Abstract
Background
Hypertension is an important cause of morbidity, which predisposes patients to major cardiovascular events and mortality. The aim of this study was to explore the association between adherence to antihypertensive medication and clinical outcomes in adult patients with cancer.
Methods and Results
Using the 2002 to 2013 Korean National Health Insurance Service–National Sample Cohort, we extracted adult patients with cancer treated with antihypertensive medications. Based on the medication possession ratio value, participants were divided into 3 groups: good (medication possession ratio ≥0.8), moderate (0.5≤ medication possession ratio <0.8), and poor (medication possession ratio <0.5) adherence groups. The primary outcomes were overall and cardiovascular mortality. The secondary outcome was cardiovascular events requiring hospitalization due to major cardiovascular diseases. Among 19 246 patients with cancer with concomitant hypertension, 66.4% were in the nonadherence group (26.3% were moderate and 40.0% were poor adherence group). Over a median of 8.4 years of follow‐up, 2752 deaths and 6057 cardiovascular events occurred. Compared with the good adherence group, the moderate and poor adherence groups had a 1.85‐fold and 2.19‐fold increased risk for overall mortality, and 1.72‐fold and 1.71‐fold elevated risk for cardiovascular mortality, respectively, after adjustment for possible confounders. Furthermore, the moderate and poor adherence groups had a 1.33‐fold and 1.34‐fold elevated risk of new‐onset cardiovascular events, respectively. These trends were consistent across cardiovascular event subtypes.
Conclusions
Nonadherence to antihypertensive medication was common in patients with cancer and was associated with worse clinical outcomes in adult patients with cancer with hypertension. More attention should be paid to improving adherence to antihypertensive medication among patients with cancer.
Keywords: adherence, cancer, cardiovascular disease, hypertension, survivorship
Subject Categories: Hypertension, Cardiovascular Disease, Cardio-Oncology
Nonstandard Abbreviations and Acronyms
- MPR
medication possession ratio
- NHIS
National Health Insurance Service
Clinical Perspective.
What Is New?
A substantial proportion (2 in 3) of patients with cancer showed nonadherence to antihypertensive medication.
Nonadherence to antihypertensive medication was associated with increased mortality and morbidity in adult patients with cancer.
What Are the Clinical Implications?
More attention should be focused on improving antihypertensive medication adherence among patients with cancer for better cardiovascular outcomes.
Hypertension is a leading risk factor for early death and disability worldwide. 1 , 2 However, the control rate of hypertension is dismally low. According to a report by the Noncommunicable Disease Risk Factor Collaboration, hypertension is under control in only 18% of men and 23% of women suffering from it, as of 2019. 1 One of the factors associated with inadequate control of hypertension is nonadherence to antihypertensive medication. 3 , 4 A recent meta‐analysis involving 13 688 patients with hypertension reported that one‐third of patients with hypertension with comorbidities were nonadherent to antihypertensive medications. 5
Recently, patients with cancer and survivors have been recognized as vulnerable subgroups who have an elevated risk of developing hypertension and its complications. 6 , 7 , 8 A cohort study reported cardiovascular disease as the most common cause of death other than cancer‐related deaths in patients with cancer. 8 , 9 Hypertension is one of the common comorbidities among patients with cancer and survivors. 10 However, hypertension is still underrecognized and undertreated both by physicians and patients during the management of patients with cancer, probably due to the main focus of curing the cancer. Furthermore, the clinical implications of nonadherence to antihypertensive medication among patients with cancer remain uncertain. Therefore, we evaluated the association between adherence to antihypertensive medication and clinical outcomes (overall/cardiovascular mortality and cardiovascular events) in patients with cancer with hypertension.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Database and Study Population
For this analysis, we used data from the Korean National Health Insurance Service (NHIS)‐National Sample Cohort database. The Korean NHIS is a compulsory health insurance system covering the entire Korean population. The NHIS‐National Sample Cohort is a representative nationwide population‐based cohort established to provide researchers and policymakers with information on the citizens' use of health insurance and services. A systematic stratified random sampling method was used, and 2.2% of the total Korean population was sampled in 2002 and followed until 2013. The database contains information about demographic characteristics and medical records (including diagnostic codes, prescriptions, and inpatient and outpatient visits). 11
From the NHIS‐National Sample Cohort 2002 to 2013 database, we extracted details of patients with cancer (n=99 354). In the current analyses, we included the following 10 most common adult solid cancers using International Classification of Diseases, Tenth Revision (ICD‐10) codes: breast cancer (C50), colon cancer (C18–C20), gastric cancer (C16), gallbladder cancer (C23), liver cancer (C22), lung cancer (C34), non‐Hodgkin's disease (C82–C86), ovarian cancer (C56), prostate cancer (C61), and renal cell carcinoma (C64). During this process, those with other cancers were excluded (n=29 778). We excluded patients with previous cancer at the baseline (2002) to confine our study population to patients with newly diagnosed cancer (n=11 339). Patients <20 or ≥85 years of age (n=1863) and those with low income (n=2181) were excluded. To minimize the effect of reverse causality, we further excluded those who died or experienced cardiovascular disease (CVD) within the first 2 years of the study (n=16 543). Additionally, we excluded patients who had not been prescribed antihypertensive medications during the whole study period (n=18 404). Ultimately, 19 246 individuals were included in the study (Figure S1). The institutional review board of Severance Hospital approved the study protocol (4‐2015‐0140) and waived the requirement of informed consent because the data provided by NHIS were anonymized.
Ascertainment of Adherence to Antihypertensive Medication
Adherence to antihypertensive medication was evaluated using the medication possession ratio (MPR), which was calculated as the number of days medication was supplied divided by the refill period. 12 Based on the MPR, participants were divided into good (MPR ≥0.8), moderate (0.5≤ MPR <0.8), and poor (MPR <0.5) adherence groups. 13 We defined nonadherence as an MPR of <0.8, as in previous studies. 12 , 13
Study Outcomes
The primary outcomes were overall and cardiovascular mortality. We identified deaths and causes by leveraging the mortality records of the National Statistical Office of Korea, linked to the NHIS database. The secondary outcomes were composite cardiovascular events or individual cardiovascular events. In our study, cardiovascular events were defined as first hospitalization due to ischemic heart disease (IHD; I20–I25), peripheral artery disease (PAD; I70–I79), cerebrovascular accident (CVA; I60–I69), and heart failure (HF; I40–I43, I50–I52). Chronic kidney disease was identified using ICD‐10 codes (N18, N19). To eliminate the effect of any previous history of CVD, we applied 2 methods. First, we adjusted for previous history of CVD (either outpatient or inpatient visits due to IHD, PAD, CVA, and HF) in the entire population (n=19 246). Second, we further excluded those who had a history of admission for CVD (IHD, PAD, CVA, and HF) and then repeated the same analyses. In the case of the second method (results after exclusion of those with prior admission history for CVD), we adjusted for the previous history of outpatient medical service use for CVD, because some patients still might have an outpatient visit history for CVD. The second method may serve as a sensitivity test. Individuals were followed up from the point of cancer diagnosis until the primary or secondary end point or the end of the study (December 31, 2013).
Statistical Analysis
Categorical variables were presented as numbers with percentages. A Cox proportional hazard regression model was constructed to identify the hazard associated with nonadherence to antihypertensive medication. Cox models applied cause‐specific hazard methods for competing risks, censoring those with competing events (other causes of death) or end of follow‐up. Crude and adjusted hazard ratios (HRs) with 95% CIs were calculated after controlling for potential confounders (age, sex, residential area, income status, CVD history, and chronic kidney disease history). Log‐log plots were performed to test the proportional hazards assumption, and we found no evidence of violating the proportional hazards assumption for MPR for various outcomes. Kaplan‐Meier curves were plotted to show the cumulative incidence of primary outcomes. The log‐rank test was used to identify differences in primary outcomes between groups. In regard to overall mortality, we performed subgroup analyses by sex (men and women) and age (<60 and ≥60 years). Statistical significance was set at a P value of <0.05. SAS version 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses.
RESULTS
Baseline Characteristics of the Study Population
Among 19 246 patients with cancer with concomitant hypertension, 62.4% were men and 42.8% were ≥65 years of age. Specifically, 8.3% were 20 to 44 years of age, 48.8% were 45 to 64 years of age, 30.2% were 65 to 74 years of age, and 12.6% were 75 to 84 years of age. The median MPR in our study cohort was 0.64 (interquartile range, 0.20–0.86). The baseline characteristics of the study population are summarized in Table S1. In the current cohort, 33.6% were in the good adherence group and 66.4% were in the nonadherence group (26.3% were moderate, and 40.0% were in the poor adherence group). The poor adherence group was younger, lived in suburban areas, and had lower income status compared with the good adherence group (Table S1). Over a median of 8.4 years of follow‐up, 2752 deaths and 6057 cardiovascular events occurred.
Association of Antihypertensive Medication Adherence and Mortality
Compared with the good adherence group, the moderate and poor adherence groups had 1.85‐fold and 2.19‐fold elevated risk, respectively, for overall mortality after controlling for possible confounding factors (age, sex, residential area, income level, previous history of CVD, and previous history of chronic kidney disease) (adjusted HR, 1.85 [95% CI, 1.66–2.06] for the moderate adherence group, and adjusted HR, 2.19 [95% CI, 1.98–2.42] for the poor adherence group; Table 1). In terms of CVD mortality, the moderate and poor adherence groups had 1.72‐fold and 1.71‐fold elevated risk, respectively, compared with the good adherence group (adjusted HR, 1.72 [95% CI, 1.32–2.24] for the moderate adherence group, and adjusted HR, 1.71 [95% CI, 1.33–2.21] for the poor adherence group).
Table 1.
Risk of Antihypertensive Nonadherence on Mortality in Patients With Cancer (n=19 246)
| Outcome | Group | No. of events | Crude | Multivariate adjusted* | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| All‐cause mortality | Good | 534 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 822 | 2.07 (1.86–2.31) | <0.001 | 1.85 (1.66–2.06) | <0.001 | |
| Poor | 1396 | 2.23 (2.02–2.47) | <0.001 | 2.19 (1.98–2.42) | <0.001 | |
| Cardiovascular mortality | Good | 92 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 141 | 2.08 (1.60–2.70) | <0.001 | 1.72 (1.32–2.24) | <0.001 | |
| Poor | 184 | 1.71 (1.33–2.20) | <0.001 | 1.71 (1.33–2.21) | <0.001 | |
HR indicates hazard ratio.
Adjusted for sex, age, residential area, income level, previous history of cardiovascular disease (either outpatient visit or hospitalization due to ischemic heart disease, peripheral artery disease, cerebrovascular accident, and heart failure), and previous history of chronic kidney disease (either outpatient visit or hospitalization due to chronic kidney disease).
The Kaplan‐Meier curve demonstrated worse overall survival in both the moderate and poor adherence groups. Specifically, the separation of the curve between the good adherence and moderate/poor adherence groups began early (within the first 0.5 years after the lag time period of 2 years) and overlap of the curves of moderate and poor adherence groups, with a slightly worse outcome for the poor adherence group (Figure 1). Generally, comparable results were obtained in each subgroup analysis of sex (men versus women) and age (<60 versus ≥60 years) (Figure 2, Table S2).
Figure 1. Cumulative Kaplan‐Meier estimates of overall survival according to antihypertensive drug adherence.
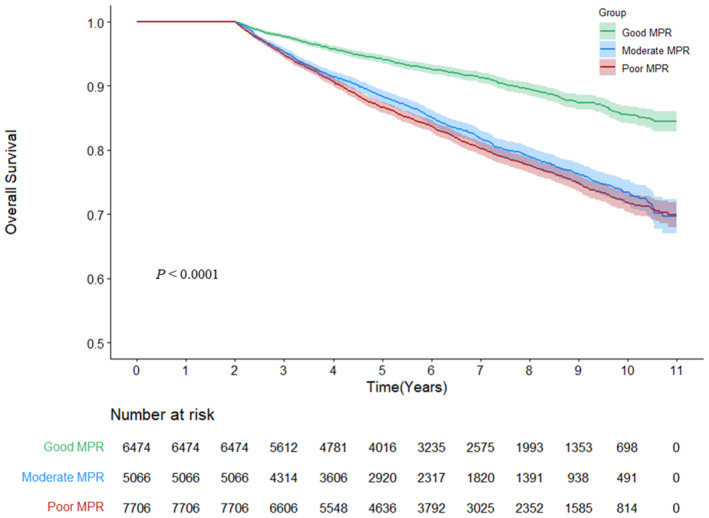
MPR indicates medication possession ratio.
Figure 2. Cumulative Kaplan‐Meier estimates of overall survival according to antihypertensive drug adherence by age and sex.
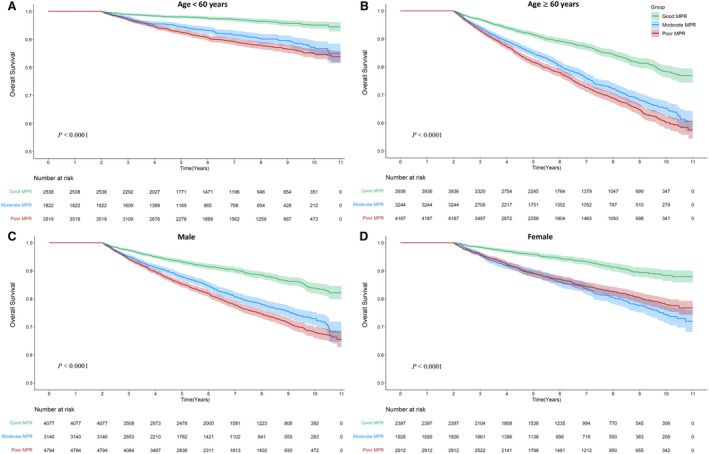
A, Cumulative Kaplan‐Meier estimates of overall survival according to antihypertensive drug adherence in patients <60 years of age. B, Cumulative Kaplan‐Meier estimates of overall survival according to antihypertensive drug adherence in patients ≥60 years of age. C, Cumulative Kaplan‐Meier estimates of overall survival according to antihypertensive drug adherence in male patients. D, Cumulative Kaplan‐Meier estimates of overall survival according to antihypertensive drug adherence in female patients. MPR indicates medication possession ratio.
Association of Antihypertensive Adherence With Future CVD Occurrence
Compared with the good adherence group, the moderate and poor adherence groups had a 1.33‐fold and 1.34‐fold elevated risk for new‐onset any CVD, respectively, after controlling for age, sex, residential area, income level, previous history of CVD, and previous history of CKD (adjusted HR, 1.33 [95% CI, 1.25–1.42] for the moderate adherence group, and adjusted HR, 1.34 [95% CI, 1.26–1.42] for the poor adherence group) (Table 2). In regard to each specific subtype of CVD (IHD, PAD, CVA, and HF), marked risk elevation was consistently observed in the moderate and poor adherence groups. For the sensitivity analyses, we performed the same analyses after excluding those with a prior history of admission for IHD, PAD, CVA, and HF. Generally, the direction and size of risks remained unchanged (Table S3).
Table 2.
Risk of Antihypertensive Nonadherence on Future CVD Occurrence in Patients With Cancer (n=19 246)
| Outcome | Group | No. of events | Crude | Multivariate adjusted* | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| New‐onset any CVD | Good | 1765 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 1817 | 1.44 (1.34–1.53) | <0.001 | 1.33 (1.25–1.42) | <0.001 | |
| Poor | 2475 | 1.23 (1.16–1.31) | <0.001 | 1.34 (1.26–1.42) | <0.001 | |
| New‐onset IHD | Good | 1197 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 1181 | 1.34 (1.23–1.45) | <0.001 | 1.25 (1.15–1.36) | <0.001 | |
| Poor | 1553 | 1.11 (1.03–1.20) | 0.0066 | 1.21 (1.13–1.31) | <0.001 | |
| New‐onset PAD | Good | 286 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 359 | 1.69 (1.45–1.97) | <0.001 | 1.54 (1.31–1.79) | <0.001 | |
| Poor | 412 | 1.23 (1.06–1.43) | 0.007 | 1.32 (1.13–1.54) | 0.0004 | |
| New‐onset CVA | Good | 727 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 828 | 1.55 (1.40–1.71) | <0.001 | 1.41 (1.28–1.56) | <0.001 | |
| Poor | 1064 | 1.26 (1.15–1.38) | <0.001 | 1.38 (1.26–1.52) | <0.001 | |
| New‐onset HF | Good | 328 | 1.00 (reference) | … | 1.00 (reference) | … |
| Moderate | 455 | 1.87 (1.62–2.15) | <0.001 | 1.65 (1.43–1.9) | <0.001 | |
| Poor | 594 | 1.56 (1.36–1.78) | <0.001 | 1.64 (1.43–1.88) | <0.001 | |
CVA indicates cardiovascular accident; CVD, cardiovascular disease; HF, heart failure; HR, hazard ratio; IHD, ischemic heart disease; and PAD, peripheral artery disease.
Adjusted for sex, age, residential area, income level, previous history of CVD (either outpatient visit or hospitalization due to IHD, PAD, CVA, and HF), and previous history of chronic kidney disease (either outpatient visit or hospitalization due to chronic kidney disease).
DISCUSSION
In this representative, nationwide population‐based cohort involving 19 246 patients with cancer with concomitant hypertension, we found that two‐thirds (66.4%) of the patients with cancer were nonadherent to their antihypertensive medications. Nonadherence to antihypertensive medication was significantly associated with adverse cardiovascular outcomes. Moreover, moderate adherence to antihypertensive medication, not to mention poor antihypertensive medication adherence, was significantly associated with an elevated risk for all‐cause and cardiovascular mortality as well as new‐onset CVD occurrence. This finding was consistent across all age and sex groups. The present study emphasizes that proper hypertension management, particularly continuous taking of antihypertensive medication, is crucial for favorable cardiovascular outcomes in patients with cancer.
Because the lifespan of patients with cancer has increased owing to advances in cancer therapy, CVD has been considered a significant health issue in cancer survivors in recent years. 14 , 15 , 16 The increased risk of CVD in these patients is largely attributable to the high prevalence of hypertension in patients with cancer compared with the general population, 7 which originates from shared risk factors as well as cancer therapy–related problems. 6 , 17 Exposure to chemotherapy (eg, vascular endothelial growth factor inhibitors, cisplatin derivatives) and some cancers (eg, renal cell carcinoma) may predispose to hypertension through nephrotoxicity, effects on endothelial function, sympathetic activity, and renin‐angiotensin system activity. 6
Despite the clinical implications, the management of hypertension and prevention of CVD in patients with cancer has received inadequate attention. 18 , 19 , 20 , 21 , 22 Adherence to antihypertensive medication is an important but underrecognized aspect in the management of hypertension. 23 A large cohort study in Korea showed poor adherence to antihypertensive medication among cancer survivors compared with the general population. 24 In that study, cancer survivors had a 15% higher risk of nonadherence to antihypertensive medication than the general population (adjusted odds ratio, 0.85 [95% CI, 0.82–0.88]). The median MPR value was lower among cancer survivors (0.84) than in the general population (0.86). The good adherence group (MPR ≥0.8) comprised 54.4% of cancer survivors and 57.5% of the general population. 24 Unfortunately, most studies, including our study, have shown even worse antihypertensive adherence status (the proportion of good adherence was <40%), particularly in those beginning antihypertensive medication, 25 younger adults, 23 , 26 and patients with cancer. In the current study, the median MPR was 0.64, and only 1 in 3 patients were in the good adherence group (33.6%). Furthermore, 40.0% of the population turned out to be in the poor adherence group (MPR <0.5).
It is important to note that the detrimental effects of nonadherence to antihypertensive medication are substantial. Previous population‐based cohort studies have reported a gradual elevation of risk according to decreased adherence to antihypertensive drugs in general patients with hypertension. 13 , 26 These associations were consistent across each subtype of CVD, such as IHD, stroke, HF, and cardiovascular death. One Italian primary care registry reported that high adherence to antihypertensive medication was associated with decreased cardiovascular events. 25 A significant decrease in the risk of a cardiovascular event was only evident in the high adherence group. Taken together, these data clearly demonstrate the risk of nonadherence of any degree. 13 , 25 , 26 Remarkably, the adverse effect of nonadherence to antihypertensive drugs was also observed in younger patients with cancer, similar to a previous study. 26 Moreover, the relative risk of nonadherence to antihypertensive drugs was higher in younger individuals, who tend to display lower compliance and do not persist with the antihypertensive therapy; this finding alarms the patients as well as the clinicians. Additionally, the current study advances previous observations by highlighting the risk of nonadherence to antihypertensive medication, which also applies to patients with cancer.
There are several possible explanations for the relationship between adherence to medication and clinical outcomes. First, good adherence to antihypertensive drugs leads to improved blood pressure (BP) control. 27 , 28 A meta‐analysis found that patients adherent to antihypertensive medication showed better BP control than those who were nonadherent. 29 Another recent study also reported that a greater BP reduction was observed in the adherent group 1 to 3 years after initiating antihypertensive medication compared with the nonadherent group. 26 Second, the poor adherence group might include those with decreased health literacy, which is often accompanied by lower income and poor education. 3 , 30 , 31 , 32 , 33 Although we adjusted for the possible confounding effect of income status and residence area, health literacy could still affect morbidity and mortality. Third, poor adherence might be associated with detrimental lifestyle factors, such as heavy drinking, smoking, physical inactivity, and/or unhealthy dietary pattern. 3 , 31
Several factors may cause nonadherence, particularly in patients with cancer, compared with the general population. First, some patients with cancer place the most importance on cancer treatment, so they may be negligent about noncancer diseases, including hypertension, and display lower adherence to the medication for these noncancer diseases. 24 , 34 In addition, it is well known that cancer survivors are generally prescribed numerous drugs that are to be taken several times a day. Polypill and complex drug regimens may reduce adherence to antihypertensive drug adherence. 4 , 24 , 31 Furthermore, depression that may affect patients with cancer can impair medication adherence. 35 These patients suffering from depression are often in poor physical condition and lack confidence in their own ability to care for themselves, which can adversely affect adherence to medications. 4 , 24 Thus, to improve medication adherence in patients with cancer, physicians should build a relationship of trust with patients and spend sufficient time in counseling them about managing their comorbidities such as hypertension. 5 , 28 In addition, it may be helpful to simplify the drug regimen by prescribing a single combination pill. Finally, a multidisciplinary team‐based approach might be helpful in evaluating and addressing the multidimensional status of patients with cancer.
Study Limitations
The present study has several potential limitations. First, causal inference is limited, and unmeasured confounders might affect the results, because our study was based on a retrospective observation. We did not have information on lifestyle factors (eg, smoking, alcohol consumption, and physical activity) and detailed information on cancer (eg, cancer staging and treatment) due to limitations in the NHIS‐National Sample Cohort database. For example, the status of cancer (advanced stage) or cancer treatment (eg, chemotherapy) might cause hypotension and negatively affect antihypertensive drug adherence and overall mortality. In this study, we attempted to minimize the confounding effect of cancer terminal status by excluding those patients who died within 2 years of the baseline. Additional analyses for several specific cancers, of which the prognosis is different, showed similar results (Figure S2). Considering that it is not possible to conduct randomized controlled trials for antihypertensive drug adherence in patients with cancer, the present analyses could provide relevant and valuable clinical information. Second, there was a lack of data on the degree of BP reduction after taking antihypertensive drugs. However, it is well known from previous studies that the good adherence group demonstrates a better BP‐lowering effect. Third, although this study used a large nationwide database, it consisted of only a limited number of eligible individuals. Therefore, the study sample size was insufficient to investigate malignancies with a relatively low incidence or the effects of individual anticancer therapies. Fourth, the method that we used to evaluate medication adherence, based on prescription refill data, might not fully capture the true adherence state. In the literature, various methods are suggested to evaluate medication adherence as follows: electronic monitoring system, monitoring drug/metabolite levels in blood or urine, patient self‐report, and prescription refill data. Although the prescription refill data (presented as MPR value in our study) enabled evaluation in a large population, it is rather an indirect method for evaluating drug adherence. 3 , 36 Finally, the results of the present study cannot be directly applicable to other populations. Subsequent studies in other countries with more detailed information on cancer (stages and histologic types) would be helpful to solidify the results.
CONCLUSIONS
A substantial proportion (2 in 3) of patients with cancer displayed nonadherence to antihypertensive medication in the current study. Nonadherence to antihypertensive medication was associated with worse all‐cause and cardiovascular mortality, as well as new‐onset CVD in adult patients with cancer with hypertension. More attention should be focused on improving antihypertensive medication adherence among patients with cancer to achieve better cardiovascular outcomes.
Source of Funding
This work was supported by the National Research Foundation of Korea grant funded by the Ministry of Science and Information and Communication Technologies (NRF‐2021R1F1A1063430), by the Catholic Medical Center Research Foundation (2022), by the Research Foundation of Internal Medicine, The Catholic University of Korea, by the Korean Society of Cardiovascular Disease Prevention, and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI19C1211). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S2
Acknowledgments
The authors express sincere gratitude to the Working Group on Cardio‐Oncology of the Korean Society of Cardiology.
This article was sent to Tochukwu M. Okwuosa, DO, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029362
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Jong‐Chan Youn, Email: jong.chan.youn@gmail.com.
Hyeon Chang Kim, Email: hckim@yuhs.ac.
REFERENCES
- 1. Zhou B, Carrillo‐Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/s0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Blobal Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/s0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung MH, Ihm SH. Improving the quality of hypertension management: multifaceted approach. Korean Circ J. 2019;49:528–531. doi: 10.4070/kcj.2019.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choudhry NK, Kronish IM, Vongpatanasin W, Ferdinand KC, Pavlik VN, Egan BM, Schoenthaler A, Houston Miller N, Hyman DJ. Medication adherence and blood pressure control: a scientific statement from the American Heart Association. Hypertension. 2022;79:e1–e14. doi: 10.1161/hyp.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta‐analysis. Medicine (Baltimore). 2017;96:e5641. doi: 10.1097/md.0000000000005641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen JB, Geara AS, Hogan JJ, Townsend RR. Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. JACC Cardio Oncol. 2019;1:238–251. doi: 10.1016/j.jaccao.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/jco.2013.49.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Youn JC, Chung WB, Ezekowitz JA, Hong JH, Nam H, Kyoung DS, Kim IC, Lyon AR, Kang SM, Jung HO, et al. Cardiovascular disease burden in adult patients with cancer: an 11‐year nationwide population‐based cohort study. Int J Cardiol. 2020;317:167–173. doi: 10.1016/j.ijcard.2020.04.080 [DOI] [PubMed] [Google Scholar]
- 9. Kim DY, Park MS, Youn JC, Lee S, Choi JH, Jung MH, Kim LS, Kim SH, Han S, Ryu KH. Development and validation of a risk score model for predicting the cardiovascular outcomes after breast cancer therapy: the CHEMO‐RADIAT score. J Am Heart Assoc. 2021;10:e021931. doi: 10.1161/jaha.121.021931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goytia EJ, Lounsbury DW, McCabe MS, Weiss E, Newcomer M, Nelson DJ, Brennessel D, Rapkin BD, Kemeny MM. Establishing a general medical outpatient clinic for cancer survivors in a public city hospital setting. J Gen Intern Med. 2009;24(Suppl 2):S451–S455. doi: 10.1007/s11606-009-1027-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service‐National Sample Cohort (NHIS‐NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 12. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018 [DOI] [PubMed] [Google Scholar]
- 13. Kim S, Shin DW, Yun JM, Hwang Y, Park SK, Ko YJ, Cho B. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension. 2016;67:506–512. doi: 10.1161/hypertensionaha.115.06731 [DOI] [PubMed] [Google Scholar]
- 14. Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, Mertens AC. Late mortality among 5‐year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/jco.2008.21.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, Stovall M, Robison LL, Hudson MM. Morbidity and mortality in long‐term survivors of Hodgkin lymphoma: a report from the childhood cancer survivor study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung WB, Youn JC, Youn HJ. Cardiovascular complications of novel anti‐cancer immunotherapy: old problems from new agents? Korean Circ J. 2020;50:743–753. doi: 10.4070/kcj.2020.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim DY, Youn JC, Park MS, Lee S, Choi SW, Ryu KH, Kim LS, Shim MS, Lee JJ, Han S. Cardiovascular outcome of breast cancer patients with concomitant radiotherapy and chemotherapy: a 10‐year multicenter cohort study. J Cardiol. 2019;74:175–181. doi: 10.1016/j.jjcc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 18. Tini G, Sarocchi M, Tocci G, Arboscello E, Ghigliotti G, Novo G, Brunelli C, Lenihan D, Volpe M, Spallarossa P. Arterial hypertension in cancer: the elephant in the room. Int J Cardiol. 2019;281:133–139. doi: 10.1016/j.ijcard.2019.01.082 [DOI] [PubMed] [Google Scholar]
- 19. Petrelli F, Ghidini A, Cabiddu M, Perego G, Lonati V, Ghidini M, Oggionni E, Galli E, Moleri G, Barni S, et al. Effects of hypertension on cancer survival: a meta‐analysis. Eur J Clin Invest. 2021;51:e13493. doi: 10.1111/eci.13493 [DOI] [PubMed] [Google Scholar]
- 20. Kidoguchi S, Sugano N, Tokudome G, Yokoo T, Yano Y, Hatake K, Nishiyama A. New concept of onco‐hypertension and future perspectives. Hypertension. 2021;77:16–27. doi: 10.1161/hypertensionaha.120.16044 [DOI] [PubMed] [Google Scholar]
- 21. Jung MH, Yi SW, An SJ, Yi JJ, Ihm SH, Han S, Ryu KH, Jung HO, Youn HJ. Associations between the triglyceride‐glucose index and cardiovascular disease in over 150,000 cancer survivors: a population‐based cohort study. Cardiovasc Diabetol. 2022;21:52. doi: 10.1186/s12933-022-01490-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, Greenbaum N, Mauch P, Lipshultz SE. Cardiovascular status in long‐term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/jco.2004.09.109 [DOI] [PubMed] [Google Scholar]
- 23. Kim HC, Cho SMJ, Lee H, Lee HH, Baek J, Heo JE. Korea hypertension fact sheet 2020: analysis of nationwide population‐based data. Clin Hypertens. 2021;27:8. doi: 10.1186/s40885-021-00166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin DW, Park JH, Park JH, Park EC, Kim SY, Kim SG, Choi JY. Antihypertensive medication adherence in cancer survivors and its affecting factors: results of a Korean population‐based study. Support Care Cancer. 2010;19:211–220. doi: 10.1007/s00520-009-0802-4 [DOI] [PubMed] [Google Scholar]
- 25. Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/circulationaha.108.830299 [DOI] [PubMed] [Google Scholar]
- 26. Lee H, Yano Y, Cho SMJ, Heo JE, Kim DW, Park S, Lloyd‐Jones DM, Kim HC. Adherence to antihypertensive medication and incident cardiovascular events in young adults with hypertension. Hypertension. 2021;77:1341–1349. doi: 10.1161/hypertensionaha.120.16784 [DOI] [PubMed] [Google Scholar]
- 27. Sohn IS, Kim CJ, Yoo BS, Kim BJ, Choi JW, Kim DI, Lee SH, Song WH, Jeon DW, Cha TJ, et al. Clinical impact of guideline‐based practice and patients' adherence in uncontrolled hypertension. Clin Hypertens. 2021;27:26. doi: 10.1186/s40885-021-00183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ernawati I, Lubada EI, Lusiyani R, Prasetya RA. Association of adherence measured by self‐reported pill count with achieved blood pressure level in hypertension patients: a cross‐sectional study. Clin Hypertens. 2022;28:12. doi: 10.1186/s40885-022-00195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta‐analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 30. Lee YM, Yu HY, You MA, Son YJ. Impact of health literacy on medication adherence in older people with chronic diseases. Collegian. 2017;24:11–18. doi: 10.1016/j.colegn.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 31. Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124:1124–1140. doi: 10.1161/circresaha.118.313220 [DOI] [PubMed] [Google Scholar]
- 32. Hassen LJ, Lenihan DJ, Baliga RR. Hypertension in the cardio‐oncology clinic. Heart Fail Clin. 2019;15:487–495. doi: 10.1016/j.hfc.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 33. Lee H, Park JH, Floyd JS, Park S, Kim HC. Combined effect of income and medication adherence on mortality in newly treated hypertension: nationwide study of 16 million person‐years. J Am Heart Assoc. 2019;8:e013148. doi: 10.1161/jaha.119.013148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahn Y, Jung MH. Cardiovascular health is the essential but overlooked aspect in the management of cancer survivors. Eur J Prev Cardiol. 2022;29:2322–2323. doi: 10.1093/eurjpc/zwac241 [DOI] [PubMed] [Google Scholar]
- 35. Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–511. doi: 10.1046/j.1525-1497.2002.00406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92:117–122. doi: 10.15386/mpr-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


