Abstract
Tellurium is a super-trace metalloid on Earth. Owing to its excellent physical and chemical properties, it is used in industries such as metallurgy and manufacturing, particularly of semiconductors and – more recently – solar panels. As the global demand for tellurium rises, environmental issues surrounding tellurium have recently aroused concern due to its high toxicity. The amount of tellurium released to the environment is increasing, and microorganisms play an important role in the biogeochemical cycling of environmental tellurium. This review focuses on novel developments on tellurium transformations driven by microbes and includes the following sections: (1) history and applications of tellurium; (2) toxicity of tellurium; (3) microbial detoxification mechanisms against soluble tellurium anions including uptake, efflux and methods of reduction, and reduced ability to cope with oxidation stress or repair damaged DNA; and (4) the characteristics and applications of tellurium nanoparticles (TeNPs) produced by microbes. This review raises the awareness of microorganisms in tellurium biogeochemical cycling and the growing applications for microbial tellurium nanoparticles.
Keywords: Tellurium, Toxicity, Resistance, Microbial transformation, Tellurium nanoparticles (TeNPs)
History and application of Tellurium
Discovery and usage
Tellurium (Te) was first discovered in 1783 from the tellurium-bearing gold mining area of the Metaliferi Mountains, in modern-day Romania (Emsley 2011). Its crustal abundance is low, averaging around 0.005 mg/kg (Wedepohl 1995). It had few early uses, though it was typically found in gold-bearing areas, most notably in the Kalgoorlie gold mining area in Western Australia where gold-bearing tellurides contain ~ 25% of the total gold endowment of this world-class system (Vielreicher et al. 2016). Tellurium is a metalloid element which, when alloyed, endows different properties to different metals. The microbiological response to tellurium was first described over a century ago, with the now-characteristic blackening brought about by the formation of tellurium nanoparticles observed by King and Davis (1914). Despite many suggestions of the potential utility of tellurium for (micro)biological applications, the role of tellurium in microbiology and pharmacology remains poorly studied (Presentato et al. 2019; Hosseini et al. 2023).
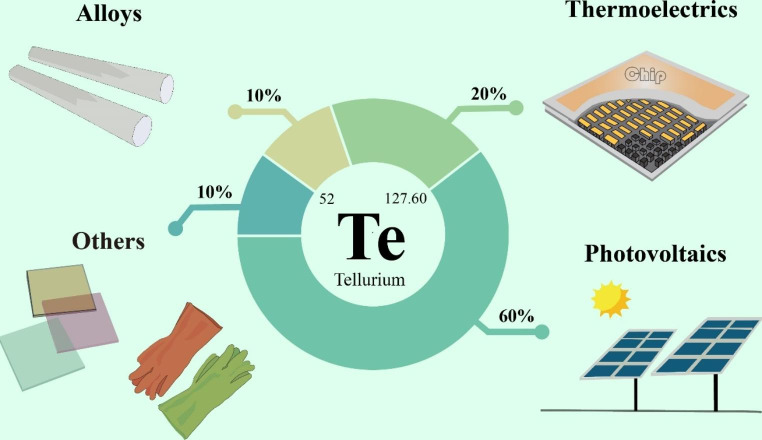
The usage of tellurium may have also increased dramatically if tellurium compounds had been used to prevent engine-knocking of combustion engines – despite their efficacy, their smell meant that insidious tetraethyl lead was used instead, to environmentally detrimental effect (Midgley Jr 1937). Today, the usage of tellurium is primarily as tellurides in solar panels (cadmium telluride) (60%) and thermoelectric devices (bismuth telluride) (20%) (Nassar et al. 2022). Other uses include in alloys of a range of metals (with steel and copper to improve machinability, with lead to improve vibration resistance) (10%), in the processing of rubber, and as a pigment in glasses and ceramics (10%)(Nuss 2019; Anderson 2022) (Fig. 1).
Fig. 1.
The applications of tellurium
Growing demand and criticality
The importance of tellurium as a commodity is increasing, primarily by virtue of the growing demand for tellurium in cadmium telluride solar panels. Cadmium telluride solar panels make up 5–10% of the solar panel market share. Despite this growing global usage of tellurium (at least 580 tonnes production in 2022, up from ~ 140 tonnes two decades ago) (Anderson 2022), only a handful of sites refine tellurium directly as a commodity, including Kankberg, Sweden (producing gold and tellurium) and more recently, the Kennecott Mine, Utah, USA (producing copper, gold, silver, molybdenum and now tellurium). Tellurium is most readily recovered from the anode slimes produced by the electrowinning of copper (Makuei and Senanayake 2018), but a move towards heap leaching for copper recovery may mean that tellurium is no longer produced from copper mining. Tellurium is often classed as a Critical Metal, particularly in the United States of America (McNulty and Jowitt 2021), where it is produced from just two refineries. The fact that Te is listed on criticality lists at all is testament to the importance of having efficient refinement pathways for by-product metal(loid)s. More than 10 times the global tellurium supply is mined every year, with the majority reporting to tailings dams and waste rock storage facilities. Mine waste storage has a high monetary cost to mine operators and potentially an environmental cost if deleterious elements leach from storage facilities (Kavlak et al. 2013; Missen et al. 2020).
Environmental issues
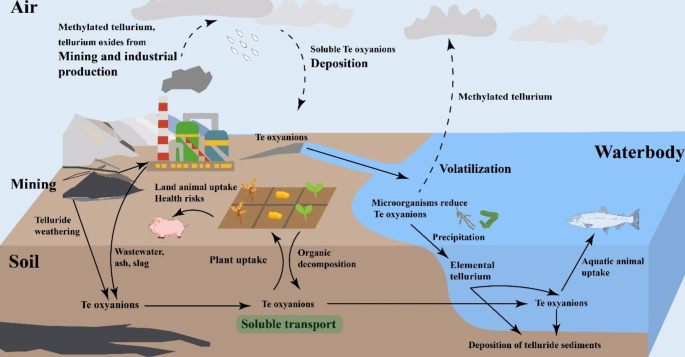
Despite its high toxicity to both microscopic and macroscopic life, tellurium’s rarity has meant that it has not left a negative environmental legacy in the manner of elements such as lead and arsenic. Tellurium is concentrated unequally in the environment, with high levels of Te in certain gold and copper deposits despite its overall low crustal abundance (Grundler et al. 2013), including extreme examples of enrichment above 0.1 wt% in ores (e.g. Börner et al. 2021) believed to be related to its mobility in (boiling) hydrothermal fluids (Cooke and McPhail 2001). Tellurium is usually present in aqueous environments in trace concentrations less than 1 µg/L (Llaver et al. 2021). Tellurium is most often one of a suite of contaminant elements present in settings such as acid mine drainage of Te-bearing pyrites (Zhan et al. 2022). However, the few studies that do exist suggest that tens of thousands of tonnes of tellurium have been released to the environment during industrial activities which process Te-rich feedstocks, with 9500 tonnes estimated to have been released to the atmosphere from copper smelters alone (Wiklund et al. 2018). Due to the extensive occurrence of tellurium and its oxyanions in various industrial and metal mining activities, tellurium has recently caused environmental pollution concerns (Alavi et al. 2020). After being released into the environment, wastewater containing tellurium ions may accumulate in soil and aquatic systems (Qin et al. 2017; Curtin et al. 2020) (Fig. 2). Tellurium is found primarily in the form of oxyanions, tellurite (TeO32−) and tellurate (TeO42−), in natural waters and weathered surface environment geological samples (Wu et al. 2014; Grygoyc and Jablonska-Czapla 2021). Soluble tellurite is more toxic than tellurate, and elemental tellurium (Te0) is less toxic than both (Yao et al. 2021). The distribution of soluble tellurium needs to be monitored in industrial settings such as copper-processing facilities.
Fig. 2.
The cycle of tellurium on the earth’s surface
Toxicity of Tellurium
General toxicity
As well as impacting plant growth and poisoning microorganisms, exposure to Te can also affect the health of humans and animals. Animal experiments have shown that exposure to tellurite accelerates liver toxicity and oxidative stress in rat liver tissues (Safhi et al. 2016). Cases of non-occupational tellurium exposure were rarely reported due to most natural settings and consumer products containing little significant tellurium. However, some groups have reported that tellurium in the soil might lead to contamination of foods. Tellurium concentrations in foods are generally < 1 mg Te / kg food and total human intake of Te per day is likely no more than 0.1 mg (Gerhardsson 2022), although it is worth noting that these concentrations nonetheless represent a Te enrichment 1–2 orders of magnitude compared to the average Te concentration in soil (average ~ 0.027 mg/kg; Ba et al. 2010), with typical values such as fresh fruits (0.185 mg/kg), cereals (0.168 mg/kg), legumes (0.382 mg/kg), potatoes (0.189 mg/kg), meat (0.686 mg/kg), nuts (1.072 mg/kg), fishes (0.803 mg/kg) and some dairy products (0.937 mg/kg) (Filippini et al. 2020; Gad and Pham 2014). As little 2 mg/kg tellurium in drinking water can pose a threat to human health (Yao et al. 2022). The amount of Te in the human body is not well-studied, but it is likely to be less than 1 mg (Emsley 2011).
Once tellurium compounds enter cells, they could induce cellular reactions including (1) interference of thiols (redox enzymes); (2) replacement of Se and S in proteins; (3) damage of cell membrane structure; and (4) increased oxidative stress (Goff et al. 2021a; Tang et al. 2022; Reddy et al. 2023). Among them, the production of reactive oxygen species (ROS) induced by tellurium oxyanions is the main factor of tellurate toxicity (Peng et al. 2022). The metabolism of tellurium in the human body remains unclear, despite being first recognized as a potential industrial poison a century ago (Shie and Deeds 1920). Recently, Duan et al. (2023) indicated a significant correlation between tellurium exposure and an increased risk of developing hypertension (high blood pressure). Animal studies showed that up to 25% of tellurium dioxide (TeO2) taken orally was absorbed by the gut; tellurium was primarily stored in the kidneys, but it accumulates in the liver, spleen, heart, lungs, brain, and bones (Hayes and Ramos 2019) (Fig. 2). Accidental ingestion of tellurium-containing metal-oxidising solutions by two children (independent of each other) led to symptoms including vomiting, difficulty swallowing, blackened tongue and lips and a garlic odour to the breath (Yarema and Curry 2005). To our knowledge, only two deaths have been recorded from tellurium ingestion due to accidental poisoning by sodium tellurite at a very high concentration of 30 mg sodium tellurite per kilogram body weight (Keall et al. 1946; Gerhardsson 2022). Symptoms included vomiting, kidney pain, loss of consciousness and a strong garlic odour. Systemic effects of acute tellurium toxicity in rats include lethargy, gastrointestinal disease, and fur changes, while chronic toxicity includes peripheral neuropathy, cerebral cortex changes, kidney and liver lesions, and reproductive effects (Gerhardsson 2022). The factors of toxicity, such as the generation of reactive oxygen species (ROS), disruption of cell membrane structure, and interference with redox enzymes, which occur in microbial cells, are also applicable in the toxicological assessment of animal cells (Safhi et al. 2018). Currently, there is a lack of research on the toxicological mechanisms of tellurium, particularly in humans and animals, compared to other group 16 counterpart, selenium, which has been more extensively studied (in part due to being a biologically essential element).
Microbial toxicity
Tellurite is toxic to both prokaryotes and eukaryotes. Tellurium compounds may act as antibacterial agents, effectively inhibiting the growth of infectious microorganisms such as Escherichia coli, Salmonella typhi and Klebsiella pneumoniae, and could be used to treat diseases such as syphilis, tuberculosis and leprosy (Goff 2020; Vavrova et al. 2021). In addition, ammonium trichloro tellurate (an organotellurium compound) also provided an option for the treatment of some symptoms caused by HIV infection (Peng and Li 2019).
Tellurite anions are toxic to most microorganisms at concentrations as low as 1 µg/ml (Arenas-Salinas et al. 2016). These soluble tellurium anions are highly toxic to most bacteria and more toxic than metals such as mercury and lead (Harrison et al. 2004; Workentine et al. 2008). Tellurite anions in high concentrations could alter balance in soil microbial communities. Kolesnikov (2019) found that the tellurium contaminated (even at 0.003 mg/kg) chernozem (black, humus-rich) soil showed a decrease in total bacterial count, reduced abundance of Azotobacter, and no significant recovery trend in the soil’s biological characteristics for 90 days after contamination. Among the soil pollution caused by silver, bismuth, tellurium and thallium, tellurium and thallium were the most ecotoxic, based on their effects on soil enzyme (catalase and dehydrogenase) activity, soil bacterial count and wheat root length (Kolesnikov et al. 2022). These studies demonstrated the high toxicity of tellurium and its significant harm to soil ecology. As tellurite anions exhibit toxicity to bacteria at low aqueous concentrations (1 µg/ml), pore water in tellurium-rich tailings contains high concentrations of salts and potentially toxic elements that might affect ectopic microbial communities (Hayes and Ramos 2019), selecting for tellurium-resistant microorganisms as a result. In addition, tellurite and tellurate extracted from semiconductor materials showed toxicity to the marine bacterium Aliivibrio fischeri (Ramos-Ruiz et al. 2016b).
Microbial detoxification mechanisms for tellurium
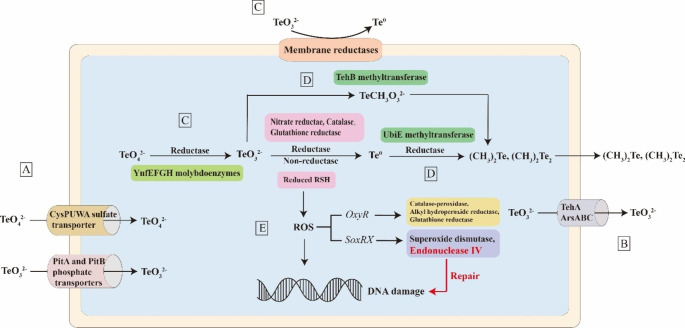
Various tellurium oxyanion detoxification mechanisms have been identified in microorganisms. The main detoxification mechanisms is reduction by either precipitation or methylation, and others include decreased intake, efflux, and reducing oxidative stress (Fig. 3).
Fig. 3.
The microbial detoxification mechanism to tellurite and tellurate. (A) reduce uptake of tellurite, (B) efflux of tellurite, (C) reduction of tellurite and tellurate to insoluble elemental tellurium [Te(0)], (D) reduction of tellurite and tellurate to methylated tellurium [Te(-II)], (E) reducing oxidative stress and DNA damage repair
Transport of tellurate
Transport is the first step in tellurium oxyanion metabolism in cells. As there are no known specific tellurite or tellurate transferases, tellurium oxyanions must enter cells via other anionic transferases. Goff and Yee (2017) discovered that in E. coli K-12, deletion of the CysPUWA sulfate transporter permease or ATPase subunits prevents tellurate entry into the cytoplasm, thus conferring higher resistance to tellurate.
Reduction of tellurate
The primary tellurium detoxification mechanism for many microorganisms is the transformation of tellurite or tellurate to elemental tellurium through biological reduction (Wu et al. 2019). In E. coli K-12, molybdopterin-containing enzymes were found to be capable of reducing tellurate to elemental tellurium (Theisen et al. 2013). A membrane-associated tellurite reductase isolated from the Erythromonas ursincola strain KR99 is also able to reduce tellurate to elemental tellurium (Maltman et al. 2017a). In addition, cysteine could also reduce tellurate to elemental tellurium in E. coli K-12 (Goff et al. 2021a).
Baesman et al. (2007) speculated that Sulfurospirillum barnesii cells might use a two-step reduction pathway to reduce tellurate, first reducing it to tellurite, and then further reducing tellurite to elemental tellurium. Ramos-Ruiz et al. (2016a) found that the reduction rate of tellurite was seven times faster than that of tellurate in a methanogenic microbial consortium. Since the reduction of tellurate requires more electrons to convert them to elemental tellurium compared to tellurite (6 per Te cation rather than 4), it takes longer to transfer the additional electrons (Maltman et al. 2017a). Therefore, tellurite accumulates relatively less during the reduction of tellurate in a community of mixed microorganisms in anaerobic, methane-producing granular sludge (Ramos-Ruiz et al. 2016a).
Transport of tellurite
Microorganisms can enhance their resistance to tellurite by reducing their tellurite intake. It is known that in the tellurite metabolism and transport system, the phosphate transporters PitA and PitB are responsible for the uptake of tellurite (Montenegro et al. 2021). Similarly, acetate transport proteins could take up tellurite in some bacteria, and the resistance of these bacteria to tellurite could increase when the competitor acetate was present (Borghese et al. 2010). In a Micromonospora strain isolated from a metal-rich environment, increasing the saturation and branched chain fatty acids may stiffen the cell membrane, resist excessive tellurium oxyanions entering the cell membrane, and avoid oxidative bursts associated with tellurite in order to cope with high concentrations (5 mM) of tellurite (Piacenza et al. 2022). Additionally, surface adsorption of tellurite could also control tellurite uptake (Goff et al. 2021b). These findings suggested that the reduced intake may contribute to tellurium oxyanion detoxification.
Efflux of tellurite out of cells is also a detoxification mechanism used by some microorganisms. In these bacteria, the tellurite resistant operon TehA encodes an intimal protein and TehB encodes a methyltransferase (Choudhury et al. 2011). E. coli TehA (EcTehA) is a typical efflux protein that could eliminate tellurite out of cells (Choudhury et al. 2011). In Aeromonas hydrophila, cytoplasmic protein TehB may accelerate the rate of reduction of tellurite to elemental tellurium (Castro et al. 2020). Additionally, the arsenite efflux system ArsABC had been reported to export tellurite (Turner et al. 1992).
Reduction of tellurite
Reduction of tellurite to element tellurium
Microorganisms convert tellurite into elemental tellurium or volatile methylated forms of tellurium through reduction as a detoxification mechanism (Ollivier et al. 2011). Microbially mediated reduction by precipitation (from soluble tellurium oxyanions to insoluble elemental tellurium) can be divided into enzymatic or non-enzymatic reduction. In terms of non-enzymatic reduction, some reducing thiols, such as glutathione, can also directly reduce tellurite to elemental tellurium (Muñoz-Diaz et al. 2022). Various organic electron shuttles (such as lawsone, menadione, anthraquinone-2-sulfonate, and anthraquinone-2,6-disulfonate) were also reported to mediate tellurite reduction (Wang et al. 2011; Borghese et al. 2020). Additionally, Fe3+ could promote electron generation and electron transfer to accelerate tellurite reduction in Shewanella oneidensis MR‑1 (Kim et al. 2013; He et al. 2021).
Numerous tellurite reductases have now been identified in microbes (Table 1). The first confirmed tellurite reductase was discovered in Mycobacterium avium (Castro et al. 2008). Subsequently, the tellurite reduction ability of nitrate reductase in E. coli, Ralstonia eutropha, Paracoccus denitrificans, and Paracoccus pantotrophus has been confirmed by both in vivo and in vitro experiments in many bacteria (Borghese et al. 2017). Calderon et al. (2006) found that the enzyme catalase in animals and Staphylococcus epidermidis could also act as a tellurite reductase. Castro et al. (2008) and Miguel et al. (2010) found that the dihydrolipoamide dehydrogenase in Aeromonas caviae ST, E. coli, Zymomonas mobilis, Streptococcus pneumoniae, and Geobacillus stearothermophilus exhibited good tellurite reduction activity. This indicated that dihydrolipoamide dehydrogenase may also be a common tellurite reductase. Glutathione reductase in Pseudomonas sp. BNF22 (Pugin et al. 2014), and 6-phosphogluconate dehydrogenase in E. coli (Sandoval et al. 2015) also showed good tellurite reduction activity. Additionally, a 117 kDa membrane protein in E. ursincola KR99 (Maltman et al. 2017a), TrxR in Bacillus sp. Y3 (Yasir et al. 2020), Mycothione reductase in Rhodococcus erythropolis PR4 (Butz et al. 2020) and Flagellin (FlaA) in Paenibacillus pabuli ALJ109b (Farias et al. 2021) were also proven to be acting as microbial tellurite reductases.
Table 1.
Microbial Te (IV) reductase
| Reductase | Cell localization | Measurement condition | Michaelis constant Km (mM) | Maximum reaction rate Vmax (U/mg) | Source | Ref. |
|---|---|---|---|---|---|---|
| Nitrate reductase | Cell membrane /periplasmic | Purified protein | 0.6 | 0.97 |
E. coli, R. eutropha, P. denitrificans, P. pantotrophus |
(Sabaty et al. 2001) |
| Catalase | Unknown | Cell extract /Partially purified proteins | 0.9 | Unknown | S. epidermidis | (Calderon et al. 2006) |
| Dihydrolipoamide dehydrogenase (E3) | Unknown | Purified protein | 0.04794 | 84.5 | A. caviae ST, E. coli, Z. mobilis, S. pneumonia, G. stearothermophilus | (Castro et al. 2008; Arenas-Salinas et al. 2016) |
| Glutathione reductase (GorA) | Cytoplasm | Purified protein | 0.08947 | 6314 | Pseudomonas sp. BNF22, E. coli | (Pugin et al. 2014; Arenas-Salinas et al. 2016) |
| 6-phosphogluconate dehydrogenase (6PGD) | Unknown | Purified protein | Unknown | Unknown | E. coli | (Sandoval et al. 2015) |
| Thioredoxin reductase (TrxB) | Unknown | Purified protein | 0.1145 | 9586 | Staphylococcus haemolyticus BNF01, E. coli | (Arenas-Salinas et al. 2016) |
| Alkyl hydroperoxide reductase (AhpF) | 0.8196 | 77,875 | ||||
| NADH: flavorubredoxin reductase (NorW) | 0.6949 | 5347 | ||||
| Putative oxidoreductase (YkgC) | 0.5171 | 2696 | ||||
| Mercuric reductase (MerA) | Unknown | Unknown | ||||
| Membrane reductase | Cell membrane | Purified protein | 3.36 | 5.15 | E. ursincola KR99 | (Maltman et al. 2017a) |
| Periplasmic reductase | Periplasmic | Purified protein | 3.9 | 5.6 | Shewanella frigidimarina ER-Te-48 | (Maltman et al. 2017b) |
| Thioredoxin-disulfide reductase (TrxR) | Unknown | Purified protein | 16.31 | 12.23 | Bacillus sp. Y3 | (Yasir et al. 2020) |
| Mycothione reductase (Mtr) | Unknown | Purified protein | 0.779 ± 0.050 | Unknown | R. erythropolis PR4 | (Butz et al. 2020) |
| Flagellin (FlaA) | Flagellum | Purified protein | Unknown | Unknown | P. pabuli ALJ109b | (Farias et al. 2021) |
Interestingly, Arenas-Salinas et al. (2016) found that most reported reductases for tellurite were flavin adenine dinucleotide (FAD) based flavin reducing proteins according to bioinformatics analysis. Therefore, they expressed and purified flavoproteins including thioredoxin reductase (TrxB), alkylhydroperoxide reductase (AhpF), glutathione reductase (GorA), mercuric reductase (MerA), NADH: flavorubredoxin reductase (NorW), dihydrolipoamide dehydrogenase (E3), and inferred oxidoreductase YkgC from E. coli and other environmental strains (Arenas-Salinas et al. 2016). The in vitro enzyme activity tests showed that these proteins could reduce tellurite to generate tellurium nanoparticles (Arenas-Salinas et al. 2016).
Currently, studies on tellurite reductases are mainly focused on aerobic conditions. The reduction mechanism of tellurite under anaerobic conditions is not very clear. A diverse community of metal(loid) oxide respiring bacteria around black smokers is known to remove tellurium (and other metalloid) oxyanions as terminal electron acceptors through anaerobic respiration (Maltman et al. 2016). However, this electron transport pathway needs further study.
Reduction of tellurite to methylated tellurium
Methylation is another method by which microbes detoxify tellurium by reduction. Primarily, tellurite methylation proceeds by conversion to dimethyl telluride [(CH3)2Te, DMTe], which is less toxic than tellurite, highly volatile and has a characteristic garlic odour (Prigent-Combaret et al. 2012). Other methylated tellurium species produced include dimethyl ditelluride [(CH3)2Te2, DMDTe] and the mixed species dimethyltellurenyl sulfide [(CH3)2TeS, DMTeS] (Ollivier et al. 2008). The most commonly identified methylation pathway was S-adenosylmethionine-dependent methylation (Choudhury 2013). DMTe has been identified as a product of tellurium bioreduction in many bacterial strains, such as Scopulariopsis brevicaulis and Pseudomonas fluorescens (Choudhury 2013). In a Penicillium strain, DMTe was not produced when only tellurium was present, but DMTe could be detected when selenium and tellurium were added together (Choudhury 2013). This suggested that the presence of selenium might induce tellurium methylation pathways.
Tellurite methylation is usually catalyzed by methyltransferase. Overexpression of the bacterial thiopurine methyltransferase (bTPMT) in E. coli can enhance its resistance to tellurite (Choudhury et al. 2011). Currently, the most well-studied tellurium methyltransferase is TehB in E. coli, which is typically arranged together with TehA (Choudhury et al. 2011). TehB could convert tellurite to TeCH3O32−, which further reacts to form DMTe (Chasteen et al. 2009; Choudhury 2013), in a process which does not produce elemental tellurium. Furthermore, Ollivier et al. (2011) found that aeration plays an important role in controlling the volatile and precipitation equilibrium of tellurite oxyanions, where elemental tellurium may be an intermediate in volatilization pathways in tellurite resistant Rhodotorula mucilaginosa. In some bacteria tellurite can be first reduced to elemental tellurium, which is then methylated by the UbiE methyltransferase to generate DMTe (Araya et al. 2004). Similarly, in the tellurite-reducing bacterium Sporosarcina sp. Te-1, the formed elemental tellurium can further be transformed into methylated organotellurium compounds (Wang et al. 2021).
Reduced oxidative stress and DNA damage repair
Tellurite or tellurate react with reduced thiols or reductases after entering the cell, leading to production of reactive oxygen species (ROS) (Calderon et al. 2006; Diaz-Vasquez et al. 2014). The production of ROS might further lead to DNA damage and affect the normal growth and reproduction of microorganisms. ROS production is the main reason why Te oxyanions are so toxic to many microbes.
Microbes can upregulate some genes including genes which promote (1) reduction of ROS or (2) repair DNA damage caused by ROS to cope with the increasing ROS. For instance, the production of ROS led to the up-regulation of global regulatory factors such as OxyR and SoxRX in E. coli; OxyR further regulated proteins such as catalase-peroxidase (KatG), alkyl hydroperoxide reductase (AhpCF), glutathione reductase (GorA), glutaredoxin A (GrxA), and thioredoxin C (TrxC) to cope with oxidative stress (Chasteen et al. 2009; Pérez et al. 2007; Zannoni et al. 2008). SoxRS, on the other hand, up-regulated proteins such as superoxide dismutase (SodA) and endonuclease IV to deal with the toxicity of ROS produced by tellurite (Chasteen et al. 2009; Choudhury 2013). KatG, AhpCF, GorA, TrxC and SodA could reduce ROS content in intracellular environments, meanwhile endonuclease IV could repair DNA damage.
Characterization and application of tellurium nanoparticles synthesized by microorganisms
Biological tellurium nanoparticles (Bio-TeNPs) based on microbes
Numerous studies reported that soluble and highly toxic tellurium oxyanions could be converted into low toxicity elemental tellurium nanoparticles through the action of bacteria, fungi or archaea (Ao et al. 2022; Srivastava et al. 2015), which is not just a laboratory phenomenon, but also detectable in nature (Missen et al. 2022). Bio-TeNPs were produced from tellurite by microorganisms in most studies, and only a few bacteria have been reported to produce Bio-TeNPs from tellurate, such as S. barnesii and Bacillus selenitireducens (Baesman et al. 2007). This might be because reduction of tellurite to elemental tellurium occurs more readily than reduction of tellurate to elemental tellurium in microorganisms (Maltman et al. 2017a). This phenomenon was similar to the trends observed for selenium oxyanion metabolism in microorganisms (Wang et al. 2022).
Literature reports of tellurium oxyanion bioreduction are dominated by bacteria (Table 2), followed by fungi and archaea (Table 3). However, fungi and archaea are more resistant to high concentrations of tellurite than bacteria (Pearion and Jablonski 1999; Hosseini et al. 2023; Wu et al. 2019; Ao et al. 2022). Higher maximum tellurite resistance might be owing to the higher metal absorption capacity, bioaccumulation ability, effective extracellular enzyme secretion and/or the filamentous structure of fungi (Barabadi et al. 2018; Kashyap et al. 2018), and archaea exhibited high levels of resistance in extreme environments with high salinity, and had the ability to resist and reduce the toxic tellurite (Srivastava et al. 2015). However, most bacteria are sensitive to tellurite at significantly lower concentrations, with even 1 µM tellurite enough to cause a toxic response. Although bacteria are tolerant to lower concentrations of tellurite than fungi and archaea, they might be a good cell factory for the production of Bio-TeNPs for further applications. This is mainly due to the fact that bacteria possess some advantages such as (1) faster growth than fungi and archaea, (2) the fermentation technology is currently more mature, and (3) produced Bio-TeNPs are easier to extract from cultures. On the other hand, fungi and archaea suitable for large-scale fermentation and rapid reduction to form Bio-TeNPs remain to be further discovered.
Table 2.
Bio-TeNPs produced by bacteria
| Biological source | Location | Percursor | Shape | Size (nm) | References |
|---|---|---|---|---|---|
| Acinetobacter pittii D120 | Unknown | Te(IV) | Rod-shaped | 60–130 | (Tang et al. 2022) |
| Aromatoleum sp. CIB | Intracellular | Te(IV) | Rod-shaped | 200 | (Alonso-Fernandes et al. 2023) |
| B. selenitireducens | Intracellular | Te(VI), Te(IV) | Rod-shaped, rosettes | 200, 1000 | (Baesman et al. 2007) |
| Bacillus sp. BZ | Cell debris | Te(IV) | Rod-shaped | 20 × 180 | (Zare et al. 2012) |
| B. Selenitireducens | Cell surfaces | Te(IV) | Rod-shaped, Rosettes | Unknown | (Wang et al. 2019) |
| Escherichia | Intracellular and extracellular | Te(IV) | Ellipse-shaped | 0.9 × 10− 3-1.8 × 10− 3 | (Nguyen et al. 2019) |
| Lactobacillus plantarum PTCC1058 | Intracellular | Te(IV) | Spheres | 45.7 | (Mirjani et al. 2015) |
| Lysinibacillus sp. ZYM-1 | Cell membrane | Te(IV) | Rod-shaped, hexagonal Te nanoplates, nanoflowers, and nanobranches | 300–500 | (Wang et al. 2018) |
| Lysinibacillus sp. EBL303 | Intracellular | Te(IV) | Spheres | 22–148 | (Hosseini et al. 2023) |
| Ochrobactrum sp. MPV1 | Intracellular | Te(IV) | Short needle-like | Unknown | (Zonaro et al. 2017) |
| Ochrobactrum sp. MPV1 | Unknown | Te(IV) | Spherical | 76.2 | (Zonaro et al. 2015) |
| Pseudomonas sp. strain BNF22 | Cytoplasm | Te(IV) | Round, porous | 60 | (Pugin et al. 2014) |
| P. pseudoalcaligenes | Cell debris | Te(IV) | Rod-shaped | 185 | (Forootanfar et al. 2015) |
| P. pabuli ALJ109b | Unknown | Te(IV) | Spheres | <100 | (Mirjani et al. 2015) |
| P. pseudoalcaligenes | Unknown | Te(IV) | Individual, rod-shaped, rosettes | 50–200 | (Shakibaie et al. 2017) |
| R. aetherivorans BCP1 | Intracellular | Te(IV) | Rod-shaped | 148 ± 104, 223 ± 116 | (Presentato et al. 2016) |
| Rhodobacter capsulatus | Extracellular | Te(IV) | Acicular | 200–700 | (Borghese et al. 2017) |
| R. aetherivorans BCP1 | Cytoplasm | Te(IV) | Spherical, rod-shaped | > 700 | (Presentato et al. 2018) |
| Raoultella | Intracellular and extracellular | Te(IV) | Rod-shaped | 1.7 × 10− 3-2.6 × 10− 3 | (Nguyen et al. 2019) |
| R. capsulatus | Extracellular | Te(IV) | Elongated needle-like | 10 ± 5 | (Borghese et al. 2020) |
| S. barnesii | Intracellular and extracellular | Te(VI), Te(IV) | Irregularly shaped spheres | < 50 | (Baesman et al. 2007) |
| Shewanella baltica | Intracellular | Te(IV) | Rod-shaped | 8–75 | (Vaigankar et al. 2018) |
| Shewanella sp. NT-1 | Intracellular | Te(IV) | Spheres | Unknown | (Sakaguchi et al. 2019) |
| Shinella sp. WSJ-2 | Intracellular | Te(IV) | Rod-shaped | 50–120 | (Wu et al. 2019) |
| Streptomyces cyaneus | Extracellular | Te(IV) | Spherical TeO2 Nps | 35–89 | (El-Sayyad et al. 2020) |
| Streptomyces graminisoli | Unknown | Te(IV) | Rods, rosette | 21.4 | (Abed et al. 2023) |
Table 3.
Bio-TeNPs produced by fungi and archaea
| Biological source | Source | Percursor | Shape | Size (nm) | Ref. | |
|---|---|---|---|---|---|---|
| Fungi | Aspergillus welwitschiae | Fungal isolate | Te(IV) | Elliptic to spherical | 60.8 | (Abo Elsoud et al. 2018) |
| Aureobasidium pullulans | Supernatants | Te(IV) | Granular | 40–70 | (Liang et al. 2019) | |
| A. pullulans | Supernatants | Te(IV) | Spherical | 5–65 | (Nwoko et al. 2021) | |
| A. niger | Hyphae | Te(IV) | Rod-shaped, spheres | 200–300, 20–100 | (Sinharoy and Lens 2022) | |
| Mortierella humilis | Supernatants | Te(IV) | Granular | 40–70 | (Liang et al. 2019) | |
| Mortierella sp. AB1 | Intracellular | Te(IV) | Rod-shaped | 100–500 | (Ao et al. 2022) | |
| Phanero chaetechrysosporium | Hyphae | Te(IV) | Needle-like | 20–465 | (Espinosa-Ortiz et al. 2017) | |
| Penicillium chrysogenum PTCC5031 | Extracellular | Te(IV) | Spherical | 33.8 | (Barabadi et al. 2018) | |
| Phoma glomerata | Supernatants | Te(IV) | Pillar and needle shapes | 40–70 | (Liang et al. 2019) | |
| P. glomerata | Supernatants | Te(IV) | Needle-shaped | 10–80 | (Liang et al. 2020) | |
| Trichoderma harzianum | Supernatants | Te(IV) | Pillar and needle shapes | 40–70 | (Liang et al. 2019) | |
| Archaea | Halococcus salifodinae BK3 | Intracellular | Te(IV) | Hexagonal needle-shaped | 10 × 44 | (Srivastava et al. 2015) |
| Haloferaxalexandrinus GUSF-1 | Cell lysate | Te(IV) | Rod-shaped | 40 × 7 | (Alvares and Furtado 2021) |
Microbial Bio-TeNPs were usually located in the cytoplasm, periplasmic space or outside the cell. Additionally, Bio-TeNPs could accumulate and be excreted from cells after forming inside the cells, which could then exist both inside and outside the cell, such as occurs in the Raoultella genus and Escherichia genus (Nguyen et al. 2019). The production of Bio-TeNPs involves two steps, reduction and precipitation, occurring either intracellularly or extracellularly, which might be related to the reductase or reducing substances at different sites (Borghese et al. 2017). So far, most microbially sourced Bio-TeNPs are derived from intracellular pathways. In terms of shape, Bio-TeNPs are formed mainly in the shape of rods, needles and spheres, but also nanoflowers, nanoplates, and even nanobranches in a few microbes (Fig. 4). Interestingly, Rhodococcus aetherivorans BCP1 converted tellurite to produce spherical tellurium nanoparticles that could further transform into nanorods with increasing exposure time (Presentato et al. 2018). Owing to electrostatic interactions, Bio-TeNPs adhered to each other and formed large rose-shaped knots in Pseudomonas pseudoalcaligenes (Shakibaie et al. 2017).
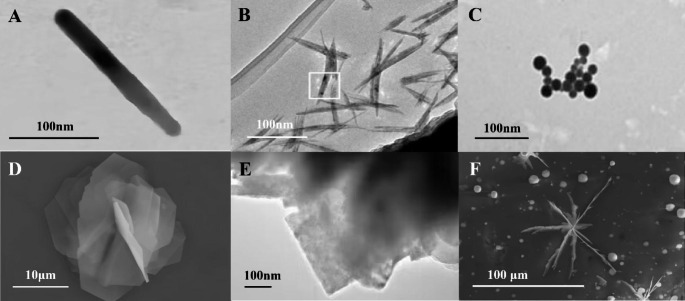
Fig. 4.
TEM images of different shapes of Bio-TeNPs. (A) Rod-like TeNPs (Shakibaie et al. 2017), (B) needle-like TeNPs (Borghese et al. 2020), (C) spherical TeNPs (Nwoko et al. 2021), and (D) nanoflower, (E) nanoplate and (F) nanobranch (Wang et al. 2018), respectively
The variety of microorganism species, growth medium and synthesis conditions all have an effect on the size, shape and dispersion of nanoparticles (Barabadi et al. 2018). The optimal metal ion concentration, pH and reaction temperature are also regarded as important factors in the formation of nanoparticles (Marooufpour et al. 2019). The links between reduction kinetics and protein structure may also lead to morphological differences in biological tellurium nanoparticles, according to the study of Lysinibacillus sp. ZYM-1 cells (Wang et al. 2018). The type of tellurate reductase presented on the cell membrane may determine the morphology of Bio-TeNPs, including the simultaneous presence of nanorods, nanoflowers, nanobranches, and nanoplates (Wang et al. 2018). The mechanism underlying the different positions and shapes of Bio-TeNPs produced by different microorganisms, as well as the variations of position and shape for Bio-TeNPs produced by the same microorganisms, are still unclear and need further investigation.
Application of microbial Bio-TeNPs
Nanomaterials have the advantages of larger surface area, higher cytocompatibility and fewer defects than other materials, and they are used in almost all fields, such as biomedicine, optoelectronics and environmental remediation (Zonaro et al. 2015; Vaigankar et al. 2018; Vahidi et al. 2021). Microbial Bio-TeNPs are typically generated under mild reaction conditions. They provide a safe, economical and environmentally friendly means to reduce the use of organic solvents and more toxic reactants in a variety of applications, reducing the production of toxic residues with great prospects for further industrial development (Borghese et al. 2020).
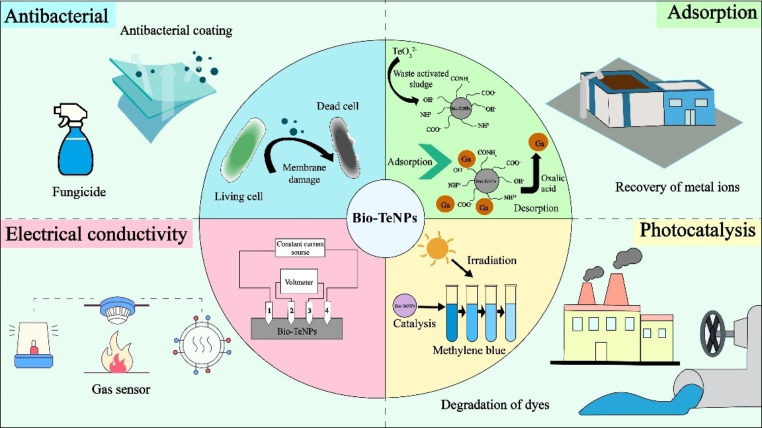
Microbial Bio-TeNPs show great potential for applications in antibacterial, adsorptive, photocatalytic and conductive electronic materials (Fig. 5). (1) Bio-TeNPs have been found to have excellent antibacterial activity (Table 4). Toxicity of Bio-TeNPs, while less than that of soluble Te oxyanions, is still significant and the Bio-TeNPs are able to destroy biofilms, produce reactive oxygen species, damage DNA and release toxic ions in specific contexts (Ghosh et al. 2021). Thus, Bio-TeNPs were found to inhibit the growth of E. coli (Pugin et al. 2014), Staphylococcus aureus (Abed et al. 2023), K. pneumoniae, Pseudomonas aeruginosa (Zare et al. 2012), Candida albicans (Zare et al. 2014), Aspergillus flavus and Aspergillus niger (El-Sayyad et al. 2020). Najimi et al. (2017) conducted a subacute evaluation of Bio-TeNPs using mice prepared by P. pseudoalcaligenes. They found that the toxicity of Bio-TeNPs was lower than that of tellurite, and in a 14-day subtoxicity study in mice, doses below 1.2 mg/kg did not cause adverse reactions. Furthermore, Abed et al. (2023) tested Bio-TeNPs in a rat intravenous infection model, which demonstrated their effectiveness against methicillin-resistant S. aureus (MRSA) and improved the survival rate of infected animals, while also showing a reasonable level of safety in terms of liver and kidney function. Bio-TeNPs are expected to be an alternative to traditional antibiotics and chemical fungicides used in antibacterial coatings of medical devices (Zonaro et al. 2017), alleviating the pressure of microbial antibiotics resistance caused by conventional antibiotics. (2) Bio-TeNPs are also good adsorbents. Bio-TeNPs produced by Shinella sp. WSJ-2 were able to remove a variety of dyes and metal ions due to electrostatic interactions (Wu et al. 2019). Bio-TeNPs can recover gallium by adsorption of up to 74 mg of Ga3+ per gram (Saikia et al. 2022). (3) Bio-TeNPs also exhibited photocatalytic ability. Bio-TeNPs achieved 90% photocatalytic degradation of the dye methylene blue in Shewanella baltica within 4 h. Therefore, they can be used as a photocatalyst for the remediation of methylene blue in industrial wastewater (Vaigankar et al. 2018). (4) Bio-TeNPs with good conductivity synthesized by R. aetherivorans BCP1 demonstrate potential for electronic applications (Presentato et al. 2018). They can be used in optoelectric, thermoelectric, piezoelectric devices, as well as gas sensors and infrared detectors. At present, the applications of Bio-TeNPs are mainly focused on antibacterial applications, while less attention has been paid to their toxicity to animals or humans. Additionally, studies on adsorption, photocatalysis and optical properties of Bio-TeNPs are still in their infancy and other applications need to be further studied.
Fig. 5.
The application of Bio-TeNPs
Table 4.
Antibacterial activity of Bio-TeNPs
| Bio-TeNPs source | Pathogens | Ref. |
|---|---|---|
| A. pittii | E. coli. | (Tang et al. 2022) |
| Bacillus sp. BZ | S. aureus, S.typhi, K. pneumonia and P. aeruginosa | (Zare et al. 2012) |
| Bacillus sp. BZ | C. albicans ATCC14053 | (Zare et al. 2014) |
| H. salifodinae BK3 | E. coli NCIM2345, P. aeruginosa MTCC2581, S. aureus MTCC737 and Micrococcus luteus | (Srivastava et al. 2015) |
| Ochrobactrum sp. MPV1 | E.coli JM109, P. aeruginosa PAO1 and S. aureus ATCC25923 | (Zonaro et al. 2015) |
| Pseudomonas sp. strain BNF22 | E. coli. | (Pugin et al. 2014) |
| P. pseudoalcaligenes | E. coli, P. aeruginosa, S. typhi, S. aureus (MRSA), C. albicans, and Candida dubliniensis | (Shakibaie et al. 2017) |
| S. cyaneus | A. flavus, A. niger, Aspergillus fumigatus, P. aeruginosa, S. aureus and K. pneumoniae | (El-Sayyad et al. 2020) |
| S. graminisoli | Methicillin-resistant S. aureus (MRSA) | (Abed et al. 2023) |
Conclusion and perspectives
In recent years, with the increasing demand for tellurium, concerns about environmental pollution and toxicity associated with tellurium have gained widespread attention. Microbes play an important role in the tellurium biogeochemical cycle. Several different mechanisms for tellurium detoxification have been identified in microorganisms. Among the detoxification mechanisms, the conversion of highly toxic tellurate and tellurite oxyanions into lower-toxicity elemental tellurium or methylated organotellurium compounds through microbial processes has been demonstrated as an environmentally friendly, feasible, and promising approach to deal with tellurite and tellurate contamination. Moreover, some microorganisms could reduce tellurite or tellurate to elemental tellurium and form Bio-TeNPs. Bio-TeNPs show great potential applications in fields including biomedicine, optoelectronics and environmental remediation. Future research is expected to focus on the following aspects: (1) tellurium pollution monitoring in the environment, (2) migration and transformation of tellurium in the natural environment, (3) tellurite and tellurate reduction mechanisms under anaerobic conditions, (4) high efficiency tellurite and tellurate reductases, (5) identification of tellurate methylase, (6) Bio-TeNPs formation and secretion mechanism, (7) the toxicity evolution of Bio-TeNPs, (8) more application directions of Bio-TeNPs.
Author contributions
YW, SY and OPM prepared the draft manuscript and the figures. XX, QG and OPM revised the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was supported by the National Natural Science Foundation of China (32000066), the Hubei Province Key R&D Program Project (2022BCE010), Hubei Province Central Government Guides Local Project, Natural Science Foundation of Hubei Province (2022CFB503), the Innovation Team Project of Hubei Education Department (T2022010), and the Open Foundation of the Hubei Key Laboratory of Edible Wild Plants Conservation and Utilization (EWPL202109).
Data availability
The data supporting the findings of this review are available in the references cited within this article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Owen P. Missen, Email: owen.missen@utas.edu.au
Xian Xia, Email: xianxia@hbnu.edu.cn.
References
- Abed NN, Abou El-Enain IMM, El-Husseiny Helal E, Yosri M. Novel biosynthesis of tellurium nanoparticles and investigation of their activity against common pathogenic bacteria. J Taibah Univ Medical Sci. 2023;18(2):400–412. doi: 10.1016/j.jtumed.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo Elsoud MM, Al-Hagar OEA, Abdelkhalek ES, Sidkey NM. Synthesis and investigations on tellurium myconanoparticles. Biotechnol Rep. 2018;18:e00247. doi: 10.1016/j.btre.2018.e00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi S, Rafieyan S, Yavari-Bafghi M, Amoozegar MA. Extremophiles: a powerful choice for bioremediation of toxic oxyanions. In: Shah MP, editor. Microbial Bioremediation & Biodegradation. Singapore: Springer Singapore; 2020. pp. 203–249. [Google Scholar]
- Alonso-Fernandes E, Fernandez-Llamosas H, Cano I, Serrano-Pelejero C, Castro L, Diaz E, Carmona M. Enhancing tellurite and selenite bioconversions by overexpressing a methyltransferase from Aromatoleum sp. CIB Microb Biotechnol. 2023;16(5):915–930. doi: 10.1111/1751-7915.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares JJ, Furtado IJ. Anti-Pseudomonas aeruginosa biofilm activity of tellurium nanorods biosynthesized by cell lysate of Haloferax alexandrinus GUSF-1 (KF796625) Biometals. 2021;34(5):1007–1016. doi: 10.1007/s10534-021-00323-y. [DOI] [PubMed] [Google Scholar]
- Anderson CS (2022) Tellurium, USGS Mineral Commodity Summaries
- Ao B, He F, Lv J, Tu J, Tan Z, Jiang H, Shi X, Li J, Hou J, Hu Y, Xia X. Green synthesis of biogenetic Te(0) nanoparticles by high tellurite tolerance fungus Mortierella sp. AB1 with antibacterial activity. Front Microbiol. 2022;13:1020179. doi: 10.3389/fmicb.2022.1020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya MA, Swearingen JW, Plishker MF, Saavedra CP, Chasteen TG, Vásquez CC. Geobacillus stearothermophilus V ubiE gene product is involved in the evolution of dimethyl telluride in Escherichia coli K-12 cultures amended with potassium tellurate but not with potassium tellurite. J Biol Inorg Chem. 2004;9(5):609–615. doi: 10.1007/s00775-004-0554-z. [DOI] [PubMed] [Google Scholar]
- Arenas-Salinas M, Vargas-Perez JI, Morales W, Pinto C, Munoz-Diaz P, Cornejo FA, Pugin B, Sandoval JM, Diaz-Vasquez WA, Munoz-Villagran C, Rodriguez-Rojas F, Morales EH, Vasquez CC, Arenas FA. Flavoprotein-mediated tellurite reduction: structural basis and applications to the synthesis of tellurium-containing nanostructures. Front Microbiol. 2016;7:1160. doi: 10.3389/fmicb.2016.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba LA, Doring M, Jamier V, Jacob C. Tellurium: an element with great biological potency and potential. Org Biomol Chem. 2010;8(19):4203–4216. doi: 10.1039/c0Ob00086h. [DOI] [PubMed] [Google Scholar]
- Baesman SM, Bullen TD, Dewald J, Zhang D, Curran S, Islam FS, Beveridge TJ, Oremland RS. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use te oxyanions as respiratory electron acceptors. Appl Environ Microbiol. 2007;73(7):2135–2143. doi: 10.1128/AEM.02558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabadi H, Kobarfard F, Vahidi H. Biosynthesis and characterization of biogenic tellurium nanoparticles by using Penicillium chrysogenum PTCC 5031: a novel approach in gold biotechnology. Iran J Pharm Res. 2018;17(Suppl2):87–97. [PMC free article] [PubMed] [Google Scholar]
- Borghese R, Zannoni D. Acetate permease (ActP) is responsible for tellurite (TeO32) uptake and resistance in cells of the facultative phototroph Rhodobacter capsulatus. Appl Environ Microbiol. 2010;76(3):942–944. doi: 10.1128/aem.02765-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese R, Brucale M, Fortunato G, Lanzi M, Mezzi A, Valle F, Cavallini M, Zannoni D. Reprint of “Extracellular production of tellurium nanoparticles by the photosynthetic bacterium Rhodobacter capsulatus. J Hazard Mater. 2017;324(Pt A):31–38. doi: 10.1016/j.jhazmat.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Borghese R, Malferrari M, Brucale M, Ortolani L, Franchini M, Rapino S, Borsetti F, Zannoni D. Structural and electrochemical characterization of lawsone-dependent production of tellurium-metal nanoprecipitates by photosynthetic cells of Rhodobacter capsulatus. Bioelectrochemistry. 2020;133:107456. doi: 10.1016/j.bioelechem.2020.107456. [DOI] [PubMed] [Google Scholar]
- Börner F, Keith M, Smith DJ, Barry TL, Neumann T, Klemd R. Fingerprinting fluid evolution by trace elements in epithermal pyrite, vatukoula Au-Te deposit, Fiji. Ore Geol Rev. 2021;137:104314. doi: 10.1016/j.oregeorev.2021.104314. [DOI] [Google Scholar]
- Butz ZJ, Hendricks A, Borgognoni K, Ackerson CJ. Identification of a TeO32 reductase/mycothione reductase from Rhodococcus erythropolis PR4. FEMS Microbiol Ecol. 2020;97(1):fiaa220. doi: 10.1093/femsec/fiaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon IL, Arenas FA, Perez JM, Fuentes DE, Araya MA, Saavedra CP, Tantalean JC, Pichuantes SE, Youderian PA, Vasquez CC. Catalases are NAD(P)H-dependent tellurite reductases. PLoS ONE. 2006;1(1):e70. doi: 10.1371/journal.pone.0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro ME, Molina R, Diaz W, Pichuantes SE, Vasquez CC. The dihydrolipoamide dehydrogenase of Aeromonas caviae ST exhibits NADH-dependent tellurite reductase activity. Biochem Biophys Res Commun. 2008;375(1):91–94. doi: 10.1016/j.bbrc.2008.07.119. [DOI] [PubMed] [Google Scholar]
- Castro L, Li J, González F, Muñoz JA, Blázquez MLJH. Green synthesis of tellurium nanoparticles by tellurate and tellurite reduction using Aeromonas hydrophila under different aeration conditions. Hydrometallurgy. 2020;196:105415. doi: 10.1016/j.hydromet.2020.105415. [DOI] [Google Scholar]
- Chasteen TG, Fuentes DE, Tantalean JC, Vasquez CC. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev. 2009;33(4):820–832. doi: 10.1111/j.1574-6976.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Choudhury HG, Beis K. Tellurite-resistance protein TehA from Escherichia coli. In: Kretsinger RH, Uversky VN, Permyakov EA, editors. Encyclopedia of Metalloproteins. New York, NY: Springer New York; 2013. pp. 2157–2160. [Google Scholar]
- Choudhury HG, Cameron AD, Iwata S, Beis K. Structure and mechanism of the chalcogen-detoxifying protein TehB from Escherichia coli. Biochem J. 2011;435(1):85–91. doi: 10.1042/bj20102014. [DOI] [PubMed] [Google Scholar]
- Cooke DR, McPhail DJEG (2001) Epithermal Au-Ag-Te mineralization Acupan, Baguio District, Philippines: numerical simulations of mineral deposition. Econ Geol 96(1):109–131. 10.2113/gsecongeo.96.1.109
- Curtin AM, Vail CA, Buckley HL. CdTe in thin film photovoltaic cells: interventions to protect drinking water in production and end-of-life. Water-Energy Nexus. 2020;3:15–28. doi: 10.1016/j.wen.2020.03.007. [DOI] [Google Scholar]
- Diaz-Vasquez WA, Abarca-Lagunas MJ, Arenas FA, Pinto CA, Cornejo FA, Wansapura PT, Appuhamillage GA, Chasteen TG, Vasquez CC. Tellurite reduction by Escherichia coli NDH-II dehydrogenase results in superoxide production in membranes of toxicant-exposed cells. Biometals. 2014;27(2):237–246. doi: 10.1007/s10534-013-9701-8. [DOI] [PubMed] [Google Scholar]
- Duan SY, Wang R, He P, Sun J, Yang HF. Associations between multiple urinary metals and the risk of hypertension in community-dwelling older adults. Environ Sci Pollut Res Int. 2023;30(31):76543–76554. doi: 10.1007/s11356-023-27797-2. [DOI] [PubMed] [Google Scholar]
- El-Sayyad GS, Mosallam FM, El-Sayed SS, El-Batal AI. Facile biosynthesis of tellurium dioxide nanoparticles by Streptomyces cyaneus melanin pigment and gamma radiation for repressing some aspergillus pathogens and bacterial wound cultures. J Clust Sci. 2020;31(1):147–159. doi: 10.1007/s10876-019-01629-1. [DOI] [Google Scholar]
- Emsley J (2011) Nature’s building blocks: an AZ guide to the elements. Oxford University Press
- Espinosa-Ortiz EJ, Rene ER, Guyot F, van Hullebusch ED, Lens PNL. Biomineralization of tellurium and selenium-tellurium nanoparticles by the white-rot fungus phanerochaete chrysosporium. Int Biodeterior Biodegradation. 2017;124:258–266. doi: 10.1016/j.ibiod.2017.05.009. [DOI] [Google Scholar]
- Farias P, Francisco R, Maccario L, Herschend J, Piedade AP, Sorensen S, Morais PV. Impact of tellurite on the metabolism of Paenibacillus pabuli AL109b with flagellin production explaining high reduction capacity. Front Microbiol. 2021;12:718963. doi: 10.3389/fmicb.2021.718963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T, Tancredi S, Malagoli C, Malavolti M, Bargellini A, Vescovi L, Nicolini F, Vinceti M. Dietary estimated intake of trace elements: risk assessment in an italian population. Expos Health. 2020;12(4):641–655. doi: 10.1007/s12403-019-00324-w. [DOI] [Google Scholar]
- Forootanfar H, Amirpour-Rostami S, Jafari M, Forootanfar A, Yousefizadeh Z, Shakibaie M. Microbial-assisted synthesis and evaluation the cytotoxic effect of tellurium nanorods. Mater Sci Eng C. 2015;49:183–189. doi: 10.1016/j.msec.2014.12.078. [DOI] [PubMed] [Google Scholar]
- Gad SC, Pham T. Tellurium. In: Wexler P, editor. Encyclopedia of Toxicology (Third Edition) Oxford: Academic Press; 2014. pp. 481–483. [Google Scholar]
- Gerhardsson L (2022) Chap. 31 - Tellurium. In: Nordberg GF, Costa M (eds) Handbook on the Toxicology of Metals (Fifth Edition). Academic Press, pp 783–794
- Ghosh S, Ahmad R, Banerjee K, AlAjmi MF, Rahman S. Mechanistic aspects of microbe-mediated nanoparticle synthesis. Front Microbiol. 2021;12:638068. doi: 10.3389/fmicb.2021.638068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff J (2020) The role of microbial sulfur metabolism in biogeochemical cycling of tellurium and selenium. 10.7282/t3-fv0d-9m67
- Goff J, Yee N. Tellurate enters Escherichia coli K-12 cells via the SulT-type sulfate transporter CysPUWA. FEMS Microbiol Lett. 2017;364(24):101093. doi: 10.1093/femsle/fnx241. [DOI] [PubMed] [Google Scholar]
- Goff JL, Boyanov MI, Kemner KM, Yee N. The role of cysteine in tellurate reduction and toxicity. Biometals. 2021;34(4):937–946. doi: 10.1007/s10534-021-00319-8. [DOI] [PubMed] [Google Scholar]
- Goff JL, Wang YW, Boyanov MI, Yu Q, Kemner KM, Fein JB, Yee N. Tellurite adsorption onto bacterial surfaces. Environ Sci Technol. 2021;55(15):10378–10386. doi: 10.1021/acs.est.1c01001. [DOI] [PubMed] [Google Scholar]
- Grundler PV, Brugger J, Etschmann BE, Helm L, Liu W, Spry PG, Tian Y, Testemale D, Pring A. Speciation of aqueous tellurium(IV) in hydrothermal solutions and vapors, and the role of oxidized tellurium species in Te transport and gold deposition. Geochim Cosmochim Acta. 2013;120:298–325. doi: 10.1016/j.gca.2013.06.009. [DOI] [Google Scholar]
- Grygoyc K, Jablonska-Czapla M. Development of a tellurium speciation study using IC-ICP-MS on soil samples taken from an area associated with the storage, processing, and recovery of electrowaste. Molecules. 2021;26(9):2651. doi: 10.3390/molecules26092651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JJ, Ceri H, Stremick CA, Turner RJ. Biofilm susceptibility to metal toxicity. Environ Microbiol. 2004;6:1220–1227. doi: 10.1111/j.1462-2920.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ramos NA. Surficial geochemistry and bioaccessibility of tellurium in semiarid mine tailings. Environ Chem. 2019;16(4):251–265. doi: 10.1071/EN18215. [DOI] [Google Scholar]
- He Y, Guo J, Song Y, Chen Z, Lu C, Han Y, Li H, Hou Y, Zhao R. Acceleration mechanism of bioavailable Fe (â…¢) on Te (IV) bioreduction of Shewanella oneidensis MR-1: Promotion of electron generation, electron transfer and energy level. J Hazard Mater. 2021;403:123728. doi: 10.1016/j.jhazmat.2020.123728. [DOI] [PubMed] [Google Scholar]
- Hosseini F, Lashani E, Moghimi H. Simultaneous bioremediation of phenol and tellurite by Lysinibacillus sp. EBL303 and characterization of biosynthesized Te nanoparticles. Sci Rep. 2023;13(1):1243. doi: 10.1038/s41598-023-28468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap PL, Rai P, Kumar R, Sharma S, Jasrotia P, Srivastava AK, Kumar S (2018) Microbial nanotechnology for climate resilient agriculture. In: Kashyap PL, Srivastava AK, Tiwari SP, Kumar S (eds) Microbes for Climate Resilient Agriculture pp279-344
- Kavlak G, Graedel TE. Global anthropogenic tellurium cycles for 1940–2010. Resour Conserv Recycl. 2013;76:21–26. doi: 10.1016/j.resconrec.2013.04.007. [DOI] [Google Scholar]
- Keall JHH, Martin NH, Tunbridge RE. Accidental poisoning due to sodium tellurite. Brit J Indust Med. 1946;3:175–176. doi: 10.1136/oem.3.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim MG, Jiang S, Lee JH, Hur HG. Promoted reduction of tellurite and formation of extracellular tellurium nanorods by concerted reaction between iron and Shewanella oneidensis MR-1. Environ Sci Technol. 2013;47(15):8709–8715. doi: 10.1021/es401302w. [DOI] [PubMed] [Google Scholar]
- King WE, Davis L. Potassium tellurite as an indicator of microbial life. Am J Public Health. 1914;4(10):917–932. doi: 10.2105/Ajph.4.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov SI. Impact of contamination with tellurium on biological properties of ordinary chernozem. Soil Sediment Contam. 2019;28(8):792–800. doi: 10.1080/15320383.2019.1666793. [DOI] [Google Scholar]
- Kolesnikov S, Minnikova T, Tsepina N, Evstegneeva N, Timoshenko A. Assessment of the ecotoxicity of Ag, Bi, Te and Tl according to the biological indicators of haplic chernozem. Appl Sci. 2022;12(24):12854. doi: 10.3390/app122412854. [DOI] [Google Scholar]
- Liang X, Perez MAM, Nwoko KC, Egbers P, Feldmann J, Csetenyi L, Gadd GM. Fungal formation of selenium and tellurium nanoparticles. Appl Microbiol Biotechnol. 2019;103(17):7241–7259. doi: 10.1007/s00253-019-09995-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Perez MAM, Zhang S, Song W, Armstrong JG, Bullock LA, Feldmann J, Parnell J, Csetenyi L, Gadd GM. Fungal transformation of selenium and tellurium located in a volcanogenic sulfide deposit. Environ Microbiol. 2020;22(6):2346–2364. doi: 10.1111/1462-2920.15012. [DOI] [PubMed] [Google Scholar]
- Llaver M, Chapana AL, Wuilloud RG (2021) Simultaneous and highly sensitive determination of selenium and tellurium species in environmental samples by on-line ionic liquid based in-situ solvent formation microextraction with hydride generation atomic fluorescence spectrometry detection. Talanta 222:121460. 10.1016/j.talanta.2020.121460 [DOI] [PubMed]
- Makuei FM, Senanayake G. Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical products - a short review. Min Eng. 2018;115:79–87. doi: 10.1016/j.mineng.2017.10.013. [DOI] [Google Scholar]
- Maltman C, Walter G, Yurkov V. A diverse community of metal(loid) oxide respiring bacteria is associated with tube worms in the vicinity of the juan de fuca ridge black smoker field. PLoS ONE. 2016;11(2):e0149812. doi: 10.1371/journal.pone.0149812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman C, Donald LJ, Yurkov V. Tellurite and tellurate reduction by the aerobic anoxygenic phototroph Erythromonas ursincola, strain KR99 is carried out by a novel membrane associated enzyme. Microorganisms. 2017;5(2):20. doi: 10.3390/microorganisms5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman C, Donald LJ, Yurkov V. Two distinct periplasmic enzymes are responsible for tellurite/tellurate and selenite reduction by strain ER-Te-48 associated with the deep sea hydrothermal vent tube worms at the Juan de Fuca Ridge black smokers. Arch Microbiol. 2017;199(8):1113–1120. doi: 10.1007/s00203-017-1382-1. [DOI] [PubMed] [Google Scholar]
- Marooufpour N, Alizadeh M, Hatami M, Asgari Lajayer B. Biological synthesis of nanoparticles by different groups of bacteria. In: Prasad R, editor. Microbial Nanobionics: volume 1, State-of-the-art. Cham: Springer International Publishing; 2019. pp. 63–85. [Google Scholar]
- McNulty BA, Jowitt SM. Barriers to and uncertainties in understanding and quantifying global critical mineral and element supply. Iscience. 2021;24(7):102809. doi: 10.1016/j.isci.2021.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley T., Jr From the periodic table to production. Indust Eng Chem. 1937;29:241–244. doi: 10.1021/ie50326a032. [DOI] [Google Scholar]
- Mirjani R, Faramarzi MA, Sharifzadeh M, Setayesh N, Khoshayand MR, Shahverdi AR. Biosynthesis of tellurium nanoparticles by Lactobacillus plantarum and the effect of nanoparticle-enriched probiotics on the lipid profiles of mice. IET Nanobiotechnol. 2015;9(5):300–305. doi: 10.1049/iet-nbt.2014.0057. [DOI] [PubMed] [Google Scholar]
- Missen OP, Ram R, Mills SJ, Etschmann B, Reith F, Shuster J, Smith DJ, Brugger J. Love is in the earth: a review of tellurium (bio)geochemistry in surface environments. Earth Sci Rev. 2020;204:103150. doi: 10.1016/j.earscirev.2020.103150. [DOI] [Google Scholar]
- Missen OP, Lausberg ER, Brugger J, Etschmann B, Mills SJ, Momma K, Ram R, Maruyama M, Fang X-Y, Melchiorre E, Ryan CG, Villalobos-Portillo EE, Castillo-Michel H, Nitta K, Sekizawa O, Shuster J, Sanyal SK, Frierdich A, Hunt S, Tsuri Y, Takahashi Y, Michibata U, Dwivedi S, Rea MAD. Natural nanoparticles of the critical element tellurium. J Hazard Mater. 2022;3:100053. doi: 10.1016/j.hazl.2022.100053. [DOI] [Google Scholar]
- Montenegro R, Vieto S, Wicki-Emmenegger D, Vasquez-Castro F, Coronado-Ruiz C, Fuentes-Schweizer P, Calderon P, Pereira R, Chavarria M (2021) The putative phosphate transporter PitB (PP1373) is involved in tellurite uptake in Pseudomonas putida KT2440. Microbiol-Sgm 167(2). 10.1099/mic.0.001002 [DOI] [PubMed]
- Muñoz-Diaz P, Jiménez K, Luraschi R, Cornejo F, Figueroa M, Vera C, Rivas-Pardo A, Sandoval JM, Vásquez C, Arenas F. Anaerobic RSH-dependent tellurite reduction contributes to Escherichia coli tolerance against tellurite. Biol Res. 2022;55(1):13. doi: 10.1186/s40659-022-00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najimi S, Shakibaie M, Jafari E, Ameri A, Rahimi N, Forootanfar H, Yazdanpanah M, Rahimi HR. Acute and subacute toxicities of biogenic tellurium nanorods in mice. Regul Toxicol Pharm. 2017;90:222–230. doi: 10.1016/j.yrtph.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Nassar NT, Kim H, Frenzel M, Moats MS, Hayes SM. Global tellurium supply potential from electrolytic copper refining. Resour Conserv Recycl. 2022;184:106434. doi: 10.1016/j.resconrec.2022.106434. [DOI] [Google Scholar]
- Nguyen VK, Choi W, Ha Y, Gu Y, Lee C, Park J, Jang G, Shin C, Cho S. Microbial tellurite reduction and production of elemental tellurium nanoparticles by novel bacteria isolated from wastewater. J Ind Eng Chem. 2019;78:246–256. doi: 10.1016/j.jiec.2019.06.006. [DOI] [Google Scholar]
- Nuss P. Losses and environmental aspects of a byproduct metal: tellurium. Environ Chem. 2019;16(4):243–250. doi: 10.1071/EN18282. [DOI] [Google Scholar]
- Nwoko KC, Liang X, Perez MA, Krupp E, Gadd GM, Feldmann J. Characterisation of selenium and tellurium nanoparticles produced by Aureobasidium pullulans using a multi-method approach. J Chromatogr A. 2021;1642:462022. doi: 10.1016/j.chroma.2021.462022. [DOI] [PubMed] [Google Scholar]
- Ollivier PRL, Bahrou AS, Marcus S, Cox T, Church TM, Hanson TE. Volatilization and precipitation of tellurium by aerobic, tellurite-resistant marine microbes. Appl Environ Microb. 2008;74(23):7163–7173. doi: 10.1128/Aem.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier PR, Bahrou AS, Church TM, Hanson TE. Aeration controls the reduction and methylation of tellurium by the aerobic, tellurite-resistant marine yeast Rhodotorula mucilaginosa. Appl Environ Microbiol. 2011;77(13):4610–4617. doi: 10.1128/AEM.00351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearion CT, Jablonski PE (1999) High level, intrinsic resistance of Natronococcus occultus to potassium tellurite. FEMS Microbiol Lett 174(1):19–23. 10.1016/S0378-1097(99)00115-9
- Peng X, Li M. Medical treatment of condyloma acuminatum, a review on the chemistry of therapeutic drugs. J Appl Virol. 2019;8(1):1–8. doi: 10.21092/jav.v8i1.106. [DOI] [Google Scholar]
- Peng W, Wang Y, Fu Y, Deng Z, Lin S, Liang R. Characterization of the tellurite-resistance properties and identification of the core function genes for tellurite resistance in Pseudomonas citronellolis SJTE-3. Microorganisms. 2022;10(1):95. doi: 10.3390/microorganisms10010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez JM, Calderón IL, Arenas FA, Fuentes DE, Pradenas GA, Fuentes EL, Sandoval JM, Castro ME, Elías AO, Vásquez CC. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS ONE. 2007;2(2):e211. doi: 10.1371/journal.pone.0000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza E, Campora S, Pavia FC, Martino DFC, Laudicina VA, Alduina R, Turner RJ, Zannoni D, Presentato A. Tolerance, adaptation, and cell response elicited by Micromonospora sp. facing tellurite toxicity: a biological and physical-chemical characterization. Int J Mol Sci. 2022;23(20):12631. doi: 10.3390/ijms232012631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presentato A, Piacenza E, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ. Rhodococcus aetherivorans BCP1 as cell factory for the production of intracellular tellurium nanorods under aerobic conditions. Microb Cell Factories. 2016;15(1):1–14. doi: 10.1186/s12934-016-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presentato A, Piacenza E, Darbandi A, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ. Assembly, growth and conductive properties of tellurium nanorods produced by Rhodococcus aetherivorans BCP1. Sci Rep. 2018;8(1):3923. doi: 10.1038/s41598-018-22320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presentato A, Turner RJ, Vásquez CC, Yurkov V, Zannoni D. Tellurite-dependent blackening of bacteria emerges from the dark ages. Environ Chem. 2019;16(4):266–288. doi: 10.1071/EN18238. [DOI] [Google Scholar]
- Prigent-Combaret C, Sanguin H, Champier L, Bertrand C, Monnez C, Colinon C, Blaha D, Ghigo JM, Cournoyer BJEM. The bacterial thiopurine methyltransferase tellurite resistance process is highly dependent upon aggregation properties and oxidative stress response. Environ Microbiol. 2012;14(10):2645–2660. doi: 10.1111/j.1462-2920.2012.02802.x. [DOI] [PubMed] [Google Scholar]
- Pugin B, Cornejo FA, Muñoz-Díaz P, Muñoz-Villagrán CM, Vargas-Pérez JI, Arenas FA, Vásquez CC. Glutathione reductase-mediated synthesis of tellurium-containing nanostructures exhibiting antibacterial properties. Appl Environ Microbiol. 2014;80(22):7061–7070. doi: 10.1128/AEM.02207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin HB, Takeichi Y, Nitani H, Terada Y, Takahashi Y. Tellurium distribution and speciation in contaminated soils from abandoned mine tailings: comparison with selenium. Environ Sci Technol. 2017;51(11):6027–6035. doi: 10.1021/acs.est.7b00955. [DOI] [PubMed] [Google Scholar]
- Ramos-Ruiz A, Field JA, Wilkening JV, Sierra-Alvarez R. Recovery of elemental tellurium nanoparticles by the reduction of tellurium oxyanions in a methanogenic microbial consortium. Environ Sci Technol. 2016;50(3):1492–1500. doi: 10.1021/acs.est.5b04074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ruiz A, Zeng C, Sierra-Alvarez R, Teixeira LH, Field JA. Microbial toxicity of ionic species leached from the II-VI semiconductor materials, cadmium telluride (CdTe) and cadmium selenide (CdSe) Chemosphere. 2016;162:131–138. doi: 10.1016/j.chemosphere.2016.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GKK, Pathak S, Nancharaiah YV. Aerobic reduction of selenite and tellurite to elemental selenium and tellurium nanostructures by Alteromonas sp. under saline conditions. Int Biodeter Biodegr. 2023;179:105571. doi: 10.1016/j.ibiod.2023.105571. [DOI] [Google Scholar]
- Sabaty M, Avazeri C, Pignol D, Vermeglio A. Characterization of the reduction of selenate and tellurite by nitrate reductases. Appl Environ Microbiol. 2001;67(11):5122–5126. doi: 10.1128/Aem.67.11.5122-5126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safhi MM, Alam MF, Khuwaja G, Islam F, Hussain S, Fageeh MM, Anwer T, Islam F. Repeated exposure of sodium tellurite on the rat liver and on the potential mechanisms of the metalloid-induced hepatotoxicity. Acta Pol Pharm. 2016;73(3):675–682. [PubMed] [Google Scholar]
- Safhi M, Alam DM, Khuwaja G, Ashafaq M, Khan A, Islam F, Anwer T, Khan G, Moni S, Islam S. Selenium in combination with tellurium protects the toxicity of tellurium in the liver mitochondria of rats. Bull environ pharmacol life sci. 2018;7:90–95. [Google Scholar]
- Saikia S, Sinharoy A, Lens PNL. Adsorptive removal of gallium from aqueous solution onto biogenic elemental tellurium nanoparticles. Sep Purif Technol. 2022;286:120462. doi: 10.1016/j.seppur.2022.120462. [DOI] [Google Scholar]
- Sakaguchi T, Kikuchi A, Romaidi Marine microbes as selenium and tellurium collector and convertor. AIP Conf Proc. 2019;2120(1):020002. doi: 10.1063/1.5115603. [DOI] [Google Scholar]
- Sandoval JM, Arenas FA, Garcia JA, Diaz-Vasquez WA, Valdivia-Gonzalez M, Sabotier M, Vasquez CC. Escherichia coli 6-phosphogluconate dehydrogenase aids in tellurite resistance by reducing the toxicant in a NADPH-dependent manner. Microbiol Res. 2015;177:22–27. doi: 10.1016/j.micres.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Shakibaie M, Adeli-Sardou M, Mohammadi-Khorsand T, ZeydabadiNejad M, Amirafzali E, Amirpour-Rostami S, Ameri A, Forootanfar H. Antimicrobial and antioxidant activity of the biologically synthesized tellurium nanorods; a preliminary in vitro study. Iran J Biotechnol. 2017;15(4):268–276. doi: 10.15171/ijb.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie MD, Deeds FEJPHR. The importance of tellurium as a health hazard in industry. A preliminary Report. Public Health Reports (1896–1970) 1920;16(35):939–954. doi: 10.2307/4575547. [DOI] [Google Scholar]
- Sinharoy A, Lens PNL. Selenite and tellurite reduction by Aspergillus niger fungal pellets using lignocellulosic hydrolysate. J Hazard Mater. 2022;437:129333. doi: 10.1016/j.jhazmat.2022.129333. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Nikhil EV, Braganca JM, Kowshik M. Anti-bacterial TeNPs biosynthesized by haloarcheaon Halococcus salifodinae BK3. Extremophiles. 2015;19(4):875–884. doi: 10.1007/s00792-015-0767-9. [DOI] [PubMed] [Google Scholar]
- Tang A, Ren Q, Wu Y, Wu C, Cheng Y. Investigation into the antibacterial mechanism of biogenic tellurium nanoparticles and precursor tellurite. Int J Mol Sci. 2022;23(19):11697. doi: 10.3390/ijms231911697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen J, Zylstra GJ, Yee N. Genetic evidence for a molybdopterin-containing tellurate reductase. Appl Environ Microbiol. 2013;79(10):3171–3175. doi: 10.1128/AEM.03996-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Hou YF, Weiner JH, Taylor DE. The arsenical atpase efflux pump mediates tellurite resistance. J Bacteriol. 1992;174(9):3092–3094. doi: 10.1128/Jb.174.9.3092-3094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidi H, Kobarfard F, Alizadeh A, Saravanan M, Barabadi HJICC. Green nanotechnology-based tellurium nanoparticles: exploration of their antioxidant, antibacterial, antifungal and cytotoxic potentials against cancerous and normal cells compared to potassium tellurite. Inorg Chem Commun. 2021;124:108385. doi: 10.1016/j.inoche.2020.108385. [DOI] [Google Scholar]
- Vaigankar DC, Dubey SK, Mujawar SY, D’Costa A. Tellurite biotransformation and detoxification by Shewanella baltica with simultaneous synthesis of tellurium nanorods exhibiting photo-catalytic and anti-biofilm activity. Ecotoxicol Environ Saf. 2018;165:516–526. doi: 10.1016/j.ecoenv.2018.08.111. [DOI] [PubMed] [Google Scholar]
- Vavrova S, Struharnanska E, Turna J, Stuchlik S. Tellurium: a rare element with influence on prokaryotic and eukaryotic biological systems. Int J Mol Sci. 2021;22(11):5924. doi: 10.3390/ijms22115924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielreicher NM, Groves DI, McNaughton NJ. The giant Kalgoorlie gold field revisited. Geosci Front. 2016;7(3):359–374. doi: 10.1016/j.gsf.2015.07.006. [DOI] [Google Scholar]
- Wang X, Liu G, Zhou J, Wang J, Jin R, Lv H. Quinone-mediated reduction of selenite and tellurite by Escherichia coli. Bioresour Technol. 2011;102(3):3268–3271. doi: 10.1016/j.biortech.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bu Y, Zhao Y, Zhang Z, Liu L, Zhou H. Morphology-tunable tellurium nanomaterials produced by the tellurite-reducing bacterium Lysinibacillus sp. ZYM-1. Environ Sci Pollut Res. 2018;25(21):20756–20768. doi: 10.1007/s11356-018-2257-y. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang X, Kislyakov IM, Dong N, Zhang S, Wang G, Fan J, Zou X, Du J, Leng Y, Zhao Q, Wu K, Chen J, Baesman SM, Liao KS, Maharjan S, Zhang H, Zhang L, Curran SA, Oremland RS, Blau WJ, Wang J. Bacterially synthesized tellurium nanostructures for broadband ultrafast nonlinear optical applications. Nat Commun. 2019;10(1):3985. doi: 10.1038/s41467-019-11898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZK, Yi XL, Liu Y, Zhou H. Complete genome sequence of a tellurate reducing bacteria Sporosarcina sp. Te-1 isolated from Bohai Sea. Mar Genomics. 2021;60:100888. doi: 10.1016/j.margen.2021.100888. [DOI] [PubMed] [Google Scholar]
- Wang D, Rensing C, Zheng S. Microbial reduction and resistance to selenium: mechanisms, applications and prospects. J Hazard Mater. 2022;421:126684. doi: 10.1016/j.jhazmat.2021.126684. [DOI] [PubMed] [Google Scholar]
- Wedepohl KH. The composition of the continental crust. Geochim Cosmochim Acta. 1995;59:1217–1232. doi: 10.1016/0016-7037(95)00038-2. [DOI] [Google Scholar]
- Wiklund JA, Kirk JL, Muir DCG, Carrier J, Gleason A, Yang F, Evans M, Keating J. Widespread atmospheric tellurium contamination in industrial and remote regions of Canada. Environ Sci Technol. 2018;52(11):6137–6145. doi: 10.1021/acs.est.7b06242. [DOI] [PubMed] [Google Scholar]
- Workentine ML, Harrison JJ, Stenroos PU, Ceri H, Turner RJ. Pseudomonas fluorescens’ view of the periodic table. Environ Microbiol. 2008;10:238–250. doi: 10.1111/j.1462-2920.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- Wu XD, Song JM, Li XG. Occurrence and distribution of dissolved tellurium in Changjiang River estuary. Chin J Oceanol Limn. 2014;32(2):444–454. doi: 10.1007/s00343-014-3161-z. [DOI] [Google Scholar]
- Wu SJ, Li TF, Xia X, Zhou ZJ, Zheng SX, Wang GJ. Reduction of tellurite in Shinella sp. WSJ-2 and adsorption removal of multiple dyes and metals by biogenic tellurium nanorods. Int Biodeterior Biodegradation. 2019;144:104751. doi: 10.1016/j.ibiod.2019.104751. [DOI] [Google Scholar]
- Yao GL, Wang K, Wang MY, Shao X, Qiu FX, Zhang T. Magnetic FeS@Lignin-derived carbon nanocomposites as an efficient adsorbent for multistage collaborative selective recovery of tellurium (IV) from wastewater. J Environ Chem Eng. 2021;9(5):106135. doi: 10.1016/j.jece.2021.106135. [DOI] [Google Scholar]
- Yao GL, Shao X, Qiu ZW, Qiu FX, Li ZD, Zhang T. Construction of lignin-based nano-adsorbents for efficient and selective recovery of tellurium (IV) from wastewater. Chemosphere. 2022;287:132058. doi: 10.1016/j.chemosphere.2021.132058. [DOI] [PubMed] [Google Scholar]
- Yarema MC, Curry SC. Acute tellurium toxicity from ingestion of metal-oxidizing solutions. Pediatrics. 2005;116(2):e319–e321. doi: 10.1542/peds.2005-0172. [DOI] [PubMed] [Google Scholar]
- Yasir M, Zhang YX, Xu ZX, Luo MZ, Wang GJ. NAD(P)H-dependent thioredoxin-disulfide reductase TrxR is essential for tellurite and selenite reduction and resistance in Bacillus sp. Y3. FEMS Microbiol Ecol. 2020;96(9):fiaa126. doi: 10.1093/femsec/fiaa126. [DOI] [PubMed] [Google Scholar]
- Zannoni D, Borsetti F, Harrison JJ, Turner RJ. The bacterial response to the chalcogen metalloids Se and Te. Adv Microb Physiol. 2008;53:1–72. doi: 10.1016/S0065-2911(07)53001-8. [DOI] [PubMed] [Google Scholar]
- Zare B, Faramarzi MA, Sepehrizadeh Z, Shakibaie M, Rezaie S, Shahverdi AR. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater Res Bull. 2012;47(11):3719–3725. doi: 10.1016/j.materresbull.2012.06.034. [DOI] [Google Scholar]
- Zare B, Sepehrizadeh Z, Faramarzi MA, Soltany-Rezaee-Rad M, Rezaie S, Shahverdi AR. Antifungal activity of biogenic tellurium nanoparticles against Candida albicans and its effects on squalene monooxygenase gene expression. Biotechnol Appl Biochem. 2014;61(4):395–400. doi: 10.1002/bab.1180. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Shen X, Chen M, Yang K, Xie H. Bioleaching of tellurium from mine tailings by indigenous Acidithiobacillus ferrooxidans. Lett Appl Microbiol. 2022;75(5):1076–1083. doi: 10.1111/lam.13569. [DOI] [PubMed] [Google Scholar]
- Zonaro E, Lampis S, Turner RJ, Qazi SJ, Vallini GJ. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front Microbiol. 2015;6:584. doi: 10.3389/fmicb.2015.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonaro E, Piacenza E, Presentato A, Monti F, Dell’Anna R, Lampis S, Vallini G. Ochrobactrum sp. MPV1 from a dump of roasted pyrites can be exploited as bacterial catalyst for the biogenesis of selenium and tellurium nanoparticles. Microb Cell Factories. 2017;16(1):215. doi: 10.1186/s12934-017-0826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this review are available in the references cited within this article.