Abstract
Background
Extracorporeal membrane oxygenation (ECMO) has been increasingly used for postcardiotomy cardiogenic shock, but without a concomitant reduction in observed in‐hospital mortality. Long‐term outcomes are unknown. This study describes patients’ characteristics, in‐hospital outcome, and 10‐year survival after postcardiotomy ECMO. Variables associated with in‐hospital and postdischarge mortality are investigated and reported.
Methods and Results
The retrospective international multicenter observational PELS‐1 (Postcardiotomy Extracorporeal Life Support) study includes data on adults requiring ECMO for postcardiotomy cardiogenic shock between 2000 and 2020 from 34 centers. Variables associated with mortality were estimated preoperatively, intraoperatively, during ECMO, and after the occurrence of any complications, and then analyzed at different time points during a patient's clinical course, through mixed Cox proportional hazards models containing fixed and random effects. Follow‐up was established by institutional chart review or contacting patients. This analysis included 2058 patients (59% were men; median [interquartile range] age, 65.0 [55.0–72.0] years). In‐hospital mortality was 60.5%. Independent variables associated with in‐hospital mortality were age (hazard ratio [HR], 1.02 [95% CI, 1.01–1.02]) and preoperative cardiac arrest (HR, 1.41 [95% CI, 1.15–1.73]). In the subgroup of hospital survivors, the overall 1‐, 2‐, 5‐, and 10‐year survival rates were 89.5% (95% CI, 87.0%–92.0%), 85.4% (95% CI, 82.5%–88.3%), 76.4% (95% CI, 72.5%–80.5%), and 65.9% (95% CI, 60.3%–72.0%), respectively. Variables associated with postdischarge mortality included older age, atrial fibrillation, emergency surgery, type of surgery, postoperative acute kidney injury, and postoperative septic shock.
Conclusions
In adults, in‐hospital mortality after postcardiotomy ECMO remains high; however, two‐thirds of those who are discharged from hospital survive up to 10 years. Patient selection, intraoperative decisions, and ECMO management remain key variables associated with survival in this cohort.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03857217.
Keywords: acute heart failure, cardiac surgery, extracorporeal membrane oxygenation, mechanical circulatory support, postcardiotomy cardiogenic shock
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Heart Failure, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- PELS‐1
Postcardiotomy Extracorporeal Life Support Study
- RVF
right ventricular failure
- V‐A ECMO
veno‐arterial extracorporeal membrane oxygenation
Clinical Perspective.
What Is New?
In adults, in‐hospital mortality after postcardiotomy extracorporeal membrane oxygenation (ECMO) is high, but postdischarge survival up to 10 years is favorable.
Common variables, such as age and preoperative cardiac arrest, are associated with survival throughout each of the steps of the in‐hospital patient stay, whereas specific variables affect the preoperative selection, intraoperative action, ECMO management, and weaning phases.
What Are the Clinical Implications?
The in‐hospital course remains the main limiting factor that needs to be addressed to improve the success of postcardiotomy ECMO.
Action could be taken to address variables associated with mortality at different time points during the dynamic ECMO clinical course to possibly enhance outcomes and develop adequate predictive models.
An adequate follow‐up of patients undergoing postcardiotomy ECMO, especially in case of postoperative complications, is advised.
Over the past decades, veno‐arterial extracorporeal membrane oxygenation (V‐A ECMO) has emerged as an essential modality of temporary mechanical circulatory support for refractory postcardiotomy cardiogenic shock. 1 , 2 The application of extracorporeal membrane oxygenation (ECMO) as bridge to recovery or more durable supportive care 3 , 4 after postcardiotomy shock has been reported between 0.4% and 3.7%, 5 with a significant and constant increase since 2007. 6 , 7 In conjunction with the growing complexity of cardiac surgical procedures, patient risk profiles, and their associated complication rates, V‐A ECMO has taken on a progressively more important role in the perioperative care of these patients. Nonetheless, morbidity and mortality rates in such patients are consistently high, 8 although reported outcomes vary in literature. 7 , 9 Even less evidence is available on long‐term outcomes and their determinants. 4 , 10 , 11 Although several studies investigated in‐hospital outcomes, data on survival of patients who underwent postcardiotomy ECMO after discharge are lacking and urgently needed. 10 , 11 Besides the evidence‐based support for the patient selection process, the intraoperative and postoperative optimization of ECMO management are required to address patient's needs and guide ECMO application. This may guarantee a more effective personalized and timely therapy, optimize use of resources, and improve in‐hospital and postdischarge outcomes.
The PELS‐1 (Postcardiotomy Extracorporeal Life Support) study includes data on adults experiencing postcardiotomy cardiogenic shock and requiring ECMO in an international group of participating hospitals. This study aimed at describing patients’ characteristics, in‐hospital outcomes, and 10‐year survival of this specific cardiac surgery population. Moreover, we investigated variables associated with in‐hospital and long‐term mortality. We considered several clinically relevant determinants preoperatively, intraoperatively, and during ECMO management, then described their association with mortality. This may provide evidence on whether development of postcardiotomy support and subsequent patient follow‐up should be tailored to these phases of ECMO support and postdischarge surveillance.
Methods
Patient Population
The PELS‐1 is an international, multicenter, retrospective observational study enrolling consecutive patients supported with ECMO in the postoperative phase (ClinicalTrials.gov: NCT03857217; registration date: February 27, 2019) in 34 centers from 16 countries (Figure S1 and Table S1).
Adult patients (aged ≥18 years) were included if they underwent postcardiotomy ECMO between January 2000 and December 2020. Inclusion criteria required cardiac surgery before ECMO (including V‐A ECMO and veno‐venous ECMO). Exclusion criteria comprised ECMO support after discharge or before surgery, ECMO support after noncardiac surgical procedures, and ECMO implantation not strictly related to cardiac surgery hospitalization. For the present analyses, characteristics and outcomes of patients who received V‐A ECMO implantation were investigated (Figure S2).
PELS‐1 was conducted in accordance with the Declaration of Helsinki. Institutional review board approval was required for all centers, of which the protocol was based on the institutional review board approval of the coordinating center (institutional review board approval number: METC‐2018‐0788; institutional review board approval date: December 19, 2018). Need for informed consent was waived on the basis of the retrospective nature of the study, the emergency of the performed procedure, and the pseudonymization of shared data. Data that support the findings of this study are available from the corresponding author on reasonable request and with the permission of all PELS‐1 participating centers.
Data Collection and Outcomes
Demographics, preoperative clinical and laboratory variables, procedural characteristics, ECMO treatment modality, cannulation strategy, in‐hospital morbidity and mortality, as well as postdischarge survival were collected from each participating hospital and included in a dedicated electronic case report form (data.castoredc.com), according to the predefined protocol and variable definitions (Data S1 and Table S2). The full data set was retained and centrally managed by the coordinating center, which had full access to all the data in the study and takes responsibility for their integrity and the data analysis. Long‐term follow‐up data were collected through the review of the most recent medical records or contact with patients at discretion of the treating center. The primary outcome of interest for the current study was all‐cause in‐hospital mortality. Secondary outcomes included in‐hospital complications and postdischarge mortality in hospital survivors.
Statistical Analysis
Demographic and clinical variables are expressed as numbers (valid percentage on available data, excluding missing values) for categorical variables and median (interquartile range [IQR]) or mean and SD for continuous variables after evaluation for normality. All descriptive statistics were performed on original data, and pairwise deletion was applied, as appropriate, after missing value analysis. Violin plots were applied to estimate the probability density function of continuous variables and represent their summary statistics. Stacked bar plots represent the distributions of levels within each categorical variable and compare them between study groups (in‐hospital survivors versus nonsurvivors). Categorical data were compared with χ2 test. Continuous variables were analyzed using Student t test or Mann‐Whitney U test, as appropriate. Overall mortality was investigated with the Kaplan‐Meier method. Patients' loss to follow‐up was included in survival analyses and was considered censored at the time of their last control.
We described the population characteristics and preoperative variables, intraoperative variables, variables while on ECMO, and postoperative complications for the whole cohort and stratified for in‐hospital survivors and nonsurvivors. To estimate the associations between determinants and in‐hospital mortality, we conducted a mixed Cox proportional hazards model, containing both fixed and random effects. The random effect was used to consider differences among centers, or centers and years. 12 We considered sets of variables deemed important clinically for the association with mortality at patient selection, intraoperative decisions, and for ECMO management, based on clinical practice and literature. 2 , 10 , 11 , 13 , 14 For the association with in‐hospital mortality, we used the following: (1) demographic data and preoperative variables; (2) demographic data and preoperative and intraoperative variables; (3) demographic data and preoperative, intraoperative, and ECMO variables; or (4) demographic data, preoperative, intraoperative, and ECMO variables, and postoperative complications. Finally, a subgroup survival analysis was performed including hospital survivors only. A multivariable model to identify variables associated with postdischarge mortality was performed using the mixed Cox proportional hazards model in the subgroup of in‐hospital survivors. The proportional hazards assumption was checked using both statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. Only variables having ≤20% missing data were considered to include in each Cox model after a multiple imputation process. Briefly, we used fully specified chained equations in the R package. 15 Mechanisms underlying missing data were investigated with sensitivity analyses. Ten imputed data sets were created and combined using between/within variance techniques to appropriately investigate uncertainty about the missing data. 15 Each model took intrinsic differences among centers using random effect into account. We report risk estimates as hazard ratios (HRs) with their 95% CIs and P values.
We considered P<0.05 as statistically significant, and hypothesis tests were 2‐sided. All data were merged from deidentified files into SPSS 26.0 (IBM, Armonk, NY) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) for data management and statistical analysis.
Results
Baseline, Surgical, and ECMO Characteristics
In total, data on 2163 patients were collected in the PELS‐1 database. Of them, 72 patients lacked data on the primary outcome and 33 received veno‐venous ECMO support. Thus, 2058 patients were included in the present analysis (Figures S2 and S3). Median age was 65.0 years (IQR, 55.0–72.0 years), with women accounting for 41% (n=843; Table 1). Hospital nonsurvivors (n=1244 [60.5%]) were older (P<0.001) and affected by a higher number of comorbidities compared with survivors (n=814 [39.5%]), as shown in Table 1. Preoperative serum creatinine (P=0.003) and EuroSCORE II values (P=0.002) were higher in nonsurvivors who presented more frequently in an unstable preoperative condition characterized by cardiogenic shock (P=0.002) or septic shock (P=0.005), or requiring mechanical ventilation (P=0.019). Preoperative cardiac arrest occurred in 189 (9.3%) of patients who were more frequently known for a history of myocardial infarction (n=68/189 [36%]; P=0.005), a recent myocardial infarction (n=34/189 [18%]; P=0.008), and peripheral vessel disease (n=39/189 [20.6%]; P=0.023) compared with those who did not experience a preoperative cardiac arrest. Moreover, 51.9% (n=97/189) of them underwent emergency surgery compared with the 23.5% (n=429/1847) of all other patients (P<0.001), and received a preoperative intra‐aortic balloon pump at a rate that was almost double compared with other patients (preoperative cardiac arrest: n=29/188 [15.4%]; no preoperative cardiac arrest: n=161/1845 [8.7%]; P=0.005). Coronary artery bypass grafting was required in 114 (60.3%) postarrest cases, and surgery as an isolated coronary artery bypass grafting procedure was required in 55 (29.1%) of these patients.
Table 1.
Preoperative Characteristics of the Overall Population
| Characteristic | Overall population (n=2058) | Survivors (n=814) | Nonsurvivors (n=1244) | P value |
|---|---|---|---|---|
| Age, y | 65.00 (55–72) | 61.75 (52.2–70) | 67.00 (58–73) | <0.001 |
| Sex | 0.463 | |||
| Women | 843 (41) | 325 (40) | ||
| Men | 1214 (59) | 488 (60) | 726 (58.4) | |
| Race or ethnicity | <0.001 | |||
| Asian | 141 (8.8) | 36 (5.5) | 105 (11.1) | |
| Black | 12 (0.8) | 5 (0.8) | 7 (0.7) | |
| Hispanic | 66 (4.1) | 27 (4.1) | 39 (4.1) | |
| White | 1232 (77.1) | 514 (78.4) | 718 (76.2) | |
| Other* | 50 (3.1) | 30 (4.6) | 20 (2.1) | |
| Unknown | 97 (6.1) | 44 (6.7) | 53 (5.6) | |
| Body mass index, kg/m2 | 26.45 (23.7–30) | 26.29 (23.5–29.4) | 26.56 (23.7–30.4) | 0.141 |
| Body surface area, m2 | 1.89 (1.7–2) | 1.91 (1.8–2.1) | 1.88 (1.7–2) | 0.010 |
| Comorbidities | ||||
| Hypertension | 1311 (66) | 489 (62.4) | 822 (68.4) | 0.007 |
| Dialysis | 178 (8.9) | 67 (8.5) | 111 (9.2) | 0.630 |
| Impaired immunity | 46 (2.9) | 21 (3.6) | 25 (2.5) | 0.219 |
| Previous myocardial infarction | 554 (26.9) | 240 (29.5) | 314 (25.2) | 0.037 |
| Myocardial infarction (last 30 d) | 233 (11.7) | 95 (12.1) | 138 (11.5) | 0.670 |
| Previous endocarditis | 161 (7.8) | 67 (8.2) | 94 (7.6) | 0.615 |
| Smoking | 470 (26.9) | 202 (30.1) | 268 (24.9) | 0.020 |
| Previous stroke | 284 (13.8) | 105 (12.9) | 179 (14.4) | 0.360 |
| Atrial fibrillation | 540 (26.3) | 200 (24.6) | 340 (27.4) | 0.167 |
| Previous pulmonary embolism | 33 (1.8) | 6 (0.8) | 27 (2.4) | 0.018 |
| Diabetes | 521 (25.3) | 177 (21.7) | 344 (27.7) | 0.003 |
| Previous transient ischemic attack | 41 (2.2) | 18 (2.5) | 23 (2.1) | 0.521 |
| Implanted pacemaker | 137 (7.3) | 48 (6.6) | 89 (7.7) | 0.364 |
| Implanted ICD | 182 (9.6) | 96 (13) | 86 (7.5) | <0.001 |
| Previous PCI | 350 (17.1) | 148 (18.3) | 202 (16.4) | 0.280 |
| Chronic obstructive pulmonary disease | 206 (10.4) | 67 (8.7) | 139 (11.5) | 0.050 |
| Peripheral artery disease | 302 (14.7) | 100 (12.3) | 202 (16.2) | 0.013 |
| Previous transplant | 75 (3.8) | 24 (3.1) | 51 (4.2) | 0.187 |
| Chronic pulmonary embolism | 41 (2.1) | 16 (2.1) | 25 (2.1) | 1.000 |
| Asthma | 23 (1.4) | 11 (1.8) | 12 (1.2) | 0.386 |
| Pulmonary hypertension (>50 mm Hg) | 428 (20.9) | 158 (19.6) | 270 (21.8) | 0.243 |
| Previous cardiac surgery | 541 (26.3) | 213 (26.2) | 328 (26.4) | 0.959 |
| Implanted LVAD | 73 (3.7) | 45 (5.7) | 28 (2.3) | <0.001 |
| Preoperative creatinine, μmol/L | 101.7 (79.6–140.6) | 98.1 (79.6–128) | 105.60 (80–148.5) | 0.003 |
| LVEF, % | 45.0 (30–60) | 44.0 (25–60) | 50.00 (31–60) | <0.001 |
| EuroSCORE II | 7.53 (3–18.5) | 6.44 (2.6–16.8) | 8.55 (3.2–20.7) | 0.002 |
| Preoperative condition | ||||
| NYHA class | 0.115 | |||
| I | 144 (7.4) | 69 (8.9) | 75 (6.4) | |
| II | 420 (21.5) | 169 (21.9) | 251 (21.3) | |
| III | 769 (39.4) | 287 (37.1) | 482 (40.8) | |
| IV | 621 (31.8) | 248 (32.1) | 373 (31.6) | |
| Preoperative cardiogenic shock | 434 (21.4) | 143 (17.9) | 291 (23.6) | 0.002 |
| Preoperative intubation | 232 (11.3) | 75 (9.2) | 157 (12.6) | 0.019 |
| Preoperative cardiac arrest | 189 (9.3) | 67 (8.3) | 122 (9.9) | 0.242 |
| Preoperative septic shock | 50 (2.5) | 10 (1.3) | 40 (3.3) | 0.005 |
| Preoperative vasopressors | 315 (15.4) | 110 (13.6) | 205 (16.6) | 0.079 |
| Preoperative acute pulmonary edema | 140 (7.1) | 51 (6.6) | 89 (7.5) | 0.474 |
| Preoperative right ventricular failure | 181 (10) | 62 (8.9) | 119 (10.8) | 0.199 |
| Preoperative biventricular failure | 123 (7.6) | 49 (8) | 74 (7.3) | 0.628 |
| Emergency surgery | 528 (25.9) | 193 (24.1) | 335 (27.1) | 0.133 |
| Urgent surgery | 451 (22.1) | 191 (23.8) | 260 (21) | 0.141 |
| Diagnosis | ||||
| Coronary artery disease | 992 (48.2) | 390 (47.9) | 602 (48.4) | 0.857 |
| Aortic vessel disease | 336 (16.3) | 109 (13.4) | 227 (18.2) | 0.003 |
| Aortic valve disease | 701 (34.1) | 226 (27.8) | 475 (38.2) | <0.001 |
| Mitral valve disease | 702 (34.1) | 247 (30.3) | 455 (36.6) | 0.004 |
| Tricuspid valve disease | 330 (16) | 113 (13.9) | 217 (17.4) | 0.032 |
| Pulmonary valve disease | 17 (0.8) | 8 (1) | 9 (0.7) | 0.620 |
| Post‐AMI ventricular septal rupture | 58 (2.8) | 25 (3.1) | 33 (2.7) | 0.588 |
| Free wall/papillary muscle rupture | 38 (1.8) | 13 (1.6) | 25 (2) | 0.616 |
| Active endocarditis | 148 (7.2) | 55 (6.8) | 93 (7.5) | 0.479 |
| Atrial septal defect | 33 (1.6) | 15 (1.8) | 18 (1.4) | 0.601 |
| Post‐LVAD right ventricular failure | 19 (0.9) | 11 (1.4) | 8 (0.6) | 0.155 |
| Other diagnosis | 260 (12.6) | 117 (14.4) | 143 (11.5) | 0.058 |
Data are reported as number (percentage; as valid percentage excluding missing values) or median (interquartile range). P values determined by χ2 test (for categorical data), Student t test (for parametric continuous data), and Mann‐Whitney U test (for nonparametric continuous data) indicate statistically significant differences between survivors and nonsurvivors. AMI indicates acute myocardial infarction; ICD, implantable cardioverter‐defibrillator; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; and PCI, percutaneous coronary intervention. *Other indicates all races or ethnicities not included in the previous list.
Nonsurvivors were more often affected by valvular or aortic vessel diseases (Table 1), which was reflected by a higher percentage of concomitant procedures, aortic surgery, and valve surgery, but also by longer cardiopulmonary bypass and cross‐clamp times (Table 2). Indications to start an ECMO support (Table 3) included failure to wean from cardiopulmonary bypass (n=788 [39.2%]), followed by cardiogenic shock (n=506 [25.2%]) and right ventricular failure (RVF; n=240 [11.9%]). Most patients received an intraoperative ECMO implantation (n=1287 [62.5%]), but nonsurvivors showed a higher percentage of cannulations in intensive care unit (n=462 [37.1%]; P<0.001). Peripheral cannulation was chosen in 965 (46.9%) patients, whereas 707 cases (34.4%) required a mixed cannulation, including both central and peripheral approaches or a dynamic approach where the cannulation setting was switched from central to peripheral or vice versa during the support time. This latter approach was particularly common in patients experiencing RVF (n=89/240 [37.1%]) compared with other indications (n=588/1770 [33.2%]; P=0.035). Use of intra‐aortic balloon pump during any time of hospitalization was reported in 30.5% (n=620) patients with no differences between survivors and nonsurvivors (P=0.109). Impella (n=9 [0.4%]) and other mechanical circulatory support devices (n=22 [1.1%]) were reported in a minority of patients. Median ECMO duration was 118 hours (IQR, 60–192 hours) with no differences between survivors (median, 116 hours; IQR, 72–168 hours) and nonsurvivors (median, 120 hours; IQR, 48–210 hours; P=0.445; Table 3 and Figure S4).
Table 2.
Procedural Characteristics
| Characteristic | Overall population (n=2058) | Survivors (n=814) | Nonsurvivors (n=1244) | P value |
|---|---|---|---|---|
| Weight of surgery | <0.001 | |||
| Unknown | 13 (0.6) | 6 (0.7) | 7 (0.6) | |
| Isolated CABG | 370 (18) | 166 (20.4) | 204 (16.4) | |
| Isolated non‐CABG | 1152 (56) | 470 (57.7) | 682 (54.8) | |
| 2 Procedures | 148 (7.2) | 61 (7.5) | 87 (7) | |
| ≥3 Procedures | 375 (18.2) | 111 (13.6) | 264 (21.2) | |
| CABG | 912 (44.3) | 351 (43.1) | 561 (45.1) | 0.389 |
| Aortic valve surgery | 714 (34.7) | 229 (28.1) | 485 (39) | <0.001 |
| Mitral valve surgery | 647 (31.5) | 224 (27.6) | 423 (34) | 0.002 |
| Tricuspid valve surgery | 275 (13.4) | 83 (10.2) | 192 (15.4) | <0.001 |
| Aortic surgery | 382 (18.6) | 124 (15.2) | 258 (20.7) | 0.002 |
| Pulmonary valve surgery | 12 (0.6) | 6 (0.7) | 6 (0.5) | 0.557 |
| LVAD | 23 (1.1) | 8 (1) | 15 (1.2) | 0.831 |
| RVAD | 6 (0.3) | 2 (0.2) | 4 (0.3) | 1 |
| Atrial septal defect repair | 38 (1.8) | 15 (1.8) | 23 (1.8) | 1 |
| Ventricular septal defect repair | 68 (3.3) | 28 (3.4) | 40 (3.2) | 0.802 |
| Ventricular surgery | 75 (3.6) | 20 (2.5) | 55 (4.4) | 0.022 |
| Rhythm surgery | 67 (3.3) | 26 (3.2) | 41 (3.3) | 1 |
| Pulmonary embolectomy | 23 (1.1) | 10 (1.2) | 13 (1) | 0.676 |
| Pulmonary endarterectomy | 48 (2.3) | 15 (1.8) | 33 (2.7) | 0.296 |
| Heart transplantation | 209 (10.2) | 130 (16) | 79 (6.4) | <0.001 |
| Off‐pump surgery | 83 (4.1) | 34 (4.3) | 49 (4) | 0.732 |
| Conversion to cardiopulmonary bypass | 25 (29.1) | 7 (19.4) | 18 (36) | 0.148 |
| Cardioplegia type | 0.178 | |||
| Blood | 706 (51.2) | 290 (54.7) | 416 (48.9) | |
| Crystalloid | 392 (28.4) | 139 (26.2) | 253 (29.8) | |
| Custodiol | 281 (20.4) | 101 (19.1) | 180 (21.2) | |
| Other | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Cardioplegia route | 0.616 | |||
| Antegrade | 927 (71.5) | 355 (73) | 572 (70.5) | |
| Retrograde | 58 (4.5) | 20 (4.1) | 38 (4.7) | |
| Antegrade+retrograde | 312 (24.1) | 111 (22.8) | 201 (24.8) | |
| Cardiopulmonary bypass time, min | 204 (139–288) | 198 (137–272) | 210 (142–300) | 0.015 |
| Cross‐clamp time, min | 99 (64–148) | 94 (62–132) | 104 (65–155) | 0.003 |
| Intraoperative transfusions | 776 (92.4) | 279 (90.9) | 497 (93.2) | 0.226 |
Data are reported as number (percentage; as valid percentage excluding missing values) or median (interquartile range). P values determined by χ2 test (for categorical data), Student t test (for parametric continuous data), and Mann‐Whitney U test (for nonparametric continuous data) indicate statistically significant differences between survivors and nonsurvivors. CABG indicates coronary artery bypass grafting; LVAD, left ventricular assist device; and RVAD, right ventricular assist device.
Table 3.
Details on ECMO
| Variable | Overall population (n=2058) | Survivors (n=814) | Nonsurvivors (n=1244) | P value |
|---|---|---|---|---|
| ECMO indication | 0.013 | |||
| Failure to wean | 788 (39.2) | 318 (40.4) | 470 (38.5) | |
| Acute pulmonary embolism | 3 (0.1) | 1 (0.1) | 2 (0.2) | |
| Arrhythmia | 43 (2.1) | 25 (3.2) | 18 (1.5) | |
| Cardiac arrest | 170 (8.5) | 61 (7.7) | 109 (8.9) | |
| Cardiogenic shock | 506 (25.2) | 177 (22.5) | 329 (26.9) | |
| Pulmonary hemorrhage | 9 (0.4) | 6 (0.8) | 3 (0.2) | |
| Right ventricular failure | 240 (11.9) | 99 (12.6) | 141 (11.5) | |
| Respiratory failure | 72 (3.6) | 29 (3.7) | 43 (3.5) | |
| Biventricular failure | 149 (7.4) | 54 (6.9) | 95 (7.8) | |
| Other | 30 (1.5) | 18 (2.3) | 12 (1) | |
| ECMO implantation timing | <0.001 | |||
| Intraoperative | 1287 (62.5) | 547 (62.7) | 740 (59.5) | |
| Intensive care unit | 716 (34.8) | 254 (31.2) | 462 (37.1) | |
| Ward | 39 (1.9) | 6 (0.7) | 33 (2.7) | |
| Catheterization laboratory | 16 (0.8) | 7 (0.9) | 9 (0.7) | |
| Chest status | 0.002 | |||
| Chest closed | 858 (57.5) | 364 (62.7) | 494 (54.2) | |
| Chest open | 634 (42.5) | 217 (37.3) | 417 (45.8) | |
| Cannulation approach | 0.006 | |||
| Only central cannulation | 341 (16.6) | 106 (13) | 235 (18.9) | |
| Only peripheral cannulation | 965 (46.9) | 400 (49.1) | 565 (45.4) | |
| Mixed/switch cannulation | 707 (34.4) | 289 (35.5) | 418 (33.6) | |
| Unknown | 45 (2.2) | 19 (2.3) | 26 (2.1) | |
| LV venting | 519 (30.8) | 190 (27.5) | 329 (33.1) | 0.014 |
| LV venting site | 0.108 | |||
| Right superior pulmonary vein | 41 (7.9) | 14 (7.4) | 27 (8.2) | |
| LV apex | 30 (5.8) | 6 (3.2) | 24 (7.3) | |
| Pulmonary artery | 15 (2.9) | 3 (1.6) | 12 (3.7) | |
| Septostomy | 2 (0.4) | 1 (0.5) | 1 (0.3) | |
| Left atrium | 38 (7.4) | 9 (4.8) | 29 (8.8) | |
| Transaortic device | 1 (0.2) | 1 (0.5) | 0 (0) | |
| Additional venous cannula | 3 (0.6) | 1 (0.5) | 2 (0.6) | |
| IABP | 387 (74.9) | 154 (81.5) | 233 (71) | |
| IABP during any time of hospitalization | 620 (30.5) | 226 (27.8) | 394 (32.2) | 0.035 |
| IABP implantation timing | 0.928 | |||
| Preoperative | 192 (31) | 69 (30.5) | 123 (31.2) | |
| Intraoperative | 428 (69) | 157 (69.5) | 271 (68.8) | |
| Distal femoral perfusion | 778 (65.8) | 332 (69) | 446 (63.5) | 0.053 |
| Anticoagulation | 0.039 | |||
| None | 187 (9.4) | 55 (7.1) | 132 (10.9) | |
| Heparin | 1785 (89.9) | 716 (92) | 1069 (88.5) | |
| Bivalirudin | 3 (0.2) | 1 (0.1) | 2 (0.2) | |
| Argatroban | 5 (0.3) | 2 (0.3) | 3 (0.2) | |
| Protamine only | 6 (0.3) | 4 (0.5) | 2 (0.2) | |
| ECMO duration, h | 118 (60–192) | 116 (72–168) | 120.00 (48–210) | 0.445 |
Data are reported as number (percentage; as valid percentage excluding missing values) or median (interquartile range). P values determined by χ2 test (for categorical data), Student t test (for parametric continuous data), and Mann‐Whitney U test (for nonparametric continuous data) indicate statistically significant differences between survivors and nonsurvivors. ECMO indicates extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; and LV, left ventricular.
In‐Hospital Outcomes, Complications, and Variables Associated With In‐Hospital Mortality
In‐hospital mortality was 60.5%, with stable rates over the study period (P=0.322; Figure S5A). In‐hospital survivors were discharged after a median of 38.0 (IQR, 26.0–60‐0) days, whereas in‐hospital death occurred at a median of 11.0 (IQR, 4–22) days after surgery (Table 4). On the basis of the different clinical profiles and hospitalization time, survivors and nonsurvivors experienced different kinds of complications (Table 4). Leg ischemia (P<0.001), cardiac arrest (P<0.001), bowel ischemia (P<0.001), RVF (P<0.001), acute kidney injury (P<0.001), septic shock (P<0.001), distributive shock (P<0.001), and multiorgan failure (P<0.001) were more frequent in nonsurvivors, whereas pneumonia (P<0.001) and pacemaker implantation (P<0.001) occurred more frequently in survivors. Acute kidney injury was more frequent in patients operated on before 2010 (n=284/452 [68.9%]) compared with those operated on since 2011 (n=785/1606 [53.3%]). In‐hospital mortality significantly differed between centers (P<0.001), types of surgeries (P<0.001), and ECMO indications (P=0.013; Tables 2 and 3 and Figure S5). The mixed Cox proportional hazards analyses identified variables associated with in‐hospital mortality at different time points of the in‐hospital clinical course (full models presented in Tables S3–S6). Main variables associated with in‐hospital mortality that remained statistically significant in each of the 4 prespecified models were age (HR, 1.02 [95% CI, 1.01–1.02]) and preoperative cardiac arrest (HR, 1.41 [95% CI, 1.15–1.73]; Table 5).
Table 4.
Details on Postoperative Outcomes
| Variable | Overall population (n=2058) | Survivors (n=814) | Nonsurvivors (n=1244) | P value |
|---|---|---|---|---|
| Intensive care unit stay, d | 13 (6–26) | 21 (13–36.5) | 9.00 (3–18) | <0.001 |
| Hospital stay, d | 20 (8–40) | 38 (26–60) | 11.00 (4–22) | <0.001 |
| Postoperative bleeding | 1156 (57.2) | 382 (48.2) | 774 (63) | <0.001 |
| Requiring rethoracotomy | 765 (39.7) | 253 (34.2) | 512 (43.2) | <0.001 |
| Cannulation site bleeding | 246 (12.2) | 73 (9.2) | 173 (14.1) | <0.001 |
| Diffuse no surgical‐related bleeding | 472 (25.4) | 139 (18.9) | 333 (29.7) | <0.001 |
| Neurological complications | ||||
| Brain edema | 84 (4.3) | 15 (1.9) | 69 (5.8) | <0.001 |
| Cerebral hemorrhage | 66 (3.4) | 22 (2.9) | 44 (3.7) | 0.37 |
| Severity | 0.276 | |||
| Minor | 21 (43.8) | 7 (58.3) | 14 (38.9) | |
| Disabling | 15 (31.3) | 4 (33.3) | 11 (30.6) | |
| Fatal | 12 (25) | 1 (8.3) | 11 (30.6) | |
| Seizure | 41 (2.1) | 16 (2.1) | 25 (2.1) | 1 |
| Stroke | 217 (10.6) | 95 (11.7) | 122 (9.9) | 0.213 |
| Severity | <0.001 | |||
| Minor | 83 (46.9) | 47 (60.3) | 36 (36.4) | |
| Disabling | 57 (32.2) | 31 (39.7) | 26 (26.3) | |
| Fatal | 37 (20.9) | 0 (0) | 37 (37.4) | |
| Vasospasm | 3 (0.2) | 1 (0.2) | 2 (0.2) | 1 |
| Arrhythmia | 624 (33) | 276 (37.3) | 348 (30.2) | 0.001 |
| Leg ischemia | 200 (10.3) | 57 (7.4) | 143 (12.2) | <0.001 |
| Cardiac arrest | 304 (16.1) | 69 (9.3) | 235 (20.4) | <0.001 |
| Pacemaker implantation | 56 (3) | 40 (5.4) | 16 (1.4) | <0.001 |
| Bowel ischemia | 107 (5.7) | 13 (1.8) | 94 (8.1) | <0.001 |
| Right ventricular failure | 389 (21) | 87 (12.1) | 302 (26.7) | <0.001 |
| Heart transplant | 111 (7.2) | 54 (9.4) | 57 (5.9) | 0.011 |
| Acute kidney injury | 1069 (56.7) | 366 (50) | 703 (61) | <0.001 |
| Pneumonia | 411 (22.2) | 196 (27.3) | 215 (19) | <0.001 |
| Septic shock | 310 (16.8) | 73 (10.2) | 237 (20.9) | <0.001 |
| Vasoplegic syndrome | 176 (9.5) | 32 (4.5) | 144 (12.7) | <0.001 |
| Acute respiratory distress syndrome | 104 (5.5) | 31 (4.2) | 73 (6.3) | 0.05 |
| Multiorgan failure | 697 (34.3) | 46 (5.7) | 651 (52.9) | <0.001 |
| Embolism | 113 (6.1) | 39 (5.4) | 74 (6.5) | 0.371 |
| Postoperative procedures | ||||
| Percutaneous coronary intervention | 48 (2.6) | 24 (3.4) | 24 (2.2) | 0.1 |
| Cardiac surgery | 413 (21.8) | 144 (19.5) | 269 (23.4) | 0.046 |
| Abdominal surgery | 85 (4.7) | 29 (4.2) | 56 (5) | 0.426 |
| Vascular surgery | 209 (11.5) | 95 (13.6) | 114 (10.2) | 0.029 |
| In‐hospital mortality | NA | |||
| Deceased on ECMO | 754 (60.6) | |||
| Deceased after weaning | 476 (38.3) | |||
| Death time unknown | 14 (1.1) | |||
| Main cause of death | NA | |||
| Multiorgan failure | 431 (37.2) | |||
| Sepsis | 85 (7.3) | |||
| Persistent heart failure | 423 (36.5) | |||
| Distributive shock syndrome | 22 (1.9) | |||
| Bleeding | 64 (5.5) | |||
| Neurological injury | 58 (5.0) | |||
| Bowel ischemia | 22 (1.9) | |||
| Other | 53 (4.6) | |||
Data are reported as number (percentage; as valid percentage excluding missing values) or median (interquartile range). P values determined by χ2 test (for categorical data), Student t‐test (for parametric continuous data), and Mann‐Whitney U test (for nonparametric continuous data) indicate statistically significant differences between survivors and nonsurvivors. ECMO, extracorporeal membrane oxygenation; NA, not applicable.
Table 5.
Mixed Cox Proportional Hazards for Significant Variables Associated With In‐Hospital Mortality
| Variable | By center | By center and year | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Model 1: demographic data and preoperative variables | ||||||||
| Age, y | 1.02 | 1.01 | 1.02 | <0.0001 | 1.02 | 1.01 | 1.02 | <0.0001 |
| Sex (reference: men) | 1.15 | 1.02 | 1.29 | 0.0280 | 1.15 | 1.01 | 1.29 | 0.0290 |
| COPD | 1.28 | 1.06 | 1.53 | 0.0086 | 1.28 | 1.06 | 1.53 | 0.0090 |
| Preoperative cardiogenic shock | 1.23 | 1.04 | 1.45 | 0.0150 | 1.23 | 1.04 | 1.45 | 0.0140 |
| Emergency surgery (vs elective) | 1.15 | 1.02 | 1.36 | 0.0430 | 1.15 | 0.97 | 1.36 | 0.1000 |
| Preoperative cardiac arrest | 1.41 | 1.15 | 1.73 | 0.0008 | 1.41 | 1.15 | 1.73 | 0.0009 |
| Preoperative right ventricular failure | 1.29 | 1.06 | 1.58 | 0.0110 | 1.29 | 1.06 | 1.58 | 0.0120 |
| Preoperative creatinine, μmol/L | 1.01 | 1.01 | 1.02 | 0.0410 | 1.01 | 1.01 | 1.02 | 0.0450 |
| Aortic vessel disease | 1.40 | 1.20 | 1.64 | <0.0001 | 1.40 | 1.20 | 1.65 | 0.0000 |
| Aortic valve disease | 1.16 | 1.02 | 1.32 | 0.0240 | 1.16 | 1.02 | 1.31 | 0.0260 |
| Model 2: demographic data and preoperative and intraoperative variables | ||||||||
| Age, y | 1.02 | 1.01 | 1.03 | <0.0001 | 1.02 | 1.01 | 1.03 | 0.0000 |
| Sex (reference: men) | 1.15 | 1.01 | 1.29 | 0.0330 | 1.14 | 1.01 | 1.29 | 0.0300 |
| COPD | 1.23 | 1.02 | 1.48 | 0.0310 | 1.23 | 1.02 | 1.48 | 0.0300 |
| Preoperative cardiogenic shock | 1.25 | 1.06 | 1.48 | 0.0073 | 1.25 | 1.06 | 1.48 | 0.0077 |
| Emergency surgery (vs elective) | 1.16 | 1.03 | 1.37 | 0.0460 | 1.16 | 0.98 | 1.37 | 0.0850 |
| Preoperative cardiac arrest | 1.45 | 1.18 | 1.77 | 0.0004 | 1.45 | 1.18 | 1.77 | 0.0004 |
| Preoperative right ventricular failure | 1.30 | 1.07 | 1.59 | 0.0090 | 1.30 | 1.07 | 1.59 | 0.0093 |
| Tricuspid valve disease | 0.74 | 0.57 | 0.97 | 0.0280 | 0.74 | 0.57 | 0.97 | 0.0280 |
| Cardiopulmonary bypass time, min | 1.01 | 1.01 | 1.02 | 0.0035 | 1.01 | 1.01 | 1.02 | 0.0004 |
| Tricuspid valve surgery | 1.49 | 1.12 | 1.99 | 0.0066 | 1.49 | 1.12 | 1.99 | 0.0066 |
| Model 3: demographic data and preoperative, intraoperative, and ECMO variables | ||||||||
| Age, y | 1.02 | 1.01 | 1.03 | <0.0001 | 1.02 | 1.01 | 1.03 | 0.0000 |
| Sex (reference: men) | 1.14 | 1.01 | 1.28 | 0.0410 | 1.14 | 1.01 | 1.28 | 0.0410 |
| COPD | 1.23 | 1.02 | 1.48 | 0.0280 | 1.23 | 1.02 | 1.48 | 0.0280 |
| Preoperative cardiogenic shock | 1.27 | 1.07 | 1.50 | 0.0055 | 1.27 | 1.07 | 1.50 | 0.0054 |
| Preoperative cardiac arrest | 1.41 | 1.14 | 1.74 | 0.0016 | 1.41 | 1.14 | 1.74 | 0.0016 |
| Preoperative right ventricular failure | 1.36 | 1.11 | 1.66 | 0.0032 | 1.36 | 1.11 | 1.66 | 0.0032 |
| Tricuspid valve disease | 0.73 | 0.56 | 0.96 | 0.0220 | 0.73 | 0.56 | 0.96 | 0.0220 |
| Cardiopulmonary bypass time, min | 1.01 | 1.01 | 1.02 | <0.0001 | 1.01 | 1.01 | 1.02 | 0.0001 |
| Tricuspid valve surgery | 1.53 | 1.15 | 2.04 | 0.0038 | 1.53 | 1.15 | 2.04 | 0.0038 |
| ECMO implanting time: postoperative (reference: intraoperative) | 1.25 | 1.06 | 1.46 | 0.0063 | 1.25 | 1.06 | 1.46 | 0.0068 |
| ECMO indication: right ventricular failure | 0.74 | 0.60 | 0.93 | 0.0093 | 0.74 | 0.60 | 0.93 | 0.0083 |
| ECMO indication: other | 0.70 | 0.54 | 0.91 | 0.0080 | 0.70 | 0.54 | 0.91 | 0.0079 |
| ECMO central cannulation | 2.86 | 1.17 | 6.98 | 0.0210 | 2.86 | 1.17 | 6.99 | 0.0210 |
| ECMO cannulation change/mixed | 2.46 | 1.01 | 5.98 | 0.0470 | 2.46 | 1.01 | 5.99 | 0.0470 |
| Model 4: demographic data, preoperative, intraoperative, and ECMO variables, and complications | ||||||||
| Age, y | 1.02 | 1.01 | 1.02 | <0.0001 | 1.02 | 1.01 | 1.02 | 0.0000 |
| Preoperative cardiac arrest | 1.34 | 1.08 | 1.66 | 0.0073 | 1.34 | 1.08 | 1.66 | 0.0078 |
| Tricuspid valve surgery | 1.53 | 1.14 | 2.05 | 0.0043 | 1.53 | 1.14 | 2.05 | 0.0044 |
| Aortic surgery | 1.32 | 1.00 | 1.75 | 0.0470 | 1.32 | 1.00 | 1.75 | 0.0470 |
| ECMO indication: right ventricular failure | 0.75 | 0.60 | 0.93 | 0.0100 | 0.75 | 0.60 | 0.93 | 0.0100 |
| ECMO indication: other | 0.68 | 0.52 | 0.88 | 0.0038 | 0.68 | 0.52 | 0.88 | 0.0038 |
| ECMO central cannulation complications | 2.71 | 1.08 | 6.79 | 0.0330 | 2.72 | 1.09 | 6.80 | 0.0330 |
| LV failure | 1.70 | 1.48 | 1.96 | <0.0001 | 1.70 | 1.48 | 1.96 | 0.0000 |
| RV failure | 1.25 | 1.08 | 1.46 | 0.0033 | 1.25 | 1.08 | 1.46 | 0.0033 |
| Cardiac arrest | 1.53 | 1.31 | 1.79 | <0.0001 | 1.53 | 1.31 | 1.79 | 0.0000 |
| Bowel ischemia | 1.28 | 1.03 | 1.60 | 0.0270 | 1.28 | 1.03 | 1.60 | 0.0270 |
| Septic shock | 0.85 | 0.72 | 0.99 | 0.0480 | 0.85 | 0.72 | 0.99 | 0.0420 |
| Pneumonia | 0.48 | 0.41 | 0.56 | <0.0001 | 0.48 | 0.41 | 0.56 | 0.0000 |
| Multiorgan failure | 3.74 | 3.27 | 4.29 | <0.0001 | 3.75 | 3.27 | 4.29 | 0.0000 |
COPD indicates chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; LV, left ventricular; and RV, right ventricular.
Long‐Term Mortality and Its Determinants
For the overall survival probability, the Kaplan‐Meier curves for 12‐month survival and postdischarge survival are shown in the Figure. Overall, 1‐, 2‐, 5‐, and 10‐year survival probabilities were 32.4% (95% CI, 30.3%–34.6%), 30.9% (95% CI, 28.8%–33.1%), 27.8% (95% CI, 25.7%–30.1%), and 19.5% (95% CI, 16.7%–22.8%), respectively. In the subgroup of hospital survivors, the median follow‐up was 2.5 years (IQR, 0.3–5.3 years). Data on survival at last follow‐up contact were available in 93.1% of in‐hospital survivors. In this subgroup, the overall 1‐, 2‐, 5‐, and 10‐year survival rates were 89.5% (95% CI, 87.0%–92.0%), 85.4% (95% CI, 82.5%–88.3%), 76.4% (95% CI, 72.5%–80.5%), and 65.9% (95% CI, 60.3%–72.0%), respectively. Older age (HR, 1.03 [95% CI, 1.02–1.05]), preoperative atrial fibrillation (HR, 1.52 [95% CI, 1.04–2.21]), emergency surgery (HR, 1.66 [95% CI, 1.07–2.55]), coronary artery bypass (HR, 1.51 [95% CI, 1.06–2.12]), aortic valve surgery (HR, 1.46 [95% CI, 1.01–2.12]), and septic shock (HR, 2.53 [95% CI, 1.42–4.53]) were associated with worse long‐term postdischarge outcome (Table 6). Postoperative acute kidney injury (HR, 1.37 [95% CI, 1.01–1.95]) was significantly associated with worse long‐term postdischarge outcome in the mixed Cox model adjusted for center only. The effect estimate remained similar (HR, 1.37 [95% CI, 0.95–1.95]) in the mixed Cox model adjusted for center and year of operation but lost statistical significance (P=0.09; Table 6).
Figure 1. Kaplan‐Meier survival curves with 95% CIs.
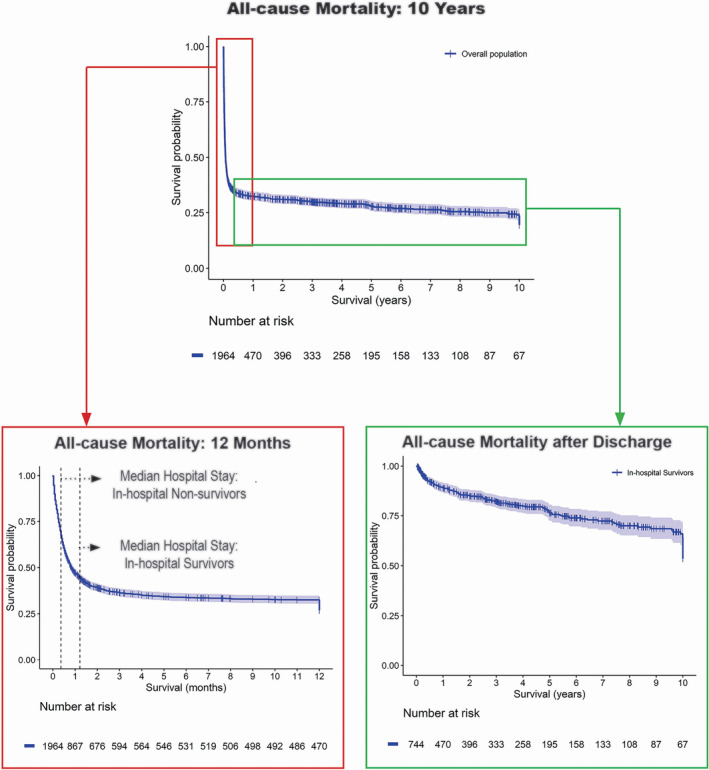
Table 6.
Mixed Cox Proportional Hazards for Postdischarge Mortality Based on Model 4
| Variable | By center | By center and year | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Age, y | 1.03 | 1.02 | 1.05 | <0.0001 | 1.03 | 1.02 | 1.05 | 0.0001 |
| Sex (reference: men) | 0.98 | 0.69 | 1.40 | 0.9100 | 0.99 | 0.69 | 1.41 | 0.9400 |
| Dialysis | 1.16 | 0.64 | 2.09 | 0.6300 | 1.22 | 0.67 | 2.23 | 0.5100 |
| Preoperative atrial fibrillation | 1.45 | 1.01 | 2.11 | 0.0420 | 1.52 | 1.04 | 2.21 | 0.0310 |
| COPD | 1.32 | 0.78 | 2.24 | 0.3000 | 1.19 | 0.68 | 2.07 | 0.5400 |
| LVEF, % | 1.00 | 0.99 | 1.01 | 0.5300 | 1.00 | 0.99 | 1.01 | 0.9100 |
| Urgent vs elective | 1.45 | 0.96 | 2.20 | 0.0800 | 1.39 | 0.92 | 2.11 | 0.1200 |
| Emergency vs elective | 1.68 | 1.04 | 2.70 | 0.0330 | 1.66 | 1.07 | 2.55 | 0.0220 |
| CABG | 1.49 | 1.05 | 2.12 | 0.0270 | 1.51 | 1.06 | 2.16 | 0.0230 |
| Aortic valve surgery | 1.41 | 1.07 | 2.24 | 0.0230 | 1.46 | 1.01 | 2.12 | 0.0450 |
| Mitral valve surgery | 1.12 | 0.76 | 1.64 | 0.5700 | 1.13 | 0.77 | 1.65 | 0.5300 |
| Complications: cerebral hemorrhage | 0.92 | 0.36 | 2.33 | 0.8600 | 0.94 | 0.37 | 2.38 | 0.8900 |
| Complications: cardiac arrest | 1.06 | 0.56 | 2.01 | 0.8500 | 1.06 | 0.56 | 2.01 | 0.8600 |
| Complications: AKI | 1.37 | 1.01 | 1.95 | 0.0480 | 1.36 | 0.95 | 1.95 | 0.0900 |
| Complications: septic shock | 2.59 | 1.45 | 4.63 | 0.0013 | 2.53 | 1.42 | 4.53 | 0.0010 |
Model 4 includes demographic data; preoperative, intraoperative, and extracorporeal membrane oxygenation variables; and complications. AKI indicates acute kidney disease; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; and LVEF, left ventricular ejection fraction.
Discussion
The PELS‐1 has 5 main findings. First, in‐hospital mortality was 60.5%, with stable rates over the study years. Second, duration of ECMO support was a median of 5 days in both survivors and nonsurvivors. Third, age and preoperative cardiac arrest are the main variables associated with in‐hospital mortality. However, different phases of the postcardiotomy ECMO support are characterized by specific variables associated with in‐hospital mortality and, thus, prediction models for patient selection, intraoperative decisions, and ECMO management should be developed separately, to aid in the decision‐making about such a temporary support. Fourth, hospital survivors appear to have a good postdischarge outcome, with 89.5% (95% CI, 87.0%–92.0%), 85.4% (95% CI, 82.5%–88.3%), 76.4% (95% CI, 72.5%–80.5%), and 65.9% (95% CI, 60.3%–72.0%) survival at 1, 2, 5, and 10 years, respectively. Finally, the overall postdischarge survival is mainly determined by patient's age, with an HR of 1.03 (95% CI, 1.02–1.05) for each additional year of age, and preexistent comorbidities, such as atrial fibrillation, emergency and type of surgery, and postoperative complications, like acute kidney injury (HR, 1.37 [95% CI, 1.01–1.95]) and septic shock (HR, 2.53 [95% CI, 1.42–4.53]).
On the basis of the increased complexity of patients undergoing cardiac surgery and the growing popularity of ECMO, its use has increased over time, but with persistently high in‐hospital mortality. 3 , 7 , 8 , 11 , 16 , 17 , 18 , 19 , 20 Resource demands for postcardiotomy V‐A ECMO are high. 2 This has led to a debate about proper patient selection to optimize resources and provide best treatments to patients who might benefit from it. Although several attempts have been made to identify best practices for postcardiotomy V‐A ECMO, robust evidence on this topic is still lacking and expert consensus recommendations have been only recently released. 2 Thus, the real‐world clinical application of postcardiotomy V‐A ECMO remains highly variable and based on individual or center‐based expertise, surgeon's choices, and inhomogeneous management strategies.
The PELS‐1 included elderly patients (median age, 65 years; 30.5% of patients aged >70 years), a high percentage of women (41%), patients on preoperative dialysis (8.9%), and patients with a history of cardiac surgery (26.3%). Despite the high preoperative risk profile of the PELS‐1 population, the current study confirmed that in‐hospital mortality of patients undergoing postcardiotomy V‐A ECMO is around 60%, as previously reported. 3 , 7 , 8 , 9 , 21 Moreover, this study demonstrates that 9.3% of included patients experienced a preoperative cardiac arrest, a variable rarely reported in this kind of population. Interestingly, these patients with a preoperative cardiac arrest are frequently known for vasculopathy and ischemic myocardial disease. They often require a preoperative intra‐aortic balloon pump and emergency coronary artery bypass grafting. Nevertheless, cardiac arrest is not the most common indication for postcardiotomy V‐A ECMO implantation. Failure to wean from cardiopulmonary bypass remains the primary indication (39.7%), followed by cardiogenic shock (25.2%) and RVF (11.9%). The latter indicates the significant impact of RVF in patients undergoing cardiac surgery. Indeed, literature reports that 2.9% of them develop clinically relevant postoperative RVF, which is associated with death, stroke, reintubation, and prolonged intensive care unit stay. 22 The current study highlights the need of further investigations to better understand the role, indication, timing, and cannulation setting for any mechanical circulatory support in postcardiotomy RVF.
Significant variability was observed within the PELS‐1 population for the cannulation approach. Indeed, the debate about the best strategy between peripheral or central cannulation is still controversial. Interestingly, 34.4% of included patients received a change in cannulation approach or underwent a mixed cannulation strategy with one central cannula combined with one peripheral cannula. This was particularly true for patients diagnosed with RVF. This finding might indicate the uncertainty about the best cannulation strategy or the dynamism of these patients undergoing V‐A ECMO whose circulatory and respiratory situation can change rapidly along the disease course. This aspect might also explain why several previous studies that investigated outcomes after central or peripheral cannulation were not able to identify a definitive answer. 16 , 23
The PELS‐1 shows that both survivors and nonsurvivors were supported with V‐A ECMO for a median of 5 days. Conflicting results have been reported on this topic, with some studies showing longer ECMO support in survivors 8 and some others showing longer support time in nonsurvivors, 7 , 11 suggesting a selection bias and the heterogeneity among ECMO policies. Whether the poor in‐hospital survival after ECMO is mainly attributable to suboptimal patient selection, an intrinsically complex disease, suboptimal weaning time, or the futility of this support remains an open question. Indeed, in many centers, 3 to 5 days of inadequate cardiac function in a patient who is not a candidate for transplant or ventricular assist device (such as elderly patients) is considered futile. 2 This common practice might reflect the effects of previous studies, which demonstrated that V‐A ECMO support >7 days is associated with increased risks of complications and higher mortality. 24 However, tools to identify potential survivors or to prevent futile treatments are still limited.
To date, published studies have focused attention on the identification of mortality prediction models mainly developed using statistical methods. 8 , 20 , 25 , 26 , 27 , 28 , 29 , 30 Nevertheless, scores and prediction models are rarely applied in the clinical practice. In fact, most of them lack external validation, are static, and do not consider the dynamism of the ECMO process and underlying disease course. Studies have reported on single tools, such as arterial lactates, 8 , 31 , 32 which become a negative prognostic factor when >6 8 , 26 or 10 31 mmol/L at ECMO initiation. Lactates are useful in unexpected emergencies, such as periarrest situations, when clinicians must decide whether to initiate rescue ECMO. However, for most patients undergoing postcardiotomy ECMO, their management does not always begin with an unexpected sudden event requiring ECMO, but it starts earlier when they are accepted for cardiac surgery. Furthermore, the concept of “prophylactic” or “early” postcardiotomy ECMO is changing the clinical scenario and increasing the use of elective ECMO in situations where lactates are still low. 2 In these cases, clinicians lack tools to identify those patients with low chances of survival, to develop preventive ECMO strategies, and to target variables associated with mortality. The current analysis proposes a stepwise approach to identify variables associated with in‐hospital mortality during different phases of the postcardiotomy ECMO clinical course: preoperative (model 1), intraoperative (model 2), during ECMO support (model 3), and when complications occur (model 4). Each of these phases is characterized by different variables to answer questions about patient's candidacy, ECMO management, and futility. Variables that remain always associated with in‐hospital mortality are age and cardiac arrest, in accordance with previous studies. 7 , 11 , 30 , 33 On top of these constant determinants, several variables with potential influence on mortality should be considered in the decision‐making process at specific time points on the in‐hospital course. Finally, in all models developed in this study, we considered the influence of the treating center and year. Indeed, center experience, local policies, differences in health care systems, changes over time, and resource allocations 34 might also impact the postcardiotomy ECMO decision‐making process.
Acknowledging that patient selection and in‐hospital mortality are the major limiting factors in the clinical success of postcardiotomy ECMO, patients who survive to discharge demonstrate a good long‐term survival. However, older age, atrial fibrillation, emergency surgery, coronary artery bypass and aortic surgery, postoperative acute kidney injury, and septic shock are associated with worse long‐term mortality. Interestingly, about 10% of discharged patients die during the first year after surgery. Chen et al previously demonstrated that patients undergoing postcardiotomy ECMO are at increased risk for all‐cause mortality and hospital readmission during the first year of follow‐up. 19 , 35 However, mortality, readmission rates, and medical expenditures are similar from the second year of follow‐up onwards. This might be explained by the influence of postoperative complications on the early postdischarge mortality, as shown by our data. Therefore, a comprehensive follow‐up program should be advised after postcardiotomy ECMO, especially during the early postdischarge time, whereas our data show that longer‐term follow‐up is characterized by reduced rate of unfavorable events. Furthermore, additional studies are required to investigate quality of life and functional status of patients who underwent postcardiotomy ECMO after discharge.
Strengths and Limitations
The structured data collection performed in the PELS‐1, the participation of 34 centers from 16 countries, and the large sample size support data robustness and statistical power. Nevertheless, PELS‐1 is observational by nature, preventing causal inferences. Data on how many adult patients received cardiac surgery at each center during the study period were not available because the analysis of ECMO implantation rates in cardiac surgery was beyond the aim of this study. Furthermore, specific data on ECMO selection criteria, protocols, weaning strategies, serial arterial lactate concentrations, longitudinal/serial data, vasopressor, and inotrope use are not captured by the database and could therefore not be included in this study. Furthermore, an in‐depth analysis of intraoperative and postoperative hemodynamic parameters, as well as coagulation parameters, anesthesia management protocols, quality of life, and rehospitalization events after discharge, was not possible. Septic shock was reported by each investigator according to the study definition. 36 However, codes for surgical site infection, bloodstream infections, antibiotics, and infectious agents are not present in the data set, and we cannot exclude a misdiagnosis of some patients who experienced persistent distributive shock or other kinds of shock accounting for persistent hemodynamic failure. The local policies for left ventricular venting differed widely among participating centers, preventing any speculation on relationships between cardiac venting and enhanced myocardial recovery/ability to wean off ECMO support. Finally, several clinical variables were collected but showed a significant amount of missing data (>20%) and were not included in the mixed Cox models.
Conclusions
The PELS‐1 shows that postcardiotomy V‐A ECMO, during an observation time of 20 years, is associated with 60% in‐hospital mortality with no improvement over time. However, 66% postdischarge survival probability up to 10 years indicates that the in‐hospital course remains the main limiting factor that needs to be addressed to improve the success of this therapeutic approach. PELS‐1 adds that common variables, such as age and preoperative cardiac arrest, affect survival throughout each of the steps of the in‐hospital patient stay, whereas specific variables affect the preoperative selection, intraoperative action, ECMO management, and ECMO weaning phases. This has implications for prediction model development in postcardiotomy ECMO. Moreover, PELS‐1 highlights the importance of preventing complications, such acute kidney injury and septic shock, based on their impact on long‐term mortality. Finally, an adequate follow‐up of patients undergoing postcardiotomy V‐A ECMO, especially in case of postoperative complications, is advised and critical for the first postdischarge year. Further studies are warranted to verify the feasibility and efficacy of these proposed interventions, particularly in the long‐term.
Appendix
PELS‐1 Investigators
Cardio‐Thoracic Surgery Department and Cardiovascular Research Institute Maastricht, Maastricht, the Netherlands (Justine Ravaux); Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria (Anne‐Kristin Schaefer, Luca Conci, Philipp Szalkiewicz); Department of Cardiac Surgery, Leipzig Heart Center, Leipzig, Germany (Jawad Khalil, Sven Lehmann); Department of Cardiac Surgery, Louis Pradel Cardiologic Hospital, Lyon, France (Jean‐Francois Obadia); Department of Cardiac Surgery, Medical Faculty, Heinrich Heine University, Duesseldorf, Germany (Nikolaos Kalampokas); Division of Cardiothoracic and Vascular Surgery, Pontchaillou University Hospital, Rennes, France (Erwan Flecher); Department of Intensive Care Adults, Erasmus MC, Rotterdam, the Netherlands (Dinis Dos Reis Miranda); Department of Intensive Care Medicine, Center of Applied Medical Research, St Vincent's Hospital, Darlinghurst, New South Wales, Australia (Kogulan Sriranjan); Departments of Medicine and Surgery, University of Maryland, Baltimore, MD (Michael A. Mazzeffi, Nazli Vedadi); SOD Cardiochirurgia Ospedali Riuniti “Umberto I–Lancisi–Salesi” Università Politecnica delle Marche, Ancona, Italy (Marco Di Eusanio); Cardiothoracic Intensive Care Unit, National University Heart Centre, National University Hospital, Singapore, Singapore (Vitaly Sorokin, Kollengode Ramanathan); Cardiac Surgery Unit, Cardiac Thoracic and Vascular Department, Niguarda Hospital, Milan, Italy (Alessandro Costetti); Department of Cardiothoracic Surgery, University Medical Center Regensburg, Regensburg, Germany (Chistof Schmid); ECMO Unit, Departamento de Anestesia, Clínica Las Condes, Las Condes, Santiago, Chile (Roberto Castillo); 2nd Department of Internal Medicine, Cardiovascular Medicine General Teaching Hospital and 1st Faculty of Medicine, Charles University in Prague, Prague, Czech Republic (Vladimir Mikulenka); and Ospedale del Cuore Fondazione Toscana “G. Monasterio,” Massa, Italy (Marco Solinas).
Sources of Funding
None.
Disclosures
Roberto Lorusso is a consultant for Medtronic, Getinge, Abiomed, and LivaNova; and advisory board member of Eurosets, Hemocue, and Xenios (honoraria are paid as research funding). Dominik Wiedemann is a consultant/proctor for Abbott and scientific advisor for Xenios. Kollengode Ramanathan has received honorarium from Baxter and Fresenius for educational lectures not related to this topic. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S6
Figures S1–S5
References 37–47
This article was sent to Julie K. Freed, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
This work was presented in part at the EuroELSO Congress, April 26 to 29, 2023.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029609
For Sources of Funding and Disclosures, see page 15.
Contributor Information
Silvia Mariani, Email: s.mariani1985@gmail.com.
the PELS‐1 Investigators:
Justine Ravaux, Anne‐Kristin Schaefer, Luca Conci, Philipp Szalkiewicz, Jawad Khalil, Sven Lehmann, Jean‐Francois Obadia, Nikolaos Kalampokas, Erwan Flecher, Dinis Dos Reis Miranda, Kogulan Sriranjan, Michael A. Mazzeffi, Nazli Vedadi, Marco Di Eusanio, Vitaly Sorokin, Kollengode Ramanathan, Alessandro Costetti, Chistof Schmid, Roberto Castillo, Vladimir Mikulenka, and Marco Solinas
References
- 1. Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, Haft J, Vercaemst L, Pappalardo F, Bermudez C, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021;67:827–844. doi: 10.1097/MAT.0000000000001510 [DOI] [PubMed] [Google Scholar]
- 2. Lorusso R, Whitman G, Milojevic M, Raffa G, McMullan DM, Boeken U, Haft J, Bermudez C, Shah A, D'Alessandro DA. 2020 EACTS/ELSO/STS/AATS expert consensus on post‐cardiotomy extracorporeal life support in adult patients. J Thorac Cardiovasc Surg. 2021;161:1287–1331. doi: 10.1016/j.jtcvs.2020.09.045 [DOI] [PubMed] [Google Scholar]
- 3. Lorusso R, Raffa GM, Alenizy K, Sluijpers N, Makhoul M, Brodie D, McMullan M, Wang IW, Meani P, MacLaren G, et al. Structured review of post‐cardiotomy extracorporeal membrane oxygenation: part 1‐adult patients. J Heart Lung Transplant. 2019;38:1125–1143. doi: 10.1016/j.healun.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meani P, Matteucci M, Jiritano F, Fina D, Panzeri F, Raffa GM, Kowalewski M, Morici N, Viola G, Sacco A, et al. Long‐term survival and major outcomes in post‐cardiotomy extracorporeal membrane oxygenation for adult patients in cardiogenic shock. Ann Cardiothorac Surg. 2019;8:116–122. doi: 10.21037/acs.2018.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallabhajosyula S, Arora S, Sakhuja A, Lahewala S, Kumar V, Shantha GPS, Egbe AC, Stulak JM, Gersh BJ, Gulati R, et al. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol. 2019;123:489–497. doi: 10.1016/j.amjcard.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 6. McCarthy FH, McDermott KM, Kini V, Gutsche JT, Wald JW, Xie D, Szeto WY, Bermudez CA, Atluri P, Acker MA, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002‐2012. Semin Thorac Cardiovasc Surg. 2015;27:81–88. doi: 10.1053/j.semtcvs.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kowalewski M, Zielinski K, Brodie D, MacLaren G, Whitman G, Raffa GM, Boeken U, Shekar K, Chen YS, Bermudez C, et al. Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock‐analysis of the extracorporeal life support organization registry. Crit Care Med. 2021;49:1107–1117. doi: 10.1097/CCM.0000000000004922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biancari F, Dalen M, Fiore A, Ruggieri VG, Saeed D, Jonsson K, Gatti G, Zipfel S, Perrotti A, Bounader K, et al. Multicenter study on postcardiotomy venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2020;159:1844–1854.e6. doi: 10.1016/j.jtcvs.2019.06.039 [DOI] [PubMed] [Google Scholar]
- 9. Kowalewski M, Raffa G, Zielinski K, Meani P, Alanazi M, Gilbers M, Heuts S, Natour E, Bidar E, Schreurs R, et al. Baseline surgical status and short‐term mortality after extracorporeal membrane oxygenation for post‐cardiotomy shock: a meta‐analysis. Perfusion. 2020;35:246–254. doi: 10.1177/0267659119865122 [DOI] [PubMed] [Google Scholar]
- 10. Biancari F, Perrotti A, Ruggieri VG, Mariscalco G, Dalen M, Dell'Aquila AM, Jonsson K, Ragnarsson S, Di Perna D, Bounader K, et al. Five‐year survival after post‐cardiotomy veno‐arterial extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2021;10:595–601. doi: 10.1093/ehjacc/zuaa039 [DOI] [PubMed] [Google Scholar]
- 11. Schaefer AK, Riebandt J, Bernardi MH, Distelmaier K, Goliasch G, Zimpfer D, Laufer G, Wiedemann D. Fate of patients weaned from post‐cardiotomy extracorporeal life support. Eur J Cardiothorac Surg. 2022;61:1178–1185. doi: 10.1093/ejcts/ezac035 [DOI] [PubMed] [Google Scholar]
- 12. Balan TA, Putter H. A tutorial on frailty models. Stat Methods Med Res. 2020;29:3424–3454. doi: 10.1177/0962280220921889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 14. Unosawa S, Sezai A, Hata M, Nakata K, Yoshitake I, Wakui S, Kimura H, Takahashi K, Hata H, Shiono M. Long‐term outcomes of patients undergoing extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. Surg Today. 2013;43:264–270. doi: 10.1007/s00595-012-0322-6 [DOI] [PubMed] [Google Scholar]
- 15. van Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 16. Raffa GM, Kowalewski M, Brodie D, Ogino M, Whitman G, Meani P, Pilato M, Arcadipane A, Delnoij T, Natour E, et al. Meta‐analysis of peripheral or central extracorporeal membrane oxygenation in postcardiotomy and non‐postcardiotomy shock. Ann Thorac Surg. 2019;107:311–321. doi: 10.1016/j.athoracsur.2018.05.063 [DOI] [PubMed] [Google Scholar]
- 17. Charlesworth M, Garcia M, Head L, Barker JM, Ashworth AD, Barnard JB, Feddy L, Venkateswaran RV. Venoarterial extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock‐a six‐year service evaluation. Artif Organs. 2020;44:709–716. doi: 10.1111/aor.13647 [DOI] [PubMed] [Google Scholar]
- 18. Brewer JM, Tran A, Yu J, Ali MI, Poulos CM, Gates J, Gluck J, Underhill D. ECMO after cardiac surgery: a single center study on survival and optimizing outcomes. J Cardiothorac Surg. 2021;16:264. doi: 10.1186/s13019-021-01638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen F, Wang L, Shao J, Wang H, Hou X, Jia M. Survival following venoarterial extracorporeal membrane oxygenation in postcardiotomy cardiogenic shock adults. Perfusion. 2020;35:747–755. doi: 10.1177/0267659120931306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu RTC, Broad JD, Osawa EA, Ancona P, Iguchi Y, Miles LF, Bellomo R. 30‐day outcomes post veno‐arterial extra corporeal membrane oxygenation (VA‐ECMO) after cardiac surgery and predictors of survival. Heart Lung Circ. 2020;29:1217–1225. doi: 10.1016/j.hlc.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 21. Biancari F, Perrotti A, Dalen M, Guerrieri M, Fiore A, Reichart D, Dell'Aquila AM, Gatti G, Ala‐Kokko T, Kinnunen EM, et al. Meta‐analysis of the outcome after postcardiotomy venoarterial extracorporeal membrane oxygenation in adult patients. J Cardiothorac Vasc Anesth. 2018;32:1175–1182. doi: 10.1053/j.jvca.2017.08.048 [DOI] [PubMed] [Google Scholar]
- 22. Levy D, Laghlam D, Estagnasie P, Brusset A, Squara P, Nguyen LS. Post‐operative right ventricular failure after cardiac surgery: a cohort study. Front Cardiovasc Med. 2021;8:667328. doi: 10.3389/fcvm.2021.667328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mariscalco G, Salsano A, Fiore A, Dalen M, Ruggieri VG, Saeed D, Jonsson K, Gatti G, Zipfel S, Dell'Aquila AM, et al. Peripheral versus central extracorporeal membrane oxygenation for postcardiotomy shock: multicenter registry, systematic review, and meta‐analysis. J Thorac Cardiovasc Surg. 2020;160:1207–1216. doi: 10.1016/j.jtcvs.2019.10.078 [DOI] [PubMed] [Google Scholar]
- 24. Mariscalco G, El‐Dean Z, Yusuff H, Fux T, Dell'Aquila AM, Jonsson K, Ragnarsson S, Fiore A, Dalen M, di Perna D, et al. Duration of venoarterial extracorporeal membrane oxygenation and mortality in postcardiotomy cardiogenic shock. J Cardiothorac Vasc Anesth. 2020;35:2662–2668. doi: 10.1053/j.jvca.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 25. Fux T, Holm M, Corbascio M, Lund LH, van der Linden J. Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock: risk factors for mortality. J Thorac Cardiovasc Surg. 2018;156:1894–1902. doi: 10.1016/j.jtcvs.2018.05.061 [DOI] [PubMed] [Google Scholar]
- 26. Biancari F, Fiore A, Jonsson K, Gatti G, Zipfel S, Ruggieri VG, Perrotti A, Bounader K, Loforte A, Lechiancole A, et al. Prognostic significance of arterial lactate levels at weaning from postcardiotomy venoarterial extracorporeal membrane oxygenation. J Clin Med. 2019;8:2218. doi: 10.3390/jcm8122218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar TK, Zurakowski D, Dalton H, Talwar S, Allard‐Picou A, Duebener LF, Sinha P, Moulick A. Extracorporeal membrane oxygenation in postcardiotomy patients: factors influencing outcome. J Thorac Cardiovasc Surg. 2010;140:330–336. doi: 10.1016/j.jtcvs.2010.02.034 [DOI] [PubMed] [Google Scholar]
- 28. Li CL, Wang H, Jia M, Ma N, Meng X, Hou XT. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. 2015;149:1445–1450. doi: 10.1016/j.jtcvs.2014.11.052 [DOI] [PubMed] [Google Scholar]
- 29. Mashiko Y, Abe T, Tokuda Y, Oshima H, Usui A. Extracorporeal membrane oxygenation support for postcardiotomy cardiogenic shock in adult patients: predictors of in‐hospital mortality and failure to be weaned from extracorporeal membrane oxygenation. J Artif Organs. 2020;23:225–232. doi: 10.1007/s10047-020-01160-5 [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Yang F, Wang X, Xie H, Fan E, Ogino M, Brodie D, Wang H, Hou X. Predicting mortality in patients undergoing VA‐ECMO after coronary artery bypass grafting: the REMEMBER score. Crit Care. 2019;23:11. doi: 10.1186/s13054-019-2307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fux T, Holm M, van der Linden J. Arterial lactate before initiation of venoarterial extracorporeal membrane oxygenation for postcardiotomy shock improves postimplant outcome prediction. J Thorac Cardiovasc Surg. 2019;157:e266–e267. doi: 10.1016/j.jtcvs.2018.12.046 [DOI] [PubMed] [Google Scholar]
- 32. Biancari F, Dell'Aquila AM, Mariscalco G. Predicting mortality after postcardiotomy venoarterial extracorporeal membrane oxygenation. Ann Transl Med. 2019;7:S100. doi: 10.21037/atm.2019.04.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biancari F, Dalén M, Fiore A, Dell'Aquila AM, Jónsson K, Ragnarsson S, Gatti G, Gabrielli M, Zipfel S, Ruggieri VG, et al. Gender and the outcome of postcardiotomy veno‐arterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2021;36:1678–1685. doi: 10.1053/j.jvca.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 34. Mesotten D, Meijs DAM, van Bussel BCT, Stessel B, Mehagnoul‐Schipper J, Hana A, Scheeren CIE, Strauch U, van de Poll MCG, Ghossein‐Doha C, et al. Differences and similarities among COVID‐19 patients treated in seven ICUs in three countries within one region: an observational cohort study. Crit Care Med. 2022;50:595–606. doi: 10.1097/CCM.0000000000005314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen SW, Tsai FC, Lin YS, Chang CH, Chen DY, Chou AH, Chen TH. Long‐term outcomes of extracorporeal membrane oxygenation support for postcardiotomy shock. J Thorac Cardiovasc Surg. 2017;154:469–477. doi: 10.1016/j.jtcvs.2017.02.055 [DOI] [PubMed] [Google Scholar]
- 36. Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 38. Adult tobacco use information . Centers for Disease Control and Prevention. Accessed May 23, 2023. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm
- 39. Global Initiative for Chronic Obstructive Lung Disease . 2023. Accessed May 23, 2023. https://goldcopd.org/
- 40. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–745. doi: 10.1093/ejcts/ezs043 [DOI] [PubMed] [Google Scholar]
- 41. Bousquet J, Mantzouranis E, Cruz AA, Ait‐Khaled N, Baena‐Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 42. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 43. Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo‐Leiro MG, Guazzi M, Harjola VP, Heymans S, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:16–37. doi: 10.1002/ejhf.1029 [DOI] [PubMed] [Google Scholar]
- 44. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, Anker SD, Atherton J, Bohm M, Butler J, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 45. Singh SSA, Dalzell JR, Berry C, Al‐Attar N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail Rev. 2019;24:805–820. doi: 10.1007/s10741-019-09794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. [DOI] [PubMed] [Google Scholar]
- 47. Shanmugam G. Vasoplegic syndrome–the role of methylene blue. Eur J Cardiothorac Surg. 2005;28:705–710. doi: 10.1016/j.ejcts.2005.07.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6
Figures S1–S5
References 37–47


