Abstract
Background
Whether the early use of sodium‐glucose cotransporter‐2 (SGLT2) inhibitors have cardioprotective effects following acute myocardial infarction is unknown. Thus, we aimed to evaluate the association between the early initiation of SGLT2 inhibitors and cardiac event rates in patients with diabetes with acute myocardial infarction undergoing percutaneous coronary intervention.
Methods and Results
Based on the National Health Insurance claims data in South Korea, patients who received percutaneous coronary intervention for acute myocardial infarction between 2014 and 2018 were analyzed. Patients given SGLT2 inhibitors or other glucose‐lowering drugs were matched based on a propensity score. The primary end point was a composite of all‐cause mortality and hospitalizations for heart failure. Major adverse cardiac events (a composite of all‐cause death, nonfatal myocardial infarction, and ischemic stroke) were compared as the secondary end point. After 1:2 propensity score matching, the SGLT2 inhibitors group (938 patients) and the no use of SGLT2 inhibitors group (1876 patients) were compared. During a median follow‐up of 2.1 years, the early use of SGLT2 inhibitors was associated with lower risks of both the primary end point (9.8% versus 13.9%; adjusted hazard ratio [HR], 0.68 [95% CI, 0.54–0.87]; P=0.002) and secondary end point (9.1% versus 11.6%; adjusted HR, 0.77 [95% CI, 0.60–0.99]; P=0.04). All‐cause mortality and hospitalizations for heart failure were also significantly lower in early users of SGLT2 inhibitors.
Conclusions
The early use of SGLT2 inhibitors in patients with diabetes treated with percutaneous coronary intervention for acute myocardial infarction was associated with a significantly lower risk of cardiovascular events, including all‐cause mortality, hospitalizations for heart failure, and major adverse cardiac events.
Keywords: acute myocardial infarction, diabetes, heart failure, mortality, sodium‐glucose cotransporter 2 inhibitors
Subject Categories: Percutaneous Coronary Intervention, Treatment, Congenital Heart Disease, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- HIRA
Health Insurance Review and Assessment Service
- MACE
major adverse cardiac events
- SGLT2
sodium‐glucose cotransporter 2
Clinical Perspective.
What Is New?
In this analysis of large national claims data, the early use of sodium‐glucose cotransporter‐2 inhibitors by patients with diabetes following percutaneous coronary intervention for acute myocardial infarction was associated with a robust reduction in not only the composite of all‐cause death and heart failure but the major ischemic composite outcome (a composite of all‐cause death, nonfatal myocardial infarction, and ischemic stroke), mainly driven by a reduction in deaths.
What Are the Clinical Implications?
Taken together with the proven cardioprotective effects of sodium‐glucose cotransporter‐2 inhibitors, our results suggest that the use of sodium‐glucose cotransporter‐2 inhibitors could expand to the acute phase of acute myocardial infarction survivors with diabetes to reduce mortality and the subsequent development of congestive heart failure.
Further research such as randomized control trials with long‐term follow‐up is warranted to evaluate the effectiveness of sodium‐glucose cotransporter‐2 inhibitors in acute myocardial infarction survivors.
Despite advances in management, survivors of acute myocardial infarction (AMI) are at a greatly increased risk for subsequent fatal and nonfatal cardiovascular events. Heart failure (HF) complicating AMI is especially common and the most powerful predictor of death; thus, it has important implications for treatment. 1 Diabetes is a well‐known risk factor for the development of coronary artery disease, 2 and patients with AMI with diabetes are at an especially high risk of cardiovascular death, HF, and subsequent major cardiovascular events (MACE). 2 , 3
In recent cardiovascular trials, sodium‐glucose cotransporter 2 (SGLT2) inhibitors were shown to reduce the risk of incident HF hospitalization in individuals with type 2 diabetes who had or were at high risk of cardiovascular disease. 4 , 5 , 6 Especially, dapagliflozin appeared to robustly reduce the risk of death, HF, and MACE in patients with diabetes with previous myocardial infarction (MI). 7 These results have prompted increased interest in the impact of SGLT2 inhibitors on AMI‐related HF or cardiovascular events. In addition, subsequent work has showed that SGLT2 inhibitors reduce the risk of death and hospitalization for patients with chronic HF, regardless of diabetes status or preservation of ejection fraction. 8 , 9 , 10 , 11 These SGLT2 investigations excluded patients with recent MI. In this regard, a further question has been raised as to whether the early use of SGLT2 inhibitors might also benefit AMI survivors after percutaneous coronary intervention (PCI). Hence, the present study aimed to determine the association between the early initiation of SGLT2 inhibitors and the rates of death, HF, and MACE in patients with diabetes treated with PCI for AMI.
METHODS
Data Source
Anonymized data and materials have been made publicly available at the Korean Health Insurance Review and Assessment Service (HIRA) database and can be accessed at https://opendata.hira.or.kr/home.do. The HIRA is a quasi‐governmental organization that systematically reviews all National Health Insurance Service claims records. 12 All records were anonymized according to relevant laws and regulations. This database covers >98% of the South Korean population and includes all health records, such as demographics, diagnoses (coded with International Classification of Diseases, Tenth Revision [ICD‐10]), drug prescriptions, and procedures. 12 We used the medical data from January 1, 2013, to August 31, 2019. Because the claims data of the HIRA are fully anonymized, this study was approved by the local institutional review board of The Catholic University of Medicine, Eunpyeong St. Mary Hospital, which waived the requirement for informed consent.
Study Population
Based on the HIRA claims database from January 2013 to August 2018, we identified patients with type 2 diabetes aged ≥18 years (Figure 1). Patients with diabetes were defined as those who were assigned the ICD‐10 codes for type 2 diabetes (ICD‐10 code: E11) and those who used anti‐diabetic medications according to the medication codes in the HIRA database within 12 months of the index day. 13 , 14 From January 2014 to August 2018, patients who underwent PCI (National Health Insurance Service electronic data interchange codes M6551, M6552, M6561–4, M6571, and M6572) for AMI (ICD‐10 codes I21.X–I22.X) 13 were enrolled with the index day defined as the date of PCI. To ensure that this was the patient's first episode of AMI, patients were excluded if the HIRA database indicated that they had a previous history of AMI (ICD‐10 codes I21.X–23.X) within 12 months of the index day (Figure 1).
Figure 1. Study flow.
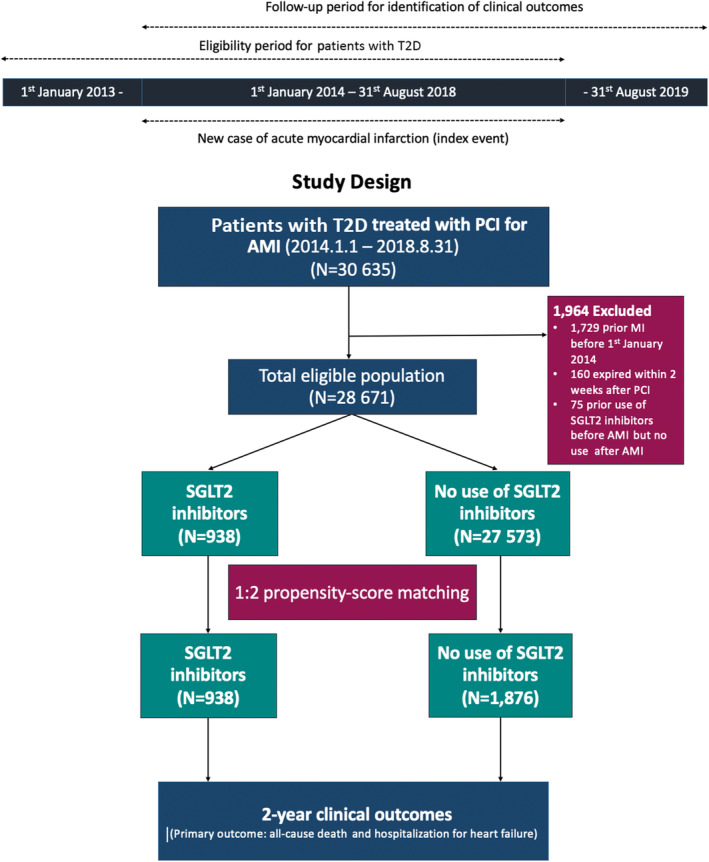
AMI indicates acute myocardial infarction; PCI, percutaneous coronary intervention; SGLT2, sodium‐glucose cotransporter‐2; and T2D, type 2 diabetes.
Patients who received SGLT2 inhibitors for >7 consecutive days within 14 days after PCI for AMI were defined as the SGLT2 inhibitors group. Otherwise, patients were assigned to the no use of SGLT2 inhibitors group. Patients who were treated with SGLT2 inhibitors before the index day were entirely excluded from the analysis. Patients who expired within 14 days after PCI were also excluded. Within 6 months of the index day, the ICD‐10 codes were used to identify other comorbidities, such as hyperlipidemia, hypertension, history of stroke, history of HF, atrial fibrillation/flutter, chronic renal disease, chronic lung disease, peripheral vascular disease, and history of malignancy. 13 , 14 Charlson comorbidity index was obtained using the ICD‐10 codes. 15 In the HIRA database, all prescribed medications were underwritten and recorded with rigorous accuracy. The use of medications, such as antiplatelet agents, statins, beta‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, calcium‐channel blockers, diuretics, and glucose‐lowing agents was assessed and identified from the prescription database of the HIRA. 12 , 13 The detailed codes of the covariates are summarized in Table S1.
Clinical Outcomes
For the evaluation of clinical outcomes, the medical claims data of the eligible population until August 31, 2019, were evaluated in the HIRA database. The primary end point was a composite of all‐cause death and hospitalizations for HF (Table S1). The secondary analysis of the ischemic end point was the composite of all‐cause death, nonfatal MI, and nonfatal ischemic stroke. All‐cause death was identified by all in‐ and outpatient claims that indicated death. 15 Hospitalization for HF was defined as a hospital admission for HF (defined using primary discharge diagnosis codes or secondary discharge diagnosis codes combined with intravenous diuretics or inotropics use). 5 , 16 To extract the diagnostic codes as naïve events, nonfatal MI was defined as emergency hospitalization lasting at least 3 days or more with a primary discharge diagnosis of AMI. 17 Nonfatal ischemic stroke was defined by the ICD‐10 diagnosis codes I63 and I64 with hospitalization and concomitant brain imaging studies using computed tomography or magnetic resonance imaging. 14 In patients with multiple events, the first event was considered to be the component of the composite outcome. To assess clinical outcomes, the patients were censored at the occurrence of an outcome event or the end of the study period (August 31, 2019), whichever came first.
Statistical Analysis
The baseline characteristics are presented as the mean±SD for continuous variables and as frequencies with percentages for categorical variables. The continuous variables were compared using the Student t‐test, and categorical variables were compared using either the chi‐squared test or Fisher exact test as appropriate, respectively. To reduce the effect of selection bias and potential confounders, we performed propensity score matching with SAS Enterprise Guide version 7.1 (SAS Institute Inc., Cary, NC). 15 , 18 The propensity scores were generated using 11 variables: age class, sex, hypertension, hyperlipidemia, atrial fibrillation/flutter, history of stroke, history of HF, chronic renal disease, history of malignancy, calcium channel blocker, and insulin use.
The patients with or without SGLT2 inhibitors were matched at a 1:2 ratio. An absolute difference (caliber) of 0.001 between the propensity scores was applied, and the closest option was used to optimize the model. 15 , 18 Standardized differences in post‐matched patient characteristics were used to assess the adequacy of propensity score matching, where a>0.2 standardized difference between the 2 groups after propensity score matching was considered a non‐negligible imbalance. No variable had >1% missing data, and a replacement for a missing value was not applied.
Event rate curves were obtained using Kaplan–Meier analysis and compared using the log‐rank test. The time to the first event was compared using Cox proportional hazards models and presented as the hazard ratio (HR) and 95% CI. Prespecified multivariable analysis adjusted for imbalanced baseline characteristics was conducted. To test the stability of the findings, we performed a sensitivity analysis using inverse propensity of treatment weighting. A 2‐tailed P value of <0.05 was considered statistically significant. All analyses were performed with SAS software Enterprise Guide version 7.1 (SAS Institute Inc.) and R software version 3.2.2 13 (R Foundation for Statistical Computing, Vienna, Austria; www.r‐project.org).
RESULTS
Study Patients
From 30 635 patients with type 2 diabetes treated with PCI for AMI from January 2014 to August 2018, 28 671 eligible patients were enrolled. A total of 938 patients were identified as receiving SGLT2 inhibitors. The SGLT2 inhibitor group consisted of 605 patients treated with dapagliflozin, (64.5%), 302 treated with empagliflozin (32.2%), and 31 treated with ipragliflozin (3.3%). The baseline characteristics of the total population are presented in Table S2. Before propensity matching, patients treated with SGLT2 inhibitors were younger, more male dominant, and had lower rates of hypertension, hyperlipidemia, atrial fibrillation/flutter, prior stroke, HF, and malignancy. The use of statins, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, and diuretics was similar, whereas the use of glucose‐lowering medications was different between the 2 groups. The Charlson comorbidity index score was lower in the SGLT2 inhibitors group.
After 1:2 propensity score matching, a total of 2814 patients were included in the analysis (938 patients in the SGLT2 inhibitors group and 1876 patients in the no use of SGLT2 inhibitors group). The mean age of the participants was 57.2 years, and 80.0% were men. The baseline characteristics were balanced between the treatment groups of patients with or without SGLT2 inhibitors except for age and the use of hypoglycemic agents (Table 1). The standardized differences for all variables except metformin use were <20%.
Table 1.
Baseline Characteristics of the Study Population After Propensity Score Matching
| SGLT2 inhibitors (n=938) | No use of SGLT2 inhibitors (n=1876) | P value | Standardized difference | |
|---|---|---|---|---|
| Age, y* | 56.4±11.3 | 57.6±11.3 | 0.01 | 0.106 |
| <65 | 717 (76.4) | 1347 (71.8) | 0.01 | 0.098 |
| ≥65 | 221 (23.6) | 529 (28.2) | ||
| Male sex* | 769 (82.0) | 1482 (79.0) | 0.06 | 0.075 |
| Hypertension* | 699 (74.5) | 1398 (74.5) | >0.99 | <0.001 |
| Hyperlipidemia | 591 (63.0) | 1182 (63.0) | >0.99 | <0.001 |
| Atrial fibrillation/flutter* | 39 (4.2) | 78 (4.2) | >0.99 | <0.001 |
| History of stroke* | 54 (5.8) | 111 (5.9) | 0.86 | 0.008 |
| History of heart failure* | 25 (2.7) | 54 (2.9) | 0.75 | 0.012 |
| Peripheral artery disease | 108 (11.5) | 174 (9.3) | 0.06 | 0.002 |
| Chronic renal disease* | 22 (2.4) | 44 (2.4) | >0.99 | 0.001 |
| Chronic lung disease | 82 (8.7) | 146 (7.8) | 0.38 | 0.035 |
| History of malignancy* | 26 (2.8) | 79 (4.2) | 0.06 | 0.076 |
| Clinical presentation | 0.13 | 0.041 | ||
| Non‐STEMI | 388 (41.4) | 739 (39.4) | ||
| STEMI | 550 (58.6) | 1137 (60.6) | ||
| CCI score | 2.8±1.3 | 2.8±1.4 | 0.86 | 0.006 |
| Discharge medications | ||||
| Aspirin | 916 (97.7) | 1822 (97.1) | 0.41 | 0.031 |
| P2Y12 inhibitors | 915 (97.6) | 1818 (96.9) | 0.34 | 0.036 |
| Beta‐blockers | 756 (80.6) | 1435 (76.5) | 0.01 | 0.099 |
| ACE inhibitors or ARBs | 679 (72.4) | 1301 (69.4) | 0.10 | 0.066 |
| MRA | 3 (0.3) | 10 (0.5) | 0.43 | 0.030 |
| Loop diuretics | 88 (9.4) | 180 (9.6) | 0.86 | 0.007 |
| Thiazide | 48 (5.1) | 96 (5.1) | >0.99 | 0.001 |
| Statin | 841 (89.7) | 1686 (89.9) | 0.86 | 0.007 |
| CCBs* | 168 (17.9) | 365 (19.5) | 0.32 | 0.039 |
| Other hypoglycemic agents | ||||
| Metformin | 649 (69.2) | 872 (46.5) | <0.001 | 0.466 |
| Sulfonylurea | 307 (32.7) | 449 (23.9) | <0.001 | 0.199 |
| DDP4 inhibitors | 238 (25.4) | 657 (35.0) | <0.001 | 0.198 |
| Thiazolidinediones | 17 (1.8) | 40 (2.1) | 0.57 | 0.021 |
| Insulin* | 19 (2.0) | 38 (2.0) | >0.99 | <0.001 |
| GLP‐1 antagonist | 0 | 0 | Not applicable | Not applicable |
| Others | 8 (0.9) | 32 (1.7) | 0.07 | 0.072 |
Values are the mean ± SD or number (%). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker, CCB, calcium‐channel blocker; CCI, Charlson comorbidities index; DPP4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium‐glucose cotransporter 2; and STEMI, ST‐segment–elevation myocardial infarction.
Variables were used to generate the propensity score.
Primary and Secondary End Points
The study population had a median follow‐up of 2.1 years (interquartile range, 1.4–2.9). At 2 years follow‐up, the early use of SGLT2 inhibitors was associated with a lower risk of the primary end point compared with no use of SGLT2 inhibitors (9.8% versus 13.9%; adjusted HR, 0.68 [95% CI, 0.54–0.87]; P=0.002; Table 2 and Figure 2). The incidence of all‐cause death was significantly lower in the SGLT2 inhibitors group compared with the no use of SGLT2 inhibitors group (3.7% versus 6.6%; adjusted HR, 0.55 [95% CI, 0.37–0.80]; P=0.002; Table 2 and Figure 3). In addition, patients treated with SGLT2 inhibitors had a significantly lower cumulative hospitalization rate for HF (7.4% versus 9.8%; adjusted HR, 0.74 [95% CI, 0.56–0.98]; P=0.03).
Table 2.
Primary and Secondary End Points
| End point, n (%)* | Unadjusted | Adjusted† | ||||||
|---|---|---|---|---|---|---|---|---|
| SGLT2 inhibitors (N=938) | No use of SGLT2 inhibitors (N=1876) | HR for SGLT2 inhibitors | 95% CI | P value | HR for SGLT2 inhibitors | 95% CI | P value | |
| Primary end point | 87 (9.8) | 237 (13.9) | 0.69 | 0.54–0.87 | 0.002 | 0.68 | 0.54–0.87 | 0.002 |
| Secondary end point | 79 (9.1) | 198 (11.6) | 0.77 | 0.60–0.99 | 0.04 | 0.77 | 0.60–0.99 | 0.04 |
| Individual outcomes | ||||||||
| All‐cause death | 34 (3.7) | 116 (6.6) | 0.55 | 0.38–0.81 | 0.002 | 0.55 | 0.37–0.80 | 0.002 |
| Hospitalization for heart failure | 68 (7.4) | 166 (9.8) | 0.75 | 0.57–0.98 | 0.04 | 0.76 | 0.56–0.98 | 0.03 |
| Nonfatal myocardial infarction | 40 (4.8) | 85 (4.9) | 0.98 | 0.68–1.40 | 0.90 | 0.97 | 0.68–1.40 | 0.88 |
| Nonfatal ischemic stroke | 13 (1.5) | 44 (2.5) | 0.61 | 0.34–1.11 | 0.10 | 0.61 | 0.33–1.10 | 0.10 |
HR indicates hazard ratio; and SGLT2, sodium‐glucose cotransporter 2.
The percentages are Kaplan–Meier estimates of the rate of the end point at 24 months.
The multivariate‐adjusted Cox proportional hazard model included age, metformin, sulfonylurea, and dipeptidyl peptidase‐4 inhibitors, which were statistically different between the 2 groups.
Figure 2. Kaplan–Meier curves of primary and secondary end points.
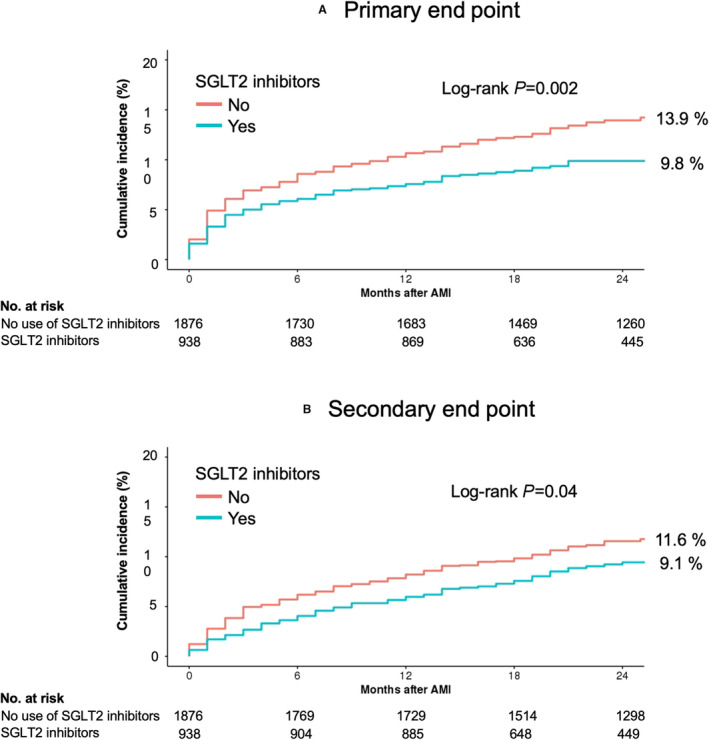
A, Shows the cumulative incidence of the primary end point (a composite of all‐cause death and hospitalizations for heart failure), and B, shows the cumulative incidence of the secondary end point of major adverse cardiovascular events (a composite of all‐cause death, nonfatal myocardial infarction, and ischemic stroke). AMI indicates acute myocardial infarction; and SGLT2, sodium‐glucose cotransporter‐2.
Figure 3. Kaplan–Meier curves of all‐cause death and cardiovascular events.
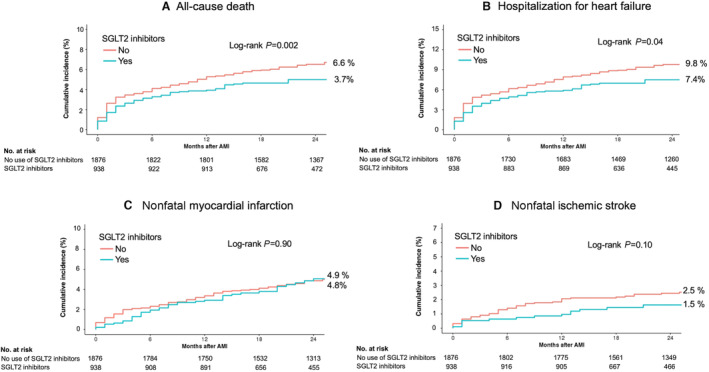
A, The cumulative incidence of all‐cause death, B, hospitalizations for heart failure, C, nonfatal myocardial infarction, and D, nonfatal ischemic stroke is presented. AMI indicates acute myocardial infarction; and SGLT2, sodium‐glucose cotransporter‐2.
Patients treated with SGLT2 inhibitors had a significantly lower rate of secondary end points compared with the patients without SGLT2 inhibitors (9.1% versus 11.6%; adjusted HR, 0.77 [95% CI, 0.60–0.99]; P=0.04; Table 2 and Figure 2). This difference was largely attributable to the lower incidence of all‐cause deaths in the SGLT2 inhibitors group. No statistical differences were observed in terms of the incidence of nonfatal MI (4.8% in the SGLT2 inhibitors group versus 4.9% in the no use of SGLT2 inhibitors group; adjusted HR, 0.97 [95% CI, 0.68–1.40]; P=0.88) and nonfatal ischemic stroke (1.5% in the SGLT2 inhibitors group versus 2.5% in the no use of SGLT2 inhibitors group; adjusted HR, 0.61 [95% CI, 0.33–1.10]; P=0.10; Table 2 and Figure 3).
The inverse probability of treatment weighting analysis (Table S3) showed consistent results, demonstrating that the SGLT2 inhibitors group was associated with a lower risk of the primary end point (12.5% versus 19.4%; adjusted HR, 0.62 [95% CI, 0.54–0.78]; P<0.001; Table S4 and Figure S1). In addition, the cumulative rate of the secondary end point was also significantly lower in patients treated with SGLT2 inhibitors (11.3% versus 14.8%; adjusted HR, 0.78 [95% CI, 0.64–0.94]; P=0.01). Similar to the propensity‐score matching analysis, the incidence of all‐cause death and hospitalizations for HF was significantly lower in the SGLT2 inhibitors group, whereas no statistical differences existed between the 2 groups in terms of nonfatal MI and nonfatal ischemic stroke (Table S4 and Figure S2).
Subgroup Analysis
The overall findings of the lower rate of composite all‐cause death and hospitalizations for HF in patients treated with SGLT2 inhibitors were consistent among subgroups that were defined according to age, sex, medication, cardiovascular risk factors, and Charlson comorbidity index scores (Figure 4). There was no evidence of a significant modulation or interaction with respect to the primary end point. In addition, the HRs of the primary and secondary end points favored each SGLT2 inhibitor (Figure S3).
Figure 4. Primary end point according to patient subgroups.
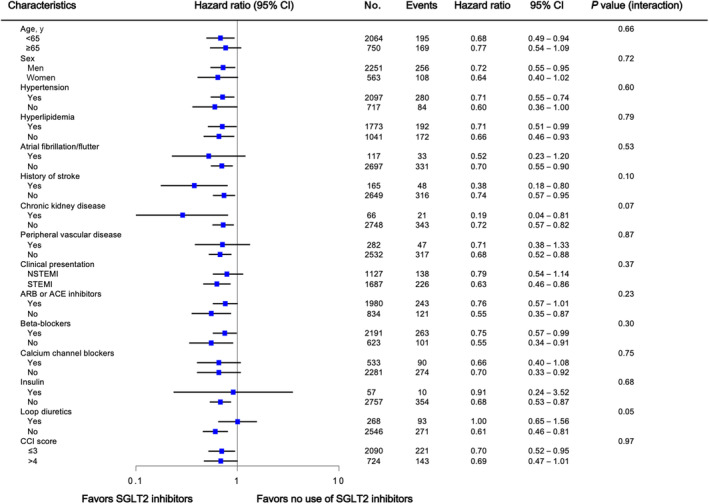
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; CCI, Charlson comorbidity index; NSTEMI, non–ST‐segment–elevation myocardial infarction; and SGLT2, sodium‐glucose cotransporter‐2.
DISCUSSION
In this observational study using a national health care service database, we found that the early use of SGLT2 inhibitors in patients with diabetes after PCI for AMI was associated with a lower risk of the composite all‐cause death and hospitalizations for HF. In addition, we observed a lower rate of MACE in patients given SGLT2 inhibitors, mainly driven by a reduction in all‐cause death, without an apparent reduction in nonfatal MI or ischemic stroke. The cardioprotective benefits of SGLT2 inhibitors were consistent across the various clinical subgroups. These findings were stable in a sensitivity analysis using inverse probability of treatment weighting.
SGLT2 inhibitors are a class of antihyperglycemic drugs with efficacy for reducing cardiovascular events, including hospital admission for HF or cardiovascular death proven by multiple randomized clinical trials. 4 , 5 The beneficial effect of SGLT2 inhibitors on events related to HF regardless of diabetes or ejection fraction was supported by data from recent randomized clinical trials of dapagliflozin or empagliflozin. 8 , 9 , 10 , 11 Accordingly, SGLT2 inhibitors are recommended for patients with type 2 diabetes and HF to reduce HF, MACE, and cardiovascular death. 19 Furthermore, the current diabetic guidelines recommend that in the setting of type 2 diabetes, SGLT2 inhibitors should be considered for individuals with established atherosclerotic cardiovascular disease or those at high risk. 19
Although recent randomized control trials with SGLT inhibitors reduced the risk of death and hospitalization for patients with chronic HF, regardless of diabetic status or preservation of ejection fraction, as with other chronic HF trials, these SGLT2 investigations excluded patients with recent MI. 8 , 9 , 10 , 11 However, in a recent randomized control trial, a clinical benefit of empagliflozin was observed for both acute de novo and decompensated chronic HF regardless of ejection fraction or diabetic status. 20 Furthermore, a study demonstrated that empagliflozin was associated with a significantly greater N‐terminal pro‐hormone of brain natriuretic peptide reduction, accompanied by a significant improvement in echocardiographic functional and structural parameters in patients with a recent MI, compared with placebo. 21 These findings raised the question of whether this benefit could be expanded to the acute phase of MI because AMI survivors are vulnerable to the development of HF and future cardiocerebrovascular events. 1 In this regard, the present study identified an association between SGLT2 inhibitors and patients with a history of type 2 diabetes who had AMI and underwent PCI in reducing all‐cause mortality and hospitalization for HF. As such, our findings suggest that the benefits seen in clinical trials and large observational studies may be extended to patients with AMI as part of clinical practice. Taken together with recent randomized clinical trial evidence indicating the cardioprotective effect of SGLT2 inhibitors regardless of diabetes, it is possible that the observed benefits of SGLT2 inhibitors could extend to a broad population of patients with AMI with or without diabetes. However, the HIRA data investigated here specifically focused on SGLT2 inhibitor effects in patients with diabetes. The substantial benefits of SGLT2 inhibitors on cardiovascular events in patients with AMI should be examined in further large clinical trials or with real‐world setting data with long‐term follow‐up periods. Some ongoing trials will provide more clear evidence on the clinical benefits of SGLT2 inhibitors in patients following AMI (eg, Dapagliflozin Effects on Cardiometabolic Outcomes in Patients With an Acute Heart Attack [DAPA‐MI], URL: https://www.clinicaltrials.gov; unique identifier: NCT04564742, and A Study to Test Whether Empagliflozin Can Lower the Risk of Heart Failure and Death in People Who Had a Heart Attack [EMPACT‐MI], URL: https://www.clinicaltrials.gov; unique identifier: NCT04509674).
The association of SGLT2 inhibitors with a robust reduction in all‐cause death and HF events in our study was generally similar to those observed in clinical trials and other observational data, despite different patient populations. A large magnitude reduction was also reported for all‐cause mortality 5 , 22 and hospitalizations for HF, 5 , 6 , 8 , 22 suggesting that these cardioprotective results would be mediated by the favorable hemodynamic effects of SLGT2 inhibitors. 23 However, with regard to the nonfatal events of MI or ischemic stroke, we did not identify any significant differences between the users of SGLT2 inhibitors and the users of other glucose‐lowering drugs. The randomized control trials showed somewhat conflicting results in terms of MI and stroke reduction. The rates of nonfatal MI were numerically lower with empagliflozin, canagliflozin, and dapagliflozin versus placebo without statistical significance, 5 , 6 , 8 while ertugliflozin showed a neutral hazard ratio. 24 The cumulative rates for nonfatal stroke numerically favored placebo versus empagliflozin 5 and canagliflozin versus placebo, 6 although none of these differences was statistically significant. Several studies using real‐world data demonstrated statistically lower rates of MI or stroke in patients treated with SGLT2 inhibitors, 25 , 26 whereas no reduction in MI or stroke was reported in other observational studies. 27 , 28 The protective effect of SGLT2 inhibitors on acute atherosclerotic vascular events needs further dedicated investigations.
AMI survivors, particularly those with the features of left ventricular dysfunction, constitute an expanding population at heightened risk for developing congestive HF or premature death. 1 This higher risk segment of the AMI population has been the focus of several international clinical trials and larger observational studies on the early use of beta‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists, which have already become established treatments for congestive HF. 29 Those medications demonstrated effectiveness in reducing the rates of cardiovascular death and the development of HF following AMI and thus, have become standard treatments for post‐MI HF as well. 1 However, these substantial improvements in AMI management have expanded the pool of MI survivors in jeopardy of developing HF during the chronic phase. In addition, despite contemporary standard‐of‐care treatment following AMI, the residual risk is intensified in patients with type 2 diabetes. 2 , 3 Therefore, preventing both the mortality and hospitalization from HF in patients with type 2 diabetes following MI remains an unmet clinical need. To our knowledge, the present study was the first large study evaluating the real‐world effectiveness of SGLT2 inhibitors on the specific outcomes of hospitalization for HF and all‐cause death in AMI survivors.
Although SGLT2 inhibitors may have potential application beyond diabetes control, the mechanisms underlying the cardioprotective effects are not yet completely understood. 23 A metabolic hypothesis has been proposed whereby metabolic substrate shifts from conventional fatty acids to ketone bodies, which SGLT2 inhibitors promote to produce, potentially contribute to improved cardiac efficiency, contractility, and cardiovascular protection. 30 Some other hypotheses include the restoration of tubuloglomerular feedback and the resultant attenuation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system activation; osmotic diuresis with a decrease in ventricular overload; inhibition of the sodium‐hydrogen exchanger pump, resulting in a decrease in myocardial calcium overload; improvement in heart fuel energetics; and increased hematocrit, resulting from hemoconcentration or an increase in red cell mass. 7 The mechanism responsible for the protection from myocardial injury attributable to ischemia is less clear, but animal models demonstrated substantial evidence of ischemic injury amelioration by SGLT2 inhibitors. An experimental study using mice showed a reduction in myocardial oxidative stress, interstitial fibrosis, and macrophage infiltration with empagliflozin. 31 Recently, an animal study found that the long‐term oral administration of canagliflozin resulted in a significant reduction in myocardial infarct size via attenuating myocardial ischemia/reperfusion injury. 32 The potential mechanisms of the cardiovascular benefits from SGLT2 inhibitors after ischemic events should be investigated in future research, including the possible myocardium‐protective effects.
Limitations
This study had several limitations. The first of these was inherent to the retrospective nature and observational design of the analyses, and therefore overall findings should be considered hypothetical and hypothesis‐generating only. Second, prescription rates of SGLT2 inhibitors were extremely low in this population, and there was no determination about initiation of SGLT2 inhibitors. Despite the use of robust (nonparsimonious) propensity score matching and additional statistical adjustments, the possibility of residual, unmeasured confounders could not be eliminated. In addition, an active comparator would be ideally required for eliminating immortal time bias and reducing residual confounding. Third, although the current study used large national claims data, the average duration of follow‐up was relatively limited, as the prescription of SGLT‐2 inhibitors in real‐world practice is still recent. Longer‐term follow‐up is required to determine whether the observed effects are sustained over time. Fourth, the propensity‐matched population was relatively young compared with the other MI cohorts, and the patients with prior MIs were excluded for precise patient selection, which might mean that the present cohort would be at relatively low risk of future cardiovascular events. Fifth, the study did not have adequate data on the causes of death or total mortality and, thus, was unable to investigate cardiovascular and any‐cause deaths. This might obscure any true treatment effect from the SGLT inhibitor use. However, there has been a debate on the use of cardiovascular‐specific mortality to assess the clinical efficacy and safety of agents because of the vague terminology on death certificates. 33 All‐cause mortality, which aggregates cardiovascular deaths and noncardiovascular deaths, is free of any potential subjectivity in classification, is clinically compelling, and is most relevant to patients. 34 Sixth, there was no information available on diabetes duration and the parameters of glycemic control such as hemoglobin A1c levels in these patients. Consequently, there could be residual confounding factors. Finally, we focused on cardiovascular outcomes only and did not assess safety.
CONCLUSIONS
In this observational analysis of large national claims data, the early use of SGLT2 inhibitors in patients with diabetes treated with PCI for AMI was associated with a significantly lower risk in not only the composite including all‐cause deaths and HF but also the ischemic composite (MACE), mainly driven by a reduction in deaths. Taken together with the recent randomized control trials showing benefits of SLGT2 inhibitors in patients with acute HF or post‐MI, 20 , 21 our results supported the likelihood that patients could derive benefit from in‐hospital SGLT2 inhibitor initiation after AMI. However, a prospective randomized clinical trial in an AMI population is required to ascertain whether SGLT2 inhibitors reduce deaths, the development of HF, and atherothrombotic vascular events.
Sources of Funding
This work was partly supported by The Research Institute of Medical Sciences. The Catholic University of Korea, Eunpyeong St. Mary's Hospital, Seoul, Republic of Korea. The sponsors played no role in this study. There was no industry involvement in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S3
This manuscript was sent to Marc A. Simon, MD, MS, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027824
For Sources of Funding and Disclosures, see page 11.
See Editorial by Nunes and Udell
References
- 1. Bahit MC, Kochar A, Granger CB. Post‐myocardial infarction heart failure. JACC Heart Fail. 2018;6:179–186. doi: 10.1016/j.jchf.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 2. Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–642. doi: 10.1016/j.jacc.2006.09.046 [DOI] [PubMed] [Google Scholar]
- 3. Ritsinger V, Nystrom T, Saleh N, Lagerqvist B, Norhammar A. Heart failure is a common complication after acute myocardial infarction in patients with diabetes: a nationwide study in the SWEDEHEART registry. Eur J Prev Cardiol. 2020;27:1890–1901. doi: 10.1177/2047487319901063 [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 7. Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516–2527. doi: 10.1161/CIRCULATIONAHA.119.039996 [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 12. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–728. doi: 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Park GM, Han S, Kim YG, Suh J, Park HW, Won KB, Ann SH, Kim SJ, Kim DW, et al. Impact of diabetes mellitus in patients undergoing contemporary percutaneous coronary intervention: results from a Korean nationwide study. PLoS One. 2018;13:e0208746. doi: 10.1371/journal.pone.0208746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Edoxaban in Asian patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol. 2018;72:838–853. doi: 10.1016/j.jacc.2018.05.066 [DOI] [PubMed] [Google Scholar]
- 15. Kim JY, Kim SH, Myong JP, Kim YR, Kim TS, Kim JH, Jang SW, Oh YS, Lee MY, Rho TH. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol. 2019;73:1123–1131. doi: 10.1016/j.jacc.2018.12.047 [DOI] [PubMed] [Google Scholar]
- 16. McCormick N, Lacaille D, Bhole V, Avina‐Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta‐analysis. PLoS One. 2014;9:e104519. doi: 10.1371/journal.pone.0104519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 18. Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;2022(45):2753–2786. doi: 10.2337/dci22-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN, Benedikt M, Wallner M, Alber H, Berger R, Lichtenauer M, Saely CH, et al. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J. 2022;43:4421–4432. doi: 10.1093/eurheartj/ehac494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jorgensen ME, Thuresson M, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamargo J. Sodium‐glucose cotransporter 2 inhibitors in heart failure: potential mechanisms of action, adverse effects and future developments. Eur Cardiol. 2019;14:23–32. doi: 10.15420/ecr.2018.34.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cannon CP, Pratley R, Dagogo‐Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 25. Kosiborod M, Birkeland KI, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Jorgensen ME, Wittbrodt ET, et al. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT2‐inhibitors versus other glucose‐lowering agents in real‐world clinical practice: results from the CVD‐REAL study. Diabetes Obes Metab. 2018;20:1983–1987. doi: 10.1111/dom.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 27. Birkeland KI, Jorgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Nystrom T, Eriksson JW, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709–717. doi: 10.1016/S2213-8587(17)30258-9 [DOI] [PubMed] [Google Scholar]
- 28. Pasternak B, Ueda P, Eliasson B, Svensson AM, Franzen S, Gudbjornsdottir S, Hveem K, Jonasson C, Wintzell V, Melbye M, et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ. 2019;366:l4772. doi: 10.1136/bmj.l4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harrington J, Petrie MC, Anker SD, Bhatt DL, Jones WS, Udell JA, Hernandez AF, Butler J. Evaluating the application of chronic heart failure therapies and developing treatments in individuals with recent myocardial infarction: a review. JAMA Cardiol. 2022;7:1067–1075. doi: 10.1001/jamacardio.2022.2847 [DOI] [PubMed] [Google Scholar]
- 30. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330 [DOI] [PubMed] [Google Scholar]
- 31. Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim‐Mitsuyama S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim VG, Bell RM, Arjun S, Kolatsi‐Joannou M, Long DA, Yellon DM. SGLT2 inhibitor, canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart. JACC Basic Transl Sci. 2019;4:15–26. doi: 10.1016/j.jacbts.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morrow DA, Wiviott SD. Classification of deaths in cardiovascular outcomes trials. Circulation. 2019;139:874–876. doi: 10.1161/CIRCULATIONAHA.118.038359 [DOI] [PubMed] [Google Scholar]
- 34. Fanaroff AC, Clare R, Pieper KS, Mahaffey KW, Melloni C, Green JB, Alexander JH, Jones WS, Harrison RW, Mehta RH, et al. Frequency, regional variation, and predictors of undetermined cause of death in cardiometabolic clinical trials: a pooled analysis of 9259 deaths in 9 trials. Circulation. 2019;139:863–873. doi: 10.1161/CIRCULATIONAHA.118.037202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


