Abstract
Background
Coronary artery calcification (CAC) is a crucial indicator of subclinical atherosclerotic cardiovascular disease. The relationship between long‐term insulin resistance (IR) trajectory and CAC has been explored in few studies. Therefore, this study aimed to investigate whether the long‐term IR time series of young adults are associated with the incidence of CAC in midlife.
Methods and Results
In a cohort study comprising 2777 participants from the CARDIA (Coronary Artery Risk Development in Young Adults) study, the homeostasis model assessment for IR was used to measure IR levels, and group‐based trajectory modeling was used to fit three 25‐year homeostasis model assessments for IR trajectories. Logistic regression was used to estimate the association between the 3 homeostasis model assessments for IR trajectories and CAC events at year 25. The results showed that among 2777 participants (mean age, 50.10±3.58 years; 56.2% women; 46.4% Black), there were 780 incident CAC events after a 25‐year follow‐up. After full adjustment, the prevalence of CAC was higher in the moderate‐ (odds ratio [OR], 1.40 [1.10–1.76]) and the high‐level homeostasis model assessments for IR trajectories (OR, 1.84 [1.21–2.78]) than in the low‐level trajectory. This association was observed in obese individuals despite the negative interaction between IR and different types of obesity (all P interactions >0.05).
Conclusions
Our study revealed that young adults with a higher level of IR were more likely to develop CAC in middle age. Furthermore, this association persisted in obese individuals. These findings highlight the importance of identifying subclinical cardiovascular risk factors and implementing primary prevention measures.
Keywords: coronary artery calcification, HOMA‐IR trajectory, insulin resistance, obesity
Subject Categories: Epidemiology, Primary Prevention, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- CARDIA
Coronary Artery Risk Development in Young Adults
- HOMA‐IR
homeostatic model assessment for insulin resistance
- IR
insulin resistance
Clinical Perspective.
What Is New?
Our study has shown that long‐term moderate or high insulin resistance (IR) in young adulthood is associated with an increased prevalence of coronary artery calcification in middle age, and this association persists in the obese.
The homeostasis model assessment for IR trajectory can identify the long‐term IR status of different adolescents and could serve as a reference factor for their future coronary artery calcification prevalence, especially among the obese.
What Are the Clinical Implications?
The results of this study highlight the importance of controlling for IR at a young age as a modifiable risk factor for coronary artery calcification.
The homeostasis model assessment for IR trajectory may provide an assessment of IR status in different subjects and guide medication and lifestyle modifications to achieve better prevention of coronary artery calcification.
Therefore, active monitoring and diagnosis of IR for an extended period (ie, primary prevention in the clinical setting) is crucial for youths.
Epidemiological studies have revealed a growing prevalence of cardiovascular risk factors among young individuals, resulting in an increased incidence rate of atherosclerotic diseases. 1 , 2 Coronary artery calcification (CAC) is one of the key markers of subclinical atherosclerotic cardiovascular disease (ASCVD). 3 The CAC score obtained by computed tomography (CT) is often used to identify individuals at high or low risk of ASCVD. 4 The 2019 European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndromes indicated that the assessment of CAC score with CT may be considered as a risk modifier in the cardiovascular risk assessment of asymptomatic subjects. 5 The 2019 American College of Cardiology/American Heart Association guidelines also suggest using CAC score measurement to guide preventive intervention decisions for patients with borderline or intermediate estimated 10‐year ASCVD risk. 6
Although some studies have shown an association between insulin resistance (IR) and cardiovascular disease, including CAC, others have yielded conflicting results. For example, 2 cohort studies of Korean individuals conducted in 2020 and 2022 found a link between IR and CAC, 7 , 8 whereas a cohort study of middle‐aged men and women with type 2 diabetes from the Multi‐Ethnic Study of Atherosclerosis did not find an association between IR and CAC. 9 However, the research on the relationship between IR and the occurrence or progress of CAC mainly focuses on middle‐aged and elderly people 9 , 10 or has a short follow‐up time. 11 Few longitudinal studies were conducted on the relationship between IR and CAC incidence and its progression in adolescents. Moreover, a single assessment of risk factors at a single time point may not reveal the causality between long‐term exposure and results. Therefore, a direct study is needed to reveal the relationship between the longitudinal trajectories of IR and CAC.
IR is often considered to be related to obesity, and it is mainly indirectly affected by the adaptive regulation of the cardiovascular system structure and function caused by obesity. 12 Obesity‐related IR is a systemic consequence caused by the expansion of poorly adapted adipose tissue. 13 However, it is unclear whether obesity‐related long‐term IR is associated with CAC.
The objective of our study was to explore the long‐term influence of IR on the incident CAC at the end point and whether there were differences in obesity‐related IR individuals. We hypothesized that participants with long‐term moderate or high levels of the homeostasis model assessment for IR (HOMA‐IR) were associated with an incremental increase in the prevalence of CAC.
Methods
This cohort study was approved by the institutional review committee of each central institution, and all participants signed written informed consent. The study strictly followed the Strengthening the Reporting of Observational Studies in Epidemiology recommendations.
All CARDIA (Coronary Artery Risk Development in Young Adults) data were obtained from the CARDIA Coordinating Center (https://www.cardia.dopm.uab.edu/contact‐cardia). Details of the National Heart, Lung, and Blood Institute policies governing the data and how to access these data are available at (https://www.cardia.dopm.uab.edu/study‐information/nhlbi‐data‐repository‐data).
Study Population
CARDIA is one of the studies focusing on age growth, cardiovascular health, and cardiovascular disease throughout the life course, including 5115 Black and White people. A detailed design of CARDIA has been published previously. 14 It was a study on the influence of cardiovascular risk factors on middle‐aged cardiovascular outcomes among young people (18–30 years old) recruited from 4 field centers in the United States (Oakland, CA; Minneapolis, MN; Chicago, IL; and Birmingham, AL). The participants were followed up every 5 years (including the second and seventh years, now up to year 30). Data collected from March 1985 to June 1986, to June 2010 to August 2011 (25‐year follow‐up) were analyzed.
For our analysis, we excluded participants who missed fasting glucose and fasting insulin measurements at baseline (n=104), had absent CAC measurements at the year‐25 examination (n=1885), and those who had <3 valid values of HOMA‐IR measurement during follow‐up visits (n=61). Moreover, participants who missed other covariate data were also excluded (n=286), including all adjusted variables described in the models. The flowchart is shown in Figure 1. Finally, 2777 participants in the CARDIA study were analyzed. We obtained research queue data with the permission of the administrator (CARDIA study; URL: https://clinicaltrials.gov/ct2/show/NCT00005130).
Figure 1. Flowchart of inclusion and exclusion criteria and studies.
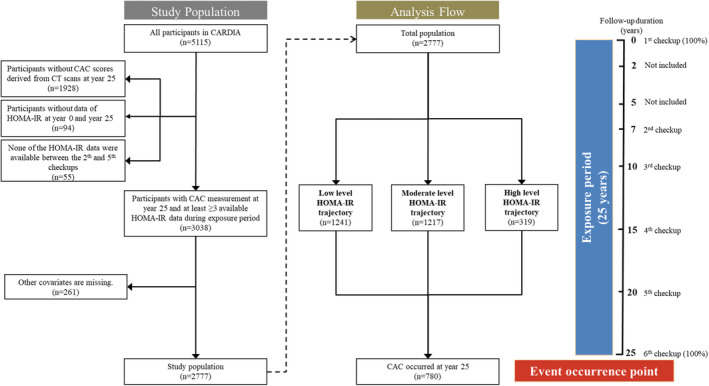
Follow‐up from 0 to 25 years was taken as the exposure period, and the 25th‐year examination was taken as the event occurrence point. Subsequently, at least 3 HOMA‐IR values obtained from the follow‐up of 2777 participants were fitted to analyze the relationship between different HOMA‐IR level trajectories and CAC score >0. CAC indicates coronary artery calcification; CARDIA, Coronary Artery Risk Development in Young Adults; CT, computed tomography; and HOMA‐IR, homeostatic model assessment for insulin resistance.
HOMA‐IR Trajectories
The index of HOMA‐IR was expressed by the formula: fasting insulin (μU/dL)×fasting glucose (mg/dL)/405. 15 We used group‐based trajectory modeling to fit different HOMA‐IR trajectories and made a comparison among the 5 groups. 16 , 17 , 18 , 19 Compared with the standard growth trajectory analysis focusing on the average population trajectory (with individual‐level random effects on time‐related regression coefficient), this method enabled us to identify groups experiencing similar levels and patterns of change from youth to middle age. We fitted the 3 trajectories from HOMA‐IR data in the follow‐up period of 0 to 25 years (years 0, 7, 10, 15, 20, 25, and no required data were found in years 2 and 5, so they were not included). Both group and average posterior probabilities assessed by the Bayesian information criterion were significant (Table S1). To sum up, the 3 different trajectory models that finally fitted were proved to be appropriate.
CAC Assessment
CAC score can not only be used as the evaluation standard of CAC severity but also as an indicator of subclinical ASCVD risk assessment. At present, coronary artery calcified plaque measured by cardiac CT is commonly used as the scoring standard of the CAC score, and the Agatston method was used to perform the CAC score in all participants in the CARDIA study. 20 Two CT scans were performed in the CARDIA study, which scanned 1 to 2 minutes for each individual, and independents were examined respectively 21 ; scores were then averaged. During the follow‐up of the years 15, 20, and 25 in the CARDIA study, there was no difference in CAC score obtained from electron beam CT and multidetector CT scanners in a previous study. 22 CAC incident was defined as CAC score >0. 23 Earlier research has shown that when a person's CAC score is >0, the CAC score tends to increase exponentially over the next decade. 24 Thus, we divided participants with CAC scores >0 into 3 groups based on severity: 1 to 100, 101 to 300, and >300. This was done to visualize more clearly the distribution of different score ranges within different HOMA‐IR trajectories. Meanwhile, we conducted sensitivity analyses on CAC score >20 Agatston units and CAC score >100 Agatston units, respectively. Additionally, the association between different HOMA‐IR trajectories and different CAC severity was analyzed.
Covariates
At every examination in the CARDIA study, all information, such as demographics, lifestyle, anthropometrics, biomarkers, and medications use, were collected with standardized protocols. 14
Age, sex, and race were queried through self‐report during follow‐up. Body mass index (BMI) was calculated using the internationally accepted formula, that is, weight (kilograms) divided by the square of height (meters). According to the definition of the World Health Organization, obesity was defined as BMI ≥30 kg/m2, and abdominal obesity was defined as waist circumference ≥102 cm for men and ≥88 cm for women. Smoking status classified participants as former, current, or never, and similar to drinking status, their status was collected through questionnaires distributed by the interviewer. Laboratory values, such as fasting glucose, fasting insulin, triglyceride, total cholesterol, and low‐density lipoprotein cholesterol, were measured using standard techniques. The blood pressure values were the average of the last 2 measurements taken from the 3 blood pressure measurements.
Statistical Analysis
Baseline characteristics analyses of 3 HOMA‐IR level trajectories were as follows: continuous variables were expressed as mean±SD or number (percentage) for categorical variables. To detect the statistical significance across groups, the χ2 test or Kruskal‐Wallis test was used as appropriate.
Binary logistic regression analysis was performed to estimate the multivariable‐adjusted association between different HOMA‐IR level trajectories and CAC using the following models: (1) adjusted for age, sex, and race; (2) adjusted the same variables in model 1 plus BMI, smoking status, and serum creatinine at the year‐25 examination; (3) adjusted for model 2 plus systolic blood pressure, diastolic blood pressure, low‐density lipoprotein cholesterol, glycosylated hemoglobin, and HOMA‐IR at the year‐25 examination. We used odds ratio (OR) and 95% CI to express the results. To understand the distribution of CAC score in different trajectories, we also divided the CAC score into 4 groups: 0, 1 to 100, 101 to 300, and >300, and observed the percentage of participants in each group.
The potential effect modification by different obesity types was explored by comparing the OR through the subgroup analysis of obesity and abdominal obesity. Meanwhile, the interaction analysis of different HOMA‐IR trajectories on obesity and abdominal obesity, respectively, was performed, and the P for interaction was obtained. Additionally, 2 sensitivity analyses were performed by using alternative thresholds to define CAC incidents. In addition, we defined the end point with a different CAC score; CAC score >20 Agatston units and CAC score >100 Agatston units were defined as CAC incident successively, and multivariable‐adjusted analyses were used to estimate the relationship between every HOMA‐IR level trajectory with CAC score >20 and CAC score >100, respectively. Moreover, we used ordered logistic regression to examine the correlation between different HOMA‐IR trajectories and CAC severity (with CAC score categorized as 0, 1–100, 101–300, or >300).
All statistical analyses were performed in SPSS version 20 (IBM, Armonk, NY), except that the HOMA‐IR trajectory was fitted using Stata 17.0 (StataCorp, College Station, TX). A 2‐sided P<0.05 was considered statistically significant.
Results
Baseline Characteristics
There were 2777 participants in the CARDIA study from 1985 to 2011 who had CAC data available at year 25 and were selected for this analysis (mean age, 50.10±3.58 years; 56.2% women; 46.4% Black). Additionally, 286 (10.3%) had a comorbidity of diabetes and 903 (32.5%) of hypertension. Baseline characteristics of the CARDIA subsample (n=2777) across the 3 trajectories of HOMA‐IR are shown in Table 1. The median values of HOMA‐IR for the different trajectories were 1.15, 2.96, and 5.92, respectively. At the year 25, among the participants in the high‐level HOMA‐IR trajectory, the proportion of participants with CAC >0 was the highest (41.2%), more were women (52.3%, P<0.001) and Black (65.8%, P=0.016), and more had hypertension (65.5%) and diabetes (44.9%). Moreover, those in the high‐level trajectory had a higher BMI (P=0.007), waist circumference, blood pressure (systolic blood pressure, diastolic blood pressure), plasma glucose, fasting insulin, triglycerides, glycosylated hemoglobin, and hypersensitive C‐reactive protein (all P<0.001 except BMI), but the high‐density lipoprotein cholesterol levels were low (P<0.001).
Table 1.
Characteristics of Participants in Different HOMA‐IR Level Trajectories at the Year‐25 Examination in the CARDIA Study
| Characteristic | Total | HOMA‐IR trajectory | P value | ||
|---|---|---|---|---|---|
| Low level (n=1241) | Moderate level (n=1217) | High level (n=319) | |||
| HOMA‐IR | 2.05 (1.20–3.52) | 1.15 (0.84–1.56) | 2.96 (2.22–4.06) | 5.92 (4.20–9.47) | <0.001 |
| CAC (%)* | 780 (28.1) | 281 (22.6) | 365 (30.2) | 134 (41.2) | <0.001 |
| Age, y | 50.10±3.58 | 50.31±3.40 | 49.78±3.70 | 50.47±3.73 | <0.001 |
| Sex | |||||
| Women (%) | 1562 (56.2) | 736 (40.7) | 656 (54.2) | 170 (52.3) | <0.001 |
| Men (%) | 1215 (43.8) | 506 (59.3) | 554 (45.8) | 155 (47.7) | |
| Race | |||||
| Black (%) | 1289 (46.4) | 444 (35.7) | 631 (52.1) | 214 (65.8) | 0.016 |
| White (%) | 1477 (53.2) | 793 (63.8) | 575 (47.5) | 109 (33.5) | |
| BMI, kg/m2 | 30.06±6.70 | 25.92±4.24 | 32.45±6.06 | 37.00±6.59 | 0.007 |
| Waist circumference, cm | 94.17±15.04 | 84.14±10.60 | 99.83±12.13 | 111.45±12.40 | <0.001 |
| SBP, mm Hg | 118.42±15.13 | 114.83±14.25 | 120.66±14.85 | 123.81±16.26 | <0.001 |
| DBP, mm Hg | 73.84±10.75 | 70.47±10.31 | 76.22±10.37 | 77.81±10.03 | <0.001 |
| Smoking currently (%) | 455 (16.4) | 220 (17.7) | 190 (15.7) | 45 (13.8) | <0.001 |
| Drinking currently (%) | 2186 (78.7) | 1038 (83.6) | 929 (76.8) | 219 (67.4) | 0.354 |
| Diabetes (%) | 286 (10.3) | 28 (2.3) | 112 (9.3) | 146 (44.9) | <0.001 |
| Hypertension (%) | 903 (32.5) | 218 (17.6) | 472 (39.0) | 213 (65.5) | <0.001 |
| Fasting glucose, mg/dL | 93 (87–101) | 89 (84–95) | 96 (90–103) | 109 (97–144) | <0.001 |
| Fasting insulin, μU/mL | 8.67 (5.33–14.17) | 5.17 (3.83–7.00) | 12.33 (9.29–16.17) | 20.67 (14.83–29.75) | <0.001 |
| Triglycerides, mg/dL | 92 (68–132) | 79 (59–105) | 105 (77–152) | 124 (88–174) | <0.001 |
| TC, mg/dL | 192.03±36.29 | 193.00±33.97 | 194.18±37.51 | 180.28±38.12 | 0.162 |
| LDL‐C, mg/dL | 110 (89–132) | 108 (89–128) | 115 (94–138) | 99 (81–123) | 0.011 |
| HDL‐C, mg/dL | 55 (45–67) | 63 (52–75) | 51 (43–61) | 46 (39–56) | <0.001 |
| Creatinine, mg/dL | 0.84 (0.73–0.98) | 0.83 (0.72–0.96) | 0.85 (0.74–1.00) | 0.83 (0.72–0.99) | <0.001 |
| CRP, μg/mL | 1.39 (0.61–3.36) | 0.77 (0.41–1.67) | 2.08 (0.95–4.61) | 3.17 (1.38–6.72) | 0.207 |
| HbA1c (%) | 5.50 (5.30–5.80) | 5.40 (5.20–5.60) | 5.60 (5.30–5.90) | 6.10 (5.70–7.10) | <0.001 |
| Antihypertension medication (%) | 736 (26.5) | 157 (12.6) | 379 (31.3) | 200 (61.5) | <0.001 |
| Lipid‐lowering therapy (%) | 420 (15.1) | 91 (7.3) | 207 (17.1) | 122 (37.5) | <0.001 |
| Medication for diabetes (%) | 196 (7.1) | 8 (0.6) | 67 (5.5) | 121 (37.2) | <0.001 |
Values are shown as mean±SD (normal distribution data), median (lower quartile–upper quartile) (nonnormal distribution data), or n (%) (categorical variable). Baseline characteristics of the 2777 eligible participants from the CARDIA study stratified follow‐up HOMA‐IR trajectories. There were significant between‐group differences in most covariates considered. BMI indicates body mass index; CAC, coronary artery calcification; CARDIA, Coronary Artery Risk Development in Young Adults; CRP, C‐reactive protein; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; and TC, total cholesterol.
CAC occurred in year 25.
We partitioned all HOMA‐IR data into 2, 3, 4, and 5 trajectories based on comparison of the Bayesian information criterion and average posterior probabilities (Table S1). The trajectories diagram of HOMA‐IR is shown in Figure 2. Finally, all participants with similar trends of HOMA‐IR level changes during the 25‐year follow‐up were classified as the same level trajectory: trajectory 1, low‐level HOMA‐IR trajectory (n=1241, 44.5%); trajectory 2, moderate‐level HOMA‐IR trajectory (n=1217, 43.4%); trajectory 3, high‐level HOMA‐IR trajectory (n=319, 12.1%).
Figure 2. HOMA‐IR level trajectories of the 25‐year follow‐up in the CARDIA study.
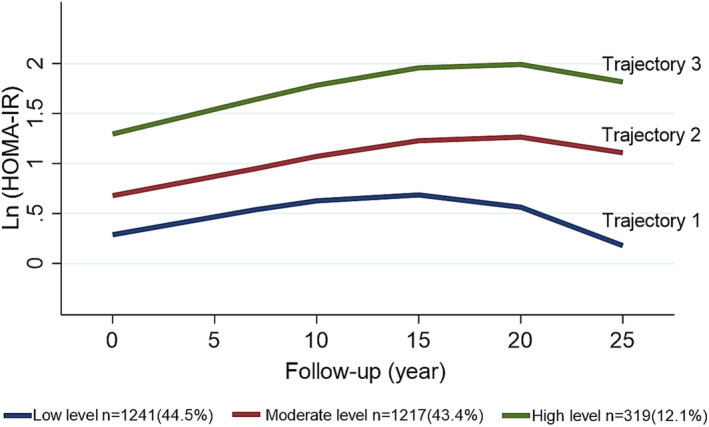
To enhance the precision and robustness of the trajectory fitting process, we utilized Ln[HOMA‐IR]. CARDIA indicates Coronary Artery Risk Development in Young Adults; HOMA‐IR, homeostatic model assessment for insulin resistance; and Ln[HOMA‐IR], natural logarithm of HOMA‐IR.
Association Between 25‐Year HOMA‐IR Level Trajectories and the Prevalence of CAC
During a follow‐up of 25 years, CAC incidents occurred in 780 (28.1%) subjects. According to the HOMA‐IR level trajectories during 25 years of follow‐up, the univariate‐ or multivariable‐adjusted estimates for the relationship of HOMA‐IR trajectories with CAC incidents are shown in Table 2. Trajectory 1 was the reference group, trajectories 2 and 3 (OR, 1.50 [95% CI, 1.25–1.80]; P<0.001; OR, 2.36 [95% CI, 1.82–3.06]; P<0.001; respectively) demonstrated significantly higher odds of CAC incidence, parallel with participants in trajectory 1. Participants in trajectory 3, with the highest levels of HOMA‐IR during the 25 years, had the highest prevalence of CAC (all P<0.05). In model 3, after fully adjusting for potential confounders, adjusted ORs (95% CI) for CAC incidence for trajectories 2 and 3, compared with the reference, were 1.40 (1.10–1.76) and 1.84 (1.21–2.78), respectively.
Table 2.
Multivariate Association of 3 HOMA‐IR Levels With Prevalence of Coronary Artery Calcification in the 25‐Year Follow‐Up Among Individuals in the CARDIA Study
| HOMA‐IR trajectory | Events/total, n/N | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Low level | 280/1241 | Reference | … | Reference | … | Reference | … | Reference | … |
| Moderate level | 370/1217 | 1.50 (1.25–1.80) | <0.001 | 1.60 (1.32–1.94) | <0.001 | 1.50 (1.20–1.87) | <0.001 | 1.40 (1.10–1.76) | 0.005 |
| High level | 130/319 | 2.36 (1.82–3.06) | <0.001 | 2.44 (1.84–3.25) | <0.001 | 2.23 (1.60–3.17) | <0.001 | 1.84 (1.21–2.78) | 0.004 |
| P for trend | <0.001 | <0.001 | <0.001 | 0.002 | |||||
Model 1, adjusted for age, sex, and race.
Model 2, adjusted for model 1 plus BMI, smoking status, and creatinine.
Model 3 adjusted for model 2 plus SBP, DBP, LDL‐C, HbA1c, and HOMA‐IR.
BMI indicates body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; and SBP, systolic blood pressure.
HOMA‐IR Trajectories and CAC Severity
The distribution of CAC scores of participants with different HOMA‐IR level trajectories is shown in Figure 3, which indicated that compared with the low HOMA‐IR trajectories, the proportion of participants with CAC score >0 in the moderate HOMA‐IR trajectories was higher. The proportions of CAC scores 1 to 100, 101 to 300, and >300 in each score hierarchy of CAC score >0 increased. Furthermore, the proportion of participants with CAC scores >0 in the high HOMA‐IR trajectories was the highest; in this group, CAC scores >100 reached 8%.
Figure 3. Distribution of the CAC score at year 25 when comparing different HOMA‐IR level trajectories.
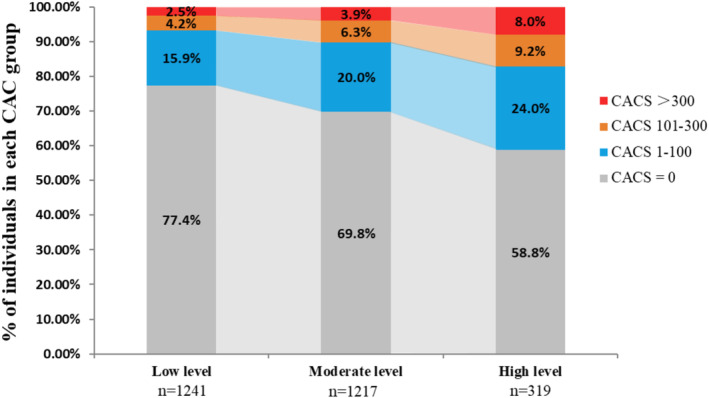
CAC indicates coronary artery calcification; CACS, coronary artery calcification score; CARDIA, Coronary Artery Risk Development in Young Adults; and HOMA‐IR, homeostatic model assessment for insulin resistance.
The results of our ordered logistic regression analysis suggest that participants with moderate and high HOMA‐IR trajectories had a significantly higher risk of higher CAC scores (1–100, 101–300, >300) compared with those with low HOMA‐IR trajectory, after adjusting for potential confounders (OR, 1.48 [95% CI, 1.18–1.86]; P=0.001; OR, 2.80 [95% CI, 1.41–3.07]; P<0.001; respectively) (Table S2).
Association of HOMA‐IR Level Trajectory and the Prevalence of CAC Under Different Types of Obesity
Interactions between HOMA‐IR level trajectory class and CAC >0 across obesity (P=0.838) and abdominal obesity (P=0.116) were evaluated (Table 3). After fully adjusting, we found that participants with moderate‐ and high‐level HOMA‐IR trajectories also showed a high prevalence of CAC in the obese group (OR, 1.64 [95% CI, 1.07–2.52]; P=0.024; OR, 1.82 [95% CI, 1.04–3.19]; P=0.036; respectively). In the nonobese group, there was no statistical significance. At the same time, among participants with abdominal obesity, only those with high‐level HOMA‐IR trajectories were associated with CAC >0 (OR, 1.90 [95% CI, 1.11–3.26]; P=0.020), whereas those with nonabdominal obesity did not show any correlation. However, for participants who were nonobese or nonabdominal obese, the relationship between the OR for estimated HOMA‐IR level trajectory and the prevalence of CAC was not statistically significant, which may be due to the small number of participants with CAC >0 in each group.
Table 3.
Relationship Among 3 HOMA‐IR Trajectories and Coronary Artery Calcification in Participants With Different Obesity Types
| HOMA‐IR trajectory | Events/total, n/N | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Obesity (P interaction = 0.159*) | |||||||||
| Subjects with obesity (BMI ≥30 kg/m2) | |||||||||
| Low level | 41/189 | Reference | … | Reference | … | Reference | … | Reference | … |
| Moderate level | 225/745 | 1.56 (1.07–2.28) | 0.021 | 1.65 (1.11–2.47) | 0.014 | 1.42 (1.09–1.85) | 0.010 | 1.64 (1.07–2.52) | 0.024 |
| High level | 104/273 | 2.22 (1.46–3.39) | <0.001 | 2.14 (1.36–3.37) | 0.001 | 4.07 (2.09–7.92) | <s0.001 | 1.82 (1.04–3.19) | 0.036 |
| Subjects without obesity (BMI <30 kg/m2) | |||||||||
| Low level | 239/1052 | Reference | … | Reference | … | Reference | … | Reference | … |
| Moderate level | 145/472 | 1.51 (1.18–1.92) | 0.001 | 1.35 (1.04–1.76) | 0.023 | 1.71 (1.14–2.57) | 0.009 | 1.17 (0.83–1.65) | 0.367 |
| High level | 26/46 | 4.42 (2.43–8.06) | <0.001 | 3.93 (2.05–7.51) | <0.001 | 2.35 (1.49–3.72) | <0.001 | 2.63 (1.09–6.36) | 0.032 |
| Abdominal obesity (P interaction = 0.171†) | |||||||||
| Subjects with abdominal obesity‡ | |||||||||
| Low level | 44/202 | Reference | … | Reference | … | Reference | … | Reference | … |
| Moderate level | 226/774 | 1.48 (1.03–2.14) | 0.037 | 1.47 (0.99–2.16) | 0.055 | 1.50 (1.01–2.22) | 0.043 | 1.42 (0.94–2.15) | 0.097 |
| High level | 119/289 | 2.51 (1.67–3.78) | <0.001 | 2.24 (1.44–3.48) | <0.001 | 2.47 (1.58–3.86) | <0.001 | 1.90 (1.11–3.26) | 0.020 |
| Subjects without abdominal obesity | |||||||||
| Low level | 236/1039 | Reference | … | Reference | … | Reference | … | Reference | … |
| Moderate level | 144/443 | 1.64 (1.28–2.10) | <0.001 | 1.39 (1.06–1.81) | 0.017 | 1.50 (1.14–1.98) | 0.003 | 1.33 (0.94–1.90) | 0.113 |
| High level | 11/30 | 1.97 (0.92–4.20) | 0.079 | 1.44 (0.64–3.24) | 0.382 | 1.50 (0.65–3.44) | 0.340 | 1.05 (0.37–3.00) | 0.928 |
Model 1, adjusted for age, sex, and race.
Model 2, adjusted for model 1 plus BMI, smoking status, and creatinine.
Model 3, adjusted for model 2 plus SBP, DBP, LDL‐C, HbA1c, and HOMA‐IR. BMI indicates body mass index; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HOMA‐IR, Homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; and SBP, systolic blood pressure.
Interaction between HOMA‐IR level trajectory and BMI ≥30.
Interaction between HOMA‐IR level trajectory and abdominal obesity.
Abdominal obesity was defined as waist circumference ≥102 cm for men and ≥88 cm for women.
Sensitivity Analyses
In sensitivity analysis, the prevalence of CAC in the moderate‐ and high‐level HOMA‐IR trajectories was higher; the results remained similar to those reported in Table S3. However, after full adjustment, the association between trajectory 2 and CAC score >20 was not significant among all individuals in the CARDIA study (OR, 1.26 [95% CI, 0.96–1.64]; P=0.097).
Discussion
In this 25‐year follow‐up prospective cohort study, we defined 3 different HOMA‐IR level trajectories from youth to middle age: low, moderate, and high. In our study, we found that long‐term IR status in youth was associated with an increased risk of developing CAC in midlife, particularly in those with a moderate or high HOMA‐IR level trajectory. Additionally, the interaction analysis in the obese population found that although IR and obesity, or IR and abdominal obesity, showed negative interaction results (all P interaction >0.05), it was observed that obese individuals on a trajectory of moderate or high HOMA‐IR levels, especially the latter, were associated with a higher prevalence of CAC.
Previous research has not demonstrated a dynamic relationship between IR and the incidence of CAC. Our study is the first to show that higher levels of IR are significantly associated with an increased risk of CAC over time, suggesting that IR may be a long‐term risk factor for cardiovascular disease. Previous studies have shown that IR was related to atherosclerosis 25 , 26 and an increment of incident CAC. 9 , 10 , 11 The existence of IR may lead to nonalcoholic fatty liver disease or multiple components of metabolic syndrome (eg, obesity, hypertension), which may affect the development of ASCVD individually or in combination. 27 , 28 , 29 In a study of 2076 young participants who developed diabetes, the baseline HOMA‐IR level was found to be positively correlated with the risk of CAC, with a 4‐year follow‐up period as the exposure period. 11 Meanwhile, in other recent studies, the CAC score was further divided into 4 categories: 0, 19, 20 to 99, and >100. It was found that after adjustment, exponential growth would occur in the next 10 years, regardless of the initial level, consistent with the formation of active coronary artery atherosclerosis, and the track of CAC score growth over time was consistent with the track of middle‐aged coronary heart disease and mortality increase. 24 However, the intermediate factors of CAC score growth over time are unknown. It is worth noting that we also observed an association between IR and CAC in obese participants (both general obesity and abdominal obesity), but no interaction between IR and different obesity types was detected. Our current analysis provides further evidence that prolonged periods of IR may increase the risk of CAC in young individuals, and the classification of HOMA‐IR trajectories does not appear to be weakened by obesity type. IR and CAC may be longitudinally correlated, with high levels of HOMA‐IR potentially contributing to the development of CAC over time. Alternatively, it is possible that higher levels of IR create an abnormal metabolic environment in the cardiovascular system, leading to an increase in the size of arterial plaque. This suggests that although obesity and IR are both components of the metabolic syndrome, IR may independently serve as a risk factor for CAC, and prolonged high levels of IR may also be harmful to the cardiovascular system. Overall, our study suggests that the impact of IR on CAC may be a dynamic, time‐dependent process, with long‐term levels of IR from young to middle age potentially serving as an important predictor and mediator of CAC in later life.
Several potential pathophysiological mechanisms may contribute to understanding the IR in the occurrence of the CAC. First, greater fasting glucose variability has been shown to stimulate cells to produce inflammatory factors, resulting in secondary pathological changes in blood vessels. 30 These changes are the most common mechanism of vascular calcification. Additionally, temporary hyperglycemia may induce osteogenesis and alkaline phosphatase activation in vascular smooth muscle cells. 31 Previous studies have shown that under high phosphorus conditions, exosomes from endothelial cells caused calcification of vascular smooth muscle cells, eventually leading to CAC. 32 It is thus obvious that being at a high level of IR for a long time may accelerate the occurrence and development of the above potential mechanisms. Thus, it is particularly important to track the longitudinal trajectory of IR, especially in those exposed to cardiovascular risk factors for an extended period, to detect potentially high‐risk patients with CAC. Clinicians should pay close attention to individuals with rising IR levels but without diabetes and suggest they undergo regular CT examinations and take preventive interventions to reduce the incidence of CAC in the future.
There were several important implications in our research for youths' cardiovascular protection. First, our study has demonstrated a significant relationship between long‐term HOMA‐IR level tracking and the occurrence of future CAC incidents, highlighting the importance of long‐term detection of IR as an effective predictor of CAC. Second, although there is no guideline for defining IR by HOMA‐IR cutoff value, we can still clearly observe the change in IR level through the trend of HOMA‐IR trajectory and treat according to the HOMA‐IR safety threshold predetermined by clinicians. Third, obesity is typically characterized by metabolic IR, 33 and identifying metabolic syndrome risk factors is an important step for risk assessment and ASCVD risk mitigation. 34 Reducing the accumulation of visceral and subcutaneous adipose tissue during young adulthood could help to decrease the occurrence of coronary events. According to the latest diabetes standard issued by the American Diabetes Association, moderate‐intensity physical activity has been shown to reduce fat accumulation in the abdomen and improve insulin sensitivity in young adults and children. 35 , 36 According to an intensive lifestyle behavior change program, young adults can improve their physical activity and reduce weight 37 to lower the risk of subclinical atherosclerosis disease under HOMA‐IR variability and obesity alone or in combination. Finally, if clinicians find that a patient's current IR level is high during diagnosis and long‐term monitoring, and shows an upward trend, timely intervention can be implemented to prevent adverse coronary outcomes in the future.
The key strengths of our study include the adoption of a prospective cohort design based on the community, a long follow‐up period, strict adherence to standardized protocols for data collection, and a comparatively large sample size. Additionally, this study used relatively innovative trajectory fitting methods that combined long‐term time series with evaluation, allowing us to identify subgroups with similar HOMA‐IR trajectories from young to middle age within the population. Most importantly, we investigated the relationship between long‐term IR in youth and CAC in midlife for the first time. However, our study had certain limitations. First, due to the observational nature of the CARDIA study and the presence of unknown potential factors, and although logistic regression was used to correct for multiple variables, our research results failed to establish a causal relationship between HOMA‐IR and CAC events; further mechanism studies are needed to address this limitation. Second, IR was evaluated using HOMA‐IR in this study instead of the hyperinsulinemic‐normoglycemic clamp test, so the correlation between IR and HOMA‐IR could not be better evaluated. Third, the measurement values of fasting glucose and fasting insulin collected by the CARDIA study every 5 years after the fifth year may not reflect the fluctuations in these indicators over shorter periods. Finally, our study did not include participants' drug use in the calibration model, because we could not confirm whether CARDIA participants' drug use was reported correctly.
Conclusions
The long‐term higher IR level was associated with the increase in the incidence rate of CAC in the general obese and abdominal obese population. Long‐term evaluation of IR in clinical practice may help identify high‐risk patients with subclinical ASCVD in the future, provide effective methods and suggestions for primary prevention, and ultimately prevent adverse cardiovascular disease outcomes from occurring.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (81900329), Guangdong Basic and Applied Basic Research Foundation (2019A1515011098, 2022A1515010416), and 2021 Guangdong Province Continuing Education Quality Improvement Project (JXJYGC2021JY0541).
Disclosures
None.
Supporting information
Data S1
Acknowledgments
The authors thank all of the participants, staff, and investigators of the CARDIA study for their contributions. Author contributions: Z.K., R.H., and X.X. contributed to the article equally. Z.K., R.H., and X.X. conceived the research idea, designed the analysis, and advised on statistical analysis methods. Z.K. drafted the article. R.H., X.X., W.L., S.W., Y.G., X.Zhang, X.Zhuang, and L.Z. contributed to the discussion and critical revision of the article for important intellectual content. All authors reviewed and approved the final article.
Z. Ke, R. Huang, and X. Xu contributed equally.
Preprint posted on Research Square November 29, 2022. doi: https://doi.org/10.21203/rs.3.rs‐2298173/v1.
This article was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028985
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Xiaodong Zhuang, Email: zhuangxd3@mail.sysu.edu.cn.
Liao Zhen, Email: liaolizhen@gdpu.edu.cn.
References
- 1. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 2. Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056 [DOI] [PubMed] [Google Scholar]
- 3. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 4. Hussain A, Ballantyne CM, Nambi V. Zero coronary artery calcium score. Circulation. 2020;142:917–919. doi: 10.1161/circulationaha.119.045026 [DOI] [PubMed] [Google Scholar]
- 5. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 6. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, Park SH, Han HW, Sung J, Jung HO, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19:34. doi: 10.1186/s12933-020-01008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song S, Choi SY, Park HE, Han HW, Park SH, Sung J, Jung HO, Sung JM, Chang HJ. Incremental prognostic value of triglyceride glucose index additional to coronary artery calcium score in asymptomatic low‐risk population. Cardiovasc Diabetol. 2022;21:193. doi: 10.1186/s12933-022-01620-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaha MJ, DeFilippis AP, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, Szklo M, Lakoski SG, Bertoni AG, Kronmal RA, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the multi‐ethnic study of atherosclerosis (MESA). Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee KK, Fortmann SP, Fair JM, Iribarren C, Rubin GD, Varady A, Go AS, Quertermous T, Hlatky MA. Insulin resistance independently predicts the progression of coronary artery calcification. Am Heart J. 2009;157:939–945. doi: 10.1016/j.ahj.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 11. Rhee EJ, Kim JH, Park HJ, Park SE, Oh HG, Park CY, Lee WY, Oh KW, Park SW. Increased risk for development of coronary artery calcification in insulin‐resistant subjects who developed diabetes: 4‐year longitudinal study. Atherosclerosis. 2016;245:132–138. doi: 10.1016/j.atherosclerosis.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 12. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315. doi: 10.1016/j.biopha.2021.111315 [DOI] [PubMed] [Google Scholar]
- 13. Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:92–107. doi: 10.1016/j.metabol.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd‐Jones DM, Lewis CE, Schreiner PJ, Shikany JM, Sidney S, Reis JP. The coronary artery risk development In young adults (CARDIA) study: JACC focus seminar 8/8. J Am Coll Cardiol. 2021;78:260–277. doi: 10.1016/j.jacc.2021.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews DR, Hosker JR, Rudenski AS, Naylor BA, Treacher DF, Turner TRC. Homeostasis model assessment: insulin resistance and fl‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 16. Nagin DS, Odgers CL. Group‐based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 17. Sharashova E, Wilsgaard T, Ball J, Morseth B, Gerdts E, Hopstock LA, Mathiesen EB, Schirmer H, Lochen ML. Long‐term blood pressure trajectories and incident atrial fibrillation in women and men: the Tromso study. Eur Heart J. 2020;41:1554–1562. doi: 10.1093/eurheartj/ehz234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu X, Huang R, Lin Y, Guo Y, Xiong Z, Zhong X, Ye X, Li M, Zhuang X, Liao X. High triglyceride‐glucose index in young adulthood is associated with incident cardiovascular disease and mortality in later life: insight from the CARDIA study. Cardiovasc Diabetol. 2022;21:155. doi: 10.1186/s12933-022-01593-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laddu DR, Rana JS, Murillo R, Sorel ME, Quesenberry CP Jr, Allen NB, Gabriel KP, Carnethon MR, Liu K, Reis JP, et al. 25‐year physical activity trajectories and development of subclinical coronary artery disease as measured by coronary artery calcium: the coronary artery risk development in young adults (CARDIA) study. Mayo Clin Proc. 2017;92:1660–1670. doi: 10.1016/j.mayocp.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeffrey CJ, Clark NJ, Wong ND, Michael M, Yadon A, Jacobs DR, Stephan S, Bild DE, Dale WO, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of multi‐ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology. 2016;234:35–43. [DOI] [PubMed] [Google Scholar]
- 21. Javaid A, Dardari ZA, Mitchell JD, Whelton SP, Dzaye O, Lima JAC, Lloyd‐Jones DM, Budoff M, Nasir K, Berman DS, et al. Distribution of coronary artery calcium by age, sex, and race among patients 30‐45 years old. J Am Coll Cardiol. 2022;79:1873–1886. doi: 10.1016/j.jacc.2022.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao SS, Pal RS, McKay CR, Gao YG, Gopal A, Ahmadi N, Child J, Carson S, Takasu J, Sarlak B, et al. Comparison of coronary artery calcium scores between electron beam computed tomography and 64‐multidetector computed tomographic scanner. J Comput Assist Tomogr. 2009;33:175–178. doi: 10.1097/RCT.0b013e31817579ee [DOI] [PubMed] [Google Scholar]
- 23. Bensenor IM, Goulart AC, Santos IS, Bittencourt MS, Pereira AC, Santos RD, Nasir K, Blankstein R, Lotufo PA. Association between a healthy cardiovascular risk factor profile and coronary artery calcium score: results from the Brazilian longitudinal study of adult health (ELSA‐brasil). Am Heart J. 2016;174:51–59. doi: 10.1016/j.ahj.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 24. Carr JJ, Jacobs DR Jr, Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. The insulin resistance atherosclerosis study (IRAS) investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.CIR.93.10.1809 [DOI] [PubMed] [Google Scholar]
- 26. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, Jacobs DR Jr, Wu C, Saad MF, Szklo M. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the multi‐ethnic study of atherosclerosis (MESA). Diabetes Care. 2007;30:2951–2956. doi: 10.2337/dc07-1042 [DOI] [PubMed] [Google Scholar]
- 28. Sung KC, Ryu S, Lee JY, Lee SH, Cheong ES, Wild SH, Byrne CD. Fatty liver, insulin resistance, and obesity: relationships with increase in coronary artery calcium over time. Clin Cardiol. 2016;39:321–328. doi: 10.1002/clc.22529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sung KC, Wild SH, Kwag HJ, Byrne CD. Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012;35:2359–2364. doi: 10.2337/dc12-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng W, Li Z, Guo W, Fan X, Zhou F, Zhang K, Ou C, Huang F, Chen M. Association between fasting glucose variability in young adulthood and the progression of coronary artery calcification in middle age. Diabetes Care. 2020;43:2574–2580. doi: 10.2337/dc20-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terao Y, Satomi‐Kobayashi S, Hirata K‐i, Rikitake Y. Involvement of rho‐associated protein kinase (ROCK) and bone morphogenetic protein‐binding endothelial cell precursor‐derived regulator (BMPER) in high glucose‐increased alkaline phosphatase expression and activity in human coronary artery smooth muscle cells. Cardiovasc Diabetol. 2015;14:14. doi: 10.1186/s12933-015-0271-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Q, Xin Z, He R, Wang T, Xu M, Lu J, Dai M, Zhang D, Chen Y, Zhao Z, et al. Age‐specific difference in the association between prediabetes and subclinical atherosclerosis: an analysis of a chinese prospective cohort study. Cardiovasc Diabetol. 2022;21:153. doi: 10.1186/s12933-022-01592-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. doi: 10.1016/j.metabol.2021.154766 [DOI] [PubMed] [Google Scholar]
- 34. Wong ND, Budoff MJ, Ferdinand K, Graham IM, Michos ED, Reddy T, Shapiro MD, Toth PP. Atherosclerotic cardiovascular disease risk assessment: an American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol. 2022;10:100335. doi: 10.1016/j.ajpc.2022.100335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: a meta‐analysis. Pediatrics. 2014;133:e163–e174. doi: 10.1542/peds.2013-2718 [DOI] [PubMed] [Google Scholar]
- 36. Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, Meléndez A, Boyle CA, Gower BA. Exercise dose and diabetes risk in overweight and obese children. JAMA. 2012;308:1103–1112. doi: 10.1001/2012.jama.10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American Diabetes Association Professional Practice C. 3 . Prevention or delay of type 2 diabetes and associated comorbidities: standards of medical care in diabetes‐2022. Diabetes Care. 2022;45:S39–S45. doi: 10.2337/dc22-S003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


