Abstract
The aim of this study is to investigate the potential impact of catheterization on intimal hyperplasia and explore the efficacy of Paclitaxel loaded PLGA nanoparticles (PTX-NPs) in preventing stenosis at the site of venous injury. Under general anesthesia, Central Venous Catheters were inserted into the rat’s right internal jugular veins (IJV) using the cut-down technique. Twenty bare catheters (C) and twenty PTX-NPs coated catheters (P) were assigned to one of four groups (C2, C4, P2, or P4) based on catheter type and expected survival time. 2 or 4 weeks after surgery, IJVs were completely harvested by formalin fixation and gelatin infusion and slides were stained with H&E (Haematoxylin and Eosin) and Masson's technique. The P2 (Paclitaxel coating, 2 weeks) group showed the most proliferation among the four groups and the P4 (Paclitaxel coating, 4 weeks) showed a tendency to decrease proliferation. Additionally, the lumen size in the P4 group was about 6% smaller than in the P2 group, and there was a lower prevalence of stenotic grade in the P4 group. Our study suggests that PTX-NPs coated catheters may be effective in preventing venous stenosis if the intended usage is prolonged, rather than for a short-term period.
Graphical abstract
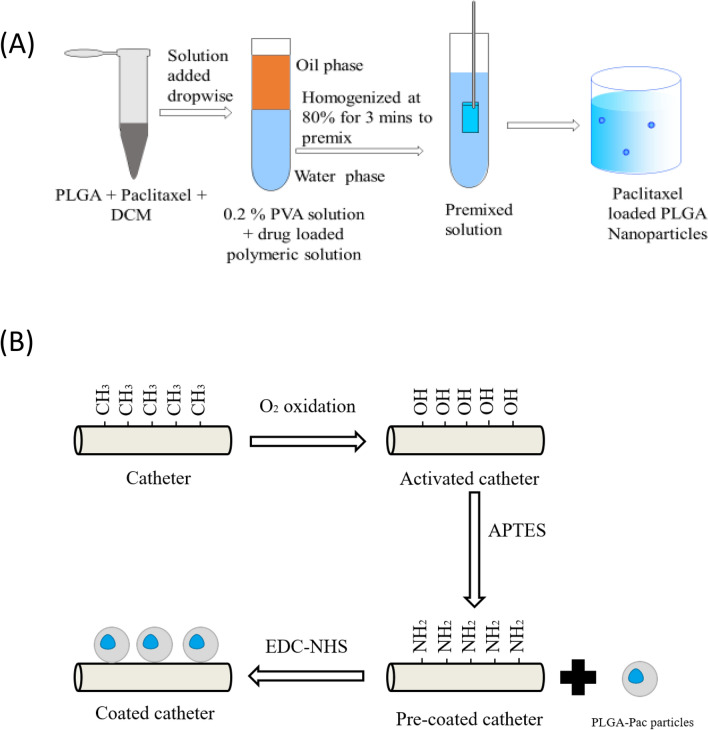
Schematic representation of catheter functionalization and coating of PTX-NPs on Catheter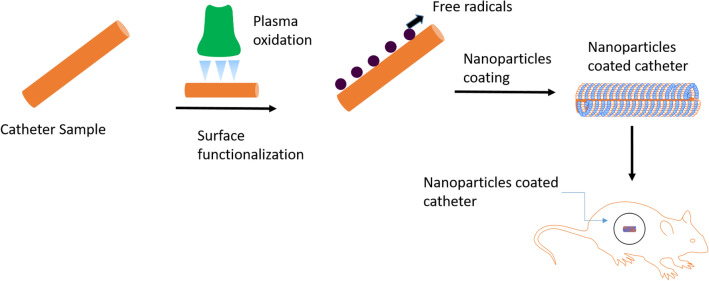
Supplementary Information
The online version contains supplementary material available at 10.1007/s13534-023-00282-y.
Keywords: Central venous catheters, Paclitaxel, Nanoparticles, Neointima, Stenosis, Intimal hyperplasia
Introduction
Central Venous Catheters (CVCs) are commonly used in hospitals for various purposes, such as end-stage renal disease, chemotherapy, nutritional support, and the resuscitation of critically ill patients [1]. The number of patients using CVCs and other devices is steadily increasing. In the US, approximately 5 million CVCs are used annually, with a complication rate during the procedure ranging from 6 to 19% [2]. Depending on the catheter function and patient needs, periods of catheterization may range from days to weeks and may even be on a permanent basis. It is well-recognized that a crucial risk factor for catheter associated infections is the duration of catheterization [3]. Infection and thrombosis are the most common early complications while venous stenosis is the most common delayed complication.
Central vein stenosis (CVS) due to indwelling catheters/devices is associated with duration of placement, active infection, large-bore catheters, and prior history of catheterization [4-6]. It is marked by neck, face, chest, and upper extremities swelling and may require treatment, if severe [7]. While CVC insertion itself is a risk factor for CVS, not all CVC recipients will develop CVS [8-10]. Besides CVC insertion, CVS can also result from fibrosing mediastinitis, post-radiation therapy, or extrinsic compression, usually from a malignant mass [7]. Venous intimal hyperplasia is cited most often as etiology, and some authors have asserted that variations in intimal caveolin-1 expression levels promote stenosis [11-13]. Therefore, the causes and treatment of CVS has been extensively researched and regularly reported.
Paclitaxel is widely used as antiproliferative drug that inhibits the growth of vascular smooth muscle cells, endothelial cells and fibroblasts. Drug-eluting stents or balloons containing paclitaxel are commonly used to treat peripheral arterial occlusive disease and myocardial infarction. Few studies have applied paclitaxel to CVS because of similar pathologic changes [14-16] while paclitaxel coating of CVCs still remains problematic.
Therefore, researchers have been investigating alternative strategies to enhance therapeutic efficacy, minimizing side effects of Paclitaxel. Using biodegradable nanocarriers is one of the most effective strategies to achieve this goal. Particularly, PLGA nanoparticles have been a promising application in the biomedical field due to their biocompatibility and biodegradability [8]. Thus, various biodegradable nanoparticles as a drug delivery system have been developed and evaluated to enhance pharmacokinetics and reduce toxicity [17].
In this context, the aim of this study is to evaluate the efficacy of Paclitaxel loaded PLGA nanoparticles (PTX-NPs) in reducing the incidence of venous stenosis. The study involved the use of PTX-NPs to coat CVCs that were implanted in rat models, and then analyzed the venous changes after placement of the PTX-NPs coated CVCs (Fig. 1).
Fig. 1.
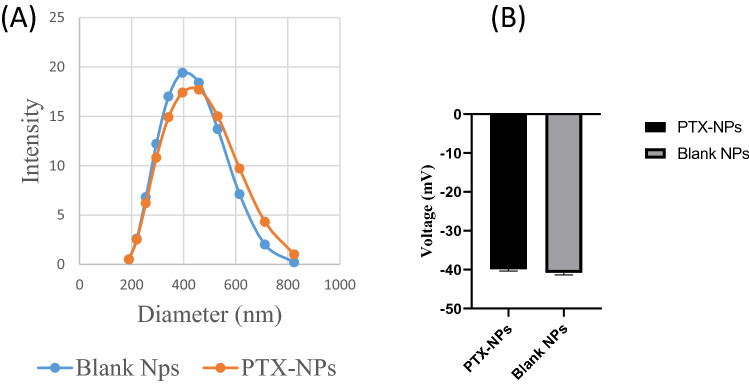
A Size of PLGA and PTX-PLGA NPs. B Zeta Potential of PLGA and PTX-NPs
Materials and methods
Materials
Commercially available Polyvinyl Alcohol (PVA), acid-terminated Poly-lactide-co-glycolide (PLGA, lactide:glycolide = 50:50, Mw = 38,000–54,000 g/mol), Dichloromethane (DCM), ethylenediamine, N- hydroxysuccinimide (NHS), Paclitaxel, and Sylgard 184 elsatomer, (3-Aminopropyl)triethoxysilane (APTES), methanol, phosphate buffered saline (PBS), Hematoxylin and Eosin (H & E) stain and ethanol were purchased from sigma aldrich and were used without purifications. Broviac® CV catheter was purchased from Teleflex Medical device company.
Paclitaxel loaded PLGA nanoparticle preparation
A schematic of nanoparticle preparation steps is provided in Fig. 2A. Paclitaxel loaded PLGA nanoparticles (PTX-NPs) were synthesized by a solvent evaporation method, as reported earlier [18]. Briefly, 83 mg PLGA and 13 mg paclitaxel was dissolved in 2 ml of dichloromethane (DCM). The solution was added dropwise in 20 ml of 0.2% (w/v) polyvinyl alcohol (PVA) solution. The resulting solution was emulsified using a Probe Sonicator (sonics Vibra cell) at 80% amplitude for 3 min. Finally, the suspension was magnetically stirred at 5000 revolutions per minute (RPM) for 4 h and centrifuged at 7000 RPM for 10 min, and washed twice with deionized (DI) water. The supernatant was discarded, and the pellets were collected. The pellet was then resuspended in DI water and freeze-dried overnight to obtain the nanoparticle powder. Size and zeta potential of PTX-NPs were measured by dynamic light scattering (Zetasizer Nano ZS90; Malvern Instruments Ltd., Malvern, UK). The amount of entrapped paclitaxel in PTX-NPs was determined by spectrophotometric estimation. 1 mg PTX-NPs were dissolved in PBS: methanol (V/V) mixture in the ratio of 7:3. Then the absorbance was measured using a Microplate reader at 230 nm [19, 20].
Fig. 2.
A Preparation of PTX-NPs. PLGA and Paclitaxel were mixed in DCM. The dissolved polymeric solution with Paclitaxel was mixed in 0.2% PVA solution. The mixed solution was homogenized at 80% for 3 min to get a homogeneous solution. After stirring for 4 h, PTX-NPs were centrifuged and harvested after purification. B Steps in paclitaxel coating of catheters. Surface hydroxyl groups (–OH) of catheter activated through oxidation; using APTES solution, NH2 attaches to catheter surface, enabling coating of nanoparticles via EDC-NHS (carbodiimide-hydrochloride)
Central venous catheter preparation
A 5 cm central venous catheter constructed specifically from formulated and processed silicone (2.7 F Single-Lumen CV catheter with Surecuff™ Tissue ingrowth cuff, 52 cm Tip to cuff length) was cut from a 50 cm long catheter. The catheter was blocked with a fish string from both sides. Plasma technique was used in order to introduce reactive groups into catheter surface using a plasma oxidation device (Femto Science Cute Plasma System) with a Pressure of 5 × 10–2 Torr and Max Power = 200 W. Furthermore, it was immersed in 3-Aminopropyltriethoxysilane (APTES) and ethanol solution. A hydrolyzed APTES solution was obtained by adding APTES to an ethanol 4% aqueous solution (V/V) and shaken at 60 RPM for 24 h in shaker. The catheter was washed three times with DI water and incubated at the oven for 30 min at 120 °C.
Catheter-APTES was directly immersed in PTX-NPs which were reacted with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-Hydroxysuccinimide (NHS) solution freshly prepared. The solution was diluted with DI water and allowed to react for 4 h rsotating in a rotator. After finishing the reaction, PTX-NPs coated catheter was then freeze-dried.
In-vitro drug release studies of PTX-NPs coated catheters
For the in-vitro release studies, a mixture of PBS: methanol in the ratio of 7:3 (V/V) was used as a medium. PTX-NPs coated catheters were placed into dialysis bags (Slide-A-Lyzer™ mini dialysis, 10 k MWCO) in the medium and stirred at 37 °C at 80 RPM. The released Paclitaxel was measured using a plate reader at every designated interval of time (0, 4, 12, 24, 48, 72, and 96 h) at 230 nm.
Selection and care of rats
Male Sprague Dawley (SD) rats (weight range, 230–250 g) were purchased (Dae Han BioLink Co Ltd, Eumseong-gun, Chungju, South Korea) at 8 weeks old. All were raised for 7 days in a designated pathogen-free (SPF) room with free access to food and water. Our experimentation protocol was approved by the Institutional Animal Care and Use Committee at the School of Medicine, Kangwon National University (IACUC No.: KW-180528-2). All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals.
Study design of rat-animal model, operative procedure, and tissue sampling
The rats were divided into four groups (n = 10 each), based on type of CVC and anticipated sacrifice, either 2 or 4 weeks after surgery. The four groups were designed as follows: (C2: uncoated, 2 weeks; P2: paclitaxel coating, 2 weeks; C4: uncoated, 4 weeks; P4: paclitaxel coating, 4 weeks).
Isoflurane was used for inhalation anesthesia. Induction was performed with 5% isoflurane for 2 min and 30 s to 4 min. Maintenance of inhalation anesthesia was achieved by administering 4% isoflurane through an anesthetic gas tube. Since there were slight differences between rats, their heart and respiratory rates were checked. The isoflurane concentration was adjusted to maintain anesthesia when the heart and respiratory rates were lower than their initial values.
Inhalation anesthesia was used to elevate the proximal right IJV using a loop of silk suture for anterior wall venotomy with 24-G needle of Midicut™, and 5 cm catheter was inserted. The study design of animal model and detailed operative procedures are described in supplementary information.
At the end of the study, the tissue surrounding the inserted catheters were carefully dissected to the level of right atrium. After fixing the tissues with gelatin and formalin all tissue harvested from IJV to SVC (Superior Vena Cava) was quickly frozen to − 20 °C for future use.
Histomorphometric analysis
Three segments of each vein were subjected to hematoxylin and eosin (H&E) stain: (1) IJV closest to catheterization, (2) SVC where catheter tip was located, and (3) a 2-cm segment proximal to catheter insertion site. Masson’s trichrome stain was also performed on IJV closest to catheterization.
Statistical analysis
All quantitative measurements involving animals were performed by two researchers, using an open-source application (Image J2; National Institutes of Health, Bethesda, MD, USA) to analyze morphometric data. Statistical computations relied on commercially available software (SPSS 25.0; IBM Corp, Armonk, NY, USA). Student’s t-test, χ2 test, and ANOVA were used to compare groups, setting significance at p < 0.05. Zeta Potential result was analyzed using Graph-pad Prism.
Results
Characterization of paclitaxel loaded PLGA nanoparticles
The physicochemical properties of PTX-NPs were measured, and the results are summarized in Table 1. The nanoparticles exhibited a narrow size distribution, with a particle size that increased from 387.6 to 403.1 nm with the loading of paclitaxel (polydispersity index, PDI without paclitaxel = 0.187, PDI with paclitaxel = 0.159). Both PLGA nanoparticles (NPs) and PTX-NPs showed negative zeta potential, (Blank NPs = − 40.2 mV and PTX-NPs = − 39.5 mV). The drug loading content and encapsulation efficiency were found to be 15.50% and 75.58%, respectively.
Table 1.
Characterization of particles
| Nanoparticles | Size (nm) | Zeta potential (mV) | Loading amount |
|---|---|---|---|
| Blank NPs | 387.6 | − 40.2 | – |
| PTX-NPs | 403.1 | − 39.5 | 16.39 ± 2.65% |
The immobilization of the nanoparticles was confirmed by fluorescence signals observed on the catheter (Fig. 3).
Fig. 3.
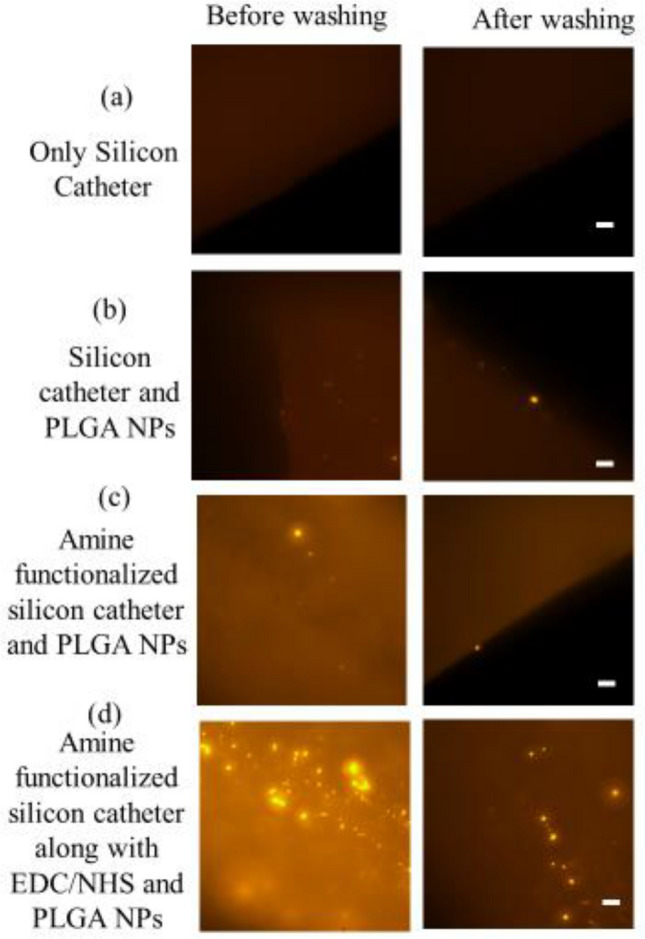
Fluorescent PLGA nanoparticles chemically conjugated on the silicon catheter. a Only silicon catheter b NPs were coated on silicon catheter c Amine functionalized silicon catheter coated with NPs by charge-charge interaction d Amine functionalized silicon catheter reacted with EDC and NHS and coated with NPs
In-vitro release profile of paclitaxel from PTX-NPs coated catheter
The in-vitro release of paclitaxel from PTX-NPs and PTX-NPs coated catheter was studied. The result showed that PTX-NPs released gradually over time, with a 15% release observed at 96 h. On the other hand, PTX-NPs coated catheter showed a delayed release, with significant paclitaxel release occurring after 24 h. Interestingly, PTX-NPs coated catheter had a lower initial releasing rate than PTX-NPs. However, as time progressed, the amount of paclitaxel released by the coated catheter was found to be similar to that of PTX-NPs, with both releasing approximately 12.5% and 15% of the drug at the 96 h (Fig. 4).
Fig. 4.
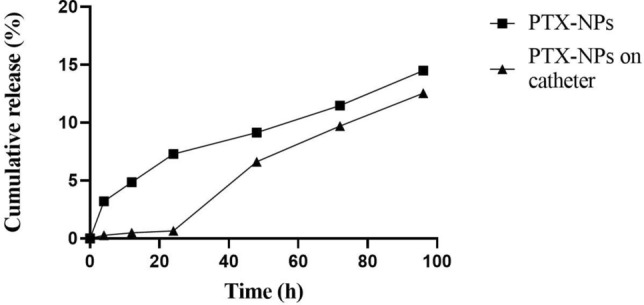
In vitro release profile of paclitaxel from PTX-NPs and PTX-NPs coated on catheter at different time point
Animal characteristics
The mean weight of rats preoperatively was not significantly different between the two groups, but the duration of operation was significantly longer in the P4 group. This was likely due to the high concentration of isoflurane concentration during general anesthesia. The other rats in P2 and P4 groups died from massive bleeding during catheterization (Table 2).
Table 2.
Mortalities and operative times during catheterization
| C2 | P2 | C4 | P4 | P-value | |
|---|---|---|---|---|---|
| Number | 10 | 9 | 10 | 8 | |
| Preoperative Wt. (g) | 327.8 ± 17.70 | 314.1 ± 10.61 | 320.1 ± 14.81 | 322.7 ± 11.95 | 0.144 |
| Operative time (s) | 799.0 ± 75.34 | 1032.2 ± 243.08 | 984.5 ± 247.92 | 950.9 ± 151.27 | 0.613 |
| Mortality | 0 | 1(10%) | 0 | 2(20%) | 0.297 |
Biodistribution studies
Table 3 highlights a comprehensive overview of the wall thickening and luminal stenosis observed in the IJV segments. It is important to note that the measurements primarily reflect the proliferation of smooth muscle, since the partial loss of intima is not an effective indicator of venous wall proliferation.
Table 3.
Vascular changes observed in four animal groups
| C2 | P2 | C4 | P4 | P-value | |
|---|---|---|---|---|---|
| Number | 10 | 9 | 10 | 8 | |
| Wall thickening (R) | 0.099 ± 0.719 | 0.155 ± 0.178 | 0.100 ± 0.070 | 0.093 ± 0.050 | 0.367 |
| Wall thickening | 0.089 ± 0.061 | 0.145 ± 0.174 | 0.091 ± 0.058 | 0.070 ± 0.044 | 0.424 |
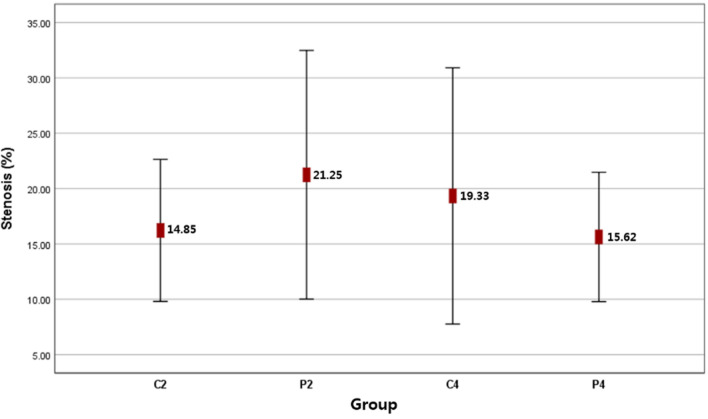
| Mean value of Stenosis (%) | 14.85 ± 7.49 | 21.25 ± 13.43 | 19.33 ± 15.06 | 15.63 ± 6.99 | 0.289 |
| Grade of stenosis | 0.688 | ||||
| Normal (0–19%) | 8 | 5 | 8 | 6 | |
| Mild (20–49%) | 2 | 3 | 1 | 2 | |
| Moderate (50–69%) | 0 | 1 | 1 | 0 | |
| Shape | 0.759 | ||||
| Eccentric | 4 | 4 | 6 | 3 | |
| Concentric | 6 | 5 | 4 | 5 |
Wall thickening(R): outer radius – inter radius of the vessel,
Wall thickening: mean of thickness of each measurement in 12 directions
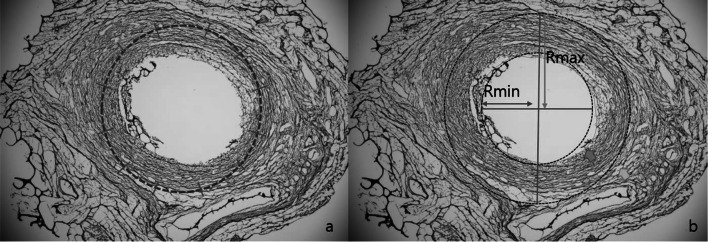
To accurately measure the growth of smooth muscles, two methods were employed. Firstly, vessel thickness was measured 12 times in a clockwise direction, and the two thickest and two thinnest regions were averaged. Secondly, the outer and inner diameter of the IJV was calculated by assuming that the venous wall had proliferated concentrically from the total area and inner area of each segment respectively (Fig. 5). Ultimately, the results by these two methods did not differ significantly.
Fig. 5.
Methods of calculating venous wall thickness: a mean of 12-point measurement; and b Rmax-Rmin method, based on presumption of concentric hyperplasia, using outer and inner diameters to calculate outer and inner areas
The study’s findings indicated that there was no significant difference between the animal groups at 2 or 4 weeks in terms of the degree of hyperplasia, as evidenced by p-value of 0.367 and 0.424 respectively. However, among the four groups the P2 group exhibited the highest degree of proliferation, while the P4 group showed a tendency towards decreased proliferation. When comparing the C2 and P2 groups, it was observed that the P2 group showed more proliferation, and it was confirmed that there was little difference in the degree of proliferation between the C4 and P4 groups. Additionally, the differences in the degree of wall thickening between the C2 and C4 groups was found to be insignificant.
There were no significant differences between the animal group in terms of lumen size, stenosis grade and severity. However, the lumen size in the P4 group was about 6% smaller than in the P2 group. Additionally, there was a lower prevalence of stenotic grade in the P4 group, but mild to moderate stenosis was observed in roughly 27% of all animals. More severe stenosis (≥ 50%) was observed in the P2 group (51%) and C4 group (56%) (Fig. 7, Table 3).
Fig. 7.
Mean value of percentage luminal stenosis in animal group; there were no significant differences
Interestingly, while there was no difference in wall thickening between the C4 and C2 groups, an increase in luminal stenosis was observed in the former group. This suggests that luminal stenosis is more strongly influenced by remodeling induced changes in the vessel lumen than by increased wall thickness.
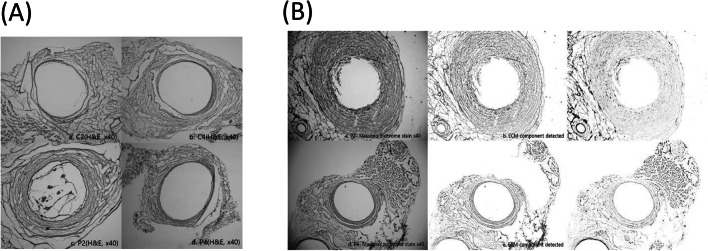
The shape of hyperplasia was found to occur in both eccentric and concentric forms, rather than appearing uniformly (p = 0.759). Masson’s trichrome staining was employed to produce differential staining of Smooth Muscle Fiber (SMF) and extracellular matrix (ECM) in tissue, with red SMF tissue being prominent and blue or greenish hues corresponding to extracellular matrix (collagen and elastin) (Fig. 6B).
Fig. 6.
Histologic views of venous cross sections in animal groups: A H & E stain (40X), group-wise (a-d) B Masson’s trichrome stain (40X), P2 and P4 groups (a, d) showing extracellular matrix (c, d) and smooth muscle fiber (e, f) components
The authors concluded that venous hyperplasia is the result of both SMF proliferation and excessive ECM. This change occurred regardless of the degree of hyperplasia (Fig. 5), suggesting that both SMF and ECM are involved in the proliferation of the venous wall. Hence, inhibition of both can be the evidence for preventing luminal stenosis at the site of venous injury.
Discussion
Recently, as the incidence of vascular disease increases, the use of CVCs is more demanding. Meanwhile, the use of current CVCs is limited due to various side effects. A common complication associated with these treatments is Intimal hyperplasia, which occurs when vascular smooth muscle cells invade and proliferate at the intima. While efforts to reduce intimal hyperplasia, have generally focused on arteriovenous grafts in dialysis patients, recent studies have shown that Paclitaxel-coated expanded polytetrafluoroethylene (ePTFE) grafts can effectively reduce juxta-anastomotic neointimal hyperplasia in this setting [21]. Given that the same mechanism is implicated in CVS, it is plausible that PTX-NPs coated catheters or devices may also provide similar benefits.
However, the present study found that tissue samples from rats treated with PTX-NPs coated catheters showed more prominent neointimal hyperplasia two weeks after treatment. This was also observed in control groups, raising the possibility that PTX-NPs coated catheters do not confer any significant advantage. Interestingly, the wall-thickening of C4 seems to be more decreased in the control group than in P2, but when Paclitaxel was used, the proliferation was suppressed by 40–50% at 4 weeks. This suggests that paclitaxel has a long-term suppressive effect of more than 4 weeks rather than a short-term of about 2 weeks. This fact is consistent with the in vitro result which showed a gradual decrease on the release rate of paclitaxel over time.
Pathologic vascular remodeling and proliferation of media occur during the first 2 weeks after vascular injury [22]. However, in our study, we have confirmed extensive multilayered hyperplasia of neointima (Fig. 6) at 4 weeks, despite evidence of constant paclitaxel release over a 2-week interval (Fig. 4). These findings suggest that PTX-NPs coated CVCs may be effective only if the time of indwelling is prolonged, unlike the efficacy of paclitaxel-coated balloons in the first 4 weeks after deployment [23]. Studies by Sousa et al. have found that 7–14 days are required for sirolimus to exert its antiproliferative cellular effects on targeted vessel walls [24]. Therefore, it is possible that a paclitaxel-coated catheter may be inadequate if the intended usage is less than 2 weeks (Fig. 7).
Paclitaxel is a promising antiproliferative agent, and is proven to be effective in preventing venous stenosis [25]. However, poor water solubility, rapid drug release, and high toxic effects limit its clinical application. Therefore, to circumvent these limitations, paclitaxel-loaded nanoparticles were used in our experiment [26]. Recently, nano-delivery systems have become advantageous owing to fewer adverse effects, the ability to control rates of drug release, and the capacity for targeted treatment [27, 28]. In our animal model, we chose nanoparticles as the potent carrier of paclitaxel, achieving a drug encapsulation of 75%. PTX-NPs released gradually and exhibited a 15% release at 96 h while PTX-NPs coated on the catheter showed a delayed release, with significant paclitaxel release occurring after 24 h. PTX-NPs coated on the catheter had a lower initial releasing rate than PTX-NPs. However, at 96 h, paclitaxel coated catheter released a similar amount (12.5%) of paclitaxel from PTX-NPs. This might be explained by the reduced surface area of PTX-NPs bounded on the catheter, limiting the hydrolysis of PLGA polymer via water molecules at initial time point.
Although nanoparticles have shown effective release of paclitaxel in vitro, more research is needed to confirm this in vivo. Additionally, daily blood samples were not possible to take in rat model; therefore, further studies on adverse effects of nanoparticles in vivo are needed.
The earlier study also mentions that the release of PTX-NPs is well known to have the ability to control the release of paclitaxel for up to 8 weeks and stably release them for 4 weeks due to PLGA being biodegraded. Intimal hyperplasia resulting from vascular injury in the late phase was inhibited owing to the aforementioned slow-releasing rate [29-31]. However, the shorter releasing period of paclitaxel in our study compared with other studies and its less clinical use due to poor solubility in blood may result in insufficient concentrations to inhibit the intimal hyperplasia. Additionally, the possibility that the paclitaxel was easily dropped out of the catheter due to physical stress when the catheter was inserted via IJV could not be excluded.
To obtain best possible results, an increasing period of tissue procurement is required, and a peel-away sheath was used to alleviate physical stress. Neointimal hyperplasia in veins is indistinct, and venous structure is poorly maintained. We encountered eccentric rather than concentric hyperplasia, making it difficult to evaluate degrees of change, which are proportional to wall thickening. Consequently, we used two different methods to measure the walls of each venous segment. However, these measurements may be skewed if hyperplastic eccentricity is extreme. By presuming that blood vessels were symmetrically thickened, we used outer and inner venous diameters to calculate outer (Amax) and inner (Amin) areas of veins, subtracting inner from outer areas to determine wall thickness. However, this method is not valid in totally collapsed vessels, so a later formula is required.
Pablo et al. [32] have proposed a method of measuring neointimal hyperplasia based on areas differential coloring by Masson’s trichrome stain. It is promising, but one expert has called for a correlation with pathologic findings. We attempted this method, but found that SMF and ECM produced a blurring of intima and media, making them impossible to distinguish. Arvydas et al. have addressed the use of digital eyes, a new technology expected to aid in accurate and rapid analysis.
In conclusion, while paclitaxel-loaded nanoparticles show promise in reducing intimal hyperplasia, our study suggests that PTX-NPs coated catheters may not be effective in the short term, and that a longer period of indwelling may be required for the drug to have a significant impact. Further research is needed to determine the optimal duration of catheter use and whether there are adverse effects associated with the use of PTX-NPs coated catheters. The use of digital eyes or other new technologies may aid in accurate and rapid analysis of neointimal hyperplasia.
Conclusion
In summary, this study aimed to investigate the effectiveness of PTX-NPs coated CVCs in reducing CVS by histological means. The result showed that there was no significant difference between the test and control groups in intimal hyperplasia after 2 or 4 weeks. However, the P4 group showed less intimal hyperplasia than the P2 group. This suggests that PTX-NPs coated catheters may be effective in preventing venous stenosis for a prolonged period, exceeding 4 weeks.
To establish the efficacy of PTX-NPs coated CVCs in reducing CVS in the long term, further research is required to determine the optimal release rate. In conclusion, this study highlights the importance of investigating the effectiveness of new technologies to reduce CVS using PTX-NPs coated catheters.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (Grant Nos.: 2017R1D1A3B03034101, 2022RIS-005, and 2022R1F1A1069516).
Author contributions
SYK: contributed in conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; SA: data analysis and interpretation; WSY: collection and assembly of data, data analysis and interpretation; WCK: collection and assembly of data, data analysis and interpretation; SBM: data analysis and interpretation; GBC: data analysis and interpretation; SK and JK: equally contributed in conception and design, administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Declarations
Conflict of interest
All of the authors has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaehong Key, Email: jkey@yonsei.ac.kr.
Seongyup Kim, Email: sykimvs@yonsei.ac.kr.
References
- 1.Forauer AR, Theoharis CG, Dasika NL. Jugular vein catheter placement: histologic features and development of catheter-related (fibrin) sheaths in a swine model. Radiology. 2006;240(2):427–434. doi: 10.1148/radiol.2402031129. [DOI] [PubMed] [Google Scholar]
- 2.Argoti-Velasco YL, et al. Proper electrocardiography-guided placement of a central venous catheter. Revista Médica del Hospital General de México. 2018;81(4):262–267. doi: 10.1016/j.hgmx.2016.09.007. [DOI] [Google Scholar]
- 3.Neoh KG, et al. Surface modification strategies for combating catheter-related complications: recent advances and challenges. J Mater Chem B. 2017;5(11):2045–2067. doi: 10.1039/C6TB03280J. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzaki A, Suminoe A, Koga Y, et al. Long-term use of peripherally inserted central venous catheters for cancer chemotherapy in children. Support Care Cancer. 2006;14:153–160. doi: 10.1007/s00520-005-0848-x. [DOI] [PubMed] [Google Scholar]
- 5.Loughran SC, Borzatta M. peripherally inserted central catheters: a report of 2506 catheter days. J Parent Enteral Nutr. 1995;19:133–136. doi: 10.1177/0148607195019002133. [DOI] [PubMed] [Google Scholar]
- 6.Tedla FM, Clerger G, et al. Prevalence of central vein stenosis in patients referred for vein mapping. CJASN. 2018;13(7):1163–8. doi: 10.2215/CJN.14001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal AK. Central vein stenosis: current concepts. Adv Chronic Kidney Dis. 2009;16(5):360–370. doi: 10.1053/j.ackd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Chopra V, Anand S, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382:311–325. doi: 10.1016/S0140-6736(13)60592-9. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y-X, Ye M, et al. Endovascular treatment of central venous stenosis and obstruction in hemodialysis patients. Chin Med J. 2013;126(3):426–430. [PubMed] [Google Scholar]
- 10.Wong FK, Kwok PC, et al. Prevention of restenosis of central venous stricture after percutaneous transluminal angioplasty and endovascular stenting by brachytherapy. Kidney Int. 1999;55:724–732. doi: 10.1046/j.1523-1755.1999.00272.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim YL, Moon SB, et al. Histological changes of the unligated vein wall adjacent to the central venous catheter after open cutdown in rats. J pediatr Surg. 2015;50(11):1928–32. doi: 10.1016/j.jpedsurg.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Jia L, et al. Correlation of caveolin-1 with vascular intimal thickness for different locations of catheter tips in a dog model. Iran J Kidney Dis. 2018;12(4):232–239. [PubMed] [Google Scholar]
- 13.Wang LH, Wei F, et al. Fibrin sheath formation and intimal thickening after catheter placement in dog model: role of hemodynamic wall shear stress. J Vasc Access. 2015;16(4):275–284. doi: 10.5301/jva.5000358. [DOI] [PubMed] [Google Scholar]
- 14.Minami Y, Kaneda H, et al. Endothelial dysfunction following drug-eluting stent implantation: a systematic review of the literature. Int J Cardiol. 2013;165(2):222–228. doi: 10.1016/j.ijcard.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 15.Singh GD, Armstrong EJ, et al. Femoropopliteal in-stent restenosis: current treatment strategies. J Cardiovasc Surg. 2014;55(3):325–333. [PubMed] [Google Scholar]
- 16.Dippel EJ, Makam P, et al. Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in-stent restenosis: initial results from the EXCITE ISR trial (EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal In-Stent Restenosis) JACC Cardiovasc Interv. 2015;8(1Pt A):92–101. doi: 10.1016/j.jcin.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Xiaolong T, et al. Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment. Nanoscale Res Lett. 2013;8(1):420. doi: 10.1186/1556-276X-8-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin A, et al. Development of paclitaxel and flurbiprofen coloaded PLGA nanoparticles: understanding critical formulation and process parameters using Plackett-Burman design. İstanbul J Pharm. 2019;49(3):161–166. [Google Scholar]
- 19.Lim HJ, et al. A novel technique for loading of paclitaxel-PLGA nanoparticles onto ePTFE vascular grafts. Biotechnol Prog. 2007;23(3):693–697. doi: 10.1021/bp060338i. [DOI] [PubMed] [Google Scholar]
- 20.Kesarwani P, Tekade RK, Jain NK. Spectrophotometric estimation of paclitaxel. Int J Adv Pharm Sci 2. 2011;29-32.
- 21.Lee BH, et al. Paclitaxel-coated expanded polytetrafluoroethylene haemodialysis grafts inhibit neointimal hyperplasia in porcine model of graft stenosis. Nephrol Dial Transplant. 2006;21(9):2432–2438. doi: 10.1093/ndt/gfl070. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Moons L, et al. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150(2):761–76. [PMC free article] [PubMed] [Google Scholar]
- 23.Regar E, Sianos G, Serruys PW. Stent development and local drug delivery. Br Med Bull. 2001;59(1):227–248. doi: 10.1093/bmb/59.1.227. [DOI] [PubMed] [Google Scholar]
- 24.Sousa JE, Costa MA, et al. Lack of neointimal proliferation after implantation of sirolimus-eluting stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103:192–5. doi: 10.1161/01.CIR.103.2.192. [DOI] [PubMed] [Google Scholar]
- 25.Kitrou PM, et al. Paclitaxel-coated balloons for the treatment of symptomatic central venous stenosis in dialysis access: results from a randomized controlled trial. J Vasc Interv Radiol. 2017;28(6):811–817. doi: 10.1016/j.jvir.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca C, Simoes S, Gaspar R. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. J Control Release. 2002;83(2):273–286. doi: 10.1016/S0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 27.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf, B. 2007;59:24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Niikura K, Iyo N, et al. Sub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiation. ACS Appl Mater Interfaces. 2013;5(9):3900–3907. doi: 10.1021/am400590m. [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Kwon OH, et al. Paclitaxel coating on ePTFE artificial graft and the release behavior. Polymer (Korea) 2011;36(3):326–31. [Google Scholar]
- 30.Tabatabaei Mirakabad FS, Joo SW, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 2014;15(2):517–535. doi: 10.7314/APJCP.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- 31.Scheller B, Hehrlein C, et al. Treatment of Coronary In-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter. N Engl J Med. 2006;355(20):2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
- 32.Pablo HM, Irene CG, et al. Quantification and statistical analysis methods for vessel wall components from stained images with Masson's trichrome. PLoS ONE. 2016;11(1):e0146954. doi: 10.1371/journal.pone.0146954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.