Abstract
The Pharmaceuticals and Personal Care Products (PPCPs) presence at harmful levels has been identified in aquatic ecosystems all over the world. Currently, PPCPs are more common in aquatic regions and have been discovered to be extremely harmful to aquatic creatures. Waste-water treatment facilities are the primary cause of PPCPs pollution in aquatic systems due to their limited treatment as well as the following the release of PPCPs. The degree of PPCPs elimination is primarily determined by the method applied for the remediation. It must be addressed in an eco-friendly manner in order to significantly improve the environmental quality or, at the very least, to prevent the spread as well as effects of toxic pollutants. However, when compared to other methods, environmentally friendly strategies (biological methods) are less expensive and require less energy. Most biological methods under aerobic conditions have been shown to degrade PPCPs effectively. Furthermore, the scientific literature indicates that with the exception of a few extremely hydrophobic substances, biological degradation by microbes is the primary process for the majority of PPCPs compounds. Hence, this review discusses about the optimistic role of microbe concerned in the degradation or transformation of PPCPs into non/less toxic form in the polluted environment. Accordingly, more number of microbial strains has been implicated in the biodegradation/transformation of harmful PPCPs through a process termed as bioremediation and their limitations.
Keywords: Microbes, Metabolic process, PPCPs, Biodegradation, Conversion pathways
1. Introduction
The surge in the global population, financial growth, and the industrial era, along with global warming has resulted in a boom in waste generation and also the implementation of particular groups of substances known as emerging contaminants in the aquatic environment [1]. The government agencies of various nations define emerging contaminants as any chemicals or microbes that are not typically explored in the natural setting but have the potential to determine their way toward the environment and lead to recognized or suspected detrimental ecological as well as human health impacts [2]. Thus, there exists a critical requirement to create methods for removing emerging contaminants. Emerging pollutants such as PPCPs, perfluorinated compounds (PFCs), microplastics, and brominated flame retardants (BFRs), in the aquatic system, can cause severe detrimental effects on aquatic biology and ultimately affect human health [3]. Adsorption (e.g., activated carbon and graphene), membrane process, oxidation process (e.g., ozonation, Fenton oxidation, UV-oxidation, electrochemical), combined methods (physical and chemical), and other approaches are used to remove PPCPs from aquatic environments [4]. However, these approaches are expensive, may cause secondary pollution, and are ineffective. Few classes of PPCPs are: pharmaceuticals, heavy metals, hormones, analgesics, antibiotics, and antidepressants, among others, and personal hygiene products such as tooth-pastes, facial creams, and so on are frequently detected in the aquatic environment [5].
The PPCPs contaminants may thus be prioritized for oversight based on knowledge gathered from surveillance data about their presence, toxic effects, possible adverse health effects, as well as public perception. While the disintegration of PPCPs in aquatic systems varies, these substances are mainly water-soluble and possess traits that enable them to pass through cell membranes as well as remain in biological tissues [4]. Nevertheless, the basic characteristics of PPCPs raise concerns regarding their hazardous effects, accumulation perspective, as well as bioactivity in an aquatic ecosystem. The PPCPs, like administered quantities on targets, have the capability of changing biochemical as well as physiological processes in a wide range of non-target organisms [6]. Hence, they have the potential to have negative effects on native as well as peripatetic species of living things exposed to PPCPs contaminated environments.
While the existence of PPCPs in the aquatic ecosystem has been observed for some time, the full extents of their existence as well as the potential hazards connected with their existence in the environment have yet to be determined [5]. Furthermore, the elimination of such pollutants in wastewater treatment facilities prior to release into the environment has been challenging owing to their minimal occurring levels and the difficulties associated with analyzing them [7]. Thus, existing treatments must be modified and upgraded in order to resolve as well as eliminate these PPCPs contaminants. Traditional wastewater treatment approaches consist of the following methods: adsorption by activated carbon (AC), filtration, ozonation, flocculation, photo-catalysis by ultraviolet irradiation, coagulation process, nanotubes of carbon (CNTs), sedimentation, ultra-sonication, chlorination, membrane filtration, and biological methods [8]. However, these techniques are insufficient for removing organic contaminants from sewage.
Besides being chemically as well as practically demanding, these procedures necessitate large systems, infrastructures as well as engineering skills, resulting in them being demanding, inefficient, lengthy, as well as expensive [9]. Additionally, the utilization of nano-adsorbents in the detoxification of PPCPs from water matrix structures has been accompanied by certain difficulties, including smaller particle sizes, secondary contaminants generation, failure for recycling or regenerating, and so on [10]. Furthermore, the application of semiconductors as enzymes in the decomposition of PPCPs possesses disadvantages as follows: they require higher UV radiation over their functions owing to their broad band gap power; several carcinogenic, such as TiO2; as well as there is a problem with recombination of electron-hole pairs upon usage. Hence, green, commercially effective, and cost-effective techniques for contamination management and avoidance are required for safeguarding the environment as well as discharges of effluent to have the smallest effect on the biosphere and ultimately human health [11]. Hence, microbes-based bioremediation is considered a more promising approach than the already existing alternative above-mentioned methods.
2. Source of PPCPs and its presence in aquatic ecosystem
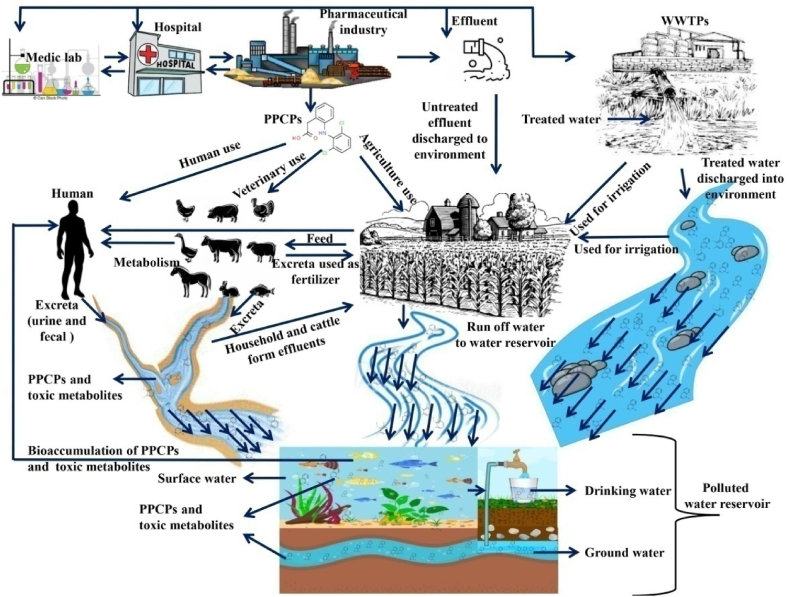
Over the last 1.5 decades, the scientific, governing, along with commercial sectors have worked hard to understand the origin, effects, as well as hazards associated with PPCPs in the aquatic environment. The day-to-day life consumption/utilization of regular drugs used for some selective disorders (e.g. diabetics, antibiotics, and so on), and beauty products (shampoos, moisturizers, deodorants, hair colors, and so on) based PPCPs cause severe environmental pollution and unintentionally residing in aquatic ecosystems from the 1970s onwards [12]. In the past two decades, PPCPs residues were found in every kind of aquatic system. Because of their hydrophilic nature and the poor efficiency of treatment plants, such PPCPs directly reach different aquatic environments [13]. Fig. 1 depicts a schematic illustration of possible PPCPs sources along with pathways. Pharmaceuticals as well as nutraceuticals are used for human and animal health issues as well as health supplement needs. Pharmaceuticals such as analgesics, antibiotics, various forms of hormones, anti-diabetics, antihypertensives, as well as a variety of other health-related substances are included [14]. These chemicals are constantly released into aquatic ecosystems from domestic as well as industrial processes. A worldwide investigation found multiple categories of PPCPs in the majority of aquatic locations. Inevitably, these statistics are based mainly on investigations carried out in various nations. The accessibility of this kind of information in those regions of the globe is due to a scarcity of studies on PPCPs along with other endocrine-disrupting pollutants in Asia and African countries [15]. Regardless of a shortage of data, the presence of certain PPCPs at concentrations that occasionally exceeded in WWTP water as well as aquatic waters has been reported [8]. Investigation on the treatment of PPCPs polluted aquatic environment, as well as the elimination of such pollutants in water treatment facilities as well as while effluent treatment prior to release into fresh water, remains substantially behind and could be lacking [16]. Table 1 shows the most typically found PPCPs in various aquatic environment as well as their prevalence levels in various countries. It is critical to remove PPCPs from aquatic environments in order to avoid the toxicity of PPCPs on aquatic organisms, which may enter the food chain and eventually reach humans.
Fig. 1.
Schematic diagram of PPCPs pollutants source and circulation in the environment [17].
Table 1.
Commonly reported PPCPs in different aquatic samples around the world.
| S.No | Name of PPCPs | Dosage: μg L-1 | Reported source | References |
|---|---|---|---|---|
| 1 | Acetaminophen | 0.35–1.52 | Cilfynydd and Coslech effluent treatment influent South Wales, UK | [8] |
| 0.56–211.38 | Cilfynydd and Coslech effluent treatment effluent, South Wales, UK | |||
| 11.3 | Effluent treatment plant, France | |||
| 5.76 | Effluent treatment plant, South Africa | [18] | ||
| 0.023–6.89 | Effluent treatment plant (influent), Korea | [19] | ||
| 5.68–74.552 | Effluent treatment plant (effluent), Korea | |||
| 2 | Aspirin | 0.02–1.56 | Cilfynydd and Coslech effluent treatment influent South Wales, UK | [8] |
| 0.84–2.49 | Cilfynydd and Coslech effluent treatment effluent, South Wales, UK | |||
| 0.012–1.43 | Surface and waste water treatment plant inffluent, Catalonia, Spain | |||
| 0.098–2.566 | Surface and waste water treatment plant effluent, Catalonia, Spain | |||
| 59.6–256 | Effluent treatment plant influent, Canada | |||
| 47.52–874 | Effluent treatment plant effluent, Canada | |||
| 3 | Bisphenol A | 0.0614–0.964 | Municipal effluent, Harbian and Tianjin city, China | [20] |
| 0.875–1.52 | Municipal effluent, Harbian and Tianjin city, China | |||
| 0.002–0.047 | Drinking water and sewage treatment plant, Germany | [21] | ||
| 0.028 | Drinking water, South Africa | [22] | ||
| 4 | Benzophenone | 1.5–8.60 | Sewage treatment plant, India | [21] |
| 5 | Carbamazepine | 0.83–4.21 | Cilfynydd and Coslech effluent treatment influent South Wales, UK | [8] |
| 0.85–2.49 | Cilfynydd and Coslech effluent treatment effluent, South Wales, UK | |||
| 1.9–2 | Wastewater treatment plant influent, Canada | |||
| 2.0–2.3 | Wastewater treatment plant effluent, Canada | |||
| 0.42 | Effluent from WWTP, Taiwan | |||
| 6 | Diclofenac | 12.4–22.3 | Effluent and influent from WWTP, South Africa | [23] |
| 15.3–19.5 | Effluent from WWTP, Germany | [8] | ||
| 0.21–0.49 | Effluent from WWTP, France | |||
| 0.119–0.285 | Influent from WWTP, UK | |||
| 0.201–0.397 | Effluent from WWTP, UK | |||
| 0.145–0.251 | Effluent and influent from WWTP, Japan | |||
| 0.145–197 | Influent from WWTP, Japan | |||
| 7 | Estrone | 0.054–0.351 | Effluent from WWTP, South Africa | [24] |
| 0.078–0.96 | Influent from WWTP, Tianjin and Harbin city, China | [20] | ||
| 0.92–0.158 | Effluent from WWTP, Tianjin and Harbin city, China | |||
| 8 | Estriol | 0.042–0.162 | Influent from WWTP, China | |
| 9 | 17-beta-Estradiol | 0.02–0.58 | Influent from WWTP, South Africa | [24] |
| 0.62–0.199 | Effluent from WWTP, South Africa | |||
| 0.0087–0.090 | Influent from WWTP, Tianjin and Harbin city, China | [20] | ||
| 0.0091–0.093 | Effluent from WWTP, Tianjin and Harbin city, China | |||
| 0.0041 | Municipal influent & effluent, USA | [8] | ||
| 10 | Ibuprofen | 0.015–4.582 | Influent from WWTP, Korea | [19] |
| 0.024–9.494 | Effluent from WWTP, Korea | |||
| 5–8 | Effluent from WWTP, Canada | [8] | ||
| 58.711–35.62 | Influent from WWTP, South Africa | [18] | ||
| 59.61–62.82 | Effluent from WWTP, South Africa | |||
| 0.14–2.1 | Influent from WWTP, UK | |||
| 0.26–2.29 | Effluent from WWTP, UK | |||
| 11 | Nalidixic acid | 1.73–25.11 | Influent from WWTP, South Africa | [23] |
| 1.98–30.84 | Effluent from WWTP, South Africa | |||
| 0.20–0.55 | Influent from WWTP, Australia | [18] | ||
| 0.22–0.75 | Effluent from WWTP, Australia | |||
| 12 | Naproxen | 0.17–0.85 | Influent from WWTP, UK | [8] |
| 0.25–1.173 | Effluent from WWTP, UK | |||
| 0.120–3.24 | Influent from WWTP, Korea | [19] | ||
| 0.180–5.938 | Effluent from WWTP, Korea | |||
| 13.50–36.52 | Influent from WWTP, South Africa | [25] | ||
| 15.68–55.00 | Effluent from WWTP, South Africa | |||
| 13 | Progesterone | 0.025–0.562 | Influent from WWTP, South Africa | [24] |
| 0.036–0.904 | Effluent from WWTP, South Africa | |||
| 14 | Sulfamethoxazole | 0.20–0.56 | Surface water, Australia | [18] |
| 0.26–1.24 | WWTP effluent, Australia | |||
| 0.35–2.00 | WWTP influent, Australia | |||
| 3.68–25.63 | WWTP influent, South Africa | |||
| 3.86–34.50 | WWTP effluent, South Africa | |||
| 0.023–0.032 | WWTP effluent, UK | |||
| 0.025–0.049 | WWTP influent, UK | |||
| 15 | Tetracyclines | 0.02–0.10 | WWTP effluent and influent, Australia | [25,26] |
| 0.02–0.06 | WWTP influent, Australia | |||
| 0.6–5.7 | Surface water, South Africa | |||
| 16 | Triclosan | 78.50–127.80 | Wastewater treatment plant influent | |
| 17 | Testosterone | 0.035–0.542 | WWTP effluent, South Africa | [24] |
| 0.026–0.635 | Wastewater influent, South Africa | |||
| 18 | Keroprofen and ibuprofen | 7-57 and 5-11 | Sediment, Msunduzi River, South Africa | [27] |
| 19 | Clofibric acid | 1–9 | Fresh water, Swiss lakes | [28] |
| 20 | Lumefantrineand artemether | 3–32 | Fresh water, Africa | [29] |
3. PPCPs classification
Pharmaceuticals are divided into active organic classes of substances as follows [30]: (a) Antibiotics (b) Steroids hormones (c) Analgesic as well as nonsteroidal anti-inflammatory drugs (NSAIDS) (d) Antiepileptics (e) Blood lipid regulators (f) β-blockers and (g) Antineoplastic. Depending on the description of use, antimicrobial agents, fungicides, disinfectants, synthetic musks, some preservatives, some sunscreen UV filters, and so on may also be referred to as drugs. Personal Care Products (PCPs) are categorized as follows: (a) Insect repellants, (b) facial ingredients, (c) soaps & detergents, (d) sunscreen UV filters, (e) triclosan, and (f) antiseptics.
4. PPCPs toxicity
The advantages of using medicines as well as numerous PPCPs as supposed are tremendous. Drug consumption causes unique biological reactions in the patient, following which 10–90% of excessive unused dosages along with certain conjugates metabolic products are eliminated and released into the surroundings [31]. In light of their poor persistence, degradation, as well as biological processes, their flow while existing in various environments may pose a threat to non-target lives found in aquatic as well as terrestrial environments. According to recent information, drinking water resources, particularly those obtaining supplies of water from PPCPs residues polluted sources as well as may require further processing to eliminate the contaminants prior to consumer's availability [8].
Because PPCPs might have negative health as well as ecological impacts on aquatic ecosystems, there has been increasing concern regarding their effects on the environment [32]. Further, PPCPs may exist in the natural environment as blends of different contaminants, causing complementary negative impacts to both aquatic as well as terrestrial creatures regardless of low amounts. The main source to worry regarding the toxic effects of PPCPs lies in the fact that they have been developed to increase their biological function at low dosages along with targeting unique metabolic processes, and enzymatic, or cell-signalling mechanisms [8,33]. The ecological preservation of such targeted molecules in particular species might raise the likelihood that these medicinal products are pharmacologically influential in organisms that do not target [34]. The notion of mode of reaction can be employed for all aquatic organisms that are accidently in contact with PPCPs in their native setting, increasing the probability of ecotoxicological impacts [35]. Fluoxetine, a depressive drug that focuses on the serotonin signalling process, was used to investigate the mode of action of the theoretical framework and it showed negative effects on vital physiological processes in oysters, such as reproduction, metabolic processes, as well as movement, at quantities nearing or considerably beneath environmental extents [36]. The capacity of PPCPs to interact with the hormonal (endocrine) system and produce unwanted homeostasis disruption remains a major issue raised via their existence in aquatic ecosystems [5]. Moreover, the toxicity caused by complicated combinations of PPCPs at modest levels may result in synergistic connections. Therefore, even if individual PPCPs exist at minimal levels and do not cause essential toxic effects while acting alone, PPCP combinations can still cause significant ecological toxicity. The antiepileptic substance carbamazepine along with the lipid-lowering drug clofibric acid was shown to have significantly greater toxic effects on Daphnia magna compared to single substances at a similar concentration [37]. It was also discovered that the synergistic action of estradiol along with 4-tert-nonylphenol can induce vitellogenin synthesis in youth rainbow trout. The influence of diclofenac, a most common drug in surface water, was studied in an investigation on brown trout [38]. Diclofenac at quantities ranging from 5 to 50 μg L−1 was found to have an effect on the kidney as well as gill integrity, as well as certain immune functions in fish (brown trout). Another investigation discovered that 17-estradiol being subjected to Leuciscus cephalus fish, led to a substantial along with a swift boost in plasma vitellogenin (Vtg) among both males as well as female Leuciscus cephalus [39]. A research team additionally found that Carssius auratus experienced a 50% reduction in plasma testosterone upon 14 days of exposure [40]. PPCPs also triggered drug resistance genes to invade aquatic organisms, as well as toxic effects on development along with hepatotoxicity. A research team found that the considerable reduction in cholesterol levels and it influenced by changed mRNA expression of lipid-metabolizing genes in the zebrafish subjected to PPCPs [41]. Researchers recently reported that ecological oestrogens, specifically BPA along with 17-estradiol (E2), disrupted normal metabolic processes of lipids in the zebrafish and caused substantially elevated lipogenesis in female fish [42]. The male species also showed early-phase sexual feminism which was followed by decreased spermatids, deposits of fat, as well as lipogenic transcription trends similar to female fish. The antibiotics comes under the category of fluoroquinolone demonstrated toxic effects on algae, fish, and crustacean, such as Lemna minor, Pseudokirchneriella subcapitata, Pimephales promelas, Microcystis aeruginosa, and Daphnia magna [43,44].
5. Antibiotic resistance
Antibiotics are commonly used in farm animals for defense, therapy, as well as growth, resulting from the selection of genes of more harmful microbes [45]. Constant antibiotic exposure has been identified as a possible cause of generated drug resistance among numerous pathogenic microbes. Steroid hormones, which are used as sex steroids, and hormonal contraceptives, can disrupt the hormonal system as well as serve as anti-androgenic ligands [46]. Endocrine disturbance has been scientifically linked to the sterilization, imposex, as well as gender equality of some vertebrates, including mollusks, fish, and numerous other aquatic creatures. Researchers reported that the occurrence of oestrogen as well as progestogens at quantities as tiny as 1.0 μg L−1 induced endocrine disruption, resulting in fish femininity as well as decreased infertility [47].
Merely a minimal dosage of 5 μg L−1 of diclofenac accumulation on tissues of rainbow trout was observed and experienced negative endocrine impacts. Conversely, zebrafish exposed to a combination of acetaminophen, venlafaxine, carbamazepine, and gemfibrozil at quantities ranging from 0.5 to 10 μg L−1 experienced tissue deterioration, a decrease in embryo creation, as well as a rise in embryo death rates [48]. Being exposed to endocrine-disrupting substances (pollutants) in aquatic environments has also been linked to hypothalamic-pituitary-gonad (HPG), hypothalamic-pituitary-thyroid (HPT), and hypothalamic-pituitary-adrenal (HPA) modulation, leading to interfering with the functioning as well as functions of different physiological characteristics in certain non-target aquatic vertebrates [49]. Another report stated that the drinking water of certain regions of South Africa's contains drugs like valproate, carbamazepine, lamotrigine, as well as levetiracetam, these are responsible for the disruption of endocrine systems in humans as well as in some fish species [50]. Furthermore, another report revealed that the NSAIDs such as Ibuprofen and Naproxen, which are frequently not removed by WWTPs, may have a negative effect on the non-target vertebrate creatures' endocrine system [8]. For instance, Oryzias latipes subjected to 0.1 μg L−1 quantity of Ibuprofen showed postponed hatching, whereas in humans 1 mg L−1 concentration of Ibuprofen can cause a rise in blood plasma extents [51].
6. Significance of reclamation of PPCPs from aquatic environment
According to one report, over 4000 distinct categories of PPCPs along with related metabolic products have been discovered in different aquatic ecosystems around the world as they have been administered as therapies for different healthcare requirements in people, animals, as well as the aquaculture sector [52]. The direct release of waste products from pharmaceutical industries, hospital waste disposals, municipal sludge, WWTP water, and excrement from humans as well as animals can cause PPCPs contamination in various forms of water bodies [52]. Creatures such as leopard frog, rainbow trout, Cyanobacteria, benthic meiofauna, green algae, Mexican shrimp, duckweed, Japanese rice fish, chironomids, beneficial soil bacteria, vultures, zebrafish, and so on are all vulnerable to PPCPs pollution through the aquatic ecosystem [17]. For instance, diphenhydramine, thioridazine, and sertraline, can cause acute poisoning in certain algal species, as well as native fish species. Moreover, painkillers medications as well as their metabolites might be toxic to amphibian species, fish varieties, as well as beneficial microbial communities [53]. Notably, phytoplankton as well as invertebrates plays a significant part in the ecosystem's as well as food chain. Hence, it must be removed from contaminated water using recyclable potential microbes for PPCPs degradation [54]. Traditional methods such as activated sludge, coagulation, flocculation, membrane bioreactor, and sedimentation are used for PPCPs removal from the polluted water. Despite the fact that the naturally occurring transformation process such as physical, chemical, as well as biological processes can remove certain quantities of PPCPs pollutants from polluted water [55]. However, a considerable quantity of the parent forms of PPCPs and associated metabolites remain in the polluted water. While the complete elimination of PPCPs and associated metabolites from aquatic systems are not possible using physical, chemical, and microbiological methods [56]. Several PPCPs and their metabolites persist in the wastewater treated by conventional treatment. For instance, so far reported microbial bioremediation processes unable to degrade or remove the carbamazepine from the polluted aquatic systems [57].
The insufficient ability of various methods to entirely eliminate the PPCPs from polluted water might be directly linked to their physicochemical attributes, parent compound-complex structure, as well as poor bioavailability [58]. It is essential to identify a successful replacement as well as a sustainable method that reduces the treatment time PPCPs pollutants. Investigators are engaging on removing the PPCPs and associated metabolites from environments using microorganisms alongside recoverable as well as reusable perspective [59]. Because microbes have more precise enzymes, they are more versatile in nature, and they are easier to manage. Selective microbes have the potential to degrade the PPCPs and their metabolites from polluted aquatic systems; however, these cannot completely degrade or eliminate the PPCPs [8].
Investigators are attempting to identify the competitive microbial strain for removing/degrading the PPCPs as well as associated metabolite products in the aquatic systems [60]. The competitive microbial strains can metabolize as well as convert the toxic form to a non-toxic form in the aquatic system. Several microbial strains and their enzymes have been used for various commercially valuable applications [61]. Among the microbes, bacteria have among the most abundant source of enzymes that are more active compared to any other creatures, including higher living things. Microbes and their active enzymes may break down both inorganic as well as inorganic blended contaminants from polluted water and yield CO2, water, CH3, biomass, and various other byproducts without disrupting the balance of the ecosystem [62]. This review provides extensive details on the different kinds of microorganisms engaged in the degradation of PPCPs from aquatic environments.
7. Metabolism of PPCPS in humans and animals
Many PPCPs are in soluble nature as well as their bioactive properties can promote biological transformation as well as depuration with their biological properties [56]. This doesn't seem to be a problem with many PPCPs as well as associated metabolite products in the aquatic ecosystem, as they do not break down easily. Several pharmaceuticals are excreted from humans and animals as a blend of the parent drug as well as associated metabolite products. The degrees of metabolism, as well as pharmacokinetics index, are determined by the proportion of quantities of the un-metabolized drug to associated metabolites determined via urine or feces analysis [63]. Tracking the PPCPs metabolic products in the aquatic environment seems limited, since the absence of established guidelines for environment-bound PPCPs metabolites, results in insufficient data on the presence, levels, behavior, as well as half-life of drug metabolites within aquatic environments. The research team reported that the carbamazepine epoxy ranged from 881 to 4025 ng L−1 in influent effluent, whereas the corresponding parent drug (carbamazepine) dosage ranged from 1.6 to 112 ng L−1 [64]. Hence, there's an obligation to comprehend ecological biotransformation, microbial metabolism, drug metabolites, as well as PCPs metabolite evaluation. This process is necessary owing to the predictable time based degradation in the PPCPs parent drug quantity in the aquatic system and associated metabolites possess detrimental properties compared to parent compound in the aquatic environment.
8. Microbial degradation of PPCPs
8.1. Bacteria
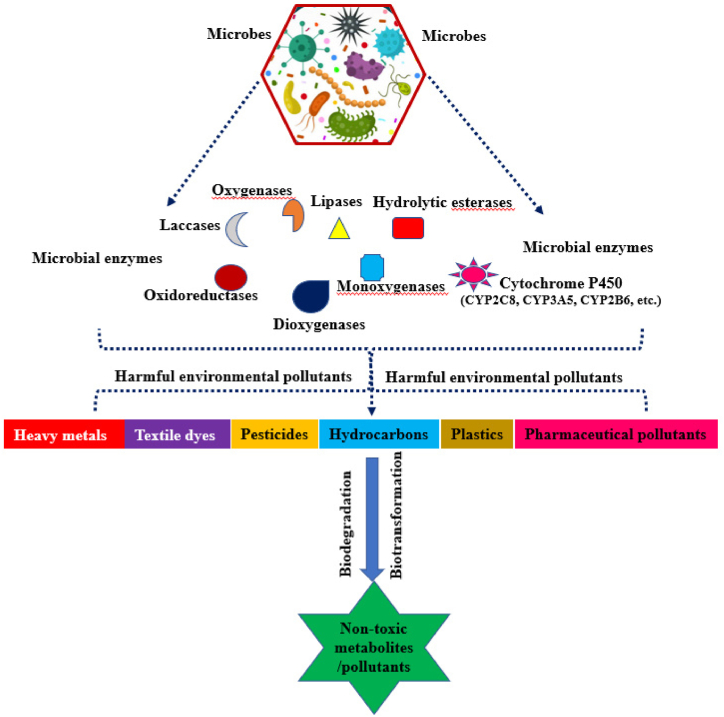
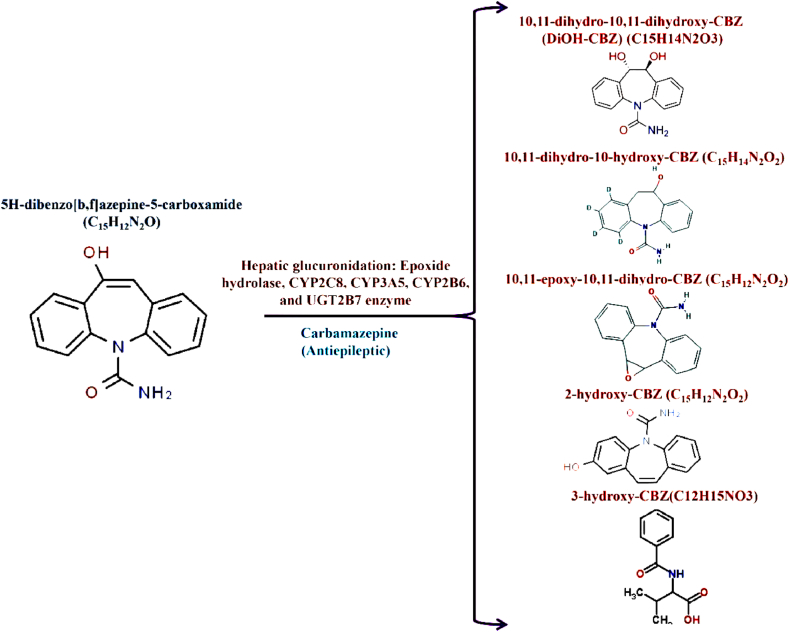
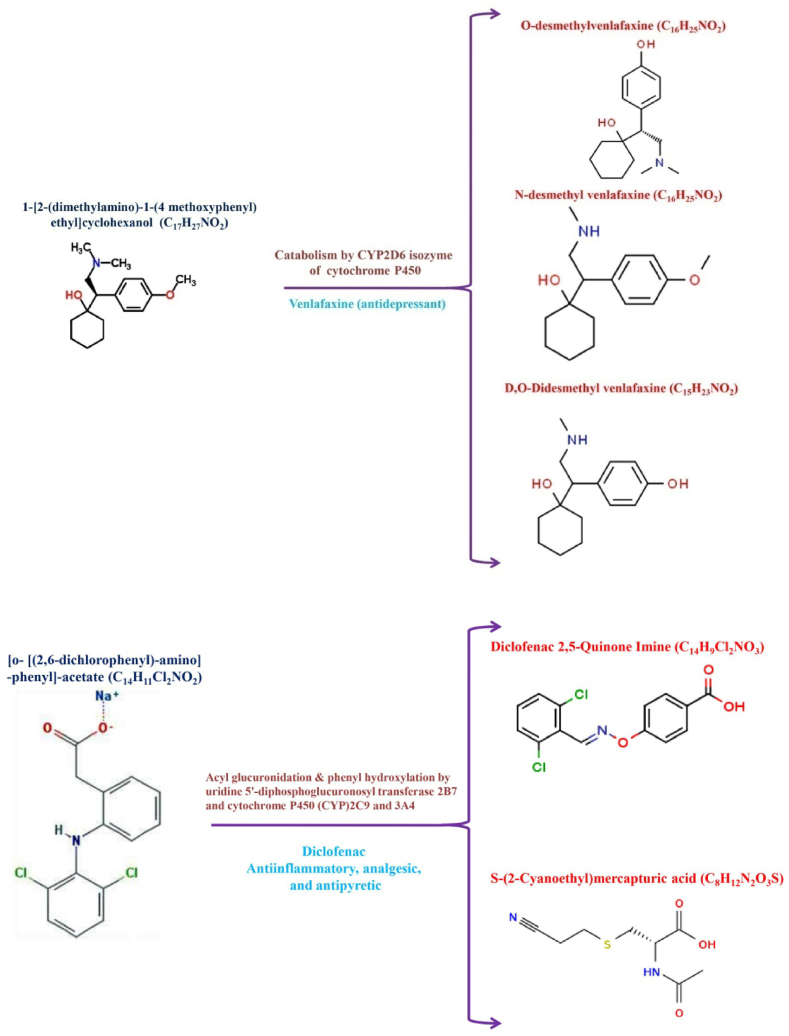
The biodegradability of PPCPs and associated metabolites through microbes, particularly bacteria, remains more difficult because the drugs have been engineered as well as synthesized that are harmful to bacteria [57]. Certain resident bacterial species are capable of decomposing pharmaceutical-specific blended pollutants by using them as C as well as N sources. Fig. 2, Fig. 3 depicts the microbial degradation and microbial enzymes involved in the degradation and transformation process/mechanisms of PPCPs in the aquatic system. The following bacterial species such as Pseudomonas putida, Geobacillus thermocatenulatus, Bacillus badius, Escherichia coli, Bacillus cereus, Rhodococcus rhodochrous, Exiguobacterium sp. RD3, and Phanerochaete chrysosporium are capable of degrading or converting PPCPs and associated metabolites in the aquatic environment [56]. Notably, the research reports that heterotrophic bacteria possess the potential to degrade clofibric acid, 4-chlorophenol, as well as α-hydroxyisobutyric acid into lactic acid [65]. Selected bacterial species that possess PPCPs have fine degradation potential and convert them into non-toxic PPCPs and associated metabolites. Another study reported that the in vitro study using Pseudomonas putida effectively degraded about 100 mg L−1 of salicylic acid (100%) in 8 h [66]. Interestingly, the bacterial species possess the potential to oxidize the bacteria group and can effectively degrade drugs through a co-metabolism process involving the major enzymes namely ammonia monooxygenase, during the NH3 malnourishment situation [67]. Notably, Pseudomonas species enumerated from polluted areas deteriorate carbamazepine up to 47% through enzymatic (cytochrome) breakdown (Fig. 4), Achromobacter denitrificans PR1 successfully breakdown the sulfonamides grouping drugs including sulfamethoxypyridazine, sulfamethazine, sulfathiazole, sulfasalazine, sulfamethoxine, and sulfapyridine as 98%, 100%, 47%, 98%, 48%, and 100% respectively in 56 h of remediation [17]. Despite this, strain is unable to degrade hypoglycemic, sulfacetamide, and sulfur-containing diuretics group drugs. Similarly, A. denitrificans PR1 effectively degraded aniline ring that yielded 3-amino-5-methylisoxazole as byproduct. Sulfamethoxazole is also degraded by Pseudomonas sp. and Proteobacteria sp. in aquatic environments [68]. Fig. 5 shows different types of microbial enzymes based metabolic reactions for PPCPs degradation. Table 2 shows possibilities for PPCP degradation/removal from aquatic systems using various known methods reported to date.
Fig. 2.
A diagram depicting the model biodegradation process by microbes and microbial enzymes on PPCPs [17].
Fig. 3.
Microbial enzymes involved in pollutant degradation.
Fig. 4.
Microbial cytochrome enzymes degrade Carbamazepine.
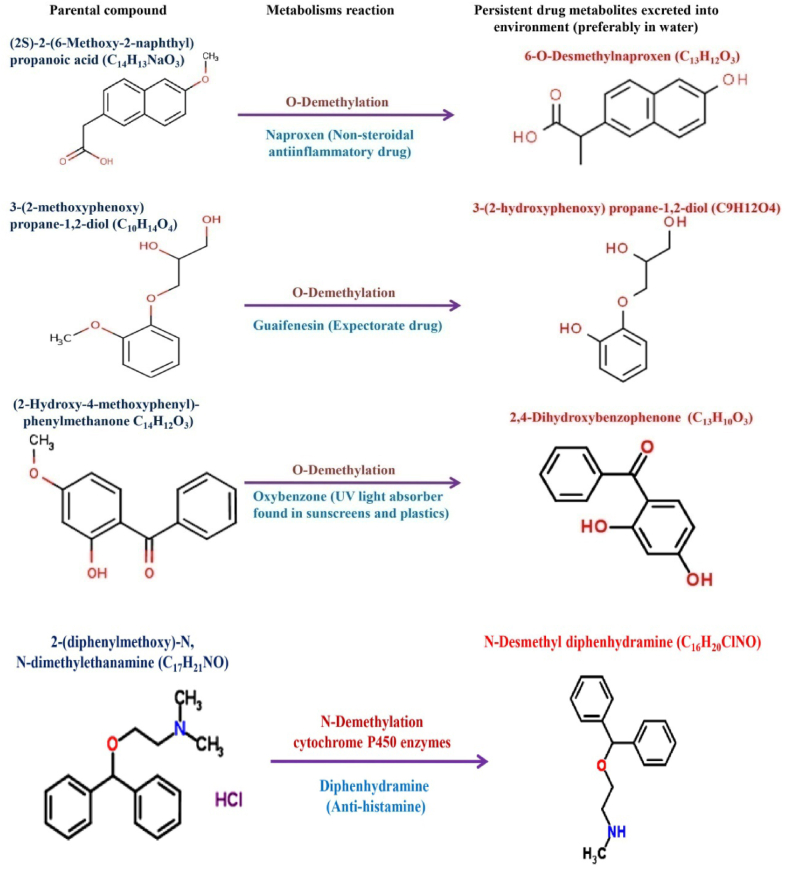
Fig. 5.
Different types of microbial metabolic reactions in PPCPs degradation.
Table 2.
Various methods reported for PPCPs degradation/removal potential-a comparison.
| Methods used | PPCPs | Source | Analysis methods | Mode of removal | Degradation/removal efficiency | References |
|---|---|---|---|---|---|---|
| Adsorption Processes | TCS, CPM, NPX, and BPA | Effluent | UPLC/UV | CNTs + Sonication, Multi-walled carbon nanotubes, Graphene oxide, and so on. | 245.47, 318, 156, and 97 mg g-1 | [69,70] |
| Biological processes | PAR, CAF, TCS, DEET, and IBP | Effluent | HPLC/DAD/LC/MS-MS/UV | Hybrid CW | 98, 98, 100, 43, 15–85% | [71,72] |
| Advanced oxidation processes | β-lactams antibiotics, DCF, OFX, ACE, MP, and CAF | Effluent | HPLC/UV, HPLC-DAD | PAA/Persulfate activated by Cu cathode + Vis irradiation/O3 + TiO2/montmorillonite nanocomposite | 60-90, 86, 99, 89, 95, 99, and 97% | [73,74] |
8.2. Fungi
Fungi are among the most efficient as well as considerable agents for the biodegradation of PPCPs and associated metabolites across different aquatic environments [75]. White-rot fungi are capable of decomposing various types of PPCPs in different dosages [76]. Fungi can synthesize various beneficial enzymes, including enzymes such as cytochrome P450, laccases, peroxidases, and ligninolytic enzymes can induce deamination, hydroxylation, formylation, oxidation, and dehalogenation reactions on PPCPs such as norfloxacin, propranolol, clofibric acid, ciprofloxacin, atenolol, ibuprofen, carbamazepine, norfloxacin, and diclofenac [77]. Fortunately, Trametes versicolor can degrade about 80% of sulfonamide antibiotics into Sulfapyridine as well as Sulfathiazole up to 72 h of bioremediation process [78]. Moreover, T. versicolor effectively degrades the ofloxacin by up to 80% in an Erlenmeyer flask approach in a short period of time. Nonetheless, it metabolizes up to 98.5% of ofloxacin content in the hospital effluent in a fluidized batch bioreactor via oxidation, hydroxylation, as well as breaking down of the piperazine, yielding known as well as unidentified metabolites. T. versicolor proactively converts pharmaceuticals belonging to microbial antibiotics, psychiatric drugs, β-blockers, as well as NSAIDs in hospital effluent in eight weeks of treatment using a fluidized-bed system [79]. T. versicolor was also able to degrade up to 97% of clofibric acid from contaminated water within 1 week of the remediation process. Another study reported that the native Basidomycetes sp. metabolizes the enrofloxacin and yielded known and unknown metabolites [56]. Phanerochaete chrysosporium degrade the Carbamazepine up to 80% and also metabolize the ibuprofen, naproxen, diazepam, carbamazepine, and diclofenac up to 50–60% [80]. T. versicolor also degrades the Iopromide up to 65% from hospital effluents and produced various forms of metabolites [81].
These fungal species can secrete the most efficient enzymes laccase as well as cytochrome P450, helps in the degradation of PPCPs in polluted water. Some other fungal species namely Ganoderma lucidium, as well as Phanerochaete chrysoporium, effectively remediate the ibuprofen after 1 week of treatment [76]. Another report states that T. versicolor alone effectively degraded the clofibric acid and carbamazepine up to 91% and 58% respectively [82]. The G. lucidium metabolize about 47% of clofibric acid. Subsequently, the Trichoderma harzianum actively degrade the carbamazepine as well as clarithromycin via co-metabolic oxidation, removing up to 57% & 72% respectively in a week of treatment. Since, these mycoremediation by potential fungi are directly related to the extra and intracellular active enzymes namely manganese peroxidase, laccase, and lignin peroxidase [83]. Some redox mediators/co-factors can enhance the degradation as well as the transformation of PPCPs and associated metabolites. For instance, the manganese peroxidase effectively degrades (99%) the carbamazepine in the presence of glucose as well as peptone [84]. T. versicolor and Pleuro tusostreatus synthesized peroxidase as well as Cytochrome P450 enzymes successfully degrades the carbamazepine, and yielded 10,11-dihydro-10,11-dihydroxycarbamazepine, acridone, and 10,11-epoxy-carbamazepine [85].
8.3. Algae
The algae-based remediation of PPCPs in an aquatic ecosystem is limited since only a few algal species have been identified and reported as having the ability to degrade/metabolize the PPCPs from the aquatic environment [86]. The algae species namely, Chlamydomonas mexicana and Scenedesmus obliquus, degraded about 35% and 28% of (1 mg L−1) carbamazepine since algae growth was impeded beyond that doses [87]. The PPCPs deterioration of algae has been proposed to be two pathways namely epoxidation and oxidation processes through cytochrome P450 [88]. The biodegradation of PPCPs (e.g. carbamazepine) by algae is thought to occur through two mechanisms [89]. First one is a metabolism pathway may be started through the epoxidation process through the cytochrome P450, yielding 10, 11-dihydro-10,11-epoxycarbamazepine as a last degraded residue. The oxidation is a second process by cytochrome P450, which results in N-hydroxycarbamazepine (N–OH-CBZ) [90]. In a comparable manner the Chlorella sorokiniana eliminated paracetamol as well as salicylic acid with 67% and 73% efficiency, correspondingly [91]. Furthermore, it may additionally degrade 60–100% of diclofenac, ibuprofen, paracetamol, and metoprolol.
9. Microbe-based bioremediation of PPCPs constraints
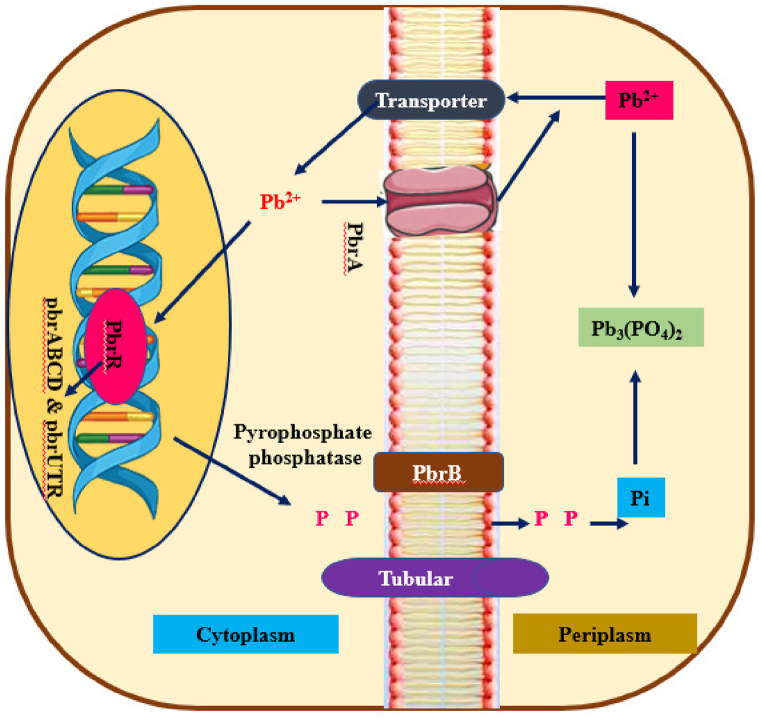
The enzymatic processes of microbial communities can immobilize, eliminate, deteriorate, change, as well as detoxify various chemical contaminants including PPCPs [92]. The microbial enzymes serve as agent, accelerating the progression of biochemical processes, which deteriorate the PPCPs pollutant across aquatic ecosystems [93]. The microbes needed several types of favorable factors for active bioremediation process towards contaminants, and their constraints are [94]: (i) The physical and chemical properties of the polluted environment (ii) the chemical composition as well as quantity of pollutants (iii) Pollutant availability to microbes. Biotic factors include (i) microbes' rivals for constrained accessible carbon resources (ii) Antagonistic relationships among microbes (iii) Microbe killing through protozoans as well as bacteriophages (iv) An imbalance between concentrations of pollutants as well as microbial communities: to degrade the contaminants via the number of microbial enzyme (v) The microbial gene expression according to emphasized environmental factors in order to create the procedure of metabolism [95]. Environmental factors such as Temperature, nutrients, O2 content, redox potential, pH, and so on may all influence microbial development as well as activity (ii) Pollutant accessibility as well as their physical and chemical characteristics (iii) A balanced dietary supplementation for active development, digestion, and reproduction decides the progress and efficiency of bioremediation processes (iv) The ideal temperature necessary for the active decomposition of resistant contaminants (v) Aerobic as well as anaerobic conditions decide the growth (viii) Harmful pollutant quantification on contaminated regions is essential owing to increased quantity of hazardous metals interfere the metabolisims of cells and are detrimental to them or slow down their decomposition process (ix) The majority of such constraints can be resolved by employing a microbial active enzyme to serve as a biocatalyst for decomposing recalcitrant contaminants in aquatic environments [96]. The metal tolerance mechanisms in bacteria facilitate the harmful recalcitrant pollutant degradation in polluted sites. Fig. 6 depicts the possible metal tolerance mechanism in bacteria with the example of Pb.
Fig. 6.
Heavy metal (Pd) tolerance and degradation process in microbes.
10. PPCPs biological degradation is aided by genetically modified organisms (GMOs)
Several environmental and nutritional demand factors restrict the bioremediation ability of wild microbes as well as their enzymes [97]. Thus microbial genes, particularly bacteria that are capable of encoding active catabolic digestive enzymes capable of degrading recalcitrant contaminants, are employed to create excellent microbial agents capable of degrading or transforming PPCPs [56]. Sophisticated genetic engineering approaches enabled this genetic information exchange. A few important aspects must be regarded when employing the rDNA technology to ensure the successful creation of GMOs [98]: 1. the sensitivity and attraction associated with the bacterial enzymes need to be altered, 2. fully comprehending the creation as well as the biochemical transformation processes, 3. tracking, and oversight of bioprocess advancement, as well as 4. The generated microbial strain should serve as biosensors for pollutant sensing as well as deterioration possibility.
The ability of microbes to degrade recalcitrant pollutants (PPCPs) is dependent upon the existence of plasmids [99]. Every plasmid can produce enzymes to break down a single pollutant, while certain plasmids break down only a few pollutants. For instance, a CAM plasmid is capable of degrading camphor, while the OCT plasmid can deteriorate hexane, decane, and octane a NAH plasmid can deteriorate naphthalene, as well as the XYL plasmid can breakdown toluene and xylene [100]. Those plasmid-based deteriorations have been used to remediate the most harmful contaminants in some real-world investigations. New plasmids are being discovered and created from different strains of bacteria that are capable of degrading a variety of recalcitrant contaminants [101]. Investigators are creating some well-known GMOs with multiple levels of remediation perspective on an extensive selection of recalcitrant contaminants. For instance, the Pseudomonas putida, deteriorates an extensive variety of recalcitrant contaminants including toluene, camphor, salicylate toluene, xylene 3-cne chlorobenxoate, and others [56]. Likewise, Alcaligenes eutrophus AE104 incorporates pEBZ141, which is used to eliminate PPCPs from wastewater from industries. The photosynthetic organism Rhodopseudomona palustris has been developed to eliminate the metal from effluent [102]. Under certain natural conditions, the transfer of genes may occur via the naturally occurring conjugation process, resulting in the development of substantial pollutant-degrading bacteria. For instance, the exceptionally recalcitrant pollutant-degrading bacterium R. eutropha CH3 was naturally conjugated with PCB gene sequences from R. eutropha A5, Achromobacter sp. LBS1C1, and A. denitrificans JB1 [103].
GMO enzymes have received greater attention because they are effective when it comes to the bioremediation of a variety of recalcitrant contaminants [104]. For instance, pRSFDuet-1 Bacillus cereus along with aldehyde dehydrogenase enzymes are able to control as well as degrade hazardous metabolic middle aldehyde compounds [105]. pGEc47B from Mycobacterium sp. HXN-150 generates CYP153A6 enzyme promoting the hydroxylation process on alkanes towards 1-alkanols. In a comparable manner, Mycobacterium sp. RP1 synthesizes the CYP151A2 enzymes, which demonstrated a remarkable capacity to break down additional amines via ring cutting [106]. As preferred expression hosts, different strains of Acinetobacter calcoaceticus, E. coli, and P. putida GPo12, are being implemented.
11. Obstacles of GMOs in PPCPs bioremediation process
Recent research reports states that the GMOs have been successful for the degradation of PPCPs merely at the in-vitro stage; though, in field trails, the degradation capability of multiple GMOs has not been as efficient [107]. Because of their lower genome stability as well as their inability to influence environmental factors, recombinant gene sequences in bacteria hosts have been much less notable in natural circumstances. Under regulated outdoor circumstances, P. fluorescens only slightly remediates the contaminants. Thus, the major challenge in creating GMOs for biological remediation remains connected with opposed field conditions. Additionally, GMOs with field-level genetic expression ability are commonly utilized by certain bacterial species such as B. subtilis, E. coli, A. calcoaceticus, and P. putida [108]. The recently created GMOs ought to possess numerous uses as well as have the capacity to thrive in an extensive variety of surroundings while expressing the gene that is needed [98]. Investigators must focus on tackling these problems in order to develop high-potential GMOs that can help solve environmental problems in an environmentally friendly manner.
12. Conclusion
This in-depth investigation described the potential scenarios of PPCPs in global aquatic environments. Numerous studies have been published concerning an extensive variety of PPCPs that are released into the aquatic systems as well as cause essential health effects and ecological systems destruction. Such substances have been around for centuries in the ecosystem, but their existences as well as detrimental impacts have only recently become apparent. Nevertheless, the acute as well as long-term effects of PPCPs pollutants in human beings, in addition to plants and animals, have not been extensively thoroughly studied. The investigation stated that water being released from WWTPs includes identifiable volume of PPCPs as well as the associated metabolites. Such PPCPs pollutants pollute the ecosystem severely as well as can cause both acute and long-term toxicity to non-targeted living things. With present methods, multiple parental types of PPCPs as well as associated metabolite products in different sort in waste water were discovered. As a result, creating suitable as well as feasible approaches to recognize contaminants as well as regularise the method of monitoring is significant.
There is scarce information regarding the toxic effects, bio-chemical transformation process, as well as environmental effects of PPCPs in aquatic environments. To circumvent current constraints in PPCPs and associated metabolites remediation using microbial strain create degradation enzymes with perform multiple tasks potential as well as Advanced Oxidation Processes (AOPs) that remove the contaminants. Since the majority of research indicated that PPCPs and associated metabolites degraded through oxidoreductase category enzymes during the oxidation process. There are currently not any permitted maximum permissible levels of PPCPs as well as their byproducts in water as well as soil in multiple nations, such as nations that are both developing and developed. As a result, every nation should decide how for tracking such pollutants based upon it's geographically well-being limitations. The subsequent process must be implemented. Generate sophisticated technologies, such as biosensors, which for detecting PPCPs and associated metabolites precisely and continuously. Special attention must be launched in developing nations to figure out the acceptable limit as well as evaluate the disinfected effluents released through industries, municipal wastewater, and so on. Remind the R&D wing by offering adequate funds for creating environmentally friendly technologies for monitoring and removing PPCPs pollutants. The bacteria and fungi screening for PPCPs removal from water should be non-pathogenic as well as should possess resistance to various PPCPs. Furthermore, depth research needs to perform to find the interaction possibilities of merging the photo-degradation as well as biodegradation with minimal costs. Establish highly effective as well as cost-effective methods for removing PPCPs and associated metabolites in the aquatic environments is required.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2014R1A6A1031189) and by an NRF grant funded by the Korean government (MSIT) (Grant No. 2021R1A2C1008368). The authors acknowledge the funding agencies of DBT and DST-FIST for providing equipment support as institutional infrastructure. The authors would like to thank the Division of Research and Innovations, Department of Biotechnology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Science, Chennai-602 105, Tamil Nadu, India for their constant support for this work.
Contributor Information
Mathiyazhagan Narayanan, Email: mathimicro@gmail.com.
Selvaraj Barathi, Email: barathiselvaraj87@gmail.com.
References
- 1.Antar M., Lyu D., Nazari M., Shah A., Zhou X., Smith D.L. Biomass for a sustainable bioeconomy: an overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021;139 [Google Scholar]
- 2.Rout P.R., Zhang T.C., Bhunia P., Surampalli R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: a review. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141990. [DOI] [PubMed] [Google Scholar]
- 3.Chapman J., Truong V.K., Elbourne A., Gangadoo S., Cheeseman S., Rajapaksha P., Latham K., Crawford R.J., Cozzolino D. Combining chemometrics and sensors: toward new applications in monitoring and environmental analysis. Chem. Rev. 2020;120:6048–6069. doi: 10.1021/acs.chemrev.9b00616. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Pérez K., Avilés-Castrillo J., Ruiz-Pulido G., Medina D.I., Parra-Saldivar R., Iqbal H.M. Nanoadsorbents in focus for the remediation of environmentally-related contaminants with rising toxicity concerns. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146465. [DOI] [PubMed] [Google Scholar]
- 5.Archer E., Petrie B., Kasprzyk-Hordern B., Wolfaardt G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere. 2017;174:437–446. doi: 10.1016/j.chemosphere.2017.01.101. [DOI] [PubMed] [Google Scholar]
- 6.Świacka K., Michnowska A., Maculewicz J., Caban M., Smolarz K. Toxic effects of NSAIDs in non-target species: a review from the perspective of the aquatic environment. Environ. Pollut. 2021;273 doi: 10.1016/j.envpol.2020.115891. [DOI] [PubMed] [Google Scholar]
- 7.Gavrilescu M., Demnerová K., Aamand J., Agathos S., Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New biotechnology. 2015;32:147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Oluwole A.O., Omotola E.O., Olatunji O.S. Pharmaceuticals and personal care products in water and wastewater: a review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC chemistry. 2020;14:1–29. doi: 10.1186/s13065-020-00714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxendale I.R., Braatz R.D., Hodnett B.K., Jensen K.F., Johnson M.D., Sharratt P., Sherlock J.P., Florence A.J. Achieving continuous manufacturing: technologies and approaches for synthesis, workup, and isolation of drug substance. May 20–21, 2014 Continuous Manufacturing Symposium. J. Pharmaceut. Sci. 2015;104:781–791. [Google Scholar]
- 10.Adeyanju C.A., Ogunniyi S., Selvasembian R., Oniye M.M., Ajala O.J., Adeniyi A.G., Igwegbe C.A., Ighalo J.O. Recent advances on the aqueous phase adsorption of carbamazepine. ChemBioEng Rev. 2022;9:231–247. [Google Scholar]
- 11.Singh N., Poonia T., Siwal S.S., Srivastav A.L., Sharma H.K., Mittal S.K. Current Directions in Water Scarcity Research. Elsevier; 2022. Challenges of water contamination in urban areas; pp. 173–202. [Google Scholar]
- 12.Khan I., Singh P., Srinivasan E. A systematic overview on treatment towards endocrine disruptors. Sustain. Energy Technol. Assessments. 2022;53 [Google Scholar]
- 13.Yang Y., Ok Y.S., Kim K.-H., Kwon E.E., Tsang Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci. Total Environ. 2017;596:303–320. doi: 10.1016/j.scitotenv.2017.04.102. [DOI] [PubMed] [Google Scholar]
- 14.Puri V., Nagpal M., Singh I., Singh M., Dhingra G.A., Huanbutta K., Dheer D., Sharma A., Sangnim T. A comprehensive review on nutraceuticals: therapy support and formulation challenges. Nutrients. 2022;14:4637. doi: 10.3390/nu14214637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.K'oreje K.O., Okoth M., Van Langenhove H., Demeestere K. Occurrence and treatment of contaminants of emerging concern in the African aquatic environment: literature review and a look ahead. J. Environ. Manag. 2020;254 doi: 10.1016/j.jenvman.2019.109752. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan R.Y., Manikandan S., Subbaiya R., Biruntha M., Govarthanan M., Karmegam N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: a review. Environ. Technol. Innov. 2021;23 [Google Scholar]
- 17.Narayanan M., El-sheekh M., Ma Y., Pugazhendhi A., Natarajan D., Kandasamy G., Raja R., Saravana Kumar R.M., Kumarasamy S., Sathiyan G., Geetha R., Paulraj B., Liu G., Kandasamy S. Current status of microbes involved in the degradation of pharmaceutical and personal care products (PPCPs) pollutants in the aquatic ecosystem. Environ. Pollut. 2022;300 doi: 10.1016/j.envpol.2022.118922. [DOI] [PubMed] [Google Scholar]
- 18.Matongo S., Birungi G., Moodley B., Ndungu P. Pharmaceutical residues in water and sediment of Msunduzi River, kwazulu-natal, South Africa. Chemosphere. 2015;134:133–140. doi: 10.1016/j.chemosphere.2015.03.093. [DOI] [PubMed] [Google Scholar]
- 19.Kim J., Yoon S., Lee S., Narumiya M., Nakada N., Han I., Tanaka H. Occurrence and fate of PPCPs wastewater treatment plants in Korea. International Proceedings of Chemical, Biological and Environmental Engineering (IPCBEE) 2012;35:57–61. [Google Scholar]
- 20.Wang Y., Wang X., Li M., Dong J., Sun C., Chen G. Removal of pharmaceutical and personal care products (PPCPs) from municipal waste water with integrated membrane systems, MBR-RO/NF. Int. J. Environ. Res. Publ. Health. 2018;15:269. doi: 10.3390/ijerph15020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Archana G., Dhodapkar R., Kumar A. Offline solid-phase extraction for preconcentration of pharmaceuticals and personal care products in environmental water and their simultaneous determination using the reversed phase high-performance liquid chromatography method. Environ. Monit. Assess. 2016;188:1–10. doi: 10.1007/s10661-016-5510-1. [DOI] [PubMed] [Google Scholar]
- 22.Van Zijl M.C., Aneck-Hahn N.H., Swart P., Hayward S., Genthe B., De Jager C. Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere. 2017;186:305–313. doi: 10.1016/j.chemosphere.2017.07.130. [DOI] [PubMed] [Google Scholar]
- 23.Agunbiade F.O., Moodley B. Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu‐Natal, South Africa. Environ. Toxicol. Chem. 2016;35:36–46. doi: 10.1002/etc.3144. [DOI] [PubMed] [Google Scholar]
- 24.Manickum T., John W. Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa) Sci. Total Environ. 2014;468:584–597. doi: 10.1016/j.scitotenv.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Amdany R., Chimuka L., Cukrowska E. Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): a laboratory calibration and field application. WaterSA. 2014;40:407–414. [Google Scholar]
- 26.Agunbiade F.O., Moodley B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ. Monit. Assess. 2014;186:7273–7291. doi: 10.1007/s10661-014-3926-z. [DOI] [PubMed] [Google Scholar]
- 27.Alexy R., Schöll A., Kümpel T., Kümmerer K. Effects and Risks; 2004. What Do We Know about Antibiotics in the Environment?, Pharmaceuticals in the Environment: Sources, Fate; pp. 209–221. [Google Scholar]
- 28.Buser H.-R., Müller M.D., Theobald N. Occurrence of the pharmaceutical drug clofibric acid and the herbicide mecoprop in various Swiss lakes and in the North Sea. Environ. Sci. Technol. 1998;32:188–192. [Google Scholar]
- 29.Miraji H., Othman O.C., Ngassapa F., Mureithi E. Scientifica; 2016. Research Trends in Emerging Contaminants on the Aquatic Environments of Tanzania; p. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitaku E., Smith D.T., Njardarson J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 31.Patnaik S., Gorain B., Padhi S., Choudhury H., Gabr G.A., Md S., Mishra D.K., Kesharwani P. Recent update of toxicity aspects of nanoparticulate systems for drug delivery. Eur. J. Pharm. Biopharm. 2021;161:100–119. doi: 10.1016/j.ejpb.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Samal K., Mahapatra S., Ali M.H. Energy Nexus; 2022. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. [Google Scholar]
- 33.Ebele A.J., Abdallah M.A.-E., Harrad S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging contaminants. 2017;3:1–16. [Google Scholar]
- 34.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.-M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautam K., Anbumani S. Current Developments in Biotechnology and Bioengineering. Elsevier; 2020. Ecotoxicological effects of organic micro-pollutants on the environment; pp. 481–501. [Google Scholar]
- 36.Howard R., Chataway J., Edwards M., Heales S., Lachmann R., Leff A., Murphy E. Toxic, metabolic and physical insults to the nervous system and inherited disorders of metabolism. Neurology: A Queen Square Textbook. 2016:729–796. [Google Scholar]
- 37.Hiba Z., Mondamert L., Remaury Q.B., Cleon A., Leitner N.K.V., Labanowski J. Occurrence of carbamazepine, diclofenac, and their related metabolites and transformation products in a French aquatic environment and preliminary risk assessment. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117052. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz S., Schmieg H., Scheurer M., Köhler H.-R., Triebskorn R. Impact of the NSAID diclofenac on survival, development, behaviour and health of embryonic and juvenile stages of brown trout, Salmo trutta f. fario. Sci. Total Environ. 2017;607:1026–1036. doi: 10.1016/j.scitotenv.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 39.Jessica D., Robert M., Frédéric S., Arnaud B., Delphine L., Jean-Pierre T., Patrick K. Do sewage treatment plant discharges substantially impair fish reproduction in polluted rivers? Sci. Total Environ. 2007;372:497–514. doi: 10.1016/j.scitotenv.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Forouhar Vajargah M., Imanpoor M.R., Shabani A., Hedayati A., Faggio C. Effect of long‐term exposure of silver nanoparticles on growth indices, hematological and biochemical parameters and gonad histology of male goldfish (Carassius auratus gibelio) Microsc. Res. Tech. 2019;82:1224–1230. doi: 10.1002/jemt.23271. [DOI] [PubMed] [Google Scholar]
- 41.Guru A., Velayutham M., Arockiaraj J. Lipid-lowering and antioxidant activity of RF13 peptide from vacuolar protein sorting-associated protein 26B (VPS26B) by modulating lipid metabolism and oxidative stress in HFD induced obesity in zebrafish larvae. Int. J. Pept. Res. Therapeut. 2022;28:74. [Google Scholar]
- 42.Li D.-L., Huang Y.-J., Gao S., Chen L.-Q., Zhang M.-L., Du Z.-Y. Sex-specific alterations of lipid metabolism in zebrafish exposed to polychlorinated biphenyls. Chemosphere. 2019;221:768–777. doi: 10.1016/j.chemosphere.2019.01.094. [DOI] [PubMed] [Google Scholar]
- 43.Janecko N., Pokludova L., Blahova J., Svobodova Z., Literak I. Implications of fluoroquinolone contamination for the aquatic environment—a review. Environ. Toxicol. Chem. 2016;35:2647–2656. doi: 10.1002/etc.3552. [DOI] [PubMed] [Google Scholar]
- 44.Song C., Zhang C., Fan L., Qiu L., Wu W., Meng S., Hu G., Kamira B., Chen J. Occurrence of antibiotics and their impacts to primary productivity in fishponds around Tai Lake, China. Chemosphere. 2016;161:127–135. doi: 10.1016/j.chemosphere.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Uddin T.M., Chakraborty A.J., Khusro A., Zidan B.R.M., Mitra S., Emran T.B., Dhama K., Ripon M.K.H., Gajdács M., Sahibzada M.U.K. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. Journal of infection and public health. 2021;14:1750–1766. doi: 10.1016/j.jiph.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Ito-Harashima S., Matano M., Onishi K., Nomura T., Nakajima S., Ebata S., Shiizaki K., Kawanishi M., Yagi T. Construction of reporter gene assays using CWP and PDR mutant yeasts for enhanced detection of various sex steroids. Gene Environ. 2020;42:1–19. doi: 10.1186/s41021-020-00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wojnarowski K., Cholewińska P., Palić D., Bednarska M., Jarosz M., Wiśniewska I. Estrogen receptors mediated negative effects of estrogens and xenoestrogens in teleost fishes. Int. J. Mol. Sci. 2022;23:2605. doi: 10.3390/ijms23052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaucic C., Dharmavathi A.L., Freeman J.L. Contemporary Chemical Approaches for Green and Sustainable Drugs. Elsevier; 2022. Using the zebrafish model system to identify the health effects of pharmaceutical pollutants; pp. 1–25. [Google Scholar]
- 49.Chen L., Wang Y., Giesy J.P., Chen F., Shi T., Chen J., Xie P. Microcystin-LR affects the hypothalamic-pituitary-inter-renal (HPI) axis in early life stages (embryos and larvae) of zebrafish. Environ. Pollut. 2018;241:540–548. doi: 10.1016/j.envpol.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Salahinejad A., Meuthen D., Attaran A., Chivers D.P., Ferrari M.C. Science of The Total Environment; 2023. Effects of Common Antiepileptic Drugs on Teleost Fishes. [DOI] [PubMed] [Google Scholar]
- 51.Pohl J., Ahrens L., Carlsson G., Golovko O., Norrgren L., Weiss J., Örn S. Embryotoxicity of ozonated diclofenac, carbamazepine, and oxazepam in zebrafish (Danio rerio) Chemosphere. 2019;225:191–199. doi: 10.1016/j.chemosphere.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 52.Anand U., Adelodun B., Cabreros C., Kumar P., Suresh S., Dey A., Ballesteros F., Jr., Bontempi E. Occurrence, transformation, bioaccumulation, risk and analysis of pharmaceutical and personal care products from wastewater: a review. Environ. Chem. Lett. 2022:1–22. doi: 10.1007/s10311-022-01498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parra-Luna M., Martín-Pozo L., Hidalgo F., Zafra-Gómez A. Common sea urchin (Paracentrotus lividus) and sea cucumber of the genus Holothuria as bioindicators of pollution in the study of chemical contaminants in aquatic media. A revision. Ecol. Indicat. 2020;113 [Google Scholar]
- 54.Xiong W., Huang X., Chen Y., Fu R., Du X., Chen X., Zhan A. Zooplankton biodiversity monitoring in polluted freshwater ecosystems: a technical review. Environmental Science and Ecotechnology. 2020;1 [Google Scholar]
- 55.Kumar R., Qureshi M., Vishwakarma D.K., Al-Ansari N., Kuriqi A., Elbeltagi A., Saraswat A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Studies in Chemical and Environmental Engineering. 2022;6 [Google Scholar]
- 56.Narayanan M., El-Sheekh M., Ma Y., Pugazhendhi A., Natarajan D., Kandasamy G., Raja R., Kumar R.S., Kumarasamy S., Sathiyan G. Environmental Pollution; 2022. Current Status of Microbes Involved in the Degradation of Pharmaceutical and Personal Care Products (PPCPs) Pollutants in the Aquatic Ecosystem. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen P.M., Afzal M., Ullah I., Shahid N., Baqar M., Arslan M. Removal of pharmaceuticals and personal care products using constructed wetlands: effective plant-bacteria synergism may enhance degradation efficiency. Environ. Sci. Pollut. Control Ser. 2019;26:21109–21126. doi: 10.1007/s11356-019-05320-w. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y., Lu G., Li J., Dang T., Xue C., Liu J., Yan Z. Multimedia distribution and trophic transfer of PPCPs in the middle and lower reaches of the Yarlung Zangbo River. Environ. Pollut. 2021;271 doi: 10.1016/j.envpol.2020.116408. [DOI] [PubMed] [Google Scholar]
- 59.Al-Baldawi I.A., Mohammed A.A., Mutar Z.H., Abdullah S.R.S., Jasim S.S., Almansoory A.F. Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: source, impacts, treatment, mechanisms, fate, and SWOT analysis. J. Clean. Prod. 2021;319 [Google Scholar]
- 60.Hassan I., Chowdhury S.R., Prihartato P.K., Razzak S.A. Wastewater treatment using constructed wetland: current trends and future potential. Processes. 2021;9:1917. [Google Scholar]
- 61.Singh R.S., Singh T., Pandey A. Microbial enzymes—an overview. Advances in enzyme technology. 2019:1–40. [Google Scholar]
- 62.Moloantoa K.M., Khetsha Z.P., Van Heerden E., Castillo J.C., Cason E.D. Nitrate water contamination from industrial activities and complete denitrification as a remediation option. Water. 2022;14:799. [Google Scholar]
- 63.Achour M., Bravo L., Sarriá B., Fredj M.B., Nouira M., Mtiraoui A., Saguem S., Mateos R. Bioavailability and nutrikinetics of rosemary tea phenolic compounds in humans. Food Res. Int. 2021;139 doi: 10.1016/j.foodres.2020.109815. [DOI] [PubMed] [Google Scholar]
- 64.Patel M., Kumar R., Kishor K., Mlsna T., Pittman C.U., Jr., Mohan D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019;119:3510–3673. doi: 10.1021/acs.chemrev.8b00299. [DOI] [PubMed] [Google Scholar]
- 65.Rasheed T., Rizwan K., Shafi S., Bilal M. Biodegradation and Biodeterioration at the Nanoscale. Elsevier; 2022. Nanobiodegradation of pharmaceutical pollutants; pp. 635–653. [Google Scholar]
- 66.Huang H., Wu K., Khan A., Jiang Y., Ling Z., Liu P., Chen Y., Tao X., Li X. A novel Pseudomonas gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresour. Technol. 2016;207:370–378. doi: 10.1016/j.biortech.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Rodríguez Y., Pérez V., López J.C., Bordel S., Firmino P.I., Lebrero R., Muñoz R. The Handbook of Polyhydroxyalkanoates; 2020. Coupling Biogas with PHA Biosynthesis; pp. 357–376. [Google Scholar]
- 68.Liu X., Chen J., Liu Y., Wan Z., Guo X., Lu S., Qiu D. Sulfamethoxazole degradation by Pseudomonas silesiensis F6a isolated from bioelectrochemical technology-integrated constructed wetlands. Ecotoxicol. Environ. Saf. 2022;240 doi: 10.1016/j.ecoenv.2022.113698. [DOI] [PubMed] [Google Scholar]
- 69.Li F., Kong Q., Chen P., Chen M., Liu G., Lv W., Yao K. Effect of halide ions on the photodegradation of ibuprofen in aqueous environments. Chemosphere. 2017;166:412–417. doi: 10.1016/j.chemosphere.2016.09.108. [DOI] [PubMed] [Google Scholar]
- 70.Sun J., Wang J., Zhang R., Wei D., Long Q., Huang Y., Xie X., Li A. Comparison of different advanced treatment processes in removing endocrine disruption effects from municipal wastewater secondary effluent. Chemosphere. 2017;168:1–9. doi: 10.1016/j.chemosphere.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Lv T., Carvalho P.N., Zhang L., Arias C.A., Chen Z., Brix H. Ibuprofen and iohexol removal in saturated constructed wetland mesocosms. Ecol. Eng. 2017;98:394–402. [Google Scholar]
- 72.Ávila C., Pelissari C., Sezerino P.H., Sgroi M., Roccaro P., García J. Enhancement of total nitrogen removal through effluent recirculation and fate of PPCPs in a hybrid constructed wetland system treating urban wastewater. Sci. Total Environ. 2017;584:414–425. doi: 10.1016/j.scitotenv.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 73.Xie R., Meng X., Sun P., Niu J., Jiang W., Bottomley L., Li D., Chen Y., Crittenden J. Electrochemical oxidation of ofloxacin using a TiO2-based SnO2-Sb/polytetrafluoroethylene resin-PbO2 electrode: reaction kinetics and mass transfer impact. Appl. Catal. B Environ. 2017;203:515–525. [Google Scholar]
- 74.Li C., Gao N., Chu W., Bond T., Wei X. Comparison of THMs and HANs formation potential from the chlorination of free and combined histidine and glycine. Chem. Eng. J. 2017;307:487–495. [Google Scholar]
- 75.Chopra S., Kumar D. Pharmaceuticals and personal care products (PPCPs) as emerging environmental pollutants: toxicity and risk assessment. Advances in animal biotechnology and its applications. 2018:337–353. [Google Scholar]
- 76.Zhuo R., Fan F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146132. [DOI] [PubMed] [Google Scholar]
- 77.Rodríguez-Rodríguez C.E., Cambronero-Heinrichs J.C., Beita-Sandí W., Durán J.E. Fungal Bioremediation. CRC Press; 2019. Removal of emerging pollutants by fungi: elimination of antibiotics; pp. 186–238. [Google Scholar]
- 78.Bilal M., Ashraf S.S., Barceló D., Iqbal H.M. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019;691:1190–1211. doi: 10.1016/j.scitotenv.2019.07.224. [DOI] [PubMed] [Google Scholar]
- 79.Hamidzadeh Z., Ghorbannezhad P., Ketabchi M.R., Yeganeh B. Biomass-derived biochar and its application in agriculture. Fuel. 2023;341 [Google Scholar]
- 80.Adeola A.O., Ore O.T., Fapohunda O., Adewole A.H., Akerele D.D., Akingboye A.S., Oloye F.F. Psychotropic drugs of emerging concerns in aquatic systems: ecotoxicology and remediation approaches. Chemistry Africa. 2022;5:481–508. [Google Scholar]
- 81.Tišma M., Žnidaršič-Plazl P., Šelo G., Tolj I., Šperanda M., Bucić-Kojić A., Planinić M. Trametes versicolor in lignocellulose-based bioeconomy: state of the art, challenges and opportunities. Bioresour. Technol. 2021;330 doi: 10.1016/j.biortech.2021.124997. [DOI] [PubMed] [Google Scholar]
- 82.Caracciolo A.B., Topp E., Grenni P. Pharmaceuticals in the environment: biodegradation and effects on natural microbial communities. A review. J. Pharmaceut. Biomed. Anal. 2015;106:25–36. doi: 10.1016/j.jpba.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 83.Shi K., Liu Y., Chen P., Li Y. Contribution of lignin peroxidase, manganese peroxidase, and laccase in lignite degradation by mixed white-rot fungi. Waste and Biomass Valorization. 2021;12:3753–3763. [Google Scholar]
- 84.Ungureanu C.V., Favier L., Bahrim G.E. Improving biodegradation of clofibric acid by Trametes pubescens through the design of experimental tools. Microorganisms. 2020;8:1243. doi: 10.3390/microorganisms8081243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asif M.B., Hai F.I., Singh L., Price W.E., Nghiem L.D. Degradation of pharmaceuticals and personal care products by white-rot fungi—a critical review. Current Pollution Reports. 2017;3:88–103. [Google Scholar]
- 86.Guedes P., Couto N., Almeida J., Rodrigues A.M., Mateus E.P., Ribeiro A.B. Electrodialytic treatment of sewage sludge: influence on microbiological community. Int. J. Environ. Sci. Technol. 2018;15:1103–1112. [Google Scholar]
- 87.Abdelfattah A., Ali S.S., Ramadan H., El-Aswar E.I., Eltawab R., Ho S.-H., Elsamahy T., Li S., El-Sheekh M.M., Schagerl M. Environmental Science and Ecotechnology; 2022. Microalgae-based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kasonga T.K., Coetzee M.A., Kamika I., Momba M.N.B. Assessing a co-culture fungal granule ability to remove pharmaceuticals in a sequencing batch reactor. Environ. Technol. 2022;43:1684–1699. doi: 10.1080/09593330.2020.1847204. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Liu J., Kang D., Wu C., Wu Y. Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: a review. Rev. Environ. Sci. Biotechnol. 2017;16:717–735. [Google Scholar]
- 90.Xiong J.-Q., Kurade M.B., Abou-Shanab R.A., Ji M.-K., Choi J., Kim J.O., Jeon B.-H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016;205:183–190. doi: 10.1016/j.biortech.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 91.Santos M.d.J.O., de Oliveira Souza C., Marcelino H.R. Algal Research; 2022. Blue Technology for a Sustainable Pharmaceutical Industry: Microalgae for Bioremediation and Pharmaceutical Production. [Google Scholar]
- 92.Narayanan M., Ali S.S., El-Sheekh M. A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: mechanisms, challenges, and future prospects. J. Environ. Manag. 2023;334 doi: 10.1016/j.jenvman.2023.117532. [DOI] [PubMed] [Google Scholar]
- 93.Kurade M.B., Ha Y.-H., Xiong J.-Q., Govindwar S.P., Jang M., Jeon B.-H. Phytoremediation as a green biotechnology tool for emerging environmental pollution: a step forward towards sustainable rehabilitation of the environment. Chem. Eng. J. 2021;415 [Google Scholar]
- 94.Azubuike C.C., Chikere C.B., Okpokwasili G.C. Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016;32:1–18. doi: 10.1007/s11274-016-2137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Y., Xiao L., Li F., Xiao M., Lin D., Long X., Wu Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules. 2018;23:2313. doi: 10.3390/molecules23092313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morsi R., Bilal M., Iqbal H.M., Ashraf S.S. Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020;714 doi: 10.1016/j.scitotenv.2020.136572. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Y., Kumar M., Sarsaiya S., Sirohi R., Awasthi S.K., Sindhu R., Binod P., Pandey A., Bolan N.S., Zhang Z. Challenges and opportunities in bioremediation of micro-nano plastics: a review. Sci. Total Environ. 2022;802 doi: 10.1016/j.scitotenv.2021.149823. [DOI] [PubMed] [Google Scholar]
- 98.Beacham T.A., Sweet J.B., Allen M.J. Large scale cultivation of genetically modified microalgae: a new era for environmental risk assessment. Algal Res. 2017;25:90–100. [Google Scholar]
- 99.Mathur P., Sanyal D., Callahan D.L., Conlan X.A., Pfeffer F.M. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ-ment and human health. Environ. Pollut. 2021;291 doi: 10.1016/j.envpol.2021.118233. [DOI] [PubMed] [Google Scholar]
- 100.Kumar N.M., Muthukumaran C., Sharmila G., Gurunathan B. 2018. Genetically Modified Organisms and its Impact on the Enhancement of Bioremediation, Bioremediation: Applications for Environmental Protection and Management; pp. 53–76. [Google Scholar]
- 101.Nagata Y., Kato H., Ohtsubo Y., Tsuda M. Lessons from the genomes of lindane‐degrading sphingomonads. Environmental Microbiology Reports. 2019;11:630–644. doi: 10.1111/1758-2229.12762. [DOI] [PubMed] [Google Scholar]
- 102.Brown B., Wilkins M., Saha R. Rhodopseudomonas palustris: a biotechnology chassis. Biotechnol. Adv. 2022 doi: 10.1016/j.biotechadv.2022.108001. [DOI] [PubMed] [Google Scholar]
- 103.Doukani K., Boukirat D., Boumezrag A., Bouhenni H., Bounouira Y. Handbook of Biodegradable Materials. Springer; 2022. Fundamentals of biodegradation process; pp. 1–27. [Google Scholar]
- 104.Ijoma G., Tekere M. Potential microbial applications of co-cultures involving ligninolytic fungi in the bioremediation of recalcitrant xenobiotic compounds. Int. J. Environ. Sci. Technol. 2017;14:1787–1806. [Google Scholar]
- 105.Wang F., Zhao J., Li Q., Yang J., Li R., Min J., Yu X., Zheng G.-W., Yu H.-L., Zhai C. One-pot biocatalytic route from cycloalkanes to α, ω‐dicarboxylic acids by designed Escherichia coli consortia. Nat. Commun. 2020;11:5035. doi: 10.1038/s41467-020-18833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmad A., Zamzami M.A., Ahmad V., Al-Thawadi S., Akhtar M.S., Khan M.J. Bacterial biological factories intended for the desulfurization of petroleum products in refineries. Fermentation. 2023;9:211. [Google Scholar]
- 107.Sharma P., Bano A., Singh S.P., Sharma S., Xia C.L., Nadda A.K., Lam S., Tong Y.W. Engineered microbes as effective tools for the remediation of polyaromatic aromatic hydrocarbons and heavy metals. Chemosphere. 2022 doi: 10.1016/j.chemosphere.2022.135538. [DOI] [PubMed] [Google Scholar]
- 108.Phian S., Nagar S., Kaur J., Rawat C.D. Microbes and Microbial Biotechnology for Green Remediation. Elsevier; 2022. Emerging issues and challenges for microbes-assisted remediation; pp. 47–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.