Abstract
Although maternal antibodies can protect against infectious disease in infancy, they can also suppress active immune responses. The effects of circulating maternal antibodies, with and without colostrum and milk antibodies, on passive protection and active immunity to human rotavirus (HRV) were examined in gnotobiotic pigs. Pigs received intraperitoneal injections of high-titer serum (immune pigs [groups 1 and 2]) from immunized sows, low-titer serum from naturally infected sows (control pigs [groups 3 and 4]), or no serum (group 5). Immune or control colostrum and milk were added to the diet of groups 2 and 4, respectively. After inoculation (3 to 5 days of age) and challenge (postinoculation day [PID] 21) with virulent HRV, the effects of maternal antibodies on protection (from diarrhea and virus shedding), and on active antibody responses (measured by quantitation of antibody-secreting cells [ASC] in intestinal and systemic lymphoid tissues by ELISPOT) were evaluated. Groups 1 and 2 had significantly less diarrhea and virus shedding after inoculation but higher rates of diarrhea and virus shedding after challenge than did groups 3 and 5. Group 1 and 2 pigs had significantly fewer immunoglobulin A (IgA) ASC in intestinal tissues at PID 21 and at postchallenge day (PCD) 7 compared to group 5. Significantly fewer IgG ASC were present in the intestines of group 2 pigs at PID 21 and PCD 7 compared to group 5. There was a trend towards fewer ASC in intestinal tissues of group 2 than group 1, from PID 21 on, with significantly fewer IgA ASC at PCD 7. IgG ASC in the duodenum and mesenteric lymph nodes of group 3 and 4 pigs were significantly fewer than in group 5 at PCD 7. These decreases in ASC emphasize the role of passive antibodies in impairing induction of ASC rather than in merely suppressing the function of differentiated B cells. To be successful, vaccines intended for populations with high titers of maternal antibodies (infants in developing countries) may require higher titers of virus, multiple doses, or improved delivery systems, such as the use of microencapsulation or immune stimulating complexes, to overcome the suppressive effects of maternal antibodies.
Rotaviruses are the leading cause of severe diarrhea in infants and young children in both developed and developing countries (17), and considerable efforts and resources have been invested in rotavirus vaccine development. Candidate rotavirus vaccines which have been tested in humans have consisted of live virus delivered orally, to mimic natural infection (39). A common observation in many reports of clinical vaccine trials, is a reduced rate of postvaccinal seroconversion in infants with high titers of passive (maternal) serum antibodies (10, 13, 19, 28, 35, 39). This suppressive effect of passive antibodies has been noted with bovine (35), rhesus (28), human-bovine (WC 3) reassortant (10), and human-rhesus reassortant rotavirus vaccines (13, 19, 39). Administration of up to three doses of oral vaccine may not overcome the suppressive effect (39). Breast-feeding has also been associated with decreased immune responses in some study populations (27, 28).
The immunological mechanisms responsible for the suppression of active immune responses by passively acquired antibodies remain unclear; proposed mechanisms include inhibition of specific B-cell function by cross-linking of Fc and surface immunoglobulin (Ig) receptors (8, 11), induction of specific suppressor T lymphocytes (16), epitope-specific inhibition of antigen processing (21), failure to elicit T-cell help (32), and idiotypic interactions (31). With live viruses, passive antibodies can also inhibit virus replication and shedding (30, 36) and thus reduce the quantity of antigen presented to the immune system. Passive antibodies not only affect the magnitude of immune responses in the short term but also may affect the nature (TH1/TH2 balance) of immune responses, with effects persisting into adult life (1). Thus, the influence of passive antibodies, both in the circulation as serum antibodies and in the intestines as milk-associated antibodies, cannot be ignored in developing effective oral vaccines against rotaviruses in infants, especially those vaccines likely to be used in the developing world, where higher levels of maternal immunity occur (28).
Gnotobiotic, caesarian-derived pigs provide an excellent model for studying the effects of passive antibodies, as they are born devoid of maternal antibodies, with a high level of immune competence, and are susceptible to infection with human rotavirus (HRV). In addition, the experimental animals are protected from natural exposure to rotavirus, a complication that can make interpretation of data from field trials difficult (19). In the present study, antibody-secreting cell (ASC) responses were studied in gnotobiotic pigs with passive serum antibodies with and without local intestinal (milk) antibodies (analogous to breast-fed and formula-fed infants, respectively). With this approach, effects on local (mucosal) active antibody responses can be distinguished from systemic effects. Insights can also be gleaned as to whether passive antibodies suppress the number of ASC generated or downregulate the function of differentiated cells. Such experimental work is essential for the development of improved vaccines which can induce protective immune responses in the presence of maternal antibodies.
MATERIALS AND METHODS
Virus.
Virulent Wa HRV (G1, P1A) rotavirus (intestinal contents from the 16th passage in gnotobiotic pigs) diluted in minimal essential medium (MEM; Life Technologies, Grand Island, N.Y.), was used for inoculation and challenge (37, 40). Attenuated Wa HRV (cell culture adapted) was propagated in monkey kidney (MA104) cells for use in enzyme-linked immunospot (ELISPOT) and virus neutralization assays.
Preparation of immune sow serum.
Rotavirus-seropositive sows (n = 2) received five or six doses of attenuated Wa HRV (∼107 fluorescent-focus-forming units [FFU]/dose) in incomplete Freund’s adjuvant (IFA) intramuscularly at 2-week intervals, followed by two doses of virulent Wa HRV (∼5 × 106 FFU/dose) orally (2-week interval). After the last immunization, serum was collected, pooled, filtered (0.22-μm-pore-size membrane filter; Millipore, Bedford, Mass.), and stored at −20°C until used. Two nonimmunized and two mock-immunized (IFA alone) rotavirus-seropositive sows in the same herd provided control normal-sow serum. Immune colostrum was collected at farrowing from 10 rotavirus-seropositive sows injected intramuscularly with attenuated Wa HRV (∼107 FFU/injection) in IFA at 4, 3, and 2 weeks before their expected parturition dates. Immune milk was collected from day 4 postpartum for 5 to 10 days. Control normal colostrum and milk were collected similarly from 10 rotavirus-seropositive sows. Colostrum and milk samples were centrifuged at 700 × g for 45 minutes (4°C), heat inactivated (56°C) for 30 min, and then centrifuged at 10,000 × g for 20 min to pellet casein aggregates. Beta-propiolactone (0.3%; 1 hour incubation at 37°C with agitation) was used to sterilize processed colostrum and milk. Titers of virus-neutralizing (VN) (plaque reduction) antibodies to Wa HRV in serum and milk administered to gnotobiotic piglets are presented in Fig. 1.
FIG. 1.
(A) VN antibody titers in pools of sow serum, colostrum, and milk prepared from immunized sows (solid bars) and nonimmunized sows (open bars). (B) GMT of VN antibodies in serum of pigs at PID 0. Pigs received intraperitoneal injections of 60 ml of immune or control serum within 18 h of derivation. A subset of pigs received 15 ml of immune or control colostrum orally twice a day from 3 to 5 days of age and 25 ml of immune or control milk twice a day for another 7 days. Solid bars indicate titers in pigs inoculated with virulent Wa HRV at PID 0, and open bars indicate titers in pigs mock inoculated at PID 0. Columns labeled with an A differ significantly from those labeled with a B (one-way ANOVA of log-transformed VN titers followed by Duncan’s multiple-range test).
Gnotobiotic pigs and experimental design.
Gnotobiotic pigs were derived by hysterectomy of near-term sows and maintained in isolation units as described previously (23). Pigs were allocated to nine groups (Table 1). Within 6 h of derivation, pigs in group 1 (immune serum) and group 2 (immune serum plus immune colostrum and milk) received intraperitoneal injections of 30 ml of maternal high-titer immune serum per pig, followed by an additional 30 ml 12 h later. Administration of these volumes of serum was found in preliminary work to result in levels of serum IgG (18 to 26 mg/ml [data not shown]) similar to those reported for naturally suckled piglets (25 to 40 mg/ml [36]). Pigs in group 3 (control normal serum) and group 4 (control normal serum plus control normal colostrum and milk) similarly received injections of serum from nonimmunized, rotavirus-seropositive (low-titer) control healthy sows. Group 2 pigs received supplements of 15 ml of immune colostrum twice a day from 3 to 5 days of age and then 25 ml of milk twice a day for another 7 days; these volumes represented approximately 25% of the diet. Group 4 pigs were fed supplements from nonimmunized rotavirus-seropositive (control normal) sows on the same schedule. Pigs in group 5 (no maternal antibody) received 60 ml of saline intraperitoneally or no injection and received no milk supplements. At 3 to 5 days of age, pigs in groups 1 to 5 were orally inoculated with 5 ml of 100 mM sodium bicarbonate (to reduce gastric acidity), followed by ∼5 × 105 FFU (∼5 × 105 50% infectious doses in gnotobiotic pigs without maternal antibodies) of virulent Wa HRV in 5 ml of MEM (37, 41, 42). Groups 6 to 9 received serum and milk preparations in the same manner as groups 1 to 4, respectively, but were mock inoculated with MEM instead of virulent virus at 3 to 5 days of age. At postinoculation day (PID) 20 to 21, pigs from all nine groups were challenged orally with ∼5 × 106 FFU (∼5 × 106 50% infectious doses) of virulent Wa HRV (37, 41). Pigs were observed daily for diarrhea, and rectal swabs were collected for assessment of virus shedding. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid. Pigs with daily fecal consistency scores of ≥2 were considered diarrheic. The cumulative fecal score was calculated as the sum of the daily fecal scores from PID 1 to 7, or from postchallenge day (PCD) 1 to 7. Blood was collected at PID 0 and at weekly intervals. Two to six pigs from each group were euthanatized, and the small intestines (duodenum and ileum), mesenteric lymph nodes (MLN), spleen, and blood were collected at the following times: PID 8, PID 20 to 21, PCD 4, and PCD 7.
TABLE 1.
Clinical disease and fecal virus shedding in gnotobiotic pigs after oral inoculation with virulent Wa HRV
| Exptl category and group no.i | n | Pigs with diarrhea
|
Pigs shedding virusa
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| % With illnesse | Protection rate (%)b | Mean duration (days)cf | Mean cumu-lative scoredf | % Sheddinge | Mean days to onsetf | Mean duration (days)f | Mean peak titer shed (FFU/ml)f | ||
| Virus inoculated | |||||||||
| 1 | 22 | 23 B | 76 | 0.3 B | 3.6 B | 64 B | 2.5 B | 2.6 B, C | 7.7 × 104 A |
| 2 | 12 | 33 B | 65 | 0.5 B | 4.1 B | 50 B | 3.6 A | 2.2 C | 2.1 × 105 A |
| 3 | 17 | 100 A | 0 | 3.5 A | 9.4 A | 100 A | 1.5 C | 4.7 A | 4.9 × 106 A |
| 4 | 11 | 91 A | 4 | 3.7 A | 9.5 A | 73 B | 3.4 A | 3.6 B | 2.5 × 106 A |
| 5 | 64 | 95 A | NAg | 3.0 A | 9.5 A | 100 A | 1.7 C | 5.7 A | 2.1 × 106 A |
| Mock inoculated control | |||||||||
| 6 | 5 | 20 B | NA | 0.4 B | 3.7 B | NDh | ND | ND | ND |
| 7 | 7 | 14 B | NA | 0.5 B | 3.9 B | ND | ND | ND | ND |
| 8 | 6 | 17 B | NA | 0.5 B | 4.3 B | ND | ND | ND | ND |
| 9 | 7 | 29 B | NA | 0.9 B | 4.3 B | ND | ND | ND | ND |
Determined by ELISA and CCIF infectivity assay.
Protection rate = [1 − (percentage of Wa HRV-inoculated pigs with diarrhea/percentage of pigs in group 5 with diarrhea)] × 100.
Duration of diarrhea determined by number of days with fecal scores of ≥2; feces were scored as follows: 0, normal; 1, pasty; 2, semiliquid; 3, liquid.
Mean cumulative score = (Σ daily fecal scores for 1 week postinoculation)/n.
Proportions in this column followed by different letters differ significantly (Fisher’s exact test).
Means in this column followed by different letters differ significantly (one-way ANOVA).
NA, not applicable.
ND, not detected (i.e., <250 FFU/ml).
Groups: 1, immune serum; 2, immune serum plus immune colostrum and milk; 3, control serum; 4, control serum plus control colostrum and milk; 5, no maternal antibodies; 6, mock inoculated, immune serum; 7, mock inoculated, immune serum plus immune colostrum and milk; 8, mock inoculated, control serum; 9, mock inoculated, control serum plus control colostrum and milk.
VN antibody assay.
A plaque reduction VN antibody assay was performed on serum and milk samples as described previously (30), with Wa HRV. VN antibody titers were expressed as the reciprocal of the serum dilution which reduced the number of plaques by >80%.
CCIF assay.
A cell culture immunofluorescence (CCIF) assay was performed to detect infectious Wa HRV as described previously (7). Briefly, rectal swabs and fecal suspensions were diluted 1:25 in MEM and were clarified by centrifugation for 20 min at 1,500 × g. The samples were further diluted to 1:100 and then serially diluted (10-fold); each dilution was assayed in duplicate on MA104 cell monolayers in 96-well plates. Rotavirus-negative and -positive fecal controls were included on each plate. Fluorescent foci within the wells were visualized and enumerated by using fluorescein isothiocyanate-conjugated hyperimmune pig antiserum to group A rotavirus and fluorescence microscopy.
Rotavirus antigen ELISA.
An antigen-capture enzyme-linked immunosorbent assay (ELISA) was performed to detect Wa HRV antigen in rectal swab fluids, feces, and intestinal contents as described previously (29). Rotavirus-negative and -positive fecal controls were included on each plate. A sample was considered positive if the mean absorbance of replicate samples on rotavirus antibody-coated wells was greater than the mean absorbance plus 3 standard deviations of samples in negative control wells.
Isolation of MNC.
Mononuclear cells (MNC) from duodenum, ileum, MLN, spleen, and blood were isolated as described previously (41). Purified MNC were resuspended in RPMI 1640 medium supplemented with 8% fetal bovine serum, 20 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 μg of gentamicin/ml, 10 μg of ampicillin/ml, and 50 μM 2-mercaptoethanol.
ELISPOT assay for virus-specific ASC.
ELISPOT assays to enumerate isotypes of rotavirus-specific ASC were conducted based on previously published methods and reagents (9, 41). Briefly, Wa HRV-infected MA104 cells in 96-well plates (Nunc-Immuno, Nalge-Nunc, Rochester, N.Y.) were acetone fixed and washed with deionized water prior to use. Single-cell suspensions of MNC from each tissue sample were added to duplicate wells (5 × 105, 5 × 104, or 5 × 103 cells/well). Plates were incubated for 12 h at 37°C in 5% CO2 and then washed and incubated with biotinylated mouse monoclonal antibody (ascites fluid) to pig IgG (hybridoma 3H7) (0.03 μg/ml), pig IgA (hybridoma 6D11) (0.04 μg/ml), or pig IgM (hybridoma 5C9) (0.35 μg/ml) (24) for 2 h at room temperature. Plates were washed, and horseradish peroxidase-conjugated streptavidin (1:30,000; Kirkegaard & Perry Laboratories Inc. [KPL], Gaithersburg, Md.) was added. After incubation for 1 h at room temperature, the plates were washed and spots were developed with a tetramethylbenzidine peroxidase substrate system (KPL). The numbers of virus-specific ASC were determined by counting blue spots in the wells and reported as the mean number of virus-specific ASC per 5 × 105 MNC.
ELISPOT assay for total IgSC.
The total number of Ig-secreting cells (IgSC) in duodenum, ileum, MLN, spleen, and peripheral blood specimens were determined by previously published methods (41, 42). Briefly, 96-well plates (Nunc-Immuno) were coated with goat anti-pig IgM (25 μg/ml; KPL), goat anti-pig IgA (30 μg/ml; Bethyl Laboratories Inc., Montgomery, Tex.), or goat anti-pig IgG (1 μg/ml; Bethyl Laboratories). Plates were washed with deionized water, and single-cell suspensions of MNC were added. After incubation for 12 h at 37°C in 5% CO2, biotinylated MAbs to porcine IgM (hybridoma 5C9), IgA (hybridoma 6D11), and IgG (hybridoma 3H7) were added to their respective plates. Reagents for color development and the method of enumeration of cells were the same as those for the rotavirus-specific ELISPOT assay described above.
Statistical analyses.
One-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test was used to assess differences in (log-transformed) VN antibody titers among groups. Percentages of pigs with diarrhea and pigs shedding virus were compared among groups by Fisher’s exact test; when significant differences were present among groups, pairwise comparisons were made by Fisher’s exact test to clarify the nature of the differences. The mean number of ASC was calculated for each treatment group at various times (PID 8, PID 21, PCD 4, and PCD 7). If significant differences were detected among groups by the Kruskal-Wallis rank sum (nonparametric) test, then pairwise differences between particular groups and the group with no maternal antibody (group 5) were tested (Kruskal-Wallis rank sum test). Differences between group 1 (immune serum) and group 2 (immune serum plus immune colostrum and milk) and between group 3 (control serum) and group 4 (control serum plus control colostrum and milk) were also tested by the Kruskal-Wallis test to assess whether colostrum and milk antibodies had immune-modulating effects beyond those of circulating antibodies alone. To evaluate whether administration of serum or feeding of colostrum and milk affected total IgSC numbers, data from groups 1 and 3 were pooled and data from groups 2 and 4 were pooled. Differences in total IgSC among (i) serum-injected pigs, (ii) serum-injected and colostrum- and milk-fed pigs, and (iii) pigs with no maternal antibody were assessed by the Kruskal-Wallis rank sum test at each time point. When differences among these groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. A significance level of 0.05 was used throughout.
RESULTS
Passive transfer of antibodies.
VN antibody titers in pooled serum, colostrum, and milk of vaccinated sows were 28-, 2.6-, and 3.4-fold higher, respectively, than titers in nonvaccinated control sows (Fig. 1). Geometric mean titers (GMT) of VN antibodies in sera of pigs receiving immune serum intraperitoneally were significantly higher than those of pigs receiving control serum (Fig. 2).
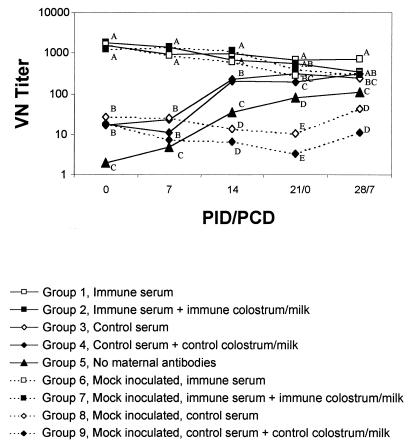
FIG. 2.
GMT of VN antibodies in piglet serum from PID 0 to PID 28. Piglets were challenge exposed at PID 21 (PCD 0). Datum points labeled with different letters at the same time point differ significantly (one-way ANOVA of log-transformed VN titers followed by Duncan’s multiple-range test).
Passive protection postinoculation.
Clinical signs and virus shedding data are summarized in Table 1. Over 90% of pigs in groups 3 (control serum), 4 (control serum plus control colostrum and milk), and 5 (no maternal antibody) had diarrhea after virus inoculation; diarrhea was present in significantly fewer (23%) group 1 (immune serum) and (33%) group 2 (immune serum plus immune colostrum and milk) pigs. The rates of diarrhea, mean number of days with diarrhea, and mean cumulative fecal scores of group 1 and 2 pigs were statistically comparable to those of age-matched mock-inoculated pigs (groups 6 to 9) and significantly less than those of groups 3 to 5.
The proportion of pigs shedding rotavirus after inoculation was significantly lower in group 1, 2, and 4 pigs (64, 50, and 73%, respectively) than in group 3 and 5 pigs (100%). Among those pigs which shed virus, the mean number of days to onset of shedding was significantly greater in group 1 pigs (immune serum) than in groups 3 (control serum) and 5 (no maternal antibody). Pigs which received immune or control milk in addition to serum (groups 2 and 4, respectively) had significantly greater mean number of days to onset of shedding than group 1 pigs. Among those pigs which shed virus, the mean duration of shedding was significantly less for group 1 and 2 pigs than for group 3 and 5 pigs. Group 4 pigs, which received control milk in addition to control serum, had significantly fewer days of virus shedding than group 3 pigs that received only control serum. Among pigs which shed virus after inoculation, mean peak virus titers in feces were lowest for group 1 and 2 pigs, but differences among groups were not significant. Group 2, which received both immune serum and immune milk, had the lowest proportion of animals shedding, the longest time to onset of shedding, and the shortest duration of shedding.
Protection postchallenge.
At PID 21 pigs were challenged with virulent Wa HRV; clinical signs and virus shedding data are summarized in Table 2. Those pigs which had been mock inoculated at PID 0 and were therefore susceptible to challenge were also exposed to Wa HRV at PID 21. Challenge data from mock-inoculated pigs that had received immune serum (group 6) were pooled with data from pigs that had received immune serum plus immune milk (group 7) for purposes of statistical analysis, as the data were comparable and the number of animals was low. Similarly, data from mock-inoculated pigs receiving control serum (group 8) or control serum plus control milk (group 9) were pooled.
TABLE 2.
Clinical disease and fecal virus shedding in gnotobiotic pigs after oral challenge with virulent Wa HRV
| Exptl category and group no.j | n | Pigs with diarrhea
|
Pigs shedding virusa
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| % With illnesse | Protection rate (%)b | Mean duration (days)cf | Mean cumu-lative scoredf | % Sheddinge | Mean days to onsetf | Mean duration (days)f | Mean peak titer shed (FFU/ml)f | ||
| Virus inoculated | |||||||||
| 1 | 12 | 42 C, D | 55 | 1.2 B | 6.3 C | 17 B | 1.5 A | 3.0 A | 3.5 × 104 A |
| 2 | 5 | 60 A, B, C | 36 | 1.8 B | 7.1 B, C | 20 B | 3.0 A | 1.0 A | 1.4 × 105 A |
| 3 | 8 | 25 C, D | 73 | 1.0 B | 4.6 C | 0 B | NAg | NA | NDh |
| 4 | 6 | 33 B, D | 65 | 1.2 B | 6.1 C | 17 B | 1.0 A | 3.0 A | 2.0 × 106 A |
| 5 | 25 | 12 D | 87 | 0.6 B | 5.0 C | 0 B | NA | NA | ND |
| Mock inoculated controli | |||||||||
| 6 | 2 | 50 A, B | NA | 2.5 A | 7.5 A | 100 A | 1.5 A | 1.0 A | 2.1 × 104 A |
| 7 | 6 | 100 A, B | NA | 4.7 A | 11.8 A | 100 A | 1.8 A | 2.7 A | 4.5 × 105 A |
| 8 | 3 | 100 A | NA | 3.7 A | 9.3 A, B | 100 A | 2.7 A | 2.3 A | 1.8 × 104 A |
| 9 | 5 | 100 A | NA | 3.4 A | 9.7 A, B | 100 A | 1.6 A | 3.2 A | 7.2 × 105 A |
Determined by ELISA and CCIF infectivity assay.
Protection rate = [1 − (percentage of Wa HRV-inoculated pigs with diarrhea/percentage of pigs in mock-inoculated groups with diarrhea)] × 100.
Duration of diarrhea determined by number of days with fecal scores of ≥2; feces were scored as follows: 0, normal; 1, pasty; 2, semi-liquid; 3, liquid.
Mean cumulative score = (Σ daily fecal scores for 1 week postchallenge)/n.
Proportions in this column followed by different letters differ significantly (Fisher’s exact test).
Means in this column followed by different letters differ significantly (one-way ANOVA).
NA, not applicable.
ND, not detectable (i.e., <250 FFU/ml).
Data from mock-inoculated groups receiving the same serum preparations were combined for statistical analysis, as the numbers of animals were low and the data were comparable.
For an explanation of groups, see Table 1, footnote i.
All mock-inoculated pigs except one (15 of 16) had diarrhea after challenge. Group 5 (no maternal antibody) had a significantly lower proportion of pigs (12%) with diarrhea than the mock-inoculated, challenged groups. Group 2 but not group 1 pigs had significantly higher rates of diarrhea than group 5 pigs. The mean duration of diarrhea was significantly longer for mock-inoculated pigs after challenge than for pigs in groups 1 to 5. Mean cumulative fecal scores did not differ significantly among groups 1 to 5.
The proportion of mock-inoculated pigs shedding rotavirus after challenge was significantly higher (100%) than that of pigs in groups 1 to 5 (0 to 20%), which did not differ significantly among the groups.
VN antibodies.
At PID 0, serum VN antibody titers of group 1 and 2 pigs given immune serum were significantly higher than those of group 3 and 4 pigs given control serum (Fig. 2). VN antibodies were not detected at PID 0 in samples from group 5 pigs, confirming their seronegative status (Fig. 2). From PID 0 to 21, the VN GMT of group 1 and 2 pigs declined 2- to 3-fold; over the same interval group 3 and 4 VN GMT increased 10- to 19-fold (Fig. 2). By PID 21, the VN antibody titers of pigs that received immune serum (group 1) were still significantly higher than titers of pigs that received control serum (group 3), and titers of pigs receiving immune serum and immune milk (group 2) were still significantly higher than titers of pigs receiving control serum and control milk (group 4). By PCD 4 to 7 these differences were no longer significant. In group 5 pigs VN antibodies were present in serum by PID 21, but titers were significantly lower than titers in group 3 and 4 pigs which had received passive antibodies from control sows.
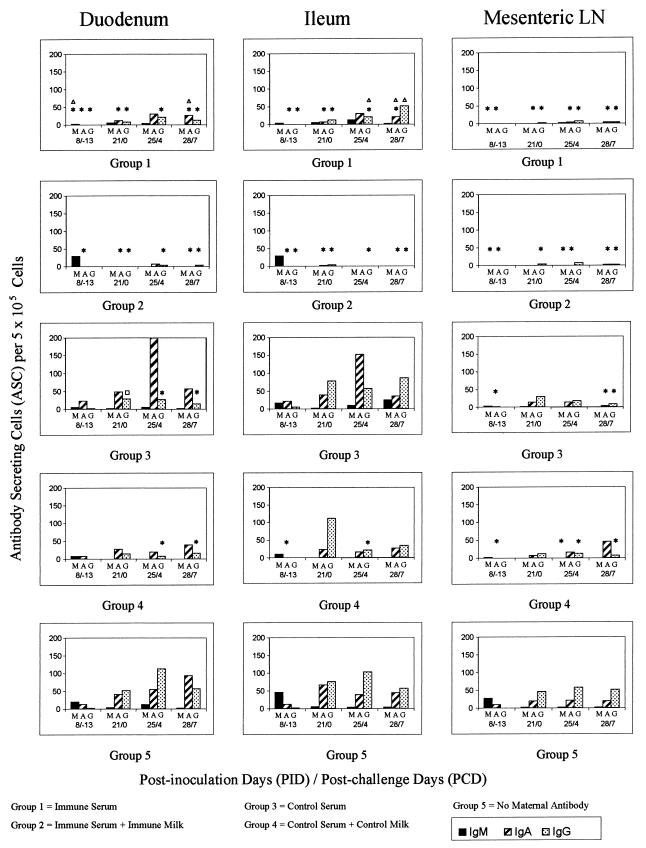
ASC responses. (i) PID 8.
ASC of all isotypes were detected in tissues of group 5 pigs, but ASC counts were low (<10 per 105 cells) in all tissues except for IgM ASC in duodenum, ileum, and MLN and IgA ASC in duodenum and ileum (Fig. 3 and 4). IgM ASC counts in duodenum and MLN of group 1 pigs were significantly lower than for group 5 pigs, whereas IgA ASC counts in duodenum, ileum, and MLN were significantly lower in both group 1 and 2 pigs than in group 5 pigs (Fig. 3). IgA ASC counts in MLN of group 3 pigs and in ileum and MLN of group 4 pigs were significantly lower than those of group 5 pigs. Data were not available for ASC numbers in mock-inoculated pigs at PID 8.
FIG. 3.
Mean ASC counts per 5 × 105 MNC in duodenum, ileum, and MLN. Symbols: ∗, group differs significantly from group 5 for the same isotype at the same time point (Kruskal-Wallis rank sum test); ▵, groups 1 and 2 differ significantly for the same isotype at the same time point (Kruskal-Wallis rank sum test); □, groups 3 and 4 differ significantly for the same isotype at the same time point (Kruskal-Wallis rank sum test).
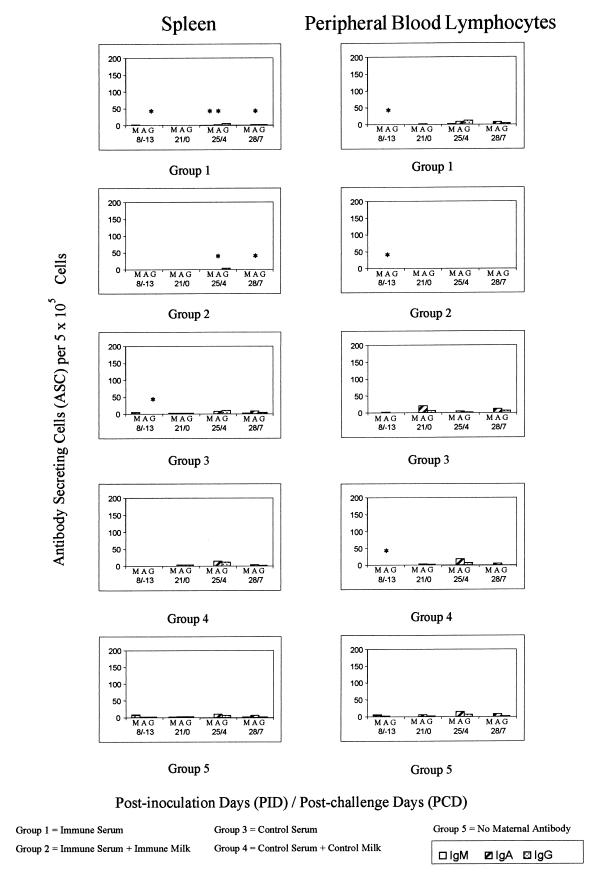
FIG. 4.
Mean ASC counts per 5 × 105 MNC in spleen and peripheral blood. For an explanation of symbols, see the legend to Fig. 3. Note the change of vertical scale from Fig. 3.
(ii) PID 21/PCD 0.
At challenge, PID 21 (= PCD 0), mean IgM ASC numbers had declined to <5 per 105 cells in all tissues of group 5 pigs; IgM ASC numbers in groups 1 to 4 did not differ significantly from these levels (Fig. 3 and 4; Table 3). By PID 21, mean IgA and IgG ASC numbers increased in the duodenum, ileum, and MLN of group 5 pigs (≥20 per 105 cells) but remained low (<10 per 105 cells) in the systemic tissues (spleen and peripheral blood) of pigs in all groups (Fig. 4). The numbers of IgG and IgA ASC in duodenum and ileum of group 1 and 2 pigs were significantly lower than those in group 5 pigs (Table 4). There were also significantly fewer IgG ASC in the MLN of group 1 and 2 pigs than those in group 5 pigs. ASC numbers of group 3 and 4 pigs did not differ significantly from those of group 5 pigs in any tissue examined. No ASC of any isotype were detected in tissues of pigs mock inoculated at PID 0 (data not shown).
TABLE 3.
Summary of ASC responses in intestinal and systemic tissues at PCD 0 and PCD 4 to 7
| Exptl category and group no.d | Time | nb | No. of ASCa
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duodenum
|
Ileum
|
MLN
|
Spleen
|
PBLe
|
|||||||||||||
| IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | |||
| Virus inoculated | |||||||||||||||||
| 1 | PID 21/PCD 0 | 5 | 4 | 11 | 9 | 5 | 7 | 14 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 1 |
| PID 25–28/PCD 4–7 | 11 | 2 | 28 | 17 | 7 | 25 | 38 | 1 | 4 | 4 | 1 | 1 | 3 | 1 | 7 | 6 | |
| 2 | PID 21/PCD 0 | 3 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| PID 25–28/PCD 4–7 | 6 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 2 | 0 | 0 | 0 | |
| 3 | PID 21/PCD 0 | 6 | 1 | 48 | 29 | 1 | 39 | 77 | 1 | 12 | 29 | 1 | 2 | 2 | 1 | 20 | 7 |
| PID 25–28/PCD 4–7 | 8 | 3 | 180 | 21 | 17 | 94 | 71 | 1 | 8 | 13 | 1 | 7 | 6 | 0 | 8 | 4 | |
| 4 | PID 21/PCD 0 | 3 | 0 | 27 | 15 | 0 | 22 | 111 | 0 | 6 | 11 | 0 | 4 | 3 | 0 | 4 | 2 |
| PID 25–28/PCD 4–7 | 6 | 0 | 29 | 12 | 0 | 21 | 28 | 0 | 31 | 10 | 0 | 9 | 7 | 0 | 11 | 4 | |
| 5 | PID 21/PCD 0 | 6 | 3 | 40 | 52 | 4 | 66 | 76 | 2 | 20 | 46 | 1 | 4 | 3 | 0 | 6 | 2 |
| PID 25–28/PCD 4–7 | 13 | 6 | 85 | 83 | 3 | 40 | 74 | 2 | 19 | 51 | 0 | 8 | 4 | 0 | 12 | 4 | |
| Mock inoculated controls | |||||||||||||||||
| 6 | PID 21/PCD 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PID 28/PCD 7 | 2 | 63 | 0 | 0 | 115 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 7 | PID 21/PCD 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NAc | NA | NA |
| PID 28/PCD 7 | 6 | 6 | 2 | 0 | 25 | 1 | 0 | 1 | 1 | 0 | 3 | 0 | 0 | 2 | 2 | 0 | |
| 8 | PID 21/PCD 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PID 28/PCD 7 | 3 | 50 | 2 | 0 | 112 | 1 | 0 | 10 | 1 | 0 | 7 | 0 | 0 | 4 | 0 | 0 | |
| 9 | PID 21/PCD 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PID 28/PCD 7 | 5 | 25 | 13 | 1 | 59 | 10 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | |
Values for the different isotypes are mean numbers of ASC per 5 × 105 MNC; for statistical analyses, see the legends to Fig. 3 and 4.
Number of pigs euthanatized in each group at each time point: PCD 4 and 7 pigs were combined for groups 1 to 5.
NA, not available.
For an explanation of groups, see Table 1, footnote i.
PBL, peripheral blood lymphocyte.
TABLE 4.
Summary of serological, clinical, and ASC data
| Group no.a | VN on PID 0bc | Postinoculationd
|
PID 21ef
|
Postchallenged
|
PCD 7e
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of pigs with diarrheag | % of pigs shedding virush | VNc | IgA ASC
|
IgG ASC
|
% of pigs with diarrhea | % of pigs shedding virus | IgA ASC
|
IgG ASC
|
||||||
| Di | Ij | D | I | D | I | D | I | |||||||
| 1 | 1,566 A | 23 B | 64 B | 684 A | 11* | 7* | 9* | 14* | 42 A, B | 17 A | 25*▵ | 20*▵ | 13* | 51▵ |
| 2 | 1,792 A | 33 B | 50 B | 543 A, B | 0* | 1* | 0* | 3* | 60 A | 20 A | 1* | 1* | 3* | 1* |
| 3 | 17 B | 100 A | 100 A | 305 B, C | 48 | 39 | 29□ | 77 | 25 A, B | 0 A | 57 | 35 | 15* | 85 |
| 4 | 11 B | 91 A | 73 B | 190 C | 27 | 22 | 15 | 111 | 33 A, B | 17 A | 39 | 25 | 16* | 35 |
| 5 | <4 C | 95 A | 100 A | 79 D | 40 | 66 | 52 | 76 | 12 B | 0 A | 94 | 43 | 56 | 56 |
For an explanation of groups, see Table 1, footnote i.
VN, VN GMT.
Means in these columns followed by different letters differ significantly (one-way ANOVA; log-transformed titers).
Proportions in these columns followed by different letters differ significantly (Fisher’s exact test).
Symbols: *, group differed significantly from group 5 for the same isotype at the same time point (Kruskal-Wallis rank sum test); ▵, groups 1 and 2 differed significantly for the same isotype at the same time point (Kruskal-Wallis rank sum test); □, groups 3 and 4 differed significantly for the same isotype at the same time point (Kruskal-Wallis rank sum test).
PID 21 = day of challenge.
Feces were scored as follows: 0, normal; 1, pasty; 2, semiliquid; 3, liquid; feces with a score of ≥2 was considered diarrheic.
Determined by ELISA and CCIF infectivity assay.
D, duodenum.
I, ileum.
(iii) PCD 4.
IgM ASC responses after challenge were minimal in group 5 pigs (Fig. 3 and 4; Table 3), and IgA ASC numbers were similar at PID 21 and PCD 4. In group 2 pigs, there were significantly fewer IgA ASC in duodenum and ileum than in group 5 pigs at PCD 4; in group 1 pigs, there were fewer IgA ASC than in group 5, but not significantly so. In group 5 pigs, IgG ASC numbers in duodenum, ileum, and MLN increased from PID 21 to PCD 4. Although IgG ASC counts increased moderately in duodenum and ileum of group 1 pigs from PID 21 to PCD 4, mean IgG ASC counts remained below 10 per 105 cells in group 2 pigs. Group 1 and 2 pigs had significantly fewer IgG ASC in duodenum and ileum than did group 5 pigs at PCD 4. Numbers of IgG ASC in duodenum of group 3 pigs and numbers of IgG ASC in duodenum, ileum, and MLN of group 4 pigs were significantly lower than those of group 5 pigs. Data were not available for ASC numbers in mock-inoculated pigs at PCD 4.
(iv) PCD 7.
Mean IgM ASC counts were <5 per 105 cells in all tissues of group 5 pigs (Fig. 3 and 4; Table 3). IgM ASC numbers in groups 1 to 4 did not differ significantly from these levels. ASC responses in pigs mock inoculated at PID 0 were dominated at PCD 7 by IgM ASC in intestinal tissues, reflecting primary exposure to rotavirus (data not shown); mean numbers of ASC of all isotypes in systemic tissues (spleen and peripheral blood) were low (<10 per 105 cells) in these pigs (data not shown). There were significantly fewer IgA ASC in duodenum, ileum, MLN, and spleen of group 1 and 2 pigs than in those of group 5 pigs. There were significantly fewer IgG ASC in duodenum and MLN of pigs in groups 1 to 4 than for group 5 (Fig. 3 and 4; Tables 3 and 4).
Effect of milk antibodies on ASC responses.
From PID 21/PCD 0 to PCD 7, mean IgA ASC in duodenum and ileum were higher for group 5 (no maternal antibody) pigs than for group 1 (immune serum) pigs, which were higher than those for group 2 (immune serum plus colostrum and milk) pigs. At PCD 7, there were significantly more IgA ASC in both the duodenum and ileum of group 1 pigs than in those of group 2 pigs (Table 4).
From PID 21 to PCD 7, mean numbers of IgG ASC in duodenum and ileum were higher for group 5 pigs than for group 1 pigs, which were higher than group 2. At PCD 7, there were significantly more IgG ASC in the ileum of group 1 pigs than in those of group 2 (Table 4).
There was a trend (P = 0.08 [data not shown]) towards higher IgG and IgA in the duodenum and ileum of group 3 pigs than in those of group 4 pigs. By PID 21, there were significantly more IgG ASC in the duodenum of group 3 pigs than in group 4 pigs (Table 4). Responses in the two groups were similar at PCD 4 and 7.
A summary of serological, clinical, and ASC data for PID 0 to PCD 7, is presented in Table 4.
Total IgSC responses. (i) PID 8.
Total IgSC data were not available for PID 8 for pigs with no maternal antibodies. Total IgA- and IgG-secreting cell numbers for pigs receiving serum alone (groups 1 and 3) were significantly higher than those for pigs receiving serum and colostrum and milk (groups 3 and 4) in duodenum, ileum, MLN, and spleen (Fig. 5 and 6; Table 5).
FIG. 5.
Mean IgSC counts per 5 × 105 MNC in duodenum, ileum, and MLN. For statistical analysis, see Table 5. NA, not available.
FIG. 6.
Mean IgSC counts per 5 × 105 MNC in spleen and peripheral blood. For statistical analysis, see Table 5. NA, not available.
TABLE 5.
Mean IgSC counts in intestinal and systemic tissues
| Time | Exptl group | ng | No. of IgSCe
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duodenum
|
Ileum
|
MLN
|
Spleen
|
PBLf
|
|||||||||||||
| IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | |||
| PID 8 | No maternal antibodiesa | 0 | NAd | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Serumb | 8 | 1,879 | 779 A | 688 A | 1,711 | 921 A | 929 A | 79 | 116 A | 93 A | 856 A | 388 A | 113 A | 26 | 19 | 39 | |
| Serum + colostrum/milkc | 4 | 409 | 100 B | 0 B | 462 | 98 B | 0 B | 54 | 6 B | 1 B | 32 B | 1 B | 1 B | 32 | 5 | 1 | |
| PID 21 | No maternal antibodies | 6 | 7 B | 1,275 A | 460 | 0 B | 2,412 A | 1,157 A | 0 B | 173 | 347 | 1 B | 54 | 42 | 0 B | 122 | 18 |
| Serum | 8 | 445 A | 1,724 A | 1,027 | 323 A | 1,682 A | 739 A | 86 A | 248 | 134 | 65 A | 86 | 145 | 46 A | 84 | 99 | |
| Serum + colostrum/milk | 4 | 14 A, B | 242 B | 67 | 35 A | 358 B | 208 B | 103 A | 155 | 100 | 73 A | 59 | 30 | 20 A | 18 | 15 | |
| PID 25/PCD 4 | No maternal antibodies | 4 | 74 | 194 B | 379 | 25 B | 669 A, B | 609 | 4 C | 155 B | 237 | 2 B | 36 B | 52 | 1 B | 71 | 29 |
| Serum | 9 | 445 | 5,147 A | 368 | 889 A | 3,456 A | 983 | 86 B | 349 A | 142 | 39 B | 74 A | 102 | 39 B | 83 | 121 | |
| Serum + colostrum/milk | 4 | 274 | 893 A, B | 59 | 1,541 A | 344 B | 320 | 1,544 A | 504 A | 177 | 565 A | 235 A | 114 | 448 A | 121 | 73 | |
| PID 28/PCD 7 | No maternal antibodies | 3 | 11 | 191 | 97 | 8 | 200 B | 77 | 6 B | 31 B | 57 | 2 | 4 B | 2 B | 0 B | 47 | 11 |
| Serum | 10 | 257 | 1,654 | 711 | 381 | 1,789 A | 2,040 | 63 A | 828 A | 426 | 30 | 321 A | 215 A | 10 B | 125 | 258 | |
| Serum + colostrum/milk | 4 | 201 | 3,176 | 280 | 65 | 381 B | 305 | 78 A | 449 A, B | 343 | 48 | 363 A | 185 A | 41 A | 219 | 149 | |
No maternal antibodies: group 5 pigs.
Serum: data from group 1 and group 3 pigs were pooled to examine the effects of administration of serum on total IgSC numbers.
Serum + colostrum/milk: data from group 2 and group 4 pigs were pooled to examine the effects of administration of serum, colostrum, and milk on total IgSC numbers.
NA, not available.
Values for the different Igs are mean numbers of IgSC per 5 × 105 MNC. Means in the same column at the same time point followed by different letters differ significantly (Kruskal-Wallis rank sum test [nonparametric]).
PBL, peripheral blood lymphocyte.
Number of pigs euthanatized in each group at each time point.
(ii) PID 21.
At PID 21, numbers of IgA-secreting cells in duodenum and of both IgA- and IgG-secreting cells in ileum were significantly higher in pigs receiving serum than in pigs receiving serum and colostrum and milk. There were significantly fewer IgM-secreting cells (in all tissues tested) in pigs receiving no maternal antibodies (group 5) than in pigs receiving serum alone.
(iii) PID 25/PCD 4 and PID 28/PCD 7.
There were significantly more IgA-secreting cells in ileum tissue of pigs receiving serum than in that of pigs receiving serum and colostrum and milk. IgM-secreting cell numbers, however, were significantly higher in colostrum- and milk-fed pigs in MLN, spleen, and peripheral blood at PID 25/PCD 4. Mean numbers of IgSC in pigs receiving no maternal antibodies (group 5) were lower than those in pigs receiving serum alone in the majority of tissues, at both time points.
DISCUSSION
In the present study pooled sera with either high (immune) or low (control normal) titers of rotavirus VN antibodies were injected intraperitoneally into newborn gnotobiotic pigs. Material injected intraperitoneally is transported cranially by lymphatic vessels and enters the vena cava and thence the blood circulation (15). Efficient uptake of the injected antibodies into the circulation was evidenced by the high titers of VN antibodies in serum of group 1 (immune serum) pigs by the time of virus inoculation at 3 to 5 days of age. Subsets of pigs also received immune or control colostrum and milk orally for the first 14 days of life.
After inoculation with virulent Wa HRV, pigs in groups 1 (immune serum) and 2 (immune serum plus colostrum and milk) had high levels of passive protection. Rates of diarrhea were comparable to rates in age-matched, mock-inoculated pigs (<33%), whereas rates in groups 3 (control serum), 4 (control serum plus colostrum and milk), and 5 (no maternal antibodies) were significantly higher (>90%). Virus shedding rates were also significantly lower in group 1 and 2 pigs than in groups 3 and 5; interestingly, group 4 pigs, which received control colostrum and milk in addition to control serum, also had a significantly lower rate of virus shedding, suggesting that even moderate titers of local gut (milk) antibodies are effective in reducing viral shedding. Mata et al. (22) have reported partial protection against rotavirus diarrhea in (exclusively) breast-fed infants in Guatemala with very high exposure to rotavirus. Velazquez et al. (34) have reported a significant relationship between duration of breast-feeding and risk of rotavirus infection. Protective effects of breast-feeding may vary with season in countries in which the disease is seasonal rather than endemic (20). The discrepancy in group 4 pigs between viral shedding data and clinical signs (significantly reduced viral shedding but an incidence of diarrhea of 91%) emphasizes the difficulty in predicting clinical protection from vaccine trials in animal models (mice, rabbits) where rotavirus does not cause clinical disease.
The protective effects seen with high titers of circulating maternal antibodies are consistent with the experimental findings of Ward et al. (36), that high titers of passive serum antibodies in colostrum-fed conventional piglets (maintained on soy-based milk replacer) are associated with reduced severity and duration of diarrhea and shorter duration of virus shedding following inoculation with homologous porcine rotavirus. Similarly, Besser et al. (4) have demonstrated protection of neonatal calves against virulent NCDV bovine rotavirus after systemic administration of specific antibodies. Besser et al. further documented the transfer of serum IgG1 into the gastrointestinal tract in neonatal calves (3); a comparable process may be responsible for the passive protection observed in the conventional piglets (36). It is unclear whether a similar transfer of serum antibodies into the intestinal lumen of human infants occurs. However, Quan et al. (26) have demonstrated high levels of Fab fragments (derived from maternal serum IgG) of high affinity in feces of neonates. The clinical importance of these Fab fragments in protection against enteric disease has not been determined, but the fragments displayed strong antibody activity to tetanus toxoid and were present in stools of week-old formula-fed babies. Bernstein et al. (2) have reported that high levels of transplacentally acquired (serum) rotavirus VN antibodies can protect infants up to 1 year of age.
Although group 1 and 2 pigs had high levels of protection after inoculation, protection against diarrhea following challenge was lower than that seen in groups 3 to 5. The duration of diarrhea and rates of virus shedding in group 1 and 2 pigs after challenge, however, were significantly lower than for naive (mock-inoculated, HRV-challenged) pigs, suggesting that a degree of active immunity developed following initial exposure to rotavirus, even in the presence of high titers of maternal antibody. Although increases in IgA and IgG ASC were evident in both the duodenum and ileum of group 1 pigs after challenge, ASC numbers remained low (<10 per 105 cells) in group 2 pigs (Fig. 3; Tables 3 and 4). The moderate level of protection seen in group 1 and 2 pigs after challenge may be attributable in part to cell-mediated immune mechanisms, as cell-mediated responses can occur in the presence of levels of passive antibodies that block humoral immune responses (33) or may be due to the continuing higher levels of circulating maternal antibodies in these pigs (Fig. 2).
Complete protection against rotavirus shedding and 73 to 87% protection against diarrhea were evident in group 3 (control serum alone) and group 5 (no maternal antibodies) pigs, respectively, following challenge (Table 2). Numerous studies have been published concerning protection against reinfection with rotavirus following natural infection. High levels of protection (93 to 100%) against symptomatic reinfection following a single (symptomatic or asymptomatic) rotavirus infection in infants have been reported by Ward and Bernstein (38) and Bernstein et al. (2) in studies conducted in the United States. Bishop et al. (6), in contrast, found neonatal infection with rotavirus to confer no protection against rotavirus infection per se but documented protection against severe clinical manifestations of rotavirus infections in an investigation conducted in Australia. Studies in Mexico (34), Guatemala (22), and India (5) suggest that protective effects of natural infection are considerably lower in developing countries; two or more rotavirus infections may be needed to confer subsequent protection (22, 34). Differences in level of rotavirus exposure, in diversity of rotavirus serotypes present locally, in virulence of local rotavirus strains, in occurrence of concurrent infections, in level of maternal antibodies, and in nutrition (or a combination of these factors) may be responsible for the spectrum of results which have been reported. The high level of protection seen in group 3 and 5 pigs after challenge in the present study may reflect in part the homotypic challenge used and the short time elapsed from induction of immunity until challenge.
Following inoculation of pigs with rotavirus (PID 21), IgG and IgA ASC responses were significantly lower in group 1 and 2 pigs than in pigs without maternal antibodies, in both duodenum and ileum. Surprisingly, significant differences in numbers of ASC persisted in these tissues even after challenge (PID 28/PCD 7 [Table 4]). These results confirm the possibility of suppression of immune responses to oral live rotavirus vaccines in infants with high titers of maternal circulating antibodies. Although in many field studies high prevaccination antibody titers are associated with lower rates of seroconversion, it is difficult in many cases to eliminate the possibility that some serum titers reflect active responses to natural exposure to rotavirus. In the present work, the use of gnotobiotic animals effectively prevents this confounding factor of unknown prior exposure to rotavirus. The significantly lower numbers of intestinal ASC in group 1 and 2 pigs, even after challenge exposure, raise the question of how many exposures to virus at what time intervals would be necessary before comparable ASC responses would be attained. Ward et al. (39) have reported that suppressive effects of prevaccination serum antibodies on serum antibody responses were evident in infants even after three doses of oral rotavirus vaccine.
The present work provides evidence of a suppressive effect of local (colostrum and milk) antibodies beyond that of circulating antibodies alone. From PID 21 to PCD 7, mean numbers of IgA and IgG ASC in duodenum and ileum were higher in group 1 (immune serum) pigs than in group 2 (immune serum plus colostrum and milk), with significantly more IgA and IgG ASC in the ileum of group 1 pigs at PCD 7 (Table 4). Kramer and Cebra (18) previously demonstrated, with enteric reovirus in a mouse model, the decreased synthesis of IgA by Peyer’s patch cells in neonatal mice suckling immune mothers. In their mouse model, circulating passive antibodies had little effect on IgA antibody synthesis, but milk antibodies had potent suppressive effects. The influence of breast feeding on immune responses of human infants to oral rotavirus vaccination is controversial (12, 27) but most likely is dependent on the titer of antibodies present and perhaps the frequency of breast-feeding and lack of supplementation with formula or other foods. Rimer et al., using logistic regression, have demonstrated negative effects of both serum and milk antibody titers on seroconversion to a rotavirus vaccine (28) and also noted that antibody titers in breast milk of mothers were higher in a developing country (Venezuela) than in a developed country (United States [New York State]).
The mechanism(s) involved in suppression of active immune responses by passive antibodies remains unclear. In the context of live oral vaccines, the effect of passive antibodies on virus replication and thus on dose of virus antigen(s) presented to the immune system must be considered. Although mean peak titers of virus shed by group 1 and 2 pigs were not significantly lower than titers for other groups, the percentage of animals shedding after inoculation and the duration of shedding were significantly lower compared to group 5 animals (no maternal antibodies). The reduced numbers of intestinal ASC in pigs with high titers of passive antibodies downplay the relative importance of feedback suppression of differentiated B cells in inhibition of antibody production and point instead to earlier events limiting the induction of proliferation of rotavirus-specific B cells.
In the present study, there were significantly more IgM-secreting cells at PID 21 in all tissues examined in pigs that received injections of serum (immune or control) than in pigs that received no maternal antibodies. This nonspecific enhancement of IgSC in general may reflect interaction of anti-idiotypic antibodies in serum with idiotypes present on neonatal B lymphocytes (14). Feeding of colostrum and milk (immune or control) to pigs which had received serum intraperitoneally was associated (PID 21) with significantly fewer IgA secreting cells in duodenum and significantly fewer IgA and IgG secreting cells in ileum, compared to pigs receiving serum alone, suggesting a nonspecific suppressive effect of components of colostrum and milk. The mechanism of this suppressive effect is unclear.
In summary, although circulating maternal antibodies can mediate a high level of passive protection against rotavirus-induced disease, active immune responses are also suppressed, as evidenced by reduced numbers of ASC in the intestine and reduced protection upon experimental challenge. Local antibodies in the intestines (colostrum and milk) can increase the level of suppression. Modified rotavirus vaccines with high titers of virus, multiple vaccine doses, and improved adjuvant and/or delivery systems may be needed to optimize protection in infants in populations with high levels of maternal antibodies. In this regard, the demonstration that microencapsulation of reovirus can bypass the suppressive effects of maternal antibodies on intestinal IgA responses in suckling mice is highly relevant (25). Immune-stimulating complexes also show promise as a means of stimulating active immune responses in the face of maternal antibodies (33).
ACKNOWLEDGMENTS
We thank Kathy Gadfield, Peggy Lewis, Christine Nielsen, and Paul Nielsen for technical assistance.
This work was supported by grants from the National Institutes of Health (RO1A133561 and RO1A137111) and the World Health Organization (GPV/V27/181/24). Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
REFERENCES
- 1.Barrios C, Brawand P, Berney M, Brandt C, Lambert P H, Siegrist C A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Euro J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein D I, Sander D S, Smith V E, Schiff G M, Ward R L. Protection from rotavirus reinfection: 2 year prospective study. J Infect Dis. 1991;164:277–283. doi: 10.1093/infdis/164.2.277. [DOI] [PubMed] [Google Scholar]
- 3.Besser T E, McGuire T C, Gay C C, Pritchett L C. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J Virol. 1988;62:2234–2237. doi: 10.1128/jvi.62.7.2234-2237.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besser T E, Gay C C, McGuire T C, Evermann J F. Passive immunity to bovine rotavirus infection associated with transfer of serum antibody into the intestinal lumen. J Virol. 1988;62:2238–2242. doi: 10.1128/jvi.62.7.2238-2242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhan M K, Lew J F, Sazawal S, Das B M, Gentsch J R, Glass R I. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–287. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- 6.Bishop R F, Barnes G L, Cipriani E, Lund J S. Clinical immunity after neonatal rotavirus infection. N Engl J Med. 1983;309:72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 7.Bohl E H, Saif L J, Theil K W, Agnes A G, Cross R F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan P L, Sinclair N R. Regulation of the immune response. V. An analysis of the function of the Fc portion of antibody in suppression of an immune response with respect to interaction with components of the lymphoid system. Immunology. 1971;21:967–981. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W K, Campbell T, VanCott J, Saif L J. Enumeration of isotype-specific antibody-secreting cells derived from gnotobiotic piglets inoculated with porcine rotaviruses. Vet Immunol Immunopathol. 1995;45:265–284. doi: 10.1016/0165-2427(94)05343-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark H F, Offit P A, Ellis R W, Eiden J J, Krah D, Shaw A R, Pichichero M, Treanor J J, Borian F E, Bell L M, Plotkin S A. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis. 1996;174:S73–S80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 11.D’Ambrosio D, Hippen K L, Minskoff S A, Mellman I, Pani G, Siminovitch K A, Cambier J C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcγRIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 12.Dennehy P H, Rodgers G C, Ward R L, Markwick A J, Mack M, Zito E T. Comparative evaluation of reactogenicity and immunogenicity of two dosages of oral tetravalent rhesus rotavirus vaccine. Pediatr Infect Dis J. 1996;15:1012–1018. doi: 10.1097/00006454-199611000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Flores J, Perez-Schael I, Blanco M, Vilar M, Garcia D, Perez M, Daoud N, Midthun K, Kapikian A. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989;27:512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson L Å, Jalil F, Ashraf R, Bernini S, Carlsson B, Cruz J R, González T, Hahn-Zoric M, Mellander L, Minoli Y, Moro G, Nave F, Zaman S, Mata L, Karlberg J, Lindblad B S. Characteristics of human milk antibodies and their effect in relation to the epidemiology of breastfeeding and infections in a developing country. In: Mestecky J, Blair C, Ogra P L, editors. Immunology of milk and the neonate. New York, N.Y: Plenum Press; 1991. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. p. 112. [Google Scholar]
- 16.Harte P G, Playfair J H L. Failure of malaria vaccination in mice born to immune mothers. II. Induction of specific suppressor cells by maternal IgG. Clin Exp Immunol. 1983;51:157–164. [PMC free article] [PubMed] [Google Scholar]
- 17.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Field’s virology. 3rd ed. New York, N.Y: Lippincott-Raven Publishers; 1996. pp. 1657–1708. [Google Scholar]
- 18.Kramer D R, Cebra J J. Role of maternal antibody in the induction of virus specific and bystander IgA responses in Peyer’s patches of suckling mice. Int Immunol. 1995;7:911–918. doi: 10.1093/intimm/7.6.911. [DOI] [PubMed] [Google Scholar]
- 19.Lanata C F, Midthun K, Black R E, Butron B, Huapaya A, Penny M E, Ventura G, Gil A, Jett-Goheen M, Davidson B L. Safety, immunogenicity, and protective efficacy of one and three doses of the tetravalent rhesus rotavirus vaccine in infants in Lima, Peru. J Infect Dis. 1996;174:268–275. doi: 10.1093/infdis/174.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Losonsky G A, deRimer H D. Rotavirus specific breast milk antibody in two populations and possible correlates of protection. In: Mestecky J, Blair C, Ogra P L, editors. Immunology of milk and the neonate. New York: Plenum Press; 1991. pp. 265–269. [DOI] [PubMed] [Google Scholar]
- 21.Manca F, Fenoglio D, LiPira G, Kunkl A, Celada F. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J Exp Med. 1991;173:37–48. doi: 10.1084/jem.173.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata L, Simhon A, Urratia J J, Kronmal R A, Fernandez R, García B. Epidemiology of rotaviruses in a cohort of 45 Guatemalan Mayan Indian children observed from birth to the age of three years. J Infect Dis. 1983;148:452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- 23.Meyer R C, Bohl E H, Kohler E M. Procurement and maintenance of germfree swine for microbiological investigation. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul P, Mengeling W L, Malstrom C E, vanDeusen R A. Production and characterization of monoclonal antibodies to porcine immunoglobulin gamma, alpha, and light chains. Am J Vet Res. 1989;50:471–475. [PubMed] [Google Scholar]
- 25.Periwal S B, Speaker T J, Cebra J J. Orally administered microencapsulated reovirus can bypass suckled, neutralizing maternal antibody that inhibits active immunization of infants. J Virol. 1997;71:2844–2850. doi: 10.1128/jvi.71.4.2844-2850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan C P, Ruffet E, Arihiro K, Pires R, Bouvet J P. High affinity serum-derived Fab fragments as another source of antibodies in the gut lumen of both neonates and adults. Scand J Immunol. 1996;44:108–114. doi: 10.1046/j.1365-3083.1996.d01-288.x. [DOI] [PubMed] [Google Scholar]
- 27.Rennels M B. Influence of breast-feeding and oral poliovirus vaccine on the immunogenicity and efficacy of rotavirus vaccines. J Infect Dis. 1996;174:S107–S111. doi: 10.1093/infdis/174.supplement_1.s107. [DOI] [PubMed] [Google Scholar]
- 28.Rimer H C, Wasserman S S, Flores J, Pichichero M E, Losonsky G A. Rotavirus-specific breast milk antibody in two populations and possible correlates of interference with rhesus rotavirus vaccine seroconversion. J Infect Dis. 1992;165:826–830. doi: 10.1093/infdis/165.5.826. [DOI] [PubMed] [Google Scholar]
- 29.Saif L J, Redman D R, Smith K L, Theil K W. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or nonimmunized cows. Infect Immun. 1983;41:1118–1131. doi: 10.1128/iai.41.3.1118-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaller J P, Saif L J, Cordle C T, Candler E, Jr, Winship T R, Smith L K. Prevention of human rotavirus-induced diarrhea in gnotobiotic piglets using bovine antibody. J Infect Dis. 1992;165:623–630. doi: 10.1093/infdis/165.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song C H, Calandra G B, Palmer C J, Miller A, Sercarz E E, Keller M A. Inhibition of offspring response to HEL-CFA by administration of anti-HEL MAB to the mother is not related to the predominant idiotype, IdXE, or specificity of the MAB. Cell Immunol. 1990;131:311–324. doi: 10.1016/0008-8749(90)90257-r. [DOI] [PubMed] [Google Scholar]
- 32.Szakal A K, Burton G F, Smith J P, Tew J G. Antigen processing and presentation in vivo. In: Spriggs D R, Koff W C, editors. Topics in adjuvant research. Boca Raton, Fla: CRC Press; 1991. pp. 11–23. [Google Scholar]
- 33.vanBinnendijk R S, Poelen M C M, vanAmerongen G, deVries P, Osterhaus A D M E. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J Infect Dis. 1997;175:524–532. doi: 10.1093/infdis/175.3.524. [DOI] [PubMed] [Google Scholar]
- 34.Velazquez R F, Matson D O, Calva J J, Guerrero M L, Morrow A L, Carter-Campbell S, Glass R I, Estes M K, Pickering L K, Ruiz-Palacios G M. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 35.Vesikari T, Joensuu J. Review of rotavirus vaccine trials in Finland. J Infect Dis. 1996;174:S81–S87. doi: 10.1093/infdis/174.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- 36.Ward L A, Rich E D, Besser T E. Role of maternally derived circulating antibodies in protection of neonatal swine against porcine group A rotavirus. J Infect Dis. 1996;174:276–282. doi: 10.1093/infdis/174.2.276. [DOI] [PubMed] [Google Scholar]
- 37.Ward L A, Yuan L, Rosen B I, Saif L J. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotavirus in a gnotobiotic pig model. Clin Diagn Lab Immunol. 1996;3:342–350. doi: 10.1128/cdli.3.3.342-350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward R L, Bernstein D I. Protection against rotavirus disease after natural rotavirus infection. J Infect Dis. 1994;169:900–904. doi: 10.1093/infdis/169.4.900. [DOI] [PubMed] [Google Scholar]
- 39.Ward R L, Knowlton D R, Zito E T, Davidson B L, Rappaport R, Mack M E. Serologic correlates of immunity in a tetravalent reassortant rotavirus vaccine trial. J Infect Dis. 1997;176:570–577. doi: 10.1086/514076. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt R G, James W D, Bohl E H, Theil K W, Saif L J, Kalica A R, Greenberg H B, Kapikian A Z, Chanock R M. Human rotavirus type 2: cultivation in vitro. Science. 1980;207:189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- 41.Yuan L, Ward L A, Rosen B I, To T L, Saif L J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan L, Kang S-Y, Ward L A, To T L, Saif L J. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330–338. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]