Abstract
Background
PARAGON‐HF (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction) suggested a potential benefit of sacubitril–valsartan in women with preserved ejection fraction. Among patients with heart failure previously treated with angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), we studied whether effectiveness of treatment with sacubitril–valsartan compared with ACEI/ARB monotherapy differed between men and women for both preserved and reduced ejection fraction.
Methods and Results
Data were derived from the Truven Health MarketScan Databases between January 1, 2011, and December 31, 2018. We included patients with a primary diagnosis of heart failure on treatment with ACEIs, ARBs, or sacubitril–valsartan on the basis of the first prescription after diagnosis. A total of 7181 patients treated with sacubitril–valsartan, 25 408 patients using an ACEI, and 16 177 patients treated with ARBs were included. A total of 790 readmissions or deaths occurred among 7181 patients in the sacubitril–valsartan group and 11 901 events in 41 585 patients treated with an ACEI/ARB. Adjusted for covariates, the hazard ratio (HR) for treatment with sacubitril–valsartan compared with an ACEI or ARB was 0.74 (95% CI, 0.68–0.80). The protective effect of sacubitril–valsartan was evident for men and women (women: HR, 0.75 [95% CI, 0.66–0.86]; P<0.01; men: HR, 0.71 [95% CI, 0.64–0.79]; P<0.01; P interaction 0.03). A protective effect for both sexes was seen only among those with systolic dysfunction.
Conclusions
Treatment with sacubitril–valsartan is more effective at reducing death and admission to the hospital for heart failure compared with ACEIs/ARBs similarly among men and women with systolic dysfunction; sex differences in the effectiveness of sacubitril–valsartan in diastolic dysfunction requires further investigation.
Keywords: heart failure, observational study, sacubitril–valsartan, sex differences
Subject Categories: Heart Failure, High Blood Pressure, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- PARAGON‐HF
Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction
- PARADIGM‐HF
Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure
- PIONEER‐HF
Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide] in Patients Stabilized From an Acute HF Episode
Clinical Perspective.
What Is New?
The evidence base for treatment in heart failure is driven by randomized controlled trials of systolic failure among men; little is known about the efficacy of currently used medications on women.
Our study uses data from 48 766 patients to demonstrate the protective effect of sacubitril–valsartan in reducing death and admission to the hospital for heart failure among women.
What Are the Clinical Implications?
Additional data are needed to investigate sex differences in diastolic dysfunction.
Our study provides evidence‐based support for new guidelines encouraging sacubitril–valsartan use in women with heart failure.
Congestive heart failure (CHF) affects ≈26 million people worldwide with significant variation by sex in incidence, pathogenesis, survival, and benefit from treatment. 1 , 2 , 3 , 4 Data from large population cohorts offer insight into the sex‐related epidemiology of CHF. In the Framingham study, the overall incidence of CHF was 5.6 and 3.3 per 1000 years among men and women, respectively. 5 Although a larger proportion of women survive, it appears that this group benefits from smaller gains in short‐ and long‐term outcomes. 6 In a Scottish population study, between 1986 and 2003, the 30‐day death rate after first hospitalization with CHF decreased from 24.4% to 16.2% in men, in contrast to a decrease from 20% to 16.9% among women. 7 , 8 These differences were also evident at 1 and 5 years. Similar trends are appreciated in other population cohorts. 9 , 10 Additionally, only the men with CHF treated by Kaiser Permanente in Oregon experienced significant improvements in death rate; the 5‐year adjusted death rate improved in men from 82.7% to 68.8% between 1970 to 1979 and 1990 to 1994 but remained the same or worse in women, with rates from 60.8% to 64.8%. 8 , 11
Current CHF management guidelines, which are based largely on trials conducted in men, may in part explain these disparate clinical outcomes. Evidence for therapies among women comes mainly from meta‐analyses, secondary analyses, and post hoc sensitivity analyses, 12 all underpowered to make robust conclusions. Recently, treatment with sacubitril–valsartan has garnered attention; its efficacy was first demonstrated by PARADIGM‐HF (Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) and since notably reinforced by the recently published PIONEER‐HF (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide] in Patients Stabilized From an Acute HF Episode) trial. 13 Both of these trials were largely conducted in men, including only about 25% women, and focused on a population with reduced ejection fraction, a syndrome more common among men. PARAGON‐HF (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction) 14 has been the only trial testing the efficacy of sacubitril–valsartan to have included an equal proportion of men and women and, interestingly, reported a statistically significant 27% relative risk reduction of cardiovascular death and total hospitalizations observed in women with preserved ejection fraction compared with no effect in men. While guidelines 15 recommend first‐line use of sacubitril–valsartan, there have been few thorough primary assessments of its effectiveness among women.
The objective of the present population‐based analysis is to compare effectiveness of sacubitril–valsartan with angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) between men and women with CHF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Dr Louise Pilote had full access to all the data in the study and takes responsibility for their integrity and the data analysis.
Study Population
Data were derived from the Truven Health MarketScan Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database (Truven Health Analytics Inc, Ann Arbor, MI) for the period January 1, 2011, to December 31, 2018. MarketScan is a family of administrative claims‐based databases containing data on almost 230 million unique patients in the United States since 1995. The database collects payment information, capturing reimbursements from health insurance plans or claim information for individuals who have both Medicare and commercial employer‐sponsored coverage. It provides access to integrated, deidentified, patient‐level data on expenses using billable codes in both the inpatient and outpatient settings and includes access to patient‐level pharmacy expenses. The database links medical and outpatient prescription drug claims and encounter data with patient enrollment data to provide individual‐specific clinical use and outcomes information. The MarketScan databases include information on outpatient pharmaceutical claims, namely, National Drug Code, date of service, and days supplied.
We created a cohort of adults, aged ≥18 years, diagnosed with CHF, and treated with ACEI, ARBs, or sacubitril–valsartan. We first selected patients with a diagnosis of CHF on the basis of the International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10), Clinical Modification codes 428 and I50, with data available to categorize either systolic or diastolic dysfunction. To maximize diagnostic specificity, we included patients with at least 2 hospitalizations for CHF in a 2‐year period on the basis of primary diagnosis in the MarketScan database. Date of the first of hospitalization was defined as the date of cohort entry. We excluded patients (1) on dual therapy or (2) who did not have a continuous enrollment in a plan with medical and pharmacy benefits for 12 months before the cohort entry.
Drug Exposure
We identified all prescriptions for ACEIs, ARBs, and sacubitril–valsartan occurring after the date of cohort entry as defined above. Patients were considered current users of a specific drug on the basis of the first prescription after cohort entry. Sacubitril–valsartan was approved by the Food and Drug Administration on July 7, 2015; therefore, data were available in MarketScan after this date. Therefore, all patients in our cohort had used either an ACEI or ARB before sacubitril–valsartan use. Follow‐up began at the date of the first prescription, and person‐time accrued until achievement of the outcome, loss of medical and pharmacy coverage, death from cardiovascular causes (obtained from hospital discharge data), or end of study follow‐up period (December 31, 2018), whichever occurred first.
CHF With Reduced Versus Preserved Ejection Fraction
To compare outcomes for CHF with reduced ejection fraction (systolic dysfunction) and preserved ejection fraction (diastolic dysfunction), we created 2 subgroups using ICD‐9 and ICD‐10 codes I50.2 and I50.3. ICD codes used are presented in Table S1. Based on these definitions, 16 159 patients (6380 men and 9779 women) with diastolic dysfunction and 32 607 patients (20 193 men and 12 414 women) with systolic dysfunction were identified. The analyses described below were conducted among men and women for the full cohort and by ejection fraction subgroups.
Outcomes
The primary outcomes of interest were (1) a composite of hospital admission for heart failure exacerbation or death from cardiovascular causes that occurred in the hospital and (2) composite of hospital admission for heart failure, death due to cardiovascular causes, stroke, myocardial infarction (MI), or cardiac arrest. The ICD codes used to derive the primary outcomes are available in Table S1. Secondarily, we studied the effect of sacubitril–valsartan versus ACEI/ARB for each MI, stroke, and cardiac arrest. Finally, we studied a composite of the following safety outcomes: hypotension, renal dysfunction, hyperkalemia, or angioedema. Data for the primary composite outcomes, secondary effectiveness outcomes, and secondary safety outcomes were obtained from hospitalization and emergency room visit records and included any of the 4 diagnosis codes recorded on the Inpatient or Outpatient Service record. Data for out‐of‐hospital death were not available in the MarketScan database.
Covariates
Baseline characteristics and covariates were assessed at the time of cohort entry and included the following: age, body mass index, Charlson comorbidity index, previous MI, atrial fibrillation, valvular heart disease, hypertension, venous thromboembolism, hypercholesterolemia, hypertriglyceridemia, diabetes, chronic kidney disease, cancer, chronic obstructive pulmonary disease, dementia, diuretic use, beta‐blocker use, and digoxin use. Data on drug use were taken in the year before the index prescription of intervention drug and group assignment. Covariates were selected a priori based on their known predictive value in CHF severity and cardiovascular death. Data for included covariates were ascertained from inpatient and outpatient claims before the index date of prescription. ICD codes for covariates are also presented in Table S1.
All analyses were conducted in accordance with local laws and regulations and received approvals from the McGill University Health Center Ethics Board. As the data were derived from the MarketScan database and anonymized, informed consent from individual patients was not obtained.
Statistical Analysis
Demographic data for users of ACEIs, ARBs, and sacubitril–valsartan are presented as mean and SD for continuous variables and as proportions for categorical variables. Data were analyzed from January 1, 2011, to December 31, 2018, on the basis of an intention‐to‐treat principle; exposure was considered as the first group to which the patient was assigned, and switching exposure groups was not considered during follow‐up. A multivariable Cox proportional hazards model was used to compare users of ACEIs/ARBs with sacubitril–valsartan users on primary and secondary effectiveness outcomes. Similarly, a time‐to‐event model was created for the composite safety outcome. All models were adjusted for the potential confounders listed above, as well as time elapsed between CHF diagnosis and first prescription of drug. Potential effect modification by sex was evaluated by including a drug‐by‐sex interaction term in the above models. Unadjusted Kaplan–Meier curves, estimated separately for men and women, were plotted to compare the incidence of the primary composite outcome across drug groups, and the corresponding unadjusted differences between groups were compared using log‐rank tests.
Sensitivity Analysis
We created a subgroup of matched patients using propensity score matching between the ACEIs/ARBs and sacubitril–valsartan cohorts. Participants were matched 1:1 using nearest neighbor without replacement with a caliper of 0.25. For a match to be made, the difference in the logits of the propensity scores for pairs of individuals from the 2 groups must be ≤0.25 times the pooled estimate of the common SD of the logits of the propensity scores. By default, a caliper of 0.25 is optimal in many settings. The logistic allowing us to get the propensity match had a good C‐statistic=0.951.
Propensity scores were generated by multivariable logistic regressions based on age, body mass index, Charlson comorbidity, creatinine at admission, previous MI, atrial fibrillation, valvular heart disease, hypertension, venous thromboembolism, hypercholesterolemia, hypertriglyceridemia, diabetes, chronic kidney disease, cancer, chronic obstructive pulmonary disease, dementia, diuretic use, beta‐blocker use, digoxin use, and time between first prescription and diagnosis of CHF. As above, Cox proportional hazards regression was used to compare users of ACEIs or ARBs with sacubitril–valsartan on the primary outcomes. There was no evidence of violation of the proportional hazard assumption with respect to the main exposure in either entire cohort or sex‐specific strata. 14 , 15
We use a P value of 0.01 to delineate significance using a conservative Bonferroni correction (P=0.05/6 for 2 outcomes and 4 subgroups). Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Our analyses were conducted on 7181 patients treated with sacubitril–valsartan, 25 408 patients using an ACEI, and 16 177 patients treated with ARBs. Demographic characteristics at the time of index prescription for users of ACEIs or ARBs versus sacubitril–valsartan are presented in Table 1. The prevalence of comorbidities, such as diabetes, hypertension, previous MI, cerebrovascular disease, and kidney disease, were similar among the 3 groups. Peripheral vascular disease, however, was far more prevalent among users of sacubitril–valsartan. Furthermore, diuretic use, often underscoring severity of symptoms in heart failure, did not differ markedly for patients taking sacubitril–valsartan compared with ACEIs/ARBs. In our cohort, a median of 2 months elapsed between diagnosis of CHF and index prescription of either an ACEI or ARB and 4.7 months between diagnosis and start of sacubitril–valsartan.
Table 1.
Characteristics of Patients With Congestive Heart Failure at the Time of Index Prescription by Drug Use
| ACEI (n=25 408) | ARB (n=16 177) | Sacubitril–valsartan (n=7181) | |
|---|---|---|---|
| Demographic characteristic | |||
| Age, y, mean (SD) | 66.3 (15.0) | 69.7 (14.0) | 60.2 (14.0) |
| Women, % | 43.9 | 53.4 | 33.4 |
| Systolic dysfunction, % | 66.4 | 56.1 | 92.8 |
| Drug use, % | |||
| Calcium channel blockers | 10.2 | 12.5 | 4.8 |
| Beta blockers | 46.1 | 38.4 | 38.7 |
| Diuretics | 61.7 | 65.6 | 66.2 |
| Nitrates | 5.8 | 6.5 | 2.8 |
| Digoxin | 13.4 | 13.3 | 13.3 |
| Clopidogrel | 4.0 | 5.0 | 4.0 |
| Statins | 25.8 | 32.0 | 22.7 |
| Warfarin | 6.0 | 7.3 | 5.6 |
| Amiodarone | 6.7 | 7.7 | 6.9 |
| Procedures at baseline, % | |||
| PCI | 5.6 | 4.7 | 6.8 |
| CABG | 3.1 | 2.1 | 3.4 |
| Comorbidities, % | |||
| Previous MI | 24.8 | 20.0 | 26.3 |
| Diabetes | 44.6 | 47.7 | 42.3 |
| COPD | 39.4 | 39.7 | 34.1 |
| Malignancy | 12.0 | 13.2 | 11.2 |
| Atrial fibrillation | 39.4 | 40.7 | 36.0 |
| Peripheral vascular disease | 19.1 | 18.5 | 41.1 |
| Hypertension | 82.7 | 87.0 | 84.2 |
| Chronic kidney disease | 24.7 | 27.7 | 23.7 |
| Cerebrovascular disease | 23.7 | 24.7 | 18.0 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass surgery; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Primary Composite Outcomes
Between index prescription and a median of 1.8 years (interquartile range, 0.47–2.4), 790 readmissions or deaths occurred among 7181 patients in the sacubitril–valsartan group (incidence rate, 11.7 per 100 person‐years of follow‐up) and 11 901 events in 41 585 patients in the ACEI/ARB group (incidence rate, 16.4 per 100 person‐years). Adjusted for covariates, the hazard ratio (HR) for treatment with sacubitril–valsartan compared with ACEI or ARB was 0.74 (95% CI, 0.68–0.80; P<0.001). For the combined composite outcome of readmissions, death, MI, stroke, or cardiac arrest, there were 929 primary events among 7181 patients taking sacubitril–valsartan (incidence rate, 14.0 per 100 person‐years) and 13 435 primary events in 41 585 patients treated with either an ACEI or ARB (incidence rate, 19.1 per 100 person‐years). As such, the incidence of the composite outcome was 12.9% and 32.3% in the sacubitril–valsartan and ACEI/ARB groups, respectively. Adjusted for covariates and time elapsed between CHF diagnosis and first prescription of drug, the HR for treatment with sacubitril–valsartan compared with an ACEI or ARB was 0.73 (95% CI, 0.68–0.79; P<0.001). The effect for both composite outcomes appears to be most present in the group with systolic dysfunction (Figure 1).
Figure 1. Sacubitril–valsartan vs ACEI/ARB for the primary composite outcomes among men and women for systolic and diastolic dysfunction.
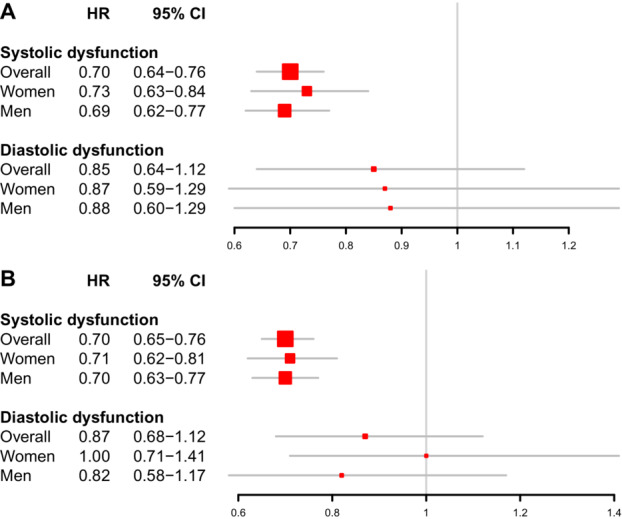
A, Readmission for heart failure or death; B, readmission for heart failure, death, stroke, MI, or cardiac arrest. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; and MI, myocardial infarction.
MI, Stroke, and Cardiac Arrest
The effect of sacubitril–valsartan was protective for all tested secondary outcomes, with the greatest risk reduction seen for MI. Between index prescription and a median of 1.6 years, 131 MIs occurred among patients taking sacubitril–valsartan compared with 1815 among patients treated with an ACEI or ARB (adjusted HR, 0.64 [95% CI, 0.51–0.81]; P=0.0001). Similarly, treatment with sacubitril–valsartan appeared to lead to reduction in strokes, but this effect was not statistically significant; 85 events occurred among users of sacubitril–valsartan versus 1337 in those treated with ACEIs or ARBs (adjusted HR, 0.82 [95% CI, 0.62–1.09]; P 0.18). Finally, the beneficial effect of sacubitril–valsartan was observed for the secondary outcome of cardiac arrest. Thirty‐two events were recorded for patients treated with sacubitril–valsartan, while 449 arrests occurred among patients taking ACEIs/ARBs (adjusted HR, 0.62 [95% CI, 0.39–0.99]; P 0.05).
Sex‐Specific Effect
We tested whether the protective effect of sacubitril–valsartan was evident and consistent in both sexes. For both composite outcomes tested, sacubitril–valsartan led to an overall reduction in events for men and women (Figure 2). When assessing the effect of sacubitril–valsartan on readmission for heart failure and death, women experienced a risk reduction of 25%, while men procured a 29% risk reduction (P sex interaction=0.03). Similarly, sacubitril–valsartan led to a risk reduction in a composite of heart failure, death, stroke, MI, and cardiac arrest by 26% among women and 29% among men (P sex interaction=0.04). As with the main effect, the protective benefit of sacubitril–valsartan appears to be most prominent in the systolic dysfunction group for both sexes (Figure 1). When assessed for individual secondary outcomes, no variation in sex for the procured benefit with sacubitril–valsartan treatment was noted (Table S2).
Figure 2. Kaplan–Meier curves comparing sacubitril–valsartan with ACEIs/ARBs for the primary composite outcomes among men and women.
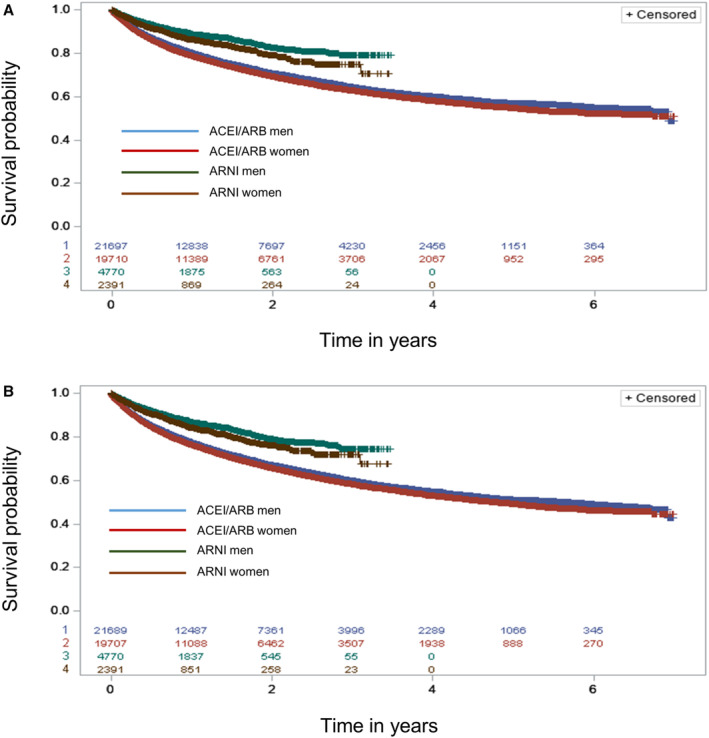
A, Readmission for heart failure or death; B, readmission for heart failure, death, stroke, MI, or cardiac arrest. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; and MI, myocardial infarction.
Sensitivity Analysis—Propensity Score Matching
The matched cohort comprised 5714 participants in the ACEI/ARB group and 5714 in the sacubitril–valsartan group. As the full cohort, the incidence of the readmission for CHF exacerbation and death was lower among patients treated with sacubitril–valsartan (12.04% versus 17.01%). Adjusted for covariates and time elapsed between CHF diagnosis and first prescription of drug, the HR for treatment with sacubitril–valsartan compared with treatment with either an ACEI or ARB was 0.75 (95% CI, 0.62–0.90; P=0.002). Similarly, when considering a composite of readmission to the hospital, death, MI, stroke, or cardiac arrest, patients using sacubitril–valsartan were at a lower risk compared with those taking an ACEI or ARB (HR, 0.73 [95% CI, 0.62–0.87]; P 0.0003). A protective effect of sacubitril–valsartan was evident for both sexes, and a sex‐specific interaction was not statistically significant (Table S3).
Safety
Among patients treated with sacubitril–valsartan, 402 primary safety events comprising either hypotension, renal dysfunction, hyperkalemia, or angioedema (5.6%) were noted compared with 5437 (13.1%) in the ACEI/ARB group. Treatment with sacubitril–valsartan was associated with fewer side events (0.63 [95% CI, 0.57–0.70]; P<0.001) (Table 2). No interaction by sex was noted.
Table 2.
Effect of Sacubitril–Valsartan Compared With ACEIs and ARBs for Composite Side Effects
| Outcome | Overall HR (95% CI) | Women HR (95% CI) | Men HR (95% CI) | Interaction P value |
|---|---|---|---|---|
| Composite of hypotension, renal dysfunction, hyperkalemia, and angioedema | 0.63 (0.57–0.70); P<0.001 | 0.65 (0.54–0.77); P<0.001 | 0.61 (0.53–0.71); P<0.001 | 0.56 |
| Systolic | 0.67 (0.59–0.76); P<0.001 | 0.74 (0.60–0.90); P 0.004 | 0.64 (0.55–0.74); P<0.001 | 0.21 |
| Diastolic | 0.58 (0.40–0.83); P 0.003 | 0.43 (0.22–0.84); P 0.01 | 0.67 (0.43–1.06); P 0.09 | 0.26 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; and HR, hazard ratio.
Discussion
We studied sacubitril–valsartan, an inhibitor of angiotensin II receptor and neprilysin, in the treatment of heart failure. Compared with patients treated with only ACEIs/ARBs, those switched to sacubitril–valsartan experienced a lower death rate from cardiovascular causes or readmission to the hospital for heart failure. The same was noted for a composite of cardiac outcomes, including readmissions, death, stroke, MI, and cardiac arrest. In contrast to previous literature, we were able to test this effect among men and women and found a risk reduction from sacubitril–valsartan to a similar degree for both sexes. Notably, the magnitude of the advantage may be limited to patients experiencing systolic dysfunction.
Based on our findings in conjunction with other small cohorts 16 and a recently published larger cohort, 17 we recommend the use of sacubitril–valsartan as first line in the treatment of heart failure. While the Canadian Cardiovascular Society guidelines 15 recommend first‐line use of sacubitril–valsartan, other guideline groups 18 , 19 continue to recommend its use as a replacement. Above and beyond the previous literature, our findings suggest that patients currently treated with ACEIs/ARBs may benefit from switching to sacubitril–valsartan. There is mounting evidence that sacubitril–valsartan leads to reduction in death and hospitalizations for acute decompensated heart failure among patients with reduced ejection fraction. To these data, we add that its use shows improved outcomes among women with a good safety profile. Although meta‐analyses of primary trials have sought to demonstrate the effectiveness of sacubitril–valsartan among patients with preserved ejection fraction, 20 especially in women, there are not yet sufficient data to replicate these findings in real‐world population‐based cohorts. It is imperative that future studies seek to validate the effectiveness of sacubitril–valsartan among patients with diastolic dysfunction and notably inquire whether the effects are present for both men and women as previously suggested by PARAGON‐HF.
There are several key strengths to our analysis. First, our data come from a large and diverse data set; MarketScan includes data from hospitals across the United States that vary significantly in their patient population and case mix, making our studied cohort very generalizable. Second, as these data come from electronic medical records, the doses and drugs chosen in each family were determined by the prescribing physicians on the basis of the patient's tolerability. Therefore, our results reflect real‐world practices and risk reduction from treatment with sacubitril–valsartan that is seen among patients outside of the strict clinical trial setting. Third, given a sufficient sample size by sex, we are the first large study to demonstrate an equivalent benefit from sacubitril–valsartan in women as men. Finally, our study provides support for switching to sacubitril–valsartan, as there appears to be clinical benefit above and beyond that acquired by ACEIs/ARBs.
Several notable limitations do exist. First, as patients were not randomly allocated, there likely exists a difference with respect to baseline risk between patients treated with ACEIs/ARBs versus those switched to sacubitril–valsartan. While our analyses were adjusted for baseline covariates, there remains a possibility of residual confounding. Additionally, a comparison of measured baseline characteristics of patients using ACEIs, ARBs, and sacubitril–valsartan confirms no significant difference in illness severity. Finally, the nonrandomized nature means causality cannot be established between treatment with an angiotensin receptor–neprilysin inhibitor and our outcomes. Our analysis does not account for time‐varying effects or competing risk of death. To overcome limitations of nonrandomized studies, we created a second cohort propensity matched on baseline characteristics as well as time. Our matched sensitivity analysis showed a similar effect size to our main cohort. Second, dosing data for ACEIs, ARBs, and sacubitril–valsartan were not available, and thus we were unable to assess and compare whether men and women received appropriately therapeutic doses. Third, we set out to understand whether sacubitril–valsartan was as effective among patients with systolic dysfunction as in patients with diastolic dysfunction and whether this effect varied by sex in both groups. Our data suggest that a vast majority of patients (92.8%) were prescribed this combined treatment only if diagnosed with systolic dysfunction. This reflects the current prescribing practices of clinicians given that guidelines largely endorse use of these drugs among patients with reduced ejection fraction. We thus did not have a sufficient sample size to reliably answer this question. Hence, future studies should focus on uncovering the effectiveness of this drug among women with preserved ejection fraction to accurately guide clinical practice given the signal for a beneficial effect in PARAGON‐HF. Finally, effect sizes ascertained from our propensity score–matched analysis represent the average treatment effect among those treated with an angiotensin receptor–neprilysin inhibitor rather than the average treatment effect in the overall cohort. 21
In conclusion, treatment with sacubitril–valsartan is more effective at reducing death and admission to the hospital for heart failure compared with ACEIs or ARBs among patients with systolic dysfunction; treatment in diastolic dysfunction requires further investigation.
Sources of Funding
This work was supported by a CIHR TEAM GRANT–Gender Outcomes International Group: to Further Well‐being Development (GOING_FWD), CIHR Funding Reference # 161904.
Disclosures
None.
Supporting information
Table S1–S3
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028865
For Sources of Funding and Disclosures, see page 8.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J. 2019;40:3859–3868. doi: 10.1093/eurheartj/ehz835 [DOI] [PubMed] [Google Scholar]
- 3. Hsich EM. Sex differences in advanced heart failure therapies. Circulation. 2019;139:1080–1093. doi: 10.1161/CIRCULATIONAHA.118.037369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sohani ZN, Alyass A, Pilote L. Clinical trials of heart failure–is there a question of sex? Can J Cardiol. 2021;37:1303–1309. doi: 10.1016/J.CJCA.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 5. Kenchaiah S, Vasan RS. Heart failure in women–insights from the Framingham heart study. Cardiovasc Drugs Ther. 2015;29:377–390. doi: 10.1007/s10557-015-6599-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheppard R, Behlouli H, Richard H, Pilote L. Effect of gender on treatment, resource utilization, and outcomes in congestive heart failure in Quebec. Canada. Am J Cardiol. 2005;95:955–959. doi: 10.1016/j.amjcard.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 7. Jhund PS, MacIntyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JWT, Capewell S, McMurray JJV. Long‐term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003. A population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172 [DOI] [PubMed] [Google Scholar]
- 8. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted county, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.CIR.98.21.2282 [DOI] [PubMed] [Google Scholar]
- 10. Tu JV, Nardi L, Fang J, Liu J, Khalid L, Johansen H. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994–2004. CMAJ. 2009;180:E118–E125. doi: 10.1503/cmaj.081197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well‐defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033 [DOI] [PubMed] [Google Scholar]
- 12. Eisenberg E, Di Palo KE, Piña IL. Sex differences in heart failure. Clin Cardiol. 2018;41:211–216. doi: 10.1002/clc.22917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018;380:539–548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 14. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 15. McDonald M, Virani S, Chan M, Ducharme A, Ezekowitz JA, Giannetti N, Heckman GA, Howlett JG, Koshman SL, Lepage S, et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37:531–546. doi: 10.1016/j.cjca.2021.01.017 [DOI] [PubMed] [Google Scholar]
- 16. Vicent L, Ayesta A, Esteban‐Fernández A, Gómez‐Bueno M, De‐Juan J, Díez‐Villanueva P, Iniesta ÁM, Rojas‐González A, Bover‐Freire R, et al. Sex influence on the efficacy and safety of sacubitril/valsartan. Cardiology. 2019;142:73–78. doi: 10.1159/000498984 [DOI] [PubMed] [Google Scholar]
- 17. Tan NY, Sangaralingham LR, Sangaralingham SJ, Yao X, Shah ND, Dunlay SM. Comparative effectiveness of sacubitril‐valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8:43–54. doi: 10.1016/j.jchf.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld JA, Masoudi FA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution set Oversight Committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 19. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 20. Salah HM, Fudim M, Al'Aref SJ, Khan MS, Almarzooq ZI, Devabhaktuni SR, Mentz RJ, Butler J, Greene SJ. Meta‐analysis of efficacy of sacubitril/valsartan in heart failure with preserved ejection fraction. Am J Cardiol. 2021;145:165–168. doi: 10.1016/j.amjcard.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 21. Stuart E. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3


