Abstract
Background
Obstructive sleep apnea (OSA) is common in heart failure with preserved ejection fraction (HFpEF). However, current evidence is equivocal regarding the potential benefits of treating OSA with positive airway pressure (PAP) therapy in HFpEF. This study assessed the association between adherence to PAP therapy and health care resource use in patients with OSA and HFpEF.
Methods and Results
Administrative insurance claims data linked with objective PAP therapy usage data from patients with OSA and HFpEF were used to determine associations between PAP adherence and a composite outcome including hospitalizations and emergency room visits. One‐year PAP adherence was based on an adapted US Medicare definition. Propensity score methods were used to create groups with similar characteristics across PAP adherence levels. The study cohort included 4237 patients (54.0% female, mean age 64.1 years); 40% were considered adherent to PAP therapy (30% intermediate adherent, 30% nonadherent). In the matched cohort, PAP‐adherent patients had fewer health care resource use visits than nonadherent patients, a 57% decrease in hospitalizations, and a 36% decrease in emergency room visits versus the year before PAP initiation. Total health care costs were lower in adherent patients than nonadherent patients ($12 732 versus $15 610, P<0.001). Outcomes for intermediately adherent patients were most similar to those for nonadherent patients.
Conclusions
Treating OSA with PAP therapy in patients with HFpEF was associated with a reduction in health care resource use. These data highlight the importance of managing concomitant OSA in patients with HFpEF, and the need for strategies to enhance PAP adherence in this population.
Keywords: health care resource use, heart failure, obstructive sleep apnea, positive airway pressure adherence
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- CMS
Centers for Medicare and Medicaid Services
- PAP
positive airway pressure
Clinical Perspective.
What Is New?
Treatment of obstructive sleep apnea in patients with heart failure with preserved ejection fraction with positive airway pressure therapy is associated with reduced risk of the composite outcome of hospitalizations and visits to the emergency room.
These observed benefits of positive airway pressure therapy also suggest the potential for cost savings for the health care system.
What Are the Clinical Implications?
Greater awareness of the importance of positive airway pressure adherence in treating obstructive sleep apnea in patients with heart failure with preserved ejection fraction is warranted.
Chronic heart failure is occurring in epidemic proportions, partly due to population aging and the improved survival of individuals after acute coronary events. 1 Chronic heart failure is often categorized based on the left ventricular ejection fraction into heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF), which occur at approximately similar frequencies. Outcomes for patients with HFpEF or HFrEF are generally equally poor, 2 with a 5‐year mortality rate of 75% and a median survival duration of 2.1 years. 3
Despite significant progress in the pharmacological and device management of HFrEF, progress regarding the management of HFpEF has been more modest, and mortality rates in the latter patient group have not improved over time. 4 Therefore, the focus is primarily on optimizing risk factors and treating comorbidities. 5 This means that there is considerable interest in new therapeutic targets for both HFpEF and HFrEF. 2 , 6 Recent studies have shown the benefit of SGLT2 (sodium‐glucose cotransporter‐2) inhibitors for the management of HFpEF. 6 , 7 However, there remains a pressing need to improve the understanding of HFpEF phenotypes, to develop novel treatments, and to implement these in clinical practice. 8
The pathogenesis of HFpEF has been the topic of intense investigation, with left ventricular hypertrophy, elevated left ventricular filling pressure, and normal or near‐normal ejection fraction being key features. The clinical syndrome of HFpEF develops from a complex interaction of several risk factors (eg, aging, obesity, hypertension) that promote molecular and cellular derangements, which in turn cause organ dysfunction and ultimately clinical symptoms. 8 , 9 The economic burden of HFpEF, particularly in terms of hospitalizations, is high. 9
Prior studies have shown that obstructive sleep apnea (OSA) is common in patients with HFpEF, although its causal role in the clinical presentation of these patients is unclear. In theory, OSA could contribute to hypertension, a common comorbidity in HFpEF, which in turn could promote ventricular hypertrophy, progressing to HFpEF over time. 10 , 11 Intermittent hypoxia induced by OSA leads to widespread stimulation of the sympathetic nervous system, the renin‐angiotensin‐aldosterone system and, importantly, a systemic inflammatory state associated with oxidative stress. 12 , 13 , 14 These pathways are also important for the consequences of hypertension, diabetes, obesity, and aging, which are common risk factors for HFpEF. 15 , 16 , 17 Furthermore, another hallmark of OSA, exaggerated intrathoracic pressure swings, can contribute to cardiac remodeling. 18 On the other hand, HFpEF could play a role in the development of sleep‐disordered breathing via upper airway edema, effects on control of breathing and other factors. 19 , 20 Furthermore, patients with OSA or HFpEF share a number of common comorbidities, including obesity and diabetes. 21
These associations require investigation in interventional studies to determine important causal pathways. In 1997 Chan et al reported that 55% of patients with HFpEF had sleep‐disordered breathing, mostly in the form of OSA. 22 Herrscher et al found that sleep‐disordered breathing was evident in 80% of patients with HFpEF, with 62% having OSA, and hypertension was quite common in those with OSA. 23 A small randomized controlled trial and an observational study of intervention with positive airway pressure (PAP) therapies for OSA (continuous PAP or adaptive servo‐ventilation) have been associated with improvements in cardiac diastolic function. 24 , 25 , 26
This study was designed to test the hypothesis that treatment of OSA would improve outcomes in patients with HFpEF. Specifically, the aim of this study was to understand the benefit of continuous PAP or automatically titrating continuous PAP, collectively referred to as PAP therapy, in patients with OSA who have HFpEF, and to determine the impact of PAP therapy initiation on health care resource use in the subsequent year.
METHODS
Data Source
We conducted a retrospective observational study of patients with HFpEF who received a new diagnosis of OSA between September 2014 and April 2019. Deidentified payer‐sourced (“closed”) administrative claims data containing more than 100 geographically dispersed health plans across the United States (licensed from Inovalon Insights LLC, Bowing MD) were linked with objective PAP usage data (AirView, ResMed Corp, San Diego, CA). The databases were linked through a tokenization process and the resulting linked database underwent a third‐party expert determination to ensure compliance with the Health Insurance Portability and Accountability Act. The study design was reviewed by an institutional review board (Advarra, Ref number Pro0004005) and deemed exempt from oversight. Because of the retrospective nature of this study, informed consent from participants was not required. The methods (eg, program code) that support the findings of this study are available from the corresponding author upon reasonable request.
Study Cohort
The study cohort consisted of adults (age ≥18 years) who completed a sleep test (Healthcare Common Procedure Coding System 95808, 95810, 95811, G0398–G0400) where an OSA diagnosis (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD‐10‐CM] G47.33, Ninth Revision, Clinical Modification [ICD‐9‐CM] 327.23) was assigned within 60 days. Patients had to have received an AirSense10 PAP device (ResMed Corp, San Diego, CA) and have at least 1 year of claims data before the first sleep test and 1 year of claims data after PAP device setup. HFpEF was identified by the presence of at least 2 health care encounter claims with a diagnosis of diastolic heart failure (ICD‐10‐CM I50.3, ICD‐9‐CM 428.3*) or at least 1 hospitalization with a primary diagnosis of diastolic heart failure in the year before device setup. Patients were excluded if claims in the year before device setup included any of the following: use of adaptive servo‐ventilation or a bilevel PAP device; PAP resupply; diagnosis of systolic heart failure, combined systolic and diastolic heart failure, central sleep apnea, nocturnal hypoventilation, pregnancy, or end‐stage renal disease; and dialysis use.
PAP Adherence
PAP usage was objectively measured by the PAP device for each night it was used over the first year. For reimbursement purposes, the US Centers for Medicare and Medicaid Services (CMS) considers a patient compliant with therapy if the PAP device is used at least 4 hours per night on 70% of the nights during a consecutive 30‐day period in the first 90 days of therapy. Three levels of adherence were evaluated in this analysis: (1) adherent patients who met CMS criteria for all 4 consecutive 90‐day time frames (quarters) within the first year; (2) nonadherent patients who did not meet CMS criteria in any of the 4 quarters; and (3) intermediate adherent patients who met CMS criteria in at least 1 but no more than 3 quarters.
Outcomes
The primary outcome was health care resource use defined by the occurrence of a composite outcome of all‐cause hospitalizations and emergency room (ER) visits. Additionally, all‐cause hospitalizations, ER visits, and cardiovascular hospitalizations were assessed individually. Cardiovascular hospitalizations were defined as a hospitalization that had 1 of the following cardiovascular diseases as the primary diagnosis: myocardial infarction, stroke, heart failure, acute coronary syndrome, arrhythmia, cardiomyopathy, or hypertension. Proxy costs for all resource use were provided by Inovalon Insights LLC based on their proprietary Proxy Financials algorithm. The algorithm is based on CMS Medicare prospective payment system fee schedules.
Covariates
The following covariates were included to account for potential differences at baseline: (1) demographics (age, sex, payer, obesity); (2) comorbidities based on ICD‐9‐CM and ICD‐10‐CM diagnosis codes in the year before the first sleep test (hyperlipidemia, hypertension, gastroesophageal reflux disease, type 2 diabetes, cancer, cerebrovascular disease, atrial fibrillation, coronary artery disease, other arrhythmias, pulmonary hypertension, psychotic disorders, depression, anxiety, other mood disorders, chronic obstructive pulmonary disease, asthma, pneumonia); (3) adherence to beta‐blocker medication; (4) presence of an implanted cardiac device based on Current Procedural Terminology, Healthcare Common Procedure Coding System, and International Classification of Diseases Procedure Coding System codes; and (5) prior year health care resource use (all‐cause hospitalizations and ER visits).
Pharmacy claims data were used to identify prescription fills of heart failure medications, including angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, mineralocorticoid receptor antagonists, angiotensin receptor neprilysin inhibitors, diuretics, SGLT‐2 inhibitors, digoxin, and vasodilators. Adherence to beta blocker medication was used as a proxy to assess the effects of healthy user behavior. With a prescription exposure window of 181 to 360 days before starting PAP therapy, patients who filled a prescription for beta blockers were labeled as “on beta blockers.” Patients with a proportion of days covered of at least 80% were labeled as “adherent to beta blockers.” Patients who were “on beta blockers” but with a proportion of days covered of less than 80% were labeled as “not adherent to beta blockers.”
Statistical Analysis
Statistical analyses were performed using R statistical software version 4.0.3, Matching R package, and PSWeight R package. 27 , 28 , 29 Baseline demographics and covariates were assessed using descriptive statistics. To control for potential confounding, a risk score for each patient was defined based on all available covariates. Model coefficients for covariates were scaled to create a single risk score for each patient. Risk‐adjusted generalized linear models with a logit link were built to determine the association between PAP adherence and predicted mean number of composite all‐cause hospitalizations and ER visits, and predicted mean number of all‐cause hospitalizations, ER visits, and cardiovascular hospitalizations individually, using the adherent and nonadherent patients. Model goodness of fit was assessed by McFadden's grouped Log‐likelihood R‐squared (LL‐R2) and 90th percentile predicted range. From models that showed a statistically significant difference between adherent and nonadherent groups, the number needed to treat (NNT) was calculated as 1/absolute risk reduction for the overall cohort. NNT represents the number of patients that would need to be adherent to PAP therapy in order to avoid 1 additional event.
Propensity score matching was used to ensure appropriate balance in baseline characteristics between the PAP adherent and nonadherent groups. A logistic regression model based on the propensity not to adhere to PAP therapy was developed using baseline covariates. From this model, coefficients were used to calculate a propensity score for each patient. Greedy matching, meaning that once a patient has been matched, they could not be used in another match, was performed using the propensity score, age group, sex, payer type, presence of atrial fibrillation, prior year all‐cause hospitalizations, and prior year ER visits. Balance across groups was assessed using standardized mean differences, with |SMD| <0.1 indicating good balance. Differences in health care resource use between matched samples after PAP setup were assessed using Wilcoxon signed‐rank tests.
Finally, to supplement the findings from propensity score matching and to include a comparison with the intermediate PAP adherence group, inverse probability treatment weighting analyses were conducted. Weights were calculated from propensity scores and applied to create a weighted pseudo‐population that mirrored the distribution of the overall cohort and was balanced across adherence groups. This approach allowed for comparison of more than 2 treatment groups, while leveraging the full sample size. Pairwise comparisons of mean number of health care visits between adherent, intermediate adherent, and nonadherent patients were conducted.
RESULTS
Baseline Characteristics
A total of 4237 patients with OSA and HFpEF were identified (54.0% female, mean age 64.1 years). The average number of comorbid conditions (not including OSA and HFpEF) was high at 6.2, with the most prevalent being hypertension (95.7% of patients), hyperlipidemia (79.5%), type 2 diabetes (57.8%), coronary artery disease (53.1%), and chronic obstructive pulmonary disease (45.1%) (Table 1). An implanted cardiac device was present in 7.2% of the cohort, with the majority of these (41.5%) having a pacemaker. Cardiovascular medication use was variable, with beta blockers and diuretics being the most commonly used agents (Table 1). Baseline characteristics in patient subgroups based on adherence group and by adherence group after inverse probability treatment weighting are shown in Tables S1 and S2, respectively.
Table 1.
Cohort Characteristics, Overall and for Matched Cohort
| Overall (n=4237) | Matched cohort | ||||
|---|---|---|---|---|---|
| Adherent (n=963) | Nonadherent (n=963) | Standardized mean difference | 95% CI | ||
| Female sex, n (%) | 2287 (54.0) | 538 (55.9) | 538 (55.9) | 0.00 | −0.09 to 0.09 |
| Age, y | 64.1±11.5 | 64.5±11.5 | 64.3±11.9 | 0.02 | −0.07 to 0.11 |
| Payer, n (%) | 0.00 | −0.09 to 0.09 | |||
| Commercial | 1833 (43.3) | 402 (41.7) | 402 (41.7) | ||
| Medicaid | 882 (20.8) | 194 (20.1) | 194 (20.1) | ||
| Medicare advantage | 1522 (35.9) | 367 (38.1) | 367 (38.1) | ||
| Obesity, n (%) | 0.07 | −0.02 to 0.15 | |||
| Morbidly obese | 2360 (55.7) | 540 (56.1) | 521 (54.1) | ||
| Obese | 1022 (24.1) | 221 (22.9) | 237 (24.6) | ||
| Overweight | 158 (3.7) | 38 (3.9) | 46 (4.8) | ||
| Healthy weight | 38 (0.9) | 13 (1.3) | 10 (1.0) | ||
| Not categorized | 659 (15.6) | 151 (15.7) | 149 (15.5) | ||
| Comorbid conditions | |||||
| Number | 6.2±2.4 | 6.1±2.2 | 6.3±2.4 | −0.06 | −0.15 to 0.03 |
| Comorbidity, n (%) | |||||
| Hypertension | 4055 (95.7) | 921 (95.6) | 930 (96.6) | 0.03 | −0.06 to 0.11 |
| Pulmonary hypertension | 1085 (25.6) | 235 (24.4) | 256 (26.6) | −0.05 | −0.14 to 0.04 |
| Atrial fibrillation | 1493 (35.2) | 334 (34.7) | 334 (34.7) | 0.00 | −0.09 to 0.09 |
| Atrial flutter | 77 (1.8) | 22 (2.3) | 12 (1.5) | 0.06 | −0.03 to 0.15 |
| Other arrhythmia | 1167 (27.5) | 271 (28.1) | 245 (25.4) | 0.06 | −0.03 to 0.15 |
| Coronary artery disease | 2249 (53.1) | 500 (51.9) | 518 (53.8) | −0.04 | −0.13 to 0.05 |
| Cerebrovascular disease | 785 (18.5) | 176 (18.3) | 185 (19.2) | −0.02 | −0.11 to 0.07 |
| Asthma | 1248 (29.5) | 271 (28.1) | 281 (29.2) | −0.02 | −0.11 to 0.07 |
| Chronic obstructive pulmonary disease | 1910 (45.1) | 424 (44.0) | 261 (47.9) | −0.08 | −0.17 to 0.01 |
| Pneumonia | 1055 (24.9) | 248 (25.8) | 227 (23.6) | 0.05 | −0.04 to 0.14 |
| Psychotic disorders | 262 (6.2) | 37 (3.8) | 68 (7.1) | −0.14 | −0.23 to 0.05 |
| Other mood disorders | 307 (7.2) | 69 (7.2) | 76 (7.9) | −0.03 | −0.12 to 0.06 |
| Depression | 1292 (30.5) | 288 (29.9) | 311 (32.3) | −0.05 | −0.14 to 0.04 |
| Anxiety | 1139 (26.9) | 251 (26.1) | 285 (29.6) | −0.08 | −0.17 to 0.01 |
| Type 2 diabetes | 2450 (95.7) | 565 (58.7) | 553 (57.4) | 0.03 | −0.06 to 0.11 |
| Hyperlipidemia | 3370 (79.5) | 776 (80.6) | 763 (79.2) | 0.03 | −0.06 to 0.12 |
| Gastroesophageal reflux disease | 1760 (41.5) | 389 (40.4) | 404 (42.0) | −0.03 | −0.12 to 0.06 |
| Cancer | 509 (12.0) | 113 (11.7) | 108 (11.2) | 0.02 | −0.07 to 0.11 |
| Heart failure variables, n (%) | |||||
| Implanted cardiac device | 306 (7.2) | 78 (8.1) | 67 (7.0) | 0.04 | −0.05 to 0.13 |
| Cardiovascular medications*, n (%) | |||||
| Angiotensin‐converting enzyme inhibitor | 1159 (32.6) | 264 (32.2) | 292 (36.0) | −0.08 | −0.18 to 0.02 |
| Angiotensin receptor blocker | 943 (26.5) | 219 (26.7) | 198 (24.4) | 0.05 | −0.04 to 0.15 |
| Angiotensin receptor neprilysin inhibitor | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0.00 | −0.10 to 0.10 |
| Beta blocker | 2181 (61.3) | 504 (61.5) | 506 (62.3) | −0.02 | −0.11 to 0.08 |
| Mineralocorticoid receptor antagonist | 456 (12.8) | 109 (13.3) | 112 (13.8) | −0.01 | −0.11 to 0.08 |
| Diuretic | 2425 (68.1) | 548 (66.8) | 562 (69.2) | −0.05 | −0.15 to 0.05 |
| Vasodilator | 321 (9.0) | 69 (8.4) | 85 (10.5) | −0.07 | −0.17 to 0.03 |
| Sodium‐glucose cotransporter‐2 inhibitor | 49 (1.4) | 7 (0.9) | 13 (1.6) | −0.07 | −0.16 to 0.03 |
| Digoxin | 111 (3.1) | 31 (3.8) | 24 (3.0) | 0.05 | −0.05 to 0.14 |
| Has Rx data, no heart failure Rx | 589 (16.5) | 139 (17.0) | 127 (15.6) | 0.04 | −0.06 to 0.13 |
| No Rx data | 677 (16.0) | 143 (14.8) | 151 (15.7) | −0.02 | −0.11 to 0.07 |
| Adherent to beta blocker†, n (%) | 0.11 | −0.04 to 0.25 | |||
| Yes | 1154 (69.8) | 262 (71.3) | 260 (66.3) | ||
| No | 499 (30.2) | 106 (28.8) | 132 (33.7) | ||
| Prior year health care visits, n (%) | |||||
| Composite | 3339 (78.8) | 769 (79.8) | 769 (79.8) | 0.00 | −0.09 to 0.09 |
| Emergency room | 2571 (60.7) | 594 (61.7) | 594 (61.7) | 0.00 | −0.09 to 0.09 |
| All‐cause hospitalization | 2017 (47.6) | 461 (47.9) | 461 (47.9) | 0.00 | −0.09 to 0.09 |
| Cardiovascular hospitalization | 856 (20.2) | 194 (20.1) | 194 (20.1) | 0.00 | −0.09 to 0.09 |
| Prior year health care visits, n per patient | |||||
| Composite | 2.33±3.00 | 2.07±2.09 | 2.14±2.30 | −0.03 | −0.12 to 0.06 |
| Emergency room | 1.54±2.55 | 1.30±1.70 | 1.33±1.75 | −0.02 | −0.11 to 0.07 |
| All‐cause hospitalization | 0.79±1.16 | 0.77±1.04 | 0.81±1.24 | −0.04 | −0.13 to 0.05 |
| Cardiovascular hospitalization | 0.24±0.52 | 0.24±0.53 | 0.23±0.52 | 0.01 | −0.08 to 0.10 |
Values are mean±SD or number of patients (%). Rx indicates prescription.
Medication percentages (other than "No Rx data") are based on patients with Rx data.
Adherence to beta blocker percentages are based on those who filled a prescription for beta blocker medication in the 181 to 360 days before starting positive airway pressure therapy.
PAP Adherence
During the first year of PAP therapy, 40% of patients were considered adherent, 30% had intermediate adherence, and 30% were nonadherent. Overall, 64.1% of the cohort met CMS compliance criteria within the first 90 days of therapy. On average, patients adherent to PAP therapy used the device on 6.6 days per week and for 7.2 hours per use day. Patients with intermediate adherence used PAP on average 3.8 days per week for 5.4 hours per use day, and nonadherent patients used PAP on 0.9 days per week for 2.9 hours per use day.
Significant predictors of adhering to PAP included older age (>55 years) and presence of cancer or morbid obesity. Significant predictors of not adhering to PAP included female sex, Medicaid or Medicare Advantage insurance (compared with commercial insurance), presence of hypertension or type 2 diabetes, and at least 1 ED visit in the year before therapy.
Risk‐Adjusted Outcomes
The risk‐adjusted model for mean number of 1‐year composite all‐cause hospitalizations and ER visits fit well (LL‐R2 of 88%) and showed a statistically significant difference across the risk range between PAP adherent and nonadherent patients (P<0.001; Figure 1). The NNT (from nonadherent to adherent) to avoid a hospitalization or ER visit was 0.8 (P<0.001). The risk‐adjusted model for mean number of 1‐year ED visits also fit well (LL‐R2 of 87%) and showed a statistically significant difference across the risk range of patients (P<0.001). The NNT to avoid an ED visit was 1.1 (P<0.001). The model fit for mean number of 1‐year all‐cause hospitalizations was good (LL‐R2 of 79%) and showed a statistically significant difference between PAP adherent and nonadherent patients (P<0.001). The NNT to avoid a hospitalization was 3.3 (P<0.001). The risk‐adjusted model for number of 1‐year cardiovascular hospitalizations was satisfactory (LL‐R2 of 57%) and showed a statistically significant difference between PAP adherent and nonadherent patients (P<0.001), but the overall number of events was low (20% of patients had at least 1 cardiovascular hospitalization in the year prior, and only 7% in the first year of PAP therapy). The NNT to avoid a cardiovascular hospitalization was 14.9 (P<0.001).
Figure 1. Effect of positive airway pressure adherence on mean number of composite hospitalizations and emergency room visits.
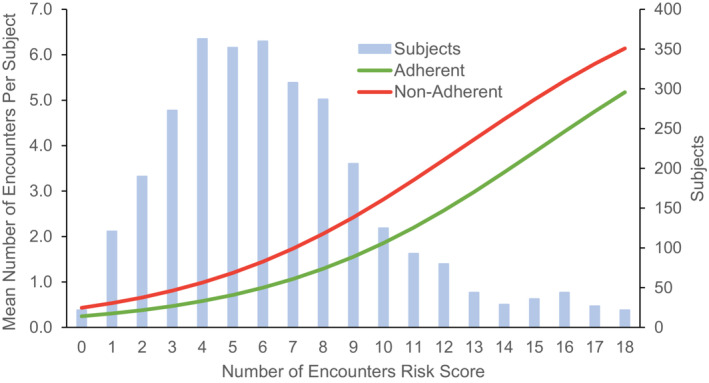
Association Between PAP Adherence and Health Care Resource Use/Costs
In the year before starting PAP, 79% of patients had an ER visit (61%) and/or a hospitalization (48%; Table 1). After 1 year, 56% of patients had an ER visit (48%) and/or a hospitalization (26%). After propensity score matching, 963 adherent and 963 nonadherent patients remained in the cohort and baseline characteristics well matched (Table 1). PAP adherent patients had significantly fewer total health care visits, including a 57% decrease in hospitalizations and a 36% decrease in ER visits in the first year of PAP compared with the previous year (Table 2, Figure 2). Total health care costs after 1 year of PAP therapy were significantly lower for adherent patients versus nonadherent patients ($12 732 versus $15 610, P<0.001), with significantly lower costs for inpatient hospitalizations ($3958 versus $6339, P<0.001) and ER visits ($717 versus $1008, P<0.001).
Table 2.
Mean Number of Health Care Resource Use Visits and Positive Airway Pressure Usage in Matched Cohort
| Overall (n=4237) | Matched cohort | |||
|---|---|---|---|---|
| Adherent (n=963) | Nonadherent (n=963) | P value | ||
| Year 1, n per patient | ||||
| Composite | 1.73±3.07 | 1.16±1.87 | 1.75±2.29 | <0.001 |
| Emergency room | 1.26±2.54 | 0.83±1.49 | 1.21±1.82 | <0.001 |
| All‐cause hospitalization | 0.47±1.12 | 0.33±0.84 | 0.53±1.08 | <0.001 |
| Cardiovascular hospitalization | 0.10±0.47 | 0.06±0.28 | 0.11±0.41 | 0.004 |
| PAP usage | ||||
| PAP hours per day | 3.7±3.0 | 6.9±1.5 | 0.4±0.6 | <0.001 |
| PAP days per week | 4.1±2.7 | 6.6±0.4 | 0.9±1.2 | <0.001 |
| PAP hours per use day | 5.4±2.3 | 7.2±1.4 | 2.9±1.7 | <0.001 |
Values are mean±SD. PAP indicates positive airway pressure.
Figure 2. Health care resource use 1 year before and 1 year after initiating positive airway pressure (PAP) therapy.
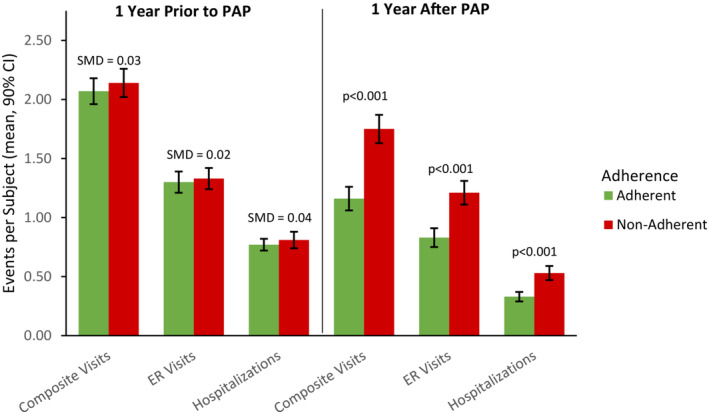
ER indicates emergency room; and SMD, standardized mean difference.
The inverse probability treatment weighting analyses confirmed significantly fewer visits for all outcomes between adherent and nonadherent patients, and between adherent and intermediate adherence patients (Table 3). Only hospitalizations were significantly lower for patients with intermediate adherence compared with those who were nonadherent. Adherent patients had lower total health care costs than intermediate adherent and nonadherent patients ($12 676 versus $16 157 and $16 173; P<0.001 and P<0.001, respectively), with significantly lower costs for inpatient hospitalizations ($3880 versus $6409 and $7025; P<0.001 and P<0.001, respectively) and ER visits ($741 versus $1142 and $1168; P<0.001 and P<0.001, respectively).
Table 3.
Mean Number of Health Care Resource Use Visits and Positive Airway Pressure Usage in the Inverse‐Probability Treatment‐Weighted Cohort
| Adherence level | P value | |||||
|---|---|---|---|---|---|---|
| Adherent (n=1701) | Intermediate (n=1250) | Nonadherent (n=1286) | A‐N | A‐I | I‐N | |
| Year 1, n per patient | ||||||
| Composite | 1.22±2.06 | 1.88±3.12 | 1.99±3.21 | <0.001 | <0.001 | 0.121 |
| Emergency room | 0.89±1.66 | 1.37±2.54 | 1.41±2.68 | <0.001 | <0.001 | 0.818 |
| All‐cause hospitalization | 0.33±0.84 | 0.51±1.23 | 0.59±1.17 | <0.001 | <0.001 | 0.006 |
| Cardiovascular hospitalization | 0.06±0.27 | 0.13±0.61 | 0.13±0.47 | 0.001 | <0.001 | 0.331 |
| PAP usage | ||||||
| PAP hours per day | 6.8±1.5 | 2.9±1.4 | 0.4±0.6 | <0.001 | <0.001 | <0.001 |
| PAP days per week | 6.6±0.5 | 3.8±1.7 | 0.9±1.2 | <0.001 | <0.001 | <0.001 |
| PAP hours per use day | 7.2±1.4 | 5.4±1.3 | 2.9±1.7 | <0.001 | <0.001 | <0.001 |
Values are mean±SD. Composite visit is a hospitalization or emergency room visit. A indicates adherent; I, intermediate adherence; N, nonadherent; and PAP, positive airway pressure.
Beta Blocker Adherence as Healthy User Effect Proxy
For patients taking beta blockers, 69.8% were categorized as adherent to the medication and 30.2% were nonadherent. Those who were adherent to beta blockers were also more likely to be adherent to PAP therapy: 74.1% of PAP adherent patients, 70.5% of PAP intermediate adherent patients, and 63.9% of PAP nonadherent patients were adherent to beta blockers. Adherence to beta blockers was included as a covariate in the risk‐adjusted models and the propensity score model and was not a significant independent predictor for any health care resource use outcome or the risk of not adhering to PAP therapy. Adherence to beta‐blockers was well balanced at baseline in both propensity score matching and inverse probability treatment weighting analyses.
DISCUSSION
Our findings are novel and important for a number of reasons. First, to our knowledge, this is the largest analysis exploring the association between OSA therapy and patients with HFpEF to date. Second, we observed that adherence to PAP therapy is associated with improvements in health care resource use, including reductions in hospitalization rate, ER visits, and cardiovascular hospitalizations. Third, we have demonstrated both clinical and economic benefits associated with treating OSA in HFpEF, particularly among patients adherent to therapy. Similar benefits of PAP adherence were seen using this linked data set in patients with HFrEF in a separate study. 30 In addition, these benefits of treating OSA with PAP therapy have also been reported in patients with comorbid chronic obstructive pulmonary disease, and type 2 diabetes. 31 , 32 Taken together, these findings encourage further study of OSA in chronic heart failure and may be clinically directive until more rigorous interventional data are available.
The population with HFpEF studied is consistent with clinical phenotypes described in the literature, being 54% female and the majority of patients (95.7%) having hypertension. Patients also had many additional comorbidities (mean 6.2 per patient), including coronary artery disease (53.1%), chronic obstructive pulmonary disease (45.1%), type 2 diabetes (57.8%), and hyperlipidemia (79.5%). These findings are consistent with the HFpEF literature and suggest that the ascertainment using ICD codes effectively captured a typical population with HFpEF for investigation.
Overall, we found that 40% of patients were adherent to PAP therapy, 30% were nonadherent, and the remaining 30% had intermediate adherence. Significant predictors of not adhering to PAP included female sex, Medicaid or Medicare Advantage insurance (versus commercial insurance), presence of hypertension or type 2 diabetes, and at least 1 ED visit in the year before therapy. Older age (>55 years), and presence of cancer or morbid obesity were associated with greater PAP adherence. Considering the high burden of medical comorbidities in this patient sample, the adherence rates were quite encouraging, and future efforts to enhance adherence using a range of patient engagement strategies are warranted. 33 , 34 , 35
Several mechanisms may explain the relationship between OSA and HFpEF. Previous research has shown that moderate to severe OSA is associated with a higher degree of diastolic dysfunction. 36 It has also been suggested that severe OSA itself impairs left ventricular diastolic function due to arterial stiffness, increased sympathetic nerve activity, and blood pressure, which are factors known to contribute to the development of HFpEF. 10 , 37 , 38 In addition, intermittent hypoxemia results in oxidative stress and increased inflammatory factors, potentially worsening cardiovascular function, and predisposing to arrhythmias. 39 , 40
PAP therapy prevents upper airway collapse, which may help mitigate the deleterious effect of OSA, 1 , 34 , 41 which results in observed clinical outcomes such as reduction in blood pressure, improvement in sleep efficiency, and reversing diastolic abnormalities. 42 , 43 , 44 These beneficial effects of PAP therapy may be mediators for the results observed in this study.
Recent studies with similar designs have shown that PAP adherence reduces the risk of cardiovascular events in older Medicare beneficiaries. In a cohort of older patients with cardiovascular disease and comorbid OSA, PAP adherence was associated with low readmission rates and a 40% reduction in health care costs. 45 , 46 Our study's results corroborate these findings. Furthermore, these data expand on the benefits of PAP adherence to patients with comorbid HFpEF and strengthens the evidence on the association between PAP adherence and reduced health care costs.
Strengths and Limitations
A strength of our analysis is that we performed a variety of complementary statistical approaches, including propensity score matched analyses and inverse probability treatment weighting analyses, all of which generated consistent findings. These strongly support the conclusion of a benefit of PAP therapy for OSA in HFpEF, independent of a diverse range of comorbidities. Additionally, the inverse probability treatment weighting analyses showed patients defined as intermediately adherent to PAP had outcomes more consistent with those who were nonadherent to therapy, highlighting the importance of consistent PAP use. The NNT analyses highlighted robust effects of PAP adherence. NNT values were 3.3 for all‐cause hospitalizations, 1.1 for ER visits, 14.9 for cardiac hospitalizations, and 0.8 for composite hospitalization/ED visit, which highlights the effectiveness of PAP therapy for these outcomes and provides strong justification for efforts to convert a nonadherent patient to an adherent one.
There is a growing body of literature highlighting new therapeutic options for HFpEF. In particular, use of SGLT2 inhibitors appears to be an important advance. These agents may be given in combination with mineralocorticoid receptor antagonists such as spironolactone, and the latter may improve underlying OSA in patients with resistant hypertension. 47 In addition, treatment with a loop diuretic could improve upper airway edema, which would be expected to improve OSA. 48 , 49 However, there are limited data on the effects of existing HFpEF therapies on sleep‐disordered breathing. We speculate that the beneficial effects seen during pharmacological management of HFpEF may, at least in part, be a function of improvements in sleep‐disordered breathing. Because our study largely predated the widespread use of SGLT2 inhibitors we cannot draw meaningful conclusions regarding the potential benefits of PAP therapy in patients with HFpEF being treated with an agent from this drug class.
Despite our study's strengths, we acknowledge a number of limitations. First, the study had a retrospective and observational design. Therefore, any findings represent correlation rather than causation. However, we believe that we have observed important associations that could lead to more rigorous research in the future. Moreover, large‐scale randomized trials with the current sample size are unlikely to occur in the foreseeable future. Second, we relied on ICD codes for classification of our patients, which was required based on our study design, and lacked information on disease severity, patient symptoms, and smoking status. However, the characteristics and demographics of our population with HFpEF were consistent with the literature. Although some misclassification may have occurred, we believe that such errors should be random and unlikely to systematically bias the results. Third, because details about the use of supplemental oxygen were not available, we were unable to assess its potential role as a confounder. Fourth, because we lack information on mortality, we studied a survivor cohort to allow us to examine the clinical and economic outcomes of interest. In addition, we observed a high prevalence of morbid obesity (55.7%) and although it is high in comparison to other comorbid cohorts with OSA, obesity was well balanced in both the propensity score matched analysis (standardized mean difference=0.07) and the inverse probability treatment weighting analysis (Tables S1 and S2). Hence it is unlikely that obesity per se influenced our findings. Future studies could address the impact of PAP therapy on hard clinical outcomes including mortality in HFpEF. Finally, because our findings are observational, outcomes associated with adherence to PAP therapy may result from the so‐called “healthy user” effect. 50 That is, PAP therapy adherence may be a marker of education, socioeconomic factors, baseline severity of the patient's symptoms, or patient motivation, meaning that any observed benefits may be a function of these other factors rather than PAP therapy per se. 31 , 51 , 52 , 53 Indeed, Platt et al 54 observed that the probability of adhering to continuous PAP was higher patients with adequate versus low usage of statin medications, although medication adherence did not fully predict PAP adherence. To investigate the possibility that the healthy user effect was mediating our observations, we conducted a number of analyses controlling for beta blocker prescription fills as a covariate. The observed benefits of PAP therapy remained robust after accounting for medication adherence suggesting that PAP therapy per se may be helpful rather than just being a marker of other health behaviors. Nevertheless, we acknowledge the potential for residual confounding and are supportive of further studies to confirm or refute our results. Despite these limitations, we believe that our findings are robust and hope that they help to raise awareness regarding OSA in HFpEF and encourage further research in this area.
CONCLUSIONS
The results of this study showed improved outcomes in patients with OSA and HFpEF who were adherent to PAP therapy during the first year after treatment initiation, with an overall reduction in health care resource use. This highlights the importance of diagnosing and treating coexistent OSA in patients with HFpEF and gives credibility to the notion that OSA may have a causal role in the progression of HFpEF.
APPENDIX
medXcloud group:
The medXcloud group is an academic‐industry collaboration involving employees and consultants of ResMed and global academic thought leaders in the fields of sleep and respiratory medicine. The medXcloud investigators include authors Peter A. Cistulli, Atul Malhotra, Jean‐Louis Pépin, Adam V. Benjafield, as well as Kimberly L. Sterling, Carlos M. Nunez, Meredith Barrett (ResMed Science Center, San Diego, CA), and Jeff Armitstead (ResMed Science Centre, Sydney, Australia).
Sources of Funding
This work was funded by ResMed.
Disclosures
P.A.C. has an appointment to an endowed academic chair at the University of Sydney that was established from ResMed funding; has received research support from ResMed, SomnoMed, and Zephyr Sleep Technologies; and is a consultant to ResMed, SomnoMed, Signifier Medical Technologies, Bayer, and Sunrise Medical. A.M. is funded by the National Institutes of Health. He reports income related to medical education from Livanova, Jazz, Zoll, and Eli Lilly. ResMed provided a philanthropic donation to UC San Diego, but A.M. has not received personal income from ResMed or medXcloud. J.‐L.P. is supported by the French National Research Agency in the framework of the Investissements d'Avenir program [grant ANR‐15‐IDEX‐02] and the e‐Health and Integrated Care and Trajectories Medicine and MIAI Artificial Intelligence chairs of excellence from the Grenoble Alpes University Foundation. He has received lecture fees or conference traveling grants from ResMed, Philips, Jazz Pharmaceuticals, Agiradom, and Bioprojet. V.K.S. is funded by the National Institutes of Health. He serves on the Sleep Number Scientific Advisory Board and as a consultant for ResMed, Jazz, Bayer, Lilly, Zoll, Apnimed, Wesper, and Huxley. K.V.C., A.S.M., F.H.S.K., and A.V.B. are all employees of ResMed. Representatives of the study sponsor were involved in the study design, collection, analysis and interpretation of data, writing of the report, and in the decision to submit the paper for publication. P.C. had final responsibility for the decision to submit for publication.
Supporting information
Tables S1–S2
Acknowledgments
Independent medical writing support was provided by Nicola Ryan. Conception and design: K.V.C., A.S.M., F.H.S.K., A.V.B., V.K.S.; Analysis: A.S.M.; interpretation: P.A.C., A.M., K.V.C., A.S.M., J.‐L.P., F.H.S.K., A.V.B., V.K.S.; drafting the first version of the article: P.A.C., A.M., V.K.S.; review and editing of the article: P.A.C., A.M., K.V.C., A.S.M., J.‐L.P., F.H.S.K., A.V.B., V.K.S.
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028733
For Sources of Funding and Disclosures, see page 10.
See Editorial by Healy et al.
Contributor Information
Peter A. Cistulli, Email: peter.cistulli@sydney.edu.au.
the medXcloud group:
Peter A. Cistulli, Atul Malhotra, Jean‐Louis Pépin, Adam V. Benjafield, Kimberly L. Sterling, Carlos M. Nunez, Meredith Barrett, and Jeff Armitstead
References
- 1. Javaheri S, Barbe F, Campos‐Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez‐Garcia MA, Mehra R, Pack AI, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194 [DOI] [PubMed] [Google Scholar]
- 3. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074 [DOI] [PubMed] [Google Scholar]
- 4. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256 [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 6. Redfield MM, Borlaug BA. Quality of life and exercise ability in heart failure with preserved ejection fraction: no time for therapeutic complacency. JAMA. 2021;326:1913–1915. doi: 10.1001/jama.2021.15874 [DOI] [PubMed] [Google Scholar]
- 7. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 8. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. doi: 10.1001/jamacardio.2016.1325 [DOI] [PubMed] [Google Scholar]
- 10. Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010 [DOI] [PubMed] [Google Scholar]
- 11. Caples SM, Garcia‐Touchard A, Somers VK. Sleep‐disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291 [DOI] [PubMed] [Google Scholar]
- 12. Lavie L. Intermittent hypoxia: the culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev Respir Med. 2008;2:75–84. doi: 10.1586/17476348.2.1.75 [DOI] [PubMed] [Google Scholar]
- 13. Lavie L. Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 14. Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci. 2012;4:1391–1403. doi: 10.2741/469 [DOI] [PubMed] [Google Scholar]
- 15. Sanderson JE, Fang F, Lu M, Ma CY, Wei YX. Obstructive sleep apnoea, intermittent hypoxia and heart failure with a preserved ejection fraction. Heart. 2021;107:190–194. doi: 10.1136/heartjnl-2020-317326 [DOI] [PubMed] [Google Scholar]
- 16. Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906 [DOI] [PubMed] [Google Scholar]
- 17. Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C‐reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03 [DOI] [PubMed] [Google Scholar]
- 18. Yu L, Li H, Liu X, Fan J, Zhu Q, Li J, Jiang J, Wang J. Left ventricular remodeling and dysfunction in obstructive sleep apnea: systematic review and meta‐analysis. Herz. 2020;45:726–738. doi: 10.1007/s00059-019-04850-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lloyd TC Jr. Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol (1985). 1990;69:1973–1980. doi: 10.1152/jappl.1990.69.6.1973 [DOI] [PubMed] [Google Scholar]
- 20. Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug‐resistant hypertension. Hypertension. 2010;56:1077–1082. doi: 10.1161/hypertensionaha.110.154427 [DOI] [PubMed] [Google Scholar]
- 21. Kohler M, West S, Stradling J. Diabetes in obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:286–287. [DOI] [PubMed] [Google Scholar]
- 22. Chan J, Sanderson J, Chan W, Lai C, Choy D, Ho A, Leung R. Prevalence of sleep‐disordered breathing in diastolic heart failure. Chest. 1997;111:1488–1493. doi: 10.1378/chest.111.6.1488 [DOI] [PubMed] [Google Scholar]
- 23. Herrscher TE, Akre H, Overland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–425. doi: 10.1016/j.cardfail.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 24. Yoshihisa A, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Impact of adaptive servo‐ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep‐disordered breathing. Eur J Heart Fail. 2013;15:543–550. doi: 10.1093/eurjhf/hfs197 [DOI] [PubMed] [Google Scholar]
- 25. Daubert MA, Whellan DJ, Woehrle H, Tasissa G, Anstrom KJ, Lindenfeld J, Benjafield A, Blase A, Punjabi N, Fiuzat M, et al. Treatment of sleep‐disordered breathing in heart failure impacts cardiac remodeling: insights from the CAT‐HF Trial. Am Heart J. 2018;201:40–48. doi: 10.1016/j.ahj.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 26. O'Connor CM, Whellan DJ, Fiuzat M, Punjabi NM, Tasissa G, Anstrom KJ, Benjafield AV, Woehrle H, Blase AB, Lindenfeld J, et al. Cardiovascular outcomes with minute ventilation‐targeted adaptive servo‐ventilation therapy in heart failure: the CAT‐HF trial. J Am Coll Cardiol. 2017;69:1577–1587. doi: 10.1016/j.jacc.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 27. R: A Language and Environment for Statistical Computing version 4.0.3. 2021. Available at: https://www.R‐project.org/. Accessed October 12, 2021.
- 28. PSweight R package version 1.1.8 ; Zhou T, Tong G, Li F, Thomas LE. PSweight: propensity score weighting analysis for causal inference with observational and randomized trials. Available at: https://cran.r‐project.org/web/packages/PSweight/index.html. Accessed March 03, 2022.
- 29. Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J Stat Softw. 2011;42:1–52. doi: 10.18637/jss.v042.i07 [DOI] [Google Scholar]
- 30. Malhotra A, Cole KV, Malik A, Pepin J, Sert Kuniyoshi F, Cistulli PA, Benjafield A, Somers V. Abstract 13554: Effect of positive airway pressure therapy for obstructive sleep apnea in heart failure patients with reduced ejection fraction—implications for healthcare resource utilization. Circulation. 2022;146:A13554. doi: 10.1161/circ.146.suppl_1.13554 [DOI] [Google Scholar]
- 31. Sterling KL, Pepin JL, Linde‐Zwirble W, Chen J, Benjafield AV, Cistulli PA, Cole KV, Emami H, Woodford C, Armitstead JP, et al. Impact of positive airway pressure therapy adherence on outcomes in patients with OSA and COPD. Am J Respir Crit Care Med. 2022;206:197–205. doi: 10.1164/rccm.202109-2035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sterling KL, Cistulli PA, Linde‐Zwirble W, Malik A, Benjafield AV, Malhotra A, Cole KV, Emami H, Woodford C, More S, et al. Association between positive airway pressure therapy adherence and health care resource utilization in patients with obstructive sleep apnea and type 2 diabetes in the United States. J Clin Sleep Med. 2022;19:563–571. doi: 10.5664/jcsm.10388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest. 2018;153:843–850. doi: 10.1016/j.chest.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pepin JL, Bailly S, Rinder P, Adler D, Benjafield AV, Lavergne F, Josseran A, Sinel‐Boucher P, Tamisier R, Cistulli PA, et al. Relationship between CPAP termination and all‐cause mortality: a French nationwide database analysis. Chest. 2022;161:1657–1665. doi: 10.1016/j.chest.2022.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woehrle H, Arzt M, Graml A, Fietze I, Young P, Teschler H, Ficker JH. Effect of a patient engagement tool on positive airway pressure adherence: analysis of a German healthcare provider database. Sleep Med. 2018;41:20–26. doi: 10.1016/j.sleep.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 36. Sidana J, Aronow WS, Ravipati G, Di Stante B, McClung JA, Belkin RN, Lehrman SG. Prevalence of moderate or severe left ventricular diastolic dysfunction in obese persons with obstructive sleep apnea. Cardiology. 2005;104:107–109. doi: 10.1159/000087128 [DOI] [PubMed] [Google Scholar]
- 37. Drager LF, Diegues‐Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, Marcondes B, Giorgi DM, Lorenzi‐Filho G, Krieger EM. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–254. doi: 10.1038/ajh.2009.246 [DOI] [PubMed] [Google Scholar]
- 38. Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045 [DOI] [PubMed] [Google Scholar]
- 39. Jelic S, Le Jemtel TH. Sleep‐disordered breathing in acute decompensated heart failure. Curr Heart Fail Rep. 2009;6:169–175. doi: 10.1007/s11897-009-0024-6 [DOI] [PubMed] [Google Scholar]
- 40. May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest. 2017;151:225–241. doi: 10.1016/j.chest.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El‐Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144:e56–e67. doi: 10.1161/cir.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 42. Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta‐analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–596. doi: 10.5664/jcsm.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pengo MF, Soranna D, Giontella A, Perger E, Mattaliano P, Schwarz EI, Lombardi C, Bilo G, Zambon A, Steier J, et al. Obstructive sleep apnoea treatment and blood pressure: which phenotypes predict a response? A systematic review and meta‐analysis. Eur Respir J. 2020;55:1901945. doi: 10.1183/13993003.01945-2019 [DOI] [PubMed] [Google Scholar]
- 44. Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, Francis A, Bohonis S, Zeglinski M, Kirkpatrick IDC, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. doi: 10.1378/chest.11-0615 [DOI] [PubMed] [Google Scholar]
- 45. Bailey MD, Wickwire EM, Somers VK, Albrecht JS. Adherence to continuous positive airway pressure reduces the risk of 30‐day hospital readmission among older adults with comorbid obstructive sleep apnea and cardiovascular disease. J Clin Sleep Med. 2022;18:2739–2744. doi: 10.5664/jcsm.10196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bock JM, Needham KA, Gregory DA, Ekono MM, Wickwire EM, Somers VK, Lerman A. Continuous positive airway pressure adherence and treatment cost in patients with obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc Innov Qual Outcomes. 2022;6:166–175. doi: 10.1016/j.mayocpiqo.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, Calhoun DA. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–537. doi: 10.1038/jhh.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32:673–680. doi: 10.1097/HJH.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 50. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–550. doi: 10.1007/s11606-010-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Donovan LM, Patel SR. The challenges of estimating causal effects of CPAP therapy from observational data. Am J Respir Crit Care Med. 2022;206:1570–1571. doi: 10.1164/rccm.202207-1413LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long‐term compliance with continuous positive airway pressure. Thorax. 2010;65:829–832. doi: 10.1136/thx.2010.135848 [DOI] [PubMed] [Google Scholar]
- 53. Jacobsen AR, Eriksen F, Hansen RW, Erlandsen M, Thorup L, Damgård MB, Kirkegaard MG, Hansen KW. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One. 2017;12:e0189614. doi: 10.1371/journal.pone.0189614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Platt AB, Kuna ST, Field SH, Chen Z, Gupta R, Roche DF, Christie JD, Asch DA. Adherence to sleep apnea therapy and use of lipid‐lowering drugs: a study of the healthy‐user effect. Chest. 2010;137:102–108. doi: 10.1378/chest.09-0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


