Abstract
Background
It is still unclear whether there is a sex difference in the prognosis of patients with hypertrophic cardiomyopathy (HCM). Therefore, we performed a meta‐analysis to elucidate the association between sex and adverse outcomes in patients with HCM.
Methods and Results
The PubMed, Cochrane Library, and Embase databases were used to search for studies on sex differences in prognosis in patients with HCM up to August 17, 2021. Summary effect sizes were calculated using a random effects model. The protocol was registered in PROSPERO (International prospective register of systematic reviews) (registration number‐ CRD42021262053). A total of 27 cohorts involving 42 365 patients with HCM were included. Compared with male subjects, female subjects had a higher age at onset (mean difference=5.61 [95% CI, 4.03–7.19]), a higher left ventricular ejection fraction (standard mean difference=0.09 [95% CI, 0.02–0.15]) and a higher left ventricular outflow tract gradient (standard mean difference=0.23 [95% CI, 0.18–0.29]). The results showed that compared with male subjects with HCM, female subjects had higher risks of HCM‐related events (risk ratio [RR]=1.61 [95% CI, 1.33–1.94], I 2=49%), major cardiovascular events (RR=3.59 [95% CI, 2.26–5.71], I 2=0%), HCM‐related death (RR=1.57 [95% CI, 1.34–1.82], I 2=0%), cardiovascular death (RR=1.55 [95% CI, 1.05–2.28], I 2=58%), noncardiovascular death (RR=1.77 [95% CI, 1.46–2.13], I 2=0%) and all‐cause mortality (RR=1.43 [95% CI, 1.09–1.87], I 2=95%), but not atrial fibrillation (RR=1.13 [95% CI, 0.95–1.35], I 2=5%), ventricular arrhythmia (RR=0.88 [95% CI, 0.71–1.10], I 2=0%), sudden cardiac death (RR=1.04 [95% CI, 0.75–1.42], I 2=38%) or composite end point (RR=1.24 [95% CI, 0.96–1.60], I 2=85%).
Conclusions
Based on current evidence, our results show significant sex‐specific differences in the prognosis of HCM. Future guidelines may emphasize the use of a sex‐specific risk assessment for the diagnosis and management of HCM.
Keywords: hypertrophic cardiomyopathy, meta‐analysis, prognosis, sex
Subject Categories: Meta Analysis, Quality and Outcomes, Mortality/Survival, Cardiovascular Disease, Women
JEL
Meta Analysis Quality and Outcomes Mortality/Survival Cardiovascular Disease Women, Sex, and Gender
Clinical Perspective.
What Is New?
Based on observational studies, female patients with hypertrophic cardiomyopathy have higher age onset, higher left ventricular ejection fraction, and higher left ventricular outflow tract gradient.
Female sex is associated with a worse prognosis in patients with hypertrophic cardiomyopathy.
There is no significant statistical association between sex and atrial fibrillation, ventricular arrhythmia, or sudden cardiac death in patients with hypertrophic cardiomyopathy.
What Are the Clinical Implications?
Future guideline may emphasize the sex‐specific risk assessment, diagnosis, or management for hypertrophic cardiomyopathy.
Nonstandard Abbreviations and Acronyms
- ASA
alcohol septal ablation
- HCM
hypertrophic cardiomyopathy
- MD
mean difference
- MYBPC3
myosin‐binding protein C3 gene
- NOS
Newcastle‐Ottawa Scale
- SCD
sudden cardiac death
Hypertrophic cardiomyopathy (HCM) is one of the most common inherited cardiovascular diseases, with a prevalence of 0.2%. 1 According to the global burden of disease, the mortality rate caused by cardiomyopathy was 0.42 in 2019. Sex‐based differences in the clinical presentation of HCM are becoming increasingly recognized. Evidence from earlier epidemiological studies showed that female subjects are underrepresented among patients with HCM; however, those diagnosed at an older age have a higher symptom burden than male subjects. 2 , 3 , 4
Subsequently, several reports showed that female subjects with HCM have a high risk of cardiovascular death and all‐cause mortality. 5 , 6 , 7 , 8 , 9 For example, an analysis by the Mayo Clinic comprising 3673 patients with 10.9 years of follow‐up showed that female sex was associated with poorer overall survival. 10 These results strongly suggest a potential sex difference in the prognosis of HCM, although several cohorts have shown no sex‐based difference in all‐cause mortality outcomes. 11 , 12 , 13 Notably, the 2020 American College of Cardiology/American Heart Association guidelines for the management of HCM have yet to provide specific comments about sex differences in prognosis. 14 Clarifying this point is important for the management and treatment of HCM. Given this background, we performed a meta‐analysis to elucidate the association between sex and outcomes of HCM.
Methods
The data sets used and analyzed in the current study are available from the corresponding author on reasonable request.
This present study reported the results by the guidelines of the Preferred Reporting Item for Systematic Review and Meta‐Analysis 2020 (Table S1). The protocol was registered with PROSPERO (http: www.york.ac.uk/inst/crd) registration number‐ CRD42021262053.
Literature Search
The PubMed, Cochrane Library, and Embase databases were used as search libraries. In addition, we searched other sources, such as the American College of Cardiology website (https://www.acc.org/) and the Circulation website (https://www.ahajournals.org/journal/circ). Without language restriction, we used the following Medical Subject Headings to retrieve advanced articles up to August 17, 2021: (1) for patients: “hypertrophic cardiomyopathy,” “hypertrophic cardiomyopathies,” and “hypertrophic obstructive cardiomyopathies,” and for exposure: “sex,” and “gender.” Table S2 shows the detailed statement of the search strategies.
Study Selection
We used the Endnote X9 database, a reference management software, to organize all the studies. All the titles and abstracts were reviewed to consider eligibility for inclusion. Then, the full‐text evaluation was performed after initial identification.
The inclusion criteria were as follows: (1) cohort studies on the association between sex and prognosis in HCM. Sex is the state of being either male or female at the biological level; HCM is an inherited cardiomyopathy characterized by asymmetric hypertrophy of the ventricles. According to the 2020 American Heart Association/American College of Cardiology guidelines for the management of hypertrophic cardiomyopathy, HCM refers specifically to a group of cardiac diseases characterized by left ventricular hypertrophy attributable to variants in the genes encoding myosin or of unknown pathogenesis. HCM in adults is diagnosed when a 2‐dimensional echocardiogram or cardiac magnetic resonance imaging shows a maximum end‐diastolic thickness of ≥15 mm anywhere in the left ventricle and there is no other cause of myocardial hypertrophy, and 13–14 mm of myocardial hypertrophy can also be diagnosed as HCM if there is a positive genetic test or if there is a family member with HCM; (2) patients in the study were adults (aged >18 years) who were diagnosed with HCM by echocardiography; and (3) studies showing odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) and their corresponding 95% CIs or providing data to calculate these risk estimates.
We excluded studies with the following conditions: (1) reviews and studies with insufficient data; and (2) articles with data on postoperative in‐hospital mortality, considering the influence of postoperative complications. Accordingly, if the same population was used in multiple studies, then we included the most informative article.
Outcome Definitions
Atrial fibrillation (AF) was defined as an irregular heart rhythm without distinct P‐waves documented on ECG. Ventricular arrhythmia involved the ventricular arrhythmia composite end point, appropriate implantable cardioverter defibrillator (ICD) therapy, ventricular tachycardia, and/or fibrillation. HCM‐related events were defined as (1) heart failure (HF) presentation, HF admission, HF worsening or progression; (2) stroke; and (3) HCM‐related composite events (related to all the above events). HCM‐related death involved (1) HF‐related death and (2) stroke death. Major cardiovascular events were defined as cardiovascular‐related death, HCM‐related cardiovascular complications, fatal arrhythmias, stroke, receiving implantable cardioverter defibrillator treatment, or undergoing heart transplantation. The details of the composite end point and noncardiovascular death of each included study are shown in Table S3.
Data Extraction and Quality Assessment
Two researchers (X.L. and Z.T.) independently extracted the information from the included literature, including author, publication year, country, sample size, duration of follow‐up, participants' information (mean age, sex, age at diagnosis, left ventricular ejection fraction [LVEF]), outcomes, and adjusted variables.
The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of observational studies. The scores range from 0 to 9 to evaluate the selection, comparability, and outcome of articles. Studies with NOS scores >7 were considered high quality. 15
Statistical Analysis
Age expressed as quartiles and medians are converted to the mean and SD to explore age differences between sexes. 16 To elucidate the baseline differences between sexes in patients with HCM, we pooled the mean age of male subjects and female subjects using the inverse‐variance method and random model, respectively.
OR is approximately equivalent to RR in retrospective studies when the incidence of the study outcome is equal to the population prevalence or the outcome is rare. 17 In addition, HR and RR have approximately the same meaning in prospective studies and can approximate each other in the same conduction. 18 Therefore, the RRs and 95% CIs were pooled by a random‐effects model. We estimated the effect size by calculating the natural logarithm of the RR (log [RR]) and its standard error (SElog [RR]). For those studies that did not provide effect size, we calculated them by events and total numbers of patients in female groups and male groups. Considering that age was the most important confounding factor, additional sensitivity analyses were performed by excluding studies without adjustment for age. In addition, we removed the univariate analysis and then performed a sensitivity analysis by removing the literature 1 by 1.
Predefined subgroup analyses included mean age (<50 years old and ≥50 years old), follow‐up time (<5 years and ≥5 years), region (America, Europe, and Asia), sample size (<1000 and ≥1000), study design (retrospective and prospective cohort), population (the methods of treatment patients involved septal myectomy or alcohol septal ablation), and NOS quality assessment (≤7 and >7).
Review Manager (RevMan) version 5.40 (The Cochrane Collaboration 2014; Nordic Cochrane Center Copenhagen, Denmark) was used for the statistical analysis. A P value of <0.05 was considered statistically significant.
Heterogeneity Test and Publication Bias
We calculated statistical P values using the Q‐test, with a P value<0.1 representing a significant difference between the 2 groups. We applied I 2 statistics to estimate the total variability due to heterogeneity. 19 Funnel plots and Egger's and Begg's tests were used to test for the presence of publication bias. Egger's and Begg's tests with a P value <0.05 were considered statistically significant.
Patient and Public Involvement
It was not appropriate or possible to involve patients or the public in the design, implementation, reporting, or dissemination plans of our research.
Results
Study Selection
As shown in Figure 1, 1525 publications and 13 conference abstracts were identified in the initial literature search (PubMed=684; Cochrane Library=3; Embase=838; other source=13). After excluding duplicates and screening the titles and abstracts, 83 remained for full‐text assessment. Thirty‐one studies were excluded for the following reasons: (1) studies without data of interest (n=4); (2) certain publication types with no data (n=7); (3) studies without appropriate methods (n=3); (4) studies that did not focus on HCM (n=6); (5) studies that did not target certain populations (n=2) or outcomes (n=8); and (6) duplicated cohorts (n=1). All the excluded studies with the corresponding reasons are shown in Table S4. Ultimately, 27 studies with 42 365 individuals (27 471 male subjects and 14 894 female subjects) were included. 2 , 5 , 6 , 7 , 9 , 10 , 11 , 13 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38
Figure 1. Flowchart of study selection in the systematic review and meta‐analysis of sex difference in the prognosis of hypertrophic cardiomyopathy.
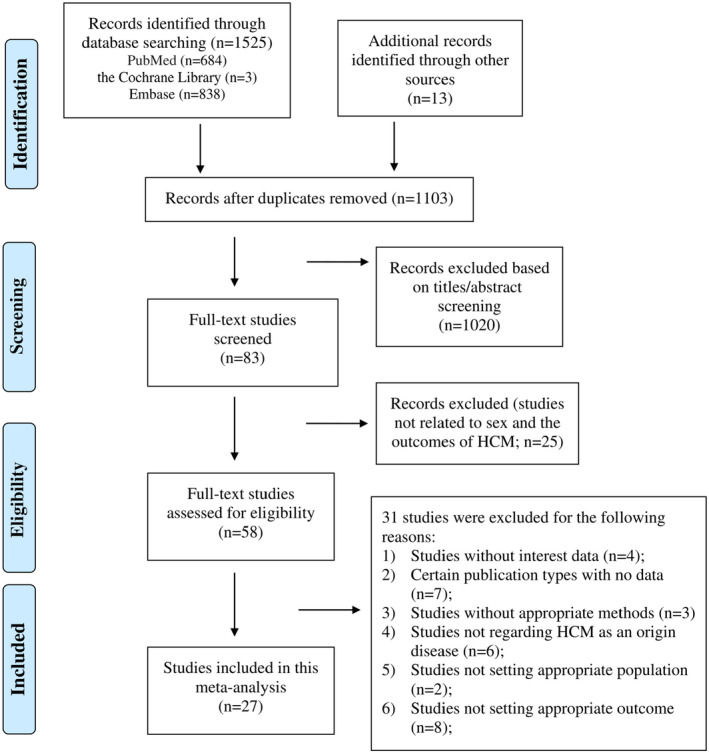
Other sources include the American College of Cardiology website and Circulation website. HCM indicates hypertrophic cardiomyopathy.
Study Characteristics
The basic characteristics of the included studies are presented in Table 1. These studies were published from 2001 to 2021, and the sample sizes varied from 50 to 9524 patients. The mean age of the patients ranged from 42 to 63 years, and the follow‐up ranged from 2.1 to 13.0 years. Eleven of them were retrospective cohort studies, and the others were prospective cohort studies. Eight studies were from North America (6 from the United States and 2 from Canada), 9 were from Europe (5 from Italy, 3 from the Netherlands, and 1 from Portugal), and 10 were from Asia (5 from China, 3 from Korea, and 2 from Japan).
Table 1.
Basic Characteristics of the Articles Included in the Meta‐Analysis of Sex Difference in the Prognosis of Hypertrophic Cardiomyopathy
| Author, year, country | Study design | Data source, study populations | Follow‐up time; sample size | Mean age, y; male, % | Baseline comorbidities or echo, female/male | End Point | Estimate effect (95% CI) or case, female/male case | Adjusted covariates | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NYHA III/IV % | ICD % | LVEF | LVOT gradient | ||||||||
| Olivotto et al, 2001, Italy 36 | PC |
Azienda Ospedaliera Careggi; and Minneapolis Heart Institute, patients with HCM |
9.1 y; 107 | 50.0; 57.0 | NA | NA | NA | NA | AF | 1.11 (0.70–1.70), male | Univariate analysis |
| Ho et al, 2004, China 25 | RC |
Queen Mary Hospital, patients with HCM |
5.8 y; 118 | 54.0; 52.5 | NA | 14.0/14.0 | 68.0/72.0 | NA | Major cardiovascular events | 5.86 (1.77–7.21) | Age at presentation, family history of HCM, NYHA class III/IV, ECG features at presentation, types of HCM |
| Woo et al, 2005, Canada 7 | PC |
Toronto General Hospital Obstructive HCM population after septal myectomy |
7.7 y; 338 | 47.0; 60.1 | NA | NA | NA | NA | HF (HF worsening) | 3.60 (2.00–6.70) | Age, history of preoperative AF, LA diameter, septal/posterior thickness ratio, concomitant CABG |
| Major cardiovascular events | 3.30 (2.00–5.40) | ||||||||||
| Olivotto et al, 2005, Italy 2 | PC |
Azienda Ospedaliera Careggi; Minneapolis Heart Institute; Tufts‐New England Medical Center, patients with HCM |
6.2 y; 969 | 42.0; 59.4 | 18.0/6.0 | NA | NA | 62.0/58.0 | HF (HF progression) | 1.50 (1.11–2.00) | Age |
| HCM‐related death | 52/58 | ||||||||||
| SCD | 26/33 | ||||||||||
| Noncardiac death | 36/22 | ||||||||||
| Lee et al, 2007, China 6 | RC |
A tertiary referral center in Taiwan, patients with HCM |
5.3 y; 163 | 60.9; 51.5 | NA | NA | NA | NA | All‐cause mortality | 2.99 (1.13–9.87) | LVOT obstruction, AF |
| Ball et al, 2011, Canada 8 | PC |
Toronto General Hospital, patients with obstructive HCM |
7.2 y; 649 | 51.0; 56.2 | NA | NA | NA | NA | HCM‐related death | 2.10 (1.20–3.60) | Age, septal thickness, resting LVOT gradient, invasive treatment |
| All‐cause mortality | 2.00 (1.30–3.20) | ||||||||||
| Wang et al, 2014, China 9 | PC |
Fuwai Hospital, patients with HCM |
4.0 y; 621 | 47.5; 74.1 | NA | 0.6/0.7 | 67.1/67.5 | 81.9/71.9 | All‐cause mortality | 2.19 (1.21–3.95) | Age, syncope (without any invasive treatment, including ICD and septal reduction therapy), SCD family history, maximum LV wall thickness, LA diameter, AF, LVOT obstruction (without septal reduction therapy) and NYHA class at enrollment. |
|
Cardiovascular death HF (chronic HF/HF progression) |
2.19 (1.17–4.09) 1.73 (1.12–2.69) |
||||||||||
| SCD | 7/12 | ||||||||||
| HF‐related death | 6/9 | ||||||||||
| Ventricular arrhythmia | 1/3 | ||||||||||
| AF | 12/28 | ||||||||||
| Stroke | 10/19 | ||||||||||
| Terauchi et al, 2015, Japan 37 | PC |
Kochi Medical School Hospital, patients with HCM |
13.0 y; 50 | 47.0; 54.0 | 17.0/0 | NA | 67.0/65.0 | NA | HCM‐related death | 3/5 | Univariate analysis |
| HCM‐related events | 11/7 | ||||||||||
| HF | 10/5 | ||||||||||
| Debonnaire et al, 2017, Netherlands 22 | PC |
Leiden University Medical Center, patients with HCM |
4.8 y; 242 | 53.0; 64.5 | NA | NA | NA | NA | AF (new‐onset AF) | 1.41 (0.75–2.63), male | Univariate analysis |
| Geske et al, 2017, United States 10 | RC |
Mayo Clinic, patients with HCM |
12.7 y; 3673 | 55.0; 54.8 | 45.0/35.0 | 6.0/7.0 | 71.0/69.0 | 36.0/23.0 | All‐cause mortality | 1.13 (1.03–1.22) | Age, NYHA Class III/IV symptoms, and history of AF, CAD, hypertension, ICD implantation, and beta receptor antagonist use |
| Ho et al, 2018, United States 5 | RC | SHARE registry, patients with HCM | 5.4 y; 4591 | 44.3; 62.9 | NA | NA | NA | NA | Composite end point | 0.88 (0.77–1.01) | Family proband status, SARC+, SARC VUS and race |
| Kubo et al, 2018, Japan 30 | PC |
Kochi Cardiomyopathy Network, patients with HCM |
6.1 y; 293 | 56.0; 67.2 | NA | NA | NA | NA | HCM‐related events | 0.93 (0.54–1.60), male | Age at registration, NYHA class III, presence of AF, maximum LV wall thickness, LVFS, and presence of LVOT obstruction |
| Van Velzen et al, 2018, Netherlands 13 | RC |
Erasmus Medical Center in Rotterdam, patients with HCM |
6.8 y; 1007 | 52.0; 61.6 | NA | 6.0/4.0 | NA | NA | All‐cause mortality | 1.25 (0.91–1.73) | Family relatedness |
| Cardiovascular death | 1.22 (0.83–1.79) | ||||||||||
| SCD | 0.75 (0.44–1.30) | ||||||||||
| HF‐related death | 1.77 (0.95–3.27) | ||||||||||
| Stroke‐ related death | 5.57 (0.55–56.8) | ||||||||||
| Noncardiac death | 2.11 (1.21–3.69) | ||||||||||
| Choi et al, 2019, Korea 21 | PC |
Two tertiary referral centers, patients with HCM |
4288 person‐years; 730 | 57.1; 75.5 | NA | NA | NA | NA | SCD | 3.83 (1.39–10.60) | HCM SCD‐risk score |
| Lorenzini et al, 2019, Italy 32 | RC |
7 European centers, patients with HCM |
6.1 y; 4893 | 49.2; 63.9 | 17.1/7.5 | 15.9/17.1 | 66.0/65.0 | 10.0/8.0 | Composite end point | 1.19 (1.06–1.30) | Age at presentation, previous VF/VT, NYHA class, EF ≤50%, MWT, LA diameter, LVOT max, AF, NSVT on Holter, family history of sudden death, syncope, septal myectomy, ASA |
| HF‐related death | 1.44 (1.25–1.59) | ||||||||||
| SCD | 0.80 (0.40–1.10) | ||||||||||
| All‐cause mortality | 2.87 (2.57–3.19) | ||||||||||
| HCM‐related death | 51/52 | ||||||||||
| Noncardiac death | 96/114 | ||||||||||
| Ghiselli et al, 2019, Italy 23 | RC |
IRCCS Sacro Cuore Don Calabria Hospital, patients with HCM |
5.9 y; 292 | 46.0; 72.3 | 11.0/6.0 | NA | 67.0/67.0 | 14.0/18.0 | Composite end point | 2.32 (1.04–5.22) | Univariate analysis |
| Jang et al, 2019, Korea 28 | PC |
Inha University Hospital, patients with nonobstructive HCM |
34.0 mo; 202 | 63.0; 69.8 | 14.8/2.1 | NA | 65.1/64.9 | NA | HF (HF presentation) | 5.01 (2.05–12.26) | Age |
| Cardiovascular death | 5.18 (1.32–20.34) | ||||||||||
| HF (HF hospitalization) | 6.86 (1.43–32.99) | ||||||||||
| Lu et al, 2019, United States 33 | RC |
Johns Hopkins HCM Registry, patients with HCM |
2.1 y; 728 | 53.3; 62.0 | 21.0/7.0 | NA | 67.0/65.0 | 35.0/26.0 | HF | 3.00 (1.10–8.40) | Age, NYHA III‐IV, LA diameter, and LV global longitudinal peak systolic strain |
| Composited end point | 1.90 (1.20–2.90) | ||||||||||
| AF | 18/12 | ||||||||||
| Ventricular arrhythmia | 5/9 | ||||||||||
| All‐cause mortality | 4/2 | ||||||||||
| Meghji et al, 2019, United States 34 | RC |
Mayo Clinic HCM population after septal myectomy |
8.2 y; 2506 | 55.1; 55.0 | 90.8/84.8 | 12.8/14.2 | 73.0/70.0 | 67.0/50.0 | All‐cause mortality | 0.98 (0.76–1.26) | Age, year of surgery, BMI, diabetes, NYHA class, amiodarone, pacemaker using, NSVT, hypertension, disopyramide, use of ACEi or angiotensin receptor blockers, presyncope, dyslipidemia, prior septal reduction, syncope, mitral valve regurgitation grade, race, β‐blocker, calcium‐channel blocker, family history of HCM and SCD, ethnicity, anteroseptal wall thickness, ICD. |
| Rowin et al, 2019, United States 11 | PC |
Tufts HCM Institution, patients with HCM |
4.7 y; 2123 | 47.2; 62.6 | 39.0/23.0 | 24.0/25.0 | 64.0/63.0 | NA | SCD | 0.92 (0.60–1.50) | Age |
| All‐cause mortality | 1.32 (0.92–1.91) | ||||||||||
| Noncardiac death | 55/46 | ||||||||||
| Cardiovascular death | 4/3 | ||||||||||
| HCM‐related death | 1.50 (0.70–3.40) | ||||||||||
| HF | 1.60 (1.20–2.10) | ||||||||||
| Stroke‐related death | 1/2 | ||||||||||
| Huurman et al, 2020, Netherlands 27 | PC |
Erasmus Medical Center HCM population after septal myectomy |
5.9 y; 162 | 52.1; 61.1 | 79.0/78.0 | 11.0/15.0 | NA | 93.0/82.0 | Composite end point | 2.32 (0.79–6.83), male | Age, NYHA class ≥III, AF, hypertension, hypercholesterolemia, diabetes, pathogenic gene variant, negative inotropic therapy, HF therapy, ICD, time from symptom onset, time from diagnosis, preoperative peak LVOT gradient, maximal wall thickness, LA diameter, LV end‐diastolic diameter, impaired systolic function, diastolic function, systolic anterior motion of the mitral valve, mitral regurgitation |
| SCD | 0/2 | ||||||||||
| All‐cause mortality | 5/10 | ||||||||||
| Huang et al, 2020, China 26 | PC |
West China Hospital, patients from HCM database with HCM |
3.2 y; 576 | 54.9; 54.9 | 46.9/30.7 | 4.2/6.0 | 66.9/66.4 | 33.0/24.0 | Cardiovascular death | 0.64 (0.32–1.30) | Univariate analysis |
| All‐cause mortality | 23/32 | ||||||||||
| Lakdawala et al, 2020, United States 31 | RC | Patients from SHARE registry with HCM | 7.7 y; 5873 | 46.7; 62.1 | 21.6/9.3 | 22.1/20.6 | 66.0/64.6 | 35.5/26.6 | HF (HF composite) | 1.85 (1.48–2.32) | Age, hypertension, and history of AF |
| All‐cause mortality | 1.45 (1.16–1.82) | ||||||||||
| Ventricular arrhythmia composite | 111/202 | ||||||||||
| AF (incident AF) | 1.21 (1.01–1.46) | ||||||||||
| Stroke | 1.48 (1.11–1.98) | ||||||||||
| HCM‐related death | 1.50 (1.13–1.99) | ||||||||||
| Montenegro Sa´ et al, 2020, Portugal 35 | RC |
Portuguese Registry of patients with HCM |
65.0 mo; 1042 | 53.3; 58.8 | 16.1/10.8 | 10.9/15.6 | 65.6/64.3 | 16.9/14.6 | All‐cause mortality | 2.05 (1.11–3.75) | Age, symptoms, HF, mitral regurgitation, diastolic dysfunction, CAD. |
| Cardiovascular death | 3.16 (1.25–7.99) | ||||||||||
| HF‐related death | 11/5 | ||||||||||
| Stroke‐related death | 2/1 | ||||||||||
| SCD | 15/18 | ||||||||||
| Wang et al, 2020, China 38 | RC |
A large tertiary hospital in North‐eastern China. HCM population after alcohol septal ablation |
7.5 y; 320 | 51.6; 49.4 | 67.3/56.3 | 6.8/5.7 | 62.0/60.0 | NA | All‐cause mortality | 1.12 (1.08–1.27) | Age, NYHA III/IV, AF, CAD, hypertension, diabetes, beta receptor antagonist use, CCB use, alcohol dose, LVEF, residual LVWT >3 mo postprocedure, reduction in LVOT gradient >3 mo postprocedure, persistent complete AVB. |
| Kim et al, 2021, Korea 29 | PC |
Korea National Health Insurance Service claims database patients with HCM |
4.4 y; 9524 | 51.7; 77.6 | NA | NA | NA | NA | Composite end point | 1.43 (1.22–1.68) | Propensity score‐matched (age, income, underlying disease, current medication, Charlson comorbidity index) |
| Cardiovascular death | 1.27 (0.91–1.78) | ||||||||||
| HF (new‐onset HF admission) | 1.54 (1.30–1.82) | ||||||||||
| All‐cause mortality | 0.91 (0.69–1.21) | ||||||||||
| Bongioanni et al, 2021, Italy 39 | RC |
Mauriziano Hospital, patients with HCM |
86.5 mo; 573 | 53.0; 61.4 | 7.9/3.0 | 8.0/7.0 | 63.0/65.0 | 34.0/24.0 | HCM‐related death | 1.52 (0.91–2.52) | Univariate analysis |
| All‐cause mortality | 32/31 | ||||||||||
| SCD | 3/13 | ||||||||||
| Stroke‐related death | 4/2 | ||||||||||
| Noncardiac death | 5/6 | ||||||||||
ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ASA, alcohol septal ablation; AVB, atrioventricular block; CABG, concomitant coronary artery bypass grafting; CAD, coronary artery disease; CCB, calcium channel blocker; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; HF, heart failure; ICD, internal cardiac defibrillator; IRCCS, Istituto di Ricovero e Cura a Carattere Scientifico; LA, left atrial; LV, left ventricular; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVOT, left ventricular outflow tract; LVWT, left ventricular wall thickness; MWT, left ventricular maximum wall thickness; NA, not applicable; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; PC, prospective cohort; RC, retrospective cohort; SARC VUS, sarcomere variant of unknown significance present; SARC, sarcomere mutation; SARC+, at least 1 pathogenic or likely pathogenic variant in any of the above sarcomere genes; SCD, sudden cardiac death; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Study Quality
Of the 27 included articles, 2 had an NOS score of 6. 22 , 26 They were univariate analyses and had a short follow‐up period (<5 years). The remaining studies were high‐quality studies with NOS scores >7 (Table S5).
Baseline Differences Between Sexes
For age and cardiac function analysis, our meta‐analysis included 11 219 female subjects and 21 672 male subjects. 2 , 6 , 9 , 10 , 11 , 13 , 23 , 25 , 26 , 27 , 28 , 29 , 31 , 32 , 33 , 35 , 37 , 38 , 39 Overall, female subjects were older at the initial diagnosis (mean difference [MD]=5.61; 95% CI: 4.03–7.19; I 2=94%; P<0.00001) (Figure 2A) and had higher LVEFs (standard MD=0.09; 95% CI: 0.02–0.15; I 2=74%; P<0.00001) and higher left ventricular outflow tract (LVOT) gradients (standard MD=0.23; 95% CI: 0.18–0.29; I 2=63%; P=0.003) (Figure 2B and 2C).
Figure 2. Forest plot showing the differences in age and cardiac function at diagnosis between sexes in patients with hypertrophic cardiomyopathy.
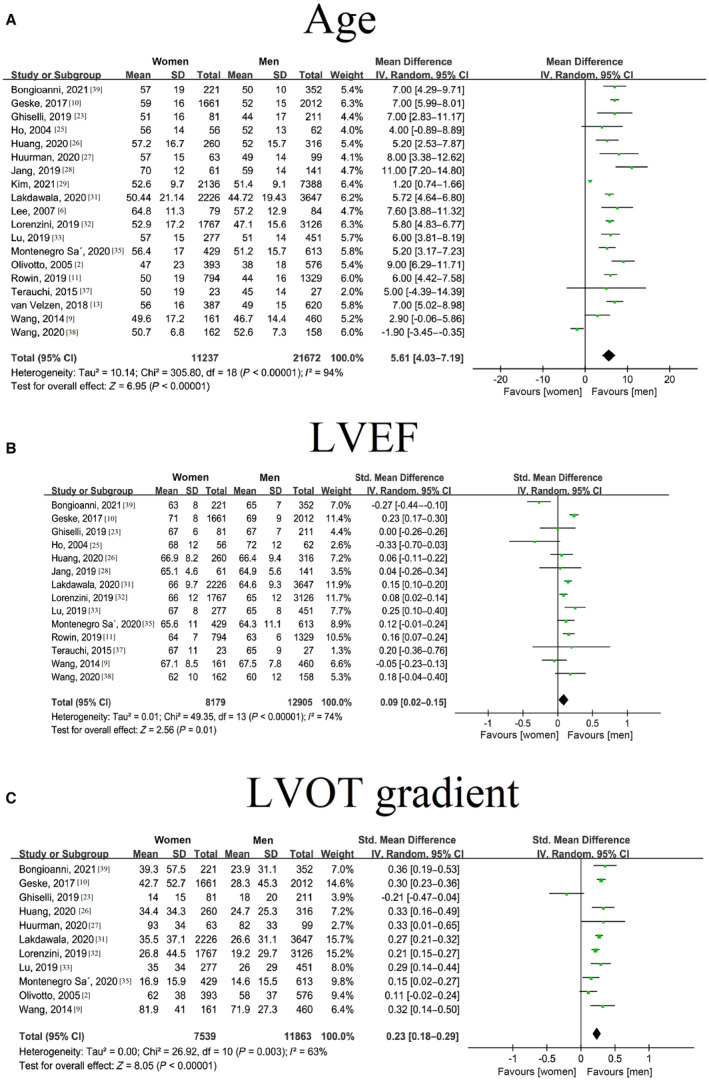
A, Diagnosis age in women and men with hypertrophic cardiomyopathy; B, Left ventricular ejection fraction in women and men with hypertrophic cardiomyopathy; C, Left ventricular outflow tract gradient in women and men with hypertrophic cardiomyopathy. HCM indicates hypertrophic cardiomyopathy; LVEF, left ventricular ejection fraction; and LVOT, left ventricular outflow tract.
Meta‐Analysis of Sex Differences in Adverse Outcomes
Atrial Fibrillation and Ventricular Arrhythmia
Five articles (7453 individuals with 4713 male subjects and 2740 female subjects) showed an association between sex and AF. 9 , 22 , 31 , 33 , 36 There was no significant difference between female and male subjects in terms of AF risk (RR=1.13 [95% CI, 0.95–1.35], I 2=5%; P=0.38), with no evidence of heterogeneity (Figure 3A). The heterogeneity did not significantly change after excluding each study one by one.
Figure 3. Forest plot for the association between sex and cardiovascular diseases in patients with hypertrophic cardiomyopathy.
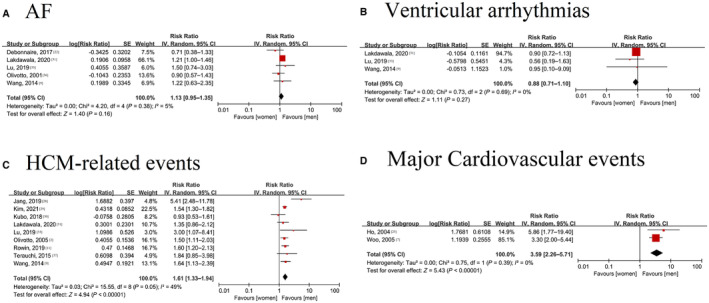
A, Forest plot for the association between sex and atrial fibrillation in patients with HCM; B, Forest plot for the association between sex and ventricular arrhythmia in patients with HCM; C, Forest plot for the association between sex and HCM‐related events in patients with HCM; D, Forest plot for the association between sex and major cardiovascular events in patients with HCM. AF indicates atrial fibrillation; and HCM, hypertrophic cardiomyopathy.
Three studies involving 7222 patients, including 4558 male subjects and 2664 female subjects, showed that female sex was not associated with a higher risk of ventricular arrhythmias (RR=0.88 [95% CI, 0.71–1.10], I 2=0%; P=0.69) (Figure 3B).
Cardiovascular Events
Our meta‐analysis included HCM‐related events and major cardiovascular events.
Nine studies on HCM‐related events involved 20 383 participants (14 216 male subjects and 6167 female subjects). 2 , 9 , 11 , 28 , 29 , 30 , 31 , 33 , 37 Female sex was associated with an increased risk of HCM‐related events (RR=1.61 [95% CI, 1.33–1.94]), with evidence of heterogeneity (I 2=49%, P=0.05). In addition, a study by Woo et al supported this result (OR=3.60 [95% CI, 1.93–6.70]). 7 The leave‐one‐out method did not significantly change the heterogeneity (I 2: 0%–55%) (Figure 3C). Further analysis showed that female sex was associated with an increased risk of HF events (RR=1.76 [95% CI, 1.49–2.07], I 2=38%; P=0.11) and stroke (RR=1.48 [95% CI, 1.13–1.94], I 2=0%; P=0.97) (Figure S1).
Two studies involving 456 patients (265 male subjects and 191 female subjects) showed the relationship between sex and major cardiovascular events. 7 , 25 Female subjects were associated with a higher risk of major cardiovascular events (RR=3.59 [95% CI, 2.26–5.71]; P=0.39) (Figure 3D), with no evidence of heterogeneity (I 2=0%).
Death
Sudden Cardiac Death
Nine studies involving 12 120 individuals with 7726 male subjects/4394 female subjects were included in the meta‐analysis of sudden cardiac death (SCD). 1 , 2 , 9 , 11 , 13 , 21 , 27 , 32 , 35 , 39 Female sex was not associated with an increased risk of SCD (RR=1.04 [95% CI, 0.75–1.42]; P=0.11), with no evidence of heterogeneity (I 2=38%, P=0.11) (Figure 4A).
Figure 4. Forest plot for the association between sex and death or composite end point in patients with hypertrophic cardiomyopathy.
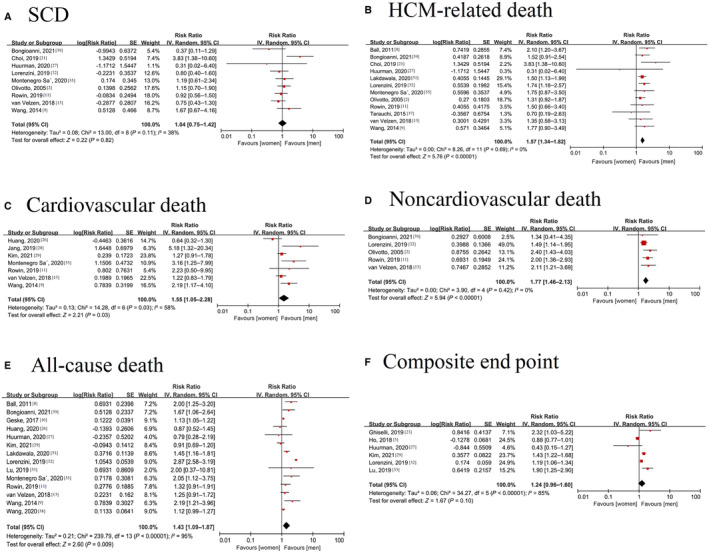
A, Forest plot for the association between sex and sudden cardiac death in patients with HCM; B, Forest plot for the association between sex and HCM‐related death in patients with HCM; C, Forest plot for the association between sex and cardiovascular death in patients with HCM; D, Forest plot for the association between sex and noncardiac death in patients with HCM; E, Forest plot for the association between sex and all‐cause mortality in HCM; F, Forest plot for the association between sex and composite end point in patients with HCM. HCM indicates hypertrophic cardiomyopathy; and SCD, sudden cardiac death.
HCM‐Related Death
Twelve studies with 18 692 participants (11 765 male subjects and 6927 female subjects) were included in the analysis of the relationship between sex and HCM‐related death. 2 , 8 , 9 , 11 , 13 , 20 , 21 , 27 , 31 , 32 , 35 , 37 There was a positive association between female sex and HCM‐related death (RR=1.57 [95% CI, 1.34–1.82]; P=0.69), with low evidence of heterogeneity (I 2=0%) (Figure 4B). Further analysis showed that female sex was associated with an increased risk of HF‐related death (RR=1.48 [95% CI, 1.29–1.70], I 2=0%; P=0.45). However, no difference was found in stroke‐related death (RR=2.71 [95% CI, 0.94–7.85], I 2=0%; P=0.72) between the sexes (Figure S1).
Cardiovascular Death
Seven studies involving 15 095 participants with 10 867 male subjects/4228 female subjects were included in the meta‐analysis of cardiovascular death. 9 , 11 , 13 , 26 , 28 , 29 , 35 The pooled results showed that female sex was associated with an increased risk of cardiovascular death in patients with HCM (RR=1.55 [95% CI, 1.05–2.28]), with evidence of heterogeneity (I 2=58%, P=0.03) (Figure 4C). The I 2 was reduced to 46% when the study by Huang et al 26 was excluded, and the results were stable (RR: 1.72 [95% CI, 1.20–2.48]; P=0.10).
Noncardiovascular Death
Five studies involving 9565 individuals with 6003 male subjects/3562 female subjects 2 , 11 , 13 , 32 , 39 were included in the meta‐analysis of noncardiovascular death. Female sex was associated with an increased risk of noncardiovascular death (RR: 1.77 [95% CI, 1.46–2.13]) (Figure 4D), with no evidence of heterogeneity (I 2=0%, P=0.42).
All‐cause Death
Fourteen articles with 31 764 individuals (20 935 male subjects/10 829 female subjects) reported all‐cause mortality. 8 , 9 , 10 , 11 , 13 , 20 , 26 , 27 , 29 , 31 , 32 , 33 , 35 , 38 Female sex was assoc iated with an increased risk of all‐cause mortality (RR=1.43 [95% CI, 1.09–1.87], I 2=95%; P<0.00001) (Figure 4E). A study by Lee et al also reported a positive relationship between sex and all‐cause mortality (OR=2.99 [95% CI, 1.13–7.91]). 6 Excluding the study by Lorenzini 32 reduced the I 2 from 95% to 53%, and the RR became 1.26 (95% CI, 1.11–1.42; P=0.01).
Composite End Point
Six studies involving 20 190 participants with 14 162 male subjects/6028 female subjects showed the composite end point. 5 , 23 , 27 , 29 , 32 , 33 The definition of the composite end point was not uniform across studies, with most being HF hospitalization or HCM‐related events, SCD, and death. There was no significant sex difference in the composite end point (RR=1.24 [95% CI, 0.96–1.60], I 2=85%; P<0.00001) (Figure 4F). By excluding the study by Ho et al 5 the heterogeneity was reduced to 68%.
Publication Bias
Publication bias tests were performed for the outcomes, with >10 studies according to the guidelines. 40 The results showed no evidence of publication bias detected by the funnel plot, Egger test, or Begg test (Egger test: HCM‐related events P=0.624; HCM‐related death P=0.922; all‐cause mortality P=0.975; Begg test: HCM‐related events P=0.754; HCM‐related death P=0.732; all‐cause mortality P=0.189) (Figures S2 and S3).
Sensitivity Analyses
We performed sensitivity analysis for HCM‐related events, HCM‐related death, and all‐cause mortality. Sensitivity analyses by excluding studies in which a univariate analysis was performed, excluding studies without age adjustment and the leave‐one‐out method generated confirmed results (Figure S4 and S5).
Subgroup Analyses
Considering the statistical power, subgroup analysis was performed only for those outcomes that were reported in >10 studies (HCM‐related events, HCM‐related death, and all‐cause mortality).
As shown in Table 2, female sex was still associated with an increased risk of worse outcomes in almost all subgroups stratified by mean age, follow‐up period, sample size, study design, population, region, and NOS quality assessment, and there was little evidence of heterogeneity between these subgroups in the meta‐regression analyses (P>0.05).
Table 2.
Subgroup Analysis for the Meta‐Analysis of Sex Difference in the Prognosis of Hypertrophic Cardiomyopathy
| Items | No. of cohorts | RR (95% CI) | I 2% | P | P | |
|---|---|---|---|---|---|---|
| Within subgroup | Between subgroup | |||||
| HCM‐related events | 9 | 1.61 (1.33–1.94) | 57 | |||
| Mean age | <50 y | 5 | 1.47 (1.19–1.82) | 65 | <0.001 | 0.49 |
| ≥50 y | 4 | 1.81 (1.04–3.14) | 73 | 0.03 | ||
| Follow‐up time | <5 y | 5 | 1.75 (1.34–2.27) | 47 | <0.001 | 0.19 |
| ≥5 y | 4 | 1.35 (1.01–1.80) | 78 | 0.04 | ||
| Sample size | <1000 | 6 | 1.68 (1.20–2.35) | 53 | 0.003 | 0.45 |
| ≥1000 | 3 | 1.43 (1.12–1.83) | 82 | 0.004 | ||
| Study design | PC | 7 | 1.61 (1.33–1.93) | 36 | <0.001 | 0.44 |
| RC | 2 | 1.34 (0.89–2.04) | 84 | 0.16 | ||
| Region | America | 2 | 1.40 (1.00–1.97) | 87 | 0.01 | 0.68 |
| Europe | 2 | 1.41 (0.69–2.86) | 60 | 0.35 | ||
| Asia | 5 | 1.71 (1.25–2.33) | 50 | <0.001 | ||
| NOS quality assessment | ≤7 | 2 | 1.63 (1.24–2.13) | 0 | <0.001 | 0.83 |
| >7 | 7 | 1.56 (1.23–1.99) | 57 | <0.001 | ||
| Excluding univariate analysis | Multivariate analysis | 7 | 1.67 (1.38–2.20) | 53 | <0.001 | 0.53 |
| Excluding without age adjustments | Adjust age | 8 | 1.56 (1.29–1.89) | 49 | <0.001 | 0.69 |
| All‐cause mortality | 14 | 1.43(1.09–1.87) | 94 | |||
| Mean age | <50 y | 4 | 1.58 (0.91–2.77) | 98 | 0.11 | 0.59 |
| ≥50 y | 11 | 1.35 (1.10–1.64) | 60 | 0.004 | ||
| Follow‐up time | <5 y | 5 | 1,20 (0.86–1.67) | 57 | 0.29 | 0.32 |
| ≥5 y | 9 | 1.52 (1.09–2.13) | 96 | 0.01 | ||
| Sample size | <1000 | 7 | 1.38 (1.03–1.84) | 58 | 0.03 | 0.83 |
| ≥1000 | 7 | 1.46 (0.97–2.19) | 97 | 0.07 | ||
| Study design | PC | 7 | 1.21 (0.98–1.50) | 60 | 0.08 | 0.20 |
| RC | 7 | 1.65 (1.07–2.55) | 96 | 0.02 | ||
| Population | Without treatment | 10 | 1.51 (1.10–2.07) | 95 | 0.01 | 0.33 |
| After treatment | 4 | 1.21 (0.89–1.65) | 70 | 0.22 | ||
| Region | America | 5 | 1.36 (1,10‐1,67) | 88 | 0.03 | 0.24 |
| Europe | 5 | 1.70 (1.05–2.75) | 66 | 0.22 | ||
| Asia | 4 | 1.11 (0.85–1.43) | 62 | 0.45 | ||
| NOS quality assessment | ≤7 | 3 | 1.27 (0.90–1.78) | 43 | 0.17 | 0.50 |
| >7 | 11 | 1.48 (1.09–2.03) | 96 | 0.01 | ||
| Excluding univariate analysis | Multivariate analysis | 10 | 1.50 (1.11–2.04) | 96 | 0.009 | 0.38 |
| Excluding without age adjustments | Adjust age | 11 | 1.49 (1.09–2.05) | 96 | 0.01 | |
| HCM‐related death | 12 | 1.33 (1.14–1.55) | 34 | |||
| Mean age | <50 y | 6 | 1.49 (1.25–1.78) | 0 | <0.001 | 0.62 |
| ≥50 y | 6 | 1.65 (1.16–2.35) | 43 | 0.006 | ||
| Follow‐up time | <5 y | 2 | 1.65 (0.98–2.79) | 0 | 0.06 | 0.77 |
| ≥5 y | 10 | 1.52 (1.24–1.85) | 29 | <0.001 | ||
| Sample size | <1000 | 7 | 1.58 (1.22–2.04) | 9 | 0.0004 | 0.72 |
| ≥1000 | 5 | 1.48 (1.16–1.89) | 26 | 0.001 | ||
| Study design | PC | 7 | 1.59 (1.21–2.10) | 9 | 0.001 | 0.71 |
| RC | 5 | 1.49 (1.18–1.87) | 26 | 0.0007 | ||
| Population | Without treatment | 11 | 1.55 (1.29–1.86) | 18 | <0.001 | 0.30 |
| After treatment | 1 | 0.31 (0.02–6.40) | 17 | 0.45 | ||
| Region | America | 3 | 1.60 (1.25–2.03) | 0 | 0.0001 | 0.67 |
| Europe | 6 | 1.43 (1.12–1.82) | 25 | 0.004 | ||
| Asia | 3 | 1.84 (1.00–3.40) | 27 | 0.05 | ||
| NOS quality assessment | ≤7 | 4 | 1.64 (0.99–2.72) | 32 | 0.06 | 0.85 |
| >7 | 8 | 1.56 (1.32–1.84) | 0 | <0.001 | ||
| Excluding univariate analysis | Multivariate analysis | 4 | 1.59 (1.06–2.38) | 64 | 0.03 | 0.83 |
| Excluding without age adjustments | Adjust age | 9 | 1.60 (1.36–1.88) | 0 | <0.001 | |
ASA indicates alcohol septal ablation; HCM, hypertrophic cardiomyopathy; NOS, Newcastle‐Ottawa Scale; PC, prospective cohort; RC, retrospective cohort; RR, risk ratio; and SM, septal myectomy.
Discussion
Main Findings
Based on the pooled analysis from 27 cohorts with 42 365 patients with HCM, the present meta‐analysis showed that (1) female subjects with HCM were older and had higher LVEFs and higher LVOT gradients at diagnosis; (2) female sex was associated with worse outcomes in patients with HCM, including cardiovascular events, but not AF or SCD; and (3) the subgroup analyses and sensitivity analyses confirmed the above results. Overall, our study showed a significant sex difference in the prognosis of HCM.
Sex‐Based Differences at Diagnosis of HCM
Our results showed that female subjects were underrepresented in our pooled cohorts, representing <40%, which was consistent with some prior findings. 2 , 10 The underlying reason for this skew is still unknown. In the HCM population, >50 sarcomere contractile protein gene mutations have been identified. 41 Some researchers have attributed it to decreased disease penetrance in female subjects, predominantly in individuals with cardiac myosin‐binding protein C3 gene (MYBPC3) variants. 37 , 42 , 43 , 44 A study showed sex differences in the clinical features of HCM caused by MYBPC3 mutation. The higher cardiac disease penetrance of MYBPC3 mutation carriers in male subjects than in female subjects was confirmed. 37 Other genetic factors, such as modifier genes on the sex chromosome, may also influence the penetrance in female subjects. This high penetrance caused by mutation allows male subjects to exhibit the disease earlier. Therefore, female subjects are older and have more serious symptoms at the time of illness onset, which affects the prognosis of HCM in women. Notably, recent results from Lakdawala et al provided novel insight into this hypothesis. 31 They showed that the sex‐based difference in the age at diagnosis was more pronounced in genetically tested patients with sarcomere‐mutated HCM (female subjects were 7.1 years older at diagnosis) than in those without sarcomere‐mutated HCM (female subjects were 3.6 years older at diagnosis). This may be related to differences in LVOT obstruction and diastolic function. However, sarcomere mutation may not be associated with systolic dysfunction (female subjects with MYBPC3 variants are 35% less likely to develop systolic dysfunction than male subjects). 31 The increased frequency and severity of LVOT obstruction in female subjects may be associated with a smaller left ventricular chamber, 45 which is consistent with our findings. The incidence of HF events was also 87% higher in female subjects when controlling for obstruction, systolic dysfunction, hypertension, and age, suggesting that diastolic dysfunction contributes to the poor prognosis of women with HCM. Indeed, sarcomere variants that cause HCM have been shown to impair relaxation in model systems spanning the spectrum from isolated sarcomere filaments to human sarcomere mutation carriers without overt HCM. 46 However, previous studies of sex‐based differences in HCM diastolic function in MYBPC3 sarcomere mutants are limited, and more research is needed to confirm this. Moreover, the disease appears to develop at similar ages in female subjects and male subjects with HCM when caused by beta myosin heavy chain 7 (MYH7) variants. Therefore, whether there is incomplete penetrance among female subjects might be a more complicated question. On the other hand, social bias, such as poor recognition of the condition by health care providers because of bias, might also be responsible, caused by lower awareness of women's diseases by their physicians.
Several large longitudinal cohorts showed worse clinical presentations in female subjects at diagnosis. Unexpectedly, the results showed that LVEFs were higher in female subjects and that LVOT gradients were lower than those in male subjects (LVEF standard MD: 0.10 [95% CI, 0.04–0.17]; LVOT gradient standard MD: 0.25 [95% CI, 0.19–0.30]). The reason for this result may be related to the small sample size of female subjects, and the exact data still need to be studied more extensively. In addition, 73% of female subjects had New York Heart Association class II to IV symptoms at the time of diagnosis compared with 53% of male subjects. Therefore, female subjects were significantly more likely to have advanced drug‐refractory HF (New York Heart Association class III/IV) than male subjects (53% and 35%, respectively). 11 Female subjects are known to have a higher prevalence of obstructive phenotypes, poorer diastolic function, and more severe HF symptoms. 2 , 10 At the same time, these results are supported by the suggestion by Abraham et al that diastolic dysfunction, not left ventricular systolic dysfunction, contributes to the worsening of symptoms in female patients with HCM. 33
Sex‐Based Differences in HCM Outcomes
Evidence from longitudinal studies showed that there might be a sex difference in the prognosis of patients with HCM, but the results were inconsistent. 47 In our results, compared with those in male subjects, the risk of HCM‐related events, HCM‐related death, cardiovascular‐related events, major cardiovascular death, noncardiovascular death, and all‐cause mortality in female patients with HCM increased by 61%, 57%, 259%, 55%, 77%, and 43%, respectively. Moreover, these results were stable in the sensitivity analysis, which confirmed the robustness of our results. Notably, there was no statistically significant difference in the composite end point. This might be because the composite end point comprised SCD and ventricular arrhythmia, which did not have a sex difference and thus might have reduced the statistical power.
It is worth noting that our meta‐analysis showed that there was no difference between sexes in SCD or ventricular arrhythmia. Considering that malignant ventricular arrhythmia is a major cause of SCD, these results are not surprising. The results reinforce the current guidelines of established clinical risk factors for HCM sudden death risk stratification, which do not include a component of sex. Based on the Sarcomeric Human Cardiomyopathy Registry study, the results in genotyped patients and full cohorts were inconsistent. Ho et al showed that female sex was associated with a decreased risk of ventricular arrhythmia composite events in genotyped cohorts (patients with HCM with a sarcomere mutation) after adjustment. 5 However, this association was not found in the overall cohort. 31 As previously reported, patients with genotyped HCM have a significantly higher composite risk of ventricular arrhythmia than patients without a sarcomere mutation, which might be attributable to the greater number of cases in the genotyped HCM cohort, higher statistical power, or other confounding factors. Considering the limited evidence from current studies, more studies are needed to verify the association between sex and ventricular arrhythmia in patients with genotyped HCM.
In patients undergoing septal myectomy, our results showed that female subjects experienced more HCM events than death (HCM‐related death or all‐cause death) (Table 2). We should interpret this result with caution considering the limited number of studies (n=1 for HCM‐related death, n=4 for all‐cause death). The conclusion that the death rate is significantly different will be more solid if larger cohorts show consistent results. In fact, the sex discrepancy is a controversial topic in contemporary literature on patients with HCM receiving surgery. Recently, Wang et al reported significantly increased mortality in female patients with HCM undergoing alcohol septal ablation based on a Chinese cohort after 10 years of follow‐up. 38 Woo showed that female subjects who underwent treatment were more likely to develop HCM‐related events, 7 and Huurman showed that the composite end point was more likely to occur in female subjects undergoing surgical treatment. 27 However, a cohort in the Netherlands showed a similar survival rate among male subjects and female subjects after surgical treatment. 27 In general, female subjects with HCM are older and have more severe symptoms, and whether female subjects, independent of the above clinical characteristics, have worse outcomes of HCM after surgical treatment remains unclear.
Age is one of the most important confounding factors in HCM outcome. In most of the included studies, female subjects were significantly older than male subjects at diagnosis. Even after age adjustment, female sex was still an independent factor for cardiovascular death, 28 all‐cause mortality,33 and HF. 2 , 31 It has also been reported that there was no sex difference in mortality after age adjustment. 26 Our results showed that even after the removal of age‐unadjusted studies, female sex was still associated with worse prognosis.
Genotype is another vital confounding factor. Survival analysis showed that compared with patients with sarcomeric variant‐negative, patients with sarcomeric variant‐positive had an earlier onset of events and higher incidences of the overall composite outcome, HF, and AF. 5 After genotype adjustment, female subjects still had a higher risk of mortality (RR=1.45) and the HF composite end point (RR=1.85). 31 However, different variants might have different influences on sex. For example, on the 2 most common genes, MYH7 and MYBPC3, the sex‐based difference in the age of diagnosis was found predominantly in individuals with MYBPC3 variants, 37 , 42 , 43 , 44 rather than in patients with MYH7 variants. 31 Therefore, there remains considerable heterogeneity within the sarcomeric variants. The interaction between sex and sarcomeric variants still needs to be clarified.
Underlying Mechanism
Although the potential mechanisms behind sex differences in patients with HCM remain unknown, several hypotheses have been proposed. Constantine and coworkers showed that there is a significant sex difference in cardiovascular physiology and morphology. 48 , 49 Compared with male subjects, female subjects have a smaller left ventricular chamber size and mass index (up to 40%). 50 Age‐related cardiac remodeling is also more pronounced in female subjects, who are initially protected from adverse cardiovascular outcomes but experience more frequent adverse outcomes after the age of 60 years. Myocardial remodeling in response to different types of ventricular overload also differs between the sexes. Female subjects experience more left ventricular hypertrophy in response to aortic stenosis, while male subjects experience more severe left ventricular dilatation following aortic regurgitation. Age‐dependent changes in diastolic ventricular function and arterial stiffness were greater in female subjects than in male subjects. Although the mechanisms behind sexual dimorphism are unclear, differences in endogenous hormones may contribute to cardiac remodeling and lifelong risk of cardiovascular disease. 48 In addition, female subjects usually have a smaller left ventricular chamber, and female patients with HCM have greater changes in the left ventricle, such as ventricular thickness and left ventricular systolic function, than male patients, which largely influences the risk of HF and LVOT obstruction between sexes. 51 , 52 On the other hand, different likelihoods of events are associated with wall thinning and cardiac remodeling. 53 There is evidence that female patients with HCM have a significantly larger degree of left ventricular remodeling. 54 The effects of left ventricle remodeling and fibrosis may cause diastolic dysfunction, which is more likely to lead to worse clinical outcomes in female subjects. Moreover, because of the bias of clinicians, more women delay their HCM diagnosis and treatment, 31 , 41 , 55 which may influence the prognosis of HCM.
Clinical Implications
The updated 2020 American Heart Association/American College Cardiology HCM Guideline for the Diagnosis and Management of HCM did not specifically comment on sex‐specific prognosis or approaches to HCM. 56 Our study can be used to help clarify the sex differences in diagnosis and prognosis in patients with HCM, highlighting the clinical importance of sex‐based differences. Further guideline updates or clinical trials may emphasize this sex difference for prognosis. In the context of SCD, our results are consistent with the current guidelines; that is, the inclusion of sex as a risk assessment factor for HCM‐SCD is not supported.
Comparison With Prior Meta‐Analyses
Sex‐related differences in patients with HCM have been reported in previous studies. 35 , 48 However, the difference in prognosis was still unclear. Consistent with our research results, a meta‐analysis shows clinical outcome differences between female subjects and male subjects. 57 Our study extends these findings. We demonstrate a sex‐specific difference in diagnosis, cardiac function, LVOT, and more comprehensive HCM outcomes, such as noncardiac death, cardiovascular death, arrhythmia, sudden death, and composite end points. Moreover, our study includes 16 more high‐quality cohorts and various subgroup analyses, which makes the results robust.
Strengths and Study Limitations
Our study systematically assessed the sex‐related prognosis of patients with HCM, adding valuable knowledge that may go into guidelines. Several limitations should be noted. First, relatively high heterogeneity was observed in the major end point; however, the heterogeneity was somewhat reduced by excluding some articles, while the results were still significant. Second, few included studies report on some outcomes (eg, major cardiovascular events), so more prospective cohorts are needed to confirm these results. Then, the component of composite end points varied across studies, which may be responsible for the inconsistent results from other outcomes. In addition, this is attributable to a lack of data, and to keep smaller heterogeneity, we select the age at diagnosis instead of age at onset for each HCM population, which may have some slight effect on the analysis of age. Finally, the meta‐analysis is based on observational studies, so causality cannot be deduced from our study.
Conclusions
Based on current evidence, our results suggest that female sex is associated with a higher risk of HCM‐related events, HCM‐related death, major cardiovascular events, cardiovascular death, noncardiovascular death, and all‐cause mortality. There is no association between sex and AF or SCD. Future guidelines may emphasize sex‐specific risk assessment, diagnosis, or management for HCM.
Sources of Funding
This work was supported by a grant from the Natural Science Foundation of Jiangxi Province (Nos. 20192ACBL21037, 202004BCJL23049, 202002BAB216022, and 20212BAB216051 to J.Z.; Nos. 20212BAB216047 and 202004BCJL23049 to P.Y.) and the National Natural Science Foundation of China (Nos. 82 160 371 and 82160371 to J.Z.; and Nos. 82100869 and 82100869 to P.Y.; No. 21 866 019 to J.M.; No. 82 100 347 to X.L.), China Postdoctoral Science Foundation (2021M703724 to X.L.), National High Technology Research and Development Program of Guangzhou (20 180 304 001 and 2019GZR110406004 to J.W.), Natural Science Foundation of Guangdong Province (2022A1515010582 to X.L.), and Natural Science Foundation of Guangzhou (202 201 011 395 to X.L.).
Disclosures
None.
Supporting information
Data S1
Tables S1–S5
Figures S1–S5
References 58–81
Acknowledgments
We acknowledge the grant support from Guangzhou Science Technology Bureau (202102010007).
Author contributions: Guarantor of the article, X. Liu; X. Liu contributed to the study concept and design and revised the draft. Z. Tan and X. Liu performed the search strategy and contributed to database research, acquisition of data, and statistical analyses. All the authors participated in data analysis and reviewed and approved the final manuscript.
H. Zhao and Z. Tan contributed equally.
This manuscript was sent to Mark W. Russell, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026270
For Sources of Funding and Disclosures, see page 19.
Contributor Information
Wengen Zhu, Email: zhuwg6@mail.sysu.edu.cn.
Xiao Liu, Email: liux587@mail.sysu.edu.cn.
References
- 1. Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120:554–569. doi: 10.1213/ane.0000000000000538 [DOI] [PubMed] [Google Scholar]
- 2. Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, Udelson JE, Cecchi F, Maron BJ. Gender‐related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:480–487. doi: 10.1016/j.jacc.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 3. Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy: clinical spectrum and treatment. Circulation. 1995;92:1680–1692. [DOI] [PubMed] [Google Scholar]
- 4. Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. [DOI] [PubMed] [Google Scholar]
- 5. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, Cirino AL, Fox JC, Lakdawala NK, Ware JS, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018;138:1387–1398. doi: 10.1161/circulationaha.117.033200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee CH, Liu PY, Lin LJ, Chen JH, Tsai LM. Clinical characteristics and outcomes of hypertrophic cardiomyopathy in Taiwan—a tertiary center experience. Clin Cardiol. 2007;30:177–182. doi: 10.1002/clc.20057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph‐Edwards A, Rakowski H. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. doi: 10.1161/01.CIR.0000162460.36735.71 [DOI] [PubMed] [Google Scholar]
- 8. Ball W, Ivanov J, Rakowski H, Wigle ED, Linghorne M, Ralph‐Edwards A, Williams WG, Schwartz L, Guttman A, Woo A. Long‐term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment. J Am Coll Cardiol. 2011a;58:2313–2321. doi: 10.1016/j.jacc.2011.08.040 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Wang J, Zou Y, Bao J, Sun K, Zhu L, Tian T, Shen H, Zhou X, Ahmad F, et al. Female sex is associated with worse prognosis in patients with hypertrophic cardiomyopathy in China. PLoS One. 2014;9:e102969. doi: 10.1371/journal.pone.0102969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geske JB, Ong KC, Siontis KC, Hebl VB, Ackerman MJ, Hodge DO, Miller VM, Nishimura RA, Oh JK, Schaff HV, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38:3434–3440. doi: 10.1093/eurheartj/ehx527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rowin EJ, Maron MS, Wells S, Patel PP, Koethe BC, Maron BJ. Impact of sex on clinical course and survival in the contemporary treatment era for hypertrophic cardiomyopathy. J Am Heart Assoc. 2019;8:e012041. doi: 10.1161/JAHA.119.012041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim M, Kim B, Choi Y‐J, Lee H‐J, Lee H, Park J‐B, Lee S‐P, Han K‐D, Kim Y‐J, Kim H‐K. Sex differences in the prognosis of patients with hypertrophic cardiomyopathy. Sci Rep. 2021;11. doi: 10.1038/s41598-021-84335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Velzen HG, Schinkel AFL, Baart SJ, Huurman R, van Slegtenhorst MA, Kardys I, Michels M. Effect of gender and genetic mutations on outcomes in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2018;122:1947–1954. doi: 10.1016/j.amjcard.2018.08.040 [DOI] [PubMed] [Google Scholar]
- 14. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020a;142:e533–e557. doi: 10.1161/cir.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Ma J, Huang L, Zhu W, Yuan P, Wan R, Hong K. Fluoroquinolones increase the risk of serious arrhythmias: a systematic review and meta‐analysis. Medicine (Baltimore). 2017;96:e8273. doi: 10.1097/md.0000000000008273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 18. Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55:893–899. doi: 10.1016/S0895-4356(02)00443-2 [DOI] [PubMed] [Google Scholar]
- 19. Liu X, Guo L, Xiao K, Zhu W, Liu M, Wan R, Hong K. The obesity paradox for outcomes in atrial fibrillation: Evidence from an exposure‐effect analysis of prospective studies. Obes Rev. 2020;21:e12970. doi: 10.1111/obr.12970 [DOI] [PubMed] [Google Scholar]
- 20. Ball W, Ivanov J, Rakowski H, Wigle ED, Linghorne M, Ralph‐Edwards A, Williams WG, Schwartz L, Guttman A, Woo A. Long‐term survival in patients with resting obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011b;58:2313–2321. doi: 10.1016/j.jacc.2011.08.040 [DOI] [PubMed] [Google Scholar]
- 21. Choi YJ, Kim HK, Lee SC, Park JB, Moon I, Park J, Kim YJ, Sohn DW, Ommen S. Validation of the hypertrophic cardiomyopathy risk‐sudden cardiac death calculator in Asians. Heart. 2019;105:1892–1897. doi: 10.1136/heartjnl-2019-315160 [DOI] [PubMed] [Google Scholar]
- 22. Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new‐onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10. doi: 10.1161/circep.116.004052 [DOI] [PubMed] [Google Scholar]
- 23. Ghiselli L, Marchi A, Fumagalli C, Maurizi N, Oddo A, Pieri F, Girolami F, Rowin E, Mazzarotto F, Cicoira M, et al. Sex‐related differences in exercise performance and outcome of patients with hypertrophic cardiomyopathy. Eur J Prev Cardiol. 2019. doi: 10.1177/2047487319886961 [DOI] [PubMed] [Google Scholar]
- 24. Gionfriddo W, Maron BJ, Madias C, Wells S, Maron MS, Rowin EJ. Abstract 13676: no difference in timing and risk for sudden death in women and men with hypertrophic cardiomyopathy. Circulation. 2019;140:A13676–A13676. doi: 10.1161/circ.140.suppl_1.13676 [DOI] [Google Scholar]
- 25. Ho HH, Lee KL, Lau CP, Tse HF. Clinical characteristics of and long‐term outcome in Chinese patients with hypertrophic cardiomyopathy. Am J Med. 2004;116:19–23. doi: 10.1016/j.amjmed.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 26. Huang FY, Shah JP, Pu XB, Hagar A, Chen SJ. Influence of gender on clinical characteristics and outcomes in chinese patients with hypertrophic cardiomyopathy. Am J Med Sci. 2020;360:517–524. doi: 10.1016/j.amjms.2020.05.017 [DOI] [PubMed] [Google Scholar]
- 27. Huurman R, Schinkel AFL, de Jong PL, van Slegtenhorst MA, Hirsch A, Michels M. Impact of sex on timing and clinical outcome of septal myectomy for obstructive hypertrophic cardiomyopathy. Int J Cardiol. 2021;323:133–139. doi: 10.1016/j.ijcard.2020.08.059 [DOI] [PubMed] [Google Scholar]
- 28. Jang JH, Shin SH, Beak YS, Ko KY, Kwon SW, Park SD, Woo SI, Kim DH, Kwan J. Impact of gender on heart failure presentation in non‐obstructive hypertrophic cardiomyopathy. Heart Vessels. 2020;35:214–222. doi: 10.1007/s00380-019-01492-0 [DOI] [PubMed] [Google Scholar]
- 29. Kim M, Kim B, Choi YJ, Lee HJ, Lee H, Park JB, Lee SP, Han KD, Kim YJ, Kim HK. Sex differences in the prognosis of patients with hypertrophic cardiomyopathy. Sci Rep. 2021;11:4854. doi: 10.1038/s41598-021-84335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubo T, Hirota T, Baba Y, Ochi Y, Takahashi A, Yamasaki N, Hamashige N, Yamamoto K, Kondo F, Bando K, et al. Patients' characteristics and clinical course of hypertrophic cardiomyopathy in a regional japanese cohort—Results From Kochi RYOMA Study. Circ J. 2018;82:824–830. doi: 10.1253/circj.CJ-17-0845 [DOI] [PubMed] [Google Scholar]
- 31. Lakdawala NK, Olivotto I, Day SM, Han L, Ashley EA, Michels M, Ingles J, Semsarian C, Jacoby D, Jefferies JL, et al. Associations between female sex, sarcomere variants, and clinical outcomes in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2021;14:e003062. doi: 10.1161/circgen.120.003062 [DOI] [PubMed] [Google Scholar]
- 32. Lorenzini M, Anastasiou Z, O'Mahony C, Guttman OP, Gimeno JR, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Garcia‐Pavia P, et al. Mortality among referral patients with hypertrophic cardiomyopathy vs the general european population. JAMA Cardiol. 2020a;5:73–80. doi: 10.1001/jamacardio.2019.4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu DY, Ventoulis I, Liu H, Kudchadkar SM, Greenland GV, Yalcin H, Kontari E, Goyal S, Corona‐Villalobos CP, Vakrou S, et al. Sex‐specific cardiac phenotype and clinical outcomes in patients with hypertrophic cardiomyopathy. Am Heart J. 2020;219:58–69. doi: 10.1016/j.ahj.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 34. Meghji Z, Nguyen A, Fatima B, Geske JB, Nishimura RA, Ommen SR, Lahr BD, Dearani JA, Schaff HV. Survival differences in women and men after septal myectomy for obstructive hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:237–245. doi: 10.1001/jamacardio.2019.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montenegro Sá F, Oliveira M, Belo A, Correia J, Azevedo O, Morais J. The sex gap in hypertrophic cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2020;73:1018–1025. doi: 10.1016/j.rec.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 36. Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–2524. doi: 10.1161/hc4601.097997 [DOI] [PubMed] [Google Scholar]
- 37. Terauchi Y, Kubo T, Baba Y, Hirota T, Tanioka K, Yamasaki N, Furuno T, Kitaoka H. Gender differences in the clinical features of hypertrophic cardiomyopathy caused by cardiac myosin‐binding protein C gene mutations. J Cardiol. 2015;65:423–428. doi: 10.1016/j.jjcc.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Zhao HW, Wang CF, Meng QK, Cui CS, Zhang XJ, Zhu Y, Fan CY, Luo DF, Chen BJ, et al. Gender disparities in clinical outcome after alcohol septal ablation for hypertrophic obstructive cardiomyopathy in the chinese han population: a cohort study. Heart Lung Circ. 2020;29:1856–1864. doi: 10.1016/j.hlc.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 39. Bongioanni S, De Rosa C, Cortese M, Mabritto B, Pizzuti A, Luceri S, Forni T, Pasquino M, Conte MR. Gender‐related differences in hypertrophic cardiomyopathy: 30 years of experience in an Italian center. Ital J Gend‐Specif Med. 2016;2:146–153. doi: 10.1723/2696.27568 [DOI] [Google Scholar]
- 40. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 [updated March 2017] The Cochrane Collaboration, 2017. https://training.cochrane.org/handbook: 9.5.2 Identifying and measuring heterogeneity. 2017.
- 41. Pelliccia F, Limongelli G, Autore C, Gimeno‐Blanes JR, Basso C, Elliott P. Sex‐related differences in cardiomyopathies. Int J Cardiol. 2019;286:239–243. doi: 10.1016/j.ijcard.2018.10.091 [DOI] [PubMed] [Google Scholar]
- 42. Lorenzini M, Norrish G, Field E, Ochoa JP, Cicerchia M, Akhtar MM, Syrris P, Lopes LR, Kaski JP, Elliott PM. Penetrance of hypertrophic cardiomyopathy in sarcomere protein mutation carriers. J Am Coll Cardiol. 2020b;76:550–559. doi: 10.1016/j.jacc.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maurizi N, Michels M, Rowin EJ, Semsarian C, Girolami F, Tomberli B, Cecchi F, Maron MS, Olivotto I, Maron BJ. Clinical course and significance of hypertrophic cardiomyopathy without left ventricular hypertrophy. Circulation. 2019;139:830–833. doi: 10.1161/circulationaha.118.037264 [DOI] [PubMed] [Google Scholar]
- 44. Semsarian C, Semsarian CR. Variable penetrance in hypertrophic cardiomyopathy: in search of the holy grail. J Am Coll Cardiol. 2020;76:560–562. doi: 10.1016/j.jacc.2020.06.023 [DOI] [PubMed] [Google Scholar]
- 45. Kim DH, Handschumacher MD, Levine RA, Choi YS, Kim YJ, Yun SC, Song JM, Kang DH, Song JK. In vivo measurement of mitral leaflet surface area and subvalvular geometry in patients with asymmetrical septal hypertrophy: insights into the mechanism of outflow tract obstruction. Circulation. 2010;122:1298–1307. doi: 10.1161/circulationaha.109.935551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garfinkel AC, Seidman JG, Seidman CE. Genetic pathogenesis of hypertrophic and dilated cardiomyopathy. Heart Fail Clin. 2018;14:139–146. doi: 10.1016/j.hfc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Siontis KC, Ommen SR, Geske JB. Sex, survival, and cardiomyopathy: differences between men and women with hypertrophic cardiomyopathy. J Am Heart Assoc. 2019;8:e014448. doi: 10.1161/jaha.119.014448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Constantine A, Dimopoulos K, Rafiq I, Vazir A. Sex differences in hypertrophic cardiomyopathy: Time to tailor risk stratification and therapy? Eur J Prev Cardiol. 2020;27:1816–1818. doi: 10.1177/2047487319890996 [DOI] [PubMed] [Google Scholar]
- 49. Fumagalli C, Olivotto I. The importance of sex differences in patients with hypertrophic cardiomyopathy—tailoring management and future perspectives. Am J Med Sci. 2020;360:433–434. doi: 10.1016/j.amjms.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 50. de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension. 1995;26:979–983. doi: 10.1161/01.hyp.26.6.979 [DOI] [PubMed] [Google Scholar]
- 51. Lin CL, Chiang CW, Shaw CK, Chu PH, Chang CJ, Ko YL. Gender differences in the presentation of adult obstructive hypertrophic cardiomyopathy with resting gradient: a study of 122 patients. Jpn Circ J. 1999;63:859–864. doi: 10.1253/jcj.63.859 [DOI] [PubMed] [Google Scholar]
- 52. Yang C, Zhang C, Yuan J, Cui J, Liu S, Hu F, Yang W, Bi X, Qiao S. Sex‐related differences in the associations between plasma free fatty acid levels and clinical features in patients with hypertrophic cardiomyopathy. Biol Sex Differ. 2016;7:63. doi: 10.1186/s13293-016-0118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maron BJ, Casey SA, Hurrell DG, Aeppli DM. Relation of left ventricular thickness to age and gender in hypertrophic cardiomyopathy. Am J Cardiol. 2003;91:1195–1198. doi: 10.1016/s0002-9149(03)00266-2 [DOI] [PubMed] [Google Scholar]
- 54. Chen YZ, Qiao SB, Hu FH, Yuan JS, Yang WX, Cui JG, Zhang Y, Zhang CL. Left ventricular remodeling and fibrosis: sex differences and relationship with diastolic function in hypertrophic cardiomyopathy. Eur J Radiol. 2015;84:1487–1492. doi: 10.1016/j.ejrad.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 55. Dimitrow PP, Czarnecka D, Jaszcz KK, Dubiel JS. Sex differences in age at onset of symptoms in patients with hypertrophic cardiomyopathy. J Cardiovasc Risk. 1997;4:33–35. doi: 10.1177/174182679700400106 [DOI] [PubMed] [Google Scholar]
- 56. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020b;76:e159–e240. doi: 10.1016/j.jacc.2020.08.045 [DOI] [PubMed] [Google Scholar]
- 57. Trongtorsak A, Polpichai N, Thangjui S, Kewcharoen J, Yodsuwan R, Devkota A, Friedman HJ, Estrada AQ. Gender‐related differences in hypertrophic cardiomyopathy: a systematic review and meta‐analysis. Pulse (Basel). 2021;9:38‐46. doi: 10.1159/000517618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Driel B, Nijenkamp L, Huurman R, Michels M, van der Velden J. Sex differences in hypertrophic cardiomyopathy: new insights. Curr Opin Cardiol. 2019;34:254–259. doi: 10.1097/hco.0000000000000612 [DOI] [PubMed] [Google Scholar]
- 59. Nijenkamp LL, Güçlü A, Appelman Y, van der Velden J, Kuster DW. Sex‐dependent pathophysiological mechanisms in hypertrophic cardiomyopathy: implications for rhythm disorders. Heart Rhythm. 2015;12:433–439. doi: 10.1016/j.hrthm.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 60. Dimitrow PP, Czarnecka D, Kawecka‐Jaszcz K, Dubiel JS. The influence of age on gender‐specific differences in the left ventricular cavity size and contractility in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2003;88:11–16; discussion 16‐17. doi: 10.1016/s0167-5273(02)00323-6 [DOI] [PubMed] [Google Scholar]
- 61. Nogales‐Romo MT, Cecconi A, Olivera MJ, Caballero P, Hernández S, Jiménez‐Borreguero LJ, Alfonso F. Sex differences in cardiac magnetic resonance features in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2020;36:1751–1759. doi: 10.1007/s10554-020-01880-y [DOI] [PubMed] [Google Scholar]
- 62. Zhang C, Liu R, Yuan J, Cui J, Hu F, Yang W, Zhang Y, Yang C, Qiao S. Gender‐related differences in the association between serum uric acid and left ventricular mass index in patients with obstructive hypertrophic cardiomyopathy. Biol Sex Differ. 2016;7:22. doi: 10.1186/s13293-016-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Frielingsdorf J, Franke A, Hess OM, Flachskampf FA. Are there sex differences in regional systolic function and wall stress in hypertrophic obstructive cardiomyopathy? A three‐dimensional echocardiography study. J Am Soc Echocardiogr. 2004;17:638–643. doi: 10.1016/j.echo.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 64. Aurigemma GP, Gaasch WH. Gender differences in older patients with pressure‐overload hypertrophy of the left ventricle. Cardiology. 1995;86:310–317. doi: 10.1159/000176895 [DOI] [PubMed] [Google Scholar]
- 65. Bos JM, Theis JL, Tajik AJ, Gersh BJ, Ommen SR, Ackerman MJ. Relationship between sex, shape, and substrate in hypertrophic cardiomyopathy. Am Heart J. 2008;155:1128–1134. doi: 10.1016/j.ahj.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lind JM, Chiu C, Ingles J, Yeates L, Humphries SE, Heather AK, Semsarian C. Sex hormone receptor gene variation associated with phenotype in male hypertrophic cardiomyopathy patients. J Mol Cell Cardiol. 2008;45:217–222. doi: 10.1016/j.yjmcc.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 67. Dimitrow PP, Czarnecka D, Strojny JA, Kawecka‐Jaszcz K, Dubiel JS. Impact of gender on the left ventricular cavity size and contractility in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2001;77:43–48. doi: 10.1016/s0167-5273(00)00401-0 [DOI] [PubMed] [Google Scholar]
- 68. Ohmoto‐Sekine Y, Suzuki J, Shimamoto R, Yamazaki T, Tsuji T, Nagai R, Ohtomo K. Gender‐specific clinical characteristics of deep Q waves in hypertrophic cardiomyopathy. Gend Med. 2007;4:274–283. doi: 10.1016/s1550-8579(07)80046-5 [DOI] [PubMed] [Google Scholar]
- 69. Movahed MR, Strootman D, Bates S, Sattur S. Prevalence of suspected hypertrophic cardiomyopathy or left ventricular hypertrophy based on race and gender in teenagers using screening echocardiography. Cardiovasc Ultrasound. 2010;8:54. doi: 10.1186/1476-7120-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ostman‐Smith I, Wettrell G, Keeton B, Holmgren D, Ergander U, Gould S, Bowker C, Verdicchio M. Age‐ and gender‐specific mortality rates in childhood hypertrophic cardiomyopathy. Eur Heart J. 2008;29:1160–1167. doi: 10.1093/eurheartj/ehn122 [DOI] [PubMed] [Google Scholar]
- 71. Sreenivasan J, Khan MS, Kaul R, Bandyopadhyay D, Hooda U, Aronow WS, Cooper HA, Panza JA, Naidu SS. Sex differences in the outcomes of septal reduction therapies for obstructive hypertrophic cardiomyopathy. JACC Cardiovasc Interv. 2021;14:930–932. doi: 10.1016/j.jcin.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 72. Carnlöf C, Insulander P, Jensen‐Urstad M, Iwarzon M, Gadler F. Atrio‐ventricular junction ablation and pacemaker treatment: a comparison between men and women. Scand Cardiovasc J. 2018;52:120–126. doi: 10.1080/14017431.2018.1446549 [DOI] [PubMed] [Google Scholar]
- 73. Condon JV, Miller KM, Le AH, Quasem M, Looney SW. Acute myocardial infarction and race, sex, and insurance types: unequal processes of care. Health Care Manag (Frederick). 2008;27:212–222. doi: 10.1097/01.HCM.0000285057.32235.5e [DOI] [PubMed] [Google Scholar]
- 74. Schulz‐Menger J, Abdel‐Aty H, Rudolph A, Elgeti T, Messroghli D, Utz W, Boyé P, Bohl S, Busjahn A, Hamm B, et al. Gender‐specific differences in left ventricular remodelling and fibrosis in hypertrophic cardiomyopathy: insights from cardiovascular magnetic resonance. Eur J Heart Fail. 2008;10:850–854. doi: 10.1016/j.ejheart.2008.06.021 [DOI] [PubMed] [Google Scholar]
- 75. Takigawa M, Kuwahara T, Takahashi A, Watari Y, Okubo K, Takahashi Y, Takagi K, Kuroda S, Osaka Y, Kawaguchi N, et al. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. Int J Cardiol. 2013;168:1984–1991. doi: 10.1016/j.ijcard.2012.12.101 [DOI] [PubMed] [Google Scholar]
- 76. Frankel DS, Tung R, Santangeli P, Tzou WS, Vaseghi M, Di Biase L, Nagashima K, Tedrow U, Bunch TJ, Tholakanahalli VN, et al. Sex and catheter ablation for ventricular tachycardia: an international ventricular tachycardia ablation center collaborative group study. JAMA Cardiol. 2016;1:938–944. doi: 10.1001/jamacardio.2016.2361 [DOI] [PubMed] [Google Scholar]
- 77. Schuldt M, Dorsch LM, Knol JC, Pham TV, Schelfhorst T, Piersma SR, Dos Remedios C, Michels M, Jimenez CR, Kuster DWD, et al. Sex‐related differences in protein expression in sarcomere mutation‐positive hypertrophic cardiomyopathy. Front Cardiovasc Med. 2021;8:612215. doi: 10.3389/fcvm.2021.612215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Luckey SW, Mansoori J, Fair K, Antos CL, Olson EN, Leinwand LA. Blocking cardiac growth in hypertrophic cardiomyopathy induces cardiac dysfunction and decreased survival only in males. Am J Physiol Heart Circ Physiol. 2007;292:H838–H845. doi: 10.1152/ajpheart.00615.2006 [DOI] [PubMed] [Google Scholar]
- 79. Brimacombe M, Walter D, Salberg L. Gender disparity in a large nonreferral‐based cohort of hypertrophic cardiomyopathy patients. J Womens Health (Larchmt). 2008;17:1629–1634. doi: 10.1089/jwh.2007.0734 [DOI] [PubMed] [Google Scholar]
- 80. Nijenkamp LLAM, Bollen IAE, Niessen HWM, dos Remedios CG, Michels M, Poggesi C, Ho CY, Kuster DWD, van der Velden J. Sex‐specific cardiac remodeling in early and advanced stages of hypertrophic cardiomyopathy. PLoS One. 2020;15. doi: 10.1371/journal.pone.0232427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marstrand P, Lakdawala NK, Day S, Ashley EA, Michels M, Pereira A, Wittekind S, Jacoby D, Ware JS, Colan SD, et al. Abstract 15857: female sex, multiple sarcomere variants and atrial fibrillation are associated with worse outcome in patients with end‐stage hypertrophic cardiomyopathy. Circulation. 2019;140:A15857–A15857. doi: 10.1161/circ.140.suppl_1.15857 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Figures S1–S5
References 58–81


