituents and anti-tumor effects of Huai’er (Vanderbylia robiniophila) Mycosystema. 2021;40:411–422. [Google Scholar]
- 19.Lv F., Li X., Wang Y. An extraction from Trametes robiniophila Murr. (Huaier) inhibits non-small cell lung cancer proliferation via targeting to epidermal growth factor receptor. Bioengineered. 2022;13:10931–10943. doi: 10.1080/21655979.2022.2066757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu Y.J., Shan L., Gao H., Zhang C.C., Qian Z.J., Wang Z.X., Xu X., Zhang X., Wang J.Y., Ma L.F., et al. Huaier suppresses the hepatocellular carcinoma cell cycle by regulating minichromosome maintenance proteins. OncoTargets Ther. 2020;13:12015–12025. doi: 10.2147/OTT.S279723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Z.D., Yang A.L., Su G.Z., Zhao Y.F., Wang Y., Chai X.Y., Tu P.F. Huaier restrains proliferative and invasive potential of human hepatoma SKHEP-1 cells partially through decreased Lamin B1 and elevated NOV. Sci. Rep. 2016;6:31298–31307. doi: 10.1038/srep31298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Qi W., Song X., Lv S., Zhang H., Yang Q. Huaier extract suppresses breast cancer via regulating tumor-associated macrophages. Sci. Rep. 2016;6:20049–20059. doi: 10.1038/srep20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan L., Li Y., Jiang H.Y., Tao Y.Q., Qian Z.J., Li L., Cai F., Ma L.F., Yu Y.C. Huaier restrains proliferative and migratory potential of hepatocellular carcinoma cells partially through decreased yes-associated protein 1. J. Cancer. 2017;8:4087–4097. doi: 10.7150/jca.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.J., Yu Z.X., Wei C., Zhang L., Song H., Chen B., Yang Q.F. Huaier aqueous extract protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NLRP3 inflammasome activation. Oncotarget. 2017;8:32937–32945. doi: 10.18632/oncotarget.16513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Z.Y., Hu X.P., Xiong H., Qiu H., Yuan X.L., Zhu F., Wang Y.H., Zou Y.M. A polysaccharide from Huaier induced apoptosis in MCF-7 breast cancer cells via downregulation of MTDH protein. Carbohydr. Polym. 2016;151:1027–1033. doi: 10.1016/j.carbpol.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 26.Fang L., Zhang Y.Z., Zang Y.W., Chai R., Zhong G.X., Li Z.Y., Duan Z.C., Ren J.C., Xu Z.H. HP-1 inhibits the progression of ccRCC and enhances sunitinib therapeutic effects by suppressing EMT. Carbohydr. Polym. 2019;223:115109–115122. doi: 10.1016/j.carbpol.2019.115109. [DOI] [PubMed] [Google Scholar]
- 27.Jian T.T., Zhang Y., Zhang G.Y., Ling J.Y. Metabolomic comparison between natural Huaier and artificial cultured Huaier. Biomed. Chromatogr. 2022;36:e5355–e5369. doi: 10.1002/bmc.5355. [DOI] [PubMed] [Google Scholar]
- 28.Li K.X., Lv J.H., Dong J., Fan D.Y., Wang Y.P., Li C.T. Chemical constituents from the ethyl acetate of fermentation liquid in Trames robiniophila. Chin. Tradit. Pat. Med. 2022;44:445–450. [Google Scholar]
- 29.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. The Gaussian 09 Package. Gaussian, Inc.; Wallingford, CT, USA: 2013. [Google Scholar]
- 30.Bruhn T., Schaumloffel A., Hemberger Y., Bringmann G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality. 2013;25:243–249. doi: 10.1002/chir.22138. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y., Zhou G.X., Kurihara H., Ye W.C., Yao X.S. Biphenyl glycosides from the fruit of Pyracantha fortuneana. J. Nat. Prod. 2006;69:1022–1024. doi: 10.1021/np0600853. [DOI] [PubMed] [Google Scholar]
- 32.Yan X.H., Liu H.L., Huang H., Li B.X., Guo Y.W. Steroids with aromatic A-rings from the Hainan soft coral Dendronephthya studeri Ridley. J. Nat. Prod. 2011;74:175–180. doi: 10.1021/np100562n. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi T., Anami D., Morikawa S., Nakagawa Y., Yamada T., Li W., Hirano T. Secoergostane-and ergostane-type steroids from Pleurotus cornucopiae var. citrinopileatus. Phytochemistry. 2023;206:113552–113559. doi: 10.1016/j.phytochem.2022.113552. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., Tian L., Huang J., Li W., Pei Y.H. Cytotoxic sterols from marine-derived fungus Pennicillium sp. Nat. Prod. Res. 2006;20:381–384. doi: 10.1080/14786410600661229. [DOI] [PubMed] [Google Scholar]
- 35.Li D.C., Yang Y., Zhang B., Liao X.J., Jiang Z.H., Xu S.H., Zhao B.X. Three New Butenolides from the Green Alga Caulerpa racemosa var. turbinate. Chem. Biodivers. 2020;17:2000022–2000030. doi: 10.1002/cbdv.202000022. [DOI] [PubMed] [Google Scholar]
- 36.Bao F.Y., Yang K.Y., Wu C.R., Gao S.Y., Wang P.H., Chen L.X., Li H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia. 2018;125:123–129. doi: 10.1016/j.fitote.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Yaoita Y., Satoh Y., Kikuchi K. A new ceramide from Ramaria botrytis (Pers.) Ricken. J. Nat. Med. 2007;61:205–207. doi: 10.1007/s11418-006-0121-8. [DOI] [Google Scholar]
- 38.Wang H., Liu T.X., Xin Z.H. A new glucitol from an endophytic fungus Fusarium equiseti Salicorn. Eur. Food Res. Technol. 2014;239:365–376. doi: 10.1007/s00217-014-2230-z. [DOI] [Google Scholar]
- 39.Shang X.Y., Li J.J., Liu M.T., Li S., Liu Y., Wang Y.F., Huang X., Jin Z.L. Cytotoxic steroids from Monascus purpureus-fermented rice. Steroids. 2011;76:1185–1189. doi: 10.1016/j.steroids.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Amagata T., Tanaka M., Yamada T., Doi M., Minoura K., Ohishi H., Yamori T., Numata A. Variation in cytostatic constituents of a sponge-derived Gymnascella dankaliensis by manipulating the carbon source. J. Nat. Prod. 2007;70:1731–1740. doi: 10.1021/np070165m. [DOI] [PubMed] [Google Scholar]
- 41.Togashi H., Mizushina Y., Takemura M., Sugawara F., Koshino H., Esumi Y., Uzawa J., Kumagai H., Matsukage A., Yoshida S., et al. 4-hydroxy-17-methylincisterol, an inhibitor of DNA polymerase-α activity and the growth of human cancer cells in vitro. Biochem. Pharm. 1998;56:583–590. doi: 10.1016/S0006-2952(98)00197-X. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W.Y., Lou L.L., Guo R., Xi Y.F., Lin B., Huang X.X., Song S.J. Diverse metabolites from corn silk with anti-Aβ1-42 aggregation activity. Fitoterapia. 2019;138:104356–104364. doi: 10.1016/j.fitote.2019.104356. [DOI] [PubMed] [Google Scholar]
- 43.Bao B.Q., Zhang P., Lee Y., Hong J.K., Lee C., Jung J. Monoindole alkaloids from a marine sponge Spongosorites sp. Mar. Drugs. 2007;5:31–39. doi: 10.3390/md502031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aiello A., Fattorusso E., Imperatore C., Irace C., Luciano P., Menna M., Santamaria R., Vitalone R. Zorrimidazolone, a bioactive alkaloid from the non-indigenous Mediterranean stolidobranch Polyandrocarpa zorritensis. Mar. Drugs. 2011;9:1157–1165. doi: 10.3390/md9061157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longeon A., Copp B.R., Quevrain E., Roue M., Kientz B., Cresteil T., Petek S., Debitus C., Bourguet-Kondracki M. Bioactive indole derivatives from the South Pacific marine sponges Rhopaloeides odorabile and Hyrtios sp. Mar. Drugs. 2011;9:879–888. doi: 10.3390/md9050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jennings L.K., Khan N.M.D., Kaur N., Rodrigues D., Morrow C., Boyd A., Thomas O.P. Brominated bisindole alkaloids from the celtic sea sponge Spongosorites calcicole. Molecules. 2019;24:3890. doi: 10.3390/molecules24213890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seiberg M., Paine C., Sharlow E., Andrade-Gordon P., Costanzo M., Eisinger M., Shapiro S.S. Inhibition of melanosome transfer results in skin lightening1. J. Investig. Dermatol. 2000;115:162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhai Y.J., Huo G.M., Zhang Q., Li D., Wang D.C., Qi J.Z., Han W.B., Gao J.M. Phaeosphaones: Tyrosinase inhibitory thiodiketopiperazines from an endophytic Phaeosphaeria fuckelii. J. Nat. Prod. 2020;83:1592–1597. doi: 10.1021/acs.jnatprod.0c00046. [DOI] [PubMed] [Google Scholar]
- 49.Pillaiyar T., Namasivayam V., Manickam M., Jung S.H. Inhibitors of melanogenesis: An updated review. J. Med. Chem. 2018;61:7395–7418. doi: 10.1021/acs.jmedchem.7b00967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in manuscript and Supplementary Materials files.