Abstract
Background
Improving health status is one of the major goals in the management of heart failure (HF). However, little is known about the long‐term individual trajectories of health status in patients with acute HF after discharge.
Methods and Results
We enrolled 2328 patients hospitalized for HF from 51 hospitals prospectively and measured their health status via the Kansas City Cardiomyopathy Questionnaire–12 at admission and 1, 6, and 12 months after discharge, respectively. The median age of the patients included was 66 years, and 63.3% were men. Six patterns of Kansas City Cardiomyopathy Questionnaire–12 trajectories were identified by a latent class trajectory model: persistently good (34.0%), rapidly improving (35.5%), slowly improving (10.4%), moderately regressing (7.4%), severely regressing (7.5%), and persistently poor (5.3%). Advanced age, decompensated chronic HF, HF with mildly reduced ejection fraction, HF with preserved ejection fraction, depression symptoms, cognitive impairment, and each additional HF rehospitalization within 1 year of discharge were associated with unfavorable health status (moderately regressing, severely regressing, and persistently poor) (P<0.05). Compared with the pattern of persistently good, slowly improving (hazard ratio [HR], 1.50 [95% CI, 1.06–2.12]), moderately regressing (HR, 1.92 [1.43–2.58]), severely regressing (HR, 2.26 [1.54–3.31]), and persistently poor (HR, 2.34 [1.55–3.53]) were associated with increased risks of all‐cause death.
Conclusions
One‐fifth of 1‐year survivors after hospitalization for HF experienced unfavorable health status trajectories and had a substantially increased risk of death during the following years. Our findings help inform the understanding of disease progression from a patient perception perspective and its relationship with long‐term survival.
Registration
URL: https://www.clinicaltrials.gov; unique identifier: NCT02878811.
Keywords: acute heart failure, death, health status, prognosis
Subject Categories: Heart Failure, Mortality/Survival, Social Determinants of Health, Lifestyle, Mental Health
Nonstandard Abbreviations and Acronyms
- China PEACE 5p‐HF
China Patient‐centered Evaluative Assessment of Cardiac Events Prospective Heart Failure
- GISSI‐HF
Gruppo Italiano per lo Studio della Sopravvivenza nella Insufficienza Cardiaca‐Heart Failure
- KCCQ‐12
Kansas City Cardiomyopathy Questionnaire−12
Clinical Perspective.
What Is New?
In a large multicenter prospective cohort of patients with acute heart failure, our study first demonstrated distinct individual trajectories of health status within 1 year of discharge and examined the associations between health status trajectories and the risk of death during the next 3 years.
Compared with the patients of persistently good trajectory, patients with slowly improving, moderately regressing, severely regressing, or persistently poor health status had an increased risk of death during the following 3 years.
Both clinical and nonclinical factors were associated with unfavorable health status trajectories, some of which are modifiable.
What Are the Clinical Implications?
Patients with unfavorable health status within 1 year of discharge have an increased risk of death compared with those with persistently good health status, which highlights the importance of regularly monitoring health status during HF hospitalization and afterward for identifying the patients at high risk of death.
Heart failure (HF) is a rapidly growing global public health problem, affecting 64.3 million people worldwide. 1 Reducing death and improving health status are both the major goals in the management of HF. 2 When patients suffer new onset or recurrence of symptoms and signs of HF, which requires urgent medical attention and results in hospitalization, they are considered as acute HF (AHF) and have severely impaired health status. 3 , 4 Understanding the long‐term trajectories in their health status is critical for improving care and decision‐making.
However, we know little about the individual trajectories of health status in AHF. Although previous studies showed rapid improvement in average health status during the first few weeks and months and subsequent stabilization of health status throughout the first year after hospital discharge, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 no study reported the individual trajectories of health status. There is scarce knowledge about the proportions of patients experiencing such typical improvement within 1 year of discharge, even the specific patterns of health status trajectories, their distribution, associated patient characteristics, and the relationships with death during the following years.
To address the knowledge gaps, using the data from a nationwide, prospective, multicenter cohort study, the China PEACE 5p‐HF (China Patient‐centered Evaluative Assessment of Cardiac Events Prospective Heart Failure) study and a latent class trajectory model, we aim to (1) characterize the heterogeneity in the trajectories of HF‐specific health status during the first year of discharge among patients with AHF, (2) identify the factors associated with unfavorable health status trajectories, and (3) examine the associations between health status trajectories during the first year and the risk of death during the next 3 years.
Methods
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
We included the patients who were enrolled in the China PEACE 5p‐HF study and had their HF‐specific health status measured at all 4 time points; that is, at admission and at 1, 6, and 12 months. 14 The China PEACE 5p‐HF study enrolled patients hospitalized for HF between August 2016 and May 2018 from 51 diverse hospitals (48 tertiary and 3 secondary hospitals) located in 20 provinces, covering all economic–geographic regions in China. Patients hospitalized for HF in these hospitals were consecutively registered, with their informed consent sought if they were local residents and aged ≥18 years. Those who signed the informed consent were enrolled and followed up at 1, 6, and 12 months after discharge, then annually. The ethics committee of Fuwai Hospital and the local ethics committees at sites approved the study.
Data Collection
Patients' demographics, socioeconomic status (ie, income, marital status, employment status, education), smoking status, depression symptoms, and cognitive function were collected by physicians using standardized questionnaires through face‐to‐face interviews during the index hospitalization. Data were directly entered into laptop computers equipped with a customized electronic data collection system that allowed real‐time offline logic checks and verified the accuracy and completeness of entered data. Information on patients' comorbidities, clinical characteristics at admission (including heart rate and blood pressure), New York Heart Association class, 12‐lead ECG, local laboratory tests, and treatments were obtained from the medical records of the index hospitalization. Left ventricular ejection fraction (LVEF) was measured during the index hospitalization by trained physicians with standard protocols. We collected blood samples at admission to perform some key analyses at the central laboratory, including NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and creatinine. We used the first local laboratory results of serum sodium during the index hospitalization.
According to LVEF, HF was classified as HF with reduced ejection fraction (LVEF ≤40%), HF with mildly reduced ejection fraction (LVEF 41%–49%), and HF with preserved ejection fraction (LVEF≥50%). Patients were defined as being on a postdischarge medication if they were prescribed the medication at discharge and taking the medication at 1‐month follow‐up. Current smokers were defined as those smoking during the past 30 days. We classified the patients into nondrinkers, moderate drinkers (0–238 g/wk), and heavy drinkers (>238 g/wk) according to their alcohol consumption during the year before the index hospitalization. 15 Exercise was defined as having at least 2 hours of regular physical activity or exercise per week during the past 6 months before the index hospitalization. The definition of comorbidities is presented in Table S1. We defined low level of income (annual household income <$1478), unemployed status (retired, unemployed, or never employed), low educational level (primary school or below), and unpartnered status (divorced, separated, widowed, and unmarried) as socioeconomic risk factors. 16 Depression status was evaluated by the Patient Health Questionnaire–2 before discharge, with scores ranging from 0 to 6 (scores ≥3 indicating depression status). 17 Cognitive function was assessed by Mini‐Cog test before discharge, with scores ranging from 0 to 5 (scores ≤2 indicating cognitive impairment). 18
HF‐Specific Health Status Assessments
Patients' health status was measured by Kansas City Cardiomyopathy Questionnaire–12 (KCCQ‐12) within 48 hours after admission of the index hospitalization and at 1, 6, and 12 months after discharge. KCCQ‐12 is a short version of the original 23‐item instrument and preserves high test–retest reliability, responsiveness, prognostic ability, and interpretability thresholds of the original KCCQ. 19 It quantifies the following health status domains: symptom frequency, physical limitations, quality of life, and social limitations. The average scores of the 4 domains generate the KCCQ‐12 overall summary scores (KCCQ‐12 score) ranging from 0 to 100. A higher score reflects possibly less symptom burden and better health status. KCCQ‐12 had been linguistically and culturally translated into Simplified Chinese and was validated. 20
Clinical Outcomes
The 2 clinical outcomes were all‐cause death and cardiovascular death. Cardiovascular death was defined as sudden cardiac death or death due to HF, cerebrovascular events, coronary heart disease, or other cardiovascular causes. If there was no evidence of an alternative nonvascular cause of death, we counted it as cardiovascular death. Causes of death were centrally adjudicated according to the supporting documents.
Statistical Analysis
Continuous variables were expressed as median and interquartile range, and categorical variables were summarized by frequencies with percentages. We compared the patient characteristics between the patients included in this study and those excluded due to missing KCCQ‐12 data using the Wilcoxon test for continuous variables and chi‐square test for categorical variables.
We constructed a latent class trajectory model to identify the individual trajectory patterns of KCCQ‐12 scores. 21 KCCQ‐12 scores, measured at admission and at 1, 6, and 12 months, were considered as a class‐defining variable in the model. The models were fitted with 1 to 7 latent classes. Model fit was determined using the Bayesian information criterion and the sample size‐adjusted Bayesian information criterion. The Bayesian information criterion measures used a correction for the sample size to account for the correlated nature of the data. 22 Lower values for absolute Bayesian information criterion indicated a better‐fitting model. After the selection of the best‐fitting models, participants were assigned to their corresponding trajectory patterns, and patient characteristics were described grouped by trajectory patterns. Trends across trajectory patterns were analyzed using chi‐square trend tests for categorical variables and linear regression for continuous variables. Next, we classified certain trajectory patterns that had low KCCQ‐12 scores throughout and did not show an improvement trend within 1 year of discharge as unfavorable health status. To identify the associated factors for trajectory patterns of unfavorable health status, we used the binomial logistic regression to generate odds ratios (ORs) and 95% CIs for the combination of unfavorable health status trajectory patterns, using the combination of favorable patterns as the reference. The characteristics were chosen on the basis of literature review. 7 , 23
We presented the cumulative incidences of death across different trajectory patterns using Kaplan–Meier analysis and compared them using log‐rank tests. Cox proportional hazards models were developed to assess the associations of trajectory patterns with death beyond the first year. We assessed the influence of noncardiovascular death as a competing risk for cardiovascular death. Considering the convenience of interpretation, we calculated hazard ratios (HRs) and 95% CIs using the trajectory pattern with the highest KCCQ‐12 scores as the reference. We adjusted potential confounding variables on the basis of previous prognostic models and clinical knowledge, which included age, sex, number of socioeconomic risk factors, drinking, exercising, new‐onset HF/decompensated chronic HF, LVEF class, comorbidities (coronary heart disease, hypertension, atrial fibrillation, diabetes, reduced renal function, chronic obstructive pulmonary disease, stroke, anemia, valvular heart disease, and cancer), systolic blood pressure at admission, NT‐proBNP at admission, serum sodium at admission, KCCQ‐12 score at admission, depression symptoms before discharge, cognitive impairment before discharge, postdischarge medication, and the number of HF rehospitalizations within 1 year of discharge. Hospital was included as a random effect to account for the clustering of patients. We also analyzed the interactions between the KCCQ‐12 trajectory patterns and age, sex, new‐onset/decompensated chronic HF, and LVEF class in the Cox models.
Several patient characteristics or biomarkers were missed with the proportions from 0.2% to 4.0%. All missing variables were imputed with median values. Tests for statistical significance were 2‐sided with a level of 0.05. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and R programming language version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
A total of 4907 patients were enrolled in China PEACE 5p‐HF Study. After excluding 889 patients who died within 1 year after the index hospitalization and 1690 with KCCQ‐12 score missing at any time point, we included 2328 patients in the current analysis. Their median (interquartile range) age was 66 (56–75) years old, and 1474 (63.3%) were men. Comorbidities were common, including coronary heart disease (60.3%), hypertension (59.8%), atrial fibrillation (36.6%), diabetes (32.2%), and reduced renal function (24.4%) (Table). Compared with the included patients, those excluded due to missing KCCQ‐12 scores had generally similar characteristics, except higher NT‐proBNP levels, being less likely to exercise or take postdischarge medications, and higher death rate beyond the first year (Table S2).
Table 1.
Characteristics by Patterns of Health Status Trajectories
| Patient characteristic | Total (n=2328) | Persistently poor (n=124) | Severely regressing (n=174) | Moderately regressing (n=172) | Slowly improving (n=241) | Rapidly improving (n=826) | Persistently good (n=791) | P trend |
|---|---|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 66 (56–75) | 69 (62–78) | 69 (61–77) | 71 (61–79) | 69 (60–77) | 65 (55–74) | 63 (53–71) | <0.01 |
| Sex, male, n (%) | 1474 (63.3) | 63 (50.8) | 101 (58.0) | 98 (57.0) | 125 (51.9) | 504 (61.0) | 583 (73.7) | <0.01 |
| SERFs, median (IQR) | 1 (1–2) | 2 (1–2) | 2 (1–3) | 2 (1–2) | 2 (1–2) | 1 (1–2) | 1 (1–2) | <0.01 |
| Current smoker, n (%) | 498 (21.4) | 18 (14.5) | 33 (19.0) | 31 (18.0) | 33 (13.7) | 172 (20.8) | 211 (26.7) | <0.01 |
| Alcohol drinker, n (%) | ||||||||
| Nondrinker | 1725 (74.1) | 113 (91.1) | 139 (79.9) | 144 (83.7) | 201 (83.4) | 607 (73.5) | 521 (65.9) | <0.01 |
| Moderate drinker | 359 (15.4) | 9 (7.3) | 24 (13.8) | 15 (8.7) | 28 (11.6) | 133 (16.1) | 150 (19.0) | <0.01 |
| Heavy drinker | 244 (10.5) | 2 (1.6) | 11 (6.3) | 13 (7.6) | 12 (5.0) | 86 (10.4) | 120 (15.2) | <0.01 |
| Exercise, n (%) | 754 (32.4) | 20 (16.1) | 41 (23.6) | 59 (34.3) | 65 (27.0) | 241 (29.2) | 328 (41.5) | <0.01 |
| SBP at admission, mm Hg, median (IQR) | 130 (120–149) | 130 (119–145) | 134 (115–150) | 130 (120–149) | 130 (117–147) | 131 (118–149) | 132 (120–150) | 0.37 |
| DBP at admission, mm Hg, median (IQR) | 80 (70–90) | 80 (70–89) | 80 (70–90) | 80 (70–90) | 80 (70–90) | 80 (70–94) | 80 (70–90) | 0.07 |
| Heart rate at admission, bpm, median (IQR) | 86 (73–101) | 82 (71–100) | 88 (75–106) | 82 (73–100) | 88 (75–101) | 89 (75–104) | 84 (72–100) | 0.32 |
| NYHA class at admission, n (%) | ||||||||
| II | 373 (16.0) | 8 (6.5) | 5 (2.9) | 33 (19.2) | 22 (9.1) | 96 (11.6) | 209 (26.4) | <0.01 |
| III | 1052 (45.2) | 55 (44.4) | 73 (42.0) | 94 (54.7) | 107 (44.4) | 374 (45.3) | 349 (44.1) | 0.61 |
| IV | 896 (38.5) | 61 (49.2) | 96 (55.2) | 42 (24.4) | 112 (46.5) | 356 (43.1) | 229 (29.0) | <0.01 |
| Unrecorded | 7 (0.3) | 0 (0.0) | 0 (0.0) | 3 (1.7) | 0 (0.0) | 0 (0.0) | 4 (0.5) | 0.91 |
| LVEF (%), median (IQR) | 44 (34–56) | 49 (39–57) | 46 (35–59) | 49 (40–57) | 48 (37–57) | 42 (32–55) | 44 (33–56) | <0.01 |
| HFrEF, n (%) | 906 (38.9) | 31 (25.0) | 62 (35.6) | 46 (26.7) | 74 (30.7) | 361 (43.7) | 332 (42.0) | <0.01 |
| HFmrEF, n (%) | 486 (20.9) | 30 (24.2) | 24 (13.8) | 39 (22.7) | 50 (20.7) | 178 (21.5) | 165 (20.9) | 0.61 |
| HFpEF, n (%) | 843 (36.2) | 54 (43.5) | 75 (43.1) | 79 (45.9) | 110 (45.6) | 256 (31.0) | 269 (34.0) | <0.01 |
| Unrecorded | 93 (4.0) | 9 (7.3) | 13 (7.5) | 8 (4.7) | 7 (2.9) | 31 (3.8) | 25 (3.2) | <0.01 |
| Prior history of HF, n (%) | 1835 (78.8) | 112 (90.3) | 145 (83.3) | 152 (88.4) | 221 (91.7) | 650 (78.7) | 555 (70.2) | <0.01 |
| Comorbidity, n (%) | ||||||||
| CHD | 1403 (60.3) | 80 (64.5) | 107 (61.5) | 103 (59.9) | 159 (66.0) | 477 (57.7) | 477 (60.3) | 0.25 |
| Hypertension | 1391 (59.8) | 80 (64.5) | 96 (55.2) | 102 (59.3) | 151 (62.7) | 499 (60.4) | 463 (58.5) | 0.67 |
| Atrial fibrillation | 851 (36.6) | 55 (44.4) | 73 (42.0) | 79 (45.9) | 114 (47.3) | 298 (36.1) | 232 (29.3) | <0.01 |
| Diabetes | 749 (32.2) | 48 (38.7) | 53 (30.5) | 61 (35.5) | 84 (34.9) | 278 (33.7) | 225 (28.4) | 0.03 |
| Reduced renal function | 568 (24.4) | 54 (43.5) | 57 (32.8) | 52 (30.2) | 79 (32.8) | 189 (22.9) | 137 (17.3) | <0.01 |
| COPD | 471 (20.2) | 38 (30.6) | 43 (24.7) | 38 (22.1) | 69 (28.6) | 155 (18.8) | 128 (16.2) | <0.01 |
| Stroke | 464 (19.9) | 32 (25.8) | 50 (28.7) | 47 (27.3) | 72 (29.9) | 130 (15.7) | 133 (16.8) | <0.01 |
| Anemia | 433 (18.6) | 42 (33.9) | 36 (20.7) | 33 (19.2) | 59 (24.5) | 156 (18.9) | 107 (13.5) | <0.01 |
| VHD | 426 (18.3) | 21 (16.9) | 40 (23.0) | 36 (20.9) | 49 (20.3) | 161 (19.5) | 119 (15.0) | 0.04 |
| Cancer | 98 (4.2) | 8 (6.5) | 8 (4.6) | 10 (5.8) | 14 (5.8) | 28 (3.4) | 30 (3.8) | 0.07 |
| Number of comorbidities, n (%) | ||||||||
| ≤2 | 966 (41.5) | 32 (25.8) | 56 (32.2) | 55 (32.0) | 64 (26.6) | 365 (44.2) | 394 (49.8) | <0.01 |
| 3–4 | 977 (42.0) | 50 (40.3) | 80 (46.0) | 80 (46.5) | 108 (44.8) | 338 (40.9) | 321 (40.6) | 0.18 |
| ≥5 | 385 (16.5) | 42 (33.9) | 38 (21.8) | 37 (21.5) | 69 (28.6) | 123 (14.9) | 76 (9.6) | <0.01 |
| Laboratory, median (IQR) | ||||||||
| NT‐proBNP at admission, pg/mL | 1144 (480–2477) | 1776 (653–3153) | 1496 (735–3184) | 1026 (472–2647) | 1340 (635–2837) | 1400 (583–2798) | 816 (338–1770) | <0.01 |
| eGFR at admission, mL/min per 1.73 m2 | 75 (60–90) | 63 (46–81) | 72 (55–88) | 71 (56–83) | 70 (55–85) | 75 (61–90) | 80 (65–94) | <0.01 |
| Serum sodium at admission, mmol/L | 140 (137–142) | 139 (137–142) | 140 (137–143) | 139 (137–142) | 139 (136–142) | 140 (137–142) | 140 (138–142) | 0.01 |
| KCCQ‐12 score at admission, median (IQR) | 47 (31–65) | 23 (12–34) | 25 (16–35) | 63 (55–72) | 33 (22–44) | 36 (26–45) | 69 (60–79) | <0.01 |
| Depression symptom before discharge, n (%) | 555 (23.8) | 70 (56.5) | 78 (44.8) | 38 (22.1) | 91 (37.8) | 198 (24.0) | 80 (10.1) | <0.01 |
| Cognitive impairment before discharge, n (%) | 659 (28.3) | 52 (41.9) | 69 (39.7) | 66 (38.4) | 78 (32.4) | 240 (29.1) | 154 (19.5) | <0.01 |
| In‐hospital ICD/CRT, n (%) | 19 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (1.5) | 7 (0.9) | 0.03 |
| Postdischarge medication*, n (%) | ||||||||
| Beta blocker | 1009 (43.3) | 39 (31.5) | 57 (32.8) | 64 (37.2) | 110 (45.6) | 368 (44.6) | 371 (46.9) | <0.01 |
| Diuretic | 1053 (45.2) | 59 (47.6) | 89 (51.1) | 62 (36.0) | 109 (45.2) | 396 (47.9) | 338 (42.7) | 0.38 |
| Aldosterone antagonists | 824 (35.4) | 46 (37.1) | 67 (38.5) | 52 (30.2) | 87 (36.1) | 311 (37.7) | 261 (33.0) | 0.41 |
| ACEI/ARB | 724 (31.1) | 32 (25.8) | 41 (23.6) | 46 (26.7) | 64 (26.6) | 275 (33.3) | 266 (33.6) | <0.01 |
| Nitrate | 405 (17.4) | 29 (23.4) | 40 (23.0) | 30 (17.4) | 52 (21.6) | 115 (13.9) | 139 (17.6) | 0.01 |
| Digoxin | 307 (13.2) | 16 (12.9) | 28 (16.1) | 14 (8.1) | 40 (16.6) | 121 (14.6) | 88 (11.1) | 0.38 |
| CCB | 213 (9.1) | 10 (8.1) | 18 (10.3) | 20 (11.6) | 20 (8.3) | 69 (8.4) | 76 (9.6) | 0.86 |
| LOS, d, median (IQR) | 9 (7–12) | 11 (9–15) | 10 (8–14) | 10 (7–12) | 11 (8–14) | 10 (7–12) | 8 (6–11) | <0.01 |
| Number of HF rehospitalizations within 1 y of discharge, mean (SD) | 0.5 (1.0) | 1.4 (1.8) | 1.0 (1.4) | 0.9 (1.4) | 0.6 (1.0) | 0.4 (0.7) | 0.3 (0.6) | <0.01 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CCB, calcium channel blocker; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; KCCQ‐12, Kansas City Cardiomyopathy Questionnaire–12; LOS, length of stay; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SERFs, socioeconomic risk factors; and VHD, valvular heart disease.
No patients used angiotensin receptor‐neprilysin inhibitor or sodium‐glucose cotransporter 2 inhibitors because of the late approval of these drugs. The first angiotensin receptor‐neprilysin inhibitor medication was approved for marketing in July 2017 and was covered by medical insurance in December 2021. The first sodium‐glucose cotransporter 2 inhibitors were approved for treating diabetes in March 2017 and for heart failure in October 2021.
Health Status Trajectory Patterns During the First Year of Discharge
The overall average KCCQ‐12 score significantly improved from admission to 1 month and 6 months and then remained stable until 12 months (Figure S1). After fitting models with 1 to 7 latent classes, the 6‐class model was considered the best fit (Table S3). According to the fitting, patients were divided into 6 patterns of health status trajectories from admission to 1 year of discharge (Figure 1). The patterns were (1) maintaining high KCCQ‐12 scores from admission to 1 year of discharge (persistently good, 791 [34.0%]); (2) low KCCQ‐12 scores at admission, a substantial increase at 1 month, and then maintaining high scores afterward (rapidly improving, 826 [35.5%]); (3) low KCCQ‐12 scores at admission, a mild increase at 1 month, and further moderate increases at 6 months and 12 months (slowly improving, 241 [10.4%]); (4) moderately high scores at admission, a mild increase at 1 month followed by a moderate decrease (moderately regressing, 172 [7.4%]); (5) low scores at admission, a substantial increase at 1 month, and a substantial decrease afterward (severely regressing; 174 [7.5%]); and (6) low scores at admission and keeping stable throughout the first year (persistently poor, 124 [5.3%]).
Figure 1. Six patterns of health status (measured by KCCQ‐12) trajectories identified by latent class trajectory model.
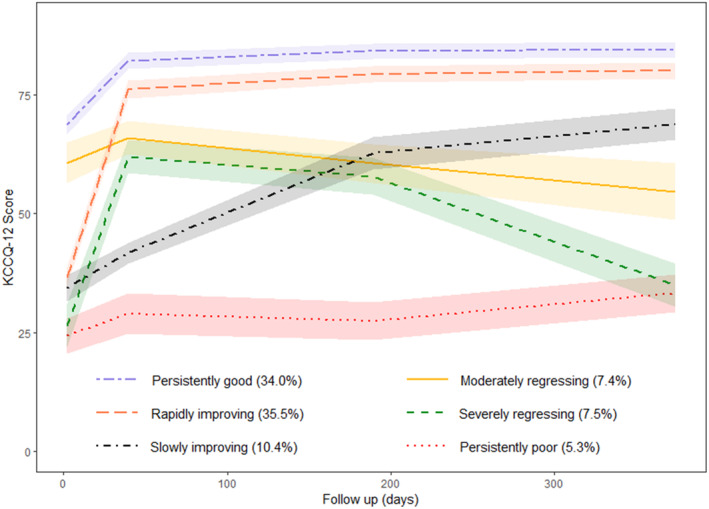
The shaded areas represent 95% CIs. KCCQ‐12 indicates Kansas City Cardiomyopathy Questionnaire‐12.
Factors Associated With the Patterns of Unfavorable Health Status Trajectories
We considered the patterns of persistently good, rapidly improving, and slowly improving as favorable health status and the other patterns as an unfavorable one. Patients with advanced age (OR, 1.10 per 5‐year increase [95% CI, 1.03–1.16]), more socioeconomic risk factors (OR, 1.17 [95% CI, 1.02–1.35 per additional socioeconomic risk factor]), decompensated chronic HF (OR, 1.43 [95% CI, 1.04–1.97]), HF with mildly reduced ejection fraction (OR, 1.38 [95% CI 1.01–1.89]), HF with preserved ejection fraction (OR, 1.82 [95% CI, 1.33–2.50]), higher levels of NT‐proBNP at admission (OR, 1.12 [95% CI, 1.02–1.23 per doubled]), depression symptoms (OR, 2.01 [95% CI, 1.58–2.55]), cognitive impairment (OR, 1.30 [95% CI, 1.02–1.67]), and each additional HF rehospitalization within 1 year of discharge (OR, 1.73 [95% CI, 1.56–1.94]) were more likely to experience the patterns of unfavorable health status. By contrast, patients who drank moderately (OR, 0.64 [95% CI, 0.44–0.94]), exercised (OR, 0.76 [95% CI, 0.59–0.98]), or took beta blockers (OR, 0.72 [95% CI, 0.57–0.93]) were less likely to experience those unfavorable patterns (Figure 2).
Figure 2. Factors associated with patterns of unfavorable health status by logistic regression.
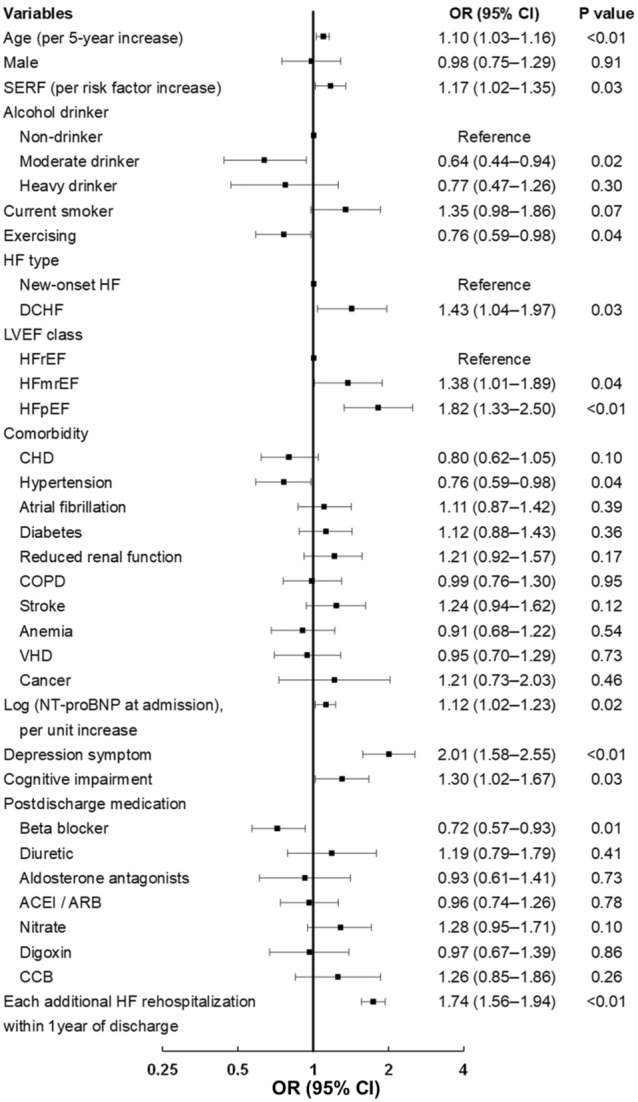
Small black squares are odds ratios, and their horizontal lines indicate the corresponding 95% CIs. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CCB, calcium channel blocker; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DCHF, decompensated chronic heart failure; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OR, odds ratio; SERF, socioeconomic risk factor; and VHD, valvular heart disease.
Association Between Health Status Trajectory Patterns Within the First Year and Death During the Following Years
During the median 3.25 years’ follow‐up beyond the first year, all‐cause and cardiovascular deaths varied by health status trajectory patterns (Figure 3). All‐cause deaths in persistently good, rapidly improving, slowly improving, moderately regressing, severely regressing, and persistently poor were 18.2%, 26.9%, 39.0%, 43.6%, 53.5%, and 58.9%, respectively (P<0.05); cardiovascular deaths were 15.0%, 20.2%, 32.0%, 32.6%, 39.7%, and 45.2%, respectively (P<0.05).
Figure 3. Cumulative incidence curves for all‐cause death (A) and cardiovascular death (B) by patterns of health status trajectories.
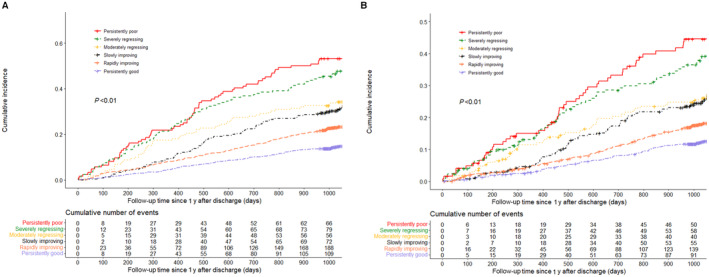
Differences in cumulative incidences across different trajectory patterns were evaluated using log‐rank tests. The red lines, green lines, golden lines, black lines, orange lines, and purple lines represent the cumulative incidences of persistently poor, severely regressing, moderately regressing, slowly improving, rapidly improving, and persistently good groups, respectively.
After adjustment, compared with persistently good, persistently poor (HR, 2.34 [95% CI, 1.55–3.53]), severely regressing (HR, 2.26 [95% CI, 1.54–3.31]), moderately regressing (HR, 1.92 [95% CI, 1.43–2.58]), and slowly improving (HR, 1.50 [95% CI, 1.06–2.12]) had increased risks of death (Figure 4). We observed a similar trend in cardiovascular death (Figure 4).
Figure 4. Risk of deaths in different patterns of health status trajectories by Cox proportional hazards model and Fine–Gray model.
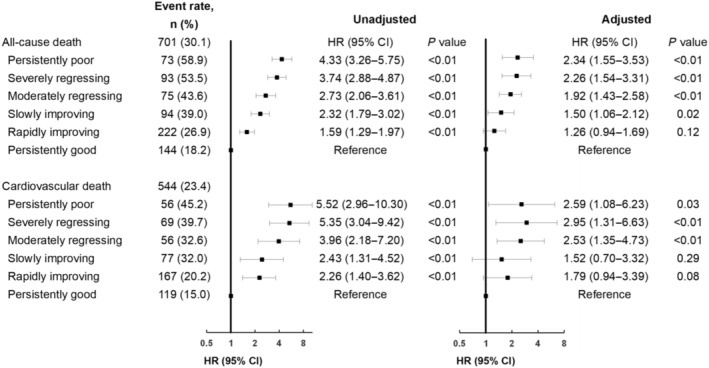
Adjusting for age, sex, number of socioeconomic risk factors, drinking, exercising, new‐onset HF/decompensated chronic heart failure, left ventricular ejection fraction class, comorbidities (coronary heart disease, hypertension, atrial fibrillation, diabetes, reduced renal function, chronic obstructive pulmonary disease, stroke, anemia, valvular heart disease, and cancer), systolic blood pressure at admission, N‐terminal pro‐B‐type natriuretic peptide at admission, serum sodium at admission, Kansas City Cardiomyopathy Questionnaire−12 score at admission, depression symptoms before discharge, cognitive impairment before discharge, postdischarge medication, and the number of heart failure rehospitalizations within 1 year of discharge. Small black squares are hazard ratios, and their horizontal lines indicate the corresponding 95% CIs. HR indicates hazard ratio.
Generally, there were no significant interactions of KCCQ‐12 trajectory patterns with most subgroups for both all‐cause and cardiovascular deaths (Tables S4 and S5).
Discussion
In this large prospective cohort of patients with AHF, we first demonstrated distinct individual trajectories of health status within 1 year of discharge, and certain patterns of trajectories were associated with an increased risk of death beyond the first year. Although a majority of patients experienced an improvement in health status after discharge, their trajectories of health status were rather heterogeneous. Approximately one‐fifth of this cohort had unsatisfactory health status during the first year. Both clinical and nonclinical factors were associated with the unfavorable health status trajectories, some of which are modifiable. Furthermore, compared with the patients of persistently good trajectory, the others had an increased risk of death during the following 3 years. Particularly, those of regressing or persistently poor trajectories had a 2‐ to 3‐fold risk.
The heterogeneity in the individual trajectories of health status was considerable. Consistent with previous studies, we noted that most patients had their health status improved substantially within the first few months of discharge. 6 , 7 , 9 , 13 However, the distinction among the health status trajectories from index admission to 1 year of discharge was significant. About 4 in 10 patients had fair (KCCQ‐12 score 50–74) or good (KCCQ‐12 score 75–100) health status at admission, one‐fifth of whom experienced moderate regressing, with their health status becoming even poorer than at admission. For most patients with poor health status (KCCQ‐12 score <50) at admission, three‐fifths witnessed rapid improvement to good status and one‐fifth slow improvement to fair status. Patients with severely regressing trajectory and those with persistently poor trajectory had similar health status at both admission and 1 year of discharge, while the former had much better health status than the latter most of the time. The heterogeneity might reflect the dynamic effects of miscellaneous factors influencing health status, such as the response to treatments or clinical events. 24 , 25 , 26 Patients with severely regressing or persistent poor health status had on average at least 1 HF rehospitalization. Our findings highlight the importance of regularly monitoring health status during HF hospitalization and afterward. Patients can be educated to self‐monitor their health status over time and seek medical help if any decrease is identified in the KCCQ‐12 score.
Both clinical and nonclinical factors were associated with unfavorable health status trajectories, some of which are modifiable. Patients with certain characteristics were more likely to have unfavorable health status trajectories; that is, unsatisfactory status in most within the first year. All these characteristics have been reported to be associated with poor health status and survival in HF, including advanced age, 23 decompensated chronic HF, 23 HF with preserved ejection fraction, 23 , 27 high NT‐proBNP level, 7 , 28 depression symptoms, 29 , 30 , 31 , 32 , 33 cognitive impairment, 34 , 35 , 36 low socioeconomic status, 37 , 38 and higher number of HF rehospitalizations. 13 In addition, patients reporting moderate consumption of alcohol before the index hospitalization were more likely to experience favorable health status trajectories. The baseline data of the GISSI‐HF (Gruppo Italiano per lo Studio della Sopravvivenza nella Insufficienza Cardiaca‐Heart Failure) trial has shown that patients with more frequent wine consumption had a significantly better perception of health status, which might be attributed to anti‐inflammation. 39 It is possible that alcohol drinking improved health status after patients were discharged from hospitalization, and the positive attitude toward life of moderate drinkers improved their prognosis. 39 Regular exercise is another protective factor for health status in our study cohort. Although exercise rehabilitation is a class I recommendation for patients with chronic HF, the evidence in particular patients is still lacking, such as Asian individuals. 2 , 40 Our findings could help identify the patients with a high risk of unfavorable health status trajectories. The modifiable characteristics associated with health status trajectories could be the therapeutic target for improving patient prognosis.
The different trajectory patterns of health status were well related to the risk of death during the following years. Previous studies reported that health status measurement at a single time point or the change between 2 time points related with clinical events within 1 year of discharge from HF hospitalization. 3 , 6 The various trajectory patterns identified in this study enriched the understanding about the association between health status and death. We found that the trajectory patterns related with the risk of death during the following 3 years in a graded fashion. Patients with severely regressing trajectory had similar health status at admission and 1 year of discharge compared with those with persistently poor trajectory, but better at 1 month and 6 months. Patients with persistently poor trajectory had a higher risk of death than those with severely regressing trajectory. Previous studies demonstrated that the use of longitudinal data of established objective predictors of death in patients with AHF (systolic blood pressure, heart rate, NT‐proBNP, etc) increased the accuracy of prognostication compared with cross‐sectional measurements. 41 , 42 In contrast with the aforementioned indicators, health status, a subjective predictor from patient perspective, reflects the multidimensional impact of a clinical condition and is one of the important outcomes concerning patients with HF. 43 In addition, the associations between the trajectory patterns and risk of death were consistent across subgroups, suggesting that our findings may be generalizable to other heterogeneous populations, although further validation is needed.
Our findings need to be interpreted cautiously considering the following points. First, approximately one‐third of patients who survived the first year of discharge did not complete all 4 health status measurements and thus were excluded from current analysis. These patients were generally similar but more likely to have characteristics associated with unfavorable health status trajectories and higher death rate than those included. The proportion of unfavorable health status may be underestimated. Second, the results are applicable only to 1‐year survivors of an episode of HF, while not to those who died during the first year. Third, our observational design could not rule out the unmeasured confounders in the analyses, such as residual congestion at discharge, although we collected comprehensive high‐quality data and centrally adjudicated all outcomes. Fourth, we included only Chinese patients. Although our study cohort represented substantial diversities in patient characteristics, life behaviors, medical resource allocation, and quality of medical care, the results may not be applicable to other populations, given that the patients in our study could be influenced by the local features in reporting their outcomes. Finally, few patients in our study used angiotensin receptor‐neprilysin inhibitor or sodium‐glucose cotransporter 2 inhibitors because of the late approval of these drugs in China. In addition, the use of an implantable cardioverter‐defibrillator or cardiac resynchronization therapy was persistently low because of physicians' choice and the high cost. 44 With adequate use of these therapies, the death rate may be further reduced. However, there is no evidence to show interactions between their effects and health status. 11 , 45 , 46 , 47 , 48 , 49 Therefore, the influence of the absence or low use of these therapies on the associations between KCCQ trajectories and death might be limited.
Conclusions
In summary, one‐fifth of 1‐year survivors after hospitalization for AHF experienced unfavorable longitudinal health status trajectories within 1 year of discharge and had a substantially increased risk of death during the following years. Moderate drinking, exercising, and beta blocker use may play a positive role in improving health status, which needs to be validated by further research. Our findings help inform the understanding of disease progression from a patient perception perspective and its relationship with long‐term survival.
Sources of Funding
This work was supported by the Fuwai Hospital Chinese Academy of Medical Sciences “High Level Hospital Clinical Research” Funding (2022‐GSP‐GG‐5), the China Academy of Chinese Medical Sciences Innovation Fund for Medical Science (2021‐I2M‐1‐009), and the National Key Technology R&D Program (2015BAI12B02) from the Ministry of Science and Technology of China.
Disclosures
Dr Jing Li reported receiving research grants, through Fuwai Hospital, from the Chinese government and Chinese Academy of Medical Sciences for work to improve the management of hypertension and blood lipids and to improve patient outcomes of cardiovascular disease and COVID‐19; receiving research agreements, through the National Center for Cardiovascular Diseases and Fuwai Hospital, from Amgen for a multicenter clinical trial assessing the efficacy and safety of omecamtiv mecarbil and for dyslipidemic patient registration; receiving a research agreement, through Fuwai Hospital, from Sanofi for a multicenter clinical trial on the effects of sotagliflozin; receiving a research agreement, through Fuwai Hospital, with the University of Oxford for a multicenter clinical trial of empagliflozin; receiving a research agreement, through the National Center for Cardiovascular Diseases, from AstraZeneca for clinical research methods training outside the submitted work; and receiving a research agreement, through the National Center for Cardiovascular Diseases, from Lilly for physician training outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1
Acknowledgments
The authors thank Prof Harlan M. Krumholz of Yale University, Prof John A. Spertus of the University of Missouri, and Prof Fredrick A. Masoudi of the University of Colorado at Denver for their instructive advice on study design. The authors appreciate the multiple contributions made by the study teams at the China National Clinical Research Center for Cardiovascular Diseases and research sites in the realms of study design and operation, particularly the data collection. The authors are grateful for the funding support provided by the Chinese government. All authors contributed to the study conception and design. The first draft of the manuscript was written by Dr Zhang, R. Ji, and Dr He. Data analysis was performed by R. Ji. X. Yan. Drs He, Zhang, Qi, and Mi contributed to the data collection. All authors commented on previous versions of the manuscript and approved the final manuscript.
This manuscript was sent to Sakima A. Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028782
For Sources of Funding and Disclosures, see page 11.
References
- 1. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 3. Hu D, Liu J, Zhang L, Bai X, Tian A, Huang X, Zhou K, Gao M, Ji R, Miao F, et al. Health status predicts short‐ and long‐term risk of composite clinical outcomes in acute heart failure. JACC Heart Fail. 2021;9:861–873. doi: 10.1016/j.jchf.2021.06.015 [DOI] [PubMed] [Google Scholar]
- 4. Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, Kamath D, Alhabib K, Bayes‐Genis A, Budaj A, et al. Health‐related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation. 2021;143:2129–2142. doi: 10.1161/CIRCULATIONAHA.120.050850 [DOI] [PubMed] [Google Scholar]
- 5. Soriano N, Ribera A, Marsal JR, Brotons C, Cascant P, Permanyer‐Miralda G. Improvements in health‐related quality of life of patients admitted for heart failure. The HF‐QoL study. Rev Esp Cardiol. 2010;63:668–676. doi: 10.1016/S0300-8932(10)70159-7 [DOI] [PubMed] [Google Scholar]
- 6. Moser DK, Yamokoski L, Sun JL, Conway GA, Hartman KA, Graziano JA, Binanay C, Stevenson LW. Improvement in health‐related quality of life after hospitalization predicts event‐free survival in patients with advanced heart failure. J Card Fail. 2009;15:763–769. doi: 10.1016/j.cardfail.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen LA, Gheorghiade M, Reid KJ, Dunlay SM, Chan PS, Hauptman PJ, Zannad F, Konstam MA, Spertus JA. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belkin M, Wussler D, Gualandro DM, Shrestha S, Strebel I, Goudev A, Maeder MT, Walter J, Flores D, Kozhuharov N, et al. Effect of a strategy of comprehensive vasodilation versus usual care on health‐related quality of life among patients with acute heart failure. ESC Heart Fail. 2021;8:4218–4227. doi: 10.1002/ehf2.13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sauser K, Spertus JA, Pierchala L, Davis E, Pang PS. Quality of life assessment for acute heart failure patients from emergency department presentation through 30 days after discharge: a pilot study with the Kansas City cardiomyopathy questionnaire. J Card Fail. 2014;20:18–22. doi: 10.1016/j.cardfail.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 10. Okello S, Abeya FC, Lumori BAE, Akello SJ, Moore CC, Annex BH, Buda AJ. Validation of heart failure quality of life tool and usage to predict all‐cause mortality in acute heart failure in Uganda: the Mbarara heart failure registry (MAHFER). BMC Cardiovasc Disord. 2018;18:232. doi: 10.1186/s12872-018-0959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kosiborod MN, Angermann CE, Collins SP, Teerlink JR, Ponikowski P, Biegus J, Comin‐Colet J, Ferreira JP, Mentz RJ, Nassif ME, et al. Effects of empagliflozin on symptoms, physical limitations and quality of life in patients hospitalized for acute heart failure–results from the EMPULSE trial. Circulation. 2022;146:279–288. doi: 10.1161/CIRCULATIONAHA.122.059725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blumer V, Greene SJ, Wu A, Butler J, Ezekowitz JA, Lindenfeld J, Alhanti B, Hernandez AF, O'Connor CM, Mentz RJ. Sex differences in clinical course and patient‐reported outcomes among patients hospitalized for heart failure. JACC Heart Fail. 2021;9:336–345. doi: 10.1016/j.jchf.2020.12.011 [DOI] [PubMed] [Google Scholar]
- 13. Vaduganathan M, Claggett BL, McMurray JJV, Solomon SD. Health status trajectories before and after hospitalization for heart failure. Circulation. 2022;145:1872–1874. doi: 10.1161/CIRCULATIONAHA.122.059282 [DOI] [PubMed] [Google Scholar]
- 14. Huang X, Yu Y, Li X, Masoudi FA, Spertus JA, Yan X, Krumholz HM, Jiang L, Li J. The China patient‐centred evaluative assessment of cardiac events (PEACE) prospective heart failure study design. BMJ Open. 2019;9:e025144. doi: 10.1136/bmjopen-2018-025144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoo JE, Shin DW, Han K, Kim D, Jeong SM, Koo HY, Yu SJ, Park J, Choi KS. Association of the frequency and quantity of alcohol consumption with gastrointestinal cancer. JAMA Netw Open. 2021;4:e2120382. doi: 10.1001/jamanetworkopen.2021.20382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ge Y, Zhang L, Gao Y, Wang B, Zheng X. Socio‐economic status and 1 year mortality among patients hospitalized for heart failure in China. ESC Heart Fail. 2022;9:1027–1037. doi: 10.1002/ehf2.13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 18. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini‐cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8:8–16. doi: 10.1161/CIRCHEARTFAILURE.114.001438 [DOI] [PubMed] [Google Scholar]
- 19. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng Y, Dong Y, Chen D, Wang L, He J. Evaluation of Kansas City cardiomyopathy questionnaire in clinical practice in patients with chronic heart failure. Chin J Cardiol. 2004;32:8–11. [Google Scholar]
- 21. Nagin DS, Odgers CL. Group‐based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 22. Jones RH. Bayesian information criterion for longitudinal and clustered data. Stat Med. 2011;30:3050–3056. doi: 10.1002/sim.4323 [DOI] [PubMed] [Google Scholar]
- 23. McNaughton CD, McConnachie A, Cleland JG, Spertus JA, Angermann CE, Duklas P, Tromp J, Lam CSP, Filippatos G, Dahlstrom U, et al. Quality of life assessed 6 months after hospitalisation for acute heart failure: an analysis from REPORT‐HF (international REgistry to assess medical practice with lOngitudinal obseRvation for treatment of heart failure). Eur J Heart Fail. 2022;24:1020–1029. doi: 10.1002/ejhf.2508 [DOI] [PubMed] [Google Scholar]
- 24. Bozkurt B, Fonarow GC, Goldberg LR, Guglin M, Josephson RA, Forman DE, Lin G, Lindenfeld J, O'Connor C, Panjrath G, et al. Cardiac rehabilitation for patients with heart failure: JACC expert panel. J Am Coll Cardiol. 2021;77:1454–1469. doi: 10.1016/j.jacc.2021.01.030 [DOI] [PubMed] [Google Scholar]
- 25. Thomas M, Khariton Y, Fonarow GC, Arnold SV, Hill L, Nassif ME, Sharma PP, Butler J, Thomas L, Duffy CI, et al. Association of changes in heart failure treatment with patients' health status: real‐world evidence from CHAMP‐HF. JACC Heart Fail. 2019;7:615–625. doi: 10.1016/j.jchf.2019.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piña IL, Camacho A, Ibrahim NE, Felker GM, Butler J, Maisel AS, Prescott MF, Williamson KM, Claggett BL, Desai AS, et al. Improvement of health status following initiation of sacubitril/valsartan in heart failure and reduced ejection fraction. JACC Heart Fail. 2021;9:42–51. doi: 10.1016/j.jchf.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 27. Warraich HJ, Kitzman DW, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Reeves GR. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail. 2018;11:e005254. doi: 10.1161/CIRCHEARTFAILURE.118.005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myhre PL, Claggett BL, Shah AM, Prescott MF, Ward JH, Fang JC, Mitchell GF, Solomon SD, Desai AS. Changes in cardiac biomarkers in association with alterations in cardiac structure and function, and health status in heart failure with reduced ejection fraction: the EVALUATE‐HF trial. Eur J Heart Fail. 2022;24:1200–1208. doi: 10.1002/ejhf.2541 [DOI] [PubMed] [Google Scholar]
- 29. Comín‐Colet J, Martín Lorenzo T, González‐Domínguez A, Oliva J, Jiménez MS. Impact of non‐cardiovascular comorbidities on the quality of life of patients with chronic heart failure: a scoping review. Health Qual Life Outcomes. 2020;18:329. doi: 10.1186/s12955-020-01566-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dekker RL, Lennie TA, Albert NM, Rayens MK, Chung ML, Wu JR, Song EK, Moser DK. Depressive symptom trajectory predicts 1‐year health‐related quality of life in patients with heart failure. J Card Fail. 2011;17:755–763. doi: 10.1016/j.cardfail.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sbolli M, Fiuzat M, Cani D, O'Connor CM. Depression and heart failure: the lonely comorbidity. Eur J Heart Fail. 2020;22:2007–2017. doi: 10.1002/ejhf.1865 [DOI] [PubMed] [Google Scholar]
- 32. Hallas CN, Wray J, Andreou P, Banner NR. Depression and perceptions about heart failure predict quality of life in patients with advanced heart failure. Heart Lung. 2011;40:111–121. doi: 10.1016/j.hrtlng.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 33. Faller H, Störk S, Schuler M, Schowalter M, Steinbüchel T, Ertl G, Angermann CE. Depression and disease severity as predictors of health‐related quality of life in patients with chronic heart failure–a structural equation modeling approach. J Card Fail. 2009;15:286–292.e2. doi: 10.1016/j.cardfail.2008.10.022 [DOI] [PubMed] [Google Scholar]
- 34. Ventoulis I, Arfaras‐Melainis A, Parissis J, Polyzogopoulou E. Cognitive impairment in acute heart failure: narrative review. J Cardiovasc Dev Dis. 2021;8:184. doi: 10.3390/jcdd8120184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pastva AM, Hugenschmidt CE, Kitzman DW, Nelson MB, Brenes GA, Reeves GR, Mentz RJ, Whellan DJ, Chen H, Duncan PW. Cognition, physical function, and quality of life in older patients with acute decompensated heart failure. J Card Fail. 2021;27:286–294. doi: 10.1016/j.cardfail.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang M, Sun D, Wang Y, Yan M, Zheng J, Ren J. Cognitive impairment in heart failure: landscape, challenges, and future directions. Front Cardiovasc Med. 2021;8:831734. doi: 10.3389/fcvm.2021.831734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3‐year follow‐up study assessing hospitalization and mortality. Eur J Heart Fail. 2010;12:1002–1008. doi: 10.1093/eurjhf/hfq114 [DOI] [PubMed] [Google Scholar]
- 38. Verma AK, Schulte PJ, Bittner V, Keteyian SJ, Fleg JL, Piña IL, Swank AM, Fitz‐Gerald M, Ellis SJ, Kraus WE, et al. Socioeconomic and partner status in chronic heart failure: Relationship to exercise capacity, quality of life, and clinical outcomes. Am Heart J. 2017;183:54–61. doi: 10.1016/j.ahj.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 39. Cosmi F, Di Giulio P, Masson S, Finzi A, Marfisi RM, Cosmi D, Scarano M, Tognoni G, Maggioni AP, Porcu M, et al. Regular wine consumption in chronic heart failure: impact on outcomes, quality of life, and circulating biomarkers. Circ Heart Fail. 2015;8:428–437. doi: 10.1161/CIRCHEARTFAILURE.114.002091 [DOI] [PubMed] [Google Scholar]
- 40. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 41. Canepa M, Siri G, Puntoni M, Latini R, Tavazzi L, Maggioni AP. Testing longitudinal data for prognostication in ambulatory heart failure patients with reduced ejection fraction. A proof of principle from the GISSI‐HF database. Int J Cardiol. 2020;313:89–96. doi: 10.1016/j.ijcard.2020.03.064 [DOI] [PubMed] [Google Scholar]
- 42. Greene SJ, Maggioni AP, Fonarow GC, Solomon SD, Böhm M, Kandra A, Prescott MF, Reimund B, Hua TA, Lesogor A, et al. Clinical profile and prognostic significance of natriuretic peptide trajectory following hospitalization for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17:98–108. doi: 10.1002/ejhf.201 [DOI] [PubMed] [Google Scholar]
- 43. Heo S, Lennie TA, Okoli C, Moser DK. Quality of life in patients with heart failure: ask the patients. Heart Lung. 2009;38:100–108. doi: 10.1016/j.hrtlng.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan X, Hua W, Xu Y, Ding L, Niu H, Chen K, Xu B, Zhang S. Incidence and predictors of sudden cardiac death in patients with reduced left ventricular ejection fraction after myocardial infarction in an era of revascularization. Heart. 2014;100:1242–1249. doi: 10.1136/heartjnl-2013-305144 [DOI] [PubMed] [Google Scholar]
- 45. Bundgaard JS, Thune JJ, Torp‐Pedersen C, Nielsen JC, Haarbo J, Rørth R, Videbæk L, Melchior T, Pedersen SS, Køber L, et al. Self‐reported health status and the associated risk of mortality in heart failure: the DANISH trial. J Psychosom Res. 2020;137:110220. doi: 10.1016/j.jpsychores.2020.110220 [DOI] [PubMed] [Google Scholar]
- 46. Kosiborod MN, Bhatt AS, Claggett BL, Vaduganathan M, Kulac IJ, Lam CSP, Hernandez AF, Martinez FA, Inzucchi SE, Shah SJ, et al. Effect of dapagliflozin on health status in patients with preserved or mildly reduced ejection fraction. JACC. 2023;81:460–473. doi: 10.1016/j.jacc.2022.11.006 [DOI] [PubMed] [Google Scholar]
- 47. Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR‐preserved trial. Circulation. 2022;145:184–193. doi: 10.1161/CIRCULATIONAHA.121.057812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, Giannetti N, Januzzi JL, Piña IL, Lam CSP, et al. Empagliflozin and health‐related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR‐reduced trial. Eur Heart J. 2021;42:1203–1212. doi: 10.1093/eurheartj/ehaa1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation. 2020;141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


