Abstract
Background
The American Heart Association recently proposed an updated definition of cardiovascular health (CVH) named as Life's Essential 8. We aimed to explore the association between this latest published CVH measurement and years lived without cardiovascular disease (CVD) among the Chinese population.
Methods and Results
We included 89 755 adults free of CVD at baseline from the Kailuan study. The CVH of each participant was scored (from 0 point to 100 points) and classified (low [0–49 points], moderate [50–79 points], and high [80–100 points]) according to Life's Essential 8, which incorporated 8 components covering health behaviors and health factors. Incident CVD was documented through follow‐ups from baseline (June 2006 to October 2007) until December 31, 2020. CVD‐free life years from age 30 to 80 years associated with different CVH scores were estimated using flexible parametric survival models. A total of 9977 incident CVDs were recorded. We observed a gradient relationship between CVH score and years lived without CVD. The age‐ and sex‐adjusted CVD‐free life years (95% CI) were 40.7 (40.3–41.0) years for low CVH, 43.3 (43.0–43.5) years for moderate CVH, and 45.5 (45.1–45.9) years for high CVH. Similar trends were noted when individual subtypes of CVD were investigated, and high CVH evaluated by health behaviors and health factors was also related to longer CVD‐free life years.
Conclusions
A higher CVH evaluated by the updated Life's Essential 8 metrics was significantly associated with a greater number of life years without CVD, indicating the importance of promoting CVH for healthy aging in China.
Keywords: cardiovascular disease, cardiovascular health, disease‐free life years, Life's Essential 8 metrics
Subject Categories: Cardiovascular Disease, Aging, Epidemiology, Lifestyle, Primary Prevention
Nonstandard Abbreviations and Acronyms
- CVH
cardiovascular health
- LE8
Life's Essential 8
- LS7
Life's Simple 7
Clinical Perspective.
What Is New?
This Chinese population‐based cohort study of 89 755 participants indicated that a higher cardiovascular health (CVH) assessed by Life's Essential 8 was significantly associated with a greater number of cardiovascular disease‐free life years between ages 30 to 80 years, ranging from 40.7 years of low CVH to 43.3 years of moderate CVH, and 45.5 years of high CVH.
What Are the Clinical Implications?
Higher CVH was related to prolonged healthy life span free from cardiovascular disease, which indicated that primordial cardiovascular disease prevention might be an effective strategy for healthy aging in China.
Cardiovascular disease (CVD) has become the leading cause of global mortality and a major contributor to disability for decades, accounting for 32.84% of total deaths and 393.1 million disability‐adjusted life years in 2019 worldwide. 1 , 2 Major modifiable risk factors contributing to CVD were identified through numerous prospective studies, and the associations of lifestyles with cardiovascular health (CVH) have been studied individually, and more recently, collectively. 3 , 4 , 5 , 6
On that basis, the American Heart Association (AHA) put forward a novel concept of ideal CVH called Life's Simple 7 (LS7) in 2010, which accelerated the transformation from a focus solely on CVD treatment to one inclusive of positive health promotion and preservation across the life course, aligning more closely with the notion of primordial prevention. 7 After more than 10 years of practice, the AHA proposed its updated approach to better assess CVH in 2022, which was named as Life's Essential 8 (LE8). 8 In brief, the indicators of LS7 included physical activity, exposure to cigarette smoking, body mass index (BMI), fasting blood glucose, total cholesterol, and blood pressure (BP), each of which was classified as poor, intermediate, or ideal. The newly proposed LE8, which enhanced and expanded the definition and methods for CVH quantification, modified the scoring algorithm of several existing indicators, used non‐high‐density lipoprotein (non‐HDL) cholesterol instead of total cholesterol, and added sleep health as the eighth metric. Thus, the LE8 included 8 metrics on both health behaviors (diet, physical activity, nicotine exposure, sleep health) and health factors (BMI, blood glucose, non‐HDL cholesterol, and BP), and the scale of each metric ranged from 0 to 100 points, allowing the generation of a new composite CVH score.
Since the proposal of LS7 in 2010, a series of population‐based researches in diverse settings have demonstrated the inverse relationships between numbers of ideal CVH metrics and risks of incident CVD. 9 , 10 , 11 , 12 However, little has been known about the exact performance of the new proposed LE8 on CVD risks, especially for those non‐US populations. 13 More importantly, most existing studies about lifestyle‐related risks either examined relative or absolute risk of CVD outcomes, and few assessed the association of health behaviors and factors with life expectancy. 14 , 15 However, uncertainty exists with regard to the association of such modifiable risk factors with CVD‐free life years, a measurement that may be more beneficial to establish public health priorities and promote healthy aging.
Therefore, we conducted the current study to quantify the extent to which CVH measured by the new LE8 definition is associated with the number of CVD‐free life years as indexed by the age at first onset of incident CVD, based on a community‐based prospective cohort of >100 000 adults from Northern China.
METHODS
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Study Population
Participants were derived from the Kailuan study, which was conducted among employees of the Kailuan company in Northern China. Detailed information on the Kailuan study has been described in previous publications. 16 , 17 , 18 In brief, 101 510 adults, including 81 110 men and 20 400 women, were recruited at the baseline survey from June 2006 to October 2007 and followed up biennially until their deaths or the latest visit of the ongoing study (December 31, 2020), whichever came first. Of 97 275 participants free from CVD at baseline, 7520 participants with incomplete information on measurements of LE8 metrics were further excluded, including 4254 for diet, 91 for physical activity, 99 for nicotine exposure, 100 for sleep health, 717 for BMI, 1875 for blood lipids, 46 for FBG, and 338 for BP. Ultimately, a total of 89 755 participants were involved in the current analyses (Figure S1).
The protocol of Kailuan study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has received ethical committee approval from both Kailuan General Hospital (Approval Number: 2006‐05) and Beijing Tiantan Hospital (Approval Number: 2010‐014‐01). Written informed consent was obtained from each patient included in the study.
Baseline Information Collection
Baseline information on sociodemographic characteristics (age at recruitment, sex, income, education attainment), lifestyles (dietary habits, tobacco smoking, physical activity, sleep duration), medical histories (previous diagnosis of CVD), and medication records (antihypertensive, antidiabetic and lipid‐lowering treatments) were collected by well‐trained local staff with a standardized questionnaire.
Physical examinations and blood biochemical tests were also conducted at the baseline survey. Weight and height were measured with lightweight clothing, and BMI was then calculated as weight in kilograms divided by height in meters squared. Three BP measurements were taken with mercury sphygmomanometers, and an average of 3 readings was used in the analyses. Overnight fasting venous blood samples were drawn for measurements of glucose and lipid levels, and non‐HDL cholesterol was calculated as total cholesterol minus HDL cholesterol.
CVH Quantification
The LE8 scoring algorithm developed by the AHA Presidential Advisory was applied to assess the CVH of each participant. Definitions and scorings for each component of CVH metrics, including both the health behavior domain (diet, physical activity, tobacco/nicotine exposure, and sleep health) and the health factor domain (BMI, blood lipids, blood glucose, and BP) were described in detail in Table S1. In simple terms, each CVH component was scored on a scale of 0 (the lowest) to 100 points (the highest), and the overall and 2 domain‐specific CVH scores were estimated as the unweighted average of individual component, also ranging from 0 to 100 points. Dietary quality was assessed with habitual consumption of salt, fatty food, and tea, each of which has been proven to be significantly associated with CVD risks in China. 19 , 20 , 21 , 22 Physical activity was scored based on minutes of physical activity per week, with <20 minutes scored 0 point, 20 to 60 minutes scored 50 points, and ≥80 minutes as 100 points. Nicotine exposure was scored based on self‐reported use of cigarettes, in which 0 points represented current smokers with ≥1 cigarette/d, 25 points represented current smokers with <1 cigarette/d, 50 points represented former smokers, and 100 points represented nonsmokers. Sleep health was evaluated based on average hours of sleep per night, with scores ranging from 0 (<4 hours) to 100 points (7 to <9 hours), in line with the AHA definition. Most health factors were scored with the clinical cutting‐off values suggested by the AHA except for BMI, the score of which was modified with overall lower cutting‐off values (≥35.0 kg/m2 scored 0 point and <23.0 kg/m2 scored 100 points) to make better adaption to Chinese population. Then, CVH status was defined in accordance with recommendations from the AHA, with 80 to 100 points as high CVH, 50 to 79 points as moderate CVH, and 0 to 49 points as low CVH.
Outcomes Ascertainment
During follow‐ups from baseline until December 31, 2020, health status of each participant was annually updated and cross‐validated through 4 complementary sources as described previously: the biennial face‐to face interviews, the medical records from the Municipal Social Insurance Institution which covered all participants in the Kailuan study, the discharge summaries from 11 hospitals in the Kailuan community and the death certificates (without specific causes of death) from provincial vital statistics offices. 23 Clinical outcomes were initially extracted from those linked sources according to the International Classification of Diseases, Tenth Revision (ICD‐10). Then, all the potential cases were further reviewed and ascertained by a central expert panel, blind to the study design.
The primary outcome of interest in the current study was incident CVD, which was defined as a composite of both stroke (including cerebral infarction, intracranial hemorrhage, and subarachnoid hemorrhage) and heart diseases (including myocardial infarction, atrial fibrillation, and heart failure). The secondary outcomes were stroke, heart diseases, and each individual end point. In accordance with the World Health Organization criteria, stroke was diagnosed and classified based on neurological signs, clinical symptoms, and neuroimages from computed tomography or magnetic resonance 24 ; myocardial infarction was diagnosed on the basis of clinical symptoms, electrocardiography and dynamic changes in levels of cardiac enzyme and other biomarkers. 25 In addition, atrial fibrillation was confirmed according to electrocardiographic documentations of absolutely irregular RR intervals and no discernible, distinct P values, 26 and heart failure was clinically diagnosed on the basis of clinical symptoms, echocardiography, chest radiography, and electrocardiography. 27
Statistical Analysis
Baseline characteristics by CVH statuses of LE8 were presented as mean±SD or frequency (percentage), as appropriate. Trends across CVH statuses were tested with linear regression models for continuous variables and Cochran‐Armitage tests for categorical variables. CVD‐free life years was defined as the number of life years between age 30 to 80 years free from diagnosis of any incident CVD. We chose age 30 years as the lower limit value, as this is typically the age at which CVD has gradually become a major health concern. For the upper limit, the current life expectancy of women (80.5 years), which was longer than men (74.7 years), was taken as a reference to cover the remaining life cycle of the middle‐aged Chinese population as much as possible. To explore the association between CVH and CVD‐free life years, hazard ratio (HR) and 95% CI were estimated using flexible parametric survival models on the cumulative hazard scale, which allowed direct estimation of the conditional cumulative hazard function. 28 , 29 The proportional hazard assumption was tested based on Schoenfeld residuals and no violations were observed (P>0.05). 30 Thus, models were generated as an extension of the traditional Weibull parametric model with link function of ln[−ln(S(t))], in which the restricted cubic spline was used to estimate the ln(t) rather than simply assumed a linear distribution. Within these models, we used restricted cubic splines with 4 internal knots (determined by the Bayes information criterion statistic) to model the baseline hazard for different CVH with age as the timescale. Then, CVD‐free life years according to each CVH category were estimated as the areas under CVD‐free survival curves from age 30 to 80 years, conditional on survival to age 30 years without incident CVD. Areas under the curve were computed via numerical integration with a spline‐based method, and we estimated CIs via bootstrapping using 100 independent replications. The main analyses were performed with 2 models. In the first model, no adjustment was made; in the second model, we adjusted sex and age at recruitment of participants.
Stratified analysis by sex, years of education, and average monthly income were further adopted to investigate the potential effect modifications of sociodemographic factors. To evaluate the robustness of our findings, 5 sensitivity analyses were conducted, including excluding 20 644 individuals exposed to dust in underground coal mines; excluding 1025 CVD occurred within the initial 2‐year follow‐up; redefining the outcome of CVD by excluding subarachnoid hemorrhage and atrial fibrillation; excluding 8253 individuals who died before developing CVD; and further adjusting for education and income levels.
All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC), stpm2 Macro, and R software, version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria), survminer and ggplot2 package. Tests were 2‐sided, with statistical significance set at P<0.05.
RESULTS
Of all the 89 755 participants included, the mean age at enrollment was 50.9 years and 79.2% were men (Table 1). In general, the overall CVH score was 65.8±11.4 points, of which diets (38.7±15.1 points) and BP (56.6±34.4 points) scored the lowest among all the health behaviors and health factors, respectively. Using categorical considerations suggested by the AHA, 8309 (9.3%) individuals were classified into the low CVH status, 72 278 (80.5%) into the moderate CVH status, and 9168 (10.2%) into the high CVH status, respectively. In addition, we observed that participants with more advantageous CVH scores were significantly younger and more possibly to be women and of higher socioeconomic status (P trend<0.0001).
Table 1.
Baseline Characteristics by CVH Status of LE8
| Characteristics | All participants | Low CVH (0–49 points) | Moderate CVH (50–79 points) | High CVH (80–100 points) | P trend |
|---|---|---|---|---|---|
| Participants, n (%) | 89 755 (100) | 8309 (9.3) | 72 278 (80.5) | 9168 (10.2) | … |
| Age at baseline, mean±SD, y | 50.9±12.3 | 51.7±10.0 | 51.5±12.2 | 45.6±13.7 | <0.0001 |
| Men, n (%) | 71 090 (79.2) | 7938 (95.5) | 58 618 (81.1) | 4534 (49.5) | <0.0001 |
| High school or above, n (%) | 18 153 (20.2) | 1361 (16.4) | 13 516 (18.7) | 3276 (35.8) | <0.0001 |
| Personal monthly income ≥800 CNY, n (%) | 12 716 (14.2) | 1318 (15.9) | 9770 (13.5) | 1628 (17.8) | <0.0001 |
| CVH scores of LE8, mean±SD, out of 100 possible points | |||||
| Overall | 65.8±11.4 | 44.1±5.1 | 66.1±7.9 | 83.8±2.9 | <0.0001 |
| Health behaviors | 60.9±15.0 | 39.9±13.4 | 61.6±13.4 | 74.4±6.9 | <0.0001 |
| Diet | 38.7±15.1 | 34.7±17.1 | 38.6±14.6 | 42.9±15.9 | <0.0001 |
| Physical activity | 53.1±24.2 | 38.5±29.7 | 53.8±23.2 | 61.1±21.5 | <0.0001 |
| Tobacco/nicotine exposure | 64.0±45.9 | 15.8±32.9 | 65.3±45.4 | 97.5±13.7 | <0.0001 |
| Sleep health | 87.7±21.9 | 70.5±29.4 | 88.6±20.8 | 96.1±11.5 | <0.0001 |
| Health factors | 70.8±17.0 | 48.3±13.5 | 70.5±14.4 | 93.2±6.5 | <0.0001 |
| BMI | 68.0±24.6 | 51.7±21.2 | 66.9±23.8 | 92.0±15.1 | <0.0001 |
| Blood lipids (non‐HDL cholesterol) | 73.2±28.5 | 48.8±27.7 | 73.5±27.8 | 93.6±15.6 | <0.0001 |
| Blood glucose | 85.3±24.2 | 64.7±30.1 | 86.1±23.3 | 98.1±8.8 | <0.0001 |
| BP | 56.6±34.4 | 28.2±31.1 | 55.7±33.2 | 89.1±15.6 | <0.0001 |
Values were represented by mean±SD for continuous variables and frequency (percentage) for categorical variables. Trends of baseline characteristics across CVH statuses were tested with linear regression for continuous variables and Cochran‐Armitage test for categorical variables. BMI indicates body mass index; BP, blood pressure; CNY, Chinese yuan; CVH, cardiovascular health; HDL, high‐density lipoprotein; and LE8, Life's Essential 8.
During follow‐up from the baseline survey to December 31, 2020, a total of 9977 cases of incident CVD, including 6031 strokes and 4577 heart diseases, were documented. As shown in Figure 1, the CVD‐free survival curves, estimated on condition survival to age 30 years without any CVD events, illustrated a graded declined CVD risk along with higher CVH scores. The HR (95% CI) for CVD decreased along with the increasing CVH scores. In comparison with the lowest scores of 0 to 29 points, the HRs (95% CI) of CVH scores of 50 to 59 points and 90 to 100 points were 0.49 (0.36–0.67) and 0.20 (0.11–0.36), respectively (Table S2). Accordingly, individuals with CVH scores of 0 to 29 points had a mean of 42.5 (40.7–44.4) CVD‐free life years between 30 and 80 years, while the estimate for those with the highest scores of 90 to 100 points reached up to 48.4 (47.6–49.1) years (Table 2). This gradient relationship remained significant after further adjustment of age at baseline and sex, and the corresponding estimates were 38.3 (36.9–40.2) and 45.6 (44.1–47.4) years, respectively.
Figure 1. Cardiovascular disease‐free survival probability by cardiovascular health scores of Life's Essential 8.
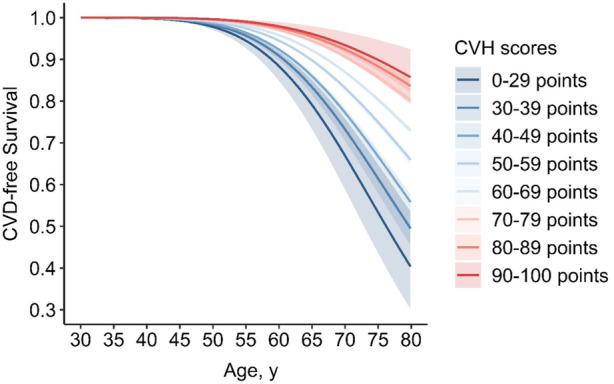
Solid lines represented point estimates of survival probability and shaded areas represented 95% CIs, which were estimated conditional on survival to age 30 years without cardiovascular disease. CVD indicates cardiovascular disease; and CVH, cardiovascular health.
Table 2.
Estimated Numbers of CVD‐Free Life Years By CVH Scores of LE8
| CVH score | No. of cases/total | CVD‐free life years from age 30 to 80 years (95% CI) | |
|---|---|---|---|
| Unadjusted | Age‐and sex‐adjusted | ||
| 0–29 points | 39/150 | 42.5 (40.7–44.4) | 38.3 (36.9–40.2) |
| 30–39 points | 284/1397 | 43.8 (43.2–44.4) | 39.8 (39.1–40.4) |
| 40–49 points | 1229/6762 | 44.7 (44.4–45.0) | 40.6 (40.1–41.0) |
| 50–59 points | 2676/18 261 | 46.0 (45.9–46.2) | 41.9 (41.5–42.2) |
| 60–69 points | 3428/29 278 | 46.9 (46.8–47.0) | 43.0 (42.6–43.2) |
| 70–79 points | 1899/24 739 | 47.7 (47.6–47.8) | 44.4 (44.1–44.7) |
| 80–89 points | 406/8814 | 48.1 (48.0–48.3) | 45.4 (45.0–45.7) |
| 90–100 points | 16/354 | 48.4 (47.6–49.1) | 45.6 (44.1–47.4) |
CVD indicates cardiovascular disease; CVH, cardiovascular health; and LE8, Life's Essential 8.
The numbers of CVD‐free life years according to overall and domain‐specific CVH statuses of LE8 were provided in Table 3 and the Kaplan–Meier plot was shown in Figure S2. Generally, the age‐ and sex‐adjusted CVD‐free life years were 40.7 (40.3–41.0) years for participants with overall low CVH, 43.3 (43.0–43.5) years for those with moderate CVH and 45.5 (45.1–45.9) years for those with high CVH. More specifically, individuals with high CVH of health behaviors had 1.2 additional years free from CVD than those with low CVH of health behaviors (44.3 [43.9–44.8] years versus 43.1 [42.7–43.4] years). Similar tendency was also observed in the HR (95% CI) of different CVH groups (Table S3). The benefits seemed to be more obvious when evaluated with health factors. Comparing high CVH of health factors with low CVH was associated with 4.6 additional CVD‐free life years (45.2 [44.9–45.5] years versus 40.6 [40.3–41.0] years).
Table 3.
Estimated Numbers of CVD‐Free Life Years By Overall and Domain‐Specific CVH Statuses of LE8 and for Major CVD Subtypes
| CVH status | No. of cases/total | CVD‐free life years from age 30 to 80 years (95% CI) | |
|---|---|---|---|
| Unadjusted | Age‐and sex‐adjusted | ||
| Overall | |||
| Low (0–49 points) | 1552/8309 | 44.5 (44.3–44.7) | 40.7 (40.3–41.0) |
| Moderate (50–79 points) | 8003/72 278 | 46.9 (46.8–47.0) | 43.3 (43.0–43.5) |
| High (80–100 points) | 422/9168 | 48.1 (48.0–48.3) | 45.5 (45.1–45.9) |
| By individual domains | |||
| Health behaviors | |||
| Low (0–49 points) | 2569/22 143 | 46.1 (45.9–46.2) | 43.1 (42.7–43.4) |
| Moderate (50–79 points) | 6971/63 549 | 46.9 (46.8–47.0) | 43.4 (43.1–43.6) |
| High (80–100 points) | 437/4063 | 47.8 (47.6–48.0) | 44.3 (43.9–44.8) |
| Health factors | |||
| Low (0–49 points) | 2191/10 414 | 45.1 (44.9–45.3) | 40.6 (40.3–41.0) |
| Moderate (50–79 points) | 5972/49 101 | 46.7 (46.6–46.8) | 43.2 (42.8–43.4) |
| High (80–100 points) | 1814/30 240 | 47.8 (47.7–47.9) | 45.2 (44.9–45.5) |
| For major CVD subtypes | |||
| Stroke | |||
| Low (0–49 points) | 988/8309 | 46.3 (46.1–46.5) | 43.2 (42.7–43.6) |
| Moderate (50–79 points) | 4810/72 278 | 48.1 (48.0–48.1) | 45.4 (45.1–45.7) |
| High (80–100 points) | 233/9168 | 48.9 (48.8–49.0) | 47.2 (46.9–47.6) |
| Heart diseases | |||
| Low (0–49 points) | 678/8309 | 47.5 (47.3–47.6) | 44.6 (44.1–45.0) |
| Moderate (50–79 points) | 3696/72 278 | 48.5 (48.5–48.6) | 46.3 (46.0–46.6) |
| High (80–100 points) | 203/9168 | 49.1 (48.9–49.2) | 47.6 (47.2–47.9) |
CVD indicates cardiovascular disease; CVH, cardiovascular health; and LE8, Life's Essential 8.
A generally consistent association was found between a high CVH of most LE8 components and longer CVD‐free life years, which was more significant among health factors compared with health behaviors. Among all the CVH components, healthy diet as well as avoidance of tobacco exposure exerted stronger protective effects in the health behavior domain, and a high CVH of BP was related to the most remarkable gains of CVD‐free life years in the health factor domain (Table S4). The associations of CVH status on CVD subtypes were further examined. Taking low CVH as reference, participants with high CVH had about 4.0 (47.2 [46.9–47.6] years versus 43.2 [42.7–43.6] years) and 3.0 (47.6 [47.2–47.9] years versus 44.6 [44.1–45.0] years) additional years free from stroke and heart diseases, respectively. Similar relationships were also observed for each individual end point of CVD (Table S5).
Results of subgroup analyses were presented in Figure 2, which indicated consistent relationships of CVH status with incident CVD across sex, years of education, and average monthly income. In addition, further excluding individuals exposed to dust in underground coal mines or incident CVD occurred within the initial 2‐year follow‐up did not considerably alter the significant association (Tables S6 and S7). Results of the other sensitivity analyses were also generally consistent with our primary findings (Tables S8 through S10).
Figure 2. Subgroup analyses for estimated number of cardiovascular disease‐free life years by cardiovascular health status of Life's Essential 8.
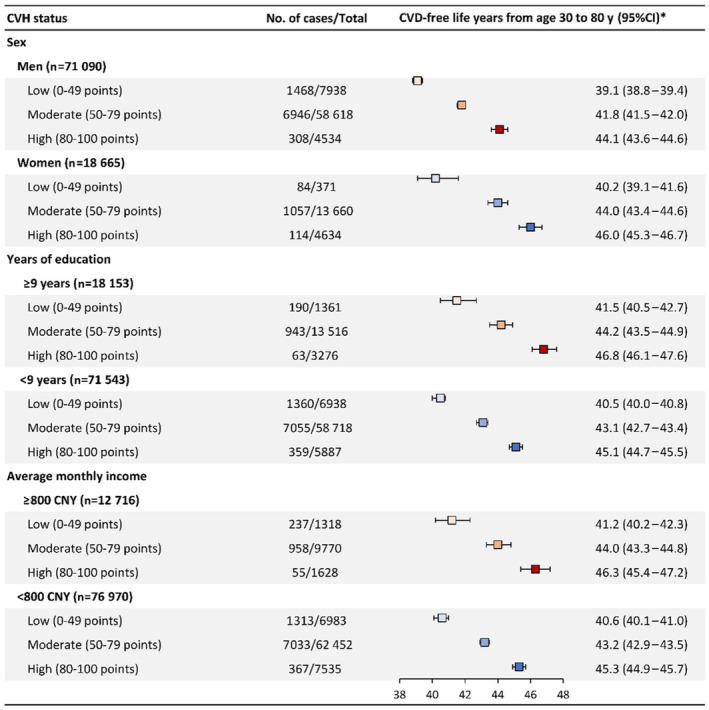
The squares represent point estimates of numbers of cardiovascular disease‐free life years, and the horizontal lines represent 95% CIs, which were estimated conditional on survival to age 30 years without cardiovascular disease and adjusted by age and sex. CNY indicates Chinese Yuan; CVD, cardiovascular disease; and CVH, cardiovascular health.
DISCUSSION
Based on this large prospective cohort study, we observed that a higher CVH evaluated by the latest‐proposed LE8 metrics was significantly associated with gains in years lived without CVD between ages 30 to 80 years. The age‐ and sex‐ adjusted CVD‐free life years ranged from 38.3 years among individuals with CVH scores of 0 to 29 points to 45.6 years among those with CVH scores of 90 to 100 points. In comparison with low CVH, moderate and high CVH classified with categorical considerations suggested by the AHA were significantly associated with about 2.6 and 4.8 additional adjusted‐years without incident CVD, respectively. Additionally, a high CVH of individual domain and component was also found to be consistently related to longer CVD‐free life years, and similar trends were observed when individual major CVD subtypes were considered instead of the total.
Although few population‐based investigations focusing on CVH metrics and CVD‐free life years were conducted before, our results were generally comparable with previous studies on the relationship between modifiable lifestyle‐related factors and CVD risks quantified by other indicators. 9 , 10 , 11 , 31 , 32 , 33 Most studies defining CVH with LS7 reported that higher numbers of ideal CVH metrics were significantly associated with reduced incidence rates and HRs of incident CVD as well as the occurrence of myocardial infarction, stroke, atrial fibrillation, and heart failure. 10 , 32 , 33 , 34 Several studies have further estimated the short‐term or lifetime CVD risks related to profiles of modifiable lifestyle‐related risk factors. The Chinese Multi‐Provincial Cohort Study, for instance, suggested that the lifetime risk of CVD up to age 80 years increased from 4.1% to 51.1% for men and from 1.9% to 38.6% for women at age 35 years, respectively, with the increasing numbers of risk factors. 35 Similarly, an individual‐level meta‐analysis, which stratified participants into mutually exclusive categories of 4 lifestyle factors (BP, cholesterol level, smoking, and diabetes), has indicated that population with an optimal risk‐factor profile had substantially lower risks of CVD mortality through the age of 80 years than those with ≥2 major risk factors. 36
Along with the release of updated LE8 scoring algorithm, the recent analysis of the National Health and Nutrition Examination Survey has proven that the LE8 score was highly correlated with the original LS7, and could reflect greater inter‐individual variations among the US population. 13 Although the association of CVH assessed by the updated LE8 scoring algorithm with CVD‐free life years has not been reported before, a previous study using the original scale of LS7 has indicated that years of life expectancy free from CVD were 38.36, 45.00, and 50.34 years in participants with consistently low, moderate, and high CVH at the index age of 35 years, respectively. 31 Our current analysis adopting LE8 metrics showed agreement with the past study and the findings were consistent across major CVD subtypes and irrespective of age, sex, and socioeconomic status.
We observed that a high CVH of health factors seemed to be associated with more obvious gains in CVD‐free life years in comparison with that of health behaviors, which was to some extent attributed to the hypothesis that the protection afforded by health behaviors may be partially achieved via optimizing the levels of health factors. These findings were consistent with findings from the project of Prediction for Atherosclerotic CVD Risk in China (China‐PAR project), which assessed CVH with the former LE7. 11 More specifically, among all the health factors, a high CVH of BP was found to imply the largest gains of life expectancy free from CVDs, indicating the importance of continuous management for hypertension. By comparison, achieving a high CVH of health behaviors was also related to a moderate extension of CVD‐free life years, less remarkable than that of health behaviors. Improving health behaviors, however, may still provide significant challenges and opportunities for CVD prevention, in consideration of the current low prevalence of ideal health behaviors.
By adopting the new approach to assess CVH of each participant, we used the more predictive non‐HDL cholesterol instead of total cholesterol and the potential influence of the sleep duration was further considered. In addition, we calculated the CVD‐free life years substituted for ubiquitous relative risk estimates, which was an absolute quantitative measurement and more intuitive for the public to understand. Other advantages of our study included the prospective cohort design, the large sample size, as well as the long‐term follow‐up.
Nevertheless, several limitations also merit discussion when interpreting the results. Because of lack of detailed dietary patterns, we modified the recommended scoring algorithm of diet quality for a better adaption to our study. Although this surrogate measurement assessed by intake of salt, fatty food, and tea intake could partially reflect the overall dietary pattern, potential influence of other dietary ingredients, such as fresh fruits and red meat, might be ignored in the current analysis. Secondly, other crucial CVD risk factors including social‐ecological determinants, psychological status, and genetic predisposition were not evaluated in our study because of lack of data. However, no significant modification effects of sociodemographic factors were observed in the stratified analyses. More evidence on the potential effects of psychological health as well as genetic susceptibility is urgently needed. Thirdly, we limited the estimation of CVD‐free years to between ages 30 to 80 years. Although this age range has taken more consideration of younger populations than previous studies and was consistent with current health policies, further research covering the entire life span would be more informative for overall improvement of CVH. 29 , 31 , 37 Fourthly, CVH as well as potential confounders were assessed with single measurements at baseline only, which could not reflect the influence of changes over the follow‐up. Studies with additional consideration for time‐varying exposures are warranted to further explore the association of CVH with numbers of CVD‐free life years. Moreover, since all the participants in our study were selected from the Kailuan community and not a representative sample for the general Chinese population, the generalizability of our findings might be limited to some extent.
In conclusion, the results of our study indicated a consistent gradient association between CVH scores measured by the updated LE8 metrics and CVD‐free life years between ages 30 to 80 years, which suggested that promoting CVH of the general population might prolong their healthy life span. These findings provided support to primordial CVD prevention as an important strategy for healthy aging in China.
Sources of Funding
This study was supported by the National Key R&D Program of China (Grant Number: 2018YFC1312402) and the Beijing Municipal Administration of Hospitals Incubating Program (Grant Number: PX2020021). Funders of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Tables S1–S10
Figures S1–S2
Acknowledgments
The authors acknowledge all staff and participants of the Kailuan study for their important participation and contribution.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029241
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Liming Lin, Email: linliming915@163.com.
Anxin Wang, Email: wanganxin@bjtth.org.
References
- 1. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sotos‐Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation. 2015;132:2212–2219. doi: 10.1161/circulationaha.115.017158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high‐income, middle‐income, and low‐income countries: the PURE study. Lancet. 2017;390:2643–2654. doi: 10.1016/s0140-6736(17)31634-3 [DOI] [PubMed] [Google Scholar]
- 5. Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Tang X, Zhang W, Qian Y, Huang Y, et al. Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. J Am Coll Cardiol. 2017;69:1116–1125. doi: 10.1016/j.jacc.2016.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd‐Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. doi: 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/circulationaha.109.192703 [DOI] [PubMed] [Google Scholar]
- 8. Lloyd‐Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/cir.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/circulationaha.111.081083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han C, Liu F, Yang X, Chen J, Li J, Cao J, Li Y, Shen C, Yu L, Liu Z, et al. Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: the China‐PAR project. Sci China Life Sci. 2018;61:504–514. doi: 10.1007/s11427-018-9281-6 [DOI] [PubMed] [Google Scholar]
- 12. Kim S, Chang Y, Cho J, Hong YS, Zhao D, Kang J, Jung HS, Yun KE, Guallar E, Ryu S, et al. Life's Simple 7 cardiovascular health metrics and progression of coronary artery calcium in a low‐risk population. Arterioscler Thromb Vasc Biol. 2019;39:826–833. doi: 10.1161/atvbaha.118.311821 [DOI] [PubMed] [Google Scholar]
- 13. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, et al. Status of cardiovascular health in US adults and children using the American Heart Association's new "Life's Essential 8" metrics: prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), 2013‐2018. Circulation. 2022;146:822–835. doi: 10.1161/circulationaha.122.060911 [DOI] [PubMed] [Google Scholar]
- 14. Sun Q, Yu D, Fan J, Yu C, Guo Y, Pei P, Yang L, Chen Y, Du H, Yang X, et al. Healthy lifestyle and life expectancy at age 30 years in the Chinese population: an observational study. Lancet Public Health. 2022;7:e994–e1004. doi: 10.1016/s2468-2667(22)00110-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, Song M, Liu G, Shin HJ, Sun Q, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368:l6669. doi: 10.1136/bmj.l6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris‐Etherton PM, Wu Y, Jin C, Huang S, Hu FB, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open. 2019;2:e194758. doi: 10.1001/jamanetworkopen.2019.4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, Li Y, Yao S, Chen S, Wu S, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75:2921–2930. doi: 10.1016/j.jacc.2020.04.038 [DOI] [PubMed] [Google Scholar]
- 18. Zhou YF, Chen S, Wang G, Chen S, Zhang YB, Chen JX, Tu ZZ, Liu G, Wu S, Pan A. Effectiveness of a workplace‐based, multicomponent hypertension management program in real‐world practice: a propensity‐matched analysis. Hypertension. 2022;79:230–240. doi: 10.1161/hypertensionaha.121.18305 [DOI] [PubMed] [Google Scholar]
- 19. Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow‐up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Liu F, Li J, Yang X, Chen J, Cao J, Wu X, Lu X, Huang J, Li Y, et al. Tea consumption and the risk of atherosclerotic cardiovascular disease and all‐cause mortality: the China‐PAR project. Eur J Prev Cardiol. 2020;27:1956–1963. doi: 10.1177/2047487319894685 [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Guo X, Lu Z, Tang J, Li Y, Xu A, Liu S. Cardiovascular diseases deaths attributable to high sodium intake in Shandong Province, China. J Am Heart Assoc. 2019;8:e010737. doi: 10.1161/jaha.118.010737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhong VW, Van Horn L, Greenland P, Carnethon MR, Ning H, Wilkins JT, Lloyd‐Jones DM, Allen NB. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all‐cause mortality. JAMA Intern Med. 2020;180:503–512. doi: 10.1001/jamainternmed.2019.6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang YH, Wang J, Chen SH, Li JQ, Lu QD, Vitiello MV, Wang F, Tang XD, Shi J, Lu L, et al. Association of longitudinal patterns of habitual sleep duration with risk of cardiovascular events and all‐cause mortality. JAMA Netw Open. 2020;3:e205246. doi: 10.1001/jamanetworkopen.2020.5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stroke–1989 . Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407 [DOI] [PubMed] [Google Scholar]
- 25. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583 [DOI] [PubMed] [Google Scholar]
- 26. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 27. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204 [DOI] [PubMed] [Google Scholar]
- 28. Royston P, Parmar MK. Flexible parametric proportional‐hazards and proportional‐odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203 [DOI] [PubMed] [Google Scholar]
- 29. Nyberg ST, Batty GD, Pentti J, Virtanen M, Alfredsson L, Fransson EI, Goldberg M, Heikkilä K, Jokela M, Knutsson A, et al. Obesity and loss of disease‐free years owing to major non‐communicable diseases: a multicohort study. Lancet Public Health. 2018;3:e490–e497. doi: 10.1016/s2468-2667(18)30139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 31. Wang L, Song L, Li D, Zhou Z, Chen S, Yang Y, Hu Y, Wang Y, Wu S, Tian Y. Ideal cardiovascular health metric and its change with lifetime risk of cardiovascular diseases: a prospective cohort study. J Am Heart Assoc. 2021;10:e022502. doi: 10.1161/jaha.121.022502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. doi: 10.1161/strokeaha.113.678839 [DOI] [PubMed] [Google Scholar]
- 33. Nayor M, Enserro DM, Vasan RS, Xanthakis V. Cardiovascular health status and incidence of heart failure in the Framingham Offspring Study. Circ Heart Fail. 2016;9:e002416. doi: 10.1161/circheartfailure.115.002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JH, Yang PS, Yu HT, Kim TH, Jang E, Uhm JS, Pak HN, Lee MH, Joung B. Association of cardiovascular health and incident atrial fibrillation in elderly population. Heart. 2021;107:1206–1212. doi: 10.1136/heartjnl-2020-318858 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Liu J, Wang W, Wang M, Qi Y, Xie W, Li Y, Sun J, Liu J, Zhao D. Lifetime risk for cardiovascular disease in a Chinese population: the Chinese Multi‐Provincial Cohort Study. Eur J Prev Cardiol. 2015;22:380–388. doi: 10.1177/2047487313516563 [DOI] [PubMed] [Google Scholar]
- 36. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nyberg ST, Singh‐Manoux A, Pentti J, Madsen IEH, Sabia S, Alfredsson L, Bjorner JB, Borritz M, Burr H, Goldberg M, et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern Med. 2020;180:760–768. doi: 10.1001/jamainternmed.2020.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S10
Figures S1–S2


