Abstract
Background
We aimed to clarify which time‐to‐maximum of the tissue residue function (Tmax) mismatch ratio is useful in predicting anterior intracranial atherosclerotic stenosis (ICAS)–related large‐vessel occlusion (LVO) before endovascular therapy.
Methods and Results
Patients with ischemic stroke who underwent perfusion‐weighted imaging before endovascular therapy for anterior intracranial LVO were divided into those with ICAS‐related LVO and those with embolic LVO. Tmax ratios of >10 s/>8 s, >10 s/>6 s, >10 s/>4 s, >8 s/>6 s, >8 s/>4 s, and >6 s/>4 s were considered Tmax mismatch ratios. Binominal logistic regression was used to identify ICAS‐related LVO, and the adjusted odds ratio (aOR) and 95% CI for each Tmax mismatch ratio increase of 0.1 were calculated. A similar analysis was performed for ICAS‐related LVO with and without embolic sources, using embolic LVO as the reference. Of 213 patients (90 women [42.0%]; median age, 79 years), 39 (18.3%) had ICAS‐related LVO. The aOR (95% CI) per 0.1 increase in Tmax mismatch ratio in ICAS‐related LVO with embolic LVO as reference was lowest with Tmax mismatch ratio >10 s/>6 s (0.56 [0.43–0.73]). Multinomial logistic regression analysis also showed the lowest aOR (95% CI) per 0.1 increase in Tmax mismatch ratio with Tmax >10 s/>6 s (ICAS‐related LVO without embolic source: 0.60 [0.42–0.85]; ICAS‐related LVO with embolic source: 0.55 [0.38–0.79]).
Conclusions
A Tmax mismatch ratio of >10 s/>6 s was the optimal predictor of ICAS‐related LVO compared with other Tmax profiles, with or without an embolic source before endovascular therapy.
Registration
clinicaltrials.gov. Identifier NCT02251665.
Keywords: embolic large‐vessel occlusion, endovascular therapy, intracranial atherosclerotic stenosis‐related large‐vessel occlusion, odds ratio, Tmax mismatch ratio
Subject Categories: Ischemic Stroke
Clinical Perspective.
What Is New?
The time‐to‐maximum tissue residue function (Tmax) mismatch ratio with the highest predictive ability for diagnosing intracranial atherosclerotic stenosis‐related large‐vessel occlusion before endovascular therapy was Tmax mismatch ratio >10 s/>6 s.
The Tmax mismatch ratio >10 s/>6 s before endovascular therapy showed a similarly high predictive ability for intracranial atherosclerotic stenosis‐related large‐vessel occlusion with embolic sources.
Cerebral blood flow and cerebral blood volume before endovascular therapy did not provide useful results for the preoperative prediction of intracranial atherosclerotic stenosis‐related large‐vessel occlusion.
What Are the Clinical Implications?
These results support the evaluation of Tmax mismatch ratios before endovascular therapy, especially Tmax mismatch ratio >10 s/>6 s, to predict the occlusion mechanism in patients with acute anterior circulation large‐vessel occlusion stroke.
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- CBV
cerebral blood volume
- CTP
computed tomography perfusion
- eTICI
extended Thrombolysis in Cerebral Infarction
- EVT
endovascular therapy
- HIR
hypoperfusion intensity ratio
- ICAS
intracranial atherosclerotic stenosis
- ICH
intracranial hemorrhage
- LVO
large‐vessel occlusion
- MR
magnetic resonance
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- PWI
perfusion‐weighted imaging
- Tmax
time‐to‐maximum tissue residue function
Intracranial atherosclerotic stenosis (ICAS)–related large‐vessel occlusion (LVO) is particularly relevant to Asian patients, in whom ICAS is more prevalent than other LVO causes. 1 , 2 , 3 In East Asia, ICAS is the cause of ≈15% to 35% of cases of acute ischemic stroke (AIS) attributable to LVO for which endovascular therapy (EVT) is performed. 4 , 5 Furthermore, acute anterior ICAS‐related LVO reportedly has a lower successful recanalization rate, longer groin puncture–to–recanalization time, and poorer outcomes than embolic LVO. 6 , 7 , 8 Previous studies have suggested that reocclusion is more likely to occur after EVT in patients with anterior ICAS‐related LVO 9 , 10 , 11 because the stenotic segment harbors unstable ruptured plaques and thrombectomy aggravates endothelial damage and platelet aggregation. 12 , 13 In addition, emboli from an obvious embolic source, such as atrial fibrillation, can lead to the occlusion of vessels with ICAS, which occurs in ≈15% of East Asian patients with AIS. 14 Therefore, identifying ICAS‐related LVO with or without an embolic source before EVT is crucial in selecting the appropriate EVT strategy. However, it is challenging to identify ICAS‐related LVO before EVT at baseline.
Perfusion‐weighted imaging (PWI) may be a helpful tool to identify hypoperfused brain tissue and collateral flow in patients with AIS attributable to anterior LVO. 15 , 16 Patients who undergo PWI before EVT show an improvement in functional disability in the early and late time windows compared with those who do not undergo PWI. 17 Previous research has demonstrated that the mismatch between PWI and diffusion‐weighted imaging is associated with infarct growth. 18 The PWI profile has been evaluated using multiple time‐to‐maximum tissue residue function (Tmax) mismatch ratios, including the hypoperfusion intensity ratio (HIR), which can easily predict the rate of collateral flow and infarct growth. 16 However, which Tmax mismatch ratio helps identify patients with acute anterior ICAS‐related LVO remains unclear. We aimed to clarify which Tmax mismatch ratio profiles are most useful in predicting anterior ICAS‐related LVO before EVT.
Methods
The data supporting the findings of this study are available from the corresponding author on reasonable request.
Study Participants
All patients with AIS who were admitted to our institute within 7 days from the last known well time were prospectively registered in the National Cerebral and Cardiovascular Center Stroke Registry. 19 , 20 , 21 We retrospectively reviewed the data of consecutive patients enrolled in this registry from January 2014 to December 2021 who met the following criteria: (1) patients with AIS attributable to occlusion of the intracranial internal carotid artery or M1/M2 segment of the middle cerebral artery, (2) patients who underwent EVT, and (3) patients who underwent computed tomography perfusion (CTP) or magnetic resonance (MR) PWI before EVT. We excluded patients with tandem occlusion (concomitant extracranial and distal intracranial artery occlusion) and patients with cerebral artery dissection. Patients who did not achieve successful recanalization (extended Thrombolysis in Cerebral Infarction [eTICI] score 22 of 2b50, 2b67, 2c, or 3) at any time during the EVT procedure or on follow‐up MR angiography within 2 weeks after EVT were also excluded. The clinical and radiological data of the patients were prospectively collected in a database and retrieved for retrospective analysis. The need for written informed consent for study registration was waived because the study was retrospective and used anonymized data. Ethics approval was obtained from the local institutional review board (M23‐073‐9, Osaka). The National Cerebral and Cardiovascular Center Stroke Registry is registered at clinicaltrials.gov (NCT02251665). The study was conducted in accordance with the principles of the Declaration of Helsinki and conformed to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 23
Mechanical Thrombectomy
All endovascular procedures were performed by neurointerventionalists certified by the Japanese Society for Neuroendovascular Therapy, 24 as recommended by the American Heart Association/American Stroke Association guidelines. 25 EVT procedures included stent‐retriever thrombectomy, contact aspiration, stent‐retriever thrombectomy combined with contact aspiration (retrieval of the stent‐retriever and aspiration catheter as a unit), and intracranial angioplasty/stenting. 26 The procedural device was selected at the treating physician's discretion but was limited to those available in Japan. The devices used are listed in Table S1. The reperfusion status after EVT was assessed using the eTICI score. 22
Clinical Data Collection
The following clinical data were collected: age, sex, prestroke modified Rankin Scale (mRS) score, baseline systolic blood pressure, baseline National Institutes of Health Stroke Scale (NIHSS) score, and medical history (current smoking status, atrial fibrillation, chronic heart failure, hypertension, diabetes, dyslipidemia, stroke or transient ischemic attack before index stroke, and ischemic heart disease). Laboratory data included blood glucose and D‐dimer concentrations. The extent of the ischemic change in the middle cerebral artery territory was graded using the Alberta Stroke Program Early Computed Tomographic Score on non–contrast‐enhanced computed tomography or diffusion‐weighted MR. The infarct volume was calculated using an apparent diffusion coefficient of <620×10−6 mm2/s on b0/b1000 MR images or a relative reduction in relative cerebral blood flow (CBF) of <30% on CTP. The infarct growth volume was determined by dividing the infarct volume by the time from the last known well time to imaging. The occlusion sites were determined using internal carotid artery angiography at baseline. Time delays included the time from the last known well time to imaging, the time from groin puncture to successful reperfusion, and the time from the last known well time to successful reperfusion. Intravenous thrombolysis was performed using the approved dose of alteplase in Japan (0.6 mg/kg). 27 The EVT details evaluated were the first‐pass effect (achievement of an eTICI score of 2c/3 after the first pass), 28 modified first‐pass effect (first‐pass eTICI score of ≥2b), final eTICI 2c/3 reperfusion, and total number of passes. The clinical variables were the mRS score at 3 months, an mRS score of 0 to 2 at 3 months, death within 3 months, any intracranial hemorrhage (ICH) within 36 hours of onset, presence of parenchymal hematoma, subarachnoid hemorrhage, and symptomatic ICH. ICH was assessed using noncontrast computed tomography or gradient‐echo MR imaging. Parenchymal hematoma was defined as type 1 or 2, as described in the hemorrhagic transformation classification system of the European Cooperative Acute Stroke Study. 29 Symptomatic ICH was defined as ICH associated with a ≥4‐point increase in the NIHSS score. 29
Board‐certified stroke neurologists (TY, Kanta T, and JK) diagnosed the causes of anterior LVO AIS as ICAS‐related LVO or embolic LVO. ICAS‐related LVO was defined as acute intracranial artery occlusion with evidence of ≥50% residual stenosis at the target arterial lesion after EVT as detected on procedural angiography, including temporary intraoperative recanalization or follow‐up angiography. 11 , 30 The degree of stenosis was measured using the following equation: Stenosis (%)=[1−(D stenosis/D normal)×100], where D stenosis is the diameter of the artery at the site of the most severe grade of stenosis, and D normal is the diameter of the proximal normal artery. 31 Moreover, ICAS‐related LVO with embolic sources was defined as acute intracranial artery occlusion with evidence of ≥50% residual stenosis and with obvious proximal embolic sources. Embolic sources in the present study were defined in accordance with the Trial of Org 10172 in Acute Stroke Treatment Classification of High‐ and Medium‐Risk Sources of Cardioembolism. 32
Imaging
CTP or MR PWI was processed on an automated image postprocessing system (RAPID; iSchemaView, Menlo Park, CA). Critically hypoperfused tissue was automatically identified using Tmax thresholds of >10 s, >8 s, >6 s, and >4 s. Tmax ratios of >10 s/>8 s, >10 s/>6 s, >10 s > 4 s, >8 s/>6 s, >8 s/>4 s, and >6 s/>4 s were considered Tmax mismatch ratios. The HIR, which measures tissue‐level collaterals, 18 , 33 was calculated as the volume of ischemic brain tissue with a Tmax delay of >10 s divided by the volume of brain tissue with a Tmax delay of >6 s. 34 When the Tmax profile was calculated as 0 mL, each Tmax volume was labeled as 1. A relative CBF of <15%, <20%, and <30%; cerebral blood volume (CBV) of <30%, <34%, and <38%; and CBV‐index profiles (as predefined in the RAPID software reports) were also obtained in the patients who underwent CTP.
Statistical Analysis
The data are summarized as median and interquartile range for continuous variables and as frequency and percentage for categorical variables. Significant differences between patients with ICAS‐related LVO and those with embolic LVO were assessed using the Mann‐Whitney U test or Fisher exact test, as appropriate. Binominal logistic regression analysis was used to evaluate the association of ICAS‐related LVO with infarct volume, each Tmax profile, and each Tmax mismatch ratio; adjusted odds ratios (ORs) with 95% CIs were calculated using embolic LVO as a reference. Significant differences between ICAS‐related LVO without embolic sources or ICAS‐related LVO with embolic sources and embolic LVO (control) were assessed using the Mann‐Whitney U test or Fisher exact test, as appropriate. Multinomial logistic regression analysis was used to identify the Tmax profiles and Tmax mismatch ratios independently associated with ICAS‐related LVO with or without embolic sources; adjusted ORs (aORs) with 95% CIs were calculated using embolic LVO as the reference. 35 The following prespecified variables reportedly associated with the occlusion mechanism were included: sex, age, atrial fibrillation, hypertension, diabetes, dyslipidemia, and baseline NIHSS score. 36 , 37 P<0.05 was considered statistically significant. All analyses were performed using the Stata/IC statistical package, version 17.0 (StataCorp LLC).
Results
Patient Characteristics
Among all 578 patients with AIS who underwent EVT, 8 were excluded because of cerebral artery dissection‐related LVO, and 21 were excluded because successful recanalization was not achieved as shown by intraoperative EVT or follow‐up MR angiography and residual stenosis could not be confirmed. Among the remaining 549 patients with anterior LVO who underwent EVT for acute intracranial internal carotid artery/middle cerebral artery M1 or M2 occlusion, 336 were excluded because of a lack of available PWI data (Figure 1). The baseline characteristics of patients who did and did not undergo PWI are shown in Table S2. Compared with patients who did not undergo PWI, patients who underwent PWI had a lower median baseline NIHSS score (16 versus 18; P<0.01), higher median Alberta Stroke Program Early Computed Tomographic Score (9 versus 10; P<0.01), and longer median time from the last known well time to imaging (167 versus 103 minutes; P<0.01).
Figure 1. Study flowchart.
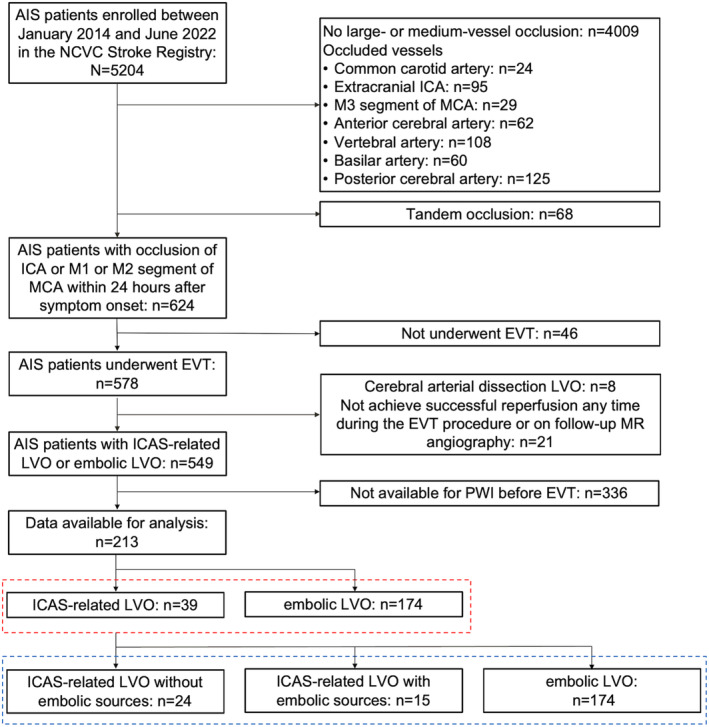
AIS indicates acute ischemic stroke; EVT, endovascular therapy; ICA, internal carotid artery; ICAS, intracranial atherosclerotic stenosis; LVO, large‐vessel occlusion; MCA, middle cerebral artery; MR, magnetic resonance; NCVC, National Cerebral and Cardiovascular Center; and PWI, perfusion‐weighted imaging.
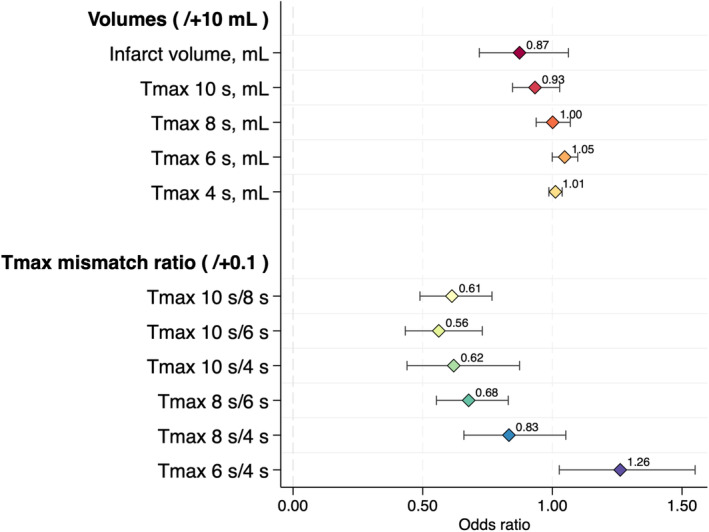
Among the remaining 213 patients (90 women [42.0%]; median age, 79 [interquartile range, 70–84] years; median NIHSS score, 16 [interquartile range, 9–23]), ICAS‐related LVO was present in 39 (18.3%). Sixty‐five percent (146/213) of patients were imaged using CTP, and the remaining were imaged using MR PWI. Compared with patients with embolic LVO, those with ICAS‐related LVO had a lower median baseline NIHSS score (10 versus 17; P<0.01), lower prevalence of atrial fibrillation (20.5% versus 65.5%; P<0.01), longer median time from groin puncture to successful reperfusion (62 versus 39 minutes; P<0.01), and higher median total number of passes (2 versus 1; P<0.01). However, the final eTICI 2c/3 reperfusion rate, mRS score at 90 days, and incidence of symptomatic ICH were not significantly different between the 2 groups. The baseline characteristics of the patients with ICAS‐related LVO and those with embolic LVO are shown in Table 1. The distribution of the mRS score at 90 days in the 2 groups is shown in Figure S1A. Compared with patients with embolic LVO, patients with ICAS‐related LVO had a significantly lower median Tmax >10 s volume (27 versus 57 mL; P<0.01), lower median Tmax ratio of >10 s/>8 s (0.48 versus 0.70; P<0.01), lower median Tmax ratio of >10 s/>6 s (HIR) (0.17 versus 0.46; P<0.01), lower median Tmax ratio of >10 s/>4 s (0.12 versus 0.47; P<0.01), and lower median Tmax ratio of >8 s/>6 s (0.44 versus 0.69; P<0.01). Patients with ICAS‐related LVO more frequently had excellent tissue‐level collaterals (HIR of ≤0.2) (56.4% versus 12.1%; aOR [95% CI], 7.79 [2.94–20.66]; P<0.01) and favorable tissue‐level collaterals (HIR of ≤0.4) (74.4% versus 37.4%; aOR [95% CI], 5.21 [1.86–14.61]; P<0.01) than patients with embolic LVO. There were no significant differences between the 2 groups in the other Tmax profiles (>8, >6, and >4 s) (Table 2). The binominal logistic regression analysis revealed a significant decrease in Tmax mismatch ratios of >10 s/>8 s (aOR per 0.1 increase [95% CI], 0.61 [0.49–0.77]; P<0.001), >10 s/>6 s (aOR per 0.1 increase [95% CI], 0.56 [0.43–0.73]; P<0.001), >10 s/>4 s (aOR per 0.1 increase [95% CI], 0.62 [0.44–0.87]; P=0.006), and >8 s/>6 s (aOR per 0.1 increase [95% CI], 0.68 [0.55–0.83]; P<0.001) in patients with ICAS‐related LVO compared with patients with embolic LVO (Table 3 and Figure 2). By contrast, the relative CBF, CBV, and CBV indexes were not significantly different between the 2 groups (Table S3).
Table 1.
Baseline Characteristics of Patients with ICAS‐Related LVO and Those With Embolic LVO
| Characteristic | ICAS‐related LVO (n=39) | Embolic LVO (n=174) | P value |
|---|---|---|---|
| Women | 14 (35.9) | 76 (43.7) | 0.47 |
| Age, y | 74 (65–82) | 79.00 (71–85) | 0.06 |
| Prestroke mRS score | 0 (0–3) | 0 (0–2) | 0.68 |
| Baseline systolic blood pressure, mm Hg | 158 (135–178) | 150 (132–167) | 0.17 |
| Baseline NIHSS score | 10 (5–17) | 17 (10–23) | <0.01 |
| Medical history | |||
| Current smoking | 8 (20.5) | 22 (12.6) | 0.21 |
| Atrial fibrillation | 8 (20.5) | 114 (65.5) | <0.01 |
| Chronic heart failure | 7 (18.4) | 61 (35.5) | 0.05 |
| Hypertension | 29 (74.4) | 122 (70.1) | 0.70 |
| Diabetes | 9 (23.1) | 35 (20.2) | 0.67 |
| Dyslipidemia | 24 (61.5) | 82 (47.7) | 0.16 |
| Stroke/TIA before index stroke | 9 (23.1) | 41 (23.6) | 1.00 |
| Ischemic heart disease | 4 (10.3) | 26 (14.9) | 0.61 |
| Laboratory data | |||
| Blood glucose, mg/dL | 120 (111–160) | 123 (109–150) | 0.98 |
| D‐dimer, /μL | 1.4 (1.0–2.9) | 1.95 (1.1–5.6) | 0.19 |
| Imaging | |||
| ASPECTS* | 9 (7–10) | 9 (7–10) | 0.64 |
| Infarct growth volume, mL/h | 0.98 (0.28–3.91) | 4.48 (0.63–13.33) | <0.01 |
| Occluded vessel | |||
| Internal carotid artery | 15 (38.5) | 36 (20.7) | 0.02 |
| M1 segment of MCA | 19 (48.7) | 71 (40.8) | 0.38 |
| M2 segment of MCA | 5 (12.8) | 67 (38.5) | <0.01 |
| Time delay | |||
| Time from LKW to imaging arrival, min | 219 (96–715) | 141 (74–397) | 0.04 |
| Time from groin puncture to successful reperfusion (eTICI score of ≥2b50), min | 62 (29–108) | 39 (27–56) | <0.01 |
| Time from LKW to successful reperfusion (eTICI score of ≥2b50), min | 396 (244–805) | 229 (158–382) | <0.01 |
| Treatment | |||
| Intravenous thrombolysis | 16 (41.0) | 75 (43.1) | 0.86 |
| First‐line thrombectomy | |||
| Stent retriever | 11 (28.2) | 43 (24.7) | <0.01 |
| Aspiration | 7 (18.0) | 33 (19.0) | |
| Combined contact aspiration and stent retriever | 6 (15.4) | 84 (48.3) | |
| Angioplasty/stenting | 10 (25.6) | 1 (0.6) | |
| Others | 3 (7.7) | 13 (7.5) | |
| Procedural variables | |||
| First‐pass effect | 13 (33.3) | 63 (36.2) | 0.85 |
| Modified first‐pass effect | 18 (46.2) | 92 (53.2) | 0.48 |
| Final eTICI ≥2b reperfusion | 33 (84.6) | 144 (82.8) | 1.00 |
| Total No. of passes | 2 (1–4) | 1 (1–2) | <0.01 |
| Clinical variables | |||
| mRS score at 90 d | 3 (1–4) | 3 (1–4) | 0.72 |
| mRS score 0–2 at 90 d | 19 (48.7) | 85 (48.9) | 1.00 |
| Death within 90 d | 1 (2.6) | 10 (5.7) | 0.69 |
| Any ICH | 13 (33.3) | 65 (37.4) | 0.72 |
| Parenchymal hematoma† | 1 (2.6) | 8 (4.6) | 1.00 |
| Subarachnoid hemorrhage | 1 (2.6) | 36 (20.7) | <0.01 |
| Symptomatic ICH‡ | 1 (2.6) | 5 (2.9) | 1.00 |
Data are presented as median (interquartile range) or number (percentage).
ASPECTS indicates Alberta Stroke Program Early Computed Tomographic Score; eTICI, extended Thrombolysis in Cerebral Infarction; ICAS, intracranial atherosclerotic stenosis; ICH, intracranial hemorrhage; LKW, last known well; LVO, large‐vessel occlusion; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and TIA, transient ischemic attack.
ASPECTS was measured using noncontrast computed tomography (n=146) and diffusion‐weighted imaging (n=67).
Parenchymal hematoma was defined as blood clots in the infarcted area with space‐occupying effects, as described in the ECASS (European Cooperative Acute Stroke Study).
Any ICH with a ≥4‐point increase in the NIHSS score from baseline.
Table 2.
Tmax Profiles and Tmax Mismatch Ratios of Patients With LVO Underlying ICAS and Those With LVO Not Underlying ICAS
| Variable | ICAS‐related LVO (n=39) | Embolic LVO (n=174) | P value* |
|---|---|---|---|
| Infarct volume, mL | 0 (0–16) | 14 (0–37) | <0.01 |
| Tmax profiles | |||
| Tmax >10 s, mL | 27 (3–75) | 57 (25–103) | <0.01 |
| Tmax >8 s, mL | 59 (17–130) | 89 (44–133) | 0.07 |
| Tmax >6 s, mL | 140 (88–204) | 134 (73–195) | 0.54 |
| Tmax >4 s, mL | 227 (148–344) | 251 (128–338) | 0.90 |
| Tmax mismatch ratio | |||
| Tmax >10 s/>8 s | 0.48 (0.20–0.64) | 0.70 (0.58–0.81) | <0.01 |
| Tmax >10 s/>6 s (HIR) | 0.17 (0.03–0.41) | 0.46 (0.31–0.59) | <0.01 |
| Tmax >10 s/>4 s | 0.12 (0.03–0.28) | 0.27 (0.15–0.34) | <0.01 |
| Tmax >8 s/>6 s | 0.44 (0.23–0.67) | 0.69 (0.55–0.75) | <0.01 |
| Tmax >8 s/>4 s | 0.26 (0.10–0.43) | 0.38 (0.27–0.46) | 0.02 |
| Tmax >6 s/>4 s | 0.69 (0.41–0.81) | 0.57 (0.47–0.67) | 0.03 |
| HIR | |||
| ≤0.2 | 22 (56.4) | 21 (12.1) | <0.01 |
| ≤0.4 | 29 (74.4) | 65 (37.4) | <0.01 |
Data are presented as median (interquartile range) or number (percentage).
HIR indicates hypoperfusion intensity ratio; ICAS, intracranial atherosclerotic stenosis; LVO, large‐vessel occlusion; and Tmax, time‐to‐maximum of the tissue residue function.
Mann‐Whitney U test.
Table 3.
Binominal Logistic Regression Analysis Investigating the Association Between the PWI Profile and ICAS‐Related LVO
| Variable | Odds ratio* | 95% CI | P value |
|---|---|---|---|
| Infarct volume, mL (+/10 mL)† | 0.87 | 0.72–1.06 | 0.174 |
| Tmax profiles (+/10 mL)† | |||
| Tmax >10 s, mL | 0.93 | 0.85–1.03 | 0.161 |
| Tmax >8 s, mL | 1.00 | 0.94–1.07 | 0.974 |
| Tmax >6 s, mL | 1.04 | 1.00–1.10 | 0.053 |
| Tmax >4 s, mL | 1.01 | 0.99–1.04 | 0.366 |
| Tmax mismatch ratio (+/0.1)‡ | |||
| Tmax >10 s/>8 s | 0.61 | 0.49–0.77 | <0.001 |
| Tmax >10 s/>6 s (HIR) | 0.56 | 0.43–0.73 | <0.001 |
| Tmax >10 s/>4 s | 0.62 | 0.44–0.87 | 0.006 |
| Tmax >8 s/>6 s | 0.68 | 0.55–0.83 | <0.001 |
| Tmax >8 s/>4 s | 0.83 | 0.66–1.05 | 0.124 |
| Tmax >6 s/>4 s | 1.26 | 1.02–1.55 | 0.027 |
HIR indicates hypoperfusion intensity ratio; ICAS, intracranial atherosclerotic stenosis; LVO, large‐vessel occlusion; PWI, perfusion‐weighted imaging; and Tmax, time‐to‐maximum of the tissue residue function.
Adjusted for sex, age, atrial fibrillation, hypertension, diabetes, dyslipidemia, and baseline National Institutes of Health Stroke Scale score.
Odds ratios are described for every increase of 10 in each continuous variable.
Odds ratios are described for every increase of 0.1 in each continuous variable.
Figure 2. Binominal logistic regression analysis of volumes and time‐to‐maximum of the tissue residue function (Tmax) mismatch ratios for prediction of intracranial atherosclerotic stenosis–related large‐vessel occlusion.
The baseline characteristics of patients with ICAS‐related LVO without embolic sources, ICAS‐related LVO with embolic sources, and embolic LVO are shown in Table S4. Compared with patients with embolic LVO, patients with ICAS‐related LVO without embolic sources (0.15 versus 0.46; P<0.01) and those with ICAS‐related LVO with embolic sources (0.21 versus 0.46; P<0.01) had a significantly lower median Tmax ratio of >10 s/>6 s (Table S5). The distribution of the mRS score at 90 days among the 3 groups is shown in Figure S1B. The multinomial logistic regression analysis revealed a significant decrease in the Tmax ratio of >10 s/>6 s in patients with ICAS‐related LVO without embolic sources (aOR per 0.1 increase [95% CI], 0.60 [0.42–0.85]; P=0.004) and in those with ICAS‐related LVO with embolic sources (aOR per 0.1 increase [95% CI], 0.55 [0.38–0.79]; P=0.001) compared with the control group (embolic LVO) (Figure 3 and Table S6). A representative case of ICAS‐related LVO treated with EVT is shown in Figure 4, and a representative case of ICAS‐related LVO with embolic sources treated with EVT is shown in Figure 5. Representative PWI profiles of ICAS‐related LVO and embolic LVO are shown in Figure S2.
Figure 3. Multinomial logistic regression analysis of volumes and time‐to‐maximum of the tissue residue function (Tmax) mismatch ratios for prediction of intracranial atherosclerotic stenosis (ICAS)–related large‐vessel occlusion (LVO) with and without embolic sources.
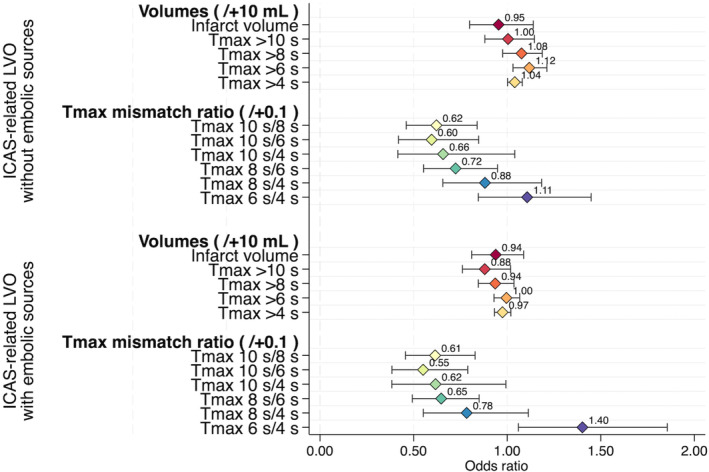
Figure 4. Representative case of intracranial atherosclerotic stenosis (ICAS)–related large‐vessel occlusion treated with endovascular therapy (EVT).
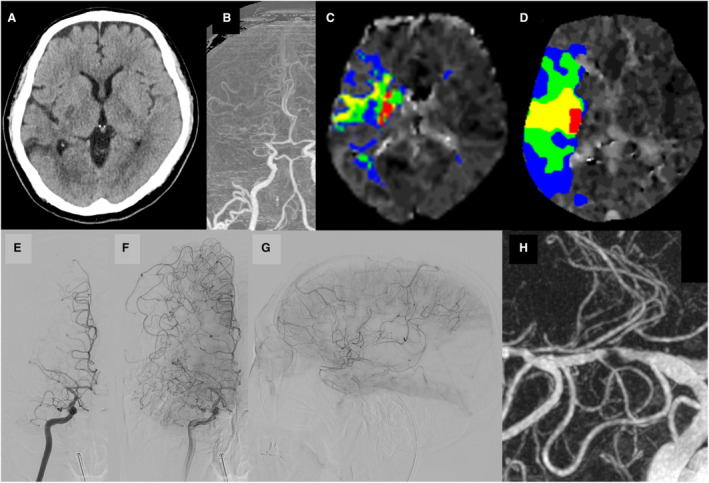
A 68‐year‐old woman without evidence of any definite proximal embolic source developed sudden left hemiplegia (National Institutes of Health Stroke Scale [NIHSS] score of 6), which was diagnosed as the cause of ICAS at the end of the procedure. A, Non–contrast‐enhanced computed tomography (CT) showed acute ischemic lesions in the basal ganglia territory of the right middle cerebral artery (MCA). B, CT angiography showed occlusion of the proximal M1 segment of the right MCA. C, Baseline CT perfusion (CTP) revealed a time‐to‐maximum of the tissue residue function (Tmax) of >10 s with 4 mL, >8 s with 18 mL, >6 s with 46 mL, and > 4 s with 123 mL and a hypoperfusion intensity ratio (HIR) of 0.1. D, Neurological deterioration was observed on day 2 (NIHSS score of 23), and CTP revealed a Tmax of >10 s with 11 mL, >8 s with 48 mL, >6 s with 122 mL, and >4 s with 270 mL and an HIR of 0.1. E, Right internal carotid artery (ICA) angiography in the early phase showed occlusion of the proximal M1 segment of the right MCA. F and G, Right ICA angiography in the late phase showed good collateral flow via leptomeningeal anastomosis (anterior view, lateral view). H, After successful recanalization with EVT, 3‐dimensional rotational angiography showed severe residual stenosis after mechanical thrombectomy and angioplasty.
Figure 5. Representative case of intracranial atherosclerotic stenosis (ICAS)–related large‐vessel occlusion (LVO) with embolic sources treated with endovascular therapy.
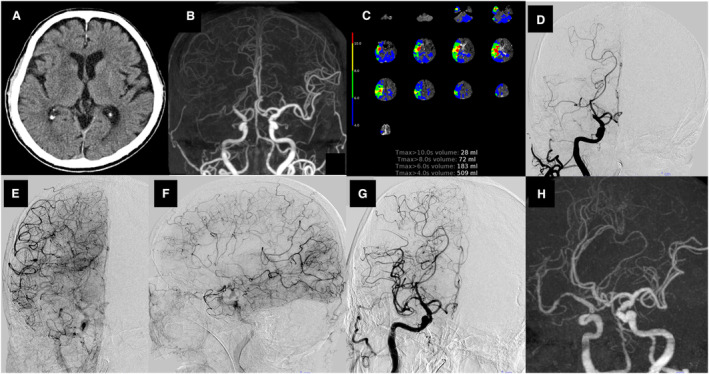
An 88‐year‐old woman with known atrial fibrillation developed sudden left hemiplegia (National Institutes of Health Stroke Scale score of 11) and was diagnosed with LVO underlying ICAS with embolic sources at the end of the procedure. A, Non–contrast‐enhanced computed tomography (CT) showed no acute ischemic lesions. B, CT angiography showed right intracranial internal carotid artery (ICA) occlusion. C, Baseline CT perfusion revealed a time‐to‐maximum of the tissue residue function of >10 s with 28 mL, >8 s with 72 mL, >6 s with 183 mL, and >4 s with 509 mL and a hypoperfusion intensity ratio of 0.15. D, Right ICA angiography in the early phase showed right intracranial ICA occlusion. E and F, Right ICA angiography in the late phase showed good collateral flow via leptomeningeal anastomosis (anterior view, lateral view). G, Right ICA angiography in the late phase showed residual severe stenosis in the M1 segment of the middle cerebral artery after successful recanalization with mechanical thrombectomy and angioplasty. H, Three‐dimensional rotational angiography showed severe residual stenosis after mechanical thrombectomy and angioplasty.
Discussion
The present study showed the potential of Tmax mismatch ratios to predict anterior ICAS‐related LVO before EVT. A Tmax mismatch ratio of >10 s/>6 s was useful as a predictive parameter of ICAS‐related LVO with or without embolic sources, showing the lowest OR and highest detection power for ICAS‐related LVO. The reason that a lower Tmax ratio of >10 s/>6 s was particularly strongly associated with ICAS‐related LVO is that ICAS‐related LVO presents with chronic hypoperfusion with good collateral flow, 16 resulting in a lower Tmax of >10 s (which is a region of large ischemic depth and poor collaterals) and a higher Tmax of >6 s (which is associated with penumbra lesions). Conversely, in many cases of embolic LVO, the infarct lesion expands relatively rapidly with proportional expansions of the Tmax >6 s and Tmax >10 s. ICAS‐related LVO with embolic sources also exhibits chronic hypoperfusion attributable to stenosis; therefore, acute embolic occlusion of the stenotic lesion can lead to large penumbra regions.
An additional finding of this study is that the Tmax mismatch ratio before EVT may predict not only ICAS‐related LVO without an embolic source but also underlying ICAS hidden in an occluded vessel despite the presence of an obvious embolic source. When patients with obvious embolic sources have the occlusion mechanism of ICAS‐related LVO, mechanical thrombectomy for hidden stenosis at the occluded site carries high risks of instant and delayed reocclusion. 10 , 11 , 38 Therefore, predicting ICAS‐related LVO with embolic sources before EVT using Tmax mismatch ratios, especially a Tmax ratio of >10 s/>6 s, may be helpful for intraoperative and postoperative monitoring. Moreover, previous studies of PWI have shown that large‐artery atherosclerosis is independently associated with a favorable HIR in patients undergoing mechanical thrombectomy, 39 , 40 supporting the results of the present study. However, studies showing an association between the Tmax mismatch ratio and the occlusion mechanism of LVO are limited. A recent study revealed that an automated CTP Tmax ratio of >4 s/>6 s with a profile of ≥2 was independent of ICAS‐related LVO. 41 In our study, a Tmax ratio of >10 s/>6 s was more predictive of not only ICAS‐related LVO without embolic sources but also ICAS‐related LVO with embolic sources than a Tmax ratio of >6 s/>4 s in a sample of comparable size (Figures 2 and 3). Tmax mismatch ratios, including a Tmax ratio of 10 s/>6 s, provide the detailed perfusion delay status of brain tissue as well as anterior LVO underlying ICAS. Our results suggested that PWI is a useful tool not only for assessing collateral flow in the anterior proximal LVO but also for predicting the mechanism of occlusion.
Our analysis showed no significant differences in CBF or CBV between ICAS‐related LVO and embolic LVO. A recent single‐center observational study found that CBV was associated with ICAS‐related LVO and could predict potential ICAS before EVT. 42 Moreover, CTP reflected hemodynamics rather than tissue fate, and poor collateral status and rapid time from onset to imaging were independently associated with overestimation of the core. 43 The significantly longer time from last known well to imaging arrival in the patients with ICAS‐related LVO group in the present results may be one reason why CBF and CBV did not differ between the 2 groups. In contrast, previous studies reported that intracranial arterial stenosis/occlusion increases the uncertainty in the accuracy of CTP to identify ischemic cores. 44 , 45 Further results from a large cohort are needed to determine whether CBF or CBV can adequately predict ICAS‐related LVO before EVT.
The present study has some limitations that should be considered. First, this was a single‐center study with a small sample size. However, although only 39 patients (18.3%) with ICAS‐related LVO were included, this proportion does not differ from other studies in the Asian population. Second, patient selection biases for PWI could not be ruled out because the prospectively collected data were retrospectively analyzed. However, the differences in the baseline NIHSS score, time delays, and successful recanalization rates between the groups in the present study are similar to those reported previously, and our study population has a certain degree of generality. 8 , 42 , 46 Third, there may be unmeasured variables, such as the anatomical features of the circle of Willis (eg, the anterior communicating artery and the posterior communicating artery), that affect the collateral circulation. Carefully designed studies are warranted to overcome these limitations.
Conclusions
Among the Tmax mismatch ratios, a Tmax ratio of >10 s/>6 s was optimal for predicting ICAS‐related LVO with or without embolic sources before EVT. Given the theoretically more detailed approach to tissue fate, the Tmax mismatch ratio may be applicable to other study cohorts.
Sources of Funding
This study was supported by the Japan Agency for Medical Research and Development (grant 22lk0201094h0004).
Disclosures
All the following conflicts are outside the submitted work. Dr Yoshimoto reports lecturer honoraria from Takeda Pharmaceutical, Nippon Boehringer Ingelheim, and Stryker. Dr Tanaka reports lecturer's fees from Daiichi‐Sankyo, Medico's Hirata, and Stryker. Dr Koge reports lecture's fees from Medtronic, Stryker, and Daiichi‐Sankyo. Dr Imamura reports lecturer's fees from Medtronic and Stryker. Dr Toyoda reports lecture honoraria from Daiichi Sankyo, Bayer, Otsuka, Novartis, Bayer, and Bristol‐Myers Squibb. Dr Koga reports honoraria from Bayer Yakuhin, Daiichi‐Sankyo, and Mitsubishi Tanabe Pharma Corporation; and research support from Daiichi‐Sankyo and Nippon Boehringer Ingelheim. Dr Ihara reports lecturer's fees from Daiichi Sankyo and Eisai; and grant support from Panasonic, GE Precision Healthcare LLC, Bristol‐Myers Squibb, and Shimadzu Corporation. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S6
Figures S1–S2
Acknowledgments
Author contributions: Study conception: Drs Yoshimoto, Inoue, Toyoda, and Ihara. Acquisition of data: Drs Yoshimoto, Inoue, Tanaka, Koge, Shiozawa, Kamogawa, Abe, and Imamura. Analysis and interpretation of data and drafting of the manuscript: Dr Yoshimoto. Editing of the manuscript for intellectual content: Drs Yoshimoto, Inoue, Tanaka, Toyoda, and Ihara. Final approval of the version to be published and agreement to be accountable for all aspects of the work: all authors.
This article was sent to Kori S. Zachrison, MD, MSc, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029899
For Sources of Funding and Disclosures, see page 10.
References
- 1. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12:1106–1114. doi: 10.1016/S1474-4422(13)70195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim JS, Bonovich D. Research on intracranial atherosclerosis from the east and west: why are the results different? J Stroke. 2014;16:105–113. doi: 10.5853/jos.2014.16.3.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura S, Sakai N, Uchida K, Yamagami H, Ezura M, Okada Y, Kitagawa K, Kimura K, Sasaki M, Tanahashi N, et al. Endovascular therapy in ischemic stroke with acute large‐vessel occlusion: Recovery by Endovascular Salvage for Cerebral Ultra‐acute Embolism Japan Registry 2. J Am Heart Assoc. 2018;7:e008796. doi: 10.1161/JAHA.118.008796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tong X, Wang Y, Fiehler J, Bauer CT, Jia B, Zhang X, Huo X, Luo G, Wang A, Pan Y, et al. Thrombectomy versus combined thrombolysis and thrombectomy in patients with acute stroke: a matched‐control study. Stroke. 2021;52:1589–1600. doi: 10.1161/STROKEAHA.120.031599 [DOI] [PubMed] [Google Scholar]
- 6. Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, Amarenco P, Carrozzella J, Spilker J, Foster LD, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014;16:27–35. doi: 10.5853/jos.2014.16.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baek JH, Kim BM, Heo JH, Kim DJ, Nam HS, Kim YD. Outcomes of endovascular treatment for acute intracranial atherosclerosis‐related large vessel occlusion. Stroke. 2018;49:2699–2705. doi: 10.1161/STROKEAHA.118.022327 [DOI] [PubMed] [Google Scholar]
- 9. Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke. 2014;16:8–17. doi: 10.5853/jos.2014.16.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwang YH, Kim YW, Kang DH, Kim YS, Liebeskind DS. Impact of target arterial residual stenosis on outcome after endovascular revascularization. Stroke. 2016;47:1850–1857. doi: 10.1161/STROKEAHA.116.013046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baek BH, Yoon W, Lee YY, Kim SK, Kim JT, Park MS. Intravenous tirofiban infusion after angioplasty and stenting in intracranial atherosclerotic stenosis‐related stroke. Stroke. 2021;52:1601–1608. doi: 10.1161/STROKEAHA.120.033551 [DOI] [PubMed] [Google Scholar]
- 12. Kang DH, Yoon W. Current opinion on endovascular therapy for emergent large vessel occlusion due to underlying intracranial atherosclerotic stenosis. Korean J Radiol. 2019;20:739–748. doi: 10.3348/kjr.2018.0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim GE, Yoon W, Kim SK, Kim BC, Heo TW, Baek BH, Lee YY, Yim NY. Incidence and clinical significance of acute reocclusion after emergent angioplasty or stenting for underlying intracranial stenosis in patients with acute stroke. AJNR Am J Neuroradiol. 2016;37:1690–1695. doi: 10.3174/ajnr.A4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong L, Lin L, Li G, Yang J, Geng Y, Lou M, Parsons M, Cheng X, Dong Q. Identification of embolic stroke in patients with large vessel occlusion: the Chinese embolic stroke score, CHESS. CNS Neurosci Ther. 2022;28:531–539. doi: 10.1111/cns.13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, Wilder MJ, Lutsep HL, Czartoski TJ, Bernstein RA, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, Straka M, Zaharchuk G, Bammer R, Lansberg MG, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 cohort. Stroke. 2014;45:1018–1023. doi: 10.1161/STROKEAHA.113.003857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhillon PS, Butt W, Podlasek A, McConachie N, Lenthall R, Nair S, Malik L, Booth TC, Bhogal P, Makalanda HLD, et al. Perfusion imaging for endovascular thrombectomy in acute ischemic stroke is associated with improved functional outcomes in the early and late time windows. Stroke. 2022;53:2770–2778. doi: 10.1161/STROKEAHA.121.038010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gawlitza M, Gragert J, Quaschling U, Hoffmann KT. FLAIR hyperintense vessel sign, diffusion‐perfusion mismatch and infarct growth in acute ischemic stroke without vascular recanalisation therapy. J Neuroradiol. 2014;41:227–233. doi: 10.1016/j.neurad.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 19. Yoshimoto T, Inoue M, Yamagami H, Fujita K, Tanaka K, Ando D, Sonoda K, Kamogawa N, Koga M, Ihara M, et al. Use of Diffusion‐Weighted Imaging‐Alberta Stroke Program Early Computed Tomography Score (DWI‐ASPECTS) and ischemic core volume to determine the malignant profile in acute stroke. J Am Heart Assoc. 2019;8:e012558. doi: 10.1161/JAHA.119.012558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshimoto T, Inoue M, Tanaka K, Kanemaru K, Koge J, Shiozawa M, Kamogawa N, Kimura S, Chiba T, Satow T, et al. Identifying large ischemic core volume ranges in acute stroke that can benefit from mechanical thrombectomy. J Neurointerv Surg. 2021;13:1081–1087. doi: 10.1136/neurintsurg-2020-016934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koge J, Tanaka K, Yoshimoto T, Shiozawa M, Kushi Y, Ohta T, Satow T, Kataoka H, Ihara M, Koga M, et al. Internal carotid artery tortuosity: impact on mechanical thrombectomy. Stroke. 2022;53:2458–2467. doi: 10.1161/STROKEAHA.121.037904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, Mitchell PJ, van der Lugt A, Menon BK, Román LS, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11:433–438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 23. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 24. Yamagami H, Hayakawa M, Inoue M, Iihara K, Ogasawara K, Toyoda K, Hasegawa Y, Ohata K, Shiokawa Y, Nozaki K, et al. Guidelines for mechanical thrombectomy in Japan, the fourth edition, March 2020: a guideline from the Japan Stroke Society, the Japan Neurological Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021;61:163–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 26. Laperque B, Labreuche J, Blanc R, Marnat G, Consoli A, Rodesch G, Saleme S, Costalat V, Bracard S, Desal H, et al. Combined use of contact aspiration and the stent retriever technique versus stent retriever alone for recanalization in acute cerebral infarction: the randomized ASTER 2 study protocol. J Neurointerv Surg. 2020;12:471–476. doi: 10.1136/neurintsurg-2019-014735 [DOI] [PubMed] [Google Scholar]
- 27. Toyoda K, Koga M, Iguchi Y, Itabashi R, Inoue M, Okada Y, Ogasawara K, Tsujino A, Hasegawa Y, Hatano T, et al. Guidelines for intravenous thrombolysis (recombinant tissue‐type plasminogen activator), the third edition, March 2019: a guideline from the Japan Stroke Society. Neurol Med Chir (Tokyo). 2019;59:449–491. doi: 10.2176/nmc.st.2019‐0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, Mueller‐Kronast N, English JD, Dabus G, Malisch TW, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49:660–666. doi: 10.1161/STROKEAHA.117.020315 [DOI] [PubMed] [Google Scholar]
- 29. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 30. Kang DH, Yoon W, Baek BH, Kim SK, Lee YY, Kim JT, Park MS, Kim YW, Kim YS, Hwang YH. Front‐line thrombectomy for acute large‐vessel occlusion with underlying severe intracranial stenosis: stent retriever versus contact aspiration. J Neurosurg. 2020;132:1202–1208. doi: 10.3171/2019.1.JNS182905 [DOI] [PubMed] [Google Scholar]
- 31. Schumacher HC, Meyers PM, Higashida RT, Derdeyn CP, Lavine SD, Nesbit GM, Sacks D, Rasmussen P, Wechsler LR. Reporting standards for angioplasty and stent‐assisted angioplasty for intracranial atherosclerosis. J Neurointerv Surg. 2010;2:324–340. doi: 10.1136/jnis.2010.002345 [DOI] [PubMed] [Google Scholar]
- 32. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 33. Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis GM, Broocks G, Flottmann F, Marks MP, Lansberg MG, Albers GW, et al. Favorable venous outflow profiles correlate with favorable tissue‐level collaterals and clinical outcome. Stroke. 2021;52:1761–1767. doi: 10.1161/STROKEAHA.120.032242 [DOI] [PubMed] [Google Scholar]
- 34. Guenego A, Mlynash M, Christensen S, Kemp S, Heit JJ, Lansberg MG, Albers GW. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol. 2018;84:616–620. doi: 10.1002/ana.25320 [DOI] [PubMed] [Google Scholar]
- 35. Greene WH. Econometric Analysis. 8th ed. Pearson; 2018:829–833. [Google Scholar]
- 36. Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease. Stroke. 2019;50:1286–1293. doi: 10.1161/STROKEAHA.118.024147 [DOI] [PubMed] [Google Scholar]
- 37. Gutierrez J, Turan TN, Hoh BL, Chimowitz MI. Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2022;21:355–368. doi: 10.1016/S1474-4422(21)00376-8 [DOI] [PubMed] [Google Scholar]
- 38. Yoshimoto T, Tanaka K, Koge J, Saito S, Yamagami H, Nakaoku Y, Ogata S, Nishimura K, Yamaguchi E, Chiba T, et al. Impact of the RNF213 p.R4810K variant on endovascular therapy for large‐vessel occlusion stroke. Stroke Vasc Intervent Neurol. 2022;2:e000396. doi: 10.1161/SVIN.122.000396 [DOI] [Google Scholar]
- 39. Ballout AA, Libman RB, Schneider JR, Ayoub MS, Wang JJ, Patsalides A, Katz JM. Hypoperfusion intensity ratio is associated with stroke mechanism in patients undergoing mechanical thrombectomy. J Stroke Cerebrovasc Dis. 2022;31:106539. doi: 10.1016/j.jstrokecerebrovasdis.2022.106539 [DOI] [PubMed] [Google Scholar]
- 40. Kim SJ, Morales JM, Yaghi S, Honda T, Scalzo F, Hinman JD, Raychev R, Sharma LK, Feldmann E, Romano JG, et al. Intracranial atherosclerotic disease mechanistic subtypes drive hypoperfusion patterns. J Neuroimaging. 2021;31:686–690. doi: 10.1111/jon.12863 [DOI] [PubMed] [Google Scholar]
- 41. Haussen DC, Bouslama M, Dehkharghani S, Grossberg JA, Bianchi N, Bowen M, Frankel MR, Nogueira RG. Automated CT perfusion prediction of large vessel acute stroke from intracranial atherosclerotic disease. Interv Neurol. 2018;7:334–340. doi: 10.1159/000487335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imaoka Y, Shindo S, Miura M, Terasaki T, Mukasa A, Todaka T. Hypoperfusion intensity ratio and CBV index as predictive parameters to identify underlying intracranial atherosclerotic stenosis in endovascular thrombectomy. J Neuroradiol. 2022;S0150‐9861(22)00164‐X. doi: 10.1016/j.neurad.2022.10.005 [DOI] [PubMed] [Google Scholar]
- 43. García‐Tornel A, Campos D, Rubiera M, Boned S, Olivé‐Gadea M, Requena M, Ciolli L, Muchada M, Pagola J, Rodriguez‐Luna D, et al. Ischemic core overestimation on computed tomography perfusion. Stroke. 2021;52:1751–1760. doi: 10.1161/STROKEAHA.120.031800 [DOI] [PubMed] [Google Scholar]
- 44. Ferreira RM, Lev MH, Goldmakher GV, Kamalian S, Schaefer PW, Furie KL, Gonzalez RG, Sanelli PC. Arterial input function placement for accurate CT perfusion map construction in acute stroke. AJR Am J Roentgenol. 2010;194:1330–1336. doi: 10.2214/AJR.09.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen X, Zou J, Bao L, Hu J, Ye G. Computed tomography perfusion imaging quality affected by different input arteries in patients of internal carotid artery stenosis. Med Sci Monit. 2019;25:9067–9072. doi: 10.12659/MSM.917995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cai Y, Gu Y, Wang Y, Wang P, Zhang L, Liu C, Chu J, Li H, Lu Z, Zhou Y, et al. A clinical prediction model for patients with acute large vessel occlusion due to underlying intracranial atherosclerotic stenosis. Clin Neuroradiol. [published online December 15, 2022]. doi: 10.1007/s00062-022-01241-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S2


