Abstract
Background
Autonomic dysfunction has been revealed in patients with acute ischemic stroke and is associated with poor prognosis. However, autonomic nervous system function assessed by heart rate variability (HRV) and its relationship with clinical outcomes in patients undergoing intravenous thrombolysis (IVT) remain unknown.
Methods and Results
Patients who did and did not undergo IVT between September 2016 and August 2021 were prospectively and consecutively recruited. HRV values were measured at 1 to 3 and 7 to 10 days after stroke to assess autonomic nervous system function. A modified Rankin scale score ≥2 at 90 days was defined as an unfavorable outcome. Finally, the analysis included 466 patients; 224 underwent IVT (48.1%), and 242 did not (51.9%). Linear regression showed a positive correlation of IVT with parasympathetic activation‐related HRV parameters at 1 to 3 days (high frequency: β=0.213, P=0.002) and with both sympathetic (low frequency: β=0.152, P=0.015) and parasympathetic activation‐related HRV parameters (high frequency: β=0.153, P=0.036) at 7 to 10 days after stroke. Logistic regression showed HRV values and autonomic function within 1 to 3 and 7 to 10 days after stroke were independently associated with 3‐month unfavorable outcomes after adjusting for confounders in patients who underwent IVT (all P<0.05). Furthermore, addition of HRV parameters to conventional risk factors significantly improved risk‐predictive ability of 3‐month outcome (the area under the receiver operating characteristic curve significantly improved from 0.784 [0.723–0.846] to 0.855 [0.805–0.906], P=0.002).
Conclusions
IVT positively affected HRV and autonomic nervous system activity, and autonomic function assessed by HRV in acute stroke phase was independently associated with unfavorable outcomes in patients undergoing IVT.
Keywords: acute ischemic stroke, autonomic function, autonomic nervous system, heart rate variability, intravenous thrombolysis, nomogram, prognosis
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ANS
autonomic nervous system
- HF
high‐frequency
- HRV
heart rate variability
- IVT
intravenous thrombolysis
- LF
low‐frequency
- NIHSS
National Institutes of Health Stroke Scale
- RMSSD
the square root of the mean of the sum of squares of the differences between adjacent normal‐to‐normal intervals
- TOAST
Trial of Org 10 172 in Acute Stroke Treatment
- TP
total power
Clinical Perspective.
What Is New?
Intravenous thrombolysis positively affected heart rate variability and autonomic nervous system activity with a different time frame in patients with acute ischemic stroke.
Lower heart rate variability values at 1 to 3 and 7 to 10 days after stroke were independently associated with unfavorable outcomes in patients with acute ischemic stroke after intravenous thrombolysis, and addition of heart rate variability parameters to conventional risk factors significantly improved the predictive ability of 3‐month unfavorable outcomes.
What Are the Clinical Implications?
Heart rate variability and autonomic nervous system function in the acute phase of stroke after intravenous thrombolysis could be considered as reliable predictors of clinical outcomes and intervention targets to improve outcomes in these patients.
Stroke is a leading cause of mortality and disability worldwide, of which ischemic stroke represents 41% to 87% of total strokes, and the economic costs of treatment and poststroke care are substantial. 1 , 2 , 3 The age‐standardized incidence rate, death rate, and disability‐adjusted life‐year rates caused by ischemic stroke remain at high levels throughout the world, 4 although the specific treatments for acute ischemic stroke, such as intravenous thrombolysis (IVT), have been innovated for several decades. 5 Previous studies have shown that nearly half of patients with stroke fail to achieve favorable outcomes even after IVT treatment. 6 Therefore, it is essential to identify a reliable predictor of IVT clinical outcomes and explore potential intervention targets to improve the prognosis of these patients.
The autonomic nervous system (ANS), comprising the sympathetic and parasympathetic nervous systems, plays a major role in maintaining homeostasis throughout the body during unpredictable and uncontrollable environmental perturbations. 7 Previous studies have reported disturbed central autonomic control in patients with ischemic stroke may result in sudden cardiac death. 8 Poststroke autonomic dysfunction observed in humans has also been replicated in animal models. 9 , 10 Additionally, autonomic function has been strongly linked to poor prognoses in patients with ischemic stroke. 11 However, to the best of our knowledge, few studies have investigated ANS function alterations in patients who undergo IVT in the hyperacute phase of ischemic stroke; hence, its predictive value remains unclear.
Heart rate variability (HRV), the beat‐by‐beat variance in heart rate, reflects the level of ANS function. It has been used in several studies, because its measurement is noninvasive, and it is more readily available for clinical application. 12 Therefore, our study used HRV analysis and aimed to explore (1) the impact of IVT on HRV and ANS function in acute ischemic stroke and (2) the association between ANS function assessed by HRV and functional outcomes in patients undergoing IVT.
METHODS
The data that support the findings of this study are available from Dr Zhen‐Ni Guo (zhen1ni2@jlu.edu.cn) or Dr Yi Yang (yang_yi@jlu.edu.cn) upon reasonable request.
Participants and the Study Protocol
This observational cohort study prospectively and consecutively recruited patients with acute ischemic stroke who were admitted to the Stroke Center at The First Hospital of Jilin University and received 0.9 mg/kg recombinant tissue plasminogen activator IVT therapy within 4.5 hours after stroke onset between September 2016 and August 2021 (IVT group). We excluded patients who (1) were <18 years of age; (2) were treated with combined endovascular and IVT therapy; (3) had a premorbid modified Rankin Scale score ≥2; (4) had a medical history of hyperthyroidism and anemia or were classified into the cardioembolic stroke category according to the TOAST (Trial of Org 10 172 in Acute Stroke Treatment) criteria, 13 which may undermine hemodynamic stability; and (5) were unable to complete all beat‐to‐beat recordings.
During the same period, patients who presented with acute ischemic stroke and who were admitted to the hospital within 3 days after stroke onset but did not receive IVT treatment or endovascular therapy were prospectively and consecutively enrolled in the non‐IVT group to obtain reference values. The exclusion criteria for the non‐IVT group were the same as those listed in 1, 3, 4, and 5 above for the IVT group.
Beat‐to‐beat recordings were obtained for each participant in the IVT and non‐IVT groups at 1 to 3 and 7 to 10 days after stroke onset. In addition, clinical outcomes were assessed using the modified Rankin Scale scores taken at 90 days after stroke from a structured telephone interview using a validated questionnaire. 14 , 15 A modified Rankin Scale score of <2 was defined as a favorable outcome, and a score of ≥2 was defined as an unfavorable outcome. This prospective observational study was registered (NCT05028868) and approved by the Ethics Committee of the First Hospital of Jilin University (2015‐156). Written informed consent was obtained from all participants, who had the right to withdraw from the study.
Data Collection
Demographic characteristics, risk factors, systolic and diastolic blood pressure and heart rate at admission, serum fasting glucose, stroke severity at admission measured using the National Institutes of Health Stroke Scale (NIHSS) score, onset to admission time, stroke subtypes classified according to the TOAST criteria, 13 and antihypertensive medication were documented in patients in the IVT and non‐IVT groups. Additionally, systolic and diastolic blood pressure, heart rate, and NIHSS scores were recorded at 1 to 3 and 7 to 10 days after stroke onset when beat‐to‐beat signals were measured. Blood pressure and heart rate were measured in the brachial artery using an automatic blood pressure monitor (Omron 711). Serum fasting glucose levels were measured the morning following admission after overnight fasting. The risk factors included cigarette smoking, alcohol consumption, hypertension, diabetes, dyslipidemia, and previous ischemic stroke. Cigarette smoking was defined as having smoked at least 1 cigarette per day for 1 year or more. 16 Alcohol consumption was defined as consuming 1 or more alcoholic drinks per day during the past year. 16 Hypertension was defined as self‐reported history of hypertension, taking oral antihypertension drugs, or receiving a clinical diagnosis of hypertension during hospitalization. 17 Diabetes was defined as having a history of diabetes, taking oral hypoglycemic agents or insulin, or receiving a clinical diagnosis of diabetes during hospitalization. 17 Dyslipidemia was defined as having a history of any type of dyslipidemia, taking oral antidyslipidemic drugs, or having at least 1 of the following findings during hospitalization: total cholesterol ≥5.18 mmol/L, triglycerides ≥1.70 mmol/L, low‐density lipoprotein cholesterol ≥3.37 mmol/L, and high‐density lipoprotein cholesterol <1.04 mmol/L. 17 , 18 In addition, the onset to recombinant tissue plasminogen activator bolus time and admission capillary blood glucose were collected for patients in the IVT group.
HRV Analysis
HRV measurements were used to assess ANS function in this study in accordance with international standards. 19 As previously reported, 20 beat‐to‐beat monitoring was measured noninvasively using a servo‐controlled plethysmograph (Finometer model 1; FMS, Rotterdam, the Netherlands) on the middle finger. All investigations were performed in a quiet examination room, with a controlled temperature ranging from 20 °C to 24 °C between 9:00 am and 10:00 am to avoid the impact of circadian rhythm on HRV, which may be a potential confounder. All participants were asked to relax in a supine position for 10 minutes in the room before the examination. Ectopic beats and artifacts were automatically detected, visually reviewed, and removed using linear interpolation. 21 Additionally, data involving ectopic beats occurring at a rate of >20% during HRV measurements were excluded. 22 The software processed beat‐to‐beat recordings and generated heart period patterns.
In the time domain, the square root of the mean of the sum of squares of the differences between adjacent normal‐to‐normal intervals (RMSSD) was analyzed. In the frequency domain, based on a fast Fourier transform, the Welch method was used to determine the power spectral density of the interval time series in the low‐frequency (LF; 0.04–0.15 Hz) and high‐frequency (HF; 0.15–0.40 Hz) ranges, as well as the total power (TP; <0.40 Hz). Physiologically and pathologically, TP was used to reflect the sum of sympathetic and parasympathetic activity, LF power was used to reflect sympathetic activity, and RMSSD and HF power were used to reflect the activation of parasympathetic cardiac regulation. 19 , 23 The RMSSD, TP, LF, and HF power components were log‐transformed to ensure a normal distribution of the parameters.
Statistical Analysis
All statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY), Stata 15.0 (StataCorp, College Station, TX), and MedCalc 19.5.6 (MedCalc Software, Ostend, Belgium). The distribution of data was assessed using a 1‐sample Kolmogorov‐Smirnov test. Normally continuous variables are expressed as mean value±SD, and the Student t test was used to compare independent samples. Nonnormally distributed variables are expressed as the median (interquartile range) and were compared using the Mann‐Whitney U test. Categorical variables were measured using frequencies, and the difference between the 2 groups was explored using the χ2 test or Fisher exact test. HRV indices measured 1 to 3 days and 7 to 10 days after stroke onset were compared using a paired‐sample t test.
Univariate and multivariable linear regression models were used to explore the effect of IVT on HRV. Residual analysis was used to evaluate the premise conditions for linear regression. The 4 models applied in the sensitivity analysis were as follows: (1) unadjusted; (2) adjusted for age and sex; (3) adjusted for age, sex, and vascular risk factors (including cigarette smoking, alcohol consumption, hypertension, diabetes, dyslipidemia, and previous ischemic stroke); and (4) adjusted for age, sex, vascular risk factors, and clinical data (including systolic and diastolic blood pressure and heart rate at admission, serum fasting glucose, NIHSS score at admission, onset to admission time, TOAST, and antihypertensive medication).
Univariate and multivariable logistic regression analyses were used to investigate the association between HRV and 3‐month clinical outcomes in patients undergoing IVT. The adjustments in the first 3 models were the same as those in linear regression. Considering the changes in vital signs and NIHSS scores along with patient conditions, systolic and diastolic blood pressure, heart rate, and NIHSS score during each period replaced the admission measures in the last model. Finally, the last model was adjusted for age, sex, vascular risk factors, and clinical data (including serum fasting glucose, onset to recombinant tissue plasminogen activator bolus time, TOAST, antihypertensive medication, and systolic and diastolic blood pressure, heart rate, and NIHSS score during each period).
To evaluate whether HRV would further increase the predictive value of conventional risk factors, 3 nomogram models were established in patients in the IVT group. Variables with P values <0.1 on univariate between‐group comparisons and those variables with established outcome‐predictive values according to the literature were eligible for inclusion in the multivariable analysis, and variables were selected using the backward elimination method. The model discrimination was measured by calculation of the area under the receiver operating characteristic curve (AUC‐ROC) and model fit was tested by the Hosmer‐Lemeshow χ2 test. To further assess model calibration, the calibration plot was undertaken for the measurement between observed and predicted probabilities. For internal validation of the predictive model, we performed 10‐fold cross‐validation. In addition, the clinical usefulness of the nomogram models was determined using decision curve analysis to quantify the net benefit. Additionally, we used AUC‐ROC, the integrated discrimination improvement, and net reclassification index to evaluate the incremental predictive value of HRV parameters beyond conventional risk factors. Comparison of the 2 ROC curves was based on the method of Delong et al. 24 All tests were 2‐tailed, and statistical significance was set at P<0.05.
RESULTS
Initially, 622 patients were screened, of whom 65 patients received endovascular therapy after IVT, 78 patients had a medical history of hyperthyroidism and anemia or were classified into the cardioembolism stroke category, and 13 patients were unable to complete all beat‐to‐beat recordings because of discharge or rejection. The final analysis included 466 patients with acute ischemic stroke, of whom 224 patients underwent IVT (IVT group: 48.1%), and 242 did not (non‐IVT group: 51.9%). Of the patients who did not receive IVT, 220 (90.9%) were not admitted to the hospital within the IVT time window. A further 22 patients had contraindications of IVT. In detail, 5 patients (2.1%) had ischemic stroke within 3 months, 4 patients (1.7%) had a history of intracranial hemorrhage, 2 patients (0.8%) had platelets <100 000/mm3, 3 patients (1.2%) had coagulopathy, 1 patient (0.4%) had severe head trauma within 3 months, 1 patient (0.4%) had intracranial surgery within 3 months, 1 patient (0.4%) had intra‐axial intracranial neoplasm, and 5 patients (2.1%) or their relatives refused to receive IVT. Table 1 shows a comparison of baseline characteristics between both groups. Patients in the IVT group had a higher proportion of dyslipidemia (IVT versus non‐IVT: 81.7% versus 74.0%, P=0.045) and a lower proportion of previous ischemic stroke history (IVT versus non‐IVT: 13.8% versus 25.2%, P=0.002). The systolic (IVT versus non‐IVT: median 157.00 versus 151.50 mm Hg, P=0.011) and diastolic blood pressure (IVT versus non‐IVT: median 92.00 versus 87.00 mm Hg, P=0.038) and NIHSS (IVT versus non‐IVT: median 6.00 versus 3.00, P<0.001) on admission were significantly higher in patients with IVT compared with those without IVT. Additionally, the TOAST distribution was significantly different between the 2 groups (P=0.003). There was a higher percentage of patients in the IVT group with large artery atherosclerosis (IVT versus non‐IVT: 37.5% versus 32.2%) and small artery occlusion (IVT versus non‐IVT: 51.3% versus 44.6%), and a lower percentage of patients with other determined causes or undetermined causes (IVT versus non‐IVT: 11.2% versus 23.1%). No differences were found in age, sex, or other vascular risk factors, including smoking, alcohol consumption, hypertension, diabetes, serum fasting glucose, heart rate, and NIHSS score on admission.
Table 1.
Comparison of Baseline Characteristics and HRV Parameters Between Patients With IVT and Without IVT
| Variables | IVT (n=224) | Non‐IVT (n=242) | χ2/t/Z | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 57.95±10.80 | 57.41±11.43 | −0.521 | 0.603 |
| Sex, men, n (%) | 182 (81.3%) | 210 (86.8%) | 2.660 | 0.103 |
| Vascular risk factors | ||||
| Cigarette smoking, n (%) | 125 (55.8%) | 134 (55.4%) | 0.009 | 0.925 |
| Alcohol consumption, n (%) | 109 (48.7%) | 112 (46.3%) | 0.264 | 0.607 |
| Hypertension, n (%) | 125 (55.8%) | 134 (55.4%) | 0.009 | 0.925 |
| Diabetes, n (%) | 53 (23.7%) | 56 (23.1%) | 0.018 | 0.895 |
| Dyslipidemia, n (%) | 183 (81.7%) | 179 (74.0%) | 4.009 | 0.045 |
| Previous ischemic stroke, n (%) | 31 (13.8%) | 61 (25.2%) | 9.487 | 0.002 |
| Clinical data | ||||
| Admission SBP, mm Hg | 157.00 (140.00–175.00) | 151.50 (135.00–168.00) | −2.534 | 0.011 |
| Admission DBP, mm Hg | 92.00 (81.00–102.00) | 87.00 (79.00–98.00) | −2.072 | 0.038 |
| Admission heart rate, bpm | 75.00 (66.00–85.00) | 75.00 (67.00–80.00) | −0.772 | 0.440 |
| Serum fasting glucose, mmol/L | 5.50 (4.80–7.13) | 5.39 (4.77–6.87) | −0.276 | 0.783 |
| Admission NIHSS score | 6.00 (4.00–10.00) | 3.00 (2.00–7.00) | −8.165 | <0.001 |
| Onset to admission time, h | 2.25 (1.52–2.91) | 8.17 (6.50–20.56) | −17.146 | <0.001 |
| TOAST | 11.628 | 0.003 | ||
| LAA | 84 (37.5%) | 78 (32.2%) | ||
| SAO | 115 (51.3%) | 108 (44.6%) | ||
| ODC or undetermined cause | 25 (11.2%) | 56 (23.1%) | ||
| Antihypertensive medication, n (%) | 99 (40.9%) | 67 (29.9%) | 6.136 | 0.013 |
| HRV variables | ||||
| 1–3 d | ||||
| SBP, mm Hg | 140.50 (121.00–161.00) | 141.00 (126.00–162.00) | −0.679 | 0.497 |
| DBP, mm Hg | 71.50 (62.00–87.00) | 74.00 (63.00–84.00) | −0.025 | 0.980 |
| Heart rate, bpm | 68.00 (61.00–76.00) | 68.00 (62.00–76.00) | −0.334 | 0.738 |
| NIHSS | 3.00 (1.00–6.75) | 3.00 (1.00–6.25) | −0.134 | 0.894 |
| RMSSD, ms, log | 1.47±0.32 | 1.36±0.23 | −4.210 | <0.001 |
| TP, ms2, log | 2.98±0.51 | 2.89±0.45 | −2.023 | 0.044 |
| LF power, ms2, log | 2.32±0.56 | 2.24±0.49 | −1.679 | 0.094 |
| HF power, ms2, log | 2.33±0.66 | 2.13±0.50 | −3.582 | <0.001 |
| 7–10 d | ||||
| SBP, mm Hg | 138.00 (118.50–153.00) | 140.00 (122.00–156.00) | 0.949 | 0.343 |
| DBP, mm Hg | 70.00 (61.00–82.00) | 70.50 (60.00–83.00) | −0.265 | 0.791 |
| Heart rate, beats/min | 68.00 (61.00–74.00) | 68.00 (62.00–75.00) | −1.023 | 0.306 |
| NIHSS | 3.00 (1.00–6.00) | 2.00 (1.00–5.00) | −1.183 | 0.237 |
| RMSSD, ms, log | 1.42±0.32 | 1.32±0.23 | −3.833 | <0.001 |
| TP, ms2, log | 2.87±0.57 | 2.76±0.42 | −2.425 | 0.016 |
| LF power, ms2, log | 2.18±0.61 | 2.06±0.46 | −2.432 | 0.015 |
| HF power, ms2, log | 2.21±0.70 | 2.03±0.52 | −3.232 | <0.001 |
Age, RMSSD, TP, LF, HF, and LF/HF are expressed as mean±SD and were analyzed using the Student t test. Admission SBP, admission DBP, admission heart rate, serum fasting glucose, admission NIHSS score, and onset to admission time are expressed as median (interquartile range) and were compared using the Mann‐Whitney U test. The other variables are expressed as n (%) and were analyzed using the χ2 test or Fisher exact test. DBP indicates diastolic blood pressure; HF, high frequency; HRV, heart rate variability; IVT, intravenous thrombolysis; LAA, large artery atherosclerosis; LF, low frequency; NIHSS, National Institutes of Health Stroke Scale; ODC, other determined cause; RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals; SAO, small artery occlusion; SBP, systolic blood pressure; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; and TP, total power.
Time Course of HRV
All HRV values, including RMSSD, TP, LF, and HF power, decreased significantly 7 to 10 days after stroke compared with those measured 1 to 3 days after stroke in the IVT and non‐IVT groups (Figure 1A). This phenomenon implies decreased ANS function in acute ischemic stroke within 7 to 10 days after stroke, regardless of IVT therapy.
Figure 1. Heart rate variability in patients with and without IVT.
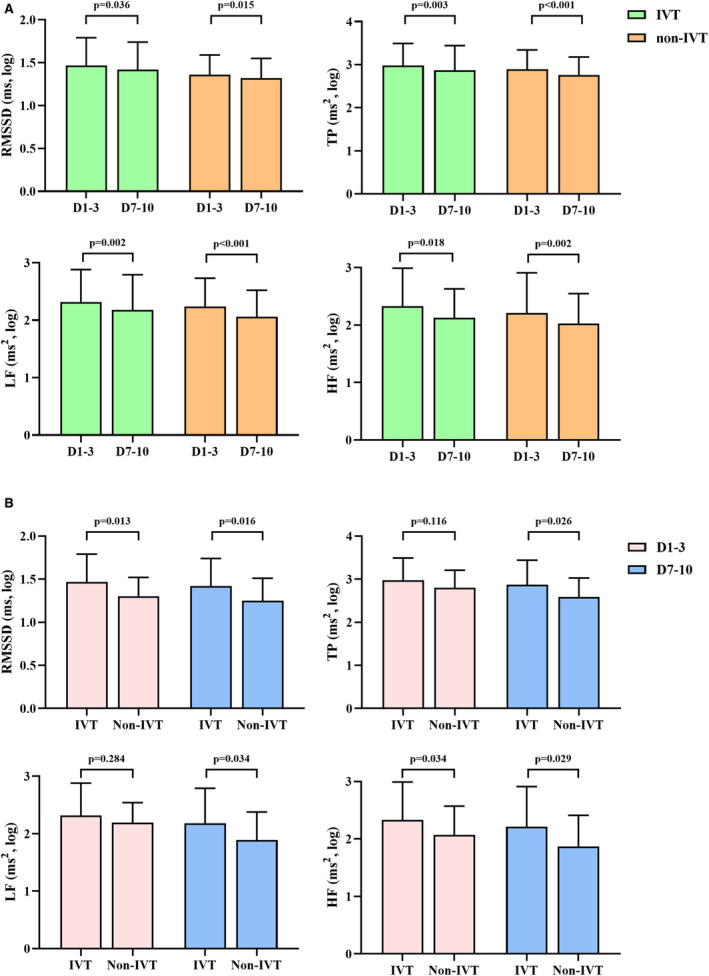
A, Time course of heart rate variability values in patients with IVT (n=224) and without IVT (n=242). B, Comparisons of heart rate variability in patients with IVT (n=224) and those without IVT who were admitted to the hospital within the IVT time window (n=22). HF indicates high frequency; IVT, intravenous thrombolysis; LF, low frequency; RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals; and TP, total power.
Impact of IVT on HRV
Table 1 shows the differences in HRV parameters between patients in the IVT and non‐IVT groups. Within 1 to 3 days after stroke onset, RMSSD, TP, and HF power were significantly higher in patients who underwent IVT than in those who did not; however, no increase was observed in LF power. This indicated that within 3 days after stroke onset, parasympathetic activity was significantly increased; however, the increase of sympathetic activity was not observed in patients who underwent IVT compared with those who did not. Within 7 to 10 days, all parameters were significantly increased in patients who underwent IVT compared with those who did not, suggesting that both sympathetic and parasympathetic activation were increased in patients who underwent IVT at 7 to 10 days.
To eliminate the impact of demographic information and vascular risk factors on HRV and ANS function, we further explored the correlation between IVT and HRV, using multivariable linear regression analysis. Table 2 shows that IVT was positively correlated with RMSSD and HF power after considering confounders within 3 days after stroke. When the measuring time was prolonged to 7 to 10 days after stroke, a positive correlation was observed between IVT and all HRV parameters, RMSSD, TP, LH, and HF.
Table 2.
Association Between Intravenous Thrombolysis and Heart Rate Variability Parameters
| Variables | Unadjusted | Adjusted age + sex | Adjusted vascular risk factors* | Adjusted stroke data† | ||||
|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | |
| 1–3 d | ||||||||
| RMSSD, ms, log | 0.107 | <0.001 | 0.110 | <0.001 | 0.108 | <0.001 | 0.128 | <0.001 |
| TP, ms2, log | 0.090 | 0.044 | 0.098 | 0.027 | 0.093 | 0.037 | 0.074 | 0.180 |
| LF power, ms2, log | 0.082 | 0.094 | 0.095 | 0.049 | 0.083 | 0.088 | 0.061 | 0.320 |
| HF power, ms2, log | 0.194 | <0.001 | 0.203 | <0.001 | 0.196 | <0.001 | 0.213 | 0.002 |
| 7–10 d | ||||||||
| RMSSD, ms, log | 0.099 | <0.001 | 0.100 | <0.001 | 0.096 | <0.001 | 0.088 | 0.008 |
| TP, ms2, log | 0.111 | 0.016 | 0.119 | 0.010 | 0.121 | 0.009 | 0.119 | 0.043 |
| LF power, ms2, log | 0.121 | 0.015 | 0.131 | 0.008 | 0.131 | 0.008 | 0.152 | 0.015 |
| HF power, ms2, log | 0.184 | <0.001 | 0.190 | <0.001 | 0.185 | <0.001 | 0.153 | 0.036 |
Univariate and multivariable linear regression analysis was used to explore the association between intravenous thrombolysis and heart rate variability parameters. HF indicates high frequency; LF, low frequency; RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; and TP, total power.
Adjusted for age, sex, and vascular risk factors (including cigarette smoking, alcohol consumption, hypertension, diabetes, dyslipidemia, and previous ischemic stroke).
Adjusted for age, sex, vascular risk factors, and clinical data (including admission systolic blood pressure, admission diastolic blood pressure, admission heart rate, serum fasting glucose, admission National Institutes of Health Stroke Scale score, onset to admission time, TOAST, and antihypertensive medication).
Furthermore, considering the influence of onset to admission time on IVT, we excluded patients who did not receive IVT because of exceeding the time window for IVT (n=220). No difference was found in terms of onset to admission time between patients with IVT (median [interquartile range]: 2.25 [1.52–2.91] hours) and those without IVT who were admitted to hospital within the time window for IVT (n=22, median [interquartile range]: 2.17 [1.46–3.37] hours, P=0.410). As shown in Figure 1B, within 1 to 3 days after stroke onset, RMSSD and HF power measured in patients with IVT were higher than those measured in patients without IVT who were admitted to the hospital within the time window for IVT; however, no difference was observed in TP and LF power. Within 7 to 10 days, all parameters were significantly higher in patients who underwent IVT compared with those who did not because of contraindication or rejection.
These results indicated that IVT was associated with increased parasympathetic activation within 3 days after stroke and was related to both increased sympathetic and parasympathetic activation 7 to 10 days after stroke. This suggests that IVT could improve ANS function in patients with acute ischemic stroke, and the time course for sympathetic and parasympathetic system function improvement was distinguished. In detail, this improvement mainly presented as enhanced parasympathetic functioning within 3 days after stroke and in both sympathetic and parasympathetic activity at 7 to 10 days after stroke.
Association Between HRV and Outcomes in Patients Who Underwent IVT
Among the 224 patients in the IVT group, 91 (40.6%) had favorable outcomes, and 133 (59.4%) had unfavorable outcomes. Table 3 compares patients' demographic characteristics and HRV parameters with different outcomes. Patients with unfavorable outcomes have a higher proportion of hypertension and higher systolic and diastolic blood pressures on admission than those with favorable outcomes.
Table 3.
Comparison of Demographic Characteristics and HRV Parameters Between Patients With Favorable and Unfavorable Outcomes in Patients With Intravenous Thrombolysis
| Variables | Favorable outcome (n=91) | Unfavorable outcome (n=133) | χ2/t/Z | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 56.85±12.51 | 58.71±9.43 | −1.268 | 0.206 |
| Sex, men, n (%) | 79 (86.8%) | 103 (77.4%) | 3.114 | 0.078 |
| Vascular risk factors | ||||
| Cigarette smoking, n (%) | 55 (60.4%) | 70 (52.6%) | 1.336 | 0.248 |
| Alcohol consumption, n (%) | 49 (53.8%) | 60 (45.1%) | 1.650 | 0.199 |
| Hypertension, n (%) | 43 (47.3%) | 82 (61.7%) | 4.544 | 0.033 |
| Diabetes, n (%) | 24 (26.4%) | 29 (21.8%) | 0.625 | 0.429 |
| Dyslipidemia, n (%) | 75 (82.4%) | 108 (81.2%) | 0.053 | 0.817 |
| Previous ischemic stroke, n (%) | 11 (12.1%) | 20 (15.0%) | 0.394 | 0.530 |
| Clinical data | ||||
| Admission SBP, mm Hg | 148.00 (131.00–171.00) | 163.00 (146.50–178.50) | −3.037 | 0.002 |
| Admission DBP, mm Hg | 86.00 (79.00–100.00) | 95.00 (83.00–104.00) | −2.796 | 0.005 |
| Admission heart rate, bpm | 74.00 (67.00–85.00) | 76.00 (65.50–85.00) | −0.051 | 0.959 |
| Admission capillary blood glucose, mmol/L | 7.20 (6.10–8.80) | 7.30 (6.30–9.70) | −1.029 | 0.304 |
| Serum fasting glucose, mmol/L | 5.26 (4.78–7.02) | 5.54 (4.80–7.24) | −1.070 | 0.285 |
| Admission NIHSS score | 5.00 (4.00–10.00) | 7.00 (4.00–10.00) | −1.896 | 0.058 |
| Onset to rt‐PA bolus time, min | 187.00 (151.00–218.00) | 187.00 (148.00–230.00) | −0.563 | 0.574 |
| TOAST | 0.415 | 0.813 | ||
| LAA | 32 (35.2%) | 52 (39.1%) | ||
| SAO | 49 (53.8%) | 66 (49.6%) | ||
| ODC or undetermined cause | 10 (11.0%) | 15 (11.3%) | ||
| Antihypertensive medication, n (%) | 22 (24.2%) | 45 (33.8%) | 2.404 | 0.121 |
| HRV parameters | ||||
| 1–3 d | ||||
| SBP, mm Hg | 134.00 (118.00–157.00) | 145.00 (125.50–166.00) | −2.299 | 0.022 |
| DBP, mm Hg | 71.00 (59.00–82.00) | 73.00 (63.00–90.50) | −2.012 | 0.044 |
| Heart rate, bpm | 65.00 (60.00–72.00) | 69.00 (63.50–78.00) | −2.601 | 0.009 |
| NIHSS | 2.00 (0.00–3.00) | 5.00 (3.00–8.00) | −7.081 | <0.001 |
| RMSSD, ms, log | 1.59±0.34 | 1.38±0.27 | 5.057 | <0.001 |
| TP, ms2, log | 3.16±0.50 | 2.86±0.48 | 4.405 | <0.001 |
| LF power, ms2, log | 2.45±0.55 | 2.23±0.55 | 3.035 | 0.003 |
| HF power, ms2, log | 2.59±0.69 | 2.15±0.58 | 5.127 | <0.001 |
| 7–10 d | ||||
| SBP, mm Hg | 139.00 (118.00–150.00) | 138.00 (120.50–157.50) | −0.844 | 0.339 |
| DBP, mm Hg | 70.00 (60.00–80.00) | 70.00 (61.00–85.00) | −0.664 | 0.507 |
| Heart rate, bpm | 67.00 (59.00–73.00) | 68.00 (62.50–74.00) | −1.381 | 0.167 |
| NIHSS | 1.00 (0.00–2.00) | 4.00 (2.00–7.00) | −7.334 | <0.001 |
| RMSSD, ms, log | 1.48±0.35* | 1.38±0.30 | 2.252 | 0.025 |
| TP, ms2, log | 2.98±0.59* | 2.79±0.54 | 2.494 | 0.013 |
| LF power, ms2, log | 2.31±0.64* | 2.10±0.57* | 2.567 | 0.011 |
| HF power, ms2, log | 2.36±0.74* | 2.11±0.66 | 2.643 | 0.009 |
DBP indicates diastolic blood pressure; HF, high frequency; HRV, heart rate variability; LAA, large artery atherosclerosis; LF, low frequency; NIHSS, National Institutes of Health Stroke Scale; ODC, other determined cause; RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals; rt‐PA, recombinant tissue plasminogen activator; SAO, small artery occlusion; SBP, systolic blood pressure; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; and TP, total power.
P<0.05 compared with that measured 1 to 3 d after stroke onset. Age, RMSSD, TP, LF, HF, and LF/HF are expressed as mean±SD and were analyzed using the Student t test. Admission SBP, admission DBP, admission heart rate, admission capillary blood glucose, admission NIHSS score, and onset to rt‐PA bolus time are expressed as median (interquartile range) and were compared using the Mann‐Whitney U test. The other variables are expressed as n (%) and were analyzed using the χ2 test or Fisher exact test. HRV indices measured 1 to 3 days and 7 to 10 days after stroke onset were compared using a paired‐sample t test.
For HRV parameters, RMSSD, TP, LF power, and HF power obtained within 1 to 3 days and 7 to 10 days after stroke in patients with unfavorable outcomes were all significantly lower than those in patients with favorable outcomes (all P<0.05). After adjusting for major covariates, lower HRV values at both 1 to 3 days and 7 to 10 days after stroke were independently associated with the 3‐month outcomes, indicating that in patients with IVT, HRV and ANS function after stroke were reliable predictors of 3‐month clinical outcomes and could be regarded as intervention targets to improve patient outcomes (Table 4).
Table 4.
The Association Between Heart Rate Variability Parameters and 3‐Month Unfavorable Outcome in Patients With Intravenous Thrombolysis
| Variables | Unadjusted | Adjusted age+sex | Adjusted vascular risk factors* | Adjusted stroke data† | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| 1–3 d | ||||||||
| RMSSD, ms, log | 0.106 (0.040–0.280) | <0.001 | 0.097 (0.036–0.259) | <0.001 | 0.087 (0.032–0.241) | <0.001 | 0.008 (0.001–0.047) | <0.001 |
| TP, ms2, log | 0.293 (0.162–0.530) | <0.001 | 0.310 (0.171–0.560) | <0.001 | 0.271 (0.145–0.505) | <0.001 | 0.080 (0.028–0.223) | <0.001 |
| LF power, ms2, log | 0.474 (0.287–0.783) | 0.004 | 0.510 (0.307–0.848) | 0.009 | 0.487 (0.288–0.822) | 0.007 | 0.301 (0.147–0.616) | <0.001 |
| HF power, ms2, log | 0.335 (0.210–0.534) | <0.001 | 0.334 (0.209–0.534) | <0.001 | 0.320 (0.197–0.519) | <0.001 | 0.113 (0.050–0.254) | <0.001 |
| 7–10 d | ||||||||
| RMSSD, ms, log | 0.388 (0.167–0.903) | 0.028 | 0.339 (0.142–0.806) | 0.014 | 0.335 (0.138–0.815) | 0.016 | 0.262 (0.080–0.854) | 0.026 |
| TP, ms2, log | 0.545 (0.333–0.890) | 0.015 | 0.551 (0.337–0.902) | 0.018 | 0.503 (0.302–0.839) | 0.008 | 0.382 (0.186–0.782) | 0.008 |
| LF power, ms2, log | 0.561 (0.356–0.883) | 0.012 | 0.579 (0.367–0.912) | 0.019 | 0.525 (0.327–0.841) | 0.007 | 0.360 (0.187–0.696) | 0.002 |
| HF power, ms2, log | 0.596 (0.401–0.886) | 0.010 | 0.571 (0.382–0.853) | 0.006 | 0.558 (0.368–0.844) | 0.006 | 0.457 (0.256–0.815) | 0.008 |
Univariate and multivariable logistic analysis were used to explore the association between HRV parameters and 3‐month unfavorable outcomes. HF indicates high frequency; LF, low frequency; OR, odds ratio; RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; and TP, total power.
Adjusted for age, sex, and vascular risk factors (including cigarette smoking, alcohol consumption, hypertension, diabetes, dyslipidemia, and previous ischemic stroke).
Adjusted for age, sex, vascular risk factors, and clinical data (including serum fasting glucose, onset to recombinant tissue plasminogen activator bolus time, TOAST, and antihypertensive medication, and systolic blood pressure, diastolic blood pressure, heart rate, and National Institutes of Health Stroke Scale score during each period).
Incremental Predictive Value of HRV in Patients Who Underwent IVT
Variables whose P value was <0.10 in univariate analyses (as shown in Table 3) and those variables with established outcome‐predictive values according to the literature (age, cigarette smoking, previous ischemic stroke, serum fasting glucose, onset to recombinant tissue plasminogen activator bolus time, and TOAST) were further selected using the backward elimination method. Finally, Model 1 included age, previous ischemic stroke, and NIHSS at 1 to 3 days after onset. Model 2 included Model 1 plus RMSSD at 1 to 3 days after onset. Model 3 included Model 2 plus NIHSS and RMSSD at 7 to 10 days after stroke. The 3 nomogram models are shown in Figure 2. The AUC‐ROC was similar in full and test models, which suggested good stability of the models. The calibration plot suggested good predictive accuracy of the nomograms. Hosmer‐Lemeshow tests showed all P values >0.05, indicating there is no statistical difference between the observed and model‐predicted probability of unfavorable outcome (Table 5).
Figure 2. Nomogram models for predicting 3‐month unfavorable outcome.
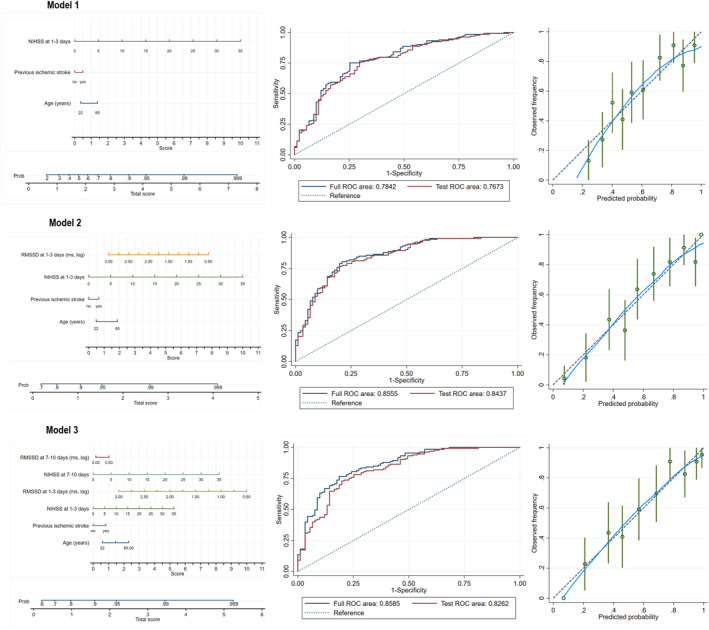
Nomograms (left), ROCs (middle), and calibration plots (right) of Model 1, Model 2, and Model 3. Model 1 included age, previous ischemic stroke, and NIHSS at 1 to 3 days after stroke. Model 2 included Model 1 plus RMSSD at 1 to 3 days after stroke. Model 3 included Model 2 plus NIHSS and RMSSD at 7 to 10 days after stroke. Each nomogram was composed of 2 areas; the upper area was designed to calculate the total score as the sum of scores for the model. In detail, a score was assigned for each variable by drawing a line upward from the corresponding values to the score line. The total score was calculated as the sum of the individual scores of each variable. The lower area was designed to get the probability (Prob) of patients to get an unfavorable functional outcome. ROCs were used to measure the discrimination of each model. The full ROC area was calculated using data from all patients in the IVT group; test ROC area was performed for 10‐fold cross‐validation. The areas under the ROC were similar in full and test models, which suggested good stability of the models. Calibration plots were used to assess model calibration, and were undertaken for the measurement between observed (y axis) and predicted probabilities (x axis). The blue line represents the performance of each nomogram model, with 95% CIs measured by Hosmer‐Lemeshow analysis (the green vertical line); the dashed line represents the reference line where an ideal nomogram would lie. The calibration plot of Model 2 and Model 3 suggested good predictive accuracy of the nomograms. NIHSS indicates National Institutes of Health Stroke Scale; RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals; and ROC, receiver operating characteristic.
Table 5.
Reclassification and Discrimination Statistics for 3‐Month Outcome After Intravenous Thrombolysis by Heart Rate Variability
| AUC‐ROC | H‐M | NRI | IDI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | χ2 | P value | Estimate (95% CI) | Z | P value | Estimate (95% CI) | Z | P value | |
| Model 1 | 0.784 (0.723–0.846) | 10.682 | 0.220 | Reference | Reference | ||||
| Model 2 | 0.855 (0.805–0.906) | 10.756 | 0.216 | 62.12 (35.46–88.78) | 4.566 | <0.001 | 14.54 (9.54–19.54) | 5.694 | <0.001 |
| Model 3 | 0.859 (0.809–0.908) | 12.587 | 0.127 | 74.03 (47.37–100.69) | 5.442 | <0.001 | 17.71 (12.71–22.71) | 6.548 | <0.001 |
Model 1 included age, previous ischemic stroke, and NIHSS at 1 to 3 d after stroke. Model 2 included model 1 plus RMSSD at 1 to 3 d after stroke. Model 3 included Model 2 plus NIHSS and RMSSD at 7 to 10 d after stroke. AUC‐ROC indicates the area under the receiver operating characteristic curve; H‐M, Hosmer‐Lemeshow χ2 test; IDI, integrated discrimination improvement; NIHSS, National Institutes of Health Stroke Scale; NRI, net reclassification index; and RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals.
For Model 2, the AUC‐ROC significantly improved with the addition of RMSSD measured 1 to 3 days after stroke onset (from 0.784 [0.723–0.846] to 0.855 [0.805–0.906], P=0.002). Moreover, the risk reclassification and discriminatory power appeared to be substantially better (net reclassification index 62.12%, P<0.001; integrated discrimination improvement 14.54%, P<0.001). Similar incremental predictive values were found in Model 3 (AUC‐ROC from 0.784 [0.723–0.846] to 0.859 [0.809–0.908], P=0.001; net reclassification index 74.03%, P<0.001; integrated discrimination improvement 17.71%, P<0.001) (Table 5). In decision curve analysis, the higher the curve, the higher the net benefit is across a given range of threshold probabilities. The results indicated that Model 2 and Model 3 showed a better clinical net benefit than Model 1 over threshold probabilities of 0.2, suggesting the net benefit gained by adding the HRV values (Model 2 and Model 3) was greater than that in Model 1 (Figure 3).
Figure 3. Decision curve analysis of the nomogram models.
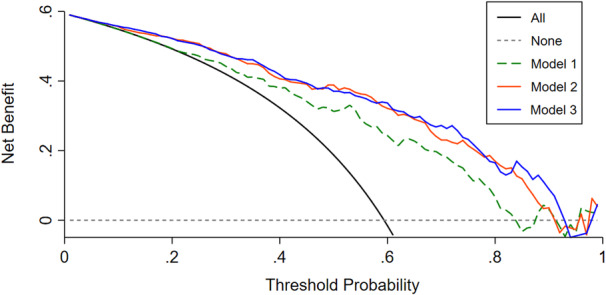
The x axis represents thresholds for risk of unfavorable outcomes, and the y axis represents net benefits hinged on different thresholds. The higher the curve, the higher the net benefit is across a given range of threshold probabilities. The black line assures that all patients develop an unfavorable outcome, whereas the gray dotted line assures that no patients develop an unfavorable outcome. The other 3 lines display the net benefit of the prediction of the 3 models, respectively. Models 2 and 3 showed a better clinical net benefit compared with Model 1 over threshold probabilities of 0.2, and the net benefit gained by adding the HRV values (Model 2 and Model 3) was greater than that in Model 1. Model 1 included age, previous ischemic stroke, and NIHSS at 1 to 3 days after stroke. Model 2 included Model 1 plus RMSSD at 1 to 3 days after stroke. Model 3 included Model 2 plus NIHSS and RMSSD at 7 to 10 days after stroke. NIHSS indicates National Institutes of Health Stroke Scale; and RMSSD, square root of the mean of the sum of the squares of differences between adjacent normal‐to‐normal intervals.
When Model 2 and Model 3 were compared, no difference was found in terms of AUC‐ROC (P=0.709) and integrated discrimination improvement (P=0.053). However, the net reclassification index was significantly increased in Model 3 compared with Model 2 (47.4%, P<0.001).
DISCUSSION
The first major finding of this study was the positive effect of IVT on ANS function assessed using HRV in patients with acute ischemic stroke. Within 3 days after stroke, improvements were primarily observed in parasympathetic activity, and at 7 to 10 days after stroke, both sympathetic and parasympathetic activity could be improved by IVT. The second major finding was that lower HRV values at 1 to 3 and 7 to 10 days after stroke were independently associated with unfavorable outcomes. Furthermore, addition of HRV parameters to conventional risk factors significantly improved the predictive ability of 3‐month unfavorable outcomes. This indicated that for inpatients who underwent IVT, HRV and ANS function after stroke were reliable predictors of 3‐month clinical outcomes and could be regarded as intervention targets to improve patient outcomes.
To the best of our knowledge, this is the first study to explore the effect of IVT on ANS function assessed by HRV in patients with acute ischemic stroke. However, a positive effect of thrombolytic therapy on cardiac autonomic tone was demonstrated earlier in patients with acute myocardial infarction. 25 , 26 , 27 Furthermore, Zabel et al reported that patients with myocardial infarction in the successful reperfusion group had preserved autonomic tone compared with those who failed reperfusion, indicating that thrombolysis‐induced reperfusion of the cardiac artery may contribute to autonomic function improvement. 27 Similarly, we considered that this improvement in patients with stroke after IVT may also be caused by partial or full recanalization of cerebrovascular tissue, during which the brain–heart axis may be the essential connector. 28 Sympathetic and parasympathetic regulation theories have been widely accepted as mechanisms of brain–heart interaction after stroke. 29 Theoretically, sympathetic and parasympathetic outflow of the ANS is controlled by the central nervous system and regulates cardiac function. Ischemic brain lesions can cause cardiac ANS dysregulation and decrease HRV. 30 Hyperacute recanalization of ischemic lesions recovers central nervous system control of the ANS and improves cardiac regulation of the ANS to some extent. 30 Our results also demonstrate that IVT is beneficial for the improvement of sympathetic and parasympathetic activity within a specific time frame. Within 3 days after stroke, ANS improvement mainly presented as enhanced parasympathetic function, and at 7 to 10 days after stroke, both sympathetic and parasympathetic activity could be increased by IVT. The mechanism underlying different times for improving sympathetic and parasympathetic system activity after IVT remain unclear. After stroke onset, the activity of both sympathetic and parasympathetic systems was decreased. Simultaneously, the balance of the sympathetic and parasympathetic system was upset, manifesting as a relative excitation of the sympathetic nervous system. 31 , 32 Interestingly, previous studies reported that thrombolytic therapy promoted the recovery of the cardiac autonomic imbalance in patients with acute myocardial infarction, 33 which suggested thrombolytic therapy may have an earlier and more significant effect on parasympathetic improvement. Similarly, in patients with stroke, we considered that IVT can also balance ANS function by improving parasympathetic activity earlier. It should be noticed that patients in the IVT group had higher admission NIHSS scores than patients in the non‐IVT group. According to a previous study, patients with higher admission NIHSS scores may have more severe autonomic dysregulation. 34 Therefore, in our study, patients in the IVT group may also have more severe ANS activity decline than patients in the non‐IVT group. At 1 to 3 days and 7 to 10 days after stroke onset, patients in the IVT group presented increased ANS activity than patients in the non‐IVT group, suggesting ANS activity was significantly improved by IVT.
Previous studies have concluded that abnormal HRV and autonomic function are associated with poor prognosis in patients with ischemic stroke. 35 , 36 , 37 Our study further indicated that this association applies to patients with ischemic stroke after thrombolytic therapy. Additionally, we established 2 HRV‐based nomogram models, Model 2 and Model 3, to evaluate whether adding HRV parameters would further increase the predictive ability of 3‐month unfavorable outcomes in patients who received IVT. We verified a substantially better discrimination power according to a significant increase in the AUC‐ROC, net reclassification index, and integrated discrimination improvement in these 2 models. However, in the decision curve analysis, the net benefit of using Model 2 or 3 over Model 1 is limited. For example, at the risk threshold of 0.2 in Models 2 and 3, the estimated net benefit is around 55%, whereas in Model 1 it is around 50%. Meaning only about 5% of patients with acute ischemic stroke who received IVT would actually benefit from implementing HRV monitoring at 1 to 3 and 7 to 10 days after stroke in everyday clinical settings. Although this might not appear to be a significant proportion, due to the large population base receiving IVT, the application of this model has the potential to benefit tens of thousands of patients, with high economic and social value. Therefore, HRV and ANS function measured at 1 to 3 days and 7 to 10 days after stroke could be considered reliable predictors of 3‐month clinical outcomes and intervention targets to improve outcomes. However, the pathophysiological mechanism underlying the relationship between autonomic function and stroke outcomes has not been fully elucidated. First, as mentioned above, ischemic brain lesions can increase the risk of arrhythmia, leading to unstable cerebral blood flow, which may enhance ischemia or cause hemorrhagic transformation and worsen clinical outcomes. 38 Second, because the sympathetic and parasympathetic nerves also modulate the inflammatory reflex, 39 poststroke autonomic dysfunction may promote the production of proinflammatory cytokines associated with secondary brain injury. In addition, cardiovascular complications, hyperglycemia, and blood–brain barrier dysfunction caused by autonomic dysfunction are also believed to influence outcomes. 40
Our study has several limitations. First, because the present study was conducted in patients during hospitalization, HRV and ANS changes after discharge (>10 days after stroke) remain unknown. A longitudinal study of multiple time point measurements could be valuable for depicting the profile of autonomic function. Second, this was an observational study in a single stroke center; thus, causal inferences cannot be made, and additional multicohort studies are needed to confirm the present study's results. Third, the stroke severity assessed by NIHSS scores at admission were significantly different between patients in the IVT and non‐IVT group, indicating baseline ANS activity may also differ between the 2 groups. 34 However, to enable patients to receive IVT as early as possible, beat‐to‐beat recordings before IVT were unavailable, and the admission HRV values and ANS function condition remain unclear. Further studies are needed to verify our conclusion. Finally, the onset to admission time between patients in the IVT and non‐IVT group differed. However, in the present clinical practice, most of the patients receive IVT if they are admitted to the hospital within the time window, because IVT is the highest recommended therapy for patients with acute ischemic stroke. It may be difficult to recruit patients who do not receive IVT but are admitted to the hospital within the time window; hence, further animal studies are needed to resolve this issue.
CONCLUSIONS
IVT positively affected HRV and autonomic nervous activity, and ANS function assessed by HRV in the acute phase of ischemic stroke was independently associated with unfavorable outcomes in patients undergoing IVT. Furthermore, HRV and ANS function in the acute phase of stroke could be considered reliable predictors of clinical outcomes and intervention targets to improve outcomes in these patients.
Sources of Funding
This project was supported by the National Natural Science Foundation of China (grant number 81971105), the Science and Technology Department of Jilin Province (YDZJ202201ZYTS677) to Dr Guo, and the Science and Technology Department of Jilin Province (YDZJ202302CXJD061), the Jilin Provincial Key Laboratory (YDZJ202302CXJD017) to Dr Yang.
Disclosures
None.
This article was sent to Neel S. Singhal, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Yi Yang, Email: zhen1ni2@jlu.edu.cn, Email: yang_yi@jlu.edu.cn.
Zhen‐Ni Guo, Email: zhen1ni2@jlu.edu.cn.
References
- 1. Collaborators GBDS . Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165260. doi: 10.1016/j.bbadis.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding Q, Liu S, Yao Y, Liu H, Cai T, Han L. Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology. 2022;98:e279–e290. doi: 10.1212/WNL.0000000000013115 [DOI] [PubMed] [Google Scholar]
- 5. Ji X. Forward thinking in stroke treatment: advances in cerebrovascular reperfusion and neurorehabilitation. Brain Circ. 2015;1:1–2. doi: 10.4103/2394-8108.166347 [DOI] [Google Scholar]
- 6. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Ronning OM, Thommessen B, Amthor KF, Ihle‐Hansen H, Kurz M, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (nor‐test): a phase 3, randomised, open‐label, blinded endpoint trial. Lancet Neurol. 2017;16:781–788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 7. Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6:1239–1278. doi: 10.1002/cphy.c150037 [DOI] [PubMed] [Google Scholar]
- 8. Soros P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012;11:179–188. doi: 10.1016/S1474-4422(11)70291-5 [DOI] [PubMed] [Google Scholar]
- 9. Cechetto DF, Wilson JX, Smith KE, Wolski D, Silver MD, Hachinski VC. Autonomic and myocardial changes in middle cerebral artery occlusion: stroke models in the rat. Brain Res. 1989;502:296–305. doi: 10.1016/0006-8993(89)90625-2 [DOI] [PubMed] [Google Scholar]
- 10. Hachinski VC, Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF. Asymmetry of sympathetic consequences of experimental stroke. Arch Neurol. 1992;49:697–702. doi: 10.1001/archneur.1992.00530310039010 [DOI] [PubMed] [Google Scholar]
- 11. Mo J, Huang L, Peng J, Ocak U, Zhang J, Zhang JH. Autonomic disturbances in acute cerebrovascular disease. Neurosci Bull. 2019;35:133–144. doi: 10.1007/s12264-018-0299-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther. 2020;24:91–102. doi: 10.1016/j.bjpt.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 14. Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.STR.0000027437.22450.BD [DOI] [PubMed] [Google Scholar]
- 15. Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified Rankin scale across multiple raters: benefits of a structured interview. Stroke. 2005;36:777–781. doi: 10.1161/01.STR.0000157596.13234.95 [DOI] [PubMed] [Google Scholar]
- 16. Zhong C, Lv L, Liu C, Zhao L, Zhou M, Sun W, Xu T, Tong W. High homocysteine and blood pressure related to poor outcome of acute ischemia stroke in chinese population. PLoS One. 2014;9:e107498. doi: 10.1371/journal.pone.0107498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, Zhou Y, Zhao X, Wang C, Liu L, et al. The China national stroke registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6:355–361. doi: 10.1111/j.1747-4949.2011.00584.x [DOI] [PubMed] [Google Scholar]
- 18. Zhang FL, Xing YQ, Wu YH, Liu HY, Luo Y, Sun MS, Guo ZN, Yang Y. The prevalence, awareness, treatment, and control of dyslipidemia in Northeast China: a population‐based cross‐sectional survey. Lipids Health Dis. 2017;16:61. doi: 10.1186/s12944-017-0453-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heart rate variability: Standards of measurement, physiological interpretation and clinical use . Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 20. Qu Y, Liu J, Guo ZN, Zhang PD, Yan XL, Zhang P, Qi S, Yang Y. The impact of remote ischaemic conditioning on beat‐to‐beat heart rate variability circadian rhythm in healthy adults. Heart Lung Circ. 2021;30:531–539. doi: 10.1016/j.hlc.2020.08.017 [DOI] [PubMed] [Google Scholar]
- 21. Webb AJS, Mazzucco S, Li L, Rothwell PM. Prognostic significance of blood pressure variability on beat‐to‐beat monitoring after transient ischemic attack and stroke. Stroke. 2018;49:62–67. doi: 10.1161/STROKEAHA.117.019107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bodapati RK, Kizer JR, Kop WJ, Kamel H, Stein PK. Addition of 24‐hour heart rate variability parameters to the cardiovascular health study stroke risk score and prediction of incident stroke: the cardiovascular health study. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.004305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kox M, Vrouwenvelder MQ, Pompe JC, van der Hoeven JG, Pickkers P, Hoedemaekers CW. The effects of brain injury on heart rate variability and the innate immune response in critically ill patients. J Neurotrauma. 2012;29:747–755. doi: 10.1089/neu.2011.2035 [DOI] [PubMed] [Google Scholar]
- 24. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 25. Chen CK, Liou YM, Lee WL, Liu JR, Cheng HJ, Yang DY, Hu WH, Ting CT. The effect of thrombolytic therapy on short‐ and long‐term cardiac autonomic activity in patients with acute myocardial infarction. Zhonghua Yi Xue Za Zhi (Taipei). 1996;58:392–399. [PubMed] [Google Scholar]
- 26. Kelly PA, Nolan J, Wilson JI, Perrins EJ. Preservation of autonomic function following successful reperfusion with streptokinase within 12 hours of the onset of acute myocardial infarction. Am J Cardiol. 1997;79:203–205. doi: 10.1016/S0002-9149(96)00715-1 [DOI] [PubMed] [Google Scholar]
- 27. Zabel M, Klingenheben T, Hohnloser SH. Changes in autonomic tone following thrombolytic therapy for acute myocardial infarction: assessment by analysis of heart rate variability. J Cardiovasc Electrophysiol. 1994;5:211–218. doi: 10.1111/j.1540-8167.1994.tb01158.x [DOI] [PubMed] [Google Scholar]
- 28. Manea MM, Comsa M, Minca A, Dragos D, Popa C. Brain‐heart axis—review article. J Med Life. 2015;8:266–271. [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain‐heart interaction: cardiac complications after stroke. Circ Res. 2017;121:451–468. doi: 10.1161/CIRCRESAHA.117.311170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tahsili‐Fahadan P, Geocadin RG. Heart‐brain axis: effects of neurologic injury on cardiovascular function. Circ Res. 2017;120:559–572. doi: 10.1161/CIRCRESAHA.116.308446 [DOI] [PubMed] [Google Scholar]
- 31. Korpelainen JT, Sotaniemi KA, Makikallio A, Huikuri HV, Myllyla VV. Dynamic behavior of heart rate in ischemic stroke. Stroke. 1999;30:1008–1013. doi: 10.1161/01.STR.30.5.1008 [DOI] [PubMed] [Google Scholar]
- 32. Chen CF, Lin HF, Lin RT, Yang YH, Lai CL. Relationship between ischemic stroke location and autonomic cardiac function. J Clin Neurosci. 2013;20:406–409. doi: 10.1016/j.jocn.2012.02.047 [DOI] [PubMed] [Google Scholar]
- 33. Lotze U, Ozbek C, Gerk U, Kaufmann H, Heisel A, Bay W, Figulla HR. Early time course of heart rate variability after thrombolytic and delayed interventional therapy for acute myocardial infarction. Cardiology. 1999;92:256–263. doi: 10.1159/000006983 [DOI] [PubMed] [Google Scholar]
- 34. Hilz MJ, Moeller S, Akhundova A, Marthol H, Pauli E, De Fina P, Schwab S. High NIHSS values predict impairment of cardiovascular autonomic control. Stroke. 2011;42:1528–1533. doi: 10.1161/STROKEAHA.110.607721 [DOI] [PubMed] [Google Scholar]
- 35. Zhao M, Guan L, Wang Y. The association of autonomic nervous system function with ischemic stroke, and treatment strategies. Front Neurol. 2019;10:1411. doi: 10.3389/fneur.2019.01411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cai K, Ni Y, Zhang Y, Shen L, Ji Q, Cao M. Heart rate variability after endovascular coiling is associated with short‐term outcomes in patients with subarachnoid hemorrhage. Neurol Res. 2018;40:856–861. doi: 10.1080/01616412.2018.1493973 [DOI] [PubMed] [Google Scholar]
- 37. Tang SC, Jen HI, Lin YH, Hung CS, Jou WJ, Huang PW, Shieh JS, Ho YL, Lai DM, Wu AY, et al. Complexity of heart rate variability predicts outcome in intensive care unit admitted patients with acute stroke. J Neurol Neurosurg Psychiatry. 2015;86:95–100. doi: 10.1136/jnnp-2014-308389 [DOI] [PubMed] [Google Scholar]
- 38. Castro P, Azevedo E, Sorond F. Cerebral autoregulation in stroke. Curr Atheroscler Rep. 2018;20:37. doi: 10.1007/s11883-018-0739-5 [DOI] [PubMed] [Google Scholar]
- 39. Pereira MR, Leite PE. The involvement of parasympathetic and sympathetic nerve in the inflammatory reflex. J Cell Physiol. 2016;231:1862–1869. doi: 10.1002/jcp.25307 [DOI] [PubMed] [Google Scholar]
- 40. Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke. 2015;10:796–800. doi: 10.1111/ijs.12573 [DOI] [PubMed] [Google Scholar]


