Abstract
Background
Splanchnic nerve modulation (SNM) is an emerging procedure to reduce cardiac filling pressures in heart failure. Although the main contributor to reduction in cardiac preload is thought to be increased venous capacitance in the splanchnic circulation, supporting evidence is limited. We examined changes in venous capacitance surrogates pre‐ and post‐SNM.
Methods and Results
This is a prespecified analysis of a prospective, open‐label, single‐arm interventional study evaluating the effects of percutaneous SNM with ropivacaine in chronic heart failure with elevated filling pressures at rest and with exercise. Patients underwent cardiopulmonary exercise testing with invasive hemodynamic assessment pre‐ and post‐SNM. Blood pressure changes with modified Valsalva maneuver and hemoconcentration, pre‐ and post‐SNM were compared using a repeated measures model. Inferior vena cava diameter and collapsibility (>50% decrease in size with inspiration), and presence of bendopnea pre‐ and post‐SNM were also compared. Fifteen patients undergoing SNM (age 58 years, 47% women, 93% with left ventricular ejection fraction ≤35%) were included. After SNM, changes in systolic blood pressure during Valsalva (peak‐to‐trough) were greater (41 versus 48 mm Hg, P=0.025). Exercise‐induced hemoconcentration was unchanged (0.63 versus 0.43 g/dL, P=0.115). Inferior vena cava diameter was reduced (1.59 versus 1.30 cm, P=0.034) with higher collapsibility (33% versus 73%, P=0.014). Bendopnea was less (47% versus 13%, P=0.025).
Conclusions
SNM resulted in increased venous capacitance, associated decreased cardiac preload, and decreased bendopnea. Minimally invasive measures of venous capacitance could serve as markers of successful SNM. Long‐term effects of SNM on venous capacitance warrant further investigation for heart failure management.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03453151.
Keywords: heart failure, splanchnic nerve modulation, sympathetic nervous system, venous capacitance
Subject Categories: Autonomic Nervous System, Hemodynamics, Pathophysiology
Nonstandard Abbreviations and Acronyms
- IVC
inferior vena cava
- PCWP
pulmonary capillary wedge pressure
- SBV
stressed blood volume
- SNM
splanchnic nerve modulation
Clinical Perspective.
What Is New?
Splanchnic nerve modulation resulted in increased venous capacitance as demonstrated by changes in the proposed surrogates: peak‐to‐trough blood pressure changes during the Valsalva maneuver, inferior vena cava diameter and its collapsibility, self‐reported bendopnea, and changes in hemoglobin level (hemoconcentration) after exercise.
We observed lack of strong correlation between changes in venous capacitance surrogates and filling pressures both pre‐ and postsplanchnic nerve modulation.
What Are the Clinical Implications?
The proposed venous capacitance surrogates may be used to identify potential candidates for splanchnic nerve modulation therapy and as markers of successful interventions targeting venous capacitance.
Venous capacitance surrogates might be helpful in managing patients with heart failure, yet further investigation is warranted.
The venous system contains ≈70% of the total blood volume. 1 Its greater compliance compared with the arterial system allows the venous circulation to function as both a conduit and a reservoir for intravascular blood volume. Veins are heavily innervated by adrenergic receptors of the sympathetic autonomic nervous system, 2 , 3 which enables the venous system to respond promptly to stimuli through changes in vascular capacitance and intravascular fluid redistribution. 4 By doing so, venous circulation plays an important role in controlling central intravascular volume and cardiac preload. The splanchnic venous compartment, which consists of veins of the abdominal visceral organs, differs from other venous vascular beds. They are more compliant and contain a much larger volume of blood in comparison to the organ volume (up to 30% of total blood volume). 5 , 6 , 7 This reservoir of venous blood is also referred to as unstressed blood volume. With sympathoadrenal stimulation, up to 800 mL of blood (unstressed blood volume) can mobilize from the splanchnic blood pool to the systemic circulation (ie, autotransfusion) within seconds, producing a rapid increase in cardiac preload. 8 This increase in venous return or stressed blood volume (SBV) generally will increase cardiac output. However, in patients with heart failure (HF), fluid redistribution from splanchnic circulation could worsen central vascular congestion and lead to HF decompensation. 9 , 10
Sympathetic nervous system–mediated changes in splanchnic venous capacitance and resultant fluid redistribution has been recognized as a potential therapeutic target in HF. Venous capacitance is reduced in HF due to an increased sympathetic tone. 11 , 12 , 13 , 14 Greater splanchnic nerve modulation is an emerging concept in the management of HF. The safety and efficacy of temporary splanchnic nerve modulation (SNM) were investigated in 2 proof‐of‐concept studies in patients with decompensated HF (Splanchnic HF‐1) 15 , 16 and chronic HF (Splanchnic HF‐2). 17 Temporary SNM lowered resting filling pressures and improved cardiac output in patients hospitalized for acute decompensated HF. In ambulatory HF, SNM reduced filling pressure at rest and during peak exercise with associated improvement in cardiac output. The observed hemodynamic changes were associated with a decrease in estimated stressed blood volume and an increase in estimated unstressed blood volume. 18 The observed hemodynamic changes post‐SNM are believed to be due to an increase in splanchnic venous capacitance, yet mechanistic evidence supporting this hypothesis is limited. We sought to investigate several noninvasive clinical surrogate measures of splanchnic venous capacitance at rest and with exercise, before and after SNM. The proposed surrogate metrics of (splanchnic) venous capacitance are as follows: blood pressure (BP) changes during Valsalva maneuver, changes in inferior vena cava (IVC) diameter, hemoconcentration during exercise, and self‐reported bendopnea.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Study Design and Population
This is a prespecified analysis of the Splanchnic HF‐2 trial (ClinicalTrials.gov NCT03453151). Splanchnic HF‐2 is a prospective, open‐label, single‐arm study evaluating the effects of SNM in ambulatory HF. Eligible patients were enrolled at Duke University Medical Center (Durham, NC) from May 2018 to June 2019. Inclusion criteria were patients with HF who had been on guideline‐directed medical therapy with persistent New York Heart Association class II/III symptoms. Patients were also required to have a resting mean pulmonary capillary wedge pressure (PCWP) ≥15 mm Hg or ≥25 mm Hg at peak exercise. Patients with chronic kidney disease stage 5 and those with increased risk of bleeding (ie, known coagulopathies, and those on oral anticoagulants or oral antiplatelet agents other than aspirin) were excluded. Qualified patients underwent a fluoroscopic‐guided percutaneous SNM at the T12/L1 level with ropivacaine. The procedure was performed bilaterally in the first 5 cases and unilaterally in the subsequent 10 cases due to symptomatic orthostatic hypotension with bilateral SNM. The expected duration of sympatholytic effects was up to 24 hours. Invasive hemodynamics were measured with patients in the supine position at rest and during exercise before and after SNM. Following resting hemodynamics, patients underwent supine cycle ergometry testing at a fixed workload of 20 W during a warm‐up phase until patients reached a steady state of expired VO2 or up to 7 minutes. After reaching steady state, patients continued to exercise, with a stepwise increase in workload of 20 W every minute until exhaustion. Hemodynamic assessment during exercise was performed at 20 W and at peak exercise. Cardiac output was assessed using the direct Fick method. Patients repeated the exercise protocol after SNM (after a 30‐minute wait period) with a recovery time of 1 to 1.5 hours between exercise studies (Figure S1). Hemodynamic tracings were reviewed in a blinded fashion. Full details on exercise protocol and hemodynamic testing can be found in the original publication. 17 The study protocols were approved by the Duke University institutional review board, and all patients provided written informed consent.
Measurements of Venous Capacitance Surrogates
In addition to invasive hemodynamics, patients underwent modified Valsalva maneuver testing at rest before and after SNM. 19 Patients used a mouthpiece with an aperture at the tip of the tube to permit the escape of a small amount of air. As changes in hemodynamics during the Valsalva maneuver greatly depend on patient's effort, patients were trained to perform the modified Valsalva maneuver before the actual test at each time point. After 1 test run and being observed at baseline to ensure mean arterial BP fluctuations to be <5 mm Hg, patients blew into the tube for 10 seconds, after which patients resumed normal breathing. Arterial BP changes were observed for the subsequent 30 seconds. Changes in arterial BP from peak (Phase I) to trough (early Phase II) were evaluated before and 1 hour after SNM while still in the catheterization laboratory.
IVC diameter was measured with ultrasound at baseline and after forceful inspiration. Changes in IVC diameters were subsequently assessed, and patients were considered to have IVC collapsibility if there was >50% decrease in IVC diameter with forceful inspiration. The IVC assessment (diameter and collapsibility) was repeated within 1 hour after SNM. Images were reviewed in a blinded fashion. To minimize the impact of prolonged fasting on IVC parameters, we calculated IVC diameter/right atrial pressure ratio, which implies IVC capacitance pre‐ and post‐SNM.
To assess for bendopnea, patients were seated and instructed to bend forward at the waist as if tying their shoes for at least 30 seconds. Patients were considered to have bendopnea if they reported shortness of breath while bending forward and breathing. The presence of bendopnea was reassessed after SNM. Patients were allowed to hydrate and eat before the assessment of bendopnea after SNM.
To assess for the degree of hemoconcentration following exercise, hemoglobin levels were measured at rest and during peak exercise to calculate changes in hemoglobin level. The measurements were reassessed after patients underwent SNM. Measurements occurred within 1.5 hours of each other.
Statistical Analysis
Continuous variables are presented using the mean and SD or median and range, as appropriate, based on the underlying distribution. Statistical comparisons between individual time points pre‐ and post‐SNM used the paired t test for continuous variables and the McNemar's test for categorical variables. BP (systolic, diastolic, and pulse pressure) with Valsalva maneuver before and after SNM were analyzed using a repeated measures model adjusted for fixed‐effects including baseline BP, test (baseline, peak or trough), time (pre‐ or post‐SNM), and all possible 2‐way interactions with a random intercept and compound‐symmetry variance structure. A similar model for hemoconcentration was also used. Model results are presented using the least‐squares (LS) mean estimate or difference with 95% CI. Post‐hoc analysis was conducted to assess the correlation between venous capacitance surrogates and changes in PCWP from resting to peak exercise pre‐ and post‐SNM using linear regression with the coefficient of determination presented (R 2). Statistical analyses were performed using SAS version 9.4 (SAS, Institute, Inc., Cary, NC). A P value <0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 15 patients were included in this analysis. The average age was 58±13 years; 7 (47%) were women, and 9 (60%) had ischemic cardiomyopathy (Table 1). Fourteen (93%) patients had left ventricular ejection fraction ≤35%. Mean NT‐proBNP (N‐terminal pro natriuretic peptide) was 2172 pmoL/L (range, 112–9319 pmoL/L). Baseline invasive hemodynamics (at rest, pre‐SNM) were the following: right atrial pressure 13.8±4.0 mm Hg, mean pulmonary artery pressure 40.5±12.3 mm Hg, and PCWP 28.3±7.6 mm Hg (Table 2). Average exercise time was 4:48±1:36 minutes pre‐SNM and 5:03±1:31 minutes post‐SNM. Cardiopulmonary exercise parameters pre‐ and post‐SNM are shown in Table 2.
Table 1.
Baseline Characteristics
| Characteristics | Values |
|---|---|
| Sex (female) | 7 (47) |
| Age, mean±SD, y | 58±13 |
| BMI, mean (range), kg/m2 | 32 (22–56) |
| Ischemic cardiomyopathy | 9 (60) |
| Comorbidities | |
| Hypertension | 5 (33) |
| Diabetes | 4 (27) |
| Atrial fibrillation | 8 (53) |
| LVEF | |
| ≤35% | 14 (93) |
| >35% | 1 (8) |
| Laboratory profiles | |
| Creatinine, mean (range), mg/dL | 1.2 (0.7–1.9) |
| BUN, mean (range), mg/dL | 32 (22–56) |
| NT‐proBNP, mean (range), pmol/L | 2172 (112–9319) |
| Guideline‐directed medical therapy | |
| Beta‐blockers | 15 (100) |
| ACE‐I/ARB | 11 (73) |
| Mineralocorticoid receptor antagonists | 10 (67) |
| Implantable cardioverter‐defibrillator | 15 (100) |
ACE‐I indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin‐receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; LVEF, left ventricular ejection fraction; and NT‐proBNP, N‐terminal pro natriuretic peptide.
Table 2.
Invasive Hemodynamics and Cardiopulmonary Exercise Functional Parameters
| Baseline | P value | Peak exercise | P value | |||
|---|---|---|---|---|---|---|
| Pre‐SNM | Post‐SNM | Pre‐SNM | Post‐SNM | |||
| Hemodynamics | ||||||
| MAP, mm Hg | 96.7±13.7 | 86.9±17.0 | 0.011 | 106.6±24.3 | 94±22.8 | 0.002 |
| RAP, mm Hg | 13.8±4.0 | 8.4±4.1 | <0.001 | 23.2±6.2 | 15.4±7.8 | <0.001 |
| mPAP, mm Hg | 40.5±12.3 | 32.7±13.8 | <0.001 | 54.1±14.4 | 45.8±17.7 | <0.001 |
| PCWP, mm Hg | 28.3±7.6 | 20.3±9.5 | <0.001 | 34.8±10.0 | 25.1±10.7 | <0.001 |
| SVR, dynes/s per cm−5 | 1947±825 | 1523±602 | <0.001 | 1189±631 | 895±312 | 0.108 |
| CI, L/min per m2 | 1.9±0.6 | 2.3±0.7 | 0.077 | 3.4±1.2 | 3.8±1.1 | 0.069 |
| CPET parameters | ||||||
| Workload, W | 33±24 | 50±30 | 0.019 | |||
| Exercise time, min | 4:48±1:36 | 5:03±1:31 | 0.181 | |||
| Peak VO2, mL/kg per min | 9.1±2.5 | 9.8±2.7 | 0.053 | |||
| VE/VCO2 slope, % | 37.1±7.6 | 35.1±6.0 | 0.067 | |||
| RER | 1.14±0.13 | 1.08±0.11 | 0.081 | |||
Data are presented as mean±SD. CI indicates cardiac index; CPET, cardiopulmonary exercise testing; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; RER, respiratory exchange ratio; SNM, splanchnic nerve modulation; and SVR, systemic vascular resistance.
Blood Pressure Changes During Valsalva Maneuver
The modified Valsalva maneuver was performed in 11 patients (Table 3). At rest, the mean heart rate was elevated after SNM (pre‐SNM 75±17 bpm and post‐SNM 79±17 bpm, P=0.042) but no significant changes in resting arterial BP were seen (pre‐SNM systolic blood pressure [SBP] 132±17 mm Hg and post‐SNM SBP 128±16 mm Hg, P=0.201). There was no difference in both SBP and diastolic blood pressure in Phase I. In the early Phase II, the SBP dropped to a lower value post‐SNM (98±27 mm Hg) as compared with pre‐SNM (112±18 mm Hg, P=0.006). No difference between pre‐ and post‐SNM diastolic blood pressure were seen in the early Phase II (68±18 mm Hg versus 62±16 mm Hg, P=0.057). Changes from peak to trough SBP during the Valsalva maneuver were greater post‐SNM (48±17 mm Hg) compared with pre‐SNM (41±19 mm Hg, LS mean difference: 7.87 [95% CI, 1.08–14.65], P=0.025) (Figure S2 and S3). There was no difference in changes from peak to trough diastolic blood pressure during the Valsalva maneuver post‐SNM (27±16 mm Hg) compared with pre‐SNM (28±22 mm Hg, LS‐mean difference: −0.33 [95% CI −11.90, 11.24], P=0.95). Pulse pressure, defined as difference between SBP and diastolic blood pressure, was unchanged from peak to trough during the Valsalva maneuver post‐SNM (19±21 mm Hg) compared with pre‐SNM (12±14 mm Hg, LS mean difference: 6.54 [95% CI, −2.88, 15.96], P=0.162).
Table 3.
Changes in Surrogates of Venous Capacitance
| Pre‐SNM | Post‐SNM | LS‐mean difference (95% CI) | P value | |
|---|---|---|---|---|
| BP changes during Valsalva maneuver | ||||
| At rest | ||||
| HR, bpm | 75±17 | 79±17 | … | 0.042 |
| SBP, mm Hg | 132±17 | 128±16 | … | 0.201 |
| DBP, mm Hg | 77±14 | 72±12 | … | 0.06 |
| PP, mm Hg | 56±14 | 53±12 | … | 0.487 |
| Phase I | ||||
| SBP, mm Hg | 153±23 | 145±20 | … | 0.079 |
| DBP, mm Hg | 95±21 | 89±22 | … | 0.104 |
| PP, mm Hg | 56±15 | 58±15 | … | 0.441 |
| Phase II (early) | ||||
| SBP, mm Hg | 112±18 | 98±27 | … | 0.006 |
| DBP, mm Hg | 68±18 | 62±16 | … | 0.057 |
| PP, mm Hg | 44±9 | 39±15 | … | 0.179 |
| Changes from Phase I (peak) to early II (trough) | ||||
| SBP, mm Hg | 41±19 | 48±17 | 7.87 (1.08 to 14.65) | 0.025* |
| DBP, mm Hg | 28±22 | 27±16 | −0.33 (−11.90 to 11.24) | 0.953* |
| PP, mm Hg | 12±14 | 19±21 | 6.54 (−2.88 to 15.96) | 0.162* |
| IVC changes | ||||
| IVC diameter (cm) at rest | 1.6±0.6 | 1.3±0.6 | … | 0.034 |
| IVC collapsibility (decrease >50% with inspiration) | 5 (33%) | 11 (73%) | … | 0.014 |
| IVC diameter/RAP at rest | 0.12±0.04 | 0.17±0.06 | … | 0.007 |
| Self‐reported bendopnea | 7 (47%) | 2 (13%) | … | 0.025 |
| Hemoconcentration | ||||
| Hemoglobin at rest, g/dL | 12.9±1.5 | 12.8±1.4 | … | 0.250 |
| Hemoglobin at peak exercise, g/dL | 13.5±1.7 | 13.2±1.6 | … | 0.007 |
| Hemoglobin changes after exercise, g/dL | 0.6±0.4 | 0.4±0.4 | −0.19 (−0.42 to 0.05) | 0.115* |
Data are presented as mean±SD. BP indicates blood pressure; bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; IVC, inferior vena cava; LS‐mean, least‐squares mean; PP, pulse pressure; RAP, right atrial pressure; SBP, systolic blood pressure; and SNM, splanchnic nerve modulation.
P values calculated from repeated measures models.
Inferior Vena Cava Changes and Self‐Reported Bendopnea
Ultrasound‐measured IVC diameter at rest was smaller post‐SNM (1.6±0.6 versus 1.3±0.6 cm, P=0.016). Incidence of IVC collapsibility was higher post‐SNM (33% versus 73%, P=0.034). The IVC diameter/right atrial pressure ratio was higher post‐SNM (0.12±0.04 versus 0.17±0.06. P=0.014). Self‐reported bendopnea rate decreased from 47% to 13% post‐SNM (P=0.025). No patient developed new bendopnea post‐SNM.
Hemoconcentration
There was no difference in hemoglobin level at rest pre‐SNM (12.9±1.5 g/dL) compared with post‐SNM (12.8±1.4 g/dL, P=0.250). Hemoglobin level was lower at peak exercise post‐SNM (13.5±1.7 versus 13.2±1.6 g/dL, P=0.007). Less hemoconcentration was found post‐SNM compared with pre‐SNM (0.6±0.4 versus 0.4±0.4 g/dL, LS mean difference: −0.19 [95% CI, −0.42, 0.05], P=0.115), but the difference was not statistically significant (Figure S4).
Intervention‐Specific Outcomes
Of 15 patients, first 5 patients underwent bilateral SNM, and the subsequent 10 patients underwent unilateral SNM. Valsalva maneuver was performed in only 1 patient in bilateral SNM. Therefore, the intervention‐specific analysis on BP changes during Valsalva maneuver was not performed. Baseline hemodynamics and changes in other venous capacitance surrogates were compared pre‐ and post‐SNM according to their interventions (Table S1).
Correlation Between Venous Capacitance Surrogates and Changes in Filling Pressures During Exercise
There was no correlation between changes in BP during Valsalva (from peak to trough) and changes in PCWP during exercise (from resting to peak exercise) both pre‐SNM (R 2=0.23, P=0.139) and post‐SNM (R 2=0.21, P=0.152) (Figure A and B). IVC diameter was correlated with changes in PCWP post‐SNM (R 2=0.28, P=0.044), but correlation pre‐SNM (R 2=0.26, P=0.053) was not found to be significant (Figure C and D). IVC collapsibility was correlated with changes in PCWP post‐SNM (R 2=0.48, P=0.004) but not pre‐SNM (R 2=0.20, P=0.093). Self‐reported bendopnea was not correlated with changes in PCWP both pre‐SNM (R 2=0.06, P=0.379) and post‐SNM (R 2=0.08, P=0.321). Hemoconcentration following exercise was not correlated with changes in PCWP both pre‐SNM (R 2=0.08, P=0.319) and post‐SNM (R 2<0.01, P=0.994) (Figure E and F).
Figure . Correlation between venous capacitance surrogates and changes in filling pressures during exercise pre‐ and post‐SNM.
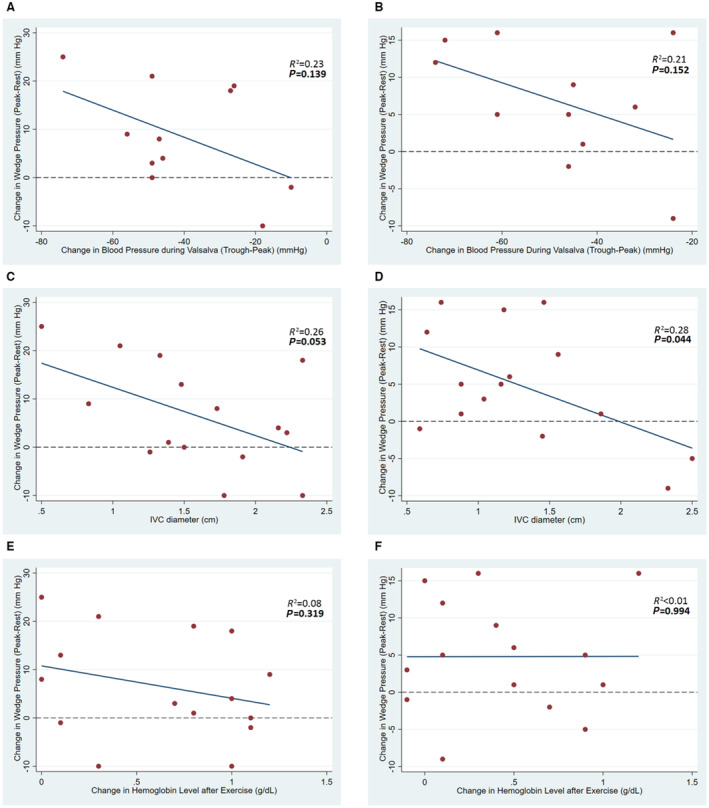
Changes in blood pressure (peak‐trough) during the Valsalva maneuver: A, Pre‐SNM, B, Post‐SNM. Inferior vena cava diameter: C, Pre‐SNM, D, Post‐SNM. Changes in hemoglobin level after exercise: E, Pre‐SNM, F, Post‐SNM. SNM indicates splanchnic nerve modulation.
Discussion
In this study, we evaluated changes in surrogate measures of venous capacitance following SNM. The primary observations from this study are as follows: (1) SNM resulted in more pronounced changes in SBP from peak to trough during Valsalva maneuver (41 versus 48 mm Hg). (2) Ultrasound‐measured IVC diameter was smaller post‐SNM (1.6 versus 1.3 cm) with higher incidence of IVC collapsibility. (3) Self‐reported bendopnea rate was lower post‐SNM (47% versus 13%). (4) Hemoglobin level at peak exercise was lower post‐SNM (13.5 versus 13.2 g/dL), but there was no difference in the degree of hemoconcentration after exercise (0.6 versus 0.4 g/dL).
Recent secondary analysis of SNM trials (Splanchnic HF‐1 and Splanchnic HF‐2) provides a mechanistic insight into physiological alteration following SNM in decompensated and ambulatory HF. SNM was shown to reduce estimated stressed blood volume up to 0.5 L, and the reduction in estimated stressed blood volume was maintained throughout exercise. 18 The feasibility of permanent surgical SNM in HF with preserved ejection fraction was also investigated (Surgical Resection of the Greater Splanchnic Nerve in Subjects Having Heart Failure With Preserved Ejection Fraction, ClinicalTrials.gov NCT0371554). Permanent SNM reduced filling pressure at rest and during exercise at 3 months, with associated improvement in functional status and quality of life. Decreased venous capacitance with a consequent increase in SBV has been proposed as 1 of the important mechanisms of cardiac filling pressure elevations leading to HF decompensation 6 , 11 and is believed to be the primary target of SNM in relieving congestion in the HF population. Our investigation demonstrating changes in extracardiac parameters as surrogates of splanchnic venous capacitance supports this hypothesis.
The Valsalva maneuver is a commonly used physiological tool to assess autonomic function but can also serve as a surrogate of central intravascular blood volume (or SBV), cardiac filling pressures, and venous capacitance. 20 , 21 , 22 , 23 During Phase I, intrathoracic pressure rises, which transmits to the periphery and consequently leads to increased arterial BP (ie, peak BP). As straining continues, venous return that serves as cardiac preload decreases and so does arterial BP. This corresponds to early Phase II, during which we observe lowest arterial BP (ie, trough BP). A drop in arterial BP (peak‐trough) is inversely related to central blood volume, where BP changes are more pronounced in patients with low central blood volume. Notably, post‐SNM, patients experienced a higher resting heart rate, lower SBP during early Phase II, and greater excursions in SBP from Phase I (peak) to early Phase II (trough) than pre‐SNM. This indicates lower central intravascular volume, with subsequent baroreflex activation (higher heart rate) after SNM, and supports our hypothesis that SNM leads to redistribution of blood into the splanchnic vascular compartment. In other words, the reduction in sympathetic tone to the splanchnic vascular compartment decreases cardiac preload and allows for greater stress‐(Valsalva) induced pressure swings. A similar observation was made after renal denervation, where the sole hemodynamic alteration postsympathetic denervation was a change in vascular compliance as measured by the Valsalva maneuver and not office or ambulatory BP. 24
IVC assessment with ultrasound is increasingly used to monitor volume status in the setting of shock or congestion. 25 IVC dimension, particularly IVC area, has been shown to correspond to changes in fluid status. 26 Degree of respirophasic changes in IVC size (ie, IVC collapsibility) has also been demonstrated to inversely correlate with central venous pressure, which is a surrogate for central intravascular volume. 27 We observed a smaller IVC diameter post‐SNM. Additionally, incidence of IVC collapsibility, defined as >50% decrease in IVC diameter with forceful inspiration, was higher post‐SNM. Both findings of IVC assessment support the effect of SNM in reducing central intravascular volume.
Bendopnea has been described as a sign of increased filling pressure in patients with advanced HF. 28 As patients lean forward, intraabdominal pressure increases and, as a consequence, fluid returns from intraabdominal (splanchnic) reservoir to the heart, which further increases cardiac filling pressure and precipitates symptoms in those whose filling pressure is already high. SNM increased splanchnic venous capacitance, and thus in theory blunted fluid shifts with forward bending, which explains the lower rate of bendopnea post‐SNM observed in this study.
Physiologically, exercise results in hemoconcentration (increase in hemoglobin during exercise), which can be observed in both healthy subjects and patients with HF. It was demonstrated that the degree of hemoconcentration appeared to be less in patients with HF (1.1±0.5 g/dL versus 0.6±0.4 g/dL). 29 Exercise‐induced hemoconcentration can be explained by 2 causes. First, with exercise, plasma volume shifts from the intravascular space (within blood vessel) to the extravascular compartment (interstitial space) as a result of exercise‐induced BP elevation and end‐organ perfusion. 30 Second, exercise activates the sympathetic nervous system, resulting in splanchnic vasoconstriction via the neuronal and hormonal pathway. The spleen is a highly blood‐rich organ, storing red blood cells at a higher hemoconcentration than the remainder of the circulating blood. 31 Sympathetic stimulation of the spleen leads to splenic vascular and capsular contraction and expulsion of highly concentrated blood (autotransfusion), which leads to systemic hemoconcentration. 32 Hypothetically, SNM, which decreases splanchnic sympathetic outflow, should result in a smaller increase in hemoglobin level during exercise and less hemoconcentration. In this study, we observed a lower hemoglobin level at peak exercise post‐SNM. Although the degree of hemoconcentration was less post‐SNM, statistical significance was not met. This finding was not entirely unforeseen because the SNM only attenuates the sympathetic stimulation component of exercise‐induced hemoconcentration, and this perhaps suggests that changes in hemoglobin level during exercise might not be an ideal surrogate of venous capacitance.
Overall, our findings using various indirect measures indicate a decrease in splanchnic venous capacitance as a key physiological alteration following SNM. We did a post‐hoc analysis to evaluate correlation between venous capacitance surrogates and changes in filling pressures during exercise. We did not expect to see a strong correlation between these variables as pressures do not always correlate with volumes or volume capacitances. 33 From our analyses, we only observed significant correlation between the IVC diameter and IVC collapsibility with pressures post‐SNM. Whether SNM renders a stronger pressure‐volume relationship is possible and needs to be further investigated.
We believe our study has important clinical implications. Because SNM selectively affects splanchnic vascular compliance (mostly venous system), we have validated a battery of minimally invasive surrogate measures pre‐ and post‐SNM that would allow us to assess vascular compliance. These measures could be used as additional markers of successful SNM or other interventions targeting venous capacitance. Considering the minimal correlation between venous capacitance and cardiac filling pressures as demonstrated in our study, it is of great importance to determine the role of venous capacitance in place of or in addition to commonly used filling pressures in clinical practice. Interval monitoring of these minimally invasive surrogates rather than invasively derived cardiac filling pressures might also be helpful in determining mid‐ or long‐term treatment response and help guide further intervention.
This study has several limitations. First, the study intervention was unblinded and subject to unmeasured bias. Despite our attempt to minimize the bias by using blinded review of hemodynamics, and ultrasound parameters, the end point of bendopnea was still susceptible to bias. Second, modified Valsalva maneuver was performed in 11 out of 15 patients. Although we did the modified Valsalva training on all patients before the actual test, the Valsalva pressures could still vary and lead to difference in BP independent of the intervention. Additionally, we modified the study intervention from bilateral to unilateral SNM after the first 5 cases, which resulted in only 1 patient in the bilateral SNM group having performed the Valsalva maneuver. This limits our ability to compare effects of the Valsalva maneuver between intervention groups. Nevertheless, the intervention‐specific analyses were derived from a much smaller sample size and subject to being underpowered. Third, the exercise protocol in this study is more strenuous than those for a general HF population, which might have led to shorter exercise time. Furthermore, the exercise was repeated on the same day post‐SNM. Changes in parameters post‐SNM suggestive of decreased central intravascular volume could have been partly due to prolonged fasting or unrecognized effects of exercise. To minimize the effect of fasting on the above, the measurements were repeated with a minimal delay in time following SNM, or as in the case of bendopnea, patients were allowed to hydrate/eat after the SNM in the recovery area, yet the reduction in bendopnea was nevertheless present. To further minimize this potential confounder, we used a repeated measures model that accounted for differences in baseline values when assessing changes during exercise. Fourth, the reported parameters were not direct measures of splanchnic venous capacitance, although physiologically correlated. They are inevitably affected by other factors such as changes in total body volume or body habitus/positions. Finally, besides the correlation between changes in venous capacitance surrogates and filling pressures, we were unable to assess the clinical significance of these observed changes due to the temporary nature of the intervention, which limits the assessment of mid‐ or long‐term clinical end points.
Conclusions
We highlight measures that could be used to test for acute or chronic changes in (splanchnic) venous capacitance and serve as (objective) surrogate metrics of technical/therapeutic success. The present study shows that SNM resulted in greater changes in peak‐to‐trough BP during the Valsalva maneuver, smaller ultrasound‐measured IVC diameter with higher incidence of collapsibility, and less bendopnea. These findings support that SNM led to increased venous capacitance with associated decrease in cardiac preload/SBV. Long‐term effects of SNM on venous capacitance warrant further investigation for the management of HF.
Sources of Funding
This study was supported by Translating Duke Health Award.
Disclosures
Dr Fudim was supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Mario Family Award, Duke Chair's Award, Translating Duke Health Award, Bayer, Bodyport, and BTG Specialty Pharmaceuticals. He receives consulting fees from Abbott, Audicor, AxonTherapies, Bodyguide, Bodyport, Boston Scientific, CVRx, Daxor, Edwards LifeSciences, Feldschuh Foundation, Fire1, Gradient, Intershunt, NXT Biomedical, Pharmacosmos, PreHealth, Shifamed, Splendo, Vironix, Viscardia, and Zoll. The remaining authors have no disclosures to report.
Supporting information
Data S1.
This manuscript was sent to Ferhaan Ahmad, MD, PhD, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028780
For Sources of Funding and Disclosures, see page 9.
References
- 1. Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–748. doi: 10.1097/ALN.0b013e3181672607 [DOI] [PubMed] [Google Scholar]
- 2. Birch DJ, Turmaine M, Boulos PB, Burnstock G. Sympathetic innervation of human mesenteric artery and vein. J Vasc Res. 2008;45:323–332. doi: 10.1159/000119095 [DOI] [PubMed] [Google Scholar]
- 3. Racchi H, Schliem AJ, Donoso MV, Rahmer A, Zúñiga A, Guzmán S, Rudolf K, Huidobro‐Toro JP. Neuropeptide Y Y1 receptors are involved in the vasoconstriction caused by human sympathetic nerve stimulation. Eur J Pharmacol. 1997;329:79–83. doi: 10.1016/s0014-2999(97)00160-x [DOI] [PubMed] [Google Scholar]
- 4. Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR, Sandler A. Raising the pressure: hemodynamic effects of splanchnic nerve stimulation. J Appl Physiol (1985). 2017;123:126–127. doi: 10.1152/japplphysiol.00069.2017 [DOI] [PubMed] [Google Scholar]
- 5. Hainsworth R. The importance of vascular capacitance in cardiovascular control. Physiology. 1990;5:250–254. doi: 10.1152/physiologyonline.1990.5.6.250 [DOI] [Google Scholar]
- 6. Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc. 2017;6:e006817. doi: 10.1161/JAHA.117.006817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenway CV, Lister GE. Capacitance effects and blood reservoir function in the splanchnic vascular bed during non‐hypotensive haemorrhage and blood volume expansion in anaesthetized cats. J Physiol. 1974;237:279–294. doi: 10.1113/jphysiol.1974.sp010482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelman S, Mushlin PS. Catecholamine‐induced changes in the splanchnic circulation affecting systemic hemodynamics. Anesthesiology. 2004;100:434–439. doi: 10.1097/00000542-200402000-00036 [DOI] [PubMed] [Google Scholar]
- 9. Burkhoff D, Tyberg JV. Why does pulmonary venous pressure rise after onset of LV dysfunction: a theoretical analysis. Am J Phys. 1993;265:H1819–H1828. doi: 10.1152/ajpheart.1993.265.5.H1819 [DOI] [PubMed] [Google Scholar]
- 10. Fudim M, Neuzil P, Malek F, Engelman ZJ, Reddy VY. Greater splanchnic nerve stimulation in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2021;77:1952–1953. doi: 10.1016/j.jacc.2021.02.048 [DOI] [PubMed] [Google Scholar]
- 11. Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4:669–675. doi: 10.1161/circheartfailure.111.961789 [DOI] [PubMed] [Google Scholar]
- 12. Gay R, Wool S, Paquin M, Goldman S. Total vascular pressure‐volume relationship in conscious rats with chronic heart failure. Am J Physiol. 1986;251:H483–H489. doi: 10.1152/ajpheart.1986.251.3.H483 [DOI] [PubMed] [Google Scholar]
- 13. Wang SY, Manyari DE, Scott‐Douglas N, Smiseth OA, Smith ER, Tyberg JV. Splanchnic venous pressure‐volume relation during experimental acute ischemic heart failure. Differential effects of hydralazine, enalaprilat, and nitroglycerin. Circulation. 1995;91:1205–1212. doi: 10.1161/01.cir.91.4.1205 [DOI] [PubMed] [Google Scholar]
- 14. Grassi G, D'Arrigo G, Pisano A, Bolignano D, Mallamaci F, Dell'Oro R, Quarti‐Trevano F, Seravalle G, Mancia G, Zoccali C. Sympathetic neural overdrive in congestive heart failure and its correlates: systematic reviews and meta‐analysis. J Hypertens. 2019;37:1746–1756. doi: 10.1097/hjh.0000000000002093 [DOI] [PubMed] [Google Scholar]
- 15. Fudim M, Ganesh A, Green C, Jones WS, Blazing MA, DeVore AD, Felker GM, Kiefer TL, Kong DF, Boortz‐Marx RL, et al. Splanchnic nerve block for decompensated chronic heart failure: splanchnic‐HF. Eur Heart J. 2018;39:4255–4256. doi: 10.1093/eurheartj/ehy682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fudim M, Jones WS, Boortz‐Marx RL, Ganesh A, Green CL, Hernandez AF, Patel MR. Splanchnic nerve block for acute heart failure. Circulation. 2018;138:951–953. doi: 10.1161/circulationaha.118.035260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fudim M, Boortz‐Marx RL, Ganesh A, DeVore AD, Patel CB, Rogers JG, Coburn A, Johnson I, Paul A, Coyne BJ, et al. Splanchnic nerve block for chronic heart failure. JACC Heart Fail. 2020;8:742–752. doi: 10.1016/j.jchf.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 18. Fudim M, Patel MR, Boortz‐Marx R, Borlaug BA, DeVore AD, Ganesh A, Green CL, Lopes RD, Mentz RJ, Patel CB, et al. Splanchnic nerve block mediated changes in stressed blood volume in heart failure. JACC Heart Fail. 2021;9:293–300. doi: 10.1016/j.jchf.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Appelboam A, Reuben A, Mann C, Gagg J, Ewings P, Barton A, Lobban T, Dayer M, Vickery J, Benger J. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015;386:1747–1753. doi: 10.1016/s0140-6736(15)61485-4 [DOI] [PubMed] [Google Scholar]
- 20. Smith ML, Beightol LA, Fritsch‐Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva's maneuver revisited: a quantitative method yielding insights into human autonomic control. Am J Phys. 1996;271:H1240–H1249. doi: 10.1152/ajpheart.1996.271.3.H1240 [DOI] [PubMed] [Google Scholar]
- 21. Korner PI, Tonkin AM, Uther JB. Reflex and mechanical circulatory effects of graded Valsalva maneuvers in normal man. J Appl Physiol. 1976;40:434–440. doi: 10.1152/jappl.1976.40.3.434 [DOI] [PubMed] [Google Scholar]
- 22. Nishimura RA, Tajik AJ. The Valsalva maneuver and response revisited. Mayo Clin Proc. 1986;61:211–217. doi: 10.1016/s0025-6196(12)61852-7 [DOI] [PubMed] [Google Scholar]
- 23. McIntyre KM, Vita JA, Lambrew CT, Freeman J, Loscalzo J. A noninvasive method of predicting pulmonary‐capillary wedge pressure. N Engl J Med. 1992;327:1715–1720. doi: 10.1056/nejm199212103272404 [DOI] [PubMed] [Google Scholar]
- 24. Saxena M, Shour T, Shah M, Wolff CB, Julu POO, Kapil V, Collier DJ, Ng FL, Gupta A, Balawon A, et al. Attenuation of splanchnic autotransfusion following noninvasive ultrasound renal denervation: a novel marker of procedural success. J Am Heart Assoc. 2018;7:7. doi: 10.1161/jaha.118.009151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaptein MJ, Kaptein EM. Inferior vena cava collapsibility index: clinical validation and application for assessment of relative intravascular volume. Adv Chronic Kidney Dis. 2021;28:218–226. doi: 10.1053/j.ackd.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 26. Ivey‐Miranda JB, Wetterling F, Gaul R, Sheridan S, Asher JL, Rao VS, Maulion C, Mahoney D, Mebazaa A, Gray AP, et al. Changes in inferior vena cava area represent a more sensitive metric than changes in filling pressures during experimental manipulation of intravascular volume and tone. Eur J Heart Fail. 2022;24:455–462. doi: 10.1002/ejhf.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ilyas A, Ishtiaq W, Assad S, Ghazanfar H, Mansoor S, Haris M, Qadeer A, Akhtar A. Correlation of IVC diameter and collapsibility index with central venous pressure in the assessment of intravascular volume in critically ill patients. Cureus. 2017;9:e1025. doi: 10.7759/cureus.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thibodeau JT, Turer AT, Gualano SK, Ayers CR, Velez‐Martinez M, Mishkin JD, Patel PC, Mammen PP, Markham DW, Levine BD, et al. Characterization of a novel symptom of advanced heart failure: bendopnea. JACC Heart Fail. 2014;2:24–31. doi: 10.1016/j.jchf.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 29. Agostoni P, Wasserman K, Guazzi M, Cattadori G, Palermo P, Marenzi G, Guazzi MD. Exercise‐induced hemoconcentration in heart failure due to dilated cardiomyopathy. Am J Cardiol. 1999;83:278–280. doi: 10.1016/s0002-9149(98)00839-x [DOI] [PubMed] [Google Scholar]
- 30. Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65:149–209. doi: 10.1152/physrev.1985.65.1.149 [DOI] [PubMed] [Google Scholar]
- 31. Crosby WH. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959;14:399–408. doi: 10.1182/blood.V14.4.399.399 [DOI] [PubMed] [Google Scholar]
- 32. Stewart IB, McKenzie DC. The human spleen during physiological stress. Sports Med (Auckland, NZ). 2002;32:361–369. doi: 10.2165/00007256-200232060-00002 [DOI] [PubMed] [Google Scholar]
- 33. Yaranov DM, Jefferies JL, Silver MA, Burkhoff D, Rao VN, Fudim M. Discordance of pressure and volume: potential implications for pressure‐guided remote monitoring in heart failure. J Card Fail. 2022;28:870–872. doi: 10.1016/j.cardfail.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


