Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) and heart failure with preserved ejection fraction (HFpEF) share common risk factors, including obesity and diabetes. They are also thought to be mechanistically linked. The aim of this study was to define serum metabolites associated with HFpEF in a cohort of patients with biopsy‐proven NAFLD to identify common mechanisms.
Methods and Results
We performed a retrospective, single‐center study of 89 adult patients with biopsy‐proven NAFLD who had transthoracic echocardiography performed for any indication. Metabolomic analysis was performed on serum using ultrahigh performance liquid and gas chromatography/tandem mass spectrometry. HFpEF was defined as ejection fraction >50% plus at least 1 echocardiographic feature of HFpEF (diastolic dysfunction, abnormal left atrial size) and at least 1 heart failure sign or symptom. We performed generalized linear models to evaluate associations between individual metabolites, NAFLD, and HFpEF. Thirty‐seven out of 89 (41.6%) patients met criteria for HFpEF. A total of 1151 metabolites were detected; 656 were analyzed after exclusion of unnamed metabolites and those with >30% missing values. Fifty‐three metabolites were associated with the presence of HFpEF with unadjusted P value <0.05; none met statistical significance after adjustment for multiple comparisons. The majority (39/53, 73.6%) were lipid metabolites, and levels were generally increased. Two cysteine metabolites (cysteine s‐sulfate and s‐methylcysteine) were present at significantly lower levels in patients with HFpEF.
Conclusions
We identified serum metabolites associated with HFpEF in patients with biopsy‐proven NAFLD, with increased levels of multiple lipid metabolites. Lipid metabolism could be an important pathway linking HFpEF to NAFLD.
Keywords: heart failure, metabolic syndrome, metabolomics, nonalcoholic fatty liver disease
Subject Categories: Heart Failure, Biomarkers, Metabolism
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TTE
transthoracic echocardiogram
Clinical Perspective.
What Is New?
Serum metabolites including lipid and amino acid metabolites are associated with the presence of heart failure with preserved ejection fraction in patients with biopsy‐proven nonalcoholic fatty liver disease.
What Are the Clinical Implications?
Although nonalcoholic fatty liver disease and heart failure with preserved ejection fraction are known to share risk factors, the pathogenic mechanisms linking the 2 diseases are unknown.
Our work points toward lipid metabolism as a potentially significant pathway.
Nonalcoholic fatty liver disease (NAFLD), which is estimated to affect one‐quarter of the world's population and the prevalence of which is projected to increase, is strongly linked to cardiovascular disease. 1 , 2 , 3 Common risk factors include diabetes, hyperlipidemia, and obesity. Not surprising, therefore, is the fact that cardiovascular disease and complications of cirrhosis represent the top causes of mortality in patients with NAFLD. 1 , 4 The link between NAFLD and coronary artery disease and related events such as myocardial infarction is well established. 5 Further, advanced fibrosis in NAFLD is independently associated with multiple forms of incident cardiovascular disease, including coronary artery disease, peripheral vascular disease, and congestive heart failure. 6
Interestingly, NAFLD appears to be more strongly associated with heart failure with preserved ejection fraction (HFpEF) than with heart failure with reduced ejection fraction. 7 The association between NAFLD and HFpEF has been explored, but it remains poorly understood. 8 , 9 Patients with HFpEF have signs or symptoms of heart failure despite normal ejection fraction. 10 Up to half of patients with HFpEF are thought to have NAFLD, 11 and among those with both HFpEF and NAFLD, advanced fibrosis may be present in up to 37.5%. 12 The prevalence of HFpEF in patients with NAFLD is less clear. As with other forms of cardiovascular disease, NAFLD and HFpEF share multiple risk factors, but the relationship between the 2 is complex. Large cohort studies have identified an independent association between NAFLD and echocardiographic features of HFpEF, including myocardial remodeling and diastolic dysfunction. 13 The longitudinal CARDIA (Coronary Artery Risk Development in Young Adults) study found that NAFLD was associated with left ventricular hypertrophy, but the association did not persist after adjustment for obesity. 14 Liver fibrosis stage is a strong predictor of overall clinical outcomes, liver‐related decompensation, and cardiovascular events in NAFLD, and may have prognostic significance in HFpEF, suggesting shared pathways of progression. 15 , 16 , 17 , 18 Elucidating common mechanisms between NAFLD and HFpEF, particularly through noninvasive means, would have important diagnostic and therapeutic implications. Metabolomics is the analysis of small molecules (metabolites) in body fluids or tissues. Metabolites are intermediates or end products of cellular processes and can include lipids, amino acids, and products of energy generation such as the tricarboxylic acid cycle. Measuring metabolites can provide a window into alterations in metabolism associated with disease states. In the context of NAFLD and HFpEF, metabolomics could further our understanding of the molecular similarities between the 2 diseases, identify biomarkers to diagnose patients with overlapping disease, and guide the development of new therapies. This is particularly important because therapeutic options are limited for HFpEF, and NAFLD has no FDA‐approved treatments.
To our knowledge, there are no published studies evaluating serum metabolites in patients with biopsy‐proven NAFLD and HFpEF. The objective of this study was to evaluate metabolites associated with the presence of HFpEF in a cohort of patients with biopsy‐proven NAFLD.
Methods
Study Population and Clinical Phenotyping
We performed a retrospective, single‐center cohort study of adults (aged ≥18 years) with biopsy‐proven NAFLD enrolled in the Duke University Health System NAFLD Biorepository and Clinical Database. The biorepository is approved by the institutional review board and contains clinical information and biospecimens including frozen liver tissue and serum from patients who underwent standard of care diagnostic liver biopsy between 2007 and 2013. Further details regarding the biorepository and its enrollment criteria have been published. 19 Participants specifically consented for genomic and metabolomic analysis of their specimens through the NAFLD biorepository. All authors had access to the study data and approved the final manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request.
For this study, we defined NAFLD as: (1) presence of >5% hepatic steatosis on liver biopsy, (2) absence of histologic and serologic evidence for other forms of chronic liver disease, and (3) little or no alcohol consumption (<20 g/d for women and <30 g/d for men). Demographic and clinical data were obtained at the time of liver biopsy. Serum samples were collected from participants at the time of liver biopsy following a 12‐hour fast. The study cohort was selected for inclusion into the metabolomic cohort based on severity of hepatic fibrosis at the time of biopsy and included participants with biopsy‐proven NAFLD at different stages of fibrosis (mild, Metavir fibrosis stage F0–1, n=50; intermediate, stage F2, n=100; advanced, stage F3–4, n=50 20 ) and included only those participants who had high quality serum and liver biopsy tissue collected at the same time. Fibrosis groups were matched for sex, age (+/− 5 years), and body mass index (BMI) (+/− 3 points). Liver biopsy specimens were independently reviewed and scored by 1 pathologist (C.G.) using nonalcoholic steatohepatitis (NASH) Clinical Research Network scoring criteria. 21
To identify patients with HFpEF, we limited the analysis to those who had transthoracic echocardiography (TTE) performed for any indication, at any time before or after liver biopsy. If >1 TTE was available for a given participant, we used the most recent exam. We established 3 definitions of HFpEF based on clinical guidelines describing heart failure as signs or symptoms of heart failure attributable to structural and functional abnormalities of the heart. 22 , 23 First, “HFpEF” was defined as ejection fraction >50% on TTE plus the presence of at least 1 echocardiographic feature of HFpEF (diastolic dysfunction, abnormal left atrial size) and at least 1 of the following heart failure signs or symptoms: jugular venous pressure >8 cm, loop diuretic treatment, peripheral or pulmonary edema, exertional symptoms, fatigue, or HFpEF on problem list. Second, “HFpEF‐no fatigue” included the same echocardiographic criteria as “HFpEF” but excluded fatigue from the heart failure signs or symptoms, given the high prevalence of fatigue among patients with chronic liver disease. 24 Finally, we defined “diastolic dysfunction” as solely echocardiographic criteria (ie, ejection fraction >50% plus either diastolic dysfunction or abnormal left atrial size). All echocardiographic criteria were ascertained based on TTE reports in the electronic medical record using manual chart review.
Clinical Data
Demographics including age, sex, BMI, smoking status, medical comorbidities, and medications were collected as part of the clinical database at the time of liver biopsy.
Metabolomics Analysis
Sample Handling and Processing
All samples were stored at −80 °C until processed on the metabolomics platform at Metabolon, Inc. (Durham, NC). Patient serum samples underwent metabolomic analysis with ultrahigh performance liquid chromatography/tandem mass spectrometry optimized for acidic species, ultrahigh performance liquid chromatography/tandem mass spectrometry for basic species, and gas chromatography/mass spectrometry. Metabolites were then identified using automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in‐source fragments as well as their associated tandem mass spectrometry spectra. This library allowed for rapid identification of metabolites in the experimental samples with high confidence. A detailed explanation of the metabolite analysis procedure and quality control process has been published, 25 , 26 and quality control details appear in Data S1.
Lipidomics Panel
Lipids were extracted in the presence of authentic internal standards using chloroform:methanol. Lipids were trans‐esterified in 1% sulfuric acid in methanol in a sealed vial under a nitrogen atmosphere at 100°C for 45 minutes. The resulting fatty acid methyl esters were extracted from the mixture with hexane containing 0.05% butylated hydroxytoluene and prepared for gas chromatography by sealing the hexane extracts under nitrogen. Fatty acid methyl esters were separated and quantified by capillary gas chromatography (Agilent Technologies 6890 Series GC) equipped with a 30‐m DB 88 capillary column (Agilent Technologies) and a flame ionization detector.
Statistical Analysis
Patient demographic, clinical, biochemical, and histologic characteristics were summarized. Categorical variables were shown as counts and percentages and comparisons between HFpEF phenotypes tested using chi‐squared test or Fisher exact test while continuous variables were analyzed using Wilcoxon test. We performed a logit link for a generalized linear model with binomial likelihood performed to evaluate the association between NAFLD without HFpEF and NAFLD with each HFpEF phenotype by metabolite while controlling for covariates: age, sex, diabetes, hypertension, BMI, and liver fibrosis stage. For each definition of HFpEF, we compared patients meeting the definition to those with TTE not meeting the definition. Statistical significance was defined as P value <0.05, and P values were subsequently adjusted for multiple comparisons using the Benjamini‐Hochberg procedure. 27 All analyses were done using R (v4.1.0).
Results
Study Participant Characteristics
Of the 200 participants with biopsy‐proven NAFLD and serum metabolite results, 187 had available clinical data. Of those, 89 had a TTE and were included in the final analyses (Figure 1). Thirty‐eight (42.7%) participants were women, and the mean age at liver biopsy was 50.4 years. The mean BMI was 36.4 kg/m2, and 36% of participants had diabetes. Four participants had evidence of significant coronary artery disease, with prior myocardial infarction, coronary artery bypass, or percutaneous coronary intervention, and 6 had documented atrial fibrillation or atrial flutter.
Figure 1. Cohort diagram.
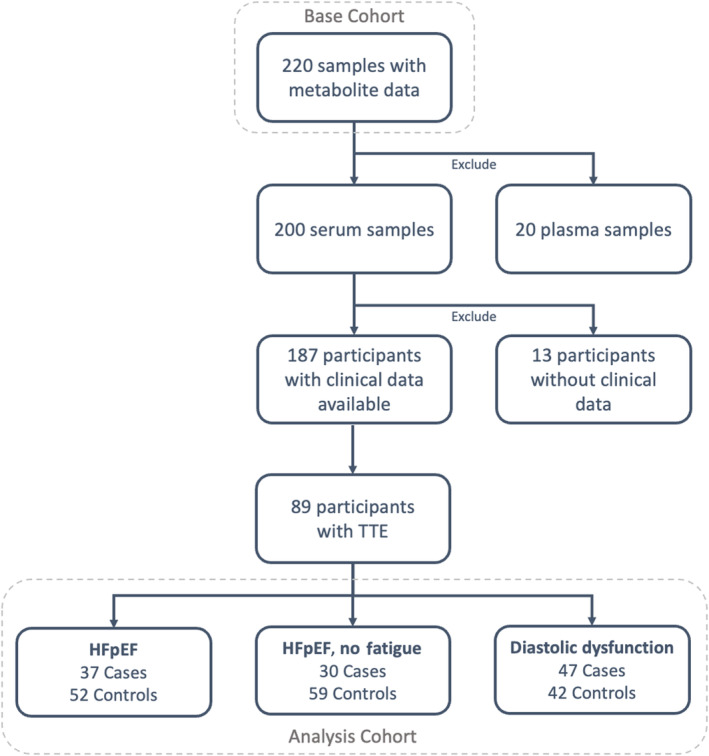
HFpEF indicates heart failure with preserved ejection fraction; and TTE, transthoracic echocardiogram.
Regarding HFpEF phenotypes, 37 met the “HFpEF” definition, 30 were classified as “HFpEF‐no fatigue,” and 47 were classified as “diastolic dysfunction.” Table 1 presents detailed demographics by HFpEF definition, and a Venn diagram depicting the number of patients who met each definition appears in Figure S1. Because only a proportion of the participants with serum metabolites had TTE information, we compared the cohort included in the final analyses to those excluded. Details are shown in Table S1. Overall, the cohorts were not statistically different by age, race, liver fibrosis stage, NAFLD Activity Score, liver blood tests, or diagnosis of hyperlipidemia. Those patients with TTE were more likely to be men, have a diagnosis of hypertension, and have a higher mean BMI and mean hemoglobin A1c.
Table 1.
Patient Characteristics
| HFpEF | HFpEF, no fatigue | Diastolic dysfunction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No (n=52) | Yes (n=37) | P value | No (n=59) | Yes (n=30) | P value | No (n=42) | Yes (n=47) | P value | |
| Age (mean, SD) | 49.0 (9.7) | 52.4 (8.7) | 0.10 | 48.9 (9.5) | 53.6 (8.4) | 0.02 | 48.6 (9.3) | 52.1 (9.3) | 0.08 |
| Women (n, %) | 22 (42.3) | 16 (43.2) | 1 | 24 (40.7) | 14 (46.7) | 0.75 | 17 (40.5) | 21 (44.7) | 0.85 |
| White race (n, %) | 46 (88.5) | 36 (97.3) | 0.13 | 53 (89.8) | 29 (96.7) | 0.26 | 36 (85.7) | 46 (97.9) | 0.03 |
| BMI (mean, SD) | 35.5 (6.4) | 37.5 (7.6) | 0.18 | 35.4 (6.7) | 38.3 (7.2) | 0.07 | 35.9 (6.5) | 36.8 (7.4) | 0.53 |
| Diabetes (n, %) | 21 (40.4) | 11 (29.7) | 0.42 | 24 (40.7) | 8 (26.7) | 0.29 | 17 (40.5) | 15 (31.9) | 0.54 |
| Hypertension (n, %) | 36 (69.2) | 31 (83.8) | 0.19 | 42 (71.2) | 25 (83.3) | 0.32 | 28 (66.7) | 39 (83.0) | 0.13 |
| Total cholesterol (mean, SD) | 181.6 (45.5) | 187.7 (44.6) | 0.55 | 186.5 (46.9) | 179.7 (41.2) | 0.53 | 187.8 (43.6) | 181.1 (46.3) | 0.52 |
| LDL (mean, SD) | 113.27 (39.2) | 108.56 (39.2) | 0.61 | 114.04 (38.4) | 105.64 (40.5) | 0.38 | 118.56 (38.5) | 104.95 (38.8) | 0.13 |
| HDL (mean, SD) | 37.4 (9.2) | 38.5 (18.5) | 0.71 | 38.9 (15.4) | 35.6 (9.3) | 0.32 | 37.7 (9.0) | 37.9 (17.0) | 0.95 |
| Statin prescribed (n, %) | 11 (21.2) | 18 (48.6) | 0.006 | 15 (25.4) | 14 (46.7) | 0.04 | 8 (19.0) | 21 (44.7) | 0.01 |
| Fibrosis stage (n, %) | 0.14 | 0.45 | 0.08 | ||||||
| Stage 0 | 0 (0.0) | 3 (8.1) | 1 (1.7) | 2 (6.7) | 0 (0.0) | 3 (6.4) | |||
| Stage 1 | 9 (17.3) | 7 (18.9) | 10 (16.9) | 6 (20.0) | 6 (14.3) | 10 (21.3) | |||
| Stage 2 | 24 (46.2) | 15 (40.5) | 25 (42.4) | 14 (46.7) | 17 (40.5) | 22 (46.8) | |||
| Stage 3 | 18 (34.6) | 9 (24.3) | 21 (35.6) | 6 (20.0) | 18 (42.9) | 9 (19.1) | |||
| Stage 4 | 1 (1.9) | 3 (8.1) | 2 (3.4) | 2 (6.7) | 1 (2.4) | 3 (6.4) | |||
BMI indicates body mass index; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
Metabolites Detected
Serum from the cohort yielded 1151 metabolites (760 identified and 361 unnamed). These metabolites derived from 8 super‐pathways: amino acids (n=157), peptides (n=55), carbohydrates (n=28), energy related (n=7), lipids (n=307), nucleotides (n=34), cofactors/vitamins (n=34), and xenobiotics (n=67). The unnamed metabolites were excluded. We also excluded metabolites for which >30% of values were missing, which was presumably attributable to levels below the limit of detection, and resulted in exclusion of 27 metabolites, or 3% of the total, as shown in Figure S2. For the remaining 656 metabolites, missing values (2.4%–2.6%, see Table S2) were imputed to half of the observed minimum value for each metabolite, and batch effects were corrected by removing mean day‐wise effects from each analyte independently after log‐transformation. 28
Association of Metabolites With NAFLD and HFpEF Phenotypes
We assessed metabolites across the 3 HFpEF phenotypes to identify metabolites unique or similar across these definitions. For the “HFpEF” phenotype, 53 metabolites were associated with the presence of HFpEF with P value <0.05. None remained significant after adjustment for multiple comparisons. The majority (39/53, 73.6%) of the significant metabolites were in lipid metabolism, and levels of these metabolites were generally increased in patients with HFpEF. Multiple glycerophosphorylcholines (GPCs, n=20) and glycerophosphorylethanolamines (GPEs, n=9) were among the most significant metabolites, and these were quantified at higher levels in patients with HFpEF compared with those without. Two metabolites of cysteine (cysteine s‐sulfate and s‐methylcysteine) were both present at significantly lower levels in patients with HFpEF. A heatmap of significant metabolites appears in Figure 2, full results are in Table S3, and a volcano plot appears in Figure S3.
Figure 2. Heat map of metabolites associated with HFpEF definition.
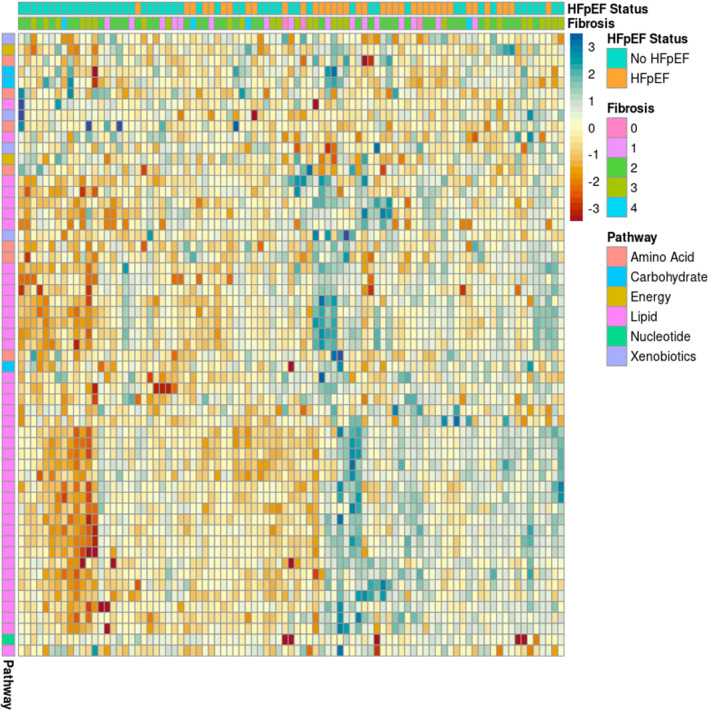
Each column is 1 subject, with the top row showing HFpEF (orange) or no HFpEF (teal). Fibrosis stage is shown in the second row. Each subsequent row represents 1 metabolite, with pathways indicated by color in the leftmost column. Increased/decreased levels (HFpEF vs no HFpEF) are shown by the colors of each box in the matrix. Negative Z‐scores are red to orange, positive Z‐scores are blue. HFpEF indicates heart failure with preserved ejection fraction.
For “HFpEF‐no fatigue,” 50 metabolites were significant to the P<0.05 level. None remained significant after adjustment for multiple comparisons. Overall, the results were similar to “HFpEF,” with robust representation of lipids (34/50, 68%), particularly GPCs and GPEs. However, lower levels of taurocholenate sulfate, a conjugated secondary bile acid, were associated with “HFpEF‐no fatigue.” Full results appear in Table S4, and a volcano plot appears in Figure S4.
For the echocardiographic definition, “diastolic dysfunction,” 39 metabolites were significantly associated with HFpEF. None remained significant after adjustment for multiple comparisons. Many metabolites overlapped with results from the other 2 HFpEF definitions and, again, most significant metabolites related to lipid metabolism (29/39, 74.3%). Several metabolites unique to this definition included biliverdin (P=0.02) and xylitol (P=0.01). Nucleotide family metabolites such as orotidine, 2′‐deoxyuridine, and orotate, precursors of pyrimidine biosynthesis, were also uniquely significant for this phenotype. Full results appear in Table S5, and a volcano plot appears in Figure S5.
Fifteen metabolites were associated with all 3 definitions of HFpEF. These included 10 lipids, 1 cysteine metabolite (S‐methylcysteine), and citrate (Table 2 and Figure 3).
Table 2.
Metabolites Significantly Associated With All 3 Definitions of HFpEF*
| Pathway | Subpathway | Metabolite |
|---|---|---|
| Lipid | Lysolipid | 1‐arachidonoyl‐GPE* (20:4)* |
| 1‐docosahexaenoylglycerophosphoethanolamine* | ||
| 2‐stearoylglycerophosphoethanolamine* | ||
| 1‐stearoyl‐GPE (18:0) | ||
| 2‐arachidonoyl‐GPE* (20:4)* | ||
| 1‐myristoyl‐GPC (14:0) | ||
| Fatty acid, dicarboxylate | azelate (nonanedioate; C9) | |
| Mole % total fatty acid | TL18:3n6 (g‐linolenic acid) | |
| Monoacylglycerol | 2‐docosahexaenoylglcyerol* | |
| 1‐arachidonylglycerol | ||
| Energy | TCA cycle | citrate |
| alpha‐ketoglutarate | ||
| Amino acid | Methionine, cysteine, SAM, and taurine metabolism | S‐methylcysteine |
| Lysine metabolism | N6‐acetyllysine | |
| Xenobiotics | Food component/plant | methyl glucopyranoside (alpha+beta) |
GPC indicates glycerophosphocholine; GPE, glycerophosphoethanolamine; SAM, S‐Adenosyl methionine; and TCA, tricarboxylic acid cycle.
Covariates: age, sex, diabetes, hypertension, body mass index, and liver fibrosis stage.
Figure 3. Venn diagram showing metabolites associated with each definition of HFpEF as well as metabolites that overlap between definitions.
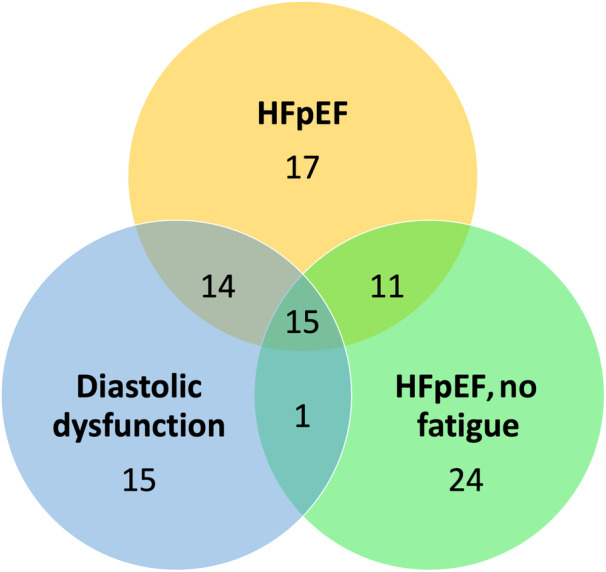
HFpEF indicates heart failure with preserved ejection fraction.
Discussion
NAFLD and cardiovascular disease have both reached epidemic proportions. Links between NAFLD and HFpEF have been increasingly recognized because of shared risk factors and pathophysiologic characteristics. 7 Despite this, common mechanisms and biomarkers able to identify patients with NAFLD at risk for, or already afflicted with, HFpEF remain unknown. Here we investigated the association between serum metabolites and HFpEF in a cohort of patients with biopsy‐proven NAFLD to identify both unique and common metabolic pathways between these diseases. To our knowledge, this is the first such analysis published. While no individual metabolites unique to those patients with NAFLD with HFpEF phenotypes survived adjustment for multiple comparisons, presumably driven by the large number of metabolites evaluated, many were significant to the P<0.05 level, and several themes persisted. First, lipid metabolism represented the most common (>65%) pathway associated with HFpEF among participants with biopsy‐proven NAFLD. Lipid metabolites were generally increased in patients with HFpEF compared with those without. Of note, more patients with HFpEF by any definition were on statin medications at the time of liver biopsy compared with those without HFpEF.
While this is the first published study to investigate the overlap between biopsy‐proven NAFLD and HFpEF, metabolomic analysis has been used in NAFLD and HFpEF alone to predict the presence and severity of disease. In NAFLD, levels of triglycerides 29 , 30 and polyunsaturated fatty acids 31 distinguished simple steatosis from NASH, and differences in bile acids separated patients with NAFLD from healthy controls. 32 Caussy et al previously reported that a combination of 10 serum metabolites in bile acid, amino acid, lipid, nucleotide, and peptide pathways could accurately identify the presence of advanced NAFLD fibrosis. 33 Additionally, our group previously found that vitamin E, serotonin, and bile acid metabolites associated with future liver‐related events in a cohort of patients with biopsy proven NAFLD and NASH. 26 In heart failure, several small studies have assessed metabolomic signatures of HFpEF compared with heart failure with reduced ejection fraction (HFrEF) and patients without heart failure. In 1 study, patients with HFpEF had several metabolomic differences, including higher serum amino acids and lower levels of several lipids including sphingomyelins and phosphatidylcholines. 34 Another study examined 63 metabolites in 752 patients (282 HFpEF, 279 HFrEF, 191 control) and found differences in long‐chain acylcarnitine levels among the groups, with higher levels seen in patients with HFpEF and patients with HFrEF compared with controls. 35 Animal models of HFpEF and metabolic syndrome demonstrate alterations in lipid metabolism with nicotinamide supplementation. 36 Overall, both NAFLD and HFpEF appear to be associated with changes in lipid and amino acid metabolism, consistent with our findings.
Several metabolites from our analysis point to areas meriting future investigation. GPCs and GPEs, both phospholipids, featured prominently in the significant metabolites associated with all 3 HFpEF definitions. GPC is a choline metabolite and acetylcholine precursor. In 1 small study of health adults, administration of GPC increased plasma growth hormone levels and increased hepatic fatty acid oxidation. 37 Choline metabolism has established links to NAFLD. A choline metabolism signature has previously been associated with steatosis severity, 38 and serum homocysteine (a choline metabolite) has been associated with histologic severity. 39 A high choline diet in an animal model of HFpEF resulted in increased myocardial fibrosis. 40 This suggests differential effects of derangements in choline metabolism in NAFLD versus HFpEF, given that choline restriction is a common method to induce steatosis in animal models of NAFLD. Although GPCs have not yet been linked to HFpEF in humans directly, 1 study of adolescents linked multiple GPCs to traditional cardiovascular risk factors including visceral adiposity. 41 GPEs, also in the phospholipid biosynthesis pathway, also featured prominently among lipid metabolites associated with HFpEF. In 1 study, differences in these metabolites were found when obese patients with and without insulin resistance had serum metabolites compared. 42 In addition, disruption of phosphoethanolamine synthesis in animal models has been shown to promote development of NASH. 43
Although fewer amino acid metabolites were associated with HFpEF relative to lipid metabolites, several are noteworthy. Cysteine metabolites (cysteine s‐sulfate and s‐methylcysteine) were present at lower levels in patients with HFpEF compared with those without. The liver metabolizes cysteine to glutathione, which is important in modulating oxidative stress. In 1 study, pediatric subjects with NAFLD had higher plasma levels of cysteine and homocysteine compared with healthy controls, but the subgroup with NASH had lower levels of cysteine and homocysteine compared with those with NAFLD not meeting histologic criteria for NASH, 44 consistent with our results. Increases in urinary cysteine excretion (specifically carboxyethyl cysteine and succinyl‐cysteine) were found in an obese mouse model of HFpEF compared with control mice. 45 It is unclear why lower levels of cysteine have been associated with HFpEF and NASH, but our findings suggest that cysteine metabolism, with its key role in addressing oxidative stress, warrants further investigation. A branched chain amino acid (BCAA) metabolite, 2‐hydroxy‐3‐methylvalerate, was also associated with HFpEF. We previously found that branched chain amino acid transaminase 1 (BCAT1) was overexpressed and hypomethylated in patients with NAFLD who experienced clinical decompensation. 46 Both human and animal data have linked BCAA to insulin resistance, and restriction of BCAA in the diet of obese mice resulted in a shift toward fatty acid metabolism in cardiac myocytes. 47 , 48
Multiple intermediates in the tricarboxylic acid cycle, including citrate (decreased) and alpha‐ketoglutarate (increased), were associated with HFpEF. Previously, increased serum levels of citrate were associated with 3‐month mortality in patients with acute heart failure, attributed to increased fatty acid beta‐oxidation. 49 Animal models have linked expression levels of liver pyruvate kinase, which makes pyruvate available for the tricarboxylic acid cycle, to steatosis severity and fibrosis in NAFLD in a sex‐specific manner. 50 In addition, deletion of a cellular citrate transporter, SLC13A5, in mice fed a high‐fat diet was found to prevent development of obesity and hepatic steatosis. 51 Carbohydrate energy extraction may have far‐reaching implications for metabolic syndrome, and derangements in this process could be 1 link between NAFLD and HFpEF.
Bile acid metabolism did not feature prominently in significant metabolites but was present. Taurocholenate sulfate was decreased in subjects with “HFpEF‐no fatigue.” Bile acids are thought to mediate the association between the gut microbiome and metabolic disease, and they may play a role in both NAFLD and HFpEF. Bile acid levels, and specifically the ratio of primary to secondary bile acids, have been associated with chronic heart failure. 52 Bile acid metabolism has been associated with severity of NAFLD, and bile acids have been used as therapeutics in NAFLD. 53 , 54 In addition, postprandial taurine‐containing bile acid levels were increased in participants with NAFLD compared with healthy controls. 55
Given the increasing awareness of the clinical and physiologic links between NAFLD and HFpEF, our analysis is well positioned to provide new insights on relevant altered metabolites between these 2 populations that have not yet been studied (patients with biopsy‐proven NAFLD and HFpEF by TTE and clinical parameters). Strengthening our results is the fact that identified metabolites overlap with those found in previous analyses of NAFLD and HFpEF independently. For example, a prospective cohort study of 156 participants with biopsy‐proven NAFLD identified 32 metabolites significantly associated with advanced fibrosis. 33 That analysis highlighted taurine and taurocholate, suggesting that taurine‐containing primary bile acids may be associated not only with progression of liver disease but also of HFpEF, as we found. In the previously mentioned metabolomic analysis of HFpEF, multiple phosphatidylcholines were significantly associated with HFpEF. 34
The limitations of this study include the small sample size, single center design, and our racially homogeneous population, a common limitation for NAFLD studies. Our ascertainment of HFpEF using retrospective chart review, particularly for clinical factors, leaves open the possibility that events or TTEs were not captured in our system or were missed due to incomplete documentation. About half of patients with serum metabolite data and clinical data available did not have TTE. Our comparison of participants with and without TTE found that participants who had TTE differed by sex, hemoglobin A1c, presence of hypertension, and BMI. Other unmeasured factors could differ between these groups. As serum metabolites were evaluated a single time at the index liver biopsy, serial measurements to indicate metabolite trends were not available, and metabolites are thought to fluctuate based on numerous factors. If heart failure developed after liver biopsy, then metabolite measurement and onset of symptoms or TTE findings would be separated in time, further limiting assessment of this relationship. Limitations in the metabolite assay itself include the possible presence of unmeasured or unnamed metabolites that could be of mechanistic or prognostic significance. Despite these limitations, these findings can provide the first step in characterizing the underlying unique and common physiology in NAFLD and HFpEF. This will be essential for establishing effective diagnostics and therapeutics given the rising prevalence and common underlying factors in both.
In summary, we conducted the first evaluation of serum metabolites associated with the presence of HFpEF by TTE and clinical criteria in a cohort of patients with biopsy‐proven NAFLD. Our findings point to the importance of lipid, amino acid, and carbohydrate metabolism in these patients. These pathways warrant further investigation to understand both the unique and overlapping features between these related disease processes, which could lend to improved development of biomarkers and treatments.
Sources of Funding
This work was supported by Metabolon, Inc (M.F.A.), AASLD Foundation Bridge Award (C.A.M.), National Institutes of Health 5T32DK007568‐29 (K.W.), and a Duke Endowment (A.M.D.).
Disclosures
Dr Guy reports consulting for CymaBay Therapeutics and NGM Biopharmaceuticals. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S5
Figures S1–S5
Acknowledgments
The authors gratefully acknowledge the contributions of Metabolon, Inc (Durham, NC) to this manuscript. KW: Conceptualization, investigation, writing—original draft; RH: Formal analysis; CH: Investigation; RM: Writing—review & editing; CG: Investigation; MFA: Conceptualization, writing—review & editing; AMD: Conceptualization, writing—review & editing; MF: Conceptualization, investigation, writing—review & editing; CAM: Conceptualization, investigation, supervision, writing—review & editing.
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029873
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 3. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun. 2019;3:1459–1471. doi: 10.1002/hep4.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, Jung SW, Du Jeong ID, Bang SJ, Shin JW, et al. Association between non‐alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68:1018–1024. doi: 10.1016/j.jhep.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 6. Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51:728–736. doi: 10.1111/apt.15660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fudim M, Zhong L, Patel KV, Khera R, Abdelmalek MF, Diehl AM, McGarrah RW, Molinger J, Moylan CA, Rao VN, et al. Nonalcoholic fatty liver disease and risk of heart failure among Medicare beneficiaries. J Am Heart Assoc. 2021;10:e021654. doi: 10.1161/JAHA.121.021654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 9. Sao R, Aronow WS. Association of non‐alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch Med Sci. 2018;14:1233–1244. doi: 10.5114/aoms.2017.68821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 11. Miller A, McNamara J, Hummel SL, Konerman MC, Tincopa MA. Prevalence and staging of non‐alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. Sci Rep. 2020;10:12440. doi: 10.1038/s41598-020-69013-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters AE, Pandey A, Ayers C, Wegermann K, McGarrah RW, Grodin JL, Abdelmalek MF, Bekfani T, Blumer V, Diehl AM, et al. Association of liver fibrosis risk scores with clinical outcomes in patients with heart failure with preserved ejection fraction: findings from TOPCAT. ESC Heart Fail. 2021;8:842–848. doi: 10.1002/ehf2.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. VanWagner LB, Wilcox JE, Colangelo LA, Lloyd‐Jones DM, Carr JJ, Lima JA, Lewis CE, Rinella ME, Shah SJ. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population‐based study. Hepatology. 2015;62:773–783. doi: 10.1002/hep.27869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, Shah SJ, Lima JAC, Lloyd‐Jones DM. Longitudinal association of non‐alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA study. J Am Heart Assoc. 2020;9:e014279. doi: 10.1161/JAHA.119.014279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shili‐Masmoudi S, Wong GL, Hiriart JB, Liu K, Chermak F, Shu SS, Foucher J, Tse YK, Bernard PH, Yip TCF, et al. Liver stiffness measurement predicts long‐term survival and complications in non‐alcoholic fatty liver disease. Liver Int. 2020;40:581–589. doi: 10.1111/liv.14301 [DOI] [PubMed] [Google Scholar]
- 16. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Keach JC, Lafferty HD, Stahler A, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018;5:262–270. doi: 10.1002/ehf2.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Chen W, Shao W, Jiang Y, Cao Z, He W, Wu M, Zhang Y, Wang J. Liver fibrosis scores and atrial fibrillation incidence in heart failure with preserved ejection fraction. ESC Heart Fail. 2022;9:3985–3994. doi: 10.1002/ehf2.14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, Murphy SK, Ashley‐Koch AE, Choi SS, Michelotti GA, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014;59:471–482. doi: 10.1002/hep.26661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C . The French METAVIR cooperative study group. Hepatology. 1994;20:15–20. doi: 10.1002/hep.1840200104 [DOI] [PubMed] [Google Scholar]
- 21. Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander‐Tetri BA, Network NCR. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807 [DOI] [PubMed] [Google Scholar]
- 23. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 24. Newton JL, Jones DE, Henderson E, Kane L, Wilton K, Burt AD, Day CP. Fatigue in non‐alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57:807–813. doi: 10.1136/gut.2007.139303 [DOI] [PubMed] [Google Scholar]
- 25. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small‐molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h [DOI] [PubMed] [Google Scholar]
- 26. Wegermann K, Howe C, Henao R, Wang Y, Guy CD, Abdelmalek MF, Diehl AM, Moyland CA. Serum bile acid, vitamin E, and serotonin metabolites are associated with future liver‐related events in nonalcoholic fatty liver disease. Hepatol Commun. 2021;5:608–617. doi: 10.1002/hep4.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Series B (Methodol) 1995;57:289–300. [Google Scholar]
- 28. Wei R, Wang J, Su M, Jia E, Chen S, Chen T, Ni Y. Missing value imputation approach for mass spectrometry‐based metabolomics data. Sci Rep. 2018;8:663. doi: 10.1038/s41598-017-19120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayo R, Crespo J, Martinez‐Arranz I, Banales JM, Arias M, Minchole I, Aller de la Fuente R, Jimenez‐Agüero R, Alonso C, de Luis DA, et al. Metabolomic‐based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun. 2018;2:807–820. doi: 10.1002/hep4.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray‐McGuire C, Schauer PR, Gupta M, Feldstein AE, Hazen SL, et al. Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring). 2009;17:1696–1701. doi: 10.1038/oby.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res. 2015;56:185–192. doi: 10.1194/jlr.P055640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, Milburn M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caussy C, Ajmera VH, Puri P, Hsu CL, Bassirian S, Mgdsyan M, Singh S, Faulkner C, Valasek MA, Rizo E, et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non‐alcoholic fatty liver disease. Gut. 2019;68:1884–1892. doi: 10.1136/gutjnl-2018-317584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One. 2015;10:e0124844. doi: 10.1371/journal.pone.0124844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunter WG, Kelly JP, McGarrah RW III, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. 2016;5:e003190. doi: 10.1161/JAHA.115.003190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abdellatif M, Trummer‐Herbst V, Koser F, Durand S, Adao R, Vasques‐Novoa F, Freundt JK, Voglhuber J, Pricolo MR, Kasa M, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13:eabd7064. doi: 10.1126/scitranslmed.abd7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawamura T, Okubo T, Sato K, Fujita S, Goto K, Hamaoka T, Iemitsu M. Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults. Nutrition. 2012;28:1122–1126. doi: 10.1016/j.nut.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 38. Corbin KD, Abdelmalek MF, Spencer MD, da Costa KA, Galanko JA, Sha W, Suzuki A, Guy CD, Cardona DM, Torquati A, et al. Genetic signatures in choline and 1‐carbon metabolism are associated with the severity of hepatic steatosis. FASEB J. 2013;27:1674–1689. doi: 10.1096/fj.12-219097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai Z, Chen J, Ding C, Wong K, Chen X, Pu L, Huang Q, Chen X, Cheng Z, Liu Y, et al. Association of hepatic global DNA methylation and serum one‐carbon metabolites with histological severity in patients with NAFLD. Obesity (Silver Spring). 2020;28:197–205. doi: 10.1002/oby.22667 [DOI] [PubMed] [Google Scholar]
- 40. Shuai W, Wen J, Li X, Wang D, Li Y, Xiang J. High‐choline diet exacerbates cardiac dysfunction, fibrosis, and inflammation in a mouse model of heart failure with preserved ejection fraction. J Card Fail. 2020;26:694–702. doi: 10.1016/j.cardfail.2020.04.017 [DOI] [PubMed] [Google Scholar]
- 41. Syme C, Czajkowski S, Shin J, Abrahamowicz M, Leonard G, Perron M, Richer L, Veillette S, Gaudet D, Strug L, et al. Glycerophosphocholine metabolites and cardiovascular disease risk factors in adolescents: a cohort study. Circulation. 2016;134:1629–1636. doi: 10.1161/CIRCULATIONAHA.116.022993 [DOI] [PubMed] [Google Scholar]
- 42. Al‐Sulaiti H, Diboun I, Agha MV, Mohamed FFS, Atkin S, Domling AS, Elrayess MA, Mazloum NA. Metabolic signature of obesity‐associated insulin resistance and type 2 diabetes. J Transl Med. 2019;17:348. doi: 10.1186/s12967-019-2096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grapentine S, Singh RK, Basu P, Sivanesan S, Mattos G, Oresajo O, Cheema J, Demeke W, Dolinsky VW, Bakovic M. Pcyt2 deficiency causes age‐dependant development of nonalcoholic steatohepatitis and insulin resistance that could be attenuated with phosphoethanolamine. Sci Rep. 2022;12:1048. doi: 10.1038/s41598-022-05140-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pastore A, Alisi A, di Giovamberardino G, Crudele A, Ceccarelli S, Panera N, Dionisi‐Vici C, Nobili V. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int J Mol Sci. 2014;15:21202–21214. doi: 10.3390/ijms151121202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baskal S, Buttner P, Werner S, Besler C, Lurz P, Thiele H, Tsikas D. Profile of urinary amino acids and their post‐translational modifications (PTM) including advanced glycation end‐products (AGEs) of lysine, arginine and cysteine in lean and obese ZSF1 rats. Amino Acids. 2022;54:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wegermann K, Henao R, Diehl AM, Murphy SK, Abdelmalek MF, Moylan CA. Branched chain amino acid transaminase 1 (BCAT1) is overexpressed and hypomethylated in patients with non‐alcoholic fatty liver disease who experience adverse clinical events: a pilot study. PLoS One. 2018;13:e0204308. doi: 10.1371/journal.pone.0204308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, et al. Branched‐chain amino acid restriction in Zucker‐fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl‐glycine export. Mol Metab. 2016;5:538–551. doi: 10.1016/j.molmet.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, Ilkayeva O, White PJ, Newgard CB. Dietary branched‐chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2020;318:E216–E223. doi: 10.1152/ajpendo.00334.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stryeck S, Gastrager M, Degoricija V, Trbusic M, Potocnjak I, Radulovic B, Pregartner G, Berghold A, Madl T, Frank S. Serum concentrations of citrate, tyrosine, 2‐ and 3‐ Hydroxybutyrate are associated with increased 3‐month mortality in acute heart failure patients. Sci Rep. 2019;9:6743. doi: 10.1038/s41598-019-42937-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chella Krishnan K, Floyd RR, Sabir S, Jayasekera DW, Leon‐Mimila PV, Jones AE, Cortez AA, Shravah V, Péterfy M, Stiles L, et al. Liver pyruvate kinase promotes NAFLD/NASH in both mice and humans in a sex‐specific manner. Cell Mol Gastroenterol Hepatol. 2021;11:389–406. doi: 10.1016/j.jcmgh.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. von Loeffelholz C, Lieske S, Neuschafer‐Rube F, Willmes DM, Raschzok N, Sauer IM, Konig J, Fromm MF, Horn P, Chatzigeorgiou A, et al. The human longevity gene homolog INDY and interleukin‐6 interact in hepatic lipid metabolism. Hepatology. 2017;66:616–630. doi: 10.1002/hep.29089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, Aukrust P, Gullestad L, Hov JR, Trøseid M. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. 2017;23:666–671. doi: 10.1016/j.cardfail.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 53. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Traussnigg S, Schattenberg JM, Demir M, Wiegand J, Geier A, Teuber G, Hofmann WP, Kremer AE, Spreda F, Kluwe J, et al. Norursodeoxycholic acid versus placebo in the treatment of non‐alcoholic fatty liver disease: a double‐blind, randomised, placebo‐controlled, phase 2 dose‐finding trial. Lancet Gastroenterol Hepatol. 2019;4:781–793. doi: 10.1016/S2468-1253(19)30184-0 [DOI] [PubMed] [Google Scholar]
- 55. Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Barritt AS IV. Altered bile acid metabolome in patients with nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318–3328. doi: 10.1007/s10620-015-3776-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Figures S1–S5


