Abstract
We report the case of a middle-aged man who presented with acute painless monocular vision loss. His medical history was remarkable for chronic total occlusion of the ipsilateral internal carotid artery (ICA) and a recent carotid endarterectomy (CEA) on the contralateral ICA. In a stepwise multidisciplinary approach assessment, we review the differential diagnosis of acute vision loss and investigate how the patient's intracranial and extracranial hemodynamic reorganization after chronic ICA occlusion may affect the clinical reasoning. Early complications of CEA and the differential diagnosis of new-onset anisocoria are also discussed.
Section 1
A 60-year-old man presented to the emergency department with a 2-hour history of acute visual loss in the right eye. His medical history was significant for arterial hypertension, tobacco use, and an asymptomatic chronic complete occlusion of the right extracranial internal carotid artery (ICA) diagnosed 5 years ago. Notably, 3 days before presentation, the patient had undergone a carotid endarterectomy (CEA) for a transient ischemic attack (TIA) resulting from left extracranial ICA stenosis (>70%). On admission, a superior nasal quadrantanopia in the right eye was observed, whereas the visual field of the left eye was fully preserved. Pupils were equal in size and responsive to light. A relative afferent pupillary defect (RAPD) was not observed. The rest of the neurologic examination was unremarkable.
Questions for Consideration:
What are the causes of acute monocular visual loss?
Where do you localize the lesion, and which underlying cause is more likely?
Section 2
The neuro-ophthalmic history of acute vision loss should assess for clinical features, such as the tempo of onset, the pattern of fluctuation, possible associated symptoms, and whether the vision loss is monocular or binocular and transient or permanent.1 Brain disorders that affect the postchiasmatic visual pathways cause contralateral homonymous defects in the visual field that the patient may falsely describe as monocular. Conversely, “true” acute monocular vision loss localizes to ocular (refractive error, corneal and lens abnormalities), retinal (arterial ischemia, venous occlusion, detachment, maculopathy, angle-closure glaucoma), or optic nerve (inflammatory or infectious optic neuritis, arteritic or nonarteritic optic neuropathy) disorders. Improvement with refraction or pinhole strongly suggests an ocular pathology, whereas positive RAPD and disproportionate color vision loss are hallmarks of optic nerve disorders. Painless vision loss suggests vascular (arterial or venous) retinal disorder or vascular nonarteritic ischemic optic neuropathy. In addition, a specific pattern of visual field loss suggests an ischemic rather than a venous etiology.
Therefore, considering our patient's medical history, hyperacute presentation, absence of associated pain, and presence of major cardiovascular risk factors, the diagnosis could be attributed to an embolic retinal infarction, although its etiology was still unclear.
The possibility of a complication due to recurrent stenosis at the site of left CEA was not convincing because this would have led to contralateral homonymous hemianopia or ipsilateral monocular vision loss. Conversely, excluding a pathogenic role of the ipsilateral ICA was more difficult. Chronic total occlusion of the extracranial ICA may result in ischemic symptoms through different pathogenic mechanisms: (1) inadequate cerebral perfusion which may lead to a hemodynamic infarction pattern, (2) detachment of an ICA embolus after spontaneous recanalization, and (3) emboli from the common or external carotid artery.2 In asymptomatic chronic total occlusion of ICA, as in our patient, redirection of blood flow and chronic compensation of secondary collaterals, such as leptomeningeal arteries, anterior and posterior communicating arteries, and the ipsilateral ophthalmic artery, result in preserved cerebrovascular reserve preventing ischemic stroke. In addition, spontaneous recanalization is very unlikely in the presence of long-standing ICA occlusion; hence, the third mechanism was the most reasonable in our patient.
Questions for Consideration:
Which first-level investigations would help confirm the diagnostic hypothesis?
Based on the clinical findings, what is the most probable diagnosis, and what treatment should be considered?
Section 3
An ophthalmologic investigation revealed normal visual acuity and intraocular pressure. The fundus examination revealed a pale optic disc in the right retinal plane and a Hollenhorst plaque in the inferior temporal branch of the central retinal artery (CRA). Therefore, our patient's acute vision loss was secondary to an arterial rather than venous cause and by an embolic rather than a hemodynamic etiology. CRA is a major branch of the ophthalmic artery that usually arises from the intracavernous segment of the ipsilateral ICA. Yet, the ophthalmic artery may serve as a collateral flow for the extracranial-intracranial circulation in the case of chronic total occlusion of the ICA; therefore, this artery was supplied by the ipsilateral ECA in our patient. Indeed, the ophthalmic artery has a rich anastomotic network originating from the facial, maxillary, and medial meningeal arteries (Figure 1).3 In addition, carotid doppler ultrasound, CT angiography of the brain and supra-aortic trunks, and echocardiography were performed and revealed the presence of an intraluminal thrombus at the carotid bulb origin of the right ECA (Figure 2). Taken altogether, although the temporal relationship with the recent CEA remained suspicious, an ischemic atheroembolism from the ipsilateral ECA was deemed responsible for the clinical manifestation. Therefore, carotid endarterectomy of the right ECA was performed as a secondary prevention therapy (Figure 2). Surprisingly, the neurologic examination the day after surgery revealed a new-onset painless anisocoria, greater in light, with a mydriatic right pupil.
Figure 1. Intracranial and Extracranial Circulation in the Lateral View.
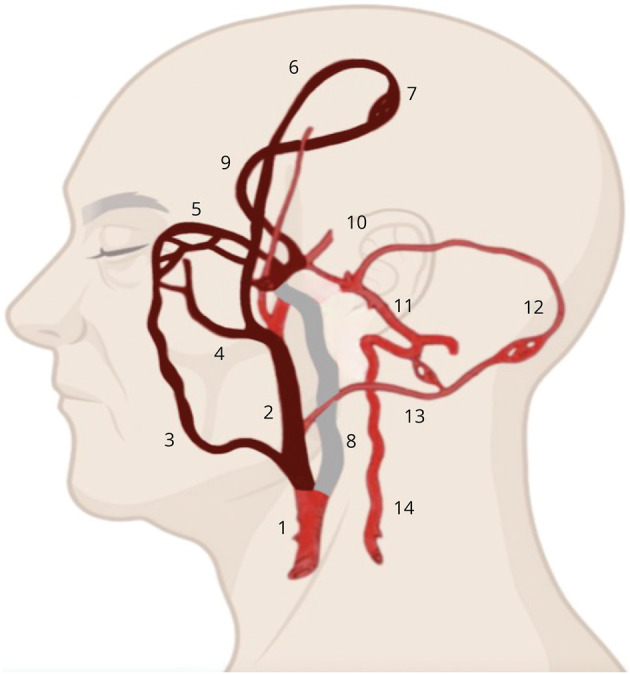
The illustration highlights the potential vascular routes that the external carotid artery thrombus may have traversed to reach our patient's ophthalmic artery (colored in dark red). The occluded extracranial internal carotid artery is colored grey. 1: common carotid artery; 2: external carotid artery; 3: facial artery; 4: internal maxillary artery; 5: ophthalmic artery; 6: middle meningeal artery; 7: leptomeningeal collaterals; 8: internal carotid artery; 9: anterior cerebral artery; 10: middle cerebral artery; 11: basilar artery; 12: posterior cerebral artery; 13: occipital artery; and 14: vertebral artery.
Figure 2. CT Angiography and Intraoperative Images.
Sagittal (A) and axial (B) CT angiography revealed an intraluminal thrombus at the origin of the ECA (yellow arrows), whereas the extracranial ICA is completely occluded (white arrows). Cerebral blood flow in the supraclinoid segment of the right internal carotid artery and its terminal branches is provided by collateral circulation (yellow asterisks), namely the ophthalmic artery (C), the anterior communicating artery, and the posterior communicating artery (D). A surgical incision at the internal carotid bulb revealed a lipid plaque resulting in complete occlusion of ICA (white arrow) and partial occlusion of ECA (yellow arrow) and CCA (E). An endarterectomy was performed, then the arteries were repaired with a Dacron patch, excluding the nonfunctional ICA (F). CCA = common carotid artery; ECA = external carotid artery; ICA = internal carotid artery.
Questions for Consideration:
Can anisocoria be considered an early complication of right CEA?
What are the possible causes of anisocoria in this patient?
Section 4
The most common complications of CEA are postoperative stroke, myocardial infarction, hyperperfusion syndrome, cervical hematoma, nerve injury, infections, and carotid restenosis.4 Cervical hematoma can lead to anisocoria secondary to Horner syndrome, which results from compression of oculosympathetic fibers ascending in the adventitia of ICA, yet this presents as ipsilateral miosis rather than mydriasis.
As a serious medical condition may herald anisocoria, a logical diagnostic approach should be made promptly.5 A detailed review of the patient's medications remains essential, especially eye drops instilled in the patient's eye. Prior ocular disease and associated symptoms, such as pain and diplopia, should be assessed. Shape, size, and location of patient's pupils should be observed. Pupillary reactivity should be evaluated. The abnormal pupil must also be identified by measuring its size in bright light (parasympathetic-dominant constriction) and dark (sympathetic-dominant dilatation). In the “anisocoria greater in bright light” scenario, as in our patient, a parasympathetic pathway dysfunction or, less commonly, asymmetric sympathetic hyperstimulation results in an abnormal ipsilateral large pupil.
Anecdotally, there have been reports of irritation of the oculosympathetic chain from carotid artery injuries, a condition known as Pourfour du Petit syndrome. This presents with persistent ipsilateral mydriasis, hyperhidrosis, and eyelid retraction referred to as reverse Horner syndrome.6
Based on anatomical location, the underlying parasympathetic-related mechanisms can be categorized in (1) preganglionic parasympathetic fiber dysfunction (III cranial nerve external fiber damage), (2) postganglionic parasympathetic fiber dysfunction (e.g., Adie tonic pupil, pharmacologic mydriasis), and (3) ocular disease (e.g., traumatic injury, close-angle glaucoma). Most of these mechanisms are suggested by a medical history, including a review of medications. Indeed, topical or aerosolized medications, including parasympatholytic cycloplegic or sympathomimetics drugs, can cause pharmacologic mydriasis, yet the most common cause is eye drops administered by ophthalmologists.
In our patient, we can infer that anisocoria did not occur as a complication of CEA but stemmed from his previous ophthalmologic visit for pharmacologic mydriasis. The physician who had performed the ophthalmologic evaluation the previous day was contacted and confirmed that he had administered significantly more eye drops to the right eye. After 24 hours of clinical observation, our patient's anisocoria had disappeared and his vision recovered, confirming the diagnosis of pharmacologic anisocoria.
Discussion
Retinal arterial ischemia is the most attributed cause of acute monocular vision loss in the elderly and often represents the heralding clinical presentation of carotid artery disease. Hollenhorst plaques are yellow/orange cholesterol emboli originating from the ipsilateral carotid artery or the aortic arch, confirming the atheroembolic mechanism of retinal embolization.7 The optimal acute management for retinal infarction is still debated. In small clinical studies, intravenous thrombolysis was effective and safe when administered early (≤4.5 hours). Nevertheless, high-quality evidence is still lacking, and the absence of a diagnostic modality that can detect salvable retinal ischemic penumbra, such as the brain CT perfusion mismatch in cerebral stroke, hinders further development of reperfusion strategies in retinal arterial ischemia.8
The patient's medical history was potentially misleading, as the recent surgical intervention raised suspicion of complications. Secondary collaterals originating from the ECA were sufficient to preserve his cerebral and retinal perfusion and arguably modify the hierarchical role of the ECA. Indeed, stenotic plaques in the ECA detected by Doppler ultrasound or CT angiography are usually neglected because no vital tissue is supplied by this vessel. Conversely, in the context of chronic occlusion of ipsilateral ICA, the ipsilateral ECA may acquire importance comparable with the internal carotid artery. Indeed, it has been demonstrated that the retina, a partial territory of the middle cerebral artery, and, to a lesser extent, of the anterior cerebral artery are supplied by the ECA in patients with chronic occlusion of ICA.9
In addition, our case highlights the importance of considering pharmacologic causes in the differential diagnosis of new-onset anisocoria. Indeed, to achieve adequate mydriasis for fundus examination, pharmacologic agents are often administered, the effects of which might last for 24–48 hours. The diagnostic hypothesis can be supported by the loss of constriction of the affected eye after the administration of 1% pilocarpine, yet if strong suspicion arises from the medical history, clinical observation until complete resolution may be a more practical approach.
Appendix. Authors

Study Funding
The authors report no targeted funding
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Prasad S, Galetta SL. Approach to the patient with acute monocular visual loss. Neurol Clin Pract. 2012;2(1):14-23. doi: 10.1212/cpj.0b013e31824cb084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu B, Li C, Guo Y, Xu K, Yang Y, Yu J. Current understanding of chronic total occlusion of the internal carotid artery. Biomed Rep. 2018;8(2):117-125. doi: 10.3892/br.2017.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279-2284. doi: 10.1161/01.str.0000086465.41263.06 [DOI] [PubMed] [Google Scholar]
- 4.Wu TY, Anderson NE, Barber PA. Neurological complications of carotid revascularisation. J Neurol Neurosurg Psychiatry. 2012;83(5):543-550. doi: 10.1136/jnnp-2011-301162 [DOI] [PubMed] [Google Scholar]
- 5.Gross JR, McClelland CM, Lee MS. An approach to anisocoria. Curr Opin Ophthalmol. 2016;27(6):486-492. doi: 10.1097/icu.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-de la Torre JR, Drake-Pérez M, Casado A, et al. Persistent isolated mydriasis as an early sign of internal carotid artery dissection: pourfour du petit syndrome. Clin Neurol Neurosurg. 2019;182:70-72. doi: 10.1016/j.clineuro.2019.04.030 [DOI] [PubMed] [Google Scholar]
- 7.Graff-Radford J, Boes CJ, Brown RD Jr. History of Hollenhorst plaques. Stroke. 2015;46:e82-e84. doi: 10.1161/strokeaha.114.007771 [DOI] [PubMed] [Google Scholar]
- 8.Mac Grory B, Lavin P, Kirshner H, Schrag M. Thrombolytic therapy for acute central retinal artery occlusion. Stroke. 2020;51(2):687-695. doi: 10.1161/strokeaha.119.027478 [DOI] [PubMed] [Google Scholar]
- 9.van Laar PJ, van der Grond J, Bremmer JP, Klijn CJ, Hendrikse J. Assessment of the contribution of the external carotid artery to brain perfusion in patients with internal carotid artery occlusion. Stroke. 2008;39(11):3003-3008. doi: 10.1161/strokeaha.108.514265 [DOI] [PubMed] [Google Scholar]



