Abstract
Purpose We aim to evaluate the diagnostic performance of the SleepImage Ring device in identifying obstructive sleep apnea (OSA) across different severity in comparison to standard polysomnography (PSG). Methods Thirty-nine patients (mean age, 56.8 ± 15.0 years; 29 [74.3%] males) were measured with the SleepImage Ring and PSG study simultaneously in order to evaluate the diagnostic performance of the SleepImage device for diagnosing OSA. Variables such as sensitivity, specificity, positive and negative likelihood ratio, positive and negative predictive value, and accuracy were calculated with PSG-AHI thresholds of 5, 15, and 30 events/h. Receiver operating characteristic curves were also built according to the above PSG-AHI thresholds. In addition, we analyzed the correlation and agreement between the apnea-hypopnea index (AHI) obtained from the two measurement devices. Results There was a strong correlation (r = 0.89, P < 0.001 and high agreement in AHI between the SleepImage Ring and standard PSG. Also, the SleepImage Ring showed reliable diagnostic capability, with areas under the receiver operating characteristic curve of 1.00 (95% CI, 0.91, 1.00), 0.90 (95% CI, 0.77, 0.97), and 0.98 (95% CI, 0.88, 1.000) for corresponding PSG-AHI of 5, 15 and 30 events/h, respectively. Conclusion The SleepImage Ring could be a clinically reliable and cheaper alternative to the gold standard PSG when aiming to diagnose OSA in adults.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13534-023-00304-9.
Keywords: Apnea-hypopnea index, Cardiopulmonary coupling, Polysomnography, Obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a clinical condition characterized by repetitive collapse of the upper airway during sleep. Nearly 1 billion people worldwide are estimated to have OSA, and it is likely to increase with the ageing population and ongoing obesity epidemic [1–3]. According to a recent population-based cohort study, almost half of the population in northeast Germany suffers from OSA [4]. Untreated OSA is associated with an increased likelihood of comorbidities, such as hypertension, heart disease, and stroke [5, 6]. However, only about 20% of patients have been diagnosed and treated, suggesting a massive diagnosis and treatment gap [7].
To date, overnight in-laboratory polysomnography (PSG) remains the gold standard for diagnosing OSA [8]; nevertheless, disadvantages such as technical complexity, labor-intensive procedures, long waiting times, and high costs limit its general availability [9]. Furthermore, patients may find the PSG device to be uncomfortable due to the multiple electrodes that are required to be attached to the face, head, and body in an unfamiliar environment for accurate measurement, which will lead to the first-night effect [10, 11]. In this case, multiple nights of recording are recommended, however, it is not practical. As a result, many portable and cheaper devices have been developed to address these issues.
Among varied devices, the cardiopulmonary coupling (CPC) technique-based portable device has been increasing in interest among researchers and physicians. The CPC technique calculates the coherence between heart/pulse rate variability and respiratory excursion derived from a single-lead electrocardiogram (ECG) or photoplethysmogram (PPG) to generate distinct CPC patterns [12, 13]. Elevated low-frequency coupling (e-LFC) was discovered to be one of the characteristic patterns substantially linked with apnea and hypopnea [14]. Therefore, the new SleepImage Ring device uses the PPG sensor to collect both the Plethysmogram (PLETH) and the oxygen saturation (SpO2) signals. From here, the device can calculate the apnea-hypopnea index (sAHI) by combining the CPC parameters and hypoxic events. Al Ashry and his colleagues analyzed the ECG and oximetry data on PSG from the database of Apnea Positive Pressure Long-term Efficacy Study and calculated the sAHI value. They found there was a strong correlation between sAHI and manually scored PSG-AHI [15]. However, there are no studies that directly validate the diagnostic performance of the portable monitoring device-SleepImage Ring for diagnosing adult OSA. We hypothesized the sAHI derived from SleepImage Ring would have a high agreement with the AHI of nurse-attended PSG in assessing OSA severity. Therefore, we aim to evaluate the diagnostic value of the SleepImage Ring device compared with PSG in identifying OSA in adults.
Materials and methods
Procedure and participants
This prospective study protocol was approved by the Ethics Committee of Charité University Medicine Berlin (EA1/093/22). Written informed consent was obtained from all participants.
Consecutive patients aged ≥ 18 years referred to the Interdisciplinary Sleep Medicine Center of Charité University Medicine Berlin for further assessment of sleep apnea between August 2022 and February 2023 were eligible for this study. The exclusion criteria were as follows: (1) finger problems related to the inability to use SleepImage Ring devices; (2) patients who do not have a mobile phone and/or cannot download the accompanying App; (3) patients with severe cognitive impairment who cannot understand how to use the device; (4) patients with previously diagnosed severe sleep disorders other than sleep apnea; (5) the SleepImage report is unavailable due to test failure.
Investigators interviewed patients for their demographic, clinical, and health behavior-related characteristics (e.g., age, sex, height, weight, smoking status, comorbidities, etc.) in a quiet setting. In addition, since some patients have been diagnosed with OSA by preliminary tests such as polygraphy, they also provided the results of their latest sleep test.
Polysomnography
All participants underwent a full-night in-laboratory PSG at the sleep center. The standard PSG examination included frontal, central, and occipital electroencephalogram, electrooculogram, chin electromyogram, tibialis anterior electromyogram, ECG, respiratory airflow (thermistor and pressure transducer), thoracic-abdominal effort (respiratory inductance plethysmography), SpO2, snoring, and body position. The recorded data were scored manually by experienced sleep technicians according to the American Academy of Sleep Medicine (AASM) manual for the Interpretation of Sleep and Related Events [11]. Apnea was defined as an airflow drop ≥ 90% of the pre-event baseline for at least 10 s. Hypopnea was defined as when the airflow drops by ≥ 30% for at least 10 s in association with either ≥ 3% oxygen desaturation or arousal. Apnea-hypopnea index (AHI) was calculated as the average number of apnea and hypopnea events per hour of sleep. According to AHI, the severity of OSA was classified as follows: mild: 5 ≤ AHI < 15 events/h, moderate: 15 ≤ AHI < 30 events/h, and severe ≥ 30 events/h.
SleepImage Ring
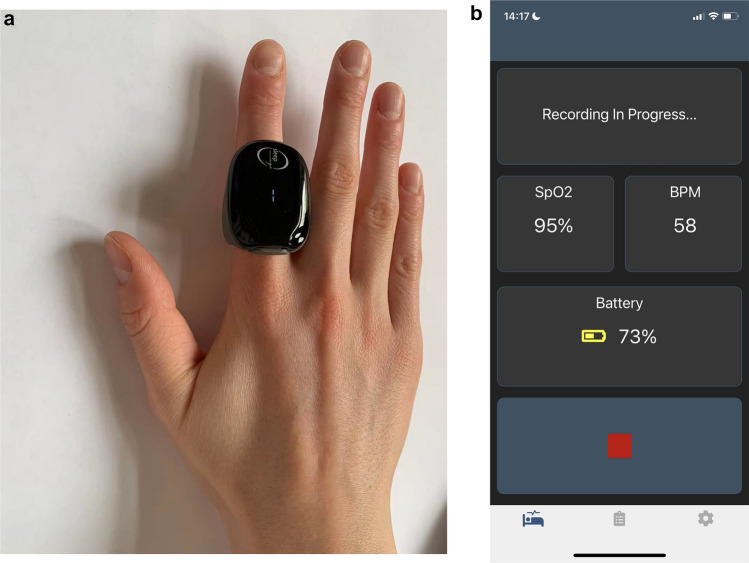
Patients wore the SleepImage Ring device whilst undergoing the PSG study. The SleepImage Ring system comprises a finger-worn recorder (Fig. 1a), a SleepImage Mobile App with a secure cloud-based portal (Fig. 1b). The Ring recorder, equipped with a PPG sensor, was used to collect PLETH and SpO2 data signals, while the mobile App was used to data transfer. The cloud-based SleepImage system (MyCardio LLC, Denver, CO, USA) automatically analyzed the coherence and cross-spectral power of pulse rate variability and respiration derived from PLETH using the CPC technique. By combining CPC data with SpO2 data, the SleepImage Ring-based AHI (sAHI) is calculated. The analytical methods of the CPC technique were described in detail previously [12]. Also, the SleepImage system complies with the EU Medical Device Directive (CE mark 0413).
Fig. 1.
Schematic of the SleepImage Ring device. a shows the finger-worn recorder, and b shows the SleepImage Mobile App
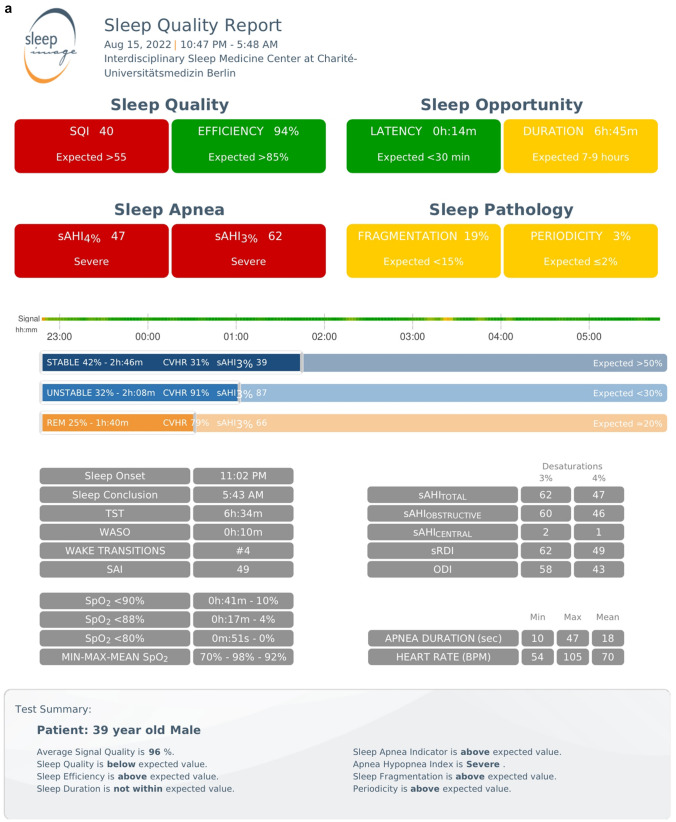
The process from monitoring to report generation involved the following steps: (1) place the thumb or index finger in the Ring recorder; (2) pair the Ring recorder with the SleepImage App using Bluetooth; (3) Click the play button on the App to start a recording before going to sleep; (4) press the stop button to end the study and upload the recording; (5) generate the sleep report automatically in the system. Figure 2 shows the final sleep report and spectrogram.
Fig. 2.
SleepImage Ring produced sleep apnea report. a shows a brief sleep apnea report, and b shows the spectrogram
Sample size calculation
According to our study hypothesis, we used the kappa test for agreement between two raters to calculate the sample size (PASS version 15.0). The primary endpoint is the presence of OSA diagnosed by PSG. According to a previous study by Seo et al. [16], a sample size of 32 subjects achieves 80% power with a significance level of 0.05 to obtain a true kappa value of 0.70.
Statistical analysis
Categorical variables are presented as frequency (percentage) and continuous variables as mean ± standard deviation or median (interquartile range), as appropriate. To evaluate the diagnostic performance of the SleepImage Ring device, we calculated the sensitivity, specificity, positive and negative predictive value, positive and negative likelihood ratio, accuracy, and Youden index with PSG-AHI thresholds of 5, 15, and 30 events/h. The Youden index (sensitivity plus specificity minus 1) was used to determine the optimal cutoff values. Receiver operating characteristic (ROC) curves were also built according to the above PSG-AHI thresholds. In addition, to assess the agreement of the AHI between the SleepImage Ring device and PSG, we utilized paired t-tests and Bland-Altman analysis. Scatterplot was created to assess the correlation between the AHI and sAHI, and the Spearman correlation coefficient was calculated due to the skewed distribution of AHI. We used the Kendall tau-b to measure the concordance of OSA severity assessed by the two methods. A value of P < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS software (IBM SPSS Statistics, IBM Corporation). Figures were generated using GraphPad Prism version 9.0.
Results
Participants
A total of 39 participants (74.4% male) completed the PSG and SleepImage Ring study. Their mean age was 56.8 ± 15.0 years (range, 27–83 years), mean body mass index (BMI) was 30.8.0 ± 7.2 kg/m2. The percentage of obesity (BMI ≥ 30 kg/m2) was 46.2% (18/39). According to the PSG results, 14 patients (35.9%) had mild OSA, 6 (15.4%) had moderate OSA, and 17 (43.6%) had severe OSA. Their median (IQR) AHI and median (IQR) lowest SpO2 were 22.4 (13.1, 56.9) events/h and 82.0 (73.0, 86.0) %, respectively. Characteristics of the participants are summarized in Table 1.
Table 1.
Demographic characteristics of participants
| Variables | Participants (N = 39) |
|---|---|
| Age, years | 56.8 ± 15.0 |
| Male: Female | 29: 10 |
| Height, cm | 174.9 ± 8.0 |
| Weight, kg | 94.0 ± 21.2 |
| BMI, kg/m2 | 30.8 ± 7.2 |
| AHI, /h | 22.4 (13.1, 56.9) |
| Total sleep time, min | 364.8 ± 69.8 |
| Sleep efficiency, % | 84.5 (69.0, 91.6) |
| Lowest SpO2 | 82.0 (73.0, 86.0) |
Values are presented as mean ± standard deviation or median (IQR) for continuous variables. AHI, apnea-hypopnea index, BMI, body mass index, SpO2, oxygen saturation
Correlation and agreement of AHI derived from two methods
There was a significantly positive correlation between the sAHI generated by SleepImage Ring and AHI obtained from PSG (Spearman correlation coefficient = 0.89, P < 0.001), along with a strong linear relationship (Fig. 3). Also, no significant difference was observed between the sAHI and AHI (30.2 ± 23.0 versus 32.9 ± 26.9, P = 0.350). The Bland-Altman plot was used to estimate agreements between the sAHI and AHI (Fig. 4), reporting a mean difference of 2.7, and the 95% limit of agreement from – 19 to 24, with 94.9% (37/39) observations in the limits of agreement. Table 2 shows the concordance of OSA severity assessed by the SleepImage Ring and PSG. We found significantly high concordance in OSA severity between the two methods (Kendall tau-b = 0.75, P < 0.001).
Fig. 3.
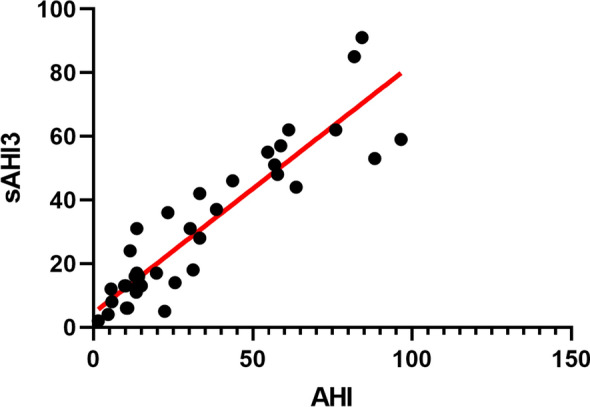
Scatterplot diagram showing the correlation between the sAHI and AHI (Spearman correlation coefficient = 0.90, P < 0.001). AHI, apnea -hypopnea index; sAHI, SleepImage apnea-hypopnea index
Fig. 4.
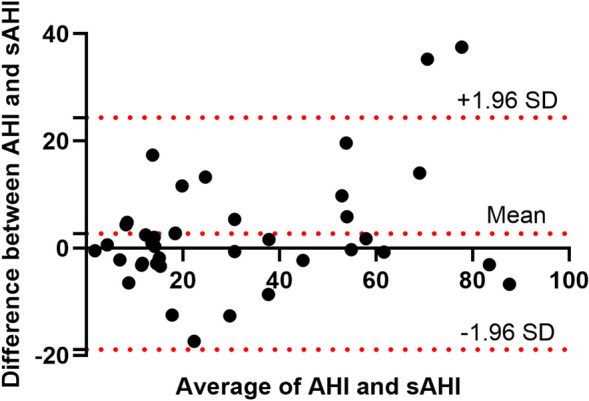
Bland-Altman plot for the level of agreement between the sAHI and AHI. AHI, apnea -hypopnea index; sAHI, SleepImage apnea-hypopnea index
Table 2.
Concordance of OSA severity measured by two methods
| OSA severity by polysomnography | ||||||
|---|---|---|---|---|---|---|
| AHI < 5 | 5 ≤ AHI < 15 | 15 ≤ AHI < 30 | AHI ≥ 30 | Total | ||
|
OSA Severity by SleepImage Ring |
AHI < 5 | 2 | 0 | 0 | 0 | 2 |
| AHI 5-<15 | 0 | 9 | 3 | 0 | 12 | |
| AHI 15-<30 | 0 | 4 | 2 | 2 | 8 | |
| AHI ≥ 30 | 0 | 1 | 1 | 15 | 17 | |
| Total | 2 | 14 | 6 | 17 | 39 | |
Same severity = 71.8%. Kendall tau-b = 0.75 (P < 0.001). OSA, obstructive sleep apnea
Diagnostic accuracy evaluation
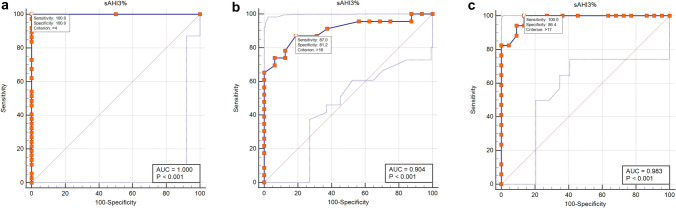
Table 3 shows the sensitivity, specificity, false-positive rate, false-negative rate, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, accuracy, Cohen’s kappa, and Youden index of the SleepImage Ring in diagnosing OSA at the PSG-AHI thresholds of 5, 15, and 30, respectively. The SleepImage Ring is accurate in detecting OSA (AHI ≥ 5) with 100% sensitivity and 100% specificity. High accuracy was also demonstrated at other two PSG-AHI thresholds: 79% (95% CI 67%, 92%) for AHI ≥ 15 and 90% (95% CI 80%, 99%) for AHI ≥ 30, respectively. ROC curves were conducted to evaluate the diagnostic ability of the SleepImage Ring (Fig. 5). When the AHI ≥ 5 events/h was set as the criteria to diagnose OSA, the area under the curve (AUC) was 1.00 (95% CI, 0.91, 1.00), the optimal cutoff value of sAHI was 4, the sensitivity and specificity were both 100%. When the AHI ≥ 15 events/h was set as the criteria to diagnose OSA, the AUC was 0.90 (95% CI 0.77, 0.97), the optimal sAHI cutoff value was 16, the sensitivity and specificity were 87% and 81%, respectively. When an AHI of ≥ 30 events/h was set as the criterion for diagnosing OSA, the AUC was 0.98 (95% CI, 0.88, 1.000), an sAHI value of 17 was optimal, yielding a sensitivity of 100% and specificity of 86%.
Table 3.
Diagnostic Performance of the Sleepimage Ring with polysomnography (N = 39)
| Variable | Values (95% CI) | ||
|---|---|---|---|
| AHI ≥ 5 (N = 37) | AHI ≥ 15 (N = 23) | AHI ≥ 30 (N = 17) | |
| Sensitivity | 1.00 (1.00, 1.00) | 0.87 (0.73, 1.00) | 0.88 (0.73, 1.00) |
| Specificity | 1.00 (1.00, 1.00) | 0.69 (0.46, 0.91) | 0.91 (0.79, 1.00) |
| False-positive rate | 0.00 (0.00, 0.00) | 0.31 (0.09, 0.54) | 0.09 (0.00, 0.21) |
| False-negative rate | 0.00 (0.00, 0.00) | 0.13 (0.00, 0.27) | 0.12 (0.00, 0.27) |
| Positive likelihood ratio | NA | 2.78 (1.32, 5.85) | 9.71 (2.56, 36.80) |
| Negative likelihood ratio | NA | 0.19 (0.06, 0.57) | 0.13 (0.03, 0.48) |
| Positive predictive value | 1.00 (1.00, 1.00) | 0.80 (0.64, 0.96) | 0.88 (0.73, 1.00) |
| Negative predictive value | 1.00 (1.00, 1.00) | 0.79 (0.57, 1.00) | 0.91 (0.79, 1.00) |
| Accuracy | 1.00 (1.00, 1.00) | 0.79 (0.67, 0.92) | 0.90 (0.80, 0.99) |
| Youden index | 1.00 (1.00, 1.00) | 0.56 (0.29, 0.82) | 0.79 (0.60, 0.99) |
| Optimal cut-off value | 4 | 16 | 17 |
NPV, Negative predictive value; PPV, Positive predictive value; LR+, Positive likelihood ratio; LR-, Negative likelihood ratio
Fig. 5.
ROC curve of diagnostic accuracy of the SleepImage Ring compared with standard polysomnography. a ROC curve for OSA diagnosis using AHI ≥ 5 events/h as the threshold; b ROC curve for OSA using AHI ≥ 15 events/h as the threshold; c ROC curve for OSA using AHI ≥ 30 events/h as the threshold. AHI, apnea -hypopnea index; sAHI, SleepImage apnea-hypopnea index; OSA, obstructive sleep apnea; ROC, receiver operating characteristic
Previous polygraphy results for reference
Of these 39 patients, 32 also provided the results of their latest polygraphy tests, and the interval between the polygraphy study and PSG was from 20 days to 18 months. Twelve patients had a time interval of less than 3 months. The Spearman correlation coefficient of AHI between polygraphy and PSG was 0.57 (P < 0.001) whereas the Spearman correlation coefficient between the SleepImage Ring and PSG was 0.91 (P < 0.001) among these 32 patients. When the diagnostic standard was set as PSG-AHI ≥ 15 events/hour, the AUCs of SleepImage Ring and polygraphy were 0.938 and 0.78, respectively (Supplementary). The difference between areas was 0.15 (P = 0.08).
Discussion
This study indicates a high level of agreement and a significantly positive correlation between the sAHI obtained from the SleepImage Ring and the AHI from in-laboratory PSG. Additionally, we also found that the SleepImage Ring had a significant sensitivity, specificity, and agreement compared with PSG in both identifying the presence of OSA and categorizing its severity. These results suggest that the SleepImage Ring could potentially be a great alternative diagnostic tool for OSA.
Since the CPC analysis was proposed by Thomas et al. in 2005, extensive clinical studies have evaluated its feasibility and accuracy in identifying OSA [17–20]. One of the validation studies was conducted by Lu et al. [20] in China, they assessed one portable device based on the CPC technique, and suggested the overall performance of this device was acceptable with the AUCs being 0.79, 0.79, and 0.86 across different OSA severity (mild, moderate, and severe). However, the device they used extracted signals from a single-lead ECG and generated AHI using the e-LFC pattern. There are two subsets in the e-LFC spectrum: narrow-band (an indicator of periodicity) and broad-band (an indicator of fragmentation) e-LFC spectra. Other causes of sleep fragmentation (e.g., fibromyalgia, depression) can produce similar patterns and thus may affect the accuracy of the AHI [21, 22]. To reduce the aforementioned limitations, a new AHI calculation method that combines SpO2 fluctuations with CPC data was proposed. Al Ashry et al. [15] analyzed ECG and pulse-oximeter tracings on PSG from a prospective clinical trial and generated the derived-AHI using the SleepImage system. The derived-AHI was validated to be comparable with the manual scoring AHI of PSG and approved by FDA both in adults and children [15, 23]. The SleepImage Ring, a new generation portable sleep monitoring device, could compute an AHI based on CPC-oximetry output from a PPG sensor and offer clinical users access to raw data, however, it has yet to be validated in a clinical context. To our knowledge, this is the first study to evaluate the diagnostic performance of the SleepImage Ring in identifying OSA and categorizing its severity.
In this study, we found a strong correlation and high agreement between the sAHI and the manually scored PSG-AHI, which is in line with the results of the technology validation study and furtherly affirms the feasibility of the SleepImage Ring in assessing OSA. We observed from the Bland-Altman plot that there is a significant difference between the AHI and sAHI in two patients (96.5 events/hour vs. 59.0 events/hour and 88.3 events/hour vs. 53.0 events/hour, respectively). Fortunately, all values indicated severe OSA, so this difference has a relatively minimal impact on clinical diagnosis and subsequent treatment. Regarding the performance testing, although both sensitivity and specificity of the SleepImage Ring are 100% in diagnosing OSA (AHI ≥ 5), only 2 patients had an AHI of less than 5 in the current study. Therefore, the false positive rate needs to be interpreted with caution. In other words, it is difficult to achieve the conclusion that this portable device can accurately rule out non-OSA patients. When we set AHI ≥ 15 events/h as criteria, the sensitivity, and specificity of the SleepImage Ring were 87% and 69%, respectively. The corresponding false-positive rate and false-negative rate were somewhat high with a total of 8 subjects categorized incorrectly. We, therefore, compared the AHI values obtained by the two methods of these eight patients and observed that the absolute difference (sAHI, AHI) between the two measures was within 0.18–3.4 events/hour in 4 of these patients. In addition, we found the optimal cutoff value of the SleepImage Ring in detecting patients with moderate-severe OSA is 16, with unchanged sensitivity (87%) and increased specificity (81%). The SleepImage Ring had a relatively high sensitivity (88%), specificity (91%), and accuracy (90%) in diagnosing severe OSA.
We also performed a comparison of ROC curves for the SleepImage Ring and polygraphy, both with PSG-AHI ≥ 15 events/h as the classification variable. It appears that the SleepImgae Ring has better diagnostic performance than polygraphy, although the differences between regions are insignificant. Considering that the time interval between polygraphy and PSG testing is relatively large, where the time interval exceeds 3 months in the vast majority (62.5%) of patients, the results of the comparison are merely for reference.
Recently, there is a growing abundance of wearable sleep-tracking devices. Relative to PSG and level 3 polygraphs, these consumer sleep trackers are easy-to-use, inexpensive, and low-burden. Among them, WatchPAT is a wrist-worn wearable device that utilizes the peripheral arterial signal for OSA diagnosis [24]. However, it is a single-use product, so it cannot be used once again. In comparison to other PPG-based ring devices, the SleepImage Ring showed commendable overall performance: Circul Ring (87% sensitivity, 83% specificity, and 0.929 AUC with PSG-AHI ≥ 5 events/hour as threshold) [25], Belun Ring (95% sensitivity, 29% specificity, and 0.934 AUC with PSG-AHI ≥ 5 events/hour as threshold)[26], Morpheus Ox (80% sensitivity, 86% specificity, and 0.909 AUC with PSG-AHI ≥ 5 events/hour as threshold) [27]. SLEEPON Go2Sleep Ring can also monitor the severity of OSA; however, no validation study has been found yet. Another very well-known sleep-tracking ring in the Western world is the Oura Ring. Note that Oura Ring is not qualified for the medical diagnosis of sleep apnea. In contrast, the SleepImage Ring enables patients to record multiple nights of sleep in a natural sleep environment with high accuracy, thereby optimizing the management of sleep apnea.
This study has several limitations. First, the sample size is relatively small, especially the sample with an AHI of less than 5 events/h. As such, the clinical question regarding whether the SleepImage Ring can accurately rule out non-OSA patient needs to be explored in a larger population. Second, this study is limited by selection bias. All participants were recruited from the ward of the hospital sleep center. While most of the patients who came to the ward for PSG examination had already been screened for OSA using level 3 portable monitoring devices in the outpatient and wanted to seek further diagnosis and treatment. Third, we did not validate the performance of the SleepImage Ring in the home context where it is meant to be utilized. Fourth, the first-night effect of PSG may disturb sleep and affect signal quality. Finally, the failure rate of the SleepImage Ring test in the current study is relatively high. The likely reason is that most of our participants are elderly patients and may not be very good at using smartphones. We propose to add the option of data uploads for consumers, such as transferring data using data cables, which may effectively ensure data records.
Conclusions
In summary, the sAHI obtained from the SleepImage Ring had a high agreement with AHI from in-laboratory PSG in assessing the presence and severity of OSA. Therefore, the SleepImage Ring has the potential to be a clinically reliable tool and cheaper alternative to the gold standard PSG when aiming to diagnose OSA in adults.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the staff of the Interdisciplinary Sleep Medical Center, Charité University Medicine Berlin, for their help with this project and thank SleepImage for providing the ring devices for this study.
Author contribution
Conception and design: Mi Lu, Thomas Penzel, and Lisa Rosenblum. Data collection: Mi Lu and Lisa Brenzinger. Data analysis: Mi Lu. Manuscript drafting: Mi Lu. Manuscript revision: Mi Lu, Matthew Salanitro, Thomas Penzel, Ingo Fietze, Martin Glos and Giuseppe Fico. All authors read and approved the final manuscript.
Funding
Parts of the study were financially supported by the EU Horizon 2020 project ODIN #101017331. Mi Lu was financially supported by the China Scholarship Council for her visiting PhD study at Charité University Medicine Berlin. The China Scholarship Council had no role in the design or conduct of this study.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Charité University Medicine Berlin (EA1/093/22).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figure(s) 1 and 2.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–98. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fietze I, Laharnar N, Obst A, Ewert R, Felix SB, Garcia C, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—results of SHIP-Trend. J Sleep Res. 2019;28(5):e12770. doi: 10.1111/jsr.12770. [DOI] [PubMed] [Google Scholar]
- 5.Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144(3):e56–e67. doi: 10.1161/CIR.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 6.Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered Breathing and Cardiovascular Disease: JACC State-of-the-art review. J Am Coll Cardiol. 2021;78(6):608–24. doi: 10.1016/j.jacc.2021.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, Safer-Zadeh E, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10(7):753–8. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–47. doi: 10.5664/jcsm.27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabil A, Marien C, LeVaillant M, Baffet G, Meslier N, Gagnadoux F. Diagnosis of sleep apnea without sensors on the patient’s face. J Clin Sleep Med. 2020;16(7):1161–9. doi: 10.5664/jcsm.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28(9):1151–61. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 13.Lu M, Penzel T, Thomas RJ. Cardiopulmonary coupling. Adv Exp Med Biol. 2022;1384:185–204. doi: 10.1007/978-3-031-06413-5_11. [DOI] [PubMed] [Google Scholar]
- 14.Thomas RJ, Mietus JE, Peng CK, Gilmartin G, Daly RW, Goldberger AL, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30(12):1756–69. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Ashry HS, Hilmisson H, Ni Y, Thomas RJ, Investigators A. Automated apnea-hypopnea index from oximetry and spectral analysis of cardiopulmonary coupling. Ann Am Thorac Soc. 2021;18(5):876–83. doi: 10.1513/AnnalsATS.202005-510OC. [DOI] [PubMed] [Google Scholar]
- 16.Seo MY, Yoo J, Hwang SJ, Lee SH. Diagnosis of obstructive sleep apnea in adults using the Cardiopulmonary Coupling-Derived Software-Generated Apnea-Hypopnea Index. Clin Exp Otorhinolaryngol. 2021;14(4):424–6. doi: 10.21053/ceo.2020.01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnusdottir S, Hilmisson H. Ambulatory screening tool for sleep apnea: analyzing a single-lead electrocardiogram signal (ECG) Sleep Breath. 2018;22(2):421–9. doi: 10.1007/s11325-017-1566-6. [DOI] [PubMed] [Google Scholar]
- 18.Hilmisson H, Lange N, Duntley SP. Sleep apnea detection: accuracy of using automated ECG analysis compared to manually scored polysomnography (apnea hypopnea index) Sleep Breath. 2019;23(1):125–33. doi: 10.1007/s11325-018-1672-0. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Sun S, Zhang M, Guo D, Liu AR, Wei Y, et al. Electrocardiogram-based sleep analysis for sleep apnea screening and diagnosis. Sleep Breath. 2020;24(1):231–40. doi: 10.1007/s11325-019-01874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu M, Fang F, Sanderson JE, Ma C, Wang Q, Zhan X, et al. Validation of a portable monitoring device for the diagnosis of obstructive sleep apnea: electrocardiogram-based cardiopulmonary coupling. Sleep Breath. 2019;23(4):1371–8. doi: 10.1007/s11325-019-01922-3. [DOI] [PubMed] [Google Scholar]
- 21.Thomas RJ, Mietus JE, Peng CK, Goldberger AL, Crofford LJ, Chervin RD. Impaired sleep quality in fibromyalgia: detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep Med. 2010;11(5):497–8. doi: 10.1016/j.sleep.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang AC, Yang CH, Hong CJ, Tsai SJ, Kuo CH, Peng CK, et al. Sleep state instabilities in major depressive disorder: detection and quantification with electrocardiogram-based cardiopulmonary coupling analysis. Psychophysiology. 2011;48(2):285–91. doi: 10.1111/j.1469-8986.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilmisson H, Berman S, Magnusdottir S. Sleep apnea diagnosis in children using software-generated apnea-hypopnea index (AHI) derived from data recorded with a single photoplethysmogram sensor (PPG): results from the Childhood Adenotonsillectomy Study (CHAT) based on cardiopulmonary coupling analysis. Sleep Breath. 2020;24(4):1739–49. doi: 10.1007/s11325-020-02049-6. [DOI] [PubMed] [Google Scholar]
- 24.Park CY, Hong JH, Lee JH, Lee KE, Cho HS, Lim SJ, et al. Clinical usefulness of watch-PAT for assessing the surgical results of obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(1):43–7. doi: 10.5664/jcsm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R, Xue J, Zhang X, Peng M, Li J, Zhou B, et al. Comparison of Ring Pulse Oximetry using reflective photoplethysmography and PSG in the detection of OSA in Chinese adults: a pilot study. Nat Sci Sleep. 2022;14:1427–36. doi: 10.2147/NSS.S367400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu W, Leung L, Kwok KC, Wu IC, Folz RJ, Chiang AA. Belun Ring platform: a novel home sleep apnea testing system for assessment of obstructive sleep apnea. J Clin Sleep Med. 2020;16(9):1611–7. doi: 10.5664/jcsm.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romem A, Romem A, Koldobskiy D, Scharf SM. Diagnosis of obstructive sleep apnea using pulse oximeter derived photoplethysmographic signals. J Clin Sleep Med. 2014;10(3):285–90. doi: 10.5664/jcsm.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.