Abstract
Sleep is an essential part of our lives and daily sleep monitoring is crucial for maintaining good health and well-being. Traditionally, the gold standard method for sleep monitoring is polysomnography using various sensors attached to the body; however, it is limited with regards to long-term sleep monitoring in a home environment. Recent advancements in wearable and nearable technology have made it possible to monitor sleep at home. In this review paper, the technologies that are currently available for sleep stages and sleep disorder monitoring at home are reviewed using wearable and nearable devices. Wearables are devices that are worn on the body, while nearables are placed near the body. These devices can accurately monitor sleep stages and sleep disorder in a home environment. In this study, the benefits and limitations of each technology are discussed, along with their potential to improve sleep quality.
Keywords: Wearable devices, Nearable devices, Sleep monitoring, Sleep stages, Sleep disorder
Introduction
Sleep is a vital aspect of our lives, constituting almost one-third of our lifetime. It is essential not only for our physical survival but also for maintaining healthy mental and physical functioning. During sleep, the body can rest and repair itself, while the brain consolidates memories and processes information [1]. However, many people struggle with sleep, facing a range of sleep-disturbing problems, including insomnia, sleep apnea, and restless legs syndrome. To address these issues, it is crucial to accurately evaluate and diagnose sleep disturbances, which can be achieved through continuous and convenient monitoring of sleep [2].
Traditionally, polysomnography (PSG) has been the standard method for quantitative sleep monitoring. However, this involves subjects visiting a sleep clinic and staying there for one or more nights while their sleep is recorded using numerous sensors attached to their body to monitor physiological signals such as electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), respiratory airflow, respiration efforts, pulse oximetry, leg movement, and body position. The recorded signals are used to evaluate each 30-second epoch of the entire sleep period, and any sleep disturbances, including apneic episodes and leg movements, can be detected.
Although PSG is an accurate method for evaluating sleep quality and diagnosing sleep-related problems, it has limitations, including spatial and temporal restrictions. In terms of spatial constraints, subjects need to visit a sleep laboratory in a clinic, which may be at a significant distance from their home. Temporally, polysomnographic recording is limited only to the nights when the subjects are staying in the clinic, making it difficult to capture a comprehensive picture of the dsleep patterns. Moreover, sleep in a clinical setting may differ from sleep in the subject’s own bedroom, which is a private and more comfortable environment. The attachment of many sensors to the body can cause discomfort and may even disrupt sleep, potentially affecting the accuracy of the recorded data. Consequently, the use of PSG for extended monitoring of sleep outside of the clinic may not be practical or feasible. Therefore, alternative methods are needed that are more convenient and suitable for long-term monitoring of sleep quality.
Sleep monitoring has become increasingly popular as people seek to improve their sleep quality and identify underlying sleep issues. To this end, many technologies have been developed to monitor sleep with maximum comfort in free-living conditions [3]. Actigraphy was the first trial used to evaluate sleep-related activities outside of a clinical environment. By recording acceleration based on a subject’s movement via a watch-type sensor, it provided information on the subject’s movements throughout their day and night activities. This method was effective in evaluating circadian rhythms and supplementing polysomnographic recordings to evaluate sleep-related disorders more accurately [4]. Actigraphy also demonstrated the usability of monitoring methods to monitor the subject’s activity in an out-of-clinic environment. Another technology, portable PSG, transfers sleep recordings to the subject’s own sleeping environment [5]. While sensors are still attached to the body surface like standard polysomnographic recording, the use of polysomnographic recording is successfully extended into an out-of-clinic environment with a minimized set of sensors.
As people increasingly seek to improve their sleep quality and identify underlying sleep issues, sleep monitoring at home has become popular. Recent advancements in sleep technology have focused on making sleep monitoring more comfortable and convenient, with sensors that can be easily wearable or installable within one’s own sleep environment at home. Sleep monitoring at home provides valuable insights into an individual’s sleep quality and quantity. Continuous monitoring of sleep throughout a length of time can detect changes in sleep states requiring prompt action, as well as gradual improvements in sleep quality caused by treatment. Detecting any signs or severity of sleep disorders can help in their early treatment and appropriate management. Sleeping in one’s own room is more comfortable and convenient compared to sleep monitoring in a clinic, providing a more accurate and realistic evaluation of the subject’s sleep in their own environment. Based on the analysis of each individual’s sleep characteristics, more effective personalized treatments, suggestions, and recommendations can be provided to maximize sleep quality. Home sleep monitoring can also be cost-effective, as it eliminates the need for subjects to visit sleep clinics and use expensive facilities, as long as the home monitoring devices provide comparable performance.
To effectively monitor sleep at home, devices need to meet certain requirements. First, they must provide accurate and reliable data that are comparable to standard PSG performance, at least within the targeted subdomain. Their performance and limitations should be verified against PSG. Second, the devices must be convenient and minimally intrusive. If subjects are required to attach multiple sensors to their body, as in PSG, the devices are unsuitable for extended use at home. Therefore, various technologies have been developed to minimize or eliminate the need for sensor attachment by incorporating sensors in wearable or separate units near the bed. Additionally, the device should be easy to use and require minimal subject involvement in the setup process. If monitoring can be performed using unconstrained technology, subjects can be conveniently monitored without interfering their normal activities and sleeping routines. Third, home monitoring devices should provide easy and reliable data access to central platforms. By connecting to a network, data accessibility can overcome the limitations of home sleep monitoring caused by spatial distance, allowing for real-time monitoring and prompt feedback to increase subject adherence. Finally, devices should be cost-effective to install and maintain for long-term use.
As the demand for sleep monitoring at home has increased, sleep technology has advanced to provide wearable and nearable devices that allow subjects to monitor their sleep. This article reviews the most recent technologies available for home sleep monitoring, discussing the benefits and limitations of each technology and its potential to improve sleep.
Stages of sleep
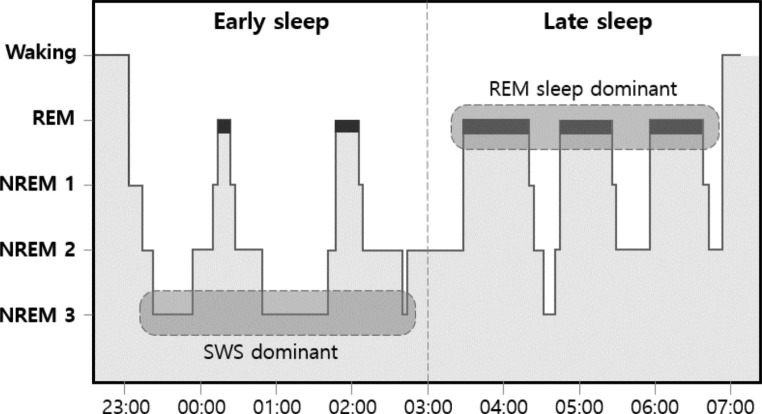
Sleep is a complex physiological process that is essential for optimizing human brain and body functions. Over the course of a night, individuals pass through different sleep states, each with distinct brain activities and body functions as dictated by sleep physiology [6]. Sleep is broadly categorized into two types: rapid eye movement (REM) sleep and non-REM sleep. Non-REM sleep is further divided into three stages, namely, N1, N2, and N3. Figure 1 shows a hypnogram depicting the variation in sleep stages over the course of one night. Each stage is characterized by specific patterns of brain activity, physiological changes, and behavioral features.
Fig. 1.
Hypnogram showing the variation of sleep stages through one night of sleep. REM: Rapid eye movement, NREM: Non-rapid eye movement, SWS: slow-wave sleep
N1 is the lightest stage of sleep, occurring primarily in the initial part of sleep and representing the transition from waking to sleeping. It is characterized by a reduction in muscle tone and slowing of brain activity compared to the waking state. This stage usually lasts for only a short period of time, typically less than 10 min.
N2 is a deeper stage of sleep than N1 and represents approximately 50% of total sleep time. During this stage, brain activity slows down and responsiveness to external stimuli further decreases. This stage is characterized by deterministic brainwaves of sleep spindles and the K-complex.
N3 is the deepest stage of sleep, also known as slow-wave sleep (SWS), and represents almost 15–25% of total sleep time. During this stage, the brain activity exhibits the lowest rhythm of delta waves, and the body is fully relaxed. This stage is critical for physical restoration of body function, memory consolidation, and strengthening of immunity.
REM sleep is a paradoxical sleep stage showing brain activity similar to that in the waking state, but with total motor inactivation. This is the sleep stage at which individuals dream and their eyes move rapidly according to dream content, giving rise to the name REM. REM sleep takes approximately 20–25% of total sleep time and is essential for cognitive processing and emotional regulation.
Sleep is a complex process that is characterized by different stages, each with unique features and functions. During the night, the sleep cycle begins with the lightest N1 stage and progresses through the N2 stage to the deepest N3 sleep stage. The cycle then shifts back to the N2 stage, and flows into the REM sleep stage. This cycling flow repeats, and four to five sleep cycles occur during a night’s sleep. SWS stages predominantly occur in the early half of sleep, while REM sleep stages occur during the latter half of sleep.
To accurately identify and classify sleep stages, the American Academy of Sleep Medicine (AASM) guidelines use multiple biological signals, including EEG, EOG, and EMG [7]. EEG is the primary method for determining all sleep stages. Alpha rhythm (8–13 Hz) is used to differentiate wakefulness from N1 sleep. Sleep spindles and k-complexes of EEG characterize N2 sleep. Moreover, slow-wave activity (0.5–2 Hz) of the EEG signal is used to determine N3 sleep. REM sleep is mainly classified based on the level of REM in the EOG and the level of chin EMG tone [7]. However, studies have shown that various biological signals, including movement level, the heart, and respiratory rate, exhibit different patterns according to the sleep stages [6, 8, 9]. Advances in processing and storage technologies have enabled the measurement of various biological signals using independent devices. Various software can analyze signals from these devices, allowing for the identification of different sleep stages. Wearable and nearable devices are particularly useful in measuring these signals outside of a hospital setting. The following sections will delve into how these devices measure biological signals and automatically identify different sleep stages.
Wearable-based sleep staging
Wearable devices are designed to be attached to an individual’s skin or worn on their body to record biological signals and information. They monitor signals and information in close proximity to the individual, making them less susceptible to noise and artifacts than nearable devices, which is discussed in the following subchapter. Additionally, wearable devices can be used for 24 h, allowing for the evaluation of sleep states as well as the relationship between daytime activity and sleep.
Headbands, watches, rings, and patch-type devices are examples of wearable devices. Headband-type devices primarily record the EEG signals and have been designed to be easily worn and to record fewer channels of EEG compared with PSG, making them more suitable for use outside of a hospital without expert assistance. In-ear EEG, which measures EEG signals from electrodes positioned inside the ear, has also been proposed by several researchers. Basic watch-type devices comprise accelerometers to record individual activities. Recent watch-type devices can record not only activities but also photoplethysmography (PPG). PPG is a measure of blood volume changes by monitoring the level of light absorption or reflection at the skin surface [10], allowing for the measurement of heartbeat interval. Respiratory sinus arrhythmia (RSA) is defined as a cyclic variation in heartbeat interval according to respiration [11]. Applying digital filters to heartbeat intervals within the respiratory frequency range can estimate the respiratory pattern. Ring-type devices have also been developed to measure the corresponding signals and information. Patch-type devices include devices attached to the skin of an individual to measure electrocardiograms (ECG), direct respiratory signals, and accelerometry signals.
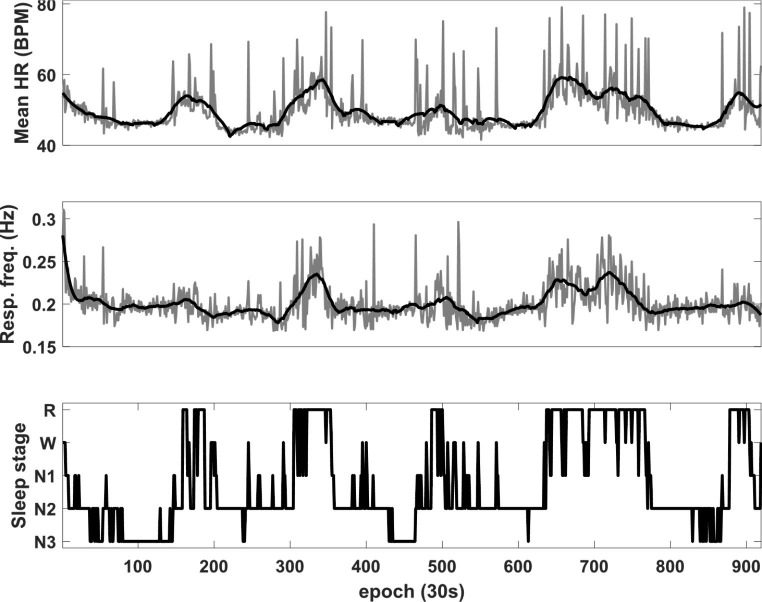
Sleep is closely tied to the activity of the autonomic nervous system (ANS), which is responsible for maintaining homeostasis in response to internal and external environmental changes [8, 9]. The ANS is composed of two branches: the sympathetic and parasympathetic, which respectively prepare the body to respond to strenuous conditions and control basic bodily functions such as rest and digestion [12]. Therefore, increased sympathetic tone is observed during daytime activity while decreased sympathetic and increased parasympathetic tones are observed during sleep [13]. However, the levels of sympathetic and parasympathetic activity vary depending on the sleep stage. During N1 to deeper sleep stages, parasympathetic tone gradually increases, and sympathetic activity shows an inverse pattern compared to parasympathetic activity [14]. In REM sleep, there are irregular fluctuations in sympathetic and parasympathetic activities [8, 9]. Thus, ANS-related biological signals and information exhibit different patterns during the various sleep stages. For example, heart and respiratory rates decrease as sleep progresses to deeper stages and show an increasing and irregular pattern during REM sleep. Therefore, signals or information from PPG, ECG, and respiratory signals related to ANS activity can be used for sleep staging. Figure 2 shows an example of biological information obtained during sleep. Table 1 provides examples of wearable devices that provide sleep-stage related information based on the analysis of biological signals. In PSG, sleep is classified into five stages: wakefulness, REM sleep, and non-REM 1, 2, and 3 (N1, N2, and N3) sleep. However, depending on the purpose, the devices can distinguish between two stages (wakefulness and sleep), three stages (wakefulness, REM sleep, and non-REM sleep), or four stages (wakefulness, REM sleep, light sleep [N1 + N2 sleep], and deep sleep [N3 sleep]).
Fig. 2.
Heart rate and respiratory frequency during sleep. Mean HR Mean heart rate, Resp. freq. Respiratory frequency, R Rapid eye movement (REM) sleep, N non-REM sleep
Table 1.
Summary of wearable devices to monitor sleep stages
| Type | Product | Signals |
|---|---|---|
| Headband | Dreem Headband | EEG (O1, O2, Fpz, F1, F8), Acc., PPG, SpO2 |
| Sleep Shepherd | EEG | |
| Muse Series | EEG, Acc., PPG, SpO2 | |
| In-ear | Nightbuds | EEG, Acc. |
| Watch | Halo Band | PPG, Acc. |
| Fibit Series | ||
| Apple Watch Series | ||
| Galaxy Watch Series | ||
| Biostrap | ||
| Actiwatch Series | Acc., Light Illuminance | |
| Whoop 4.0 | Skin Temp., Acc., PPG, SpO2 | |
| Ring | Oura | PPG, Acc., Skin Temp. |
| Sleepon | PPG, SpO2 | |
| Etc. | WatchPAT 300 | PAT, PPG, SpO2, Acc., Body Position, Sound (snoring), Resp. (chest movement) |
EEG electroencephalogram, Acc. Accelerometry signals, PPG photoplethysmogram, Skin Temp. Skin temperature, PAT peripheral arterial tone, Resp. Respiration
Devices like headbands and in-ear devices measure EEG signals, which are commonly used in the manual scoring of PSG to classify different sleep stages with high accuracy. However, these devices usually record fewer channels of EEG signals compared to PSG, making them more convenient to use. In PSG, different sleep stages are classified based on EEG patterns measured in various regions. For instance, wakefulness and N1 sleep stage are distinguished by the level of alpha rhythm (8–13 Hz) recorded over the occipital regions, while the N3 sleep stage is determined by the level of slow-wave activity (0.5–2 Hz) measured over the frontal regions. Additionally, REM sleep is scored mainly based on REM patterns observed in EOG signals. Therefore, it is essential to extract valuable features from fewer EEG channels to classify different sleep stages. While determining REM sleep may be challenging using only EEG signals because it is primarily scored using EOG and EMG patterns, recording EEG in the frontal regions can capture eye movements and potentially improve performance in detecting REM sleep. These devices mostly use dry electrodes to measure EEG signals, which are known to be weak due to noise and artifacts. Therefore, advanced signal processing methods are needed to extract features and determine sleep stages accurately.
In contrast, basic watch-type devices can determine sleep stages between wakefulness and sleep based on the level of activity using an accelerometer. While some studies have explored methods to classify sleep into more than two classes, sleep is mainly characterized by a decrease in activity, making it challenging to determine several sleep stages using only the level of activity. These devices measure only the activity level, which is beneficial for battery consumption; moreover, they can measure activity during the daytime, making them useful for determining circadian rhythms based on long-term data [15]. Long-term data can also help identify sleep patterns, such as sleep regularity and social jet lag [16, 17].
Watch-type devices equipped with PPG sensors can accurately determine sleep stages based on heart rate variability (HRV). The ANS plays a key role in sleep regulation, and ANS activity changes as we transition between different sleep stages [8, 9]. For instance, sympathetic activity gradually decreases from light (N1) to deep (N3) sleep, while parasympathetic activity increases. REM sleep is characterized by fluctuations in both sympathetic and parasympathetic activity [8, 9]. PPG sensors can detect variations in blood flow caused by heartbeats and HRV parameters can be extracted using different methods, including time, frequency, and nonlinear analyses [13, 14]. Respiratory patterns can also be estimated from HRV data, using the RSA phenomenon [11]. Some devices can measure peripheral arterial tone (PAT), which is related to sympathetic tone, and has been found to differ between non-REM and REM sleep [18, 19]. These features can be used to classify sleep into four stages, and the devices can be worn on the wrist, providing high user comfort and mobility. However, motion artifacts and signal quality issues can affect the accuracy of sleep stage classification, and advanced signal-processing techniques are needed to overcome these challenges.
Patch-type devices that measure ECG and respiratory signals are less susceptible to motion artifacts, as they are typically worn close to the heart and lungs. These devices can also use accelerometry signals to estimate sleep posture and distinguish between wakefulness and sleep [20].
While various wearable devices for sleep monitoring have been commercialized (see Table 1), few studies have reviewed their accuracy in classifying sleep stages. Table 2 summarizes the performance of different wearable devices in sleep stage scoring using biological signals.
Table 2.
Performances of reported sleep staging using the signals from wearable devices
| Signals / Models | # of Stages |
# of Rec. |
Performance | Ref. | ||
|---|---|---|---|---|---|---|
| Agree. (%) | Kappa | |||||
|
EEG (O1, O2, Fpz, F7, F8), Acc. PPG, SpO2 / LSTM |
5 | 25 | 83.5 | 0.75 | [21] | |
|
EEG (F4, C4, O2) / MLP neural network |
5 | 154 | 89 | - | [22] | |
| EEG (Fpz-Cz, Pz-Oz) / CNN | 5 | 20 | 86 | - | [23] | |
| EEG (Fpz-Cz) / CNN + BiRNN | 5 | 61 | 84.3 | 0.79 | [24] | |
| EEG (F4-EOG) / CNN + LSTM | 5 | 62 | 86.2 | 0.80 | [25] | |
| EEG (Fpz-Cz) / LSTM | 5 | 12 | 86.7 | - | [26] | |
| EEG (In-ear) | Support Vector Machine | 5 | 16 | 74.1 | 0.61 | [27] |
| Random Forest | 5 | 80 | 80.6 | 0.73 | [28] | |
| Acc. |
Generalized Estimating Equation |
2 | 77 | 86.3 | - | [29] |
| Rule-based model | 2 | 228 | 84.0 | - | [30] | |
| ECG | LSTM | 4 | 584 | 71.9 | 0.57 | [31] |
| LSTM | 4 | 584 | 77 | 0.61 | [32] | |
| LSTM | 5 | 373 | 71.2 | 0.52 | [33] | |
| CNN | 4 | 993 | 77 | 0.66 | [34] | |
| PPG | Bayesian linear discriminant | 4 | 215 | 59.3 | 0.42 | [35] |
| LSTM | 4 | 60 | 69.8 | 0.55 | [31] | |
| CNN + Gated Recurrent Unit | 5 | 894 | 64.1 | 0.51 | [36] | |
| PPG, Acc. / MLP | 3 | 219 | 72.3 | 0.28 | [37] | |
| PAT, PPG, Acc. / Rule-based model | 4 | 227 | 66.0 | 0.48 | [38] | |
# of Rec. number of data points used in the study, Agree. agreement, Ref. reference, EEG electroencephalogram, Acc. Accelerometry signals, ECG electrocardiogram, PPG photoplethysmogram, Skin Temp. Skin temperature, PAT Peripheral arterial tone, LSTM Long-Short Term Memory, MLP multilayer perceptron, CNN convolutional neural network, BiRNN Bidirectional recurrent neural network, SVM support vector machine
Nearable-based sleep staging
Nearable devices monitor biological signals and information from a distance without direct contact with individuals. Their main advantage is that they can measure signals and information in an unobtrusive manner. Unlike wearable devices, which can be detached, nearable devices are mostly positioned near an individual’s sleeping environment and can only monitor sleep status. Examples of nearable devices include radar-, microphone-, and film-based systems that are installed on the bed.
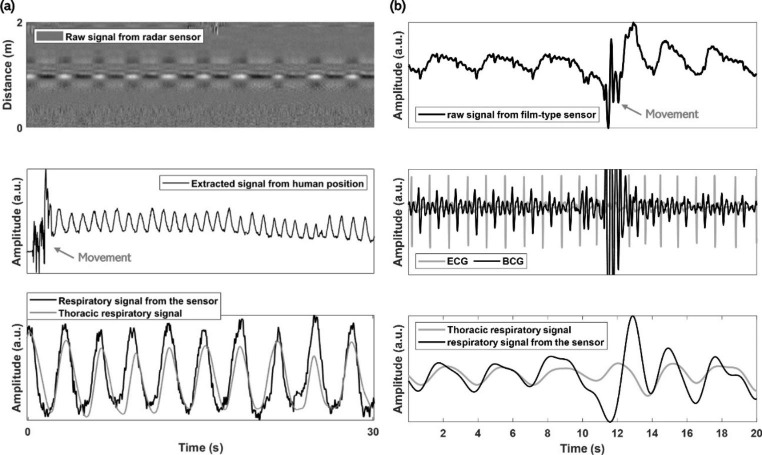
Radar-based systems measure information related to the modulation characteristics between transmitted and received radio frequency (RF) signals caused by the displacement of objects [39, 40]. Respiration and heartbeat cause displacement of the chest and the surface near the heart; moreover, radar systems can detect respiratory and heart-activity-related signals. However, raw radar signals may contain unintended components, called clutter, which must be eliminated [39]. Preprocessing techniques, such as filtering the signal received from the target distance, can be applied to monitor heartbeat- and respiratory signals. Although radar-based systems are advantageous for measuring biological signals over long distances, without direct contact, the presence of unintended objects between the radar system and target object can make it challenging to measure the desired signal. Additionally, signal quality can be influenced by an individual’s posture during sleep.
Microphone-based systems estimate respiratory information by recording breathing-related sound signals. The amplitude and frequency differences of the recorded sound signal between the inhalation and exhalation phases are used to estimate respiratory information [41]. Although previous studies recorded sound signals close to the nose and neck positions [42], recent studies have reported that respiratory information can be obtained using a microphone set more than 1 m away from an individual [43]. One advantage of this system is that sounds can be recorded using built-in microphone devices, such as smartphones and artificial intelligence speakers. However, sound recording quality depends on hardware specifications, and respiratory sounds and environmental noise must be distinguished.
Film-based sensors, which can be installed on a mattress or between mattress layers, are used to measure biological signals. These sensors generate electrical signals when an external force is exerted on them [44]. When the heart beats, slight vibrations are generated and transmitted through the medium to the mattress, where they can be detected by sensors as an external force. This induces an electrical signal called a ballistocardiogram (BCG). Additionally, the force generated by changes in thoracic volume during respiration is delivered to the sensor, allowing the respiratory signal to be measured. Thus, film-type systems can monitor the heart and respiratory activities simultaneously with only one sensor. Examples of biological signals measured using nearable devices are shown in Fig. 3.
Fig. 3.
Examples of biological signals from nearable devices. (a) Data from radar system and (b) data from film-type sensor. ECG electrocardiogram, BCG ballistocardiogram
Although film-type devices record signals in an unobtrusive manner, the quality of the signals is known to be good because the vibrations derived from the heart and respiratory activities are transmitted to the sensors through a medium such as the individual’s body or the mattress. However, the sensors measure heart- and respiration-related activities as well as motion artifacts in the same manner. This makes it difficult to obtain heart- and respiration-related information during individual movements. Nonetheless, movement is an important characteristic for identifying wakefulness, and the quantification of movement can be used to determine the relationship between wakefulness and sleep.
Table 3 summarizes examples of commercialized nearable devices used to monitor sleep, while Table 4 provides a summary of sleep stage scoring using signals from nearby devices. Although the principles of signal measurement differ for nearable devices, they obtain similar information, such as movement-, heart-, and respiration-related information, with the exception of microphone-based systems. Nearable devices typically estimate sleep in four stages based on HRV and respiratory rate variability. Previous studies have been based on rule-based and conventional machine learning methods, but recent studies have introduced automatic sleep staging using deep neural network-based methods. Sleep is known to show continuity, with the current sleep status influenced by the past sleep status. Therefore, one strategy is to develop methods that reflect the characteristics of sleep continuity, such as the use of long- and short-term memory networks, to determine sleep stages.
Table 3.
Summary of nearable devices to monitor sleep stages
| Type | Product/Solution | Signals |
|---|---|---|
| Bed-installed | Beddit | Ballistocardiogram, Respiratory activity, Movement |
| Withings | ||
| Eight Sleep Tracker | ||
| SleepIQ | ||
| Sleepme | ||
| Fullpower | ||
| Radar | S+ | Respiration-related activity, Movement |
| Somnofy | ||
| Halo Rise | ||
| Circadia | Heart- and Respiration-related activity, Movement | |
| Microphone | Sleep Cycle | Respiration-related sound, Movement |
Table 4.
Performances of reported sleep staging using the signals from nearable devices
| Type | Signals / Models | # of Stages |
# of Rec. |
Performance | Ref. | ||
|---|---|---|---|---|---|---|---|
| ACC (%) | Kappa | ||||||
| Radar |
Sig_hba., Sig_ra., Mov. / LSTM |
4 | 51 | 82.6 | 0.73 | [45] | |
|
Sig_hba., Sig_ra., Mov. / K-Nearest Neighbor |
4 | 13 | 81.0 | - | [46] | ||
| RF spectrogram / Conditional Adversarial Discriminator | 4 | 100 | 79.8 | 0.70 | [47] | ||
| Resp. Mov. / LSTM | 4 | 71 | 76.0 | 0.63 | [48] | ||
| - | 4 | 40 | 70.0 | 0.53 | [49] | ||
| Microphone | Resp.-related sound | Feedforward neural network | 3 | 250 | 86.9 | 0.69 | [50] |
|
CNN + LSTM +Transformer |
4 | 1481 | - | 0.52 | [51] | ||
| Film / bed installed | BCG, Resp. Mov. / LSTM | 4 | 60 | 73.9 | 0.55 | [52] | |
| BCG, Resp. Mov. / DNN | 2 | 45 | 86.0 | 0.45 | [53] | ||
| Resp. Mov / Rule-based model | 4 | 25 | 70.9 | 0.48 | [54] | ||
| BCG, Resp. Mov. / - | 4 | 102 | 79.0 | 0.68 | [55] | ||
| BCG, Resp. Mov. / - | 4 | 85 | 64.5 | 0.46 | [56] | ||
# of Rec. number of data points, ACC. accuracy, Ref. reference, Sig_hba. signal, reflecting the heartbeat activity from the radar signal, Sig_ra. signal reflecting the respiratory activity from the radar signal, Mov. Movement, RF radio frequency, Resp. Respiration, BCG ballistocardiogram, LSTM Long-Short Term Memory, CNN convolutional neural network, DNN deep neural network
Finally, the microphone-based system automatically scores sleep stages with time-frequency features from the recorded sound signals, and the time-frequency patterns of the sound signals differ according to the sleep stages.
Sleep disorders
Sleep-related disorders are numerous, with their accurate diagnosis and treatment requiring a quantitative evaluation of sleep dynamics [57]. Sleep disorders have a profound impact on a person’s wellbeing and affect both physical and mental health as well as productivity and safety. Home monitoring of sleep disorders is crucial for improving sleep quality and overall health.
Insomnia is the most prevalent sleep disorder, affecting approximately 30% of adults. This condition is characterized by difficulty in falling or staying asleep despite having the opportunity to sleep, leading to daytime fatigue, lack of energy, difficulty concentrating, and irritability [58]. The treatment of insomnia depends on identifying the underlying cause through the analysis of sleep patterns and addressing psychological, medical, and sleep hygiene factors. Insomnia is often associated with other medical or psychiatric conditions, including depression, which presents in 80% of cases. Objective sleep monitoring is critical for the quantitative evaluation of insomnia. Insomniacs typically have longer sleep, frequent awakenings during the night, and reduced total sleep time, leading to a decrease in sleep efficiency. Reduced total sleep time is also associated with reduced slow-wave and REM sleep, which are essential for maintaining healthy physical and mental function. Monitoring sleep parameters through wearable and nearable devices over extended periods can provide a more detailed picture of a patient’s sleep dynamics and help tailor treatment to their individual needs.
Sleep disorders have a significant impact on a person’s quality of life and can affect physical and mental health, productivity, and safety. Monitoring the occurrence and improvement of sleep disorders at home is important for overall well-being. Obstructive sleep apnea (OSA) is another common sleep disorder, with a prevalence estimated to be between 3 and 7% worldwide. This sleep disorder is characterized by repetitive episodes of partial or complete obstruction of the upper airway during sleep, which can lead to decreased airflow and blood oxygen levels [59]. Symptoms often include loud snoring, gasping, or choking during sleep, which can result in morning headaches and daytime sleepiness. OSA is a serious health condition that can lead to complications such as high blood pressure, heart disease, stroke, and diabetes. The severity of OSA is determined by the apnea and hypopnea index (AHI), which represents the average number of apnea and hypopnea events per hour. Apnea or hypopnea events are detected by respiratory sensors, and a single episode is detected when the period of breathing cessation lasts longer than 10 s. The AHI is used to classify the severity of the disease, with an AHI < 5 per hour considered normal, and patients with severe disease having an AHI > 30 per hour. OSA can also have a significant impact on autonomic balance, which refers to the balance between the sympathetic and parasympathetic autonomic tones of the nervous system. The reduction in blood oxygen levels during apnea and hypopnea (AH) events triggers a reflex response in the ANS, resulting in an increase in sympathetic tone and a decrease in parasympathetic tone. This increase in sympathetic tone during sleep can lead to elevated blood pressure, increased heart rate, and other cardiovascular effects, with a higher risk of serious complications. While AH events are typically detected with respiratory sensors in standard polysomnographic recordings, alternative and compatible methods based on accompanying sounds or shifts in autonomic balance can be used for detection and evaluation.
Restless leg syndrome (RLS) is a neurological disorder that causes an uncontrollable urge to move the legs, disrupting sleep architecture. Periodic leg movements during sleep (PLMS) are used to diagnose RLS. Narcolepsy, another neurological disorder, leads to excessive daytime sleepiness and sudden sleep episodes. Sleep latency can be, evaluated using PSG and the multiple sleep latency test (MSLT) during the daytime. Parasomnias, which are characterized by abnormal behavior during sleep, such as walking, talking, or night terrors, and circadian rhythm disorders, which disrupt the natural sleep-wake cycle, are also common sleep disorders. In REM sleep behavior disorder, muscle inactivation during REM sleep is absent, causing people to physically act out their dreams.
To effectively evaluate, diagnose, and treat sleep disorders, advanced home sleep monitoring technology is necessary. Wearable and nearable devices are valuable tools for monitoring sleep disorders in a home environment. In the following sections, from among the various sleep disorders, studies related to OSA are discussed, along with a variety of devices that monitor and treat the disease.
Wearable-based sleep disorder monitoring
It is important to monitor sleep disorders at home because these conditions can have serious health consequences if left untreated. OSA is a sleep disorder characterized by repeated episodes of partial or complete blockage of the upper airway during sleep. This can lead to disrupted sleep and low blood oxygen levels, which can increase the risk of heart disease, stroke, and other health problems. Continuous home monitoring is required to prevent the occurrence of OSA and manage it after medical treatment.
Technologies for monitoring OSA in a home environment include detecting AH events and using them to screen OSA severity, and most wearable devices provide a method for screening the severity of OSA. The severity of OSA is divided into four levels based on the AHI value, which is defined as the number of AH events per unit hour. The normal group is defined as an AHI value of less than 5, the mild group is defined as an AHI value of 5 or more and less than 15, the moderate group is defined as an AHI value of 15 or more and less than 30, and the severe group is defined as an AHI value of 30 or more. Wearable systems for screening the severity of OSA provide classification performance by setting the AHI value for classifying groups as a cutoff value (Table 5).
Table 5.
Performances of reported OSA monitoring using the signals from wearable devices
| Type | Signals | # of Rec. |
Classification type | Performance | Ref. |
|---|---|---|---|---|---|
| Patch | ECG + Acc. | 96 | Respiratory event | ACC = 83.0%, k = 0.53 | [60] |
| OSA severity (AHI ≥ 15) | ACC = 87.0%, k = 0.72 | ||||
| ECG + Acc. | 189 | Respiratory event | ACC = 82.0%, k = 0.57 | [61] | |
| OSA severity (AHI ≥ 15) | ACC = 81.0%, k = 0.63 | ||||
| ECG + Acc. | 119 |
OSA severity (AHI ≥ 5,15,30) |
AUC = 0.80, AUC = 0.87, AUC = 0.87 |
[62] | |
| ECG + Acc.+SpO2 | 115 |
AH events (OSA,CSA,HYP, NOR) OSA severity |
ACC = 92.3% ACC = 89.3% |
[63] | |
| Belt | Nasal pressure + oximetry + pulse | 50 | OSA severity (AHI ≥ 10,20) |
SENS = 0.977, SPEC = 1.0, SENS = 0.969, SPEC = 1.0 |
[64] |
| Piezoelectric at ABD and THO | 34 | AH events (NOR, OSA, CSA) | ACC = 81.8% | [65] | |
| Bio-impedance | 25 | AH events (AH, N) | ACC = 72.8% | [66] | |
| Watch |
PAT + OS +HR + actigraphy |
38 |
OSA severity (AHI ≥ 5,15,30) |
ACC = 97.0%, ACC = 100%, ACC = 100% |
[67] |
| PPG | 250 |
OSA severity (AHI ≥ 5,15,30) |
AUC = 0.84, AUC = 0.86, AUC = 0.85 |
[68] | |
| PPG | 102 |
OSA severity (AHI ≥ 5,15,30) |
AUC = 0.81, AUC = 0.88, AUC = 0.84 k = 0.507 |
[69] | |
| Ring | OS + PPG + Acc. | 50 |
OSA severity (AHI ≥ 5,15,30) |
AUC = 0.934, k = 0.292 AUC = 0.908, k = 0.719 AUC = 0.946, k = 0.319 |
[70] |
| PPG + Acc. | 207 |
OSA severity (AHI ≥ 5,15,30) |
ACC = 86.0%, k = 0.574 AUC = 76.0%, k = 0.544 AUC = 91.0%, k = 0.784 |
[71] |
# of Rec. number of data used in the study, ACC. accuracy, Ref. reference, k kappa coefficient, Acc., Accelerometer, OSA Obstructive sleep apnea, CSA central sleep apnea, HYP hypopnea, NOR normal breathing, PAT peripheral arterial tonometry, OS oxygen saturation, HR heart rate, PPG photoplethysmography, SVM support vector machine, LSTM Long-Short Term Memory, CNN convolutional neural network
Patch-type wearable devices are a type of non-invasive device that can be used to monitor OSA at home. These devices are small, lightweight, and can be worn on the skin like a band-aid. They contain sensors that can detect a range of physiological signals, including heart rate, oxygen saturation, and breathing patterns. The data collected by these devices can be analyzed to detect AH events and diagnose OSA severity. It is based on the chest wall motion from accelerometer and ECG-derived respiration (EDR) signal to detect AH events [60–62]. Respiratory events are difficult to classify only using a wearable-patch, and when the SpO2 signal is measured together, respiratory events such as obstructive, central, and hypopnea can be distinguished [63]. Although such a device can be used in a home environment, using it for a long time is difficult because it must be attached to the skin.
Belt-type wearable devices are another type of wearable device used for monitoring OSA at home. These devices are typically worn around the chest or abdomen and use sensors to monitor breathing patterns, chest movements, and HRV. Respiratory sensors such as respiratory inductance plethysmography (RIP) [64], piezoelectric band [65], and bio-impedance [66] are used to measure chest movement and detect sleep apnea events. RIP measures respiratory effort and respiratory volumes during breathing. Piezoelectric bands measure mechanical strain and pressure changes. These sensors are made up of piezoelectric materials that produce an electrical charge when exposed to mechanical stress or pressure, which can be detected and measured. Since belt-type devices are worn on the chest and abdomen, they can measure the respiratory effort signals and distinguish between central and obstructive apnea.
Watch-type wearable devices have gained popularity in recent years for monitoring sleep and detecting sleep apnea with the increasing use of smartwatches. These devices typically use accelerometers and PPG sensors to track movement and HRV during sleep [68, 69]. Some devices also include additional sensors, such as electrocardiography sensors and pulse oximeters, to improve the accuracy of sleep monitoring [67]. Watch-type wearable devices provide a promising option for monitoring OSA at home, offering convenience and a less obtrusive way to collect data compared to other wearable devices.
Ring-type wearable devices are a relatively new technology for monitoring OSA. This type of device is worn on the finger and monitors OSA by measuring oxygen saturation, PPG, and accelerometer signals [70, 71]. Table 5 summarizes the performances of different wearable devices with regards to AH events detection and OSA severity classification using biological signals.
Nearable-based sleep disorders monitoring
Nearable devices can monitor OSA without direct contact with individuals. The use of nearable devices for monitoring OSA at home has several advantages. It is a non-contact method that allows for continuous monitoring without disturbing the sleep of the person being monitored. Additionally, it is a portable, convenient, and cost-effective method for OSA monitoring in a home environment.
Radar-type nearable devices can measure vital signs and physiological parameters in a non-invasive and unobtrusive manner. Respiration events can be detected using radar signals reflected from a person, and radar can also measure heartbeats. Several types of radar are used to monitor OSA as a nearable device, such as Frequency-modulated continuous wave (FMCW) [76, 77], Doppler [75], and ultra-wideband (UWB) radar [72–74]. Primarily, methods using UWB radar to monitor OSA have been reported in recent years. Compared to FMCW and Doppler radar, UWB radar has several advantages. It provides higher range resolution with lower transmit power and higher bandwidth, resulting in lower interference with other radio frequency devices as well as better penetration through walls and other obstacles. Additionally, UWB radar can be more suitable for short-range applications, such as indoor monitoring, because of its lower power consumption. Non-contact radar sensors can be placed under the mattress or in the room and use radio waves to detect movements caused by breathing. The sensor records changes in the reflected signal caused by chest and abdominal movements during breathing, and algorithms analyze the recorded data to identify respiratory events such as apnea and hypopnea. By analyzing the detected events, the system can diagnose and monitor OSA, allowing for convenient, cost-effective, and non-intrusive monitoring that can improve patient compliance and outcomes.
Smartphone-based devices are another type of nearable device used for monitoring OSA at home [43, 78–80]. These devices typically use smartphone built-in sensors, such as the accelerometer, gyroscope, and microphone, to measure movement, snoring sounds, and breathing patterns during sleep. A variety of apps are available for download that can record and analyze sleep data, including the presence of snoring and the frequency of apnea events.
Film/bed installed devices can be used to monitor OSA. This type of device is unobtrusive and placed under the bedsheet or mattress, making it easy to use and non-invasive for the user. It measures signals such as heart and respiratory rates, body movements, and other physiological parameters related to sleep and breathing, providing comprehensive data for sleep analysis and OSA diagnosis. This device can record data continuously over long periods, providing a more accurate representation of an individual’s sleep patterns and potential OSA symptoms than a one-night sleep study in a clinic. However, the quality of the measured signal can be easily affected by motion, and pre-processing of artifacts is required to extract reliable physiological information. Most studies [81–83] using film-type sensors for AH events detection and OSA screening are performed after removing artifacts (Table 6).
Table 6.
Performances of reported OSA monitoring using the signals from nearable devices
| Type | Signals | # of Rec. |
Classification type | Performance | Ref. |
|---|---|---|---|---|---|
| Radar | UWB | 36 | AH events (AH/N) | ACC = 93.0%, k = 0.728 | [72] |
|
OSA severity (avg. cutoff ≥ 5,15,30) |
ACC = 98.0%, k = 0.96 | ||||
| 176 |
OSA severity (AHI ≥ 5,15,30) |
SENS = 1, SPEC = 1, AUC = 1 | [73] | ||
| SENS = 0.97, SPEC = 0.96, AUC = 0.991 | |||||
| SENS = 0.95, SPEC = 0.99, AUC = 0.994 | |||||
| 94 |
OSA severity (AHI ≥ 5,15,30) |
Corr.=0.927 ACC = 93.0 ACC = 91.0 ACC = 100.0 |
[74] | ||
| Doppler | 12 | AH events (AH/N) |
SENS = 86.0% SPEC = 91.0% ACC = 92.0% |
[75] | |
| FMCW | 10 |
AH events (Apneic/Not-apneic) |
ACC = 95.53%, k = 0.68 | [76] | |
| 44 | AH events |
Binary (AH/N), k = 0.715 Multi (A/H/N), k = 0.648 |
[77] | ||
| Mobilephone | Audio, LC | 50 |
OSA severity (AHI ≥ 15) |
SENS = 0.70, SPEC = 0.94, Corr.=0.94 | [78] |
| 13 |
AH events *ref: ApneaLinkTM |
SENS = 87.96%, SPEC = 75.22%, ACC = 81.79% |
[79] | ||
| Audio, LN | 128 |
OSA severity (AHI ≥ 15) |
SENS = 88.3%, SPEC = 80.0%, ACC = 84.2%, AUC = 0.92 | [80] | |
| 1315 (phone 297) |
AH events (3class-A/H/N, 2class-AH/N) |
ACC = 85.9%, k = 0.66 ACC = 88.8%, k = 0.71 |
[43] | ||
|
OSA severity (AHI ≥ 5,15,30) |
AUC = 0.93, AUC = 0.85, AUC = 0.94 |
||||
| Film/Bed installed | PVDF | 26 |
AH events (apneic/non-apneic) |
ACC = 85.5%, k = 0.60 | [81] |
|
OSA severity (AHI ≥ 5,15,30) |
ACC = 96.2, k = 0.84, ACC = 96.2, k = 0.92, ACC = 92.3, k = 0.85 |
||||
| 24 | OSA severity (AHI ≥ 5) |
SENS = 100%, SPEC = 82%, ACC = 96% |
[82] | ||
| Load cell | 14 |
OSA severity (4 class, Normal/Mild /Moderate/Severe) |
ACC = 74.3% | [83] |
# of Rec. number of data used in the study, Ref. reference, ACC. accuracy, SENS sensitivity, SPEC specificity, AUC area under the curve, k kappa coefficient, Corr. Correlation coefficient, LC located chest of subject, LN located near to the subject
Discussion and conclusion
Advances in technology have enabled sleep monitoring using wearable/nearable devices outside hospitals. The performance of sleep monitoring can vary depending on the type of sensor and the algorithms used to remove noise and artifacts for both wearable and nearable devices. Although the performance of wearable/nearable devices can vary depending on sensor configuration and algorithms, the wearable devices are attached to the user’s body; therefore, they can fundamentally measure biosignals more accurately than nearable devices. Furthermore, in recent years, deep learning models have been widely used in sleep monitoring research. To utilize deep learning in the development of sleep monitoring models, the problem of imbalance between data classes must be solved. In the case of sleep stages, the proportion of N3 sleep is less than that of other stages of sleep, and in the case of sleep apnea events, the number of apnea-hypopnea events is relatively less than that of normal breathing. When training a model, the ratio between classes must be appropriately checked before applying methods to the imbalance problem. To elaborate, a method of augmenting the number of deficient data classes and increasing the weight of a class during training or methods as an ensemble of several models can be used to prevent overfitting of the model to a specific class.
In sleep stages monitoring studies, various methods have been reported to monitor sleep stages using biological information that cannot be used to score sleep stages in standard PSG. However, a previous study has reported an inter-rater variability of approximately 83% in PSG manual scoring [7]. Recent studies have reported performance results for automatic sleep stage scoring with an accuracy range of 60–83% for 4-level sleep stages, even without using biological information used in PSG scoring. Performance values have improved with the introduction of various novel methods. However, further work is still required to approach a clinical standard of performance with approximately 90% accuracy [84]. Several devices have been commercialized, but few validation studies have reported the automatic scoring of sleep stages using these devices, and long-term data studies are needed to evaluate their accuracy. It is important to accurately detect the total recording time (TRT) in practical use, which is used to determine sleep stage-related parameters, such as sleep efficiency obtained with 100*(total sleep time/TRT). The accurate detection of TRT should be addressed in further studies. Recently, various repositories for sleep data have become available, such as sleepdata.org [85] and Physionet [86], which can be the helpful in selecting potential biological signals to be recorded using wearable and nearable devices as well as in developing automatic methods of sleep stages.
Wearable and nearable-based devices are used not only in the sleep stage but also in monitoring sleep disorders. These devices have been studied for screening OSA severity as well as detecting AH events. Compared to the PSG test, these technologies show high accuracies, and can be widely used for the prevention and prognosis of OSA through long-term monitoring. The current research focuses on developing accurate sensor devices; however, further research involving personalized interventions, longitudinal monitoring, and standardization is needed to ensure the accuracy, reliability, and clinical utility of the devices. These advancements hold great potential to enhance the diagnosis, management, and overall quality of life for individuals with OSA.
Wearable devices have the advantage of being able to measure biosignals more accurately than nearable devices because sensors are attached to the body, but they are difficult to use for a long time. Nearable devices are convenient for long-term monitoring in terms of usability but are more susceptible to noise and artifacts. There are advantages and disadvantages for each device type, and technologies are being developed such that they can complement each other. Healthy sleep is an essential element for our physical and mental health, and it is important to monitor sleep and respond quickly when abnormal symptoms appear. In this respect, the utilization of wearable and nearable devices is expected to increase with continuous development of these technologies.
Author contributions
All authors contributed to the study conception and design. Writing-original draft preparation: All authors. Writing-review and editing: All authors.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1A5A1015596). This research was supported by the Ministry of Science and ICT (MSIT), Korea under the Information Technology Research Center (ITRC) support program (IITP-2023-RS-2022-00156225) supervised by the Institute for Information, and Communications Technology Planning and Evaluation (IITP). The present Research has been conducted by the Research Grant of Kwangwoon University in 2023.
Declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study does not involve any human participants hence, the ethical approval is not required.
Consent to publish
All authors have approved the content for publications.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heenam Yoon, Email: h-yoon@smu.ac.kr.
Sang Ho Choi, Email: shchoi@kw.ac.kr.
References
- 1.Moorcroft WH. Understanding sleep and dreaming. New York: Springer US; 2013. [Google Scholar]
- 2.Cay G, Ravichandran V, Sadhu S, Zisk AH, Salisbury AL, Solanki D, Mankodiya K. Recent Advancement in Sleep Technologies: a Literature Review on Clinical Standards, sensors, apps, and AI methods. IEEE Access. 2022;10:104737–56. doi: 10.1109/ACCESS.2022.3210518. [DOI] [Google Scholar]
- 3.Park KS, Choi SH. Smart Technologies toward Sleep Monitoring at Home. Biomed Eng Lett. 2019;9:73–85. doi: 10.1007/s13534-018-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadeh A. The role and validity of actigraphy in Sleep Medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for Sleep Apnea diagnosis in a Population Based Cohort using synchronized Home Polysomnography. Sleep. 2006;29:367–74. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA, Dement WC. Normal human sleep : An Overview.
- 7.Rosenberg RS, Van Hout S. The American Academy of Sleep Medicine Inter-Scorer Reliability Program: respiratory events. J Clin Sleep Med. 2014;10:447–54. doi: 10.5664/jcsm.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper RM, Verrier RL. Cardiovascular Physiology and coupling with respiration: Central and Autonomic Regulation. Princ Pract Sleep Med. 2017;6th Ed:132–41. doi: 10.1016/B978-0-323-24288-2.00013-1. [DOI] [Google Scholar]
- 9.Lanfranchi PA, Somers VK. Cardiovascular Physiology: Autonomic Control in Health and in Sleep Disorders. 2010.
- 10.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28. 10.1088/0967-3334/28/3/R01. [DOI] [PubMed]
- 11.Yasuma F, Hayano JI. Respiratory sinus arrhythmia: why does the Heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–90. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- 12.Bergström RM. Physiology of the autonomic nervous system. Acta Anaesthesiol Scand. 1964;8:17–20. doi: 10.1111/j.1399-6576.1964.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Electrophysiology TF, of the, E.S Of C. the N.A. Heart Rate Variability: Standards of Measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 14.Penzel T, Kantelhardt JW, Grote L, Peter J, Bunde A. Comparison of detrended fluctuation analysis and spectral analysis for Heart Rate Variability in Sleep and Sleep Apnea. IEEE Trans Biomed Eng. 2003;50:1143–51. doi: 10.1109/TBME.2003.817636. [DOI] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 16.Fischer D, Klerman EB, Phillips AJK. Measuring sleep regularity: theoretical Properties and practical usage of existing Metrics. Sleep. 2021;44. 10.1093/sleep/zsab103. [DOI] [PMC free article] [PubMed]
- 17.Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, Czeisler CA. Irregular Sleep/Wake patterns are Associated with poorer academic performance and delayed circadian and Sleep/Wake timing. Sci Rep. 2017;7:3216. doi: 10.1038/s41598-017-03171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herscovici S, Pe’er A, Papyan S, Lavie P. Detecting REM sleep from the Finger: an automatic REM sleep algorithm based on peripheral arterial tone (PAT) and actigraphy. Physiol Meas. 2007;28:129–40. doi: 10.1088/0967-3334/28/2/002. [DOI] [PubMed] [Google Scholar]
- 19.Lavie P, Schnall RP, Sheffy J, Shlitner A. Peripheral vasoconstriction during REM sleep detected by a New Plethysmographic Method. Nat Med. 2000;6:606–6. doi: 10.1038/76135. [DOI] [PubMed] [Google Scholar]
- 20.Yoon H, Hwang S, Jung D, Choi S, Joo K, Choi J, Lee Y, Jeong D-U. Kwangsuk Park. Estimation of Sleep Posture Using a Patch-Type Accelerometer Based Device. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE, August 2015:4942–4945. [DOI] [PubMed]
- 21.Arnal PJ, Thorey V, Debellemaniere E, Ballard ME, Bou Hernandez A, Guillot A, Jourde H, Harris M, Guillard M, Van Beers P, et al. The Dreem Headband compared to Polysomnography for Electroencephalographic Signal Acquisition and Sleep Staging. Sleep. 2020;43. 10.1093/sleep/zsaa097. [DOI] [PMC free article] [PubMed]
- 22.Hussain I, Hossain MA, Jany R, Bari MA, Uddin M, Kamal ARM, Ku Y, Kim JS. Quantitative evaluation of EEG-Biomarkers for prediction of sleep stages. Sensors. 2022;22:3079. doi: 10.3390/s22083079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilamala A, Madsen KH, Hansen LK. Deep Convolutional Neural Networks for Interpretable Analysis of EEG Sleep Stage Scoring. In Proceedings of the 2017 IEEE 27th International Workshop on Machine Learning for Signal Processing (MLSP); IEEE, September 2017:1–6.
- 24.Mousavi S, Afghah F, Acharya UR. SleepEEGNet: Automated Sleep Stage Scoring with sequence to sequence Deep Learning Approach. PLoS ONE. 2019;14:e0216456. doi: 10.1371/journal.pone.0216456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supratak A, Dong H, Wu C, Guo Y, DeepSleepNet A model for Automatic Sleep Stage Scoring based on raw Single-Channel EEG. IEEE Trans Neural Syst Rehabil Eng. 2017;25:1998–2008. doi: 10.1109/TNSRE.2017.2721116. [DOI] [PubMed] [Google Scholar]
- 26.Michielli N, Acharya UR, Molinari F. Cascaded LSTM recurrent neural network for automated sleep stage classification using Single-Channel EEG signals. Comput Biol Med. 2019;106:71–81. doi: 10.1016/j.compbiomed.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Alqurashi YD, Morrell MJ, Mandic DP. Hearables: Automatic overnight sleep monitoring with standardized In-Ear EEG sensor. IEEE Trans Biomed Eng. 2020;67:203–12. doi: 10.1109/TBME.2019.2911423. [DOI] [PubMed] [Google Scholar]
- 28.Mikkelsen KB, Tabar YR, Kappel SL, Christensen CB, Toft HO, Hemmsen MC, Rank ML, Otto M, Kidmose P. Accurate whole-night sleep monitoring with dry-contact Ear-EEG. Sci Rep. 2019;9:16824. doi: 10.1038/s41598-019-53115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring sleep: Accuracy, Sensitivity, and specificity of wrist actigraphy compared to Polysomnography. Sleep. 2013;36:1747–55. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for Sleep-Wake Assessment in Sleep Apnea Patients. Sleep. 2004;27:1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 31.Radha M, Fonseca P, Moreau A, Ross M, Cerny A, Anderer P, Long X, Aarts RM. A deep transfer Learning Approach for Wearable Sleep Stage classification with Photoplethysmography. npj Digit Med. 2021;4:135. doi: 10.1038/s41746-021-00510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radha M, Fonseca P, Moreau A, Ross M, Cerny A, Anderer P, Long X, Aarts RM. Sleep stage classification from Heart-Rate Variability using long short-term memory neural networks. Sci Rep. 2019;9:14149. doi: 10.1038/s41598-019-49703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Y, Qi X, Wang H, Liu Z, Wang G, Yan XA, Multi-Class Automatic sleep staging Method based on long short-term Memory Network using single-lead electrocardiogram signals. IEEE Access. 2019;7:85959–70. doi: 10.1109/ACCESS.2019.2924980. [DOI] [Google Scholar]
- 34.Sridhar N, Shoeb A, Stephens P, Kharbouch A, Shimol D, Ben Burkart J, Ghoreyshi A, Myers L. Deep learning for automated sleep staging using Instantaneous Heart Rate. npj Digit. Med. 2020;3:106. doi: 10.1038/s41746-020-0291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonseca P, Weysen T, Goelema MS, Møst EIS, Radha M, Lunsingh Scheurleer C, van den Heuvel L, Aarts RM. Validation of Photoplethysmography-Based sleep staging compared with polysomnography in healthy middle-aged adults. Sleep. 2017;40. 10.1093/sleep/zsx097. [DOI] [PubMed]
- 36.Korkalainen H, Aakko J, Duce B, Kainulainen S, Leino A, Nikkonen S, Afara IO, Myllymaa S, Töyräs J, Leppänen T. Deep learning enables sleep staging from Photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43. 10.1093/sleep/zsaa098. [DOI] [PMC free article] [PubMed]
- 37.Walch O, Huang Y, Forger D, Goldstein C. 2019. Sleep Stage Prediction with Raw Acceleration and Photoplethysmography Heart Rate Data Derived from a Consumer Wearable Device. [DOI] [PMC free article] [PubMed]
- 38.Hedner J, White DP, Malhotra A, Herscovici S, Pittman SD, Zou D, Grote L, Pillar G. Sleep staging based on autonomic signals: a Multi-Center Validation Study. J Clin Sleep Med. 2011;07:301–6. doi: 10.5664/JCSM.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi SH, Yoon H. Convolutional neural networks for the real-time monitoring of vital Signs based on Impulse Radio Ultrawide-Band Radar during Sleep. Sensors. 2023;23:3116. doi: 10.3390/s23063116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman T, Adams AT, Ravichandran RV, Zhang M, Patel SN, Kientz JA, Choudhury T, DoppleSleep:. A Contactless Unobtrusive Sleep Sensing System Using Short-Range Doppler Radar. In Proceedings of the Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing; ACM: New York, NY, USA, September 7 2015:39–50.
- 41.Doheny EP, O’Callaghan BPF, Fahed VS, Liegey J, Goulding C, Ryan S, Lowery MM. Estimation of respiratory rate and Exhale Duration using Audio signals recorded by Smartphone Microphones. Biomed Signal Process Control. 2023;80:104318. doi: 10.1016/j.bspc.2022.104318. [DOI] [Google Scholar]
- 42.Nam Y, Reyes BA, Chon KH. Estimation of respiratory rates using the built-in microphone of a smartphone or headset. IEEE J Biomed Heal Informatics. 2016;20:1493–501. doi: 10.1109/JBHI.2015.2480838. [DOI] [PubMed] [Google Scholar]
- 43.Le VL, Kim D, Cho E, Jang H, Reyes RD, Kim H, Lee D, Yoon IY, Hong J, Kim JW. Real-time detection of Sleep Apnea based on Breathing sounds and prediction reinforcement using home noises: Algorithm Development and Validation. J Med Internet Res. 2023;25:e44818. doi: 10.2196/44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura T, Chen W. Seamless Healthcare Monitoring: Advancements in Wearable, Attachable, and Invisible Devices. Seamless Healthc. Monit. Adv. Wearable, Attach. Invis. Devices 2017.
- 45.Kwon HB, Choi SH, Lee D, Son D, Yoon H, Lee MH, Lee YJ, Park KS. Attention-based LSTM for non-contact sleep stage classification using IR-UWB radar. IEEE J Biomed Heal Informatics. 2021;25:3844–53. doi: 10.1109/JBHI.2021.3072644. [DOI] [PubMed] [Google Scholar]
- 46.Hong H, Zhang L, Gu C, Li Y, Zhou G, Zhu X. Noncontact Sleep Stage Estimation using a CW Doppler Radar. IEEE J Emerg Sel Top Circuits Syst. 2018;8:260–70. doi: 10.1109/JETCAS.2017.2789278. [DOI] [Google Scholar]
- 47.Zhao M, Yue S, Katabi D, Jaakkola TS, Bianchi MT. Learning Sleep Stages from Radio Signals: A Conditional Adversarial Architecture. 34th Int. Conf. Mach. Learn. ICML 2017 2017;8:6205–6214.
- 48.Toften S, Pallesen S, Hrozanova M, Moen F, Grønli J. Validation of Sleep Stage classification using Non-Contact Radar Technology and Machine Learning (Somnofy®) Sleep Med. 2020;75:54–61. doi: 10.1016/j.sleep.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Zaffaroni A, Doheny EP, Gahan L, Ivanov Y, Kilroy H, O’Mahony N, O’Rourke D. Non-Contact Estimation of Sleep Staging. 2018:77–80.
- 50.Dafna E, Tarasiuk A, Zigel Y. Sleep staging using Nocturnal Sound Analysis. Sci Rep. 2018;8:13474. doi: 10.1038/s41598-018-31748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong J, Tran HH, Jung J, Jang H, Lee D, Yoon IY, Hong JK, Kim JW. End-to-end sleep staging using Nocturnal sounds from Microphone chips for Mobile Devices. Nat Sci Sleep. 2022;14:1187–201. doi: 10.2147/NSS.S361270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi SH, Kwon HB, Jin HW, Yoon H, Lee MH, Lee YJ, Park KS. Long short-term memory networks for unconstrained sleep stage classification using Polyvinylidene Fluoride Film Sensor. IEEE J Biomed Heal Informatics. 2020;24:3606–15. doi: 10.1109/JBHI.2020.2979168. [DOI] [PubMed] [Google Scholar]
- 53.Siyahjani F, Garcia Molina G, Barr S, Mushtaq F. Performance evaluation of a Smart Bed Technology against Polysomnography. Sensors. 2022;22:2605. doi: 10.3390/s22072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang S, Lee Y, Jeong D, Park K. Unconstrained sleep stage estimation based on Respiratory Dynamics and Body Movement. Methods Inf Med. 2016;55:545–55. doi: 10.3414/ME15-01-0140. [DOI] [PubMed] [Google Scholar]
- 55.Ding F, Cotton-Clay A, Fava L, Easwar V, Kinsolving A, Kahn P, Rama A, Kushida C. Polysomnographic validation of an Under-Mattress monitoring device in estimating Sleep Architecture and Obstructive Sleep Apnea in adults. Sleep Med. 2022;96:20–7. doi: 10.1016/j.sleep.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Tal A, Shinar Z, Shaki D, Codish S, Goldbart A. Validation of contact-free sleep monitoring device with comparison to Polysomnography. J Clin Sleep Med. 2017;13:517–22. doi: 10.5664/jcsm.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavlova K, Latreille M, Sleep Disorders V. Am J Med. 2019;132:292–9. doi: 10.1016/j.amjmed.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Laval U, City Q, Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379:1129–70. doi: 10.1016/S0140. [DOI] [PubMed] [Google Scholar]
- 59.Punjabi NM. The Epidemiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008;5:136–143. [DOI] [PMC free article] [PubMed]
- 60.Yeo M, Byun H, Lee J, Byun J, Rhee HY, Shin W, Yoon H. Respiratory event detection during Sleep using Electrocardiogram and Respiratory related signals: using Polysomnogram and Patch-Type Wearable device data. IEEE J Biomed Heal Informatics. 2021 doi: 10.1109/JBHI.2021.3098312. [DOI] [PubMed] [Google Scholar]
- 61.Yeo M, Byun H, Lee J, Byun J, Rhee HY, Shin W, Yoon H. Robust method for screening sleep apnea with single-lead ECG using deep residual network: evaluation with Open Database and Patch-Type Wearable device data. IEEE J Biomed Heal Informatics. 2022;26:5428–38. doi: 10.1109/JBHI.2022.3203560. [DOI] [PubMed] [Google Scholar]
- 62.Hsu YS, Chen TY, Wu D, Lin CM, Juang JN, Liu W. Te Screening of Obstructive Sleep Apnea in Patients who snore using a Patch-Type device with Electrocardiogram and 3-Axis Accelerometer. J Clin Sleep Med. 2020;16:1149–60. doi: 10.5664/jcsm.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang HC, Wu HT, Huang PC, Ma HP, Lo YL, Huang YH. Portable sleep apnea syndrome screening and event detection using long short-term memory recurrent neural network. Sensors. 2020;20:1–17. doi: 10.3390/s20216067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng SSS, Chan TO, To KW, Ngai J, Tung A, Ko FWS, Hui DSC. Validation of a Portable Recording device (ApneaLink) for identifying patients with suspected obstructive sleep Apnoea Syndrome. Intern Med J. 2009;39:757–62. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 65.Lin YY, Wu HT, Hsu CA, Huang PC, Huang YH, Lo YL. Sleep apnea detection based on thoracic and Abdominal Movement signals of wearable Piezoelectric bands. IEEE J Biomed Heal Informatics. 2017;21:1533–45. doi: 10.1109/JBHI.2016.2636778. [DOI] [PubMed] [Google Scholar]
- 66.Van Steenkiste T, Groenendaal W, Dreesen P, Lee S, Klerkx S, De Francisco R, Deschrijver D, Dhaene T. Portable detection of apnea and hypopnea events using Bio-Impedance of the chest and deep learning. IEEE J Biomed Heal Informatics. 2020;24:2589–98. doi: 10.1109/JBHI.2020.2967872. [DOI] [PubMed] [Google Scholar]
- 67.Choi JH, Lee B, Lee JY, Kim HJ. Validating the Watch-PAT for diagnosing obstructive sleep apnea in adolescents. J Clin Sleep Med. 2018;14:1741–7. doi: 10.5664/jcsm.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papini GB, Fonseca P, van Gilst MM, Bergmans JWM, Vullings R, Overeem S. Wearable monitoring of sleep-disordered breathing: estimation of the apnea–hypopnea index using wrist-worn reflective photoplethysmography. Sci Rep. 2020;10:1–15. doi: 10.1038/s41598-020-69935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Wang W, Guo Y, Zhang H, Chen Y, Xie L. A single-center validation of the Accuracy of a photoplethysmography-based Smartwatch for Screening Obstructive Sleep Apnea. Nat Sci Sleep. 2021;13:1533–44. doi: 10.2147/NSS.S323286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu W, Leung L, Kwok KC, Wu IC, Folz RJ, Chiang AA. Belun Ring platform: a Novel Home Sleep Apnea Testing System for Assessment of Obstructive Sleep Apnea. J Clin Sleep Med. 2020;16:1611–7. doi: 10.5664/jcsm.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao R, Xue J, Zhang X, Peng M, Li J, Zhou B, Zhao L, Penzel T, Kryger M, Dong XS, et al. Comparison of Ring Pulse Oximetry using reflective photoplethysmography and PSG in the detection of OSA in chinese adults: a pilot study. Nat Sci Sleep. 2022;14:1427–36. doi: 10.2147/NSS.S367400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwon HB, Son D, Lee D, Yoon H, Lee MH, Lee YJ, Choi SH, Park KS, Choi SH. Hybrid CNN-LSTM Network for Real-Time apnea-hypopnea event detection based on IR-UWB radar. IEEE Access. 2022;10:17556–64. doi: 10.1109/ACCESS.2021.3081747. [DOI] [Google Scholar]
- 73.Zhou Y, Shu D, Xu H, Qiu Y, Zhou P, Ruan W, Qin G, Jin J, Zhu H, Ying K, et al. Validation of Novel Automatic Ultra-Wideband Radar for Sleep Apnea Detection. J Thorac Dis. 2020;12:1286–95. doi: 10.21037/jtd.2020.02.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang S, Kim DK, Lee Y, Lim YH, Park HK, Cho SH, Cho SH. Non-contact diagnosis of obstructive sleep apnea using impulse-radio Ultra-Wideband Radar. Sci Rep. 2020;10. 10.1038/s41598-020-62061-4. [DOI] [PMC free article] [PubMed]
- 75.Baboli M, Singh A, Soll B, Boric-Lubecke O, Lubecke VM. Wireless Sleep Apnea Detection using continuous Wave Quadrature Doppler Radar. IEEE Sens J. 2020;20:538–45. doi: 10.1109/JSEN.2019.2941198. [DOI] [Google Scholar]
- 76.Zhuang Z, Wang F, Yang X, Zhang L, Fu CH, Xu J, Li C, Hong H. Accurate Contactless Sleep Apnea Detection Framework with Signal Processing and Machine Learning Methods. Methods. 2022;205:167–78. doi: 10.1016/j.ymeth.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Choi JW, Kim DH, Koo DL, Park Y, Nam H, Lee JH, Kim HJ, Hong SN, Jang G, Lim S, et al. Automated detection of Sleep Apnea-Hypopnea events based on 60 GHz frequency-modulated Continuous-Wave Radar using Convolutional recurrent neural networks: a preliminary Report of a prospective cohort study. Sensors. 2022;22. 10.3390/s22197177. [DOI] [PMC free article] [PubMed]
- 78.Nakano H, Hirayama K, Sadamitsu Y, Toshimitsu A, Fujita H, Shin S, Tanigawa T. Monitoring sound to Quantify Snoring and Sleep Apnea Severity using a smartphone: Proof of Concept. J Clin Sleep Med. 2014;10:73–8. doi: 10.5664/jcsm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castillo-Escario Y, Ferrer-Lluis I, Montserrat JM, Jane R. Entropy Analysis of Acoustic signals recorded with a smartphone for detecting Apneas and Hypopneas: a comparison with a Commercial System for Home Sleep Apnea diagnosis. IEEE Access. 2019;7:128224–41. doi: 10.1109/ACCESS.2019.2939749. [DOI] [Google Scholar]
- 80.Tiron R, Lyon G, Kilroy H, Osman A, Kelly N, O’Mahony N, Lopes C, Coffey S, McMahon S, Wren M, et al. Screening for obstructive sleep apnea with Novel Hybrid Acoustic Smartphone App Technology. J Thorac Dis. 2020;12:4476–95. doi: 10.21037/jtd-20-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hwang SH, Lee HJ, Yoon HN, Jung DW, Lee YJG, Lee YJ, Jeong DU, Park KS. Unconstrained sleep apnea monitoring using Polyvinylidene Fluoride Film-Based Sensor. IEEE Trans Biomed Eng. 2014;61:2125–34. doi: 10.1109/TBME.2014.2314452. [DOI] [PubMed] [Google Scholar]
- 82.Mora GG, Kortelainen JM, Hernández ERP, Tenhunen M, Bianchi AM, Méndez MO. Evaluation of pressure Bed Sensor for Automatic SAHS Screening. IEEE Trans Instrum Meas. 2015;64:1935–43. doi: 10.1109/TIM.2014.2366976. [DOI] [Google Scholar]
- 83.Mosquera-Lopez C, Leitschuh J, Condon J, Hagen CC, Rajhbeharrysingh U, Hanks C, Jacobs PG. Design and evaluation of a Non-Contact Bed-Mounted sensing device for automated in-home detection of obstructive sleep apnea: a pilot study. Biosensors. 2019;9. 10.3390/bios9030090. [DOI] [PMC free article] [PubMed]
- 84.Perez-Pozuelo I, Zhai B, Palotti J, Mall R, Aupetit M, Garcia-Gomez JM, Taheri S, Guan Y, Fernandez-Luque L. The future of Sleep Health: A Data-Driven Revolution in Sleep Science and Medicine. npj Digit Med. 2020;3:42. doi: 10.1038/s41746-020-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dean DA, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, Sahoo SS, Jayapandian CP, Cui L, Morrical MG, et al. Scaling up Scientific Discovery in Sleep Medicine: the National Sleep Research Resource. Sleep. 2016;39:1151–64. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldberger AL, Amaral LAN, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet. Circulation. 2000;101. 10.1161/01.CIR.101.23.e215. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Walch O, Huang Y, Forger D, Goldstein C. 2019. Sleep Stage Prediction with Raw Acceleration and Photoplethysmography Heart Rate Data Derived from a Consumer Wearable Device. [DOI] [PMC free article] [PubMed]