Abstract
Pulmonary fibrosis is a sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection that currently lacks effective preventative or therapeutic measures. Post-viral lung fibrosis due to SARS-CoV-2 has been shown to be progressive on selected patients using imaging studies. Persistent infiltration of macrophages and monocytes, a main feature of SARS-CoV-2 pulmonary fibrosis, and long-lived circulating inflammatory monocytes might be driving factors promoting the profibrotic milieu in the lung. The upstream signal(s) that regulates the presence of these immune cells (despite complete viral clearance) remains to be explored. Current data indicate that much of the stimulating signals are localized in the lungs. However, an ongoing low-grade systemic inflammation in long Coronavirus Disease 2019 (COVID-19) symptoms suggests that certain non-pulmonary regulators such as epigenetic changes in hematopoietic stem cells might be critical to the chronic inflammatory response. Since nearly one-third of the world population have been infected, a timely understanding of the underlying pathogenesis leading to tissue remodeling is required. Herein, we review the potential pathogenic mechanisms driving lung fibrosis following SARS-CoV-2 infection based upon available studies and our preliminary findings (Graphical abstract).
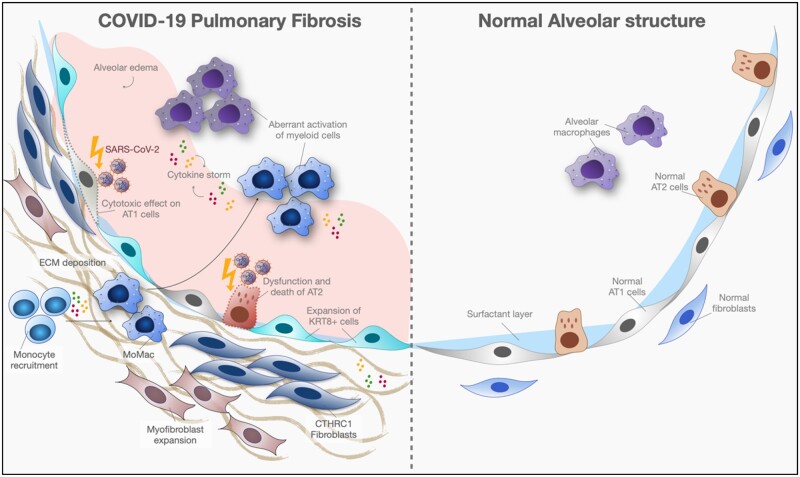
Graphical Abstract
Schematic illustration of cellular interaction in COVID-19 PF.
Introduction
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has affected hundreds of millions of people and caused over 6 million deaths to date worldwide. After acute infection, some patients develop postacute sequelae SARS-CoV-2 (PASC) symptoms that affect many organ systems, in which the long-term impact is creating an enormous burden to public health.1 With the lungs being the primary organ of infection, it is unsurprising that appreciable amount patients develop variable degrees of pulmonary fibrosis (PF) that can result in respiratory failure and death.2 For up to 12 months of follow-up, multiple studies reported the prevalence of 4.8–35%, notably higher among critically ill patients.3 Interestingly, emerging data suggest areas of overlap between idiopathic pulmonary fibrosis (IPF) and SARS-CoV-2 PF.4,5 In this review, we summarize evolving research addressing the underlying pathogenic mechanisms of PF in SARS-CoV-2 infection and discuss potential early cellular and molecular targets that might prevent this devastating pulmonary complication in COVID-19 survivors.
Lung pathology and spatial architectures of subacute and chronic phase of COVID-19
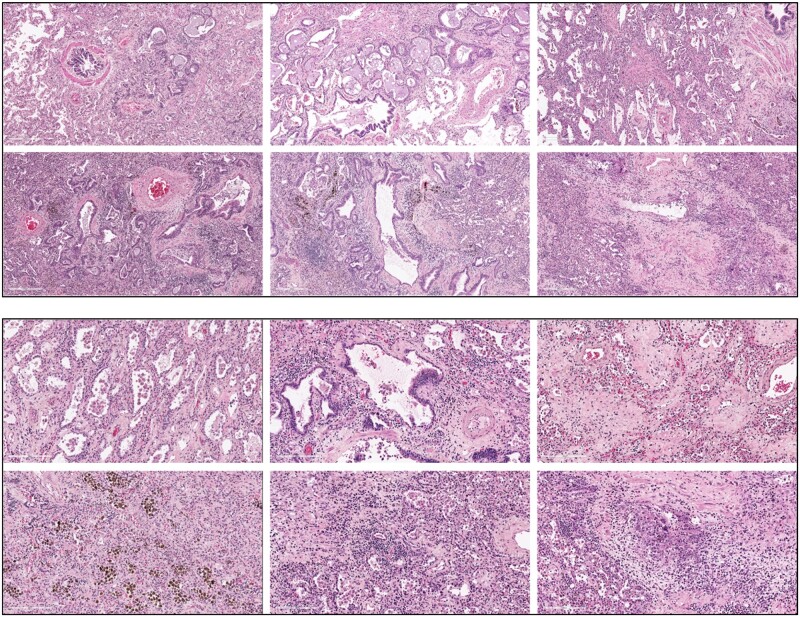
There is limited information about the pathological characteristics of lung fibrosis in SARS-CoV-2 infection. One study described usual interstitial pneumonia pattern of fibrosis, similar to that seen in IPF.6 In contrast, another reported lung explants from postacute COVID-19 PF patients demonstrated a non-specific interstitial pneumonia (NSIP) and other patterns of fibrosis that include traction bronchiectasis and bronchiectasis with evidence of ongoing injury and interstitial fibroblastic proliferation.7,8 Our institution performed 20 cases of lungs transplantation for post-COVID-19 PF with chronic respiratory failure, and interestingly, the key pathology findings of most cases were also NSIP (unpublished data, Figure 1). Specifically, a diffuse interstitial fibrosis with inflammatory cell infiltration and peribronchiolar metaplasia near fibrotic foci were the main features. Interstitial hemosiderin-laden macrophages were observed indicating prior lung hemorrhage (Figure 1, lower left panel). The classical histology description of NSIP conforms to inflammatory-driven fibroproliferative nature of SARS-CoV-2 PF.
Figure 1.
Diffuse interstitial fibrosis with extensive peribronchiolar metaplasia and inflammatory cell infiltration in COVID-19 lung fibrosis. Hematoxylin and eosin staining of six COVID-19 pulmonary fibrosis explanted lungs.
Widespread inflammatory cellular infiltration was one of the specific features in COVID-19 lung fibrosis that may uniquely drive fibroproliferative response in SARS-CoV-2 infection. Indeed, persistent macrophage infiltration and progressive expansion of mesenchymal fibroblasts surrounding the alveolar walls were more pronounced in SARS-CoV-2 than in H1N1 pneumonia and absent in bacterial infection.9 Lung progenitor failure was theorized as a profibrotic characteristic in COVID-19 PF. Specifically, the infected alveolar progenitor epithelial cells expressed higher apoptotic and inflammatory markers (cysteine-aspartic acid protease 3 (CASP3), phosphorylated signal transducer and activator of transcription 3 (pSTAT3) and interleukin-6 (IL-6)). They also lack of interaction with immune or mesenchyme cells suggesting an unresponsive or unstimulated state of epithelial cells to repair the damaged lungs.9 Lastly, single-cell transcriptomic profiling of SARS-CoV-2 explanted lungs identified fibrogenic pathways reminiscent to those found in IPF.2,5 Hence, it is conceivable that dysregulated lung repair in COVID-19 is a result of inflammatory-driven mesenchyme/fibroblast expansion and inhibited alveolar regeneration.
To this end, available data suggest that the SARS-CoV-2 PF histologically resembles NSIP, but signaling pathways are activated that overlap with those found in IPF. With a limited follow-up timeframe, COVID-19 PF was radiographically progressive. However, clinical characterization in a longer observational term remains to be determined. Nevertheless, studying the mechanisms that cause fibrosis in COVID-19 survivors may have broader implications and provide insight into other types of lung fibrosis such as IPF.
Potential mechanisms of COVID-19 PF
Comprehensive multiomic analysis of biological samples from convalescent COVID-19 patients with PF has provided valuable information regarding the underlying pathogenetic mechanisms of SARS-CoV-2 PF.2,9–13 Much of the literature focuses on fibrosis identified in lungs obtained from fatal COVID-19 cases. However, as lung transplantation is now an acceptable option for those with post-COVID PF,14 studies are increasingly using COVID-19 lung explants to understand the mechanisms driving PF after SARS-CoV-2 infection. A major challenge here is the lack of suitable animal models to validate the functions of those identifiable targets. Nonetheless, a mouse-adapted SARS-CoV-2 MA10 infection in aged mice has shown significant promise as a model of PASC lung fibrosis.15 Like other types of PF, multiple lung cells including lung epithelia, immune cells and lung fibroblasts, are implicated in SARS-CoV-2 PF. Functional studies of these cells in SARS-CoV-2 preclinical models are scarce. The chronic MA10 mouse infection indicated an early anti-viral EIDD-2801 (Emory Institute of Drug Design) plus an early anti-fibrotic agent (nintedanib) reduced clinical severity, lung damages and chronic pulmonary lesions, as well as decreased peak fibrotic disease, respectively.15 Here, we described current findings to support potential pathogenic mechanism of lung fibrosis associated with SARS-CoV-2 viral infection.
Cellular mechanisms
Monocytes and macrophages
A delayed or impaired type I interferon (IFN) response during acute SARS-CoV-2 infection appears to drive the aberrant activation of monocytes and macrophages, and together, these myeloid cells trigger a hyperinflammatory or ‘cytokine storm’ phenomenon.10,16,17 This cytokine storm leads to widespread diffuse alveolar damage and extensive lung destruction consequently triggering the fibroproliferation.9 Substantial expansion of both interstitial macrophage (CD14+CD16+CD206+CD163+CD123+ cells) and fibroblasts is one explanation for the higher Ashcroft fibrotic score in COVID-19 compared with influenza infection or bacterial pneumonia.9 Similarly, profibrotic monocyte-derived macrophages (MDM or MoMac) expand in the lungs after SARS-CoV-2 infection and were colocalized with mesenchymal cells and area of collagen deposition.2,5 The additional characterization of macrophages revealed the accumulation of CD163+ macrophages concomitant with collagen deposition.5 Moreover, six subtypes of monocyte/macrophage population with distinct transcriptomic profiles were identified in bronchoalveolar lavage (BAL) fluid (FCN1-monocytes expressing ficolin-M, alarmins (S100A8, S100A12), and inflammatory cytokines (IL1B, IL6, CXCL8, and CCR2); Mono/Macrophages (transitional state); CD163/LGMN-macrophages; AM1; AM2; and proliferating AM, during early and late COVID-19 ARDS in response to tissue injuries. In lung tissues, similar monocyte/macrophage populations were colocalized with mesenchymal cells and area of collagen deposition during late phase of ARDS indicating their putative profibrotic role.5 Furthermore, an in vitro functional study showed that SARS-CoV-2 stimulated profibrotic transcriptional and proteomic changes in human peripheral blood mononuclear cells (PBMC). These profibrotic MoMacs expressing SPP1, ILRN, MMP9, CHI3L1 and PLA2G7 were abundant in explanted COVID-19 lungs.2
Together with infiltrated MoMac in the lungs, excessive circulating monocytes likely fuel an inflammatory state and drive fibroblast proliferation.18 Thus, it is plausible that the persistent infiltration of profibrotic MoMacs promote chronic inflammatory state in damaged lung tissues leading to progressive lung architectural destruction and dysregulation of lung remodeling.
Aberrant lung epithelial cells
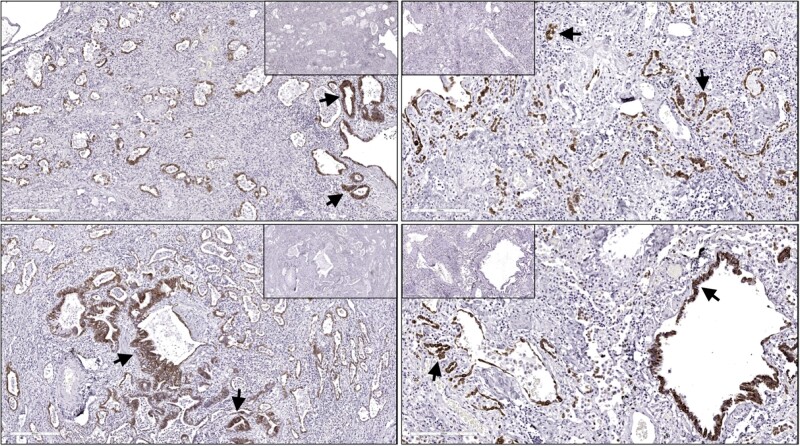
Like IPF, the loss of alveolar type 2 (AT2) cells is one of common characteristics in SARS-CoV-2 fibrotic lungs. Moreover, the remaining AT2 cells express pathologic profibrotic signature molecules including pSTAT3 and the receptor tyrosine kinase and proto-oncogene KIT, and contained high levels of signaling proteins such as IL-6, arginase 1 and apoptotic marker CASP3.9 However, the intercellular interaction between these aberrant AT2 cells and other profibrotic cell types was found to be minimal.9 The other striking feature similar to IPF lungs is an accumulation transitional alveolar epithelial cells that expressed canonical markers (KRT8/CLDN4/CDKN1A), and non-canonical markers (KRT5/TP63/KRT17)9,13 in response to alveolar damages. These findings were observed in our explanted COVID-19 lungs (Figure 2). A cell fate determination of these transitional cell is ongoing, although most believe that they could represent the inability of AT2 cells to fully differentiate into AT1 cells for proper alveolar repair and regeneration or evidence of a protracted injury and repair cycle.13 Speculatively, in a chronic SARS-CoV-2 MA10 mouse infectious model, these aberrant Krt8+ epithelial progenitor can replenish AT2 cells during alveolar regeneration.15 In summary, the loss of AT2 epithelial progenitors and the accumulation of alveolar transitional cells are the main pathological findings that the correlation between their dynamic changes and structure alteration during fibroproliferative process is under intense investigation.
Figure 2.
Expansion of Keratin 8 positive epithelial cells in COVID-19 lung explants. KRT8 immunostaining. DAB-stained cells indicate KRT8+ cells (see arrows). IgG stain is represented in low magnification. DAB=3,3’-diaminobenzidine.
Pathological fibroblasts
An expansion of lung fibroblasts, specifically myofibroblasts, and other elements of the mesenchyme were the main histological findings of late- or end-stage fibrotic COVID-19 lungs.2,8,9 Whether this accumulation is simply occurring in response to extensive tissue damage, or these fibroblasts are indeed pathologic fibroblasts that perpetuate fibrotic process remains unclear.
Senescent characteristics in lung fibroblasts after SARS-CoV-2 infection have been reported,19 but other profibrotic features in these cells such as invasiveness capability have yet to be explored. Nonetheless, these fibroblasts strongly expressed Collagen Triple Helix Repeat Containing protein 1 (CTHRC1), a recently described marker for these cells, and profibrotic genes including COL1A1 and COL3A1.17 Current evidence suggests that the accumulation of fibroblasts was driven by immune-mediated processes mediated by macrophages, profibrotic MoMac, NK cells, and T cells, and that the majority of lung fibroblasts were highly proliferative.5,9 As such, the current evidence points to fibroblasts as an effector responding to the inflammatory environment.
Profibrotic signaling pathway
Cellular senescence
Cellular senescence is a key profibrotic pathway in IPF, which is also detected in COVID-19 PF. Senescence signature genes are expressed in aberrant epithelial cells in explanted COVID-19 PF lungs.2 Moreover, the sustained presence of senescence-associated secretory phenotypes such as IL-6, IL-1β, TNF, among other factors present in circulation and the lung environment further implicates cellular senescence in COVID-19 PF.13,20 Indeed, SARS-CoV-2 infected epithelia and lung fibroblasts promote paracrine senescence of adjacent bronchial and alveolar epithelial cells.19 Further evidence of SARS-CoV-2 induced-senescence driving lung pathology has been explored in a preclinical model in which it was shown that early treatment with senolytic agents such as navitoclax, a dasatinib/quercetin cocktail or fisetin ameliorated both morbidity and mortality.21
Inflammation regulatory pathways
Chronic persistent inflammation is the main feature of SARS-CoV-2, especially in severe cases with PF.2 However, transcriptomic profiling of circulating monocytes indicated lower responsiveness to IFN signaling caused a delay resolution of ARDS in severe COVID-19.10 An impaired or dysregulated IFN response during early infection appears to trigger significant downstream deleterious effects leading to hyperimmune activation and cytokine storm. Specifically, in SARS-CoV-2 PF, lower circulating IFN-γ (i.e. type II IFN) may be a risk factor for persistent fibrosis.22 Another study also suggested that the development of PF in post-COVID-19 patients was associated with lower circulating IFN-β and higher IL-1α and TGF-β.23
Epigenetic mechanisms
Bromodomain-containing protein 4 (BRD4) is one of the key epigenetic transcriptional regulators in pathologic tissue remodeling.24 Pharmacologic targeting of BRD4 during SARS-CoV-2 infection has been shown to prevent viral entry via modulation of angiotensin-converting enzyme 2 (ACE2) and Transmembrane Protease, Serine 2 (TMPRSS2) receptor expression in bronchial epithelial cells.25 In addition, epigenetic mechanisms appear to be regulating inflammatory responsiveness in myeloid cells during COVID-19. Epigenetic reprogramming in hematopoietic stem and progenitor cells (HSPC) imprints differentiated innate immune cells including monocytes, and this reprogramming is associated with the severity of SARS-CoV-2 infection.26 Specifically, it was noted that there are persistent epigenetic changes in Activator Protein 1 (AP-1) and Interferon Regulatory Factor (IRF) transcriptional activity in HSPC and monocytes characterized by high AP-1 activity and low IRF activity lasting up to 12 months after infection in severe COVID-19 patients. It is not presently clear whether BRD4 contributes to this transcriptional dysregulation, but these epigenetic changes could contribute to fibrosis in PASC and other complications in long-COVID-19. These findings suggest that a hyper-inflammatory state and subsequent PF could be attenuated by Bromodomain and Extraterminal protein (BET) inhibitors albeit the timing of administration appears to be paramount of maximal benefit.27
Summary and conclusion
PF is one of the most devastating respiratory complications of SARS-CoV-2 infection. The true prevalence and long-term clinical course of SARS-CoV-2 PF particularly among patients with less severe forms of the disease remain elusive. The lack of suitable animal models to fully understand the pathophysiology of SARS-CoV-2 PF significantly impairs efforts to identify therapeutic targets. Nevertheless, extensive data derived from autopsied and explanted lungs have highlighted the significance of the inflammatory process in the severity of COVID-19, and this process is viewed as the main culprit in PASC conditions including PF.
We currently have no way to predict those that will develop PASC PF. Clearly, the severity of illness is a strong predictor so those with obesity, advanced age and immunosuppressed states clearly are at risk. However, developing specific biomarkers would be beneficial to identify patients to attempt early interventions to disrupt fibroproliferation. In that regard, research is needed to identify novel therapeutics to treat those with PF as a long-term sequelae of SARS-CoV-2 infection. Anti-fibrotic agents such as nintedanib and pirfenidone have shown some initial promise, but a larger study is required for conclusive results. The use of anti-inflammatory agents during any infection is a delicate balance between circumventing excessive organ damage and triggering uncontrolled infection. To date, corticosteroids and pathway-specific therapy (i.e. IL-6 and JAK inhibitors) impact clinical course, but there is insufficient data regarding their impact on PF. Available data suggest that targeting inflammatory pathways expressed in myeloid cells (e.g. MoMacs) might be a novel therapeutic strategy in SARS-CoV-2 PF. Cellular senescence is another prominent pathologic process that is directly linked to inflammation, and therapeutic targeting of this pathway in the preclinical setting is efficacious. Lung transplantation is also now an option for those with PASC PF with chronic respiratory failure, but long-term survival remains to be determined and these patients will have to contend with transplant-associated complications.
Funding
NIH-1K08HL141590-01A1 (T.P.); Sunshine Biotech and Cedars-Sinai Medical Center (M.E. and C.M.H.); NIH R01 HL155759, R01 HL137076 and R01 HL151646 (P.C.).
Conflict of interest: None declared.
Contributor Information
T Parimon, From the Cedars-Sinai Medical Center, Women’s Guild Lung Institute, 127 San Vicente Blvd, Los Angeles, CA 90048, USA; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Cedars-Sinai Medical, Center, 8700 Beverly Blvd, Los Angeles, CA 90048, USA.
M Espindola, From the Cedars-Sinai Medical Center, Women’s Guild Lung Institute, 127 San Vicente Blvd, Los Angeles, CA 90048, USA; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Cedars-Sinai Medical, Center, 8700 Beverly Blvd, Los Angeles, CA 90048, USA.
A Marchevsky, Pathology Department, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048, USA.
R Rampolla, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Cedars-Sinai Medical, Center, 8700 Beverly Blvd, Los Angeles, CA 90048, USA.
P Chen, From the Cedars-Sinai Medical Center, Women’s Guild Lung Institute, 127 San Vicente Blvd, Los Angeles, CA 90048, USA; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Cedars-Sinai Medical, Center, 8700 Beverly Blvd, Los Angeles, CA 90048, USA.
C M Hogaboam, From the Cedars-Sinai Medical Center, Women’s Guild Lung Institute, 127 San Vicente Blvd, Los Angeles, CA 90048, USA; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Cedars-Sinai Medical, Center, 8700 Beverly Blvd, Los Angeles, CA 90048, USA.
References
- 1. Mehandru S, Merad M.. Pathological sequelae of long-haul COVID. Nat Immunol 2022; 23:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med 2020; 12: eabe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299:E177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fadista J, Kraven LM, Karjalainen J, Andrews SJ, Geller F, Baillie JK, et al. Shared genetic etiology between idiopathic pulmonary fibrosis and COVID-19 severity. EBioMedicine 2021; 65:103277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, et al. ; Deutsche COVID-19 OMICS Initiative (DeCOI). SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021; 184:6243–61.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konopka KE, Perry W, Huang T, Farver CF, Myers JL.. Usual interstitial pneumonia is the most common finding in surgical lung biopsies from patients with persistent interstitial lung disease following infection with SARS-CoV-2. EClinicalMedicine 2021; 42:101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flaifel A, Kwok B, Ko J, Chang S, Smith D, Zhou F, et al. Pulmonary pathology of end-stage COVID-19 disease in explanted lungs and outcomes after lung transplantation. Am J Clin Pathol 2022; 157:908–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aesif SW, Bribriesco AC, Yadav R, Nugent SL, Zubkus D, Tan CD, et al. Pulmonary pathology of COVID-19 following 8 weeks to 4 months of severe disease: a report of three cases, including one with bilateral lung transplantation. Am J Clin Pathol 2021; 155:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, et al. The spatial landscape of lung pathology during COVID-19 progression. Nature 2021; 593:564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao C, Bora SA, Parimon T, Zaman T, Friedman OA, Palatinus JA, et al. Cell-type-specific immune dysregulation in severely ill COVID-19 patients. Cell Rep 2021; 34:108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang S, Yao X, Ma S, Ping Y, Fan Y, Sun S, et al. A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat Cell Biol 2021; 23:1314–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He J, Cai S, Feng H, Cai B, Lin L, Mai Y, et al. Single-cell analysis reveals bronchoalveolar epithelial dysfunction in COVID-19 patients. Protein Cell 2020; 11:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021; 595:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roach A, Chikwe J, Catarino P, Rampolla R, Noble PW, Megna D, et al. Lung transplantation for Covid-19–related respiratory failure in the United States. N Engl J Med 2022; 386:1187–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinnon KH, Leist SR, Okuda K, Dang H, Fritch EJ, Gully KL, et al SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci Transl Med eabo5070. https://www.science.org/doi/epdf/10.1126/scitranslmed.abo5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022; 606:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, et al A molecular single-cell lung atlas of lethal COVID-19. Nature 2021; 595:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fava VM, Bourgey M, Nawarathna PM, Orlova M, Cassart P, Vinh DC, et al. A systems biology approach identifies candidate drugs to reduce mortality in severely ill patients with COVID-19. Sci Adv 2022; 8:eabm2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuji S, Minami S, Hashimoto R, Konishi Y, Suzuki T, Kondo T, et al. SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response. Nat Aging 2022; 2:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D'Agnillo F, Walters K-A, Xiao Y, Sheng Z-M, Scherler K, Park J, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med 2021; 13:eabj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 2021; 599:283–9. [DOI] [PubMed] [Google Scholar]
- 22. Hu Z-J, Xu J, Yin J-M, Li L, Hou W, Zhang L-L, et al. Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients. Front Immunol 2020; 11:585647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colarusso C, Maglio A, Terlizzi M, Vitale C, Molino A, Pinto A, et al. Post-COVID-19 who develop lung fibrotic-like changes have lower circulating levels of IFN-β but higher levels of IL-1α and TGF-β. Biomedicines 2021; 9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang X, Peng R, Phillips JE, Deguzman J, Ren Y, Apparsundaram S, et al. Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am J Pathol 2013; 183:470–9. [DOI] [PubMed] [Google Scholar]
- 25. Qiao Y, Wang XM, Mannan R, Pitchiaya S, Zhang Y, Wotring JW, et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci U S A 2020; 118:e2021450118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheong J-G, Ravishankar A, Sharma S, Parkhurst CN, Nehar-Belaid D, Ma S, et al. Epigenetic memory of COVID-19 in innate immune cells and their progenitors. 2022: bioRxiv. 2022.02.09.479588. https://doi.org/10.1101/2022.02.09.479588 [Google Scholar]
- 27. Chen IP, Longbotham JE, McMahon S, Suryawanshi RK, Carlson-Stevermer J, Gupta M, et al. Viral E protein neutralizes BET protein-mediated post-entry antagonism of SARS-CoV-2. Cell Reports 2021; 40:111088. [DOI] [PMC free article] [PubMed]