Abstract
As the blood–brain barrier (BBB) hinders efficient drug delivery to the brain, drug delivery via the intranasal pathway, bypassing the BBB, has received considerable attention. However, intranasal administration still has anatomical and physiological limitations, necessitating further solutions to enhance effectiveness. In this study, we used transcranial magnetic stimulation (TMS) on fluorescent magnetic nanoparticles (MNPs) of different sizes (50, 100, and 300 nm) to facilitate MNP’s transportation and delivery to the brain parenchyma. To validate this concept, anesthetized rats were intranasally injected with the MNPs, and TMS was applied to the center of the head. As the result, a two-fold increase in brain MNP delivery was achieved using TMS compared with passive intranasal administration. In addition, histological analysis that was performed to investigate the safety revealed no gross or microscopic damages to major organs caused by the nanoparticles. While future studies should establish the delivery conditions in humans, we expect an easy clinical translation in terms of device safety, similar to the use of conventional TMS. The strategy reported herein is the first critical step towards effective drug transportation to the brain.
Keywords: Intranasal administration, Transcranial magnetic stimulation, Magnetic force, Magnetic targeted delivery, Magnetic drug targeting
Introduction
Drug delivery to the brain is one of the most challenging unmet needs for treating neurological diseases. The blood–brain barrier (BBB) is a unique structure surrounding the brain and primarily protects the central nervous system (CNS) from noxious substances present in the circulatory system [1, 2]. However, the BBB also significantly impedes drug delivery by preventing the entry of 100% large- and more than 98% small-molecule therapeutics [3, 4]. Consequently, drug development for brain diseases has the poorest success rate out of that for all other diseases [5]. Typically, drugs are administered orally or intravenously; however, they are mostly blocked by the BBB before reaching the brain [2, 6]. Accordingly, numerous neurologically active substances and drugs developed for similar non-CNS diseases were effective in the pre-clinical stage but failed to treat CNS diseases during clinical trials.
Contrary to oral or intravenous drug administration, intranasal administration allows for direct drug delivery to the brain from the nose, avoiding the need to cross the BBB [3, 4, 7–13]. Neuronal bundles from the olfactory epithelium reach the CNS via numerous pores in the cribriform plate of the ethmoid bone [7, 8]. Therefore, pharmaceutical agents can pass through the pores along the olfactory nerves, reaching the brain tissue through the olfactory bulb [3, 4, 7–10, 13]. The olfactory region is the only part of the body where the CNS is in contact with the peripheral environment and has recently attracted attention as an effective neurological drug delivery pathway[3]. Thus, the direct nose-to-brain pathway has significant potential in the development of noninvasive modalities that can bypass the BBB, thereby allowing for the direct delivery of pharmaceutical agents to brain tissues. Additionally, this pathway avoids drug degradation through systemic clearance, and the large surface area of the olfactory epithelium allows for effective drug absorption [3, 7, 10, 14, 15]. Despite these evident advantages, there are limitations to intranasal administration. The residence time of therapeutics is short owing to rapid mucociliary clearance, thereby significantly reducing the amount of therapeutics reaching the brain [6, 7]. To reduce mucociliary clearance, increase therapeutic agent bioavailability, and protect against enzymatic degradation in the nasal cavity, various studies have attempted to improve drug delivery via the intranasal pathway using nanocarriers [7, 8, 10–12, 14, 15]. Additionally, the release of therapeutic agents from nanoparticles can be controlled by physical stimuli, pH, temperature, or chemical interactions [7, 16]. However, even with the use of a nanocarrier, delivery efficiency to the olfactory regions in the upper and posterior part of the nose is extremely low (< 1%) [7, 17]. Therefore, the development of new strategies to significantly enhance the effectiveness of intranasal drug delivery to the brain is crucial for the treatment of neurological diseases.
Magnetic drug targeting (MDT) technologies may be a promising strategy to enhance drug accumulation in target tissues as magnetic drug carriers can be attracted to the target areas with externally applied magnetic forces [18–24]. Magnetic nanoparticles (MNPs) can be manipulated by the gradients of externally applied magnetic fields, such that they are pulled, pushed, and concentrated into tissues [25]. Therefore, MDT strategies may guide magnetic nanocarriers to the olfactory region and increase therapeutic residence time via applied magnetic forces. Currently, MDT employs permanent magnets to generate MNP-manipulating magnetic forces because magnet utilization is cost-effective, versatile, and does not require complicated circuits or sophisticated control algorithms [18–22]. However, the use of static magnets restricts efficacy to shallow targets, such as superficial tumors or in small-animal experiments. As the static magnetic field strength rapidly decreases as the distance from the magnet increases, MNP delivery to relatively deep targets distant from the magnetic surface is difficult [22, 26]. Therefore, a fundamental limitation exists; MNPs cannot be directed to deep regions or a central target between an array of magnets, regardless of their arrangement [18, 23]. To reach deeper targets, several studies have investigated magnetic materials implanted in the body [27]. However, magnetic implants are neither suitable for every biological condition nor patient, as the positioning of the magnets requires additional procedures [23]. Therefore, targeting MNPs to deep tissues, particularly the brain parenchyma, using permanent magnets is still impracticable. Additionally, although the technique elicits significant effects in small animals, the replication of such a magnet system on a clinical scale, capable of generating sufficiently strong magnetic forces, is challenging [28].
In this study, we propose that externally applied time-varying magnetic fields generated by transcranial magnetic stimulation (TMS) devices offer a practical solution to overcome the limitations of current MDT methods and improve the intranasal delivery of injected MNPs to brain tissues. When placed over the scalp, the TMS coil generates time-varying magnetic fields that can pass through the skull and induce magnetic fields in the brain tissues. The induced magnetic field intensity is easily controlled by the amplitude of currents flowing in the coil. Moreover, magnetic field distribution is determined by either the design or placement of the coil [29–31]. In addition, TMS can induce a magnetic field sufficient for nerve stimulation in deep brain tissues, further supporting the notion that TMS may allow MNP delivery to deep brain regions [31]. However, herein, TMS was only used to apply a magnetic force which is proportional to the gradient of the induced magnetic field to guide MNPs, as opposed to neurostimulation. TMS-induced neurostimulation is regarded as an undesired effect during drug delivery, and thus, the identification of the proper intensity to facilitate MNP delivery without causing neurostimulation was considered in this study. To the best of our knowledge, this is the first study attempting to deliver MNPs while utilizing a clinical TMS system; therefore, we aimed to identify the ideal conditions to successfully facilitate intranasal transport of MNPs to the brain parenchyma. As TMS use is widely accepted in clinical practice and its safety has been established, this technology can be easily adapted to human application after validating its effectiveness. To accomplish this goal, this study provides novel insights acting as an initial critical step.
Methods
Materials
MNPs selected for this study were commercially available from Micromod, Germany. These MNPs with diameters of approximately 50, 100, and 300 nm were cross-linked with dextran iron oxide composite particles, with an iron oxide core of circa of 75–80% (w/w) and a polydispersity index of < 0.2, as provided by the manufacturer. The MNP saturation magnetization values provided were 110, 76, and 70 Am2/kg for diameter of 50, 100, and 300 nm, respectively. Furthermore, the MNP optical properties were far-red fluorescent dye (DY-730), with the excitation and emission wavelengths of 732 and 758 nm, respectively.
In vivo study design
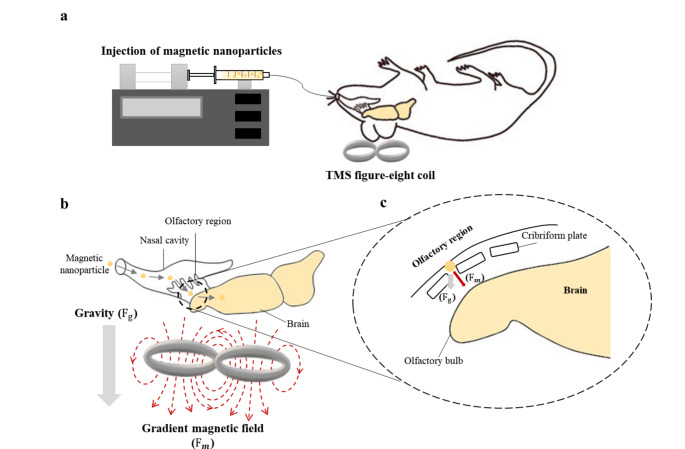
All animal research procedures were approved by the Pohang University of Science and Technology Institutional Animal Care and Use Committee (POSTECH-2021-0117). Sprague–Dawley male rats, aged 8–10 weeks and weighing 230–320 g, were used (n = 64). Rats were anesthetized by intraperitoneal urethane injection (1.2–1.5 g/kg) before a solution containing fluorescent MNPs (150 µL) was injected via the nasal cavity using a syringe pump (NE-4000, New Era Pump Systems Inc., USA) and polyethylene tube (SP-10, Natsume Seisakusho Co., Ltd., Japan) (Fig. 1a). The external magnetic field was applied during the injection to attract the MNPs through the TMS coil (Brain-Stim-A, REMED, South Korea) placed over the head of each rat (Fig. 1b). The magnetic force applied to the MNPs in the external magnetic field was calculated as follows:
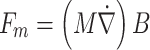 |
Fig. 1.
a Schematic of the experimental setup. b Representation of the flow of the magnetic nanoparticles from the nasal cavity to the brain through the application of external forces. c Zoomed-in view of the magnetic particles passing through the cribriform plate
where M denotes the magnetization of particles and 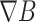 is the magnetic field gradient (T/m) [32].
is the magnetic field gradient (T/m) [32].
Before the experiment commenced, the threshold intensity was defined based on motor-evoked potential (MEP) measurements. In TMS, MEP is used as an indicator for motor cortex excitability [33, 34], and the resting motor threshold (rMT) refers to the lowest TMS intensity that evokes MEP. During TMS neurostimulation, the rMT stimulus intensity usually ranges from 80 to 130% of the rMT [33, 35]. However, in this study, TMS is used to guide MNPs through a generated external magnetic field, not neurostimulation; thus, a considerably lower rMT intensity was selected. MEPs were recorded in four rats by inserting a 26G stainless steel needle electrode (EL450, BIOPAC Systems Inc., USA), connected to the data acquisition system (MP160, BIOPAC Systems Inc.), into the first dorsal internal muscle of the right forelimb during TMS application to the contralateral motor cortex (Brain-Stim-A, REMED). This device provides a magnetic intensity range (0–100%) that is adjustable in 5% increments. In this study, the maximum intensity of 50% rMT was used for attracting MNPs.
During the MNP delivery experiment, the rat was placed in supine position with the center of the TMS coil placed at the center of the head. The TMS-induced external magnetic field was generated during MNP injection to enhance particle transfer via the cribriform plate to the brain via both gravity and magnetic forces (Fig. 1b, c). The sham group was injected with MNPs under the same conditions but in the absence of TMS; thus, the particles were only affected by gravity (passive delivery). Because this is the first study on intranasally injected MNP delivery to the brain using TMS, we determined the stimulation intensity and injection rate of fluorescent MNPs (diameter, 50 nm) to establish their ideal brain delivery conditions. Particle size can significantly affect the fate of nanoparticles once they are introduced into the body [14, 24, 25]. Thus, the biodistribution of fluorescent MNPs was investigated using 50- (synomag-CLD-far redF, Micromod, Germany), 100- (BNF-Dextran-far redF, Micromod), and 300-nm (nanomag-CLD-far redF, Micromod) particles. All particles were purchased from the same manufacturer and have equal optical properties. Immediately after the experiment, the rats were euthanized via heart puncture.
Fluorescence imaging and statistics
After euthanization, major organs, including the brain, heart, lungs, liver, kidneys, spleen, pancreas, and small intestine, were rapidly harvested. Following fixation with 4% paraformaldehyde (PC2031, BIOSESANG, South Korea), fluorescence signals from the organs were analyzed using a fluorescence-labeled organism bio-imaging instrument (FOBI, NeoScience, South Korea). To detect fluorescence signals, a filter containing 750 ± 25 nm excitation filters, 810 ± 20 nm filters, and 785 nm dichroic mirror was used. The total fluorescence intensity of the region of interest (ROI) in each organ was measured by NIR channel with 730 nm excitation and 820 nm emission filter. Individual groups were compared using a one-way t-test. All data are presented as the mean ± SD, and statistical significance was set at p < 0.05.
Histological evaluation
For histological evaluation, the harvested organs were dehydrated in 30% sucrose solution before being embedded in tissue-freezing medium at − 20 °C. All organs were cryosectioned in 6 μm-thick slices. The pathological sections were stained with hematoxylin and eosin (H&E) to investigate tissue damage and scanned using a Scanscope AT (Aperio, USA).
Computational simulation
Particle movement in the magnetic fields generated by a TMS coil or magnet was compared using the COMSOL Multiphysics software version 5.6 (COMSOL Inc., USA) under the assumption that they have the same dimensions and generate the same peak magnetic field intensity. The typical figure-eight TMS coil shape was modeled with a diameter of 2 × 73.5 mm with a wire thickness of 10 mm. For size consistency, the magnet was modeled as a block with a width of 93.5 mm, depth of 187 mm, and height of 20 mm. The background domain was modeled as a sphere with a radius of 200 mm; the materials were designated as air, and the outer layer as an infinite element domain. The human head phantom was modeled as a sphere with a radius of 85 mm and an outer layer of 15 mm [29]. The material of the outer layer was set as skull bone with a conductivity of 0.02 S/m, whereas the inner sphere was set as brain tissue with a conductivity of 0.37 S/m [36]. Finally, the particles were released along the x-axis of the inner sphere.
Results
Brain delivery and biodistribution analyses
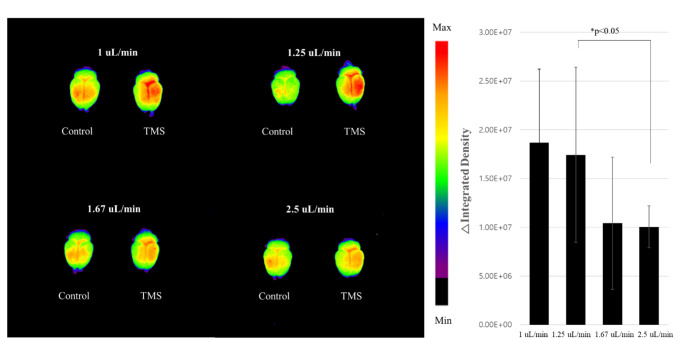
The MEP was first measured in four rats, and the average rMT of the TMS device was identified. Based on this measurement, 25% and 50% of the average rMT were determined for a TMS intensity of dragging MNPs. A stronger magnetic field results in better MNP transportation; however, the maximum intensity of our experiment was set to 50% rMT, which is considerably lower than the TMS intensity used for neurostimulation in clinical practice [33, 35]. Here, 150 µL of MNPs was injected at a rate of 1 µL/min for 150 min during the application of a TMS-generated magnetic field. As shown in Fig. 2, our results demonstrate that fluorescence signals from the fluorescent MNPs in the brain were significantly increased in the TMS group than in the sham control group, even when only 25% rMT was applied. When 50% rMT was applied, the fluorescence signal in the brain tissues of the TMS group presented a near two-fold increase compared to that in the sham group. Subsequent experiments were conducted using 50% rMT to deliver more MNPs to the brain.
Fig. 2.

Fluorescence signals of the brain dependent on TMS intensity during intranasal injection of 50 nm sized fluorescent MNPs for 150 min at 1 µL/min (n = 4 per group)
Additionally, we investigated the MNP delivery efficiency dependent on flow rate and total stimulation time. The total volume of the MNP solution was fixed at 150 µL because it is the maximum injectable volume without overflowing the rat nasal cavity. The fluorescence in the brain was compared at an MNP injection rate and TMS exposure time of 2.5 µL/min and 60 min, 1.67 µL/min and 90 min, 1.25 µL/min and 120 min, and 1 µL/min and 150 min, respectively. Moreover, fluorescence intensity was compared between the TMS and sham control groups according to each injection rate. As shown in Fig. 3, similar results were observed for the relatively fast (2.5 and 1.67 µL/min) and relatively slow injection rates (1.25 and 1 µL/min). A significant difference was observed between the 2.5 µL/min injection rate for 60 min and the 1.25 µL/min injection rate for 120 min. Accordingly, the 1.25 µL/min MNP injection rate for 120 min was selected for subsequent experiments as it provides the best trade-off between shorter duration and effective MNP delivery.
Fig. 3.
Brain fluorescence signals and their difference between control and TMS groups, depending on the injection rate of the fluorescence magnetic nanoparticles (n = 4 per group, except for the “1.25 µL/min” group with n = 8)
Finally, the fluorescence intensities of the brain and other organs were investigated relative to MNP size. In the brain, results suggest that a higher transmission efficacy is associated with smaller particles (Fig. 4). When comparing the difference in fluorescence signals between the TMS group and the control, a significant transmission increase was demonstrated by the 50-nm particles compared with that of the 100-nm and 300-nm particles (Fig. 4a). Although biodistribution is dependent on particle size, there was no significant difference between the organs of the sham and TMS groups, except for the brain. Moreover, the greatest fluorescence intensity in all the groups was expressed in the liver and kidneys (Fig. 4b).
Fig. 4.
a Brain fluorescence signals and their differences between control and TMS groups, depending on the fluorescent magnetic nanoparticles size and b biodistribution analysis of organs of interest (n = 4 per group, except for the “50 nm” group with n = 8)
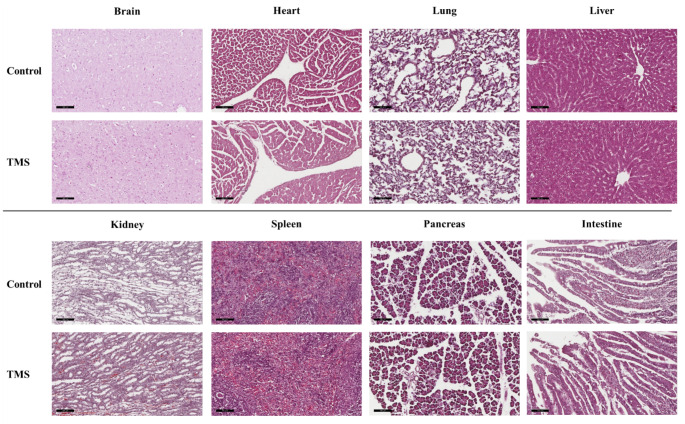
Histological analysis
To evaluate whether MNPs can cause tissue damage, inflammation, or lesion, we reviewed H&E-stained tissue sections from the brain, heart, lung, liver, kidney, pancreas, spleen, and intestine. As shown in Fig. 5, no gross or microscopic anomalies were observed in any of the organs. Moreover, these presented no evident signs of inflammation or fibrosis. Despite the relatively high levels of nanoparticles in the liver and kidney, no significant lesions were observed. Overall, these results indicate that our experimental protocol is well tolerated and represents a potential brain-targeting drug delivery strategy [37].
Fig. 5.
Histological evaluation of hematoxylin and eosin-stained tissue sections from major organs of control and TMS-treated group (200×, scale bar 100 μm)
Simulation of particle trajectories
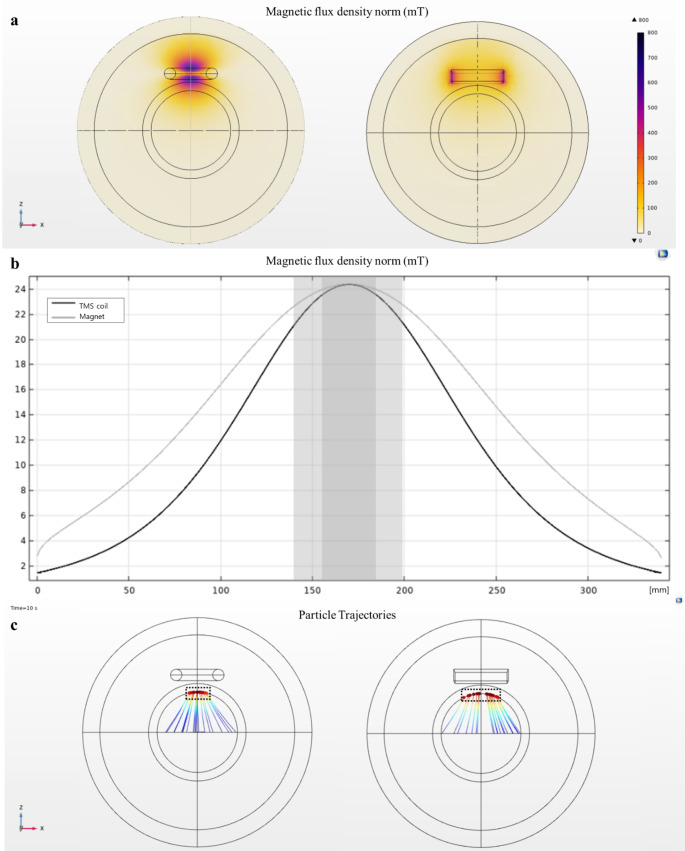
Particle movement generated by the coils and magnets was analyzed with identical total dimension and maximum magnetic field intensity reaching the target area. When forming an identical peak magnetic flux density in the middle of the phantom sphere, the magnet generated the strongest magnetic field at the edges, whereas the coil generated the strongest magnetic field at the center (Fig. 6a). This may be ascribed to the characteristic of the figure-eight coil, where the field size is strongest at the center point where a pair of coils meets. Furthermore, the magnetic field generated from the coil was strongly distributed beyond the skull, whereas the magnetic field generated from the magnet formed around the surface. When examining the field strength along the z-axis, the gradient of the magnetic field formed by the coil was considerably steeper than that of the magnet (Fig. 6b). Using this field distribution, we tracked the movement of particles released along the x-axis in the middle of the phantom sphere. As indicated in Fig. 6c, the particle distribution that reached the upper region of the phantom sphere under the magnetic field generated by the coil was twice as narrow as that generated by the magnet.
Fig. 6.
Comparison of simulation results between the magnet and figure-eight coil. a Magnetic field distribution when peak intensity is the same as 500 mT and zoomed-in view of the hemisphere with a field range of 100–500 mT. b Field strength along the z-axis of the phantom sphere. c Particle accumulation tracking in the upper region
Discussion
Developing CNS drugs and enhancing their transportation to deep brain tissues has been a primary goal of pharmaceutical approaches in brain disorder treatment. In this study, we proposed a novel strategy to enhance the intranasal drug delivery to the deep brain tissues using TMS-induced magnetic fields to attract MNPs. Compared to conventional MDT methods that use permanent magnets, TMS is significantly advantageous: magnetic field intensity can easily be controlled, and various field distributions can be formed through the design and placement of the coil. When using external magnets, field distribution and intensity regulation are particularly difficult [5, 23]. Because of these limitations, the use of MDT is currently restricted to superficial targets. Moreover, animal-to-human translation is difficult, owing to the considerably larger working distance required in humans and, thus, stronger magnetic fields. Contrarily, TMS is already used in clinical settings for several neurological diseases, demonstrating that it can safely induce strong magnetic fields in the brain parenchyma without any additional scaled-up equipment to adapt from animal models to humans.
In this study, a TMS device was used to generate strong magnetic fields for attracting MNPs to brain tissues. Because TMS induces time-varying magnetic fields, as opposed to static magnets, several parameters had to be optimized for successful MNP delivery to the brain. TMS can generate high-intensity fields because it is generally used for clinical neurostimulation (magnetic field range: 1–2 T; electric field: >100 V/m) [38]. However, in this study, TMS was utilized to generate a magnetic field able to move MNPs, and nerve stimulation had to be avoided. Therefore, we used an intensity lower than 50% rMT. The fluorescence intensity in the brain was significantly increased in the TMS group than in the sham group (Fig. 2). Additionally, it was confirmed that the MNPs were transmitted more efficiently as the magnetic field strength increased. Compared to the sham group, the fluorescence signal in the brain increased 1.5- and 1.8-fold when TMS was applied at 25% and 50% rMT, respectively. Considering that TMS uses 80–130% rMT for neurostimulation [33, 35], these results indicate that the proposed modality can deliver particles to the brain parenchyma with a relatively weak pulsed magnetic field compared with that used in the clinic. Moreover, it was confirmed that the TMS intensity used in this study did not elicit MEPs in rats. After determining the intensity for MNP delivery, delivery efficiency was evaluated based on flow rate. The faster the infusion rate, the better to reduce total treatment time; however, our results indicated that the amount of MNPs delivered to the brain was significantly decreased at injection rates faster than 1.25 µL/min (Fig. 3). A clear trend arose; the slower the injection rate, the greater the fluorescence intensity in the brain. Because TMS generates a short-pulsed rather than static magnetic field, a rapid flow rate might result in the magnetic particle being insufficiently exposed to evoke a response. Lastly, the biodistribution of fluorescence intensity based on MNP size was evaluated. Smaller magnetic nanoparticles are considerably easier to magnetize than larger ones [24]. Our results clearly demonstrate that smaller MNPs are more efficiently delivered to the brain (Fig. 4). Furthermore, olfactory axons have a diameter < 100 nm, and only nanoparticles smaller than 100 nm can be transported passively via these axons to the brain [4]. However, through the TMS coil-induced magnetic force, we observed a significant increase in fluorescent signals using 50-, 100-, and 300-nm MNPs. This indicates that even large-sized nanoparticles can pass through the cribriform plate under the influence of the magnetic force. Moreover, there were no significant differences between organs in the sham and TMS groups other than the brain. Similar to previous studies, the highest MNP concentration was observed in the liver and kidneys [39, 40]; these accumulations may be subsequently degraded or filtered out during extended investigations.
We herein demonstrated the possibility of TMS utilization in MDT to the brain by minimizing the associated magnetic field intensity (≤ 50%). Subsequent studies may utilize TMS to control magnetic field vectors, thereby maneuvering the movement of MNP-conjugated drugs. Moreover, as TMS coil-induced magnetic field focality and depth may be adjusted by coil design [29, 36], MNPs can be more precisely manipulated through various coil designs. The figure-eight coil is the most widely used and provides focal magnetic fields at the center of the coil [29, 30]. From the magnetic field distribution and particle trajectory simulation, we confirmed that the figure-eight coil can generate a deeper and narrower magnetic field distribution than the magnet. Moreover, at identical coil and magnet dimension and peak magnetic field intensity, MNPs were focused at the center of the figure-eight coil, while distributed over the entire surface of the magnet (Fig. 6). In the future, additional TMS effects can also be expected, such as modulating neuro-excitability by selecting parameters according to diseases [41] or controlling drug release through electromagnetic signal interactions [42]. In future studies, we aim to conduct pharmaceutical experiments with MNP-conjugated drugs. For example, Parkinson’s disease is a movement disorder caused by selective loss of dopaminergic neurons in the substantia nigra pars compacta. The representative treatment for Parkinson’s disease is to enhance the level of dopamine in the brain, and the MNP-conjugated drugs suggested by our study can be a strong candidate to increase the efficiency of dopamine delivery to specific regions of the brain as a novel treatment option. However, this short-term discrete model study could not evaluate whether the particles are removed by biodegradation or systematic clearance; therefore, long-term biodistribution observations are required in future studies. Despite these limitations, we believe this study provides a new approach to brain-targeted magnetic drug delivery for improving delivery efficiency.
In this study, we proposed the use of the TMS coil to overcome the limitations of magnet use in existing MDT methods and to efficiently deliver drugs to the brain tissues. To demonstrate this, magnetic fields were applied by placing the TMS coil at the center of the rat head in concomitance with fluorescent magnetic particle injection. This study revealed that TMS-induced pulsed magnetic fields significantly increase the amount of MNPs delivered to the brain compared to the sham group, in which passive nasal delivery was employed. We thus suggest a novel application of the TMS device and expect that the translation of these results to the clinic will be easily achieved in the future because TMS is already widely used in clinical practice. Our findings could be extended to drug delivery studies aimed at treating brain diseases with MNP-conjugated drugs, thereby providing a novel pathway for treating brain disorders with greater efficiency.
Acknowledgements
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2022M3C1A3081294), National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C2005385 and 2022R1A2C2092821), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03047902).
Author contributions
All authors contributed intellectually to the research. Eunbi Ye and Eunkyoung Park designed this study. The experiments were performed by Eunbi Ye and supported by Eunseon Kim. Jung Eun Lee provided advices for the experiments. All results were analyzed by Eunbi Ye, who also prepared the manuscript. Sung-Min Park, Eunkyoung Park, and Seung Ho Yang reviewed the manuscript critically for its intellectual content. All authors have read and approved the manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Statements and Declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
All animal research procedures have been approved by the Pohang University of Science and Technology Institutional Animal Care and Use Committee (POSTECH-2021-0117).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eunbi Ye and Eunkyoung Park contributed equally to this work as first authors.
Contributor Information
Seung Ho Yang, Email: 72ysh@catholic.ac.kr.
Sung-Min Park, Email: sungminpark@postech.ac.kr.
References
- 1.van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012;7:383–94. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast. 2015;2015:708306. doi: 10.1155/2015/708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvaraj K, Gowthamarajan K, Karri VVSR. Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif Cells Nanomed Biotechnol. 2018;46:2088–95. doi: 10.1080/21691401.2017.1420073. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Minko T. Nanotherapeutics for nose-to-brain drug delivery: an approach to bypass the blood brain barrier. Pharmaceutics. 2021;13. 10.3390/pharmaceutics13122049. [DOI] [PMC free article] [PubMed]
- 5.Mohseni M, Connell JJ, Payne C, Patrick PS, Baker R, Yu Y, Siow B, Zaw-Thin M, Kalber TL, Pankhurst QA, Lythgoe MF. Scalable magnet geometries enhance tumour targeting of magnetic nano-carriers. Mater Des. 2020;191:108610. doi: 10.1016/j.matdes.2020.108610. [DOI] [Google Scholar]
- 6.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa CP, Moreira JN, Sousa Lobo JM, Silva AC. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta Pharm Sin B. 2021;11:925–40. doi: 10.1016/j.apsb.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379:146–57. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Chapman CD, Frey WH 2nd, Craft S, Danielyan L, Hallschmid M, Schiöth HB, et al. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30:2475–84. 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed]
- 10.Sabir F, Ismail R, Csoka I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: Status quo and outlook. Drug Discov Today. 2020;25:185–94. doi: 10.1016/j.drudis.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Youssef NAHA, Kassem AA, Farid RM, Ismail FA, El-Massik MAE, Boraie NA. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int J Pharm. 2018;548:609–24. doi: 10.1016/j.ijpharm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Alam S, Khan ZI, Mustafa G, Kumar M, Islam F, Bhatnagar A, Ahmad FJ. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomedicine. 2012;7:5705–18. doi: 10.2147/IJN.S35329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander A, Saraf S. Nose-to-brain drug delivery approach: a key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen Res. 2018;13:2102–4. doi: 10.4103/1673-5374.241458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alavian F, Shams N. Oral and intra-nasal administration of nanoparticles in the cerebral ischemia treatment in animal experiments: considering its advantages and disadvantages. Curr Clin Pharmacol. 2020;15:20–9. doi: 10.2174/1574884714666190704115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali J, Ali M, Baboota S, Sahani JK, Ramassamy C, Dao L. Bhavna. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr Pharm Des. 2010;16:1644–53. doi: 10.2174/138161210791164108. [DOI] [PubMed] [Google Scholar]
- 16.Seo HI, Cheon YA, Chung BG. Graphene and thermo-responsive polymeric nanocomposites for therapeutic applications. Biomed Eng Lett. 2016;6:10–5. doi: 10.1007/s13534-016-0214-6. [DOI] [Google Scholar]
- 17.Si XA, Xi J, Kim J, Zhou Y, Zhong H. Modeling of release position and ventilation effects on olfactory aerosol drug delivery. Respir Physiol Neurobiol. 2013;186:22–32. doi: 10.1016/j.resp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Hour FQ, Moghadam AJ, Shakeri-Zadeh A, Bakhtiyari M, Shabani R, Mehdizadeh M. Magnetic targeted delivery of the SPIONs-labeled mesenchymal stem cells derived from human Wharton’s jelly in Alzheimer’s rat models. J Control Release. 2020;321:430–41. doi: 10.1016/j.jconrel.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Thébault CJ, Ramniceanu G, Boumati S, Michel A, Seguin J, Larrat B, Mignet N, Menager C, Doan BT. Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P. J Control Release. 2020;322:137–48. doi: 10.1016/j.jconrel.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Xi J, Zhang Z, Si XA. Improving intranasal delivery of neurological nanomedicine to the olfactory region using magnetophoretic guidance of microsphere carriers. Int J Nanomedicine. 2015;10:1211–22. doi: 10.2147/IJN.S77520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raut SL, Kirthivasan B, Bommana MM, Squillante E, Sadoqi M. The formulation, characterization and in vivo evaluation of a magnetic carrier for brain delivery of NIR dye. Nanotechnology. 2010;21:395102. doi: 10.1088/0957-4484/21/39/395102. [DOI] [PubMed] [Google Scholar]
- 22.Alexiou C, Jurgons R, Schmid R, Hilpert A, Bergemann C, Parak F, Iro H. In vitro and in vivo investigations of targeted chemotherapy with magnetic nanoparticles. J Magn Magn Mater. 2005;293:389–93. doi: 10.1016/j.jmmm.2005.02.036. [DOI] [Google Scholar]
- 23.Nacev A, Weinberg IN, Stepanov PY, Kupfer S, Mair LO, Urdaneta MG, Shimoji M, Fricke ST, Shapiro B. Dynamic inversion enables external magnets to concentrate ferromagnetic rods to a central target. Nano Lett. 2015;15:359–64. doi: 10.1021/nl503654t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen LB, Thomsen MS, Moos T. Targeted drug delivery to the brain using magnetic nanoparticles. Ther Deliv. 2015;6:1145–55. doi: 10.4155/tde.15.56. [DOI] [PubMed] [Google Scholar]
- 25.Rogers HB, Anani T, Choi YS, Beyers RJ, David AE. Exploiting size-dependent drag and magnetic forces for size-specific separation of magnetic nanoparticles. Int J Mol Sci. 2015;16:20001–19. doi: 10.3390/ijms160820001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinkai M. Functional magnetic particles for medical application. J Biosci Bioeng. 2002;94:606–13. doi: 10.1016/S1389-1723(02)80202-X. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Pacheco R, Marquina C, Gabriel Valdivia J, Gutiérrez M, Soledad Romero M, Cornudella R, et al. Magnetic nanoparticles for local drug delivery using magnetic implants. J Magn Magn Mater. 2007;311:318–22. doi: 10.1016/j.jmmm.2006.11.192. [DOI] [Google Scholar]
- 28.Al-Jamal KT, Bai J, Wang JT, Protti A, Southern P, Bogart L, Heidari H, Li X, Cakebread A, Asker D, Al-Jamal WT, Shah A, Bals S, Sosabowski J, Pankhurst QA. Magnetic drug targeting: preclinical in vivo studies, mathematical modeling, and extrapolation to humans. Nano Lett. 2016;16:5652–60. doi: 10.1021/acs.nanolett.6b02261. [DOI] [PubMed] [Google Scholar]
- 29.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno S, Sekino M. Figure-eight coils for magnetic stimulation: from focal stimulation to deep stimulation. Front Hum Neurosci. 2021;15:805971. doi: 10.3389/fnhum.2021.805971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samoudi AM, Tanghe E, Martens L, Joseph W. Deep transcranial magnetic stimulation: improved coil design and assessment of the induced fields using MIDA model. BioMed Res Int. 2018;2018:7061420. doi: 10.1155/2018/7061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shevkoplyas SS, Siegel AC, Westervelt RM, Prentiss MG, Whitesides GM. The force acting on a superparamagnetic bead due to an applied magnetic field. Lab Chip. 2007;7:1294–302. doi: 10.1039/b705045c. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Lee AY. Transcranial magnetic stimulation parameters as neurophysiological biomarkers in Alzheimer’s disease. Annals Clin Neurophysiol. 2021;23:7–16. doi: 10.14253/acn.2021.23.1.7. [DOI] [Google Scholar]
- 34.Ter Braack EM, de Goede AA, van Putten MJAM. Resting motor threshold, MEP and TEP variability during daytime. Brain Topogr. 2019;32:17–27. doi: 10.1007/s10548-018-0662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmoller J, Carpenter LL, Cincotta M, Chen R, Daskalakis JD, Di Lazzaro V, Fox MD, George MS, Gilbert D, Kimiskidis VK, Koch G, Ilmoniemi RJ, Lefaucheur JP, Leocani L, Lisanby SH, Miniussi C, Padberg F, Pascual-Leone A, Paulus W, Peterchev AV, Quartarone A, Rotenberg A, Rothwell J, Rossini PM, Santarnecchi E, Shafi MM, Siebner HR, Ugawa Y, Wassermann EM, Zangen A, Ziemann U, Hallett M. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCann H, Pisano G, Beltrachini L. Variation in reported human head tissue electrical conductivity values. Brain Topogr. 2019;32:825–58. doi: 10.1007/s10548-019-00710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kuhl AA. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7:4557–76. [PMC free article] [PubMed] [Google Scholar]
- 38.Grehl S, Martina D, Goyenvalle C, Deng ZD, Rodger J, Sherrard RM. In vitro magnetic stimulation: a simple stimulation device to deliver defined low intensity electromagnetic fields. Front Neural Circuits. 2016;10:85. doi: 10.3389/fncir.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MJ, Veiseh O, Bhattarai N, Sun C, Hansen SJ, Ditzler S, Knoblaugh S, Lee D, Ellenbogen R, Zhang M, Olson JM. Rapid pharmacokinetic and biodistribution studies using cholorotoxin-conjugated iron oxide nanoparticles: a novel non-radioactive method. PLoS ONE. 2010;5:e9536. doi: 10.1371/journal.pone.0009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye E, Lee S, Park W, Park E, Cho DW, Jang J, Park SM. In vitro study of neurochemical changes following low-intensity magnetic stimulation. IEEE Access. 2020;8:194363–72. doi: 10.1109/ACCESS.2020.3033029. [DOI] [Google Scholar]
- 42.Samanta D, Hosseini-Nassab N, Zare RN. Electroresponsive nanoparticles for drug delivery on demand. Nanoscale. 2016;8:9310–7. doi: 10.1039/c6nr01884j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.