Abstract
Due to limited chemotherapeutic options for leishmaniasis, novel synthetic compounds are gaining attention for evaluation against leishmaniasis. This study aimed to synthesize the compound's Schiff bases of Vanillin to investigate and evaluate their anti-leishmanial potentials against intracellular protozoan parasites Leishmania tropica. In the current study, the phenomena of synergism by designing Schiff bases with Vanillin enhances their desired importance. A total of five compounds Schiff bases of Vanillin were synthesized using different aromatic amines and Vanillin. The structural analysis of all the compounds was done through FT-IR (Fourier Transformer-Infrared), thin layer chromatography, and spectroscopic techniques such as 13C-NMR, mass spectrometry, and 1H-NMR. The antimicrobial properties of all the compounds ZI-1, ZI-2, BS-1, KH-1, and FA-2 against promastigotes and amastigotes forms of L. tropica were analyzed at three different concentrations 25, 50, and 100 µg/ml. The in-vitro MTT assay was performed to calculate the percent inhibition, IC50 values, and their cytotoxicity. The highest percent inhibition values against promastigote form of L. tropica were BS-1 53.78% at 25 µg/ml, ZI-2 66.95% at 50 µg/ml, and again ZI-2 76.92% at 100 µg/ml. Similarly, the highest percent inhibition values against intracellular amastigote stage were BS-1 55.77% at 25 µg/ml, ZI-2 67.78% at 50 µg/ml and again ZI-2 84.93% 100 µg/ml. The highest potency was recorded for BS-1 in both stages, with IC50 values of 9.83 and 4.27 µg/ml against promastigotes and intracellular amastigotes, respectively. The percent hemolysis as toxicity; the lowest percent hemolysis was recorded for ZI-1 at three different concentrations of 25, 50, 100 µg/ml of 2.60, 3.50, and 6.31, respectively. These results suggested that all the compounds exhibited anti-leishmanial activity, with BS-1 as the most potent. Further studies are suggested to increase the activity of compounds with structural modifications by the addition of some other synergistic, novel, and analogue compounds.
Keywords: Leishmania tropica, Novel Schiff bases, Macrophages, Amastigotes, Promastigotes
Introduction
Leishmaniasis is vector-borne obligate intracellular, hemoflagellate, intra-macrophage protozoal infection (Khokhar et al. 2022; Oryan et al. 2008) with clinical manifestation of visceral integration, mucosal damages, and self-healing ulcers on the skin (Ali et al. 2012). The transmission of disease to humans from the bite of sand flies and sometimes accidentally in laboratories. According to World Health Organization (WHO) leishmaniasis is a reemerging neglected disease with tropical serious clinical manifestation, broad spectrum, and potentially fatal. With three main clinical forms, cutaneous, mucocutaneous, and visceral leishmaniasis affecting the community’s poorest section and become a serious third-world problem causing permanent disfigurement due to resistance (WHO). The parasite Leishmania lifestyle is digenetic, extracellular, and intracellular (Sohail et al. 2021; Arevalo et al. 2007). In protozoal infections following malaria leishmaniasis resulting 70,000 deaths/year, new cases about 1.5 to 2 million/year, the number of infected people approximately 12-15 million worldwide, and 350 million people at risk in about 98 countries (Khan et al. 2022; Reithinger et al. 2007). Without a vaccine scientific community is combating this dreadful disease by developing chemotherapeutic agents. In this context, many natural plants extract products are gaining attention to reduce the toxic effects and repair the damage done with modern treatments (Rahman et al. 2004). Due to the safe nature and low-price availability of medicinal plants used extensively in developing countries. Many drugs derived from medicinal plants as therapeutics against leishmaniasis could be significant (Accioly et al. 2012; Corral et al. 2016). In this study, due to the alarming situation in the country, we searched for highly prevalent compounds to minimize resistance to drugs, long durations of treatment, cost, and serious side effects of first-line antimonial (Balasegaram et al. 2012; Shemshadi et al. 2015).
Any compound having good biological/antimicrobial properties is ideal for the desired objectives, Schiff's bases organic compound proved as a broad range of significant biological/antimicrobial activities, which is antiinflammatory, antipyretic, antioxidant, anti-corrosive, antiproliferative, antibacterial, antimalarial, antifungal, and antiviral (Przybylski et al. 2009). Similarly, Vanillin (4-hydroxy-3- methoxybenzaldehyde) organic compound primarily obtained from the vanilla bean extract (Blank et al. 1992) contributes to the industries with their pleasant smell for the usage in beverages, food, air fresheners, pharmaceuticals, cooking, candles, fragrances, incense, potpourri, and perfumes. The broad range profile of schiff bases and uses of Vanillin makes it an excellent candidate to use against the parasite Leishmania tropica.
The phenomena of synergism by combining two or more compounds to increase their effect greater than the sum of their separate effects, Schiff bases and Vanillin are combined to prepare the compound Schiff bases of Vanillin (SBOV). The current study was designed to formulate the compound SBOV and to check their in-vitro efficacy against L. tropica.
Materials and methods
Materials
Vanillin, Amines, distilled water, Sulfuric acid (H2SO4), ethyl alcohol, Dimethyl sulfoxide (DMSO) melting point apparatus Gallen Kamp apparatus in Celsius scale (°C), Buchi rotary evaporator, thin layer chromatography (TLC) on Kieselgel 60 F254 (Merck), Nuclear Magnetic Resonance (NMR) Bruker instrument of 300 MHz and 75 MHz, FT-IR Schimadzu Fourier Transform Infrared Spectrophotometer Model 270, Media RPMI-1640 (Bio-west), medium 199 (Capricorn), heat-inactivated fetal bovine serum (hi-FBS) (Sigma Aldrich), strepto-pen, 50 ml tubes, 96-well plate (Coster), histopaque-1077® (Sigma Aldrich), tips and micropipette were used in the experiments.
Procedure for drug preparation
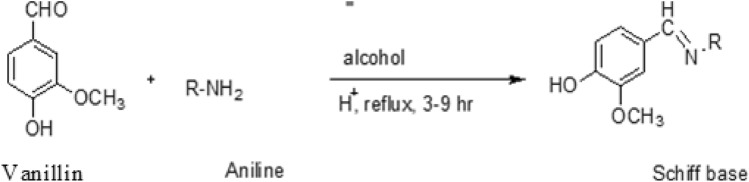
Vanillin (0.01M) and different substituted aromatic amines (0.01M) were taken in a tonic flask, added a few drops of concentrated H2SO4, ethyl alcohol (15–20 ml), and using a magnetic bar to the stirrer reaction mixture at 100 °C for 2–9 hour. After completion of the reaction, solvent was removed under reduced pressure by a rotary evaporator. The crude was washed with ethyl alcohol. Separated solid was filtered and recrystallized from ethyl alcohol (Fig. 1).
Fig. 1.
Synthesis of Schiff basis of Vanillin
Parasite culture
The KHW23 strain of L.tropica was sub-cultured on RPMI-1640 (Biowest) supplemented with heat inactivated-fetal Bovine Serum (Sigma Aldrich), Antibiotics Penicillin (100U/ml)/Streptomycin (0.1mg/ml) (Caisson laboratories), and PH 7.2 at 24 ± 1 °C, from already maintained culture in Department of Microbiology, Institute of Basic Medical Science, Khyber Medical University, Peshawar. After seven days of incubation, the smear was prepared on a clean glass slide and observed under the microscope.
Preparation of stock solutions
The stock solutions of the following compounds of Schiff bases of Vanillin were prepared
4-((4-fluorophenylimino)methyl)-2-methoxyphenol (ZI-1).
2-methoxy-4-((4-nitrophenylimino)methyl)phenol (ZI-2).
2-(4-hydroxy-3-methoxybenzylideneamino)phenol (BS-1).
4-((2-bromophenylimino)methyl)-2-methoxyphenol (KH-1).
4-((2-chloro-4-methylphenylimino) methyl)-2-methoxyphenol (FA-2).
The same procedure was used for all compounds i.e., the stock solution was prepared by adding 1mg of the drug (compound) in 1ml of DMSO as solvent where the concentration of DMSO was kept less than 1%.
Promastigote assay
As previously described, the in-vitro anti-promastigote activity was carried out for all the compounds (Nabi et al. 2012). In a 96-well microtiter plate 100µl complete culture containing promastigote (1×106), compounds at three different (25, 50, 100µg/ml) concentrations were added to each well and incubated for 72 h, PH 7.2 at 25 ± 1 °C, 0.1% DMSO as negative while amphotericin-B as positive control. All the experiments were performed in triplicate. MTT assay was performed by adding MTT reagent to each well and incubated again for three h at 25 ± 1 °C. Finally, the reaction was stopped by adding DMSO, and the absorbance was measured by an ELISA reader (Biotek Instrument) at 570 nm. The data were then subjected to Microsoft excel for the calculation of percent inhibition and IC50 value.
Isolation and infection of Macrophages
Peripheral Blood Mononuclear Cell (PBMC) were isolated from human blood using Histopaque-1077® (Sigma Aldrich) by density gradient centrifugation. The freshly isolated PBMC was seeded in a complete medium of RPMI-1640 for maturation to get macrophages. Percent viability was performed by diluting the cells in medium and adding trypan blue solution, finally counting the cell under a light microscope. Viability was calculated with the following formula:
Washed promastigotes with PBS were added to macrophages and incubated at 34 ± 1 °C with 5% CO2 for 24h. The addition of parasite to the culture of macrophages was 1×106 parasite at MOI (Multiplicity of Infection) 1:10 macrophage parasite.
Amastigotes assay
The activity of amastigote inside macrophages was carried out for all the compounds as previously described (Khan et al. 2022a, b). In a 96-well microtiter plate 100µl of complete culture containing amastigotes inside macrophages (106 cells/ml), compounds at three different (25, 50, 100µg/ml) concentrations were added to each well and incubated for 72 h, PH 7.2 at 25 ± 1 °C, 0.1% DMSO as negative while saponin as positive control. All the experiments were performed in triplicate. MTT assay was performed by adding MTT reagent to each well and incubate again for three hours at 25 ± 1 °C. Finally, the reaction was stopped by adding DMSO, and the absorbance was measured by an ELISA reader (Biotek Instruments) at 570 nm. The data were then subjected to Microsoft excel for the calculation of percent inhibition and IC50 value.
In-vitro hemolysis assay
Hemocompatibility is one of the important criteria for any compound that limits the clinical use in contact with blood. Red blood cells were isolated from human blood by removing plasma through centrifugation at 14,000 for 5 minutes to know the hemolytic properties. After centrifugation, the supernatant was discarded, and the pellet (erythrocyte) was about 200μL collected by mixing and shaking with phosphate buffer solution (1x PBS: PH 7.2). The collected RBC (100μL), test compound at three different concentrations (25, 50, 100µg/ml) was added to each well and incubated for 60 minutes at 37 °C. The absorbance of released hemoglobin was measured by an ELISA reader (Biotek Instrument) at 540 nm, PBS as negative, and Triton-X as a positive control. The Percent hemolysis is calculated from the following formula:
Results
Parasite culturing and macrophages infection
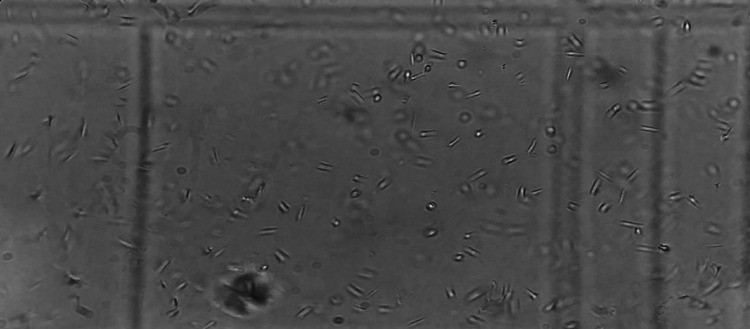
Freshly isolated strain of L. tropica was cultured in RPMI 1640 medium and was observed for promastigote form under light microscope at 40X (Fig. 2). The intracellular amastigote form of L.tropica inside the macrophages is also shown in Fig. 3.
Fig. 2.
Promastigote form of Leishmania tropica under Light Microscope at 40x
Fig. 3.
GIEMSA stained Macrophages infected with parasite Leishmania tropica
Synthesized Schiff bases of vanillin:
The synthesized compounds are following with general properties and IR-spectra.
4-((4-fluorophenylimino) methyl)-2-methoxyphenol (ZI-1)
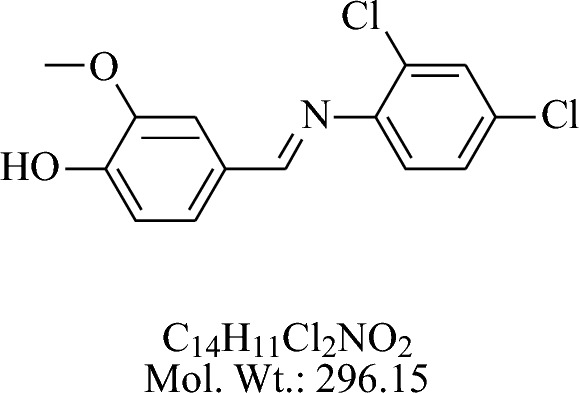
The compound 4-((4-fluorophenylimino)methyl)-2- methoxyphenol (ZI-1) 2-4-Di chloro anillin treated with vanillin in acidic medium using glacial acetic acid by the general procedure as described above is shown in Fig. 4.
Fig. 4.
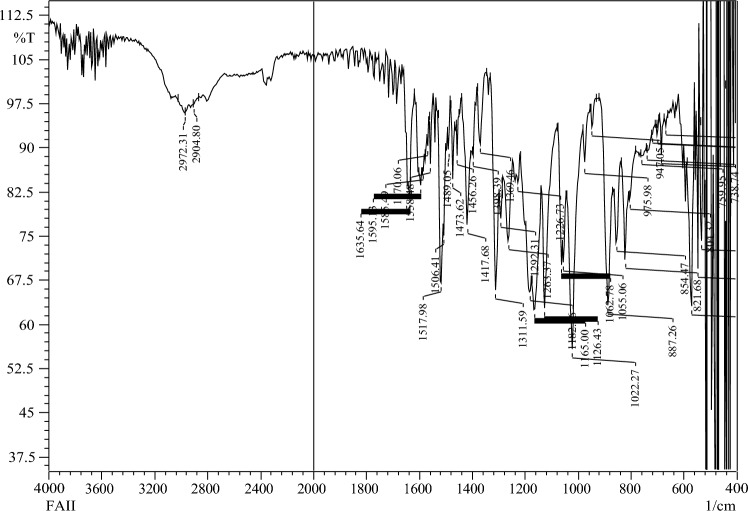
IR-spectra of ZI-1
Yield: 75% (A), 50 % (B). White brown crystal. MP = 208-216 °C. Rf = 0.62(ethyl acetate:n .hexane 3:7). FTIR (KBr):2936 (CH), 1653 (C=N) 1536 (Ar) cm-1.
-
(B)
2-methoxy-4-((4-nitrophenylimino) methyl) phenol (ZI-2)

The compound 2-methoxy-4-((4-nitrophenylimino)methyl)phenol (ZI-2) was synthesized from vanillin and 4-nitro aniline is presented in Fig. 5. The compound was recrystallized from ethyl alcohol.
Fig. 5.
IR-spectra of ZI-2
Yield: 75% (A), 68% (B). Yellow solid. M.P = 190-192 °C. Rf = 0.68 (ethyl acetate: hexane, 3:7) FTIR (KBr): 2939 (CH), 1655 (C=N) 1535 (Ar) cm-1.
-
(C)
2-(4-hydroxy-3-methoxybenzylideneamino) phenol (BS-1)
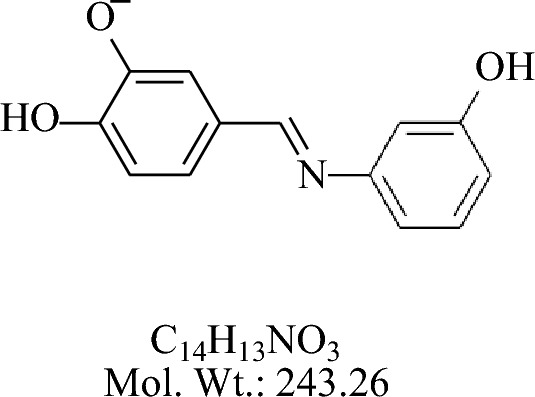
The compound 2-(4-hydroxy-3-methoxybenzylideneamino) phenol (BS-1) was synthesized by the reaction of 3-amino phenol with vanillin are shown in Fig. 6.
Fig. 6.
IR-spectra of BS-1
Yield: 50% (A), 75 % (B). Dark gum. Rf = 0.66 (ethyl acetate: .hexae 3:7) FTIR (KBr):2934 (CH), 1652(C=N) 1539(Ar) cm-1.
-
(D)
4-((2-bromophenylimino) methyl)-2-methoxyphenol (KH-1)
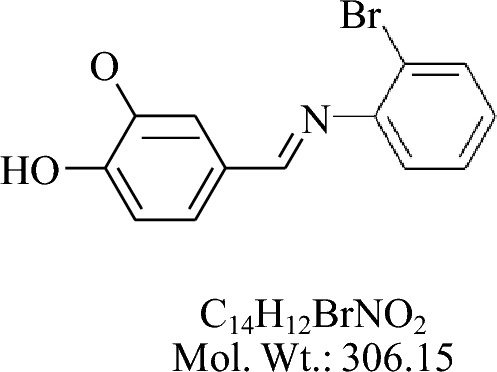
The compound 4-((2-bromophenylimino) methyl)-2-methoxyphenol (KH-1) was synthesized by the reaction of vanillin and 2-Br aniline by the general procedures as described above.
Yield: 20 % (A), 80% (B). White crystal. MP = 203-210. Rf = 0.67 (ethyl acetate. hexane, 3:7) FTIR (KBr):2936 (CH), 1656 (C=N) 1530 (Ar) cm-1 (Fig. 7).
-
(E)
4-((2-chloro-4-methylphenylimino) methyl)-2-methoxyphenol (FA-2)

Fig. 7.
IR-spectra of KH-1
The synthesis of 4-((2-chloro-4-methylphenylimino) methyl)-2-methoxyphenol (FA-2) was carried out by using 2-chloro-4-methyle aniline by the general procedure as described above. The compound was yellow solid.
Yield: 70% (A), 80% (B). MP = 238-239 °C. Rf = 0.87 (ethylacetate: n. hexane 3:7) FTIR (KBr):2936 (CH), 1656 (C=N) 1530 (Ar), 912 (C-Cl) cm-1 (Fig. 8).
Fig. 8.
IR-spectra of FA-2
Anti-promastigote assay
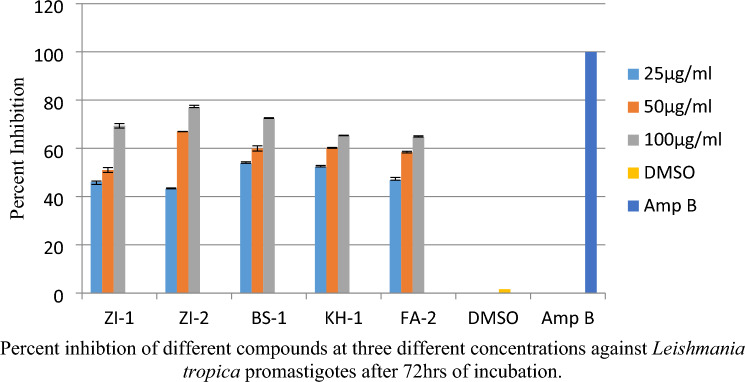
For promastigote assay three different concentrations of the compound were evaluated, the optical density was measured by MTT method. The percent inhibition for all the five compounds ZI-1, ZI-2, BS-1, KH-1, and FA-2 in three different concentrations, at 25 µg/ml percent inhibition values were 46.04, 43.22, 53.78, 52.27, 46.79, at 50 µg/ml percent inhibition values were 51.01, 66.95, 59.95, 60.18, 58.42 and at 100 µg/ml percent inhibition values were 69.39, 76.92, 72.39. 65.26, 64.65 respectively, as shown in the Fig. 9. Where DMSO was used as a negative control while amphotericin-B was used as a positive control.
Fig. 9.
Percent inhibition of Schiff bases of vanillin against promastigotes of Leishmania tropica
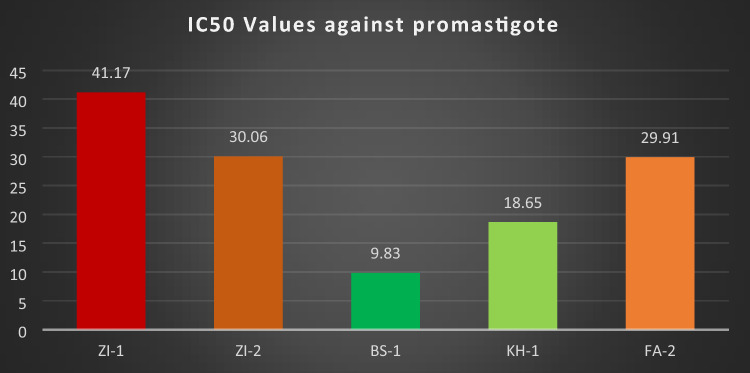
The IC50 (half maximal inhibitory concentration) value calculated for all the compounds, ZI-1, ZI-2, BS-1, KH-1 and FA-2 against promastigote stage were 41.17, 30.06, 9.83, 18.65 and 29.91 respectively. BS-1 showed good IC50 value followed by KH-1, FA-2, ZI-2, and ZI-1 as shown in the Fig. 10.
Fig. 10.
IC50 value of ZI-1, ZI-2, BS-1, KH-1, and FA-2 against promastigotes of Leishmania tropica
Anti-amastigotes assay
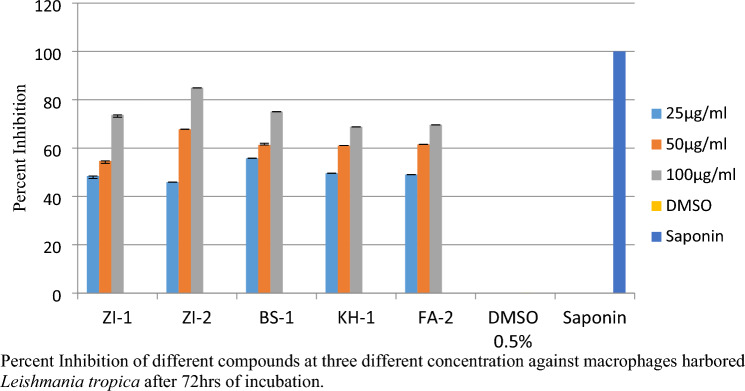
For anti-amastigotes assay three different concentrations of the compound were evaluated, the optical density was measured by MTT method. The percent inhibition for all the five compounds ZI-1, ZI-2, BS-1, KH-1 and FA-2 in three different concentrations; at 25 µg/ml percent inhibition values were 48.42, 45.89, 55.77, 49.58, 49.02 at 50 µg/ml percent inhibition values were 54.69, 67.78, 61.29, 61.06, 61.52and at 100 µg/ml percent inhibition values were 73.74, 84.93, 75.06. 68.79, 69.60 respectively as shown in the Fig. 11. DMSO was used as negative and Saponin as positive control.
Fig. 11.
Percent Inhibition of schiff bases of vanillin against macrophages harbored Leishmania tropica
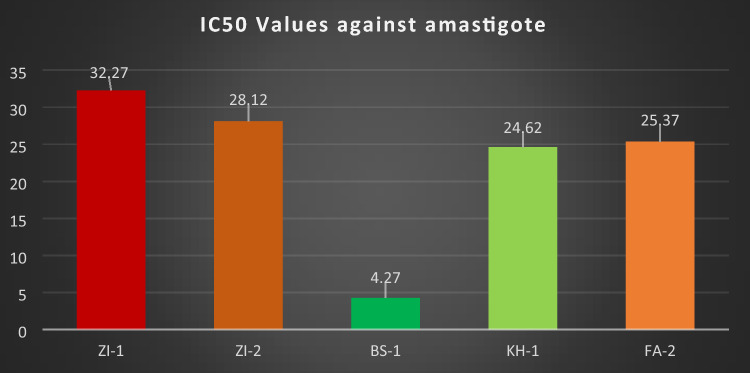
The IC50 (half maximal inhibitory concentration) value calculated for all the compounds against amastigote inside macrophages for ZI-1, ZI-2, BS-1, KH-1 and FA-2 were 32.27, 28.12, 4.27, 24.62 and 25.37 respectively. BS-1 showed good IC50 value followed by KH-1, FA-2, ZI-2, and ZI-1 as shown in the Fig. 12.
Fig. 12.
IC50 value of ZI-1, ZI-2, BS-1, KH-1 and FA-2 against macrophages harbored Leishmania tropica
In-vitro cytotoxicity assay
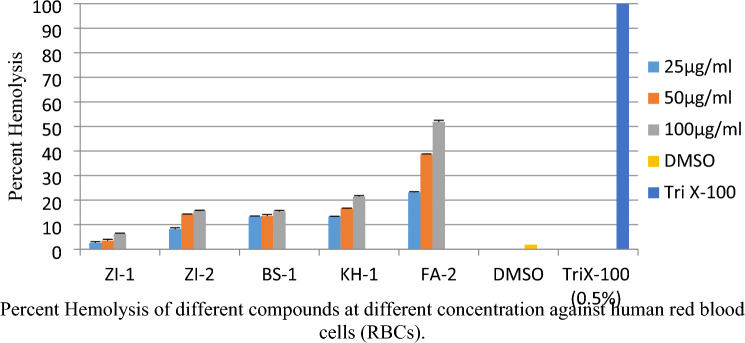
The percent hemolysis for all the five compounds were evaluated in three different concentrations. Percent hemolysis of ZI-1, ZI-2, BS-1, KH-1, and FA-2 starting from the lowest 25 µg/ml were 2.60, 8.22, 13.33,13.33, 23.12 at 50 µg/ml 3.50, 14.33, 13.52, 16.64, 38.63 and the highest concentration 100 µg/ml were 6.31, 15.64, 15.44, 21.45 and 51.89 respectively as shown in the Fig. 13. DMSO was used as a negative control while Triton-X 100 (0.5%) as positive control.
Fig. 13.
Percent hemolysis of Schiff bases of vanillin against human RBCs
Discussion
Protozoal diseases are affecting the world, and leishmaniasis, a neglected tropical disease, is contributing to the burden by targeting the poorest section of population, mostly in developing countries. (Tasdemir et al. 2006). Due to the undeterred use of first-line pentavalent antimonials, the drugs either lost or decreased their efficacy, finally leading to resistance in some endemic areas (Moreno et al. 2011). Thus, new and more effective drugs are urgently needed. The growing interest in medicinal plants and natural products emphasized Schiff bases of Vanillin as an alternative therapy for CL. In this innovation schiff bases of Vanillin can provide better therapeutic agents in designing drugs as several studies have already tackled the screening of plant extracts against leishmaniasis (Teran et al. 2019). This study clearly indicated that schiff bases of Vanillin significantly inhibited the growth of promastigote and amastigotes form of L.tropica during in-vitro and ex-vivo experimentations.
Schiff bases are essential organic compounds that attract the researcher’s attention due to their biological properties. Schiff bases ligands preparation is an easy, simple, and -step condensation process for primary amines and aldehydes. The second compound to form a complex with Schiff bases is Vanillin (Ayodhya et al. 2019).
In this study, we evaluated the efficacy of schiff bases of Vanillin against promastigotes and amastigotes forms of L.tropica. Interestingly, all the schiff bases of Vanillin significantly decreased the number of both forms of the parasite inside macrophage in comparison to the control (amphotericin-B). The IC50 values for ZI-1, ZI-2, BS-1, KH-1, and FA-2 against promastigote stage were 41.17, 30.06, 9.83, 18.65 and 29.91 respectively in comparison to the standard amphotericin-B (IC50=0.17 ± 0.01), which estimate that the positive control amphotericin-B remained more effective. The comparison of IC50 values between ZI-1, ZI-2, BS-1, KH-1, and FA-2 showed that BS-1 inhibit the growth of promastigote stage of Leishmania tropica better in all the concentrations as compared to other. The IC50 value of BS-1 (IC50 = 9.83 µg/ml) which is 2, 3, 3 and 4 folds effective than KH-1, FA-2, ZI-2, and ZI-1 respectively. We conclude that BS-1 is more effective than those of the rest compounds against promastigote stage of parasite.
Like promastigote stage, all the compounds ZI-1, ZI-2, BS-1, KH-1, and FA-2 inhibited amastigote growth inside macrophage with IC50 values of 32.27, 28.12, 4.27, 24.62 and 25.37 respectively in comparison to saponin. The comparison of IC50 values between ZI-1, ZI-2, BS-1, KH-1 and FA-2 showed that BS-1 inhibited the growth of amastigote stage of Leishmania tropica significantly. As the IC50 value for BS-1 (IC50 = 4.27 µg/ml) which is 5, 5, 6 and 7 folds effective than KH-1, FA-2, ZI-2, and ZI-1 respectively. We conclude that BS-1 is more effective than those of the rest compounds against amastigote stage of parasite inside macrophages. Remarkably, BS-1 inhibited the parasite in amastigote stage inside macrophage with IC50 value of 4.27 µg/ml is two folds higher than the promastigote stage 9.83 µg/ml which indicate that compound BS-1 have macrophage modulation properties. The similar study was reported before and addressed that intracellular amastigote are more sensitive against Pentostam and glucantime than promastigotes (Callahan et al. 1997). Another study reported that Pentavalent antimony Sb (V) reduction to their active trivalent antimony Sb (III) requires host enzyme which justifying that amastigotes are more susceptible as compare to promastigote stage (extracellular) (Callahan et al. 1994) (Shaked-Mishan et al. 2001). The sensitivity of amastigote was reported by (Abbas and Zghair. 2015) according to that study IC50 of SbV and AmBisome against amastigote was 0.88 and 0.75 μg/ml respectively, as compared to promastigote stage 5.42 mg/ml and 2.14 μg/ml, respectively. Reported compounds capable to bind with mammalian/eukaryotic G protein coupled receptors which is absent in trypanosomatids/Leishmania genome (El-Sayed et al. 2005). The inhibition of parasite Leishmania tropica with schiff bases of Vanillin possibly targeting the likely ligand proteins i.e. G protein coupled receptors and host cell signaling pathways (De Muylder et al. 2011). All these studies indicates that their activity is dependent on macrophages functioning include targeting the host factors which is essential for the development of parasite Leishmania tropica. Thus, all these pathways can be used to redesign the conventional drugs and as emerging drug discovery against microbial infections (Schwegmann and Brombacher. 2008; Jayaswal et al. 2010).
Similarly hemolytic assay for all the compounds ZI-1, ZI-2, BS-1, KH-1 and FA-2 where the percent hemolysis at 25 µg/ml were 2.60, 8.22, 13.33,13.33, 23.12, at 50 µg/ml 3.50, 14.33, 13.52, 16.64, 38.63, and at 100 µg/ml were 6.31, 15.64, 15.44, 21.45 and 51.89 respectively. Dramatically, all the compounds were too toxic except ZI-1, which showed lower percent hemolysis in all three different concentrations which was a promising result for an ideal therapeutic agent against L. tropica. The standard for measuring of toxicity was hemolytic > 5%, slight hemolysis range of 2-5 % and non-hemolytic range was <2 % according to “American Society for Testing and Materials Designation” (Neun and Dobrovolskaia. 2011). Our results offered that the use of SBOV especially BS-1 in topical formulations could be an effective anti-leishmanial therapy.
Conclusion
This study was conducted to investigate the anti-leishmanial and cytotoxicity properties of Schiff bases of Vanillin with the objective of searching for novel therapeutic compounds against leishmaniasis. The anti-leishmanial activity of all the compounds exhibited lower to moderate activity compared to the standard amphotericin-B drug. BS-1 and KH-1 showed lower IC50 values, which were more potent and highly active than other compounds against promastigote and macrophage-harbored Leishmania tropica. Most of the compounds showed higher toxicity towards the normal human red blood cells (>5% concentration), suggesting only the topical use of the compounds. Hence it is concluded that BS-1 possesses more anti-leishmanial potential than other SBOVs which was evaluated in this study. The structural modifications may further make these compounds more effective in reducing toxicities and enhancing their anti-leishmanial potentials.
Acknowledgements
We are thankful to Mr. Yousaf Khan and Mr. Karim Dil for their technical support.
Declarations
Conflict of interest
We have no conflict of interest to disclose regarding this manuscript. As a corresponding author, I confirm that the manuscript has been read and approved for submission by all the named authors.
Ethical Approval
All experiments and sampling procedures were ethically performed following standard protocols approved by Ethics Committee of Institute of Basic Medical Sciences, Khyber Medical University Peshawar, Pakistan under reference No. Dir/KMU-EB/IV/000513.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas ZM, Zghair KH. Efficacy of amphotericine B drug against promastigote and axenic amastigote of Leishmania tropica in Vitro. Iraqi J Sci. 2015;56:1343–1349. [Google Scholar]
- Accioly MP, Bevilaqua CM, Rondon FC, de Morais SM, Machado LK, Almeida CA, de Andrade Jr HF, Cardoso RP. Leishmanicidal activity in vitro of Musa paradisiaca L. and Spondias mombin L. fractions. Vet Parasitol. 2012;187:79–84. doi: 10.1016/j.vetpar.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Ali N, Mekuria AH, Requena JM, Engwerda C. Immunity to visceral leishmaniasis. J Trop Med. 2012;13:780809. doi: 10.1155/2012/780809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- Ayodhya D, Veerabhadram G. Fabrication of Schiff base coordinated ZnS nanoparticles for enhanced photocatalytic degradation of chlorpyrifos pesticide and detection of heavy metal ions. J Materiomics. 2019;5:446–454. doi: 10.1016/j.jmat.2019.02.002. [DOI] [Google Scholar]
- Balasegaram M, Ritmeijer K, Lima MA, Burza S, Ortiz Genovese G, Milani B, Gaspani S, Potet J, Chappuis F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerg Drugs. 2012;17:493–510. doi: 10.1517/14728214.2012.748036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I, Ha Sen A, Grosch W. Original paper zyxwvutsrqponmlkj Potent odorants of the roasted powder and brew of Arabica coffee zyxwvutsrqpo. Z Lebensm Unters Forsch. 1992;195:239–245. doi: 10.1007/BF01202802. [DOI] [Google Scholar]
- Callahan HL, Roberts WL, Rainey PM, Beverley SM. The PGPA gene of Leishmania major mediates antimony (SbIII) resistance by decreasing influx and not by increasing efflux. Mol Biochem Parasitol. 1994;68:145–149. doi: 10.1016/0166-6851(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Callahan HL, Portal AC, Devereaux R, Grogl MA. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/AAC.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral MJ, Benito-Peña E, Jiménez-Antón MD, Cuevas L, Moreno-Bondi MC, Alunda JM. Allicin induces calcium and mitochondrial dysregulation causing necrotic death in Leishmania. PLoS Negl Trop Dis. 2016;10:e0004525. doi: 10.1371/journal.pntd.0004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muylder G, Ang KK, Chen S, Arkin MR, Engel JC, McKerrow JH. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis. 2011;19:e1253. doi: 10.1371/journal.pntd.0001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Sci. 2005;15:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- Jayaswal S, Kamal MA, Dua R, Gupta S, Majumdar T, Das G, Kumar D, Rao KV. Identification of host-dependent survival factors for intracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog. 2010;6:e1000839. doi: 10.1371/journal.ppat.1000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RU, Khan M, Sohail A, Ullah R, Iqbal A, Ahmad B, Khan IU, Tariq A, Ahmad M, Said A, Ullah S. Efficacy of pentamidine-loaded chitosan nanoparticles as a novel drug delivery system for Leishmania tropica. Trop Biomed. 2022;39:1–7. doi: 10.47665/tb.39.4.003. [DOI] [PubMed] [Google Scholar]
- Khan TA, Mnasri A, Al Nasr IS, Özdemir I, Gürbüzd N, Hamdi N, Koko WS. Activity of benzimidazole derivatives and their N-heterocyclic carbene silver complexes against leishmania major promastigotes and amastigotes. Biointerface Res Appl Chem. 2022;13:132–133. [Google Scholar]
- Khokhar M, Shereen MA, Khan M, Khan RU, Sohail A, Khan IU, Khan IU, Khattak S. In vitro efficacy of polymer coated miltefosine drug against leishmania tropica. J Parasit Dis. 2022;46:366–376. doi: 10.1007/s12639-021-01452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno D, Plano D, Baquedano Y, Jiménez-Ruiz A, Antonio Palop J, Sanmartín C. Antileishmanial activity of imidothiocarbamates and imidoselenocarbamates. Parasitol Res. 2011;108:233–239. doi: 10.1007/s00436-010-2073-x. [DOI] [PubMed] [Google Scholar]
- Nabi S, Ahmed N, Mj K, Bazai Z, Yasinzai M, Ym A-K. In vitro antileishmanial, antitumor activities and phytochemical studies of methanolic extract and its fractions of Juniperus Excelsa Berries. World Appl Sci J. 2012;19:1495–1500. [Google Scholar]
- Neun BW, Dobrovolskaia MA. Method for analysis of nanoparticle hemolytic properties in vitro. Characterization of nanoparticles intended for drug delivery. Methods Mol Biol. 2011;627:215–224. doi: 10.1007/978-1-60327-198-1_23. [DOI] [PubMed] [Google Scholar]
- Oryan A, Mehrabani D, Owji SM, Motazedian MH, Hatam GH, Asgari Q. Morphologic changes due to cutaneous leishmaniosis in BALB/c mice experimentally infected with Leishmania major. J Appl Anim Res. 2008;34:87–92. doi: 10.1080/09712119.2008.9706946. [DOI] [Google Scholar]
- Przybylski P, Huczyński AW, Pyta KK, Brzezinski B, Bartl F. Biological properties of Schiff bases and azo derivatives of phenols. Curr Org Chem. 2009;13:124–148. doi: 10.2174/138527209787193774. [DOI] [Google Scholar]
- Rahman MA, Mossa JS, Al-Said MS, Al-Yahya MA. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2004;75:149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous Leishmaniasis. Lancet Infec Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Schwegmann A, Brombacher F. Host-directed drug targeting of factors hijacked by pathogens. Sci Sign. 2008;1:8–13. doi: 10.1126/scisignal.129re8. [DOI] [PubMed] [Google Scholar]
- Shaked-Mishan P, Ulrich N, Ephros M, Zilberstein D. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J Biol Chem. 2001;276:3971–3976. doi: 10.1074/jbc.M005423200. [DOI] [PubMed] [Google Scholar]
- Shemshadi B, Ranjbar-Bahadory S, Ahmadi H. Effect of Caparis spinosa root extract on promastigotes and amastigotes of Leishmania major. Archi of Advan in Biosci. 2015;6:21–34. [Google Scholar]
- Sohail A, Khan RU, Khan M, Khokhar M, Ullah S, Ali A, Bilal H, Khattak S, Khan M, Ahmad B. Comparative efficacy of amphotericin B-loaded chitosan nanoparticles and free amphotericin B drug against Leishmania tropica. Bull Natl Res Cent. 2021;45:1–9. doi: 10.1186/s42269-021-00644-5. [DOI] [Google Scholar]
- Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, Rüedi P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother. 2006;50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran R, Guevara R, Mora J, Dobronski L, Barreiro-Costa O, Beske T, Pérez-Barrera J, Araya-Maturana R, Rojas-Silva P, Poveda A, Heredia-Moya J. Characterization of antimicrobial, antioxidant, and leishmanicidal activities of Schiff base derivatives of 4-aminoantipyrine. Mol. 2019;24:2696. doi: 10.3390/molecules24152696. [DOI] [PMC free article] [PubMed] [Google Scholar]