Abstract
Background
Previous results provide supportive but not conclusive evidence for the use of omega‐3 fatty acids to reduce blood lipids and prevent events of atherosclerotic cardiovascular disease, but the strength and shape of dose–response relationships remain elusive.
Methods and Results
This study included 90 randomized controlled trials, reported an overall sample size of 72 598 participants, and examined the association between omega‐3 fatty acid (docosahexaenoic acid, eicosapentaenoic acid, or both) intake and blood lipid changes. Random‐effects 1‐stage cubic spline regression models were used to study the mean dose–response association between daily omega‐3 fatty acid intake and changes in blood lipids. Nonlinear associations were found in general and in most subgroups, depicted as J‐shaped dose–response curves for low‐/high‐density lipoprotein cholesterol. However, we found evidence of an approximately linear dose–response relationship for triglyceride and non‐high‐density lipoprotein cholesterol among the general population and more evidently in populations with hyperlipidemia and overweight/obesity who were given medium to high doses (>2 g/d).
Conclusions
This dose–response meta‐analysis demonstrates that combined intake of omega‐3 fatty acids near linearly lowers triglyceride and non‐high‐density lipoprotein cholesterol. Triglyceride‐lowering effects might provide supportive evidence for omega‐3 fatty acid intake to prevent cardiovascular events.
Keywords: 1‐stage regression, hyperlipidemia, long‐chain fatty acids, non‐HDL cholesterol, triglyceride
Subject Categories: Lipids and Cholesterol, Metabolism, Atherosclerosis
Clinical Perspective.
What Is New?
Intake of omega‐3 fatty acids of more than 2 g/d appears to have a near‐linear association with reductions in triglyceride and non‐high‐density lipoprotein cholesterol.
Omega‐3 polyunsaturated fatty acid supplementation at lower doses is associated with an increased level of low‐density lipoprotein cholesterol.
What Are the Clinical Implications?
A medium dose of omega‐3 fatty acids is potentially needed for the management of dyslipidemia, and a higher dose may afford more benefits for people who are at high risk of developing cardiovascular diseases.
The recommendation for omega‐3 fatty acid supplementation to reduce cardiovascular disease risks could be supported in patients with a high level of triglyceride in the context of guideline‐directed statin therapies.
Nonstandard Abbreviations and Acronyms
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- ω3 PUFA
omega‐3 polyunsaturated fatty acid
Despite the enforced lipid‐lowering measures over the past decade, global cardiovascular disease (CVD)‐caused deaths rose by almost 20% from 2010 to 2020. Between 2015 and 2018, in the United States alone, dyslipidemia prevalence ranged from 17% to 38%, determined by either total cholesterol ≥200 mg/dL, low‐density lipoprotein cholesterol (LDL‐C) ≥130 mg/dL, triglyceride ≥150 mg/dL, or high‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL. 1
With the hope of protecting the population with hyperlipidemia from CVD events, high‐intensity statin therapy targeting LDL‐C was recommended for the treatment of blood cholesterol. 2 , 3 Another strategy is to lower the triglyceride level or triglyceride‐rich lipoprotein. 4 , 5 Supplementation of omega‐3 polyunsaturated fatty acids (ω3 PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), is one of the lipid‐lowering approaches. 6 Researchers have long seen ω3 PUFA intake as a potential strategy to address vascular conditions, but there have also been concerns. ω3 PUFAs could reduce serum triglyceride concentration by approximately 15% to 30% 6 , 7 , 8 , 9 but could not affect or even increase LDL‐C levels. 9 , 10 , 11 , 12 , 13 Previous systematic reviews and meta‐analyses have been unable to reveal a significant dose–response relationship. 12 , 14 Some aggregated data have brought more uncertainty 6 , 9 , 13 , 15 rather than a solid conclusion. These past meta‐analyses examined the dose–response relationship using pooled linear meta‐regression 9 , 12 , 13 , 16 without taking into account the correlations among effects at different dose levels. 17
Extrapolation of the causal relationship between ω3 PUFA intake and vascular risk remains controversial, both in large randomized controlled trials (RCTs) and in many extensive meta‐analyses. ω3 PUFA intake has been associated with a reduced risk of major cardiovascular events, primarily in 2 trials: JELIS (Japan Eicosapentaenoic Acid Lipid Intervention Study) 18 and REDUCE‐IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial). 19 However, many previous 20 , 21 , 22 , 23 and recently completed clinical studies 24 , 25 showed that ω3 PUFA supplementation did not offer significant favorable impacts on cardiovascular events. Moreover, JELIS was often challenged for its selection of patients with a relatively high background of fish consumption, 26 and REDUCE‐IT was revisited for the use of mineral oil as a comparator, 27 , 28 , 29 respectively. A few meta‐analyses found a statistically significant CVD risk reduction, 30 , 31 but more results showed insufficient evidence of a possible protective effect. 32 , 33 , 34 , 35 , 36 , 37 Neither linear assumption‐driven meta‐regressions 38 , 39 , 40 , 41 , 42 nor stratified dose analyses 42 , 43 have conclusively estimated the dose–response relationship between ω3 PUFA intake and relative risk reduction, raising the possibility of a nonlinear dose–response curve. 30
This necessitates a rigorous examination of the dose–response effects of ω3 PUFAs on lipid changes among RCTs. We and others have used a 1‐stage cubic spline regression model 17 to perform dose–response meta‐analyses in 3 systematic reviews of blood pressure. 44 , 45 , 46 The 1‐stage spline mixed model allows us to fully capture the nonlinear dose–response relationship and reflect heterogeneity in studies with <3 exposure levels. 17 Following a comprehensive review of the literature, this study aims to more precisely characterize the dose–response effect between ω3 PUFAs (DHA, EPA, or both) and lipid profile, including triglyceride, LDL‐C, HDL‐C, non‐HDL‐C, and apolipoprotein B (apoB), in the general population and relevant subgroups.
Methods
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) reporting guidelines for the meta‐analysis of randomized trials (Table S1). The data that support the findings of this study are available from the corresponding author upon reasonable request. This meta‐analysis was carried out with data from previously published trials. Therefore, the approval of the ethics review or the institutional review board is not applicable.
Literature Retrieval
The literature retrieval was performed for articles published before June 2022, using the PubMed and EMBASE databases (Table S2). Additional searches were carried out to screen the reference lists of relevant studies, reviews, and meta‐analyses for more studies. Two authors (T.W. and N.Z.) independently reviewed each study and discrepancies were resolved through discussion. The prespecified eligibility criteria were parallel RCTs that examined the association between intake of DHA/EPA (combined or individual) and lipid changes (triglyceride, LDL‐C, HDL‐C, non‐HDL‐C, and apoB) in adults (aged ≥18 years). The exclusion criteria are (1) concurrent controls were lacking; (2) the duration of the intervention was <4 weeks; (3) studies were carried out in pregnant and nursing women; and (4) trials with a small sample size (<20 in each arm), not providing statistical power greater than 70% to measure a reduction of 53.2 mg/dL (0.6 mmol/L) in triglyceride after treatment with fish oil compared with a control intervention, given the SD of 66.5 mg/dL (0.75 mmol/L) and the 2‐tailed significance level at 0.05. 47 , 48
Assessment of the methodological quality was performed independently using the Cochrane Risk‐of‐Bias tool RoB2. 49 Two authors (T.W. and X.L.) independently assessed the risk of bias in the domains of randomization (random sequence generation), blinding (allocation concealment, blinding of participants and personnel, and blinding of outcome assessors), missing outcome(s) (incomplete outcome data), measurement (method and measurement bias), and selection of results (reporting bias).
Data Extraction
Information from the included study was extracted independently by 2 authors (T.W. and X.Z.) and confirmed by the other 2 authors (Y.S. and B.L.) using a standardized form. The effects of each exposure dose were collected individually in our study. In experiments with multiple follow‐up time points, only changes in lipid levels were extracted at the end of treatment versus before treatment. If the SD was not provided directly, we calculated it from SE, interquartile range, or CIs. 50
Exposure and Outcome Assessment
Most studies used a combined supplementation of EPA and DHA. Exposure levels were expressed by combined DHA + EPA or DHA/EPA alone. In some cases, DHA/EPA dose was considered separately, even when mixed EPA + DHA formulation was administered. If possible, the achieved change in red blood cell (RBC) omega index, the percentage of EPA plus DHA of total fatty acid in the RBC membrane, was extracted. This index serves as a biomarker of absorbed and integrated fish oil and reflects long‐term exposure levels. 51 , 52 We determined the net mean difference in lipid profile (ΔLipidbetween) between the exposure levels of each RCT as the difference at the end of the intervention minus the corresponding pretreatment value (ΔLipidintra‐group). The numerical values of triglyceride, LDL‐C, HDL‐C, and non–HDL‐C are given in mg/dL and mmol/L. To convert to mg/dL, the values in mmol/L for LDL‐C, HDL‐C, and non–HDL‐C are multiplied by 38.6 and for triglyceride by 88.6. 2 Circulating non‐HDL‐C is used as an outcome to represent all atherogenic lipoproteins, such as cholesterol‐containing LDL‐C/intermediate‐density lipoprotein and primarily triglyceride‐containing very low‐density lipoprotein. The non‐HDL‐C analysis includes only trials that reported non‐HDL‐C data. ApoB‐containing lipoproteins, including very low‐density lipoproteins, triglyceride‐rich remnant particles, and LDL, are central causal factors in the progression of atherosclerotic plaque. 2 , 3 ApoB quantitation is performed as an outcome to predict the overall atherogenic lipid profile.
Publication Bias Assessment
Publication bias was examined visually using funnel plots to assess the SE as a function of effect size, along with Egger's regression test to examine small‐study bias using R metafor. 53 We also used the trim‐and‐fill method to estimate the number of potential missing studies due to publication bias. A leave‐one‐out strategy was applied for sensitivity analyses, where we repeatedly ran the dose–response analysis to assess the missing study's influence on overall lipid changes.
Dose–Response Analysis
The control dose (0 g/d) was used as a reference for all analyses as described in our previous blood pressure analysis. 46 A 1‐stage random‐effects dose–response model 17 was established to predict the average dose–response relationship between DHA + EPA administration and changes in lipid levels. We tested the linearity assumption underlying the dose–response relationship by fitting a restricted cubic spline model with 3 knots (10th, 50th, and 90th percentiles) of doses. 54 The included studies were pooled into a continuous dose–response curve, and then estimates of lipid changes were calculated at given doses (that is, 1, 2, 3, 4, and 5 g/d). Furthermore, subgroup analyses were performed by stratifying studies according to preexisting hyperlipidemia status (total cholesterol ≥200 mg/dL [5.2 mmol/L] or triglyceride ≥150 mg/dL [1.7 mmoL/L]), patients with hyperlipidemia taking lipid‐lowering medications (yes versus no), baseline mean body mass index (≥25 or <25 kg/m2), preexisting coronary heart disease (CHD) (yes versus no), mean age (≥50 or <50 years), duration of treatment (>13 or ≤13 weeks), and use of EPA/DHA only. The 1‐stage cubic spline regression model was conducted using the dosresmeta R packages (https://github.com/alecri/dosresmeta). 17 , 55 , 56
Results
Study Characteristics
The systematic search retrieved 2385 relevant articles after removing duplicated 727 items. The title and abstract review further excluded 2086 articles. A full‐text examination of 299 articles yielded 90 eligible RCTs. 20 , 21 , 24 , 47 , 48 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 A PRISMA flow diagram of the literature screening can be seen in Figure 1. Study characteristics of included trials are shown in Table S3. These trials, published between 1990 and 2022, reported an overall sample size of 72 598 participants with a range of the mean age between 25.7 and 70.0 years and a range of the mean body mass index between 22.8 and 34.6 kg/m. 2 These trials were carried out in Europe (n=36, 40.0%), Asia (n=35, 38.9%), North America (n=18, 20.0%), and Oceania (n=1, 1.1%). Most trials (82/90) included both men and women, 8 included only men, and no trial included only women. Fifty‐two (57.8%) trials were reported with hyperlipidemia, and 11 (12.2%) trials were restricted to participants without hyperlipidemia. Among those 52 trials with hyperlipidemia, patients were regularly treated with lipid‐lowering medications (statins or fibrates) in 25 (48.1%) trials in addition to ω3 PUFA and in 17 (32.7%) trials with ω3 PUFA alone. Eighteen (20.0%) trials were conducted in participants with preexisting CHD and 46 (51.1%) trials in participants without CHD. The median duration of the intervention was 13.0 weeks (interquartile range, 8.5–26.0), and the duration was >13.0 weeks in 40 (44.4%) trials and <13.0 weeks in 50 (55.6%) trials. The most commonly used control/comparator was olive oil, along with the remainder consisting of various vegetable oils, such as safflower, sunflower, corn, soybean, and palm oils. Some controls were statin or fibrate alone or lipid‐lowering medication plus olive oil. Sixty‐three out of 90 trials reported the combined effects of DHA and EPA, with an average combined dose of 2.26 (interquartile range, 1.52–3.10, range, 0.30–6.90) g/d, DHA dose of 1.07 (interquartile range, 0.52–1.51, range, 0.12–3.68) g/d, and EPA dose of 1.48 (interquartile range, 0.82–1.83, range, 0.18–4.10) g/d (Figure S1); only 22 and 5 trials observed the effects of EPA or DHA alone, respectively.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram of systematic literature search and screening for randomized controlled trials published through June 2022 that met the study inclusion and exclusion criteria.
DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid; and RCT, randomized controlled trial.
Overall Dose–Response Analysis for Lipid Changes
The calculated mean changes and SEs of the included trials were visualized by scatterplots (Figure S2). The model performance comparison indicated that the restricted cubic spline model fits the overall data better than the linear or quadratic model (Figure S3). Table 1 and Table S4 summarize the overall effects of the combined application of DHA + EPA on mean changes in lipid profile. An approximately linear relationship for both triglyceride and non‐HDL‐C suggests that increasing combined supplementation is associated with greater reductions in both instances compared with the control groups (combined application dose of 0 g/d), with a steeper gradient for triglyceride than for non‐HDL‐C across the entire dose range (Figure 2). The mean change in triglyceride was −42.61 (95% CI, −53.41 to −31.80) mg/dL for 2 g/d and −68.90 (95% CI, −98.40 to −39.40) mg/dL for 3 g/d of DHA + EPA. The mean change in non‐HDL for 2 g/d of DHA + EPA was −4.13 (95% CI, −9.20 to 0.95) mg/dL and −8.31 (95% CI, −11.78 to −4.83) mg/dL at 3 g/d (Table 1). Significant nonlinear dose–response relationships were found between DHA + EPA intake and LDL‐C or HDL‐C changes. The J‐shaped curve for LDL‐C change peaked at 1.75 g/d intake with a moderate LDL‐C increment of 2.91 (95% CI, 0.34−5.47) and HDL‐C increment of 3.48 (95% CI, 1.09−5.86) mg/dL, respectively. The similar J‐shaped curve for the HDL‐C change indicated a limited increase (Figure 2). These findings provided strong evidence for the intake of DHA + EPA to reduce triglyceride and non‐HDL‐C levels, but not LDL‐C levels, in a nearly linear manner in the overall population. 6 , 8 , 12
Table 1.
Estimated Average Dose–Response Relationship Between DHA + EPA Consumption (g/d) and Lipid Reduction (mg/dL) ‡
| Lipid | Participants | N* | 1.0 g/d | 2.0 g/d | 3.0 g/d | |||
|---|---|---|---|---|---|---|---|---|
| MD | (95% CI) | MD | (95% CI) | MD | (95% CI) | |||
| Triglyceride | All | 86 | −19.21 | (−32.01 to 6.41) | −42.61 | (−53.41 to 31.80) | −68.90 | (−98.40 to 39.40) |
| LDL‐C | All | 80 | 2.91 | (0.34 to 5.47) | 3.48 | (1.09 to 5.86) | 2.43 | (−0.36 to 5.22) |
| HDL‐C | All | 87 | 1.36 | (0.47 to 2.25) | 1.69 | (0.78 to 2.61) | 1.32 | (−0.97 to 3.60) |
| Non‐HDL‐C | All | 22 | −1.18 | (−6.24 to 3.89) | −4.13 | (−9.20 to 0.95) | −8.31 | (−11.78 to 4.83) |
| Hyperlipidemia status | ||||||||
| Triglyceride | Yes | 49 | −23.05 | (−43.59 to 2.51) | −49.89 | (−63.28 to 36.49) | −80.58 | (−150.43 to 10.74) |
| No | 11 | −17.24 | (−31.01 to 3.48) | −27.36 | (−45.82 to 8.89) | −32.58 | (−50.72 to 14.43) | |
| LDL‐C | Yes | 48 | 2.82 | (−1.25 to 6.90) | 4.17 | (0.09 to 8.24) | 4.01 | (0.50 to 7.51) |
| No | 10 | 7.79 | (1.83 to 13.75) | 7.64 | (1.15 to 14.14) | 2.48 | (−6.33 to 11.29) | |
| HDL‐C | Yes | 51 | 1.96 | (0.59 to 3.34) | 2.38 | (0.62 to 4.13) | 1.15 | (0.05 to 2.26) |
| No | 10 | 3.43 | (1.22 to 5.63) | 2.92 | (−0.84 to 6.69) | −0.30 | (−10.56 to 9.96) | |
| Non‐HDL‐C† | Yes | 21 | −0.89 | (−6.37 to 4.58) | −3.74 | (−9.57 to 2.09) | −8.24 | (−11.80 to 4.68) |
| Participants with hyperlipidemia taking lipid‐lowering medication | ||||||||
| Triglyceride | Yes | 22 | 1.93 | (−15.04 to 18.90) | −27.96 | (−44.08 to 11.84) | −98.23 | (−201.25 to 4.79) |
| No | 17 | −18.97 | (−46.12 to 8.19) | −52.75 | (−71.38 to 34.12) | −100.71 | (−160.80 to 40.61) | |
| LDL‐C | Yes | 24 | 1.21 | (−1.49 to 3.92) | 1.06 | (−2.79 to 4.91) | −0.83 | (−3.84 to 2.17) |
| No | 15 | −0.41 | (−3.77 to 2.95) | 3.02 | (−0.07 to 6.12) | 10.13 | (5.57 to 14.70) | |
| HDL‐C | Yes | 24 | −0.56 | (−2.92 to 1.79) | 0.64 | (−1.41 to 2.69) | 4.09 | (−9.20 to 17.38) |
| No | 17 | 4.15 | (0.63 to 7.66) | 4.98 | (0.64 to 9.32) | 2.65 | (0.01 to 5.28) | |
| Non‐HDL‐C | Yes | 13 | 1.44 | (−7.38 to 10.27) | −1.90 | (−11.46 to 7.67) | −9.59 | (−13.90 to 5.27) |
| No | 3 | −1.87 | (−7.72 to 3.98) | −3.52 | (−11.49 to 4.46) | −4.88 | (−10.31 to 0.55) | |
| Baseline mean body mass index | ||||||||
| Triglyceride | ≥25 kg/m2 | 53 | −25.54 | (−42.03 to 9.04) | −46.86 | (−58.64 to 35.08) | −65.27 | (−91.38 to 39.17) |
| <25 kg/m2 | 22 | −5.76 | (−24.62 to 13.10) | −9.23 | (−23.58 to 5.12) | −11.47 | (−82.65 to 59.72) | |
| LDL‐C | ≥25 kg/m2 | 52 | 4.15 | (0.41 to 7.89) | 5.00 | (1.74 to 8.27) | 3.56 | (0.34 to 6.79) |
| <25 kg/m2 | 20 | 1.00 | (−2.62 to 4.62) | −1.42 | (−3.50 to 0.67) | −5.83 | (−13.76 to 2.10) | |
| HDL‐C | ≥25 kg/m2 | 55 | 1.56 | (0.76 to 2.36) | 1.78 | (0.82 to 2.75) | 1.08 | (0.15 to 2.01) |
| <25 kg/m2 | 21 | 1.76 | (−5.20 to 8.73) | 4.69 | (−1.47 to 10.85) | 8.20 | (−12.01 to 28.41) | |
| Non‐HDL‐C† | ≥25 kg/m2 | 18 | 1.19 | (−5.32 to 7.69) | −1.78 | (−8.32 to 4.76) | −7.61 | (−11.31 to 3.90) |
DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MD, mean difference; and non‐HDL‐C, non‐high‐density lipoprotein cholesterol.
Numbers may not sum to group totals due to missing data or unspecified subgroups in the trials.
Due to the unavailability of data, only 1 subgroup estimate was performed in the absence or presence of hyperlipidemia, overweight/obesity (≥25 kg/m2), and preexisting coronary heart disease.
The complete dose–response outcomes are presented in Table S4.
Figure 2. Dose–response relationship between changes in lipids and combined intake of DHA + EPA.
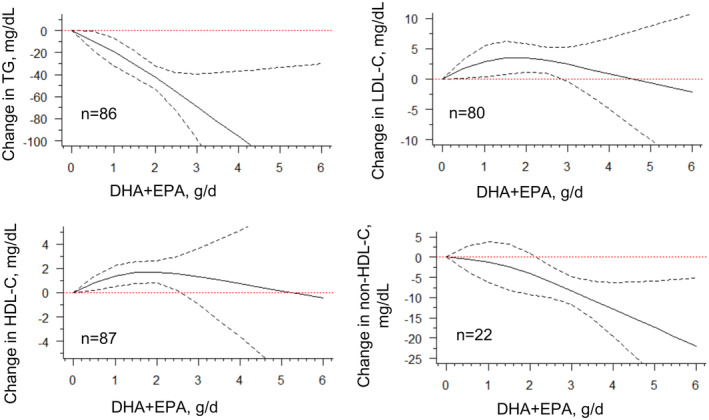
Marginal average dose–response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/d as the reference. Studies included n=86 for TG, n=80 for LDL‐C, n=87 for HDL‐C, and n=22 for non‐HDL‐C. DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and TG, triglyceride.
The amount and ratio of EPA and DHA differ in various supplements, resulting in different bioavailability and absorption rates. 142 , 143 , 144 Therefore, we analyzed the lipid response to the achieved percentage change in the RBC omega index. Achieved change in the RBC omega index was negatively and almost linearly associated with changes in triglyceride and non‐HDL‐C but positively associated with changes in HDL‐C, over a wide range of RBC omega index changes (0%−300%). These trends were not observed in the LDL‐C change with marginally null effects throughout the entire exposure range (Table S5 and Figure S4).
Subgroup Analyses for Lipid Changes
In subgroup studies stratified by prespecified hyperlipidemic status at entry, the approximately linear trend for triglyceride change was found only in the population with hyperlipidemia but not in the population without hyperlipidemia, where the effect stably plateaued at roughly 40 mg/dL. The non‐HDL‐C change was almost the same as the overall effects because 21 out of 22 trials were among participants with hyperlipidemia (Table S4 and Figure 3).
Figure 3. Dose–response relationship between changes in lipids and combined intake of DHA + EPA of studies stratified by hyperlipidemia status.
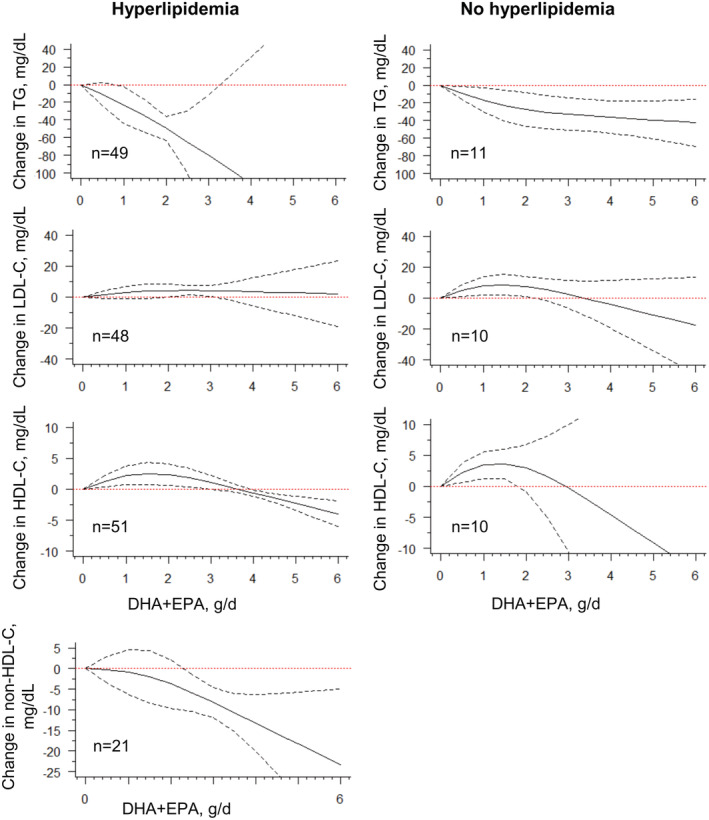
Marginal average dose–response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/day as reference, in participants with or without hyperlipidemia. n=the number of the included study. DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and TG, triglyceride.
Similar results for triglyceride were obtained in participants with hyperlipidemia, whether they were treated with lipid‐lowering medication or not if they had ingested more than 2 g/d ω3 PUFAs. However, participants who received lipid‐lowering medication had a much steeper curve in non‐HDL‐C than those who did not. ω3 PUFA increased LDL‐C level significantly with a dose greater than 2 g/d, consistent with previous findings. 7 , 9 , 12 , 13 Moreover, this dose–response trend is independent of baseline LDL‐C levels (≥130 versus <130 mg/dL, data not shown). Fatty acids combined with statins, compared with fatty acid monotherapy, could synergistically increase HDL‐C levels at a dose greater than 2 g/d (Table S4 and Figure 4).
Figure 4. Subgroup analysis for changes in lipids and combined intake of DHA + EPA among hyperlipidemic participants.
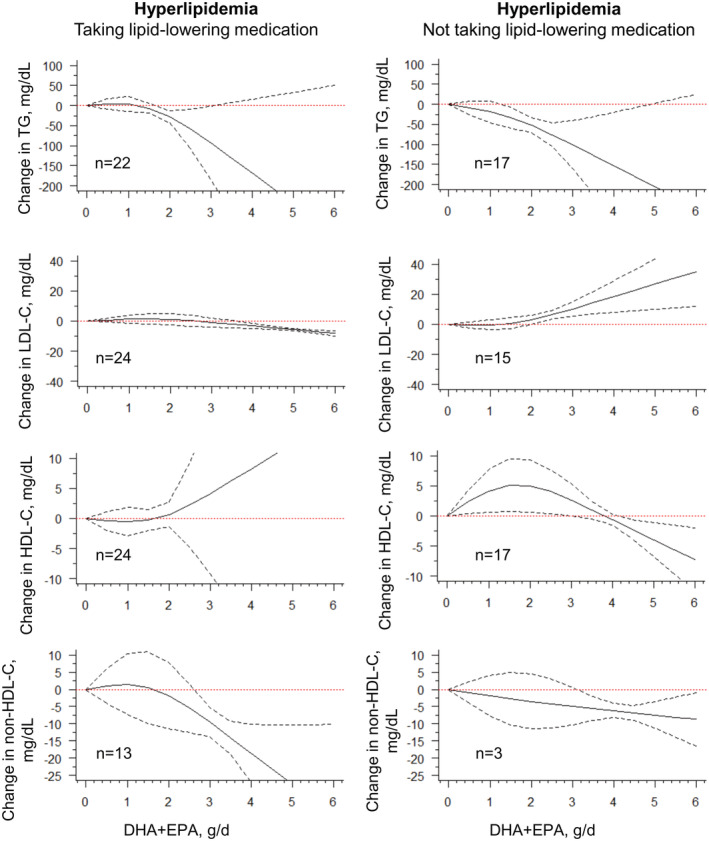
Marginal average dose–response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/day as reference, in participants taking or not taking lipid‐lowering medications. n=the number of the included study. DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and TG, triglyceride.
When we stratified according to baseline mean body mass index (<25 versus ≥25 kg/m2), we found stronger triglyceride effects of ω3 PUFA monotherapy in participants with higher background body mass index, classified as overweight/obesity (Table S4 and Figure 5). Similar findings were also observed when stratified by preexisting CHD (yes versus no), where those with preexisting CHD saw greater reductions after the dose reached 2 g/d (Table S4 and Figure S5). Moreover, DHA + EPA supplementation demonstrated greater responses to lower triglyceride levels among patients with hyperlipidemia and CHD (Figure S6). This could warrant secondary prevention of EPA + DHA for CHD. 34 , 145 When we considered baseline mean age (<50 versus ≥50 years) and trial duration (4–13 weeks versus >13 weeks), the dose–response relationship demonstrated mild variations in triglyceride and non‐HDL‐C differences between age and trial duration and with little evidence to support other lipid‐altering efficacy, compared with the overall effects (Figures S7 and S8).
Figure 5. Dose–response relationship between changes in lipids and combined intake of DHA + EPA of the studies stratified by overweight/obesity classified by the baseline mean of body mass index (BMI).
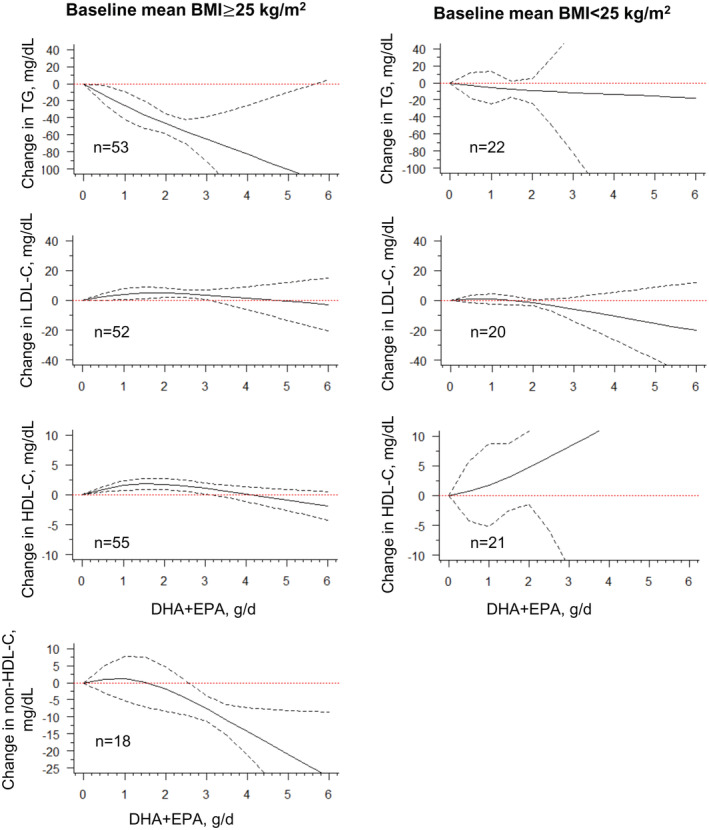
Marginal average dose–response curve (solid line) with 95% point‐wise CIs (dashed lines) estimated by a 1‐stage random‐effects restricted cubic spline model, using 0 g/day as reference, among participants with a mean BMI ≥25 or <25 kg/m2. n=the number of the included study. BMI indicates body mass index; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and TG, triglyceride.
There is an apparent need to differentiate the role of DHA and EPA in conferring lipid and vascular impacts. 11 , 146 , 147 , 148 Our classification of the retrieved experiments using DHA/EPA as individual fatty acids revealed that the magnitude of triglyceride decrease is similar in treatment with DHA and EPA alone (Table S4 and Figure S9). The effects of DHA on HDL‐C appeared to reach the plateau after a dose of 2 g/d. DHA is more likely to be associated with an increase in LDL‐C compared with EPA alone (Table S4 and Figure S9). When the dosage of DHA/EPA intake was considered separately, as shown in Figure S10, there was still an approximately linear relationship in triglyceride reduction, though the slope became gradual. The dose–response effects of non‐HDL‐C stabilized after the separate DHA/EPA dose of more than 2 g/d. In multiple subgroup analyses, separate EPA seemed to show weaker lipid‐lowering effects than separate DHA, which exerted greater triglyceride‐lowering effects in participants with hyperlipidemia, overweight/obesity, and CHD across the entire dose range (Figures S11‐14). These disparities between separate DHA and EPA were not evident for responses of non‐HDL‐C (Figures S11‐14). With the removal of all EPA/DHA monotherapies (Figure S15), the dose responses are consistent with the previous data (Figure 2). Collectively, combined supplementation of DHA and EPA appeared to exert a robust effect on triglyceride reduction but not other serum lipids.
Lastly, except for a nearly linear dose–response association between apoB and the RBC omega index, J‐shaped curvilinear trends are commonly seen in general or in various subgroup responses (Figures S16 and S17).
Risk of Study Bias and Publication Bias
After evaluating all trials included in the lipid profile study, 3 trials were classified as high and another 3 as moderate risk of bias, and the remaining trials were classified as low risk of bias (Table S6). The exclusion of moderate‐ and high‐risk biased trials did not appreciably change the shape of the dose–response curve (results not shown). The funnel plot and Egger's regression test indicated asymmetry only in the overall triglyceride models (z=−3.37, P<0.001) but not in the pooled HDL‐C, LDL‐C, and non‐HDL‐C models (Figure S18). This suggests that publication bias, if present due to the effects of the small study, did not strongly affect our overall findings. Leave‐one‐out sensitivity analyses in 1‐stage regression models proved that overall effects were not driven by a small number of specific trials but reflected the global effect of all included trials (Figure S19).
Discussion
In this dose–response meta‐analysis using a 1‐stage method, we examined the strength and shape of the lipid‐lowering effects of DHA + EPA supplementation with up‐to‐date literature. We found evidence of an approximately linear dose–response relationship for triglyceride and non‐HDL‐C reduction among the general population and especially in populations with hyperlipidemia and overweight/obesity. These inverse correlations were more prominent in participants receiving basal lipid‐lowering medications or with preexisting CHD, given the intake dose was higher than 2 g/d.
The current meta‐analysis differs from others in the statistical methodology used and the consideration of a nonlinear relationship. Previous dose–response models using pooled meta‐regression method were conducted based on the assumption that a linear causal relationship existed, 9 , 12 , 13 , 16 without taking into account the correlations at different dose levels. The current 1‐stage model is more flexibly capable of estimating nonlinear dose–response curves based on aggregated data with <3 exposure levels. 17 Moreover, 1‐stage dose–response meta‐analysis does not assume a particular shape for the relationship, allowing for nonlinear relations between exposure and outcome, which includes linear, U‐shape, and J‐shape curvilinear models. Therefore, we think that either a near‐linear or a nonlinear relationship is entirely driven by the data instead of an assumption given by the investigators. We have now provided a pictorial presentation to illustrate that triglyceride and non‐HDL‐C reduction confers biological plausibility in a dose‐dependent manner in an atherosclerosis setting and other cardiometabolic complications.
Though LDL is widely recognized as the dominant atherogenic factor, 149 the current analysis suggested that LDL‐C, a surrogate of LDL particle concentration, did not appear to be targeted by EPA and DHA in the treatment of dyslipidemia, an outcome in agreement with many published meta‐analysis results. 4 , 17 , 18 , 19 Previous meta‐regression analyses assumed a linear relationship between ω3 PUFA intake and triglyceride changes among RCT studies. 9 , 13 Without any assumption, our current 1‐stage dose–response analyses coincided with a nearly linear association between triglyceride reduction and ω3 PUFA intake. Using the continuous dose–response curve, we have estimated the optimal dose for triglyceride reduction in various subgroup analyses. For example, medium to high doses (>2 g/d) were predicted to exert significant triglyceride‐lowering effects among hyperlipidemic participants. These dose predictions cannot be performed in previous meta‐analyses that failed to reveal a significant dose–response relationship 12 , 14 and brought uncertainty. 6 , 9 , 13 , 15 Moreover, the triglyceride‐lowering potency was proportionally mirrored in non‐HDL‐C reduction with moderate gradients. To our best knowledge, this is the first dose–response meta‐analysis of the relationship between ω3 PUFA intake and non‐HDL‐C changes, an indicator of the cholesterol content of all atherogenic lipoproteins. 100 , 150 , 151
The association between triglyceride‐lowering and ω3 PUFA intake could causally lead to the reduction of cardiovascular risks in patients with high triglyceride, as previously reported in trials 18 , 19 and meta‐analyses. 38 Furthermore, our findings indicate that statin and fish oil synergistically offer benefits in reducing non‐HDL‐C compared with fish oil alone. High‐dose eicosapentaenoic ethyl ester combined with baseline statins can lead to a remarkable decline in first and recurrent events in high‐risk patients with hypertriglyceridemia. 18 , 19 However, patients with hyperlipidemia without baseline lipid‐lowering medication may suffer from increased serum LDL‐C levels and decreased HDL‐C levels. A possible explanation for this synergy is that people who qualify for triglyceride‐lowering trials, despite statin therapy, have hypertriglyceridemia that is differently affected by fish oil. 134 Further subgroup analyses demonstrated greater responses in patients with preexisting CHD and overweight/obesity when treated with ω3 PUFA, indicating fish oil's potential benefits in secondary prevention. However, the most recently completed RESPECT‐EPA (Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid) 152 showed that among Japanese patients with chronic coronary artery disease treated with statin therapy, additional EPA may be associated with a minimal reduction in adverse cardiovascular outcomes (10.9% of the icosapent ethyl group versus 14.9% of the control group, P=0.055) after 6 years of follow‐up. 153 DHA is biochemically and pharmacologically different from EPA in membrane incorporation, lipoprotein oxidation, and generation of specialized pro‐resolving lipid mediators. 146 , 148 Although DHA/EPA as individual fatty acids revealed a similar magnitude of decrease in triglyceride in our analysis, increases in LDL‐C were significantly greater in participants treated with DHA alone than in those treated with EPA alone, which was consistent with previous synthesized results. 11 This may explain why EPA + DHA combination treatment in various trials did not demonstrate an effect on reducing cardiovascular risk. 23 , 24 , 25 , 133 However, the available studies of DHA and EPA monotherapy, especially for DHA (n=5), are limited, with many studies at an EPA dose of 1.8 g/d or below. The wide CIs in higher dose ranges lead to unstable models, which would warrant more high‐dose monotherapy studies in the future.
Our current dose–response analyses recommend taking more than 2 g/d ω3 PUFA from a pharmacokinetic perspective, securing the active substances absorbed to reach the systemic circulation or tissues, such as the cell membrane. Clinical trials revealed that ω3 PUFA supplementation of <1 g/d resulted in a very limited reduction in atherosclerotic CVD risk of major vascular events and CVD‐caused deaths. 22 , 23 , 133 , 154 Conversely, patients with hypertriglyceridemia treated with a medium‐to‐high dose of icosapent ethyl were less likely to develop ischemic events, including CVD death. 18 , 19 However, taking into account the selection of the target population with a higher level of ω3 PUFA in JELIS 26 or the use of mineral oil as a comparator in REDUCE‐IT, 27 we still need more conclusive evidence from well‐designed trials to examine the potency of ω3 PUFA supplementation to prevent cardiovascular events.
Exposure and outcome measurements play a critical role in the estimation of valid causal relationships. We used a prestandardized protocol for dose intake (exposure level) in our data extraction process, excluding trials of DHA/EPA supplementation through diet, where the exposure level was hardly determined by the accurate fraction of pure DHA/EPA amount over the food consumed daily. Exposure levels were examined from 3 different perspectives: total combined doses of DHA + EPA, individual use of DHA/EPA (monotherapy), and separate doses. To precisely reflect the exposure level, we further included the achieved omega‐3 index change in the RBC membrane. The outcome measurement was also taken into account in our risk of bias assessment. All included trials have demonstrated detailed measurement protocols (such as automatic biochemistry measurement and standardized staff training, etc.) to obtain stable lipid profile readouts, though some of these studies were not designed to test the effect on lipids as the primary outcome. Intrinsically significant variations among original trials, such as the device for lipid measurement and the year of study (conducted 1990–2022), are likely to bring some uncertainty to our results and potentially weaken the conclusion. Although we attempted to examine the influence of these factors on our overall findings in subgroup analyses, we acknowledge that it is not possible to account for this heterogeneity directly in our analyses. The overall risk of bias did not divert from our expectations.
There are several limitations. First, the current study was carried out with study‐level data but not individual data. This weakness may be compensated for by 1‐stage methods that allow for the estimation of a nonlinear trend that accounts for the correlation between studies. Another effort was made by subgrouping strategies, considering the status of hyperlipidemia (with or without lipid‐lowering medications), overweight/obesity, CHD, age, and duration. Second, we did not consider the influence of diabetes and metabolic syndrome on the lipid profile as possible cofounders. Unlike meta‐regression analysis, the 1‐stage dose–response could not handle multivariate or network analyses. Third, our current study was limited to the dose–response relationship between DHA/EPA supplementation and serum lipid changes. We did not perform further analyses to reveal whether changes in lipid profiles would result in a reduction in end point risk. We did not explain why comparable associations were evident for EPA‐ and DHA‐only to lower serum triglyceride, but purified high‐dose EPA had generally shown more robust benefits compared with mixed EPA + DHA in cardiovascular event trials. The mechanisms for end point prevention appear to be attributed to the pleiotropic effects in addition to serum lipid regulation. 38 , 148 Fourth, intrinsically significant data sparsity in the original trials might bring some uncertainty to our results and potentially weaken the conclusion. For example, because of a limited number of studies, a wider CI in a higher dose range is very evident, and the discrepancy between EPA and DHA is still unclear. Future well‐designed studies with an appropriate comparator/placebo and population selection examining DHA/EPA‐only effects should further investigate these issues.
Conclusions
The use of the new model reveals a nearly linear response at doses greater than 2 g/d of DHA + EPA supplementation in overall and subgroup analyses in the performance of triglyceride and non‐HDL‐C reduction. Individuals who are at high risk for developing CVD, such as those with hyperlipidemia and overweight/obesity, may be more responsive to the beneficial impacts of ω3 PUFA. This research helps improve our understanding of the moderate effects of omega‐3 fatty acids on lipid reduction and CVD prevention.
Sources of Funding
This work was supported by the Macau Science and Technology Development Fund (FDCT) (0123/2020/A and 0053/2021/A1) and the Faculty Research Grants of Macau University of Science and Technology (FRG‐21‐038‐SP).
Disclosures
None.
Supporting information
Data S1
Acknowledgments
The authors sincerely thank Edward Li for his diligent proofreading of this article.
This article was sent to Daniel Edmundowicz, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029512
For Sources of Funding and Disclosures, see page 12.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 4. Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar‐Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, et al. Triglyceride‐rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies‐a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris‐Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
- 6. Leslie MA, Cohen DJ, Liddle DM, Robinson LE, Ma DW. A review of the effect of omega‐3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015;14:53. doi: 10.1186/s12944-015-0049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris WS. n‐3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S [DOI] [PubMed] [Google Scholar]
- 8. Skulas‐Ray AC, West SG, Davidson MH, Kris‐Etherton PM. Omega‐3 fatty acid concentrates in the treatment of moderate hypertriglyceridemia. Expert Opin Pharmacother. 2008;9:1237–1248. doi: 10.1517/14656566.9.7.1237 [DOI] [PubMed] [Google Scholar]
- 9. Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta‐analysis. Int J Cardiol. 2009;136:4–16. doi: 10.1016/j.ijcard.2008.03.092 [DOI] [PubMed] [Google Scholar]
- 10. Sharp RP, Gales BJ, Sirajuddin R. Comparing the impact of prescription omega‐3 fatty acid products on low‐density lipoprotein cholesterol. Am J Cardiovasc Drugs. 2018;18:83–92. doi: 10.1007/s40256-017-0253-0 [DOI] [PubMed] [Google Scholar]
- 11. Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low‐density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol. 2012;6:5–18. doi: 10.1016/j.jacl.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 12. Bernstein AM, Ding EL, Willett WC, Rimm EB. A meta‐analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL‐cholesterol and LDL‐cholesterol in persons without coronary heart disease. J Nutr. 2012;142:99–104. doi: 10.3945/jn.111.148973 [DOI] [PubMed] [Google Scholar]
- 13. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega‐3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 14. Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta‐analysis. Curr Atheroscler Rep. 2011;13:474–483. doi: 10.1007/s11883-011-0210-3 [DOI] [PubMed] [Google Scholar]
- 15. Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58 [DOI] [PubMed] [Google Scholar]
- 16. Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063 [DOI] [PubMed] [Google Scholar]
- 17. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One‐stage dose‐response meta‐analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–1596. doi: 10.1177/0962280218773122 [DOI] [PubMed] [Google Scholar]
- 18. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
- 19. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 20. Investigators OT, Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, et al. n‐3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859 [DOI] [PubMed] [Google Scholar]
- 21. Group RaPSC , Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, et al. n‐3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409 [DOI] [PubMed] [Google Scholar]
- 22. Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, Clemons TE, Fine LJ, Chew EY. Effect of long‐chain omega‐3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age‐Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. 2014;174:763–771. doi: 10.1001/jamainternmed.2014.328 [DOI] [PubMed] [Google Scholar]
- 23. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, et al. Marine n‐3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, et al. Effect of high‐dose omega‐3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. Jama. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DWT, Tveit A, Fagerland MW, Solheim S, et al. Effects of n‐3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. 2021;143:528–539. doi: 10.1161/CIRCULATIONAHA.120.052209 [DOI] [PubMed] [Google Scholar]
- 26. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885 [DOI] [PubMed] [Google Scholar]
- 27. Ridker PM, Rifai N, MacFadyen J, Glynn RJ, Jiao L, Steg PG, Miller M, Brinton EA, Jacobson TA, Tardif JC, et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin‐1beta, interleukin‐6, C‐reactive protein, oxidized low‐density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein‐associated phospholipase A2: a REDUCE‐IT biomarker substudy. Circulation. 2022;146:372–379. doi: 10.1161/CIRCULATIONAHA.122.059410 [DOI] [PubMed] [Google Scholar]
- 28. Wilkins JT, Lloyd‐Jones DM. Icosapent ethyl supplementation and cardiovascular prevention—implications of evolving data. JAMA Cardiology. 2022;7:1185–1186. doi: 10.1001/jamacardio.2022.3701 [DOI] [PubMed] [Google Scholar]
- 29. Nissen SE. When is a placebo not a placebo. JAMA Cardiol. 2022;7:1183–1184. doi: 10.1001/jamacardio.2022.3698 [DOI] [PubMed] [Google Scholar]
- 30. Bernasconi AA, Wiest MM, Lavie CJ, Milani RV, Laukkanen JA. Effect of omega‐3 dosage on cardiovascular outcomes: an updated meta‐analysis and meta‐regression of interventional trials. Mayo Clin Proc. 2021;96:304–313. doi: 10.1016/j.mayocp.2020.08.034 [DOI] [PubMed] [Google Scholar]
- 31. Maki KC, Palacios OM, Bell M, Toth PP. Use of supplemental long‐chain omega‐3 fatty acids and risk for cardiac death: an updated meta‐analysis and review of research gaps. J Clin Lipidol. 2017;11(1152–1160):e1152. doi: 10.1016/j.jacl.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 32. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374 [DOI] [PubMed] [Google Scholar]
- 33. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, et al. Associations of Omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, Summerbell CD, Worthington HV, Song F, et al. Omega‐3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2020;3:CD003177. doi: 10.1002/14651858.CD003177.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 fatty acids and cardiovascular outcomes: systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2012;5:808–818. doi: 10.1161/CIRCOUTCOMES.112.966168 [DOI] [PubMed] [Google Scholar]
- 37. Kwak SM, Myung SK, Lee YJ, Seo HG; Korean Meta‐analysis Study G . Efficacy of omega‐3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta‐analysis of randomized, double‐blind, placebo‐controlled trials. Arch Intern Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262 [DOI] [PubMed] [Google Scholar]
- 38. Marston NA, Giugliano RP, Im K, Silverman MG, O'Donoghue ML, Wiviott SD, Ference BA, Sabatine MS. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid‐lowering therapeutic classes: a systematic review and meta‐regression analysis of randomized controlled trials. Circulation. 2019;140:1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marik PE, Varon J. Omega‐3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. doi: 10.1002/clc.20604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sethi A, Bajaj A, Khosla S, Arora RR. Statin use mitigate the benefit of omega‐3 fatty acids supplementation‐a meta‐regression of randomized trials. Am J Ther. 2016;23:e737–e748. doi: 10.1097/MJT.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 41. Hu Y, Hu FB, Manson JE. Marine omega‐3 supplementation and cardiovascular disease: an updated meta‐analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8:e013543. doi: 10.1161/JAHA.119.013543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Markozannes G, Ntzani EE, Tsapas A, Mantzoros CS, Tsiara S, Xanthos T, Karpettas N, Patrikios I, Rizos EC. Dose‐related meta‐analysis for omega‐3 fatty acids supplementation on major adverse cardiovascular events. Clin Nutr. 2022;41:923–930. doi: 10.1016/j.clnu.2022.02.022 [DOI] [PubMed] [Google Scholar]
- 43. Casula M, Olmastroni E, Gazzotti M, Galimberti F, Zambon A, Catapano AL. Omega‐3 polyunsaturated fatty acids supplementation and cardiovascular outcomes: do formulation, dosage, and baseline cardiovascular risk matter? An updated meta‐analysis of randomized controlled trials. Pharmacol Res. 2020;160:105060. doi: 10.1016/j.phrs.2020.105060 [DOI] [PubMed] [Google Scholar]
- 44. Filippini T, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, Moreira P, Malavolti M, Orsini N, Whelton PK, et al. Potassium intake and blood pressure: a dose‐response meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2020;9:e015719. doi: 10.1161/JAHA.119.015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Filippini T, Malavolti M, Whelton PK, Naska A, Orsini N, Vinceti M. Blood pressure effects of sodium reduction: dose‐response meta‐analysis of experimental studies. Circulation. 2021;143:1542–1567. doi: 10.1161/CIRCULATIONAHA.120.050371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X, Ritonja JA, Zhou N, Chen BE, Li X. Omega‐3 polyunsaturated fatty acids intake and blood pressure: a dose‐response meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2022;11:e025071. doi: 10.1161/JAHA.121.025071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maki KC, Orloff DG, Nicholls SJ, Dunbar RL, Roth EM, Curcio D, Johnson J, Kling D, Davidson MH. A highly bioavailable omega‐3 free fatty acid formulation improves the cardiovascular risk profile in high‐risk, statin‐treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. 2013;35(1400–1411):e1401–e1403. doi: 10.1016/j.clinthera.2013.07.420 [DOI] [PubMed] [Google Scholar]
- 48. Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, Wu Y, Chen JL, Kang C, Shu FR, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One. 2015;10:e0133496. doi: 10.1371/journal.pone.0133496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 50. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions (version 6.2, updated February 2021). Cochrane Database of Systematic Reviews. 2021. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 51. Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long‐term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63:1008–1015. doi: 10.1038/ejcn.2009.19 [DOI] [PubMed] [Google Scholar]
- 52. Harris WS, Von Schacky C. The omega‐3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030 [DOI] [PubMed] [Google Scholar]
- 53. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 54. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crippa A, Orsini N. Multivariate dose‐response meta‐analysis: the dosresmeta R package. J Stat Soft. 2016;72:1–15. doi: 10.18637/jss.v072.c01 [DOI] [Google Scholar]
- 56. Orsini N. Weighted mixed‐effects dose–response models for tables of correlated contrasts. Stata J. 2021;21:320–347. [Google Scholar]
- 57. Flaten H, Hostmark AT, Kierulf P, Lystad E, Trygg K, Bjerkedal T, Osland A. Fish‐oil concentrate: effects on variables related to cardiovascular disease. Am J Clin Nutr. 1990;52:300–306. doi: 10.1093/ajcn/52.2.300 [DOI] [PubMed] [Google Scholar]
- 58. Hendra TJ, Britton ME, Roper DR, Wagaine‐Twabwe D, Jeremy JY, Dandona P, Haines AP, Yudkin JS. Effects of fish oil supplements in NIDDM subjects. Controlled study. Diabetes Care. 1990;13:821–829. doi: 10.2337/diacare.13.8.821 [DOI] [PubMed] [Google Scholar]
- 59. Reis GJ, Silverman DI, Boucher TM, Sipperly ME, Horowitz GL, Sacks FM, Pasternak RC. Effects of two types of fish oil supplements on serum lipids and plasma phospholipid fatty acids in coronary artery disease. Am J Cardiol. 1990;66:1171–1175. doi: 10.1016/0002-9149(90)91093-l [DOI] [PubMed] [Google Scholar]
- 60. Bonaa KH, Bjerve KS, Nordoy A. Docosahexaenoic and eicosapentaenoic acids in plasma phospholipids are divergently associated with high density lipoprotein in humans. Arterioscler Thromb. 1992;12:675–681. doi: 10.1161/01.atv.12.6.675 [DOI] [PubMed] [Google Scholar]
- 61. Kaul U, Sanghvi S, Bahl VK, Dev V, Wasir HS. Fish oil supplements for prevention of restenosis after coronary angioplasty. Int J Cardiol. 1992;35:87–93. doi: 10.1016/0167-5273(92)90059-c [DOI] [PubMed] [Google Scholar]
- 62. Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, Weiner BH, Slack JD, Kellett MA, Raizner AE, et al. Do fish oils prevent restenosis after coronary angioplasty? Circulation. 1994;90:2248–2257. doi: 10.1161/01.cir.90.5.2248 [DOI] [PubMed] [Google Scholar]
- 63. Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC. Controlled trial of fish oil for regression of human coronary atherosclerosis. HARP research group. J Am Coll Cardiol. 1995;25:1492–1498. doi: 10.1016/0735-1097(95)00095-l [DOI] [PubMed] [Google Scholar]
- 64. Shimizu H, Ohtani K, Tanaka Y, Sato N, Mori M, Shimomura Y. Long‐term effect of eicosapentaenoic acid ethyl (EPA‐E) on albuminuria of non‐insulin dependent diabetic patients. Diabetes Res Clin Pract. 1995;28:35–40. doi: 10.1016/0168-8227(95)01056-j [DOI] [PubMed] [Google Scholar]
- 65. Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of dietary supplementation with n‐3 fatty acids on coronary artery bypass graft patency. Am J Cardiol. 1996;77:31–36. doi: 10.1016/s0002-9149(97)89130-8 [DOI] [PubMed] [Google Scholar]
- 66. Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol‐lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–659. doi: 10.1093/ajcn/66.3.649 [DOI] [PubMed] [Google Scholar]
- 67. Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M, Berglund L, Osmundsen K. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 68. Sirtori CR, Paoletti R, Mancini M, Crepaldi G, Manzato E, Rivellese A, Pamparana F, Stragliotto E. N‐3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian fish oil multicenter study. Am J Clin Nutr. 1997;65:1874–1881. doi: 10.1093/ajcn/65.6.1874 [DOI] [PubMed] [Google Scholar]
- 69. Borthwick L; Group UKS . The effects of an omega‐3 ethyl ester concentrate on blood lipid concentrations in patients with hyperlipidaemia. Clin Drug Investig. 1998;15:397–404. doi: 10.2165/00044011-199815050-00004 [DOI] [PubMed] [Google Scholar]
- 70. Nordoy A, Bonaa KH, Nilsen H, Berge RK, Hansen JB, Ingebretsen OC. Effects of simvastatin and omega‐3 fatty acids on plasma lipoproteins and lipid peroxidation in patients with combined hyperlipidaemia. J Intern Med. 1998;243:163–170. doi: 10.1046/j.1365-2796.1998.00297.x [DOI] [PubMed] [Google Scholar]
- 71. Johansen O, Brekke M, Seljeflot I, Abdelnoor M, Arnesen H. N‐3 fatty acids do not prevent restenosis after coronary angioplasty: results from the CART study. Coronary Angioplasty Restenosis Trial. J Am Coll Cardiol. 1999;33:1619–1626. doi: 10.1016/s0735-1097(99)00054-6 [DOI] [PubMed] [Google Scholar]
- 72. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega‐3 fatty acids on coronary atherosclerosis. A randomized, double‐blind, placebo‐controlled trial. Ann Intern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003 [DOI] [PubMed] [Google Scholar]
- 73. Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, Beilin LJ. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–1094. doi: 10.1093/ajcn/71.5.1085 [DOI] [PubMed] [Google Scholar]
- 74. Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, France M. An omega‐3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85:544–548. doi: 10.1136/heart.85.5.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Finnegan YE, Minihane AM, Leigh‐Firbank EC, Kew S, Meijer GW, Muggli R, Calder PC, Williams CM. Plant‐ and marine‐derived n‐3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr. 2003;77:783–795. doi: 10.1093/ajcn/77.4.783 [DOI] [PubMed] [Google Scholar]
- 76. Hamazaki K, Itomura M, Huan M, Nishizawa H, Watanabe S, Hamazaki T, Sawazaki S, Terasawa K, Nakajima S, Terano T, et al. n‐3 long‐chain FA decrease serum levels of TG and remnant‐like particle‐cholesterol in humans. Lipids. 2003;38:353–358. doi: 10.1007/s11745-003-1069-x [DOI] [PubMed] [Google Scholar]
- 77. Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, Clausen P, Rasmussen BF, Schmidt EB, Tholstrup T, et al. Effects of trans‐ and n‐3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr. 2004;58:1062–1070. doi: 10.1038/sj.ejcn.1601934 [DOI] [PubMed] [Google Scholar]
- 78. Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, Arnesen H. Influence of long‐term intervention with dietary counseling, long‐chain n‐3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long‐standing hyperlipidemia. Am J Clin Nutr. 2005;81:583–589. doi: 10.1093/ajcn/81.3.583 [DOI] [PubMed] [Google Scholar]
- 79. Maki KC, Van Elswyk ME, McCarthy D, Hess SP, Veith PE, Bell M, Subbaiah P, Davidson MH. Lipid responses to a dietary docosahexaenoic acid supplement in men and women with below average levels of high density lipoprotein cholesterol. J Am Coll Nutr. 2005;24:189–199. doi: 10.1080/07315724.2005.10719465 [DOI] [PubMed] [Google Scholar]
- 80. Geppert J, Kraft V, Demmelmair H, Koletzko B. Microalgal docosahexaenoic acid decreases plasma triacylglycerol in normolipidaemic vegetarians: a randomised trial. Br J Nutr. 2006;95:779–786. doi: 10.1079/bjn20051720 [DOI] [PubMed] [Google Scholar]
- 81. Lee KW, Blann AD, Lip GY. Effects of omega‐3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post‐myocardial infarction. Thromb Res. 2006;118:305–312. doi: 10.1016/j.thromres.2005.07.018 [DOI] [PubMed] [Google Scholar]
- 82. Sanders TA, Gleason K, Griffin B, Miller GJ. Influence of an algal triacylglycerol containing docosahexaenoic acid (22: 6n‐3) and docosapentaenoic acid (22: 5n‐6) on cardiovascular risk factors in healthy men and women. Br J Nutr. 2006;95:525–531. doi: 10.1079/bjn20051658 [DOI] [PubMed] [Google Scholar]
- 83. Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, Ballantyne CM, Ginsberg HN; Investigators COopO‐wS . Efficacy and tolerability of adding prescription omega‐3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8‐week, randomized, double‐blind, placebo‐controlled study. Clin Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 84. Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R. Eicosapentaenoic acid reduces the progression of carotid intima‐media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191:162–167. doi: 10.1016/j.atherosclerosis.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 85. Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C‐reactive protein in metabolic syndrome. Diabetes Care. 2007;30:144–146. doi: 10.2337/dc06-1179 [DOI] [PubMed] [Google Scholar]
- 86. Kaul N, Kreml R, Austria JA, Richard MN, Edel AL, Dibrov E, Hirono S, Zettler ME, Pierce GN. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J Am Coll Nutr. 2008;27:51–58. doi: 10.1080/07315724.2008.10719674 [DOI] [PubMed] [Google Scholar]
- 87. Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub‐analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200:135–140. doi: 10.1016/j.atherosclerosis.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 88. Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega‐3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes patients. East Mediterr Health J. 2008;14:305–313. [PubMed] [Google Scholar]
- 89. Ebrahimi M, Ghayour‐Mobarhan M, Rezaiean S, Hoseini M, Parizade SM, Farhoudi F, Hosseininezhad SJ, Tavallaei S, Vejdani A, Azimi‐Nezhad M, et al. Omega‐3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto‐immunity. Acta Cardiol. 2009;64:321–327. doi: 10.2143/AC.64.3.2038016 [DOI] [PubMed] [Google Scholar]
- 90. Hartwich J, Malec MM, Partyka L, Perez‐Martinez P, Marin C, Lopez‐Miranda J, Tierney AC, Mc Monagle J, Roche HM, Defoort C, et al. The effect of the plasma n‐3/n‐6 polyunsaturated fatty acid ratio on the dietary LDL phenotype transformation–insights from the LIPGENE study. Clin Nutr. 2009;28:510–515. doi: 10.1016/j.clnu.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 91. Khandelwal S, Demonty I, Jeemon P, Lakshmy R, Mukherjee R, Gupta R, Snehi U, Niveditha D, Singh Y, van der Knaap HC, et al. Independent and interactive effects of plant sterols and fish oil n‐3 long‐chain polyunsaturated fatty acids on the plasma lipid profile of mildly hyperlipidaemic Indian adults. Br J Nutr. 2009;102:722–732. doi: 10.1017/S0007114509297170 [DOI] [PubMed] [Google Scholar]
- 92. Nomura S, Inami N, Shouzu A, Omoto S, Kimura Y, Takahashi N, Tanaka A, Urase F, Maeda Y, Ohtani H, et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet‐derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. 2009;20:16–22. doi: 10.1080/09537100802409921 [DOI] [PubMed] [Google Scholar]
- 93. Rizza S, Tesauro M, Cardillo C, Galli A, Iantorno M, Gigli F, Sbraccia P, Federici M, Quon MJ, Lauro D. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis. 2009;206:569–574. doi: 10.1016/j.atherosclerosis.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Satoh N, Shimatsu A, Kotani K, Himeno A, Majima T, Yamada K, Suganami T, Ogawa Y. Highly purified eicosapentaenoic acid reduces cardio‐ankle vascular index in association with decreased serum amyloid A‐LDL in metabolic syndrome. Hypertens Res. 2009;32:1004–1008. doi: 10.1038/hr.2009.145 [DOI] [PubMed] [Google Scholar]
- 95. Bays HE, McKenney J, Maki KC, Doyle RT, Carter RN, Stein E. Effects of prescription omega‐3‐acid ethyl esters on non–high‐density lipoprotein cholesterol when coadministered with escalating doses of atorvastatin. Mayo Clin Proc. 2010;85:122–128. doi: 10.4065/mcp.2009.0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hallund J, Madsen BO, Bugel SH, Jacobsen C, Jakobsen J, Krarup H, Holm J, Nielsen HH, Lauritzen L. The effect of farmed trout on cardiovascular risk markers in healthy men. Br J Nutr. 2010;104:1528–1536. doi: 10.1017/S0007114510002527 [DOI] [PubMed] [Google Scholar]
- 97. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial G . n‐3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603 [DOI] [PubMed] [Google Scholar]
- 98. Neil HA, Ceglarek U, Thiery J, Paul S, Farmer A, Holman RR. Impact of atorvastatin and omega‐3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: a randomised placebo controlled factorial trial. Atherosclerosis. 2010;213:512–517. doi: 10.1016/j.atherosclerosis.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 99. Zhang J, Wang C, Li L, Man Q, Song P, Meng L, Du ZY, Froyland L. Inclusion of Atlantic salmon in the Chinese diet reduces cardiovascular disease risk markers in dyslipidemic adult men. Nutr Res. 2010;30:447–454. doi: 10.1016/j.nutres.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 100. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the multi‐center, placebo‐controlled, randomized, double‐blind, 12‐week study with an open‐label extension [MARINE] trial). Am J Cardiol. 2011;108:682–690. doi: 10.1016/j.amjcard.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 101. Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Kita T, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18:99–107. doi: 10.5551/jat.5876 [DOI] [PubMed] [Google Scholar]
- 102. Kim SH, Kim MK, Lee HY, Kang HJ, Kim YJ, Kim HS. Prospective randomized comparison between omega‐3 fatty acid supplements plus simvastatin versus simvastatin alone in Korean patients with mixed dyslipidemia: lipoprotein profiles and heart rate variability. Eur J Clin Nutr. 2011;65:110–116. doi: 10.1038/ejcn.2010.195 [DOI] [PubMed] [Google Scholar]
- 103. Krysiak R, Gdula‐Dymek A, Okopien B. The effect of bezafibrate and omega‐3 fatty acids on lymphocyte cytokine release and systemic inflammation in patients with isolated hypertriglyceridemia. Eur J Clin Pharmacol. 2011;67:1109–1117. doi: 10.1007/s00228-011-1063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Krysiak R, Gdula‐Dymek A, Okopien B. Monocyte‐suppressing effect of bezafibrate but not omega‐3 fatty acids in patients with isolated hypertriglyceridaemia. Basic Clin Pharmacol Toxicol. 2011;109:23–29. doi: 10.1111/j.1742-7843.2011.00675.x [DOI] [PubMed] [Google Scholar]
- 105. Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei CL. Effects of n‐3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:870–879. doi: 10.1016/j.jacc.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 106. Sanders TA, Hall WL, Maniou Z, Lewis F, Seed PT, Chowienczyk PJ. Effect of low doses of long‐chain n‐3 PUFAs on endothelial function and arterial stiffness: a randomized controlled trial. Am J Clin Nutr. 2011;94:973–980. doi: 10.3945/ajcn.111.018036 [DOI] [PubMed] [Google Scholar]
- 107. Schuchardt JP, Neubronner J, Kressel G, Merkel M, von Schacky C, Hahn A. Moderate doses of EPA and DHA from re‐esterified triacylglycerols but not from ethyl‐esters lower fasting serum triacylglycerols in statin‐treated dyslipidemic subjects: results from a six month randomized controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2011;85:381–386. doi: 10.1016/j.plefa.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 108. Takaki A, Umemoto S, Ono K, Seki K, Ryoke T, Fujii A, Itagaki T, Harada M, Tanaka M, Yonezawa T, et al. Add‐on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: a randomized controlled study. J Atheroscler Thromb. 2011;18:857–866. doi: 10.5551/jat.7260 [DOI] [PubMed] [Google Scholar]
- 109. Tierney AC, McMonagle J, Shaw DI, Gulseth HL, Helal O, Saris WH, Paniagua JA, Golabek‐Leszczynska I, Defoort C, Williams CM, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome–LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond). 2011;35:800–809. doi: 10.1038/ijo.2010.209 [DOI] [PubMed] [Google Scholar]
- 110. Agouridis AP, Kostapanos MS, Tsimihodimos V, Kostara C, Mikhailidis DP, Bairaktari ET, Tselepis AD, Elisaf MS. Effect of rosuvastatin monotherapy or in combination with fenofibrate or omega‐3 fatty acids on lipoprotein subfraction profile in patients with mixed dyslipidaemia and metabolic syndrome. Int J Clin Pract. 2012;66:843–853. doi: 10.1111/j.1742-1241.2012.02972.x [DOI] [PubMed] [Google Scholar]
- 111. Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin‐treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984–992. doi: 10.1016/j.amjcard.2012.05.031 [DOI] [PubMed] [Google Scholar]
- 112. Derosa G, Cicero AF, Fogari E, D'Angelo A, Bonaventura A, Romano D, Maffioli P. Effects of n‐3 PUFAs on postprandial variation of metalloproteinases, and inflammatory and insulin resistance parameters in dyslipidemic patients: evaluation with euglycemic clamp and oral fat load. J Clin Lipidol. 2012;6:553–564. doi: 10.1016/j.jacl.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 113. Koh KK, Quon MJ, Shin KC, Lim S, Lee Y, Sakuma I, Lee K, Han SH, Shin EK. Significant differential effects of omega‐3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis. 2012;220:537–544. doi: 10.1016/j.atherosclerosis.2011.11.018 [DOI] [PubMed] [Google Scholar]
- 114. Satoh‐Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, Kono S, Takaya T, Ono K, Wada H, et al. Highly purified eicosapentaenoic acid increases interleukin‐10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35:2631–2639. doi: 10.2337/dc12-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Flock MR, Skulas‐Ray AC, Harris WS, Etherton TD, Fleming JA, Kris‐Etherton PM. Determinants of erythrocyte omega‐3 fatty acid content in response to fish oil supplementation: a dose‐response randomized controlled trial. J Am Heart Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hlais S, El‐Bistami D, El Rahi B, Mattar MA, Obeid OA. Combined fish oil and high oleic sunflower oil supplements neutralize their individual effects on the lipid profile of healthy men. Lipids. 2013;48:853–861. doi: 10.1007/s11745-013-3819-x [DOI] [PubMed] [Google Scholar]
- 117. Tani S, Nagao K, Matsumoto M, Hirayama A. Highly purified eicosapentaenoic acid may increase low‐density lipoprotein particle size by improving triglyceride metabolism in patients with hypertriglyceridemia. Circ J. 2013;77:2349–2357. doi: 10.1253/circj.cj-12-1401 [DOI] [PubMed] [Google Scholar]
- 118. Maki KC, Yurko‐Mauro K, Dicklin MR, Schild AL, Geohas JG. A new, microalgal DHA‐ and EPA‐containing oil lowers triacylglycerols in adults with mild‐to‐moderate hypertriglyceridemia. Prostaglandins Leukot Essent Fatty Acids. 2014;91:141–148. doi: 10.1016/j.plefa.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 119. Oh PC, Koh KK, Sakuma I, Lim S, Lee Y, Lee S, Lee K, Han SH, Shin EK. Omega‐3 fatty acid therapy dose‐dependently and significantly decreased triglycerides and improved flow‐mediated dilation, however, did not significantly improve insulin sensitivity in patients with hypertriglyceridemia. Int J Cardiol. 2014;176:696–702. doi: 10.1016/j.ijcard.2014.07.075 [DOI] [PubMed] [Google Scholar]
- 120. Scorletti E, Bhatia L, KG MC, Clough GF, Nash K, Hodson L, Moyses HE, Calder PC, Byrne CD; Study W . Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology. 2014;60:1211–1221. doi: 10.1002/hep.27289 [DOI] [PubMed] [Google Scholar]
- 121. Toyama K, Nishioka T, Isshiki A, Ando T, Inoue Y, Kirimura M, Kamiyama T, Sasaki O, Ito H, Maruyama Y, et al. Eicosapentaenoic acid combined with optimal statin therapy improves endothelial dysfunction in patients with coronary artery disease. Cardiovasc Drugs Ther. 2014;28:53–59. doi: 10.1007/s10557-013-6496-3 [DOI] [PubMed] [Google Scholar]
- 122. Mansoori A, Sotoudeh G, Djalali M, Eshraghian MR, Keramatipour M, Nasli‐Esfahani E, Shidfar F, Alvandi E, Toupchian O, Koohdani F. Effect of DHA‐rich fish oil on PPARgamma target genes related to lipid metabolism in type 2 diabetes: a randomized, double‐blind, placebo‐controlled clinical trial. J Clin Lipidol. 2015;9:770–777. doi: 10.1016/j.jacl.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 123. Ahn J, Park SK, Park TS, Kim JH, Yun E, Kim SP, Lee HW, Oh JH, Choi JH, Cha KS, et al. Effect of n‐3 polyunsaturated fatty acids on regression of coronary atherosclerosis in statin treated patients undergoing percutaneous coronary intervention. Korean Circ J. 2016;46:481–489. doi: 10.4070/kcj.2016.46.4.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bays HE, Hallen J, Vige R, Fraser D, Zhou R, Hustvedt SO, Orloff DG, Kastelein JJ. Icosabutate for the treatment of very high triglycerides: a placebo‐controlled, randomized, double‐blind, 12‐week clinical trial. J Clin Lipidol. 2016;10(181–191):e181–e182. doi: 10.1016/j.jacl.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 125. Derosa G, Cicero AF, D'Angelo A, Borghi C, Maffioli P. Effects of n‐3 pufas on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. Biofactors. 2016;42:316–322. doi: 10.1002/biof.1277 [DOI] [PubMed] [Google Scholar]
- 126. Koh KK, Oh PC, Sakuma I, Lee Y, Han SH, Shin EK. Vascular and metabolic effects of omega‐3 fatty acids combined with fenofibrate in patients with hypertriglyceridemia. Int J Cardiol. 2016;221:342–346. doi: 10.1016/j.ijcard.2016.07.038 [DOI] [PubMed] [Google Scholar]
- 127. Sawada T, Tsubata H, Hashimoto N, Takabe M, Miyata T, Aoki K, Yamashita S, Oishi S, Osue T, Yokoi K, et al. Effects of 6‐month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly‐diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol. 2016;15:121. doi: 10.1186/s12933-016-0437-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Su TC, Hwang JJ, Huang KC, Chiang FT, Chien KL, Wang KY, Charng MJ, Tsai WC, Lin LY, Vige R, et al. A randomized, double‐blind, placebo‐controlled clinical trial to assess the efficacy and safety of ethyl‐ester omega‐3 fatty acid in Taiwanese hypertriglyceridemic patients. J Atheroscler Thromb. 2017;24:275–289. doi: 10.5551/jat.34231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tani S, Nagao K, Kawauchi K, Yagi T, Atsumi W, Matsuo R, Hirayama A. The ratio of eicosapentaenoic acid (EPA) to arachidonic acid may be a residual risk marker in stable coronary artery disease patients receiving treatment with statin following EPA therapy. Am J Cardiovasc Drugs. 2017;17:409–420. doi: 10.1007/s40256-017-0238-z [DOI] [PubMed] [Google Scholar]
- 130. Tani S, Nagao K, Yagi T, Atsumi W, Hirayama A. Impact of adding eicosapentaenoic acid to statin therapy on plasma pentraxin 3 level in patients with stable coronary artery disease: a 6‐month, randomized controlled study. Am J Cardiovasc Drugs. 2017;17:49–59. doi: 10.1007/s40256-016-0195-y [DOI] [PubMed] [Google Scholar]
- 131. Toth S, Sajty M, Pekarova T, Mughees A, Stefanic P, Katz M, Spisakova K, Pella J, Pella D. Addition of omega‐3 fatty acid and coenzyme Q10 to statin therapy in patients with combined dyslipidemia. J Basic Clin Physiol Pharmacol. 2017;28:327–336. doi: 10.1515/jbcpp-2016-0149 [DOI] [PubMed] [Google Scholar]
- 132. Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S, Matsui M, Ikeno E, Hirono O, Miyawaki H, Yashiro Y, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70:537–544. doi: 10.1016/j.jjcc.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 133. Group ASC , Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, et al. Effects of n‐3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 134. Kim CH, Han KA, Yu J, Lee SH, Jeon HK, Kim SH, Kim SY, Han KH, Won K, Kim DB, et al. Efficacy and safety of adding Omega‐3 fatty acids in statin‐treated patients with residual hypertriglyceridemia: ROMANTIC (rosuvastatin‐OMAcor iN residual hyperTrIglyCeridemia), a randomized, double‐blind, and placebo‐controlled trial. Clin Ther. 2018;40:83–94. doi: 10.1016/j.clinthera.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 135. Oscarsson J, Onnerhag K, Riserus U, Sunden M, Johansson L, Jansson PA, Moris L, Nilsson PM, Eriksson JW, Lind L. Effects of free omega‐3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non‐alcoholic fatty liver disease: a double‐blind, randomized, placebo‐controlled study. J Clin Lipidol. 2018;12(1390–1403):e1394. doi: 10.1016/j.jacl.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 136. Stroes ESG, Susekov AV, de Bruin TWA, Kvarnstrom M, Yang H, Davidson MH. Omega‐3 carboxylic acids in patients with severe hypertriglyceridemia: EVOLVE II, a randomized, placebo‐controlled trial. J Clin Lipidol. 2018;12:321–330. doi: 10.1016/j.jacl.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 137. Zhou Q, Zhang Z, Wang P, Zhang B, Chen C, Zhang C, Su Y. EPA+DHA, but not ALA, improved lipids and inflammation status in hypercholesterolemic adults: a randomized, double‐blind, placebo‐controlled trial. Mol Nutr Food Res. 2019;63:e1801157. doi: 10.1002/mnfr.201801157 [DOI] [PubMed] [Google Scholar]
- 138. Fukumoto K, Takemoto Y, Yoshikawa J, Norioka N, Iguchi T, Namikawa H, Tochino Y, Yoshiyama M, Shuto T. Predictors of endothelial function improvement in patients with mild hypertriglyceridemia without evidence of coronary artery disease treated with purified eicosapentaenoic acid. Atherosclerosis. 2020;309:27–32. doi: 10.1016/j.atherosclerosis.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 139. Jun JE, Jeong IK, Yu JM, Kim SR, Lee IK, Han KA, Choi SH, Kim SK, Park HK, Mok JO, et al. Efficacy and safety of omega‐3 fatty acids in patients treated with statins for residual hypertriglyceridemia: a randomized, double‐blind, placebo‐controlled clinical trial. Diabetes Metab J. 2020;44:78–90. doi: 10.4093/dmj.2018.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kita Y, Watanabe M, Kamon D, Ueda T, Soeda T, Okayama S, Ishigami K, Kawata H, Horii M, Inoue F, et al. Effects of fatty acid therapy in addition to strong statin on coronary plaques in acute coronary syndrome: an optical coherence tomography study. J Am Heart Assoc. 2020;9:e015593. doi: 10.1161/JAHA.119.015593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Guo XF, Wang C, Yang T, Ma WJ, Zhai J, Zhao T, Xu TC, Li J, Liu H, Sinclair AJ, et al. The effects of fish oil plus vitamin D3 intervention on non‐alcoholic fatty liver disease: a randomized controlled trial. Eur J Nutr. 2022;61:1931–1942. doi: 10.1007/s00394-021-02772-0 [DOI] [PubMed] [Google Scholar]
- 142. Schuchardt JP, Schneider I, Meyer H, Neubronner J, von Schacky C, Hahn A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega‐3 fatty acid formulations–a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011;10:145. doi: 10.1186/1476-511X-10-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dyerberg J, Madsen P, Moller JM, Aardestrup I, Schmidt EB. Bioavailability of marine n‐3 fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids. 2010;83:137–141. doi: 10.1016/j.plefa.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 144. Offman E, Marenco T, Ferber S, Johnson J, Kling D, Curcio D, Davidson M. Steady‐state bioavailability of prescription omega‐3 on a low‐fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: the ECLIPSE II study. Vasc Health Risk Manag. 2013;9:563–573. doi: 10.2147/VHRM.S50464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Casula M, Soranna D, Catapano AL, Corrao G. Long‐term effect of high dose omega‐3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: a meta‐analysis of randomized, placebo controlled trials [corrected]. Atheroscler Suppl. 2013;14:243–251. doi: 10.1016/S1567-5688(13)70005-9 [DOI] [PubMed] [Google Scholar]
- 146. Brinton EA, Mason RP. Prescription omega‐3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis. 2017;16:23. doi: 10.1186/s12944-017-0415-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Toth PP, Chapman MJ, Parhofer KG, Nelson JR. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am Heart J. 2022;17:100148. doi: 10.1016/j.ahjo.2022.100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega‐3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40:1135–1147. doi: 10.1161/ATVBAHA.119.313286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, Magni P, Marz W, Masana L, Mello ESA, et al. Practical guidance for combination lipid‐modifying therapy in high‐ and very‐high‐risk patients: a statement from a European Atherosclerosis Society task force. Atherosclerosis. 2021;325:99–109. doi: 10.1016/j.atherosclerosis.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 150. Sasaki J, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Itakura H, Hishida H, Kita T, et al. Relationship between coronary artery disease and non‐HDL‐C, and effect of highly purified EPA on the risk of coronary artery disease in hypercholesterolemic patients treated with statins: sub‐analysis of the Japan EPA Lipid Intervention Study (JELIS). J Atheroscler Thromb. 2012;19:194–204. doi: 10.5551/jat.8326 [DOI] [PubMed] [Google Scholar]
- 151. Kastelein JJ, Maki KC, Susekov A, Ezhov M, Nordestgaard BG, Machielse BN, Kling D, Davidson MH. Omega‐3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94–106. doi: 10.1016/j.jacl.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 152. Nishizaki Y, Miyauchi K, Iwata H, Inoue T, Hirayama A, Kimura K, Ozaki Y, Murohara T, Ueshima K, Kuwabara Y, et al. Study protocol and baseline characteristics of randomized trial for evaluation in secondary prevention efficacy of combination therapy‐statin and eicosapentaenoic acid: RESPECT‐EPA, the combination of a randomized control trial and an observational biomarker study. Am Heart J. 2022;257:1–8. doi: 10.1016/j.ahj.2022.11.008 [DOI] [PubMed] [Google Scholar]
- 153. Gupta K, Hirsch JR, Kalsi J, Patel V, Gad MM, Virani SS. Highlights of cardiovascular disease prevention studies presented at the 2022 American Heart Association scientific sessions. Current Atherosclerosis Reports. 2023;25:31–41. doi: 10.1007/s11883-022-01079-7 [DOI] [PubMed] [Google Scholar]
- 154. Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, et al. OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


