Abstract
Toxoplasma gondii (T. gondii) is a parasite that obtains the iron it needs for its own metabolism from the host-cell iron pool. In this work, we aimed to investigate if iron supplementation or deficiency affected the course of T. gondii infection. Eighty mice were divided into four groups, each with 20 animals: Group (I): Uninfected control group. Group (II): Infected control group: injected with Phosphate buffered saline. Group (III): Infected group: received iron sucrose treatment. Group (IV): Infected group: treated with deferoxamine. Quantitative PCR studies were performed on days 3 and 8 post-infection to detect the expression of iron metabolism genes (hamp and ferroprotin) and immune-histochemical analysis to study the percentage of TNF-α and TGF-β tissue expression. Iron supplementation induced progressions of infection evident by increased tissue expression of pro-inflammatory cytokine TNF-α and downregulation of TGF-β which is mostly linked to suppression of the inflammatory process caused by T. gondii. Increased expression of TGF-β and decreased expression of TNF-α was noticed when iron deprivation occurred. On day 3, we noticed increased expression in the hamp gene with iron supplementation while it decreases when the iron supply is low. On the contrary, iron deficiency increased ferroprotin gene expression whereas supplementing decreased it. On day 8, the level of expression of these genes returned to normal levels. These observations document the potential role of iron in controlling toxoplasmosis infection and indicate that the transcription of hamp and ferroprotin in T. gondii-infected cells appears to be regulated by a sophisticated indirect mechanism.
Keywords: T. gondii, q-PCR, Immunohistochemistry, TGF-β, TNF-α
Introduction
Toxoplasma gondii (T. gondii) is a common intracellular parasite that causes severe pathology in people with impaired immune systems (Pamukcu et al. 2021) and possibly fatal illness in innately affected children (Cannella et al. 2014). Nearly 190,000 people worldwide are affected by congenital toxoplasmosis each year, resulting in a large disease burden of 1.2 million disability-adjusted life years (Saad et al. 2020). Toxoplasmosis is the second most frequent cause of death due to food-borne disease (Innes et al. 2019). Eating fruits and vegetables that have been contaminated with oocysts from cat faces or raw or semi-raw meat that has cysts is the source of the spreading of the infection (Robert et al. 2012). More than 40 million Americans have this parasite, according to the Centers for Disease Control and Prevention (CDC), which listed toxoplasmosis as one of the neglected parasitic illnesses that need public health action control (Daher et al. 2021).
For both the prevention and treatment of toxoplasmosis, pyrimethamine, and sulfadiazine are indicated (Montazeri et al. 2018). These drugs are not advised for use in patients who are immunocompromised or pregnant (Dunay et al. 2018). Despite their effectiveness, side effects such as pyrimethamine-related hematological toxicity and bone marrow suppression, as well as sulfadiazine-related hypersensitivity and allergic skin reactions are frequently reported (Georgiev 1994). Given the inefficacy of these drugs to treat chronic toxoplasmosis and the documented observations of drug resistance (Silva et al. 2019), the development of new toxoplasmosis therapies is imperative. Nevertheless, the major challenge to developing such new drugs is finding substances that can reach the protozoan within the host cell at concentrations harmful to the parasite but not to the host (Portes et al.2015).
Iron is a vital component of the body and is involved in numerous bodily systems (Gunawardena and Dunlap 2012). Numerous vital cellular functions such as oxygen binding and transport, Adenosine triphosphate (ATP) production, deoxyribonucleic acid (DNA) creation, and repair (Santana-Codina and Mancias 2018) and heme biosynthesis depend on iron (Bergmann et al. 2020). Although excess iron has the potential to be damaged due to its capacity to produce free oxygen radicals, iron is necessary for many different cell processes. It is therefore strictly regulated at the cellular and systemic levels to avoid both insufficiency and overload (Wang and Babitt 2019). Mammals don't have an active excretory system; therefore, their bodies regulate their iron levels by limiting intestinal iron absorption and continually recycling and utilizing cellular iron. Cells are safeguarded from free iron toxicity by a number of safety mechanisms, including export through Ferroprotein (Camaschella 2013). The Ferroportin-hepcidin axis (FPN1-HAMP), is one of the underlying processes (Soares and Weiss 2015).
These transport channels are essential for host defense and decrease microbial survival and virulence; hence they must not be interfered with (Weinberg 2009). According to elemental analyses, about 5% of the atoms in eukaryotes are iron atoms (Al-Sandaqchi et al. 2018). In the literature, there is limited research on the effect of iron on different parasites. The iron chelator: deferoxamine (DFO), which is frequently used to treat iron overload, could be an effective treatment for T. cruzi infection (Arantes et al. 2007). Mice treated with DFO displayed a reduction in the proliferation of L. infantum in the spleen and liver (Malafaia et al. 2011). Moreover, it was discovered that using DFO to lower iron availability caused a pronounced and dose-dependent suppression of plasmodium formation (Portugal et al. 2011). To prevent the spread of infections, iron chelators may be used in combination with treatments that target the iron uptake routes of the microorganisms to reduce their ability to survive (Chhabra et al. 2020). Due to the T. gondii requirement for proliferation and the uptake of iron by the enterocytes prior to spreading to other organs, we set out to investigate the impact of iron addition or deprivation on the outcome of T. gondii infection in experimentally infected mice using immunohistochemical analysis and molecular assay.
Material and methods
Animal and parasites
Eighty laboratory-bred male Swiss albino mice about eight weeks old weighing (20-25gm) were involved in this study and classified into four groups (20 mice each): Group (I): Non-infected control-negative group. Group (II): Toxoplasmosis-infected control-positive group that was injected with vehicle (phosphate buffered saline) (PBS) intraperitoneally (I.P) one day before infection and for an additional seven day post-infection (p.i). Group (III): Infected group received iron sucrose (iron supplementation group). Group (IV): Infected group received deferoxamine (iron deprivation group). T. gondii strain (ME-49 strain) was obtained from the Parasitology Department, Faculty of Medicine, Zagazig University.
Experimental design
Except for the control negative group, all mice of other groups were infected orally on day 0. Infection was induced by administering 25 cysts per mouse orally using a stomach tube. This infection was obtained from another mouse brain that was infected 45–60 days before (Fuentes-Castro et al. 2017). Iron supplementation and deprivation interventions were done one day before infection and for an additional seven-day post-infection. On day 3 p.i, 10 mice from all groups were sacrificed by cervical dislocation for quantitative PCR (qPCR) assay on small intestine (S.I) samples for the expression genes involved in iron absorption. On day 8 p.i remaining animals of all groups were sacrificed, and S.I samples were collected for qPCR and immunohistochemical analysis to detect tissue expression of TNF-α and TGF-β cytokines.
Drugs
For iron supplementation
Iron sucrose; sucroferric oxyhydroxide or iron saccharate (C12H29Fe5Na2O23) (scrofer, Amoun pharmaceutical company, Egypt) (80 mg\kg) was used as ampule I.P one day before infection and for an additional seven-day postinfection (Kuo et al. 2014).
For iron chelation or deprivation Deferoxamine; Desferrioxamine B mesylate or Desferrioxamine mesylate (C26H52N6O11S) (Desferal, Novartis, Egypt) (300 mg\ kg) was used as vials I.P one day before infection and for an additional seven-day post-infection (Oliveira et al. 2020).
Techniques used to study the effect of iron supplementation or deprivations on toxoplasmosis infection outcome
Immunohistochemistry to detect tissue expression of TNF-α and TGF-β
The standard immunohistochemical methods were adopted by Bancroft and Gamble (2008). Briefly, small intestine tissue sections were mounted on positively charged glass slides (Biogenex, USA), and deparaffinized in xylene. After xylene was removed by absolute ethanol, slides were placed in an unsealed plastic container filled with sufficient antigen retrieval solution (Citrate buffer solution, pH 6) and microwaved for 5 min at power 10. The container was removed and allowed to cool for 15 min and the slides were washed in deionized water several times and then placed in phosphate buffer saline (PBS) for 5 min. Tissue sections were incubated with an endogenous peroxidase-blocking reagent containing hydrogen peroxide and sodium azide (DAKO peroxidase blocking reagent, Cat. No. S 2001). Excess buffer was blotted off, and the slides were allowed to dry except for the tissue section. The supersensitive primary monoclonal antibody against both TNF-α and the transforming growth factor-β (TGF-β) was added to the sections and incubated for 60 min at room temperature then, the slides were rinsed in PBS, incubated with a biotin-streptavidin (BSA) system. After that, 1–2 drops of the ready-to-use DAKO EnVision system were applied for 20 min at room temperature and rinsed again with PBS. Diaminobenzidine (DAB) was used as a chromogen. The slides were mixed for 10–20 min until a desirable brown color was obtained then counterstained with Mayer’s hematoxylin. The average grayscale of the positive cells was automatically calculated. The immune reactive intensity was expressed by the average grayscale. Values < 160 were considered low, 160–170 medium, and 170–180 high (Hashish and Kamal 2015). Quantitative scoring method of TNFα and TGF-β: immune-activity quantitation was made by digital image analysis through the image J analysis software on five fields from each slide. This software could measure the total positive stained brown color/areas throughout the unstained cells. From this data, an index (positively stained cells per a total of 1000 cells) can be computed.
Relative quantification of mRNA by qPCR
Small intestine sample mRNA was harvested using TRIzol reagent according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA, USA). RNA concentration was determined (GeneQuant 1300 spectrophotometer, GE Healthcare, Uppsala, Sweden), and complementary DNA (cDNA) was synthesized using 5 ng/mL mRNA through reverse transcription reaction following the manufacturer’s instructions (Promega, Madison, WI, USA). Quantitative PCR (qPCR) assays were performed using GoTAq® qPCR (Master Mix, Promega, Madison, WI, USA) in Applied Biosystems 7500 Real-Time PCR System (Life Technologies). Assays were performed at 40 cycles with melting temperature (TM) at 60 ˚C (30 s) Samples Ct data (cycle threshold) were normalized to the expression of reference gene control (ß- actin) and the relative expression of each studied gene was analyzed by the 2-ΔΔCt method (Livak and Schmittgen 2001). The sequences of the analyzed genes were ferroportin, F, 50-CTGTGTTTCTGGTGGAACTCTATGG-30, and R, 50-TCTTATCCACCCAGTCACCAATG -30; and hamp, F, 50-AGCCTGAGCAGCACCACCT-30, and R, 50- CAATGTCTGCCCTGCTTTCTT-30. (Olivera et al. 2020). ß-actin F: CAGCCTTCCTTCTTG GGTAT, R: TGGCATAGAGGTCTTTACGG (Dou et al. 2013).
Ethical consideration
Mice were maintained in accordance with the research protocols following the recommendations of the National Institutes of Health (NIH) guide for the care and use of laboratory animals, Faculty of Medicine, Zagazig University. All surgeries were done under anesthesia and all efforts were made to ensure minimal animal suffering. As T. gondii is a bio-safety level 2 (Bl-2) pathogen, appropriate precautions were followed when handling the parasite. Care was taken to avoid infection of assisting personnel during the parasite-animal passage. The study protocol was approved by the Parasitology Department Review Board and ZU-IACUC committee (ZU-IACUC/3/F/34/2021).
Statistical analysis
Quantitative values of the measured parameters were expressed as mean ± standard deviation (SD). Data were analyzed by two-way ANOVA with Tukey's post-hock test to determine the significance of differences between studied groups using Statistical Package for Social Sciences (SPSS), version 25.0. All statistical tests were considered significant at p ≤ 0.05 and highly significant at p ≤ 0.01.
Results
Immunohistochemical results
Tumor necrosis factor alpha (TNF-α)
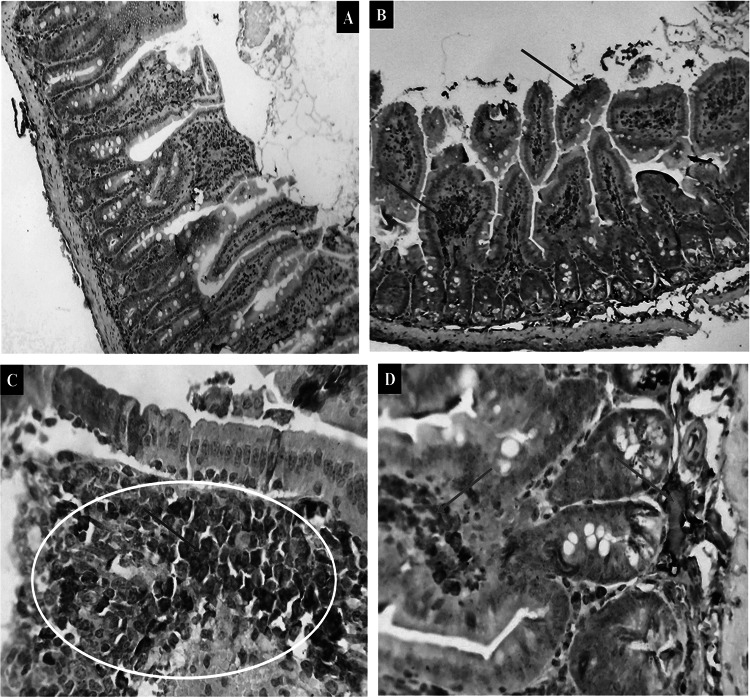
Expressions of TNF- α were mostly linked to the inflammatory process caused by T. gondii. Intestinal tissues of GI showed a negative reaction (Fig. 1A). Note the brownish immune reaction of the immune cells that ordinarily reside in the gut. GII exhibited a little cytoplasmic reaction (Fig. 1B). Tissues from group GIV with light immune reactivity showed almost the same immune reactivity (Fig. 1D). The intestinal mucosa had considerable brownish cytoplasmic reactivity, and submucosal inflammatory cells were seen in GIII, which also displayed comparable but stronger immunological reactivity (Fig. 1C).
Fig. 1.
Photomicrographs showed cytoplasmic immune reactivity of the intestinal tissues against TNF-α in the different experimental groups (green arrows and the yellow circle). A The expression is almost negative in (GI) X200. B moderate in (GII) X200. C strong in (GIII) X400. D mild in GIV X400
Transforming growth factor beta (TGF-β)
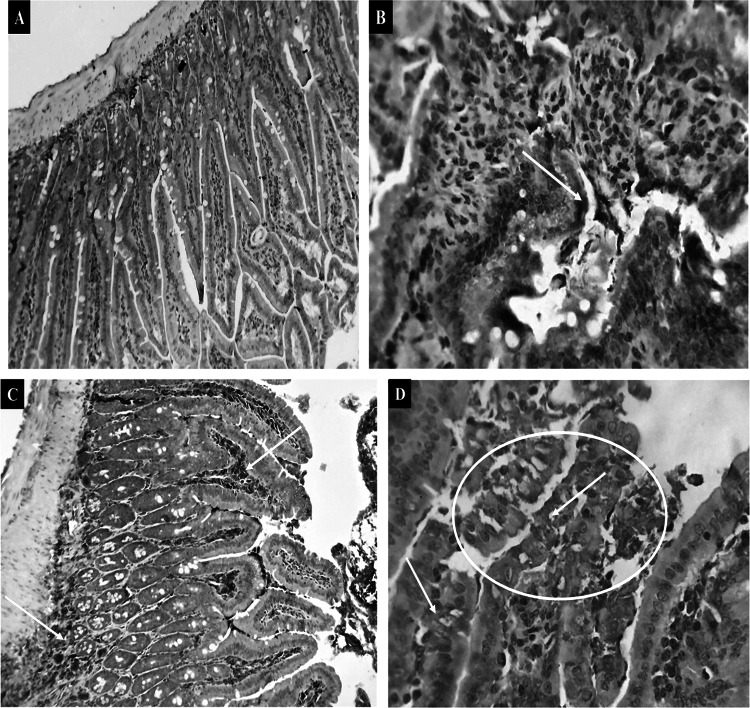
Aside from a slight brownish immunostaining of the intestinal cells, which generally infiltrates inflammatory mucosal and submucosal round cells, intestinal mucosa cells in the non-infected control group (GI) showed negative immune reactivity for TGF- β. In contrast, the immune reactive staining reaction was typical of GII, where a significant proportion of the intestinal mucosa and glandular epithelium as well as infiltrated or aggregated round cells had a considerable brownish cytoplasmic affinity to the employed marker. Intestinal sections of GIII denoted mild immune reactivity to TGF-β except for some intestinal submucosal round cells that were mildly stained. GIV demonstrated a strong number of reactive intestinal mucosal and glandular cells beside the infiltrated or aggregated round cells (Fig. 2).
Fig. 2.
Photomicrographs showed the cytoplasmic reactivity of the intestinal tissues against TGF-β in the different experimental groups (green arrows and the yellow circle). A The expression is almost negative in (GI) X200. B moderate in (GII) X400. C mild in (GIII) X 200. D strong in (GIV) X 400
*Morphometric Findings
Estimation of immune-reactive cells of TNF- α and TGF- β in various groups was done using Image J software analysis. Following GIII in order of largest number of TNF- α immune reactive cells were GII and GIV. The percentage of positive cells in the negative control group was the lowest. According to TGF- β findings, GIV (the iron deprivation group) had the most positively stained cells, followed by GII, GIII, and GI, in that order (Table 1).
Table 1.
The percentage of TNF-α and TGF-β positive cells
| TGF-β | TNF-α | Group |
|---|---|---|
| 6.78133 | 5.42333 | GI |
| 35.4867 | 43.5133 | GII |
| 15.99333 | 65.725 | GIII |
| 43.07733 | 11.41667 | GIV |
II. Quantitative real-time PCR (qPCR)
In this work, the rate of expression of genes (HAMP and ferroportin) involved in iron absorption was examined on small intestinal (S.I.) samples on days three and eight following infection. Results from Day 3 showed that GIII had much higher hamp expression than the other groups. GIV had greater levels of ferroportin expression as compared to other research groups (Table 2, Figs. 3, 4).
Table 2.
Day 3 qPCR results of HAMP and ferroportin levels among the studied groups
| Variable | Control GI | Toxoplasma GII | Iron GIII | Deferoxamine GIV | f | P value |
|---|---|---|---|---|---|---|
|
Fold change HAMP: Mean ± SD Range |
0.91 ± 0 0.89–0.93 |
8.9 ± 0 8.94–8.94 |
12.4 ± 5 11.8–12.8 |
3.6 ± 0.32 3.2- 3.9 |
293.7 | 0.000** |
|
Fold change Ferroportin: Mean ± SD Range |
1.05 ± 0 0.98–1.12 |
0.56 ± 0 0.57–0.57 |
0.28 ± 0.01 0.22–0.34 |
0.83 ± 0.04 0.78–0.86 |
89.4 | 0.000** |
**Highly significant = P < 0.001
Fig. 3.
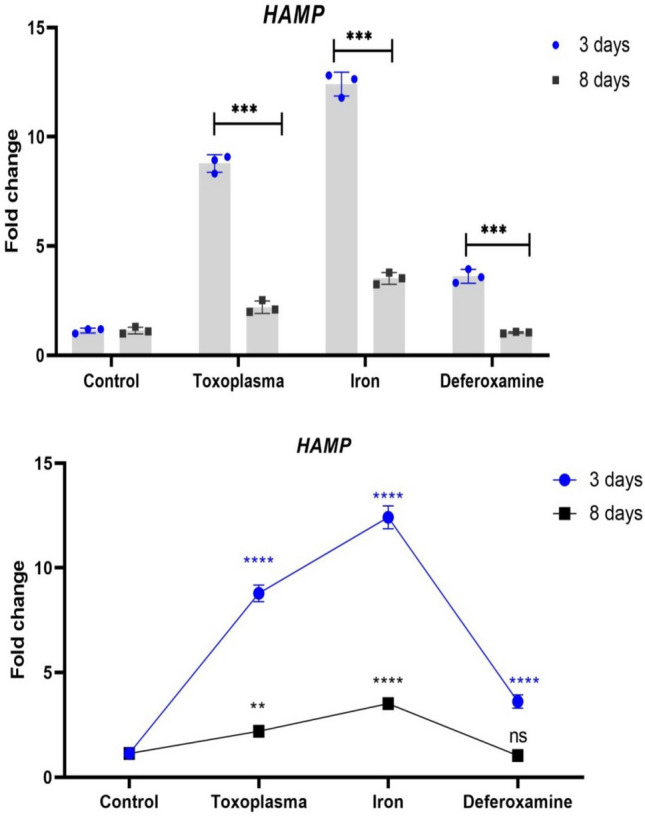
Differences in expression of HAMP between 3 and 8 days in each group. Each dot/ squar refers to the mean of 3replicates and error bar indicate the standard error. The stars refer to the significant differences in HAMP expression between each group and the controls at the respective time point as estimated by two-way ANOVA with Tukey’s post-hock test
Fig. 4.
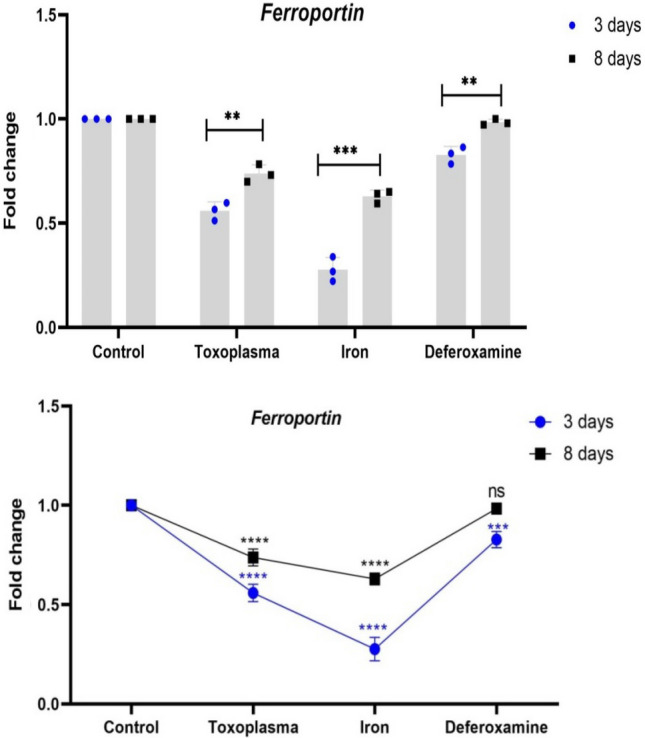
Differences in expression of ferroprotin between 3 and 8 days in each group. Each dot/ square refers to the mean of 3replicates and error bar indicate the standard error. The stars refer to the significant differences in ferroprotin expression between each group and the controls at the respective time point as estimated by two-way ANOVA with Tukey's post-hock test
On day 8, the expression level of hamp and ferroportin genes return to be around the level of the control group. There was a highly statistically significant difference between all studied groups as regard Fold change HAMP and Fold change Ferroportin (P < 0.001) (Table 3).
Table 3.
Day 8 qPCR results data of hamp and ferroportin among the studied groups:
| Variable | Control GI | Toxoplasma GII | Iron GIII | Deferoxamine GIV | f | P value |
|---|---|---|---|---|---|---|
|
Fold change HAMP: Mean ± SD Range |
0.915 ± 0 0.9–0.93 |
2.1 ± 0 2.1–2.1 |
3.5 ± 0.27 3.25–3.78 |
1 ± 0.036 1–1.07 |
127.1 | 0.000* |
|
Fold change Ferroportin: Mean ± SD Range |
1.06 ± 0 0.96–1.17 |
0.73 ± 0 0.73–0.73 |
0.63 ± 0.03 0.59–0.65 |
0.98 ± 0.01 0.97–1 |
174.6 | 0.000* |
**Highly significant = P < 0.001
Table 4 shows that among the iron group; there was a highly statistically significant difference between days 3 and 8 as regard the fold change of HAMP (p < 0.001). There was a statistically significant difference between days 3 and 8 as regard the fold change of ferroportin (p < 0.05). In the deferoxamine group, there was a statistically significant difference between days 3 and 8 as regard fold change of hamp and ferroportin (p < 0.05) (Table 5).
Table 4.
Comparison between days 3 and 8 among the Iron group:
| Variable | Day 3 | Day 8 | Mean difference | Paired t-test | P value |
|---|---|---|---|---|---|
|
Fold change HAMP: Mean ± SD |
12.4 ± 5.5 | 3.5 ± 0.27 | 8.89 ± 0.3 | 50.3 | 0.000** |
|
Fold change Ferroportin: Mean ± SD |
0.28 ± 0.01 | 0.63 ± 0.03 | − 0.35 ± 0.08 | − 7.1 | 0.019* |
**Highly significant (P < 0.001) *Significant (P < 0.05)
Table 5.
Comparison between days 3 and 8 among the deferoxamine group:
| Variable | Day 3 | Day 8 | Mean difference | Paired t-test | P value |
|---|---|---|---|---|---|
|
fold change HAMP: Mean ± SD |
3.6 ± 0.32 | 1 ± 0.036 | 2.6 ± 0.33 | 13.6 | 0.005* |
|
Fold change Ferroportin: Mean ± SD |
0.83 ± 0.04 | 0.98 ± 0.01 | 0.156 ± 0.05 | 5.1 | 0.036* |
*Significant (P < 0.05)
Discussion
Over one-third of the world's people are infected by the common pathogen T. gondii (Cerutti et al. 2020). In apicomplexan parasites, iron consumption is still poorly understood. Any warm-blooded species as well as any nucleated cell can become infected by T. gondii, exposing them to a variety of iron availability. We were concerned about the use of iron by T. gondii and the consequences of the host interfering with iron storage.
In the current study, immunohistochemical labeling of both TNF-α (a pro-inflammatory cytokine) and TGF-β (anti-inflammatory cytokine) levels has been demonstrated in intestinal tissues of different studied groups. Regarding TNF-α, a notable expression was detected in the intestine of Toxoplasma-infected mice in GII. This finding agrees with Bessa et al. (2012) and Meira et al. (2014) who claimed that TNF-α is crucial for causing effector functions to be activated against T. gondii during all phases of infection. According to Akdis et al. (2016), high concentrations of this cytokine trigger the Th1 cellular response, which in turn activates macrophages, encourages the creation of microbicidal substances like nitric oxide, and aids in the removal and prevention of tachyzoite reproduction. Blanchard et al. (2015) stated that TNF-α exerts a significant influence on the resistance to parasite reactivation during toxoplasmosis. Further, the reduction of parasite replication by TNF-α would seem to be important for macrophage activation, but this effect can only be achieved in conjunction with IFN-γ. Both the acute and chronic phases of the disease are affected by this protective effect in mice (Filisetti and Candolfi 2004).
Bout et al. (1999) found that T. gondii-infected enterocytes produce IL-l, IL-6, and TNF-α that can orient the adaptive immune response. In our study, after sucroferric oxyhydroxide supplementation, expression of TNF-α significantly increased in the intestine of mice in GIII. This observation agreed with Liline et al. (2022) who demonstrated that TNF-α expression was increased especially with iron supply and coupled with more inflammation and cell death. The observation of high levels of TNF-α in mice led to iron retention within the reticuloendothelial system and served to emphasize the crucial function of cytokines in inducing alterations in iron homeostasis in vivo (Alvarez et al. 1996). Importantly, the drop in TNF-α level in the desferrioxamine B mesylate treated group explains the effectiveness of this substance in preventing infection. Aghabi et al. (2021) stated that DFO chelates iron, which reduces inflammation and parasite survival. Additionally, it was mentioned that deferoxamine prevented T. gondii from growing in the small intestine. The reduced parasite burden found in the organ may be due to the parasites' need for iron for development (Gail et al. 2004). On the other hand, Olivera et al. (2020) reported that regardless of whether the mice were given DFO or iron treatment, the T. gondii infection elevated the TNF-α level systemically.
The transforming growth factor-β (TGF-β) family is a large and still growing group of structurally related cytokines. The members of the TGF-β superfamily are highly conserved through evolution and are present in nearly all multicellular organisms (Schmierer and Hill 2007). Production of TGF-β by antigen-presenting cells leads to the differentiation of induced regulatory T cells (iTregs) (Chen et al. 2010) which inhibit the protective T cell responses of the host and contributes to disease progression. This fact highlights the importance of this cytokine during parasitic infections (Salama et al. 2021).
In this study, we reported that expression of TGF-β increased in the enterocytes of mice in response to iron chelation (GIV) compared to control-positive mice. These observations agree with Misumi et al. (2008) who observed that DFO is associated with enhanced expression of TGF-β in experimentally infected rats. Moreover, Qayoom et al. (2019) also mentioned that deferoxamine played an important role in controlling infection and was associated with increased expression of TGF-β, especially in experimentally infected rats. Interestingly, Lykens et al. (2010) and Marchioro et al. (2018) observed that TGF-β levels were increased in the serum of infected pregnant women with T. gondii when compared with those of uninfected pregnant women and this was associated with the absence of congenital transmission. It should be remembered that this cytokine regulates the immune response by stimulating regulatory T cells. However, Raouf-Rahmati et al. (2021) demonstrated that toxoplasmosis induces a highly specific local immunoregulatory process as evidenced by the up-regulation of IL-10 and the downregulation of TGF-β mRNA. This could indicate an attempt to prevent unnecessary tissue damage. Excessive production and/or activation of TGF-β may modulate cellular survival, growth, migration, and invasion. Therefore, the expression levels of TGF-β are often upregulated in a wide spectrum of pathological conditions; emerging studies have suggested that TGF-β is also critical for tissue regeneration (Li et al. 2017). TGF-β acts as an immune regulatory cytokine. Its role in mucosal immunity and induction of Th17 may be important to reduce inflammation, nonetheless, based on the circumstances, it may increase host susceptibility to the parasite (Akdis et al. 2016).
In the current work, qPCR was used to examine how the T. gondii infection affected the (mRNA) expression of genes related to iron metabolism. Iron regulatory proteins post-transcriptionally control genes encoding proteins that modulate iron uptake, recycling, and storage and are regulated by iron. Hepcidin (hamp) is a 25 amino acid peptide hormone (Gwamaka et al. 2012). Hepcidin controls serum iron levels through ferroportin (FPN) degradation in iron-absorptive enterocytes and iron-recycling macrophages (Camaschella et al. 2020). In mice and rats, hamp expression has risen in the liver during an acute phase reaction (Pigeon et al. 2001). This liver hormone is crucial for iron-mediated mammalian defenses (Ganz 2011). By binding to the iron export protein ferroportin, this hormone suppresses iron efflux from macrophages, hepatocytes, and enterocytes, acting as a negative regulator of iron metabolism. Ferroportin degradation leads to cellular iron retention and decreased iron availability (Tandara and Salamunić 2012). Our research showed that on day 3 post-infection, hamp expression significantly increased in response to iron supplement, but ferroportin expression was downregulated. In contrast, hamp expression was downregulated whereas ferroprotin expression was elevated in control negative mice. In agreement with our results, Nemeth et al. (2004) observed that ferroportin is localized on the surface of absorptive intestinal enterocytes, and an increase in hepcidin induces its internalization and degradation. Theurl et al. (2009) explained that the reduction in ferroportin expression in enterocytes induced by hepcidin causes a decrease in the amount of iron absorbed resulting in a reduction of circulating iron levels, which is made worse by the restriction of iron export from macrophages.
In this study, a substantial drop in hamp expression in enterocytes RNA was seen with increased ferroportin expression when iron chelation was utilized in GIV. Oliveira et al. (2020) noted that increased hamp expression levels might cause ferroportin breakdown in enterocytes, promoting iron buildup within the cell, and hence T. gondii proliferation and infection progression. Abuga et al. (2022) have linked hypoferritinemia to low hepcidin levels even in the presence of inflammation. Kautz et al. (2014) mentioned that erythroferrone, which is formed in erythroblasts, has been found as blocking hepcidin and so providing more iron for hemoglobin synthesis in scenarios like stress erythropoiesis, which occurs in reaction to hypoferritinemia. Interestingly, Sagar et al. (2021) explained that inflammatory disorders restrict iron absorption due to elevated levels of circulating hamp. The increased production of hamp causes ubiquitination of ferroportin leading to its degradation, thereby retaining iron in the spleen, duodenal enterocytes, macrophages, and hepatocytes. Hamp inhibitors and antagonists play a consequential role in ameliorating inflammation-associated anemia. Also, Aghabi et al. (2021) reported that T. gondii responds to iron deficiency by altering the expression of genes responsible for iron metabolism to boost the parasite growth l. On the other hand, (Almeida et al. 2019) opposed this finding as no statistical difference was observed in hamp, and ferroportin mRNA expression levels during T. gondii infection.
In our investigation, when the infection progresses on day 8, the expression of the hamp and ferroportin genes returns to levels comparable to the control group. A similar observation was noticed by Oliveira et al. (2020). They reported an increase in hepcidin and a decrease in ferroprotin mRNA expression levels, suggesting that it could be a host-defense mechanism trying to retain the parasite proliferation in a more precocious phase of parasite entry. However, as the infection progresses, the parasite itself could be involved in the return of the expression of these genes to levels of non-infected animals, for its own benefit. Loreal et al. (2014) found an ultimate decrease in the iron export process mediated by the ferroportin of the reticulo-endothelial system. Thus, the interplay of hamp and ferroportin efficiently controls the flux of iron into plasma and the delivery of iron to iron-consuming tissues. Interestingly, non-iron metals influence hepcidin production at the transcriptional level, and it is repressed or increased in response to a variety of stimuli. The hypoferremic response to infection, for example, is regulated by the hamp, which is released from the liver in response to pro-inflammatory cytokines and the unfolded protein response inside the endoplasmic reticulum (Drakesmith and Prentice 2012).
In response to infections, neutrophils, and macrophages also synthesize hamp, and thus iron availability is modulated (Peyssonnaux et al. 2007). Furthermore, hamp-independent mechanisms that drive hypoferremic response to infection include cytokines such as IFN- γ, TNF- α, interleukin-1 (IL-1), and interleukin-6 (IL-6) that increase iron-withholding defenses by altering iron metabolism (Nairz et al. 2010). Kim and Nemeth (2015) stated that when the infection progresses and the intracellular iron level rises, cytoplasmic ferritin sequesters the iron. Moreover, iron regulatory proteins 1 and 2 regulate iron levels by binding to iron response elements in iron-depleted environments, enhancing the stability of mRNA of proteins related to iron absorption, and suppressing translation of numerous targets associated with iron sequestration or storage. Thus, depending on iron levels, iron regulatory proteins affect post-transcriptional regulation in two ways: as a translation enhancer and as a ferroportin inhibitor.
In conclusion, the current investigation highlighted the crucial role of iron as a nutrient for T. gondii growth, particularly in the small intestine following oral infection. This study found experimental evidence of an effect of iron supplementation on toxoplasmosis outcome, as well as the expression of the hamp and ferroprotin genes. Our results reported increased expression in the hamp gene with iron supplementation while it decreases when the iron supply is low. On the contrary, iron deficiency increased ferroprotin gene expression whereas supplementing decreased it. Our findings suggest that hamp and ferroprotin expressions in T. gondii-infected cells appear to be mediated by complicated indirect pathways involved in release of thus far uncharacterized secreted factors. Additional experiments are necessary to clarify these points.
Author contributions
All authors contributed to the research conception and design. Material preparation was performed by EM, and data collection and analyses were performed by SHY, EM, and ESE. The study was finalized by FAMSA. The first draft of the manuscript was written by AIMI and all authors reviewed the previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
All authors confirm that No fund has been received for this paper.
Declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abuga KM, Muriuki JM, Uyoga SM, Mwai K, Makale J, et al. Hepcidin regulation in Kenyan children with severe malaria and non-typhoidal Salmonella bacteremia. Haematologica. 2022;107(7):589. doi: 10.3324/haematol.2021.279316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghabi D, Sloan M, Dou Z, Guerra AJ, Harding CR. The vacuolar iron transporter mediates iron detoxification in Toxoplasma gondii. Biorxiv. 2021 doi: 10.1101/2021.09.08.458725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Almeida MPO, Ferro EAV, Briceño MPP, Oliveira MC, Barbosa BF, et al. Susceptibility of human villous (bewo) and extravillous (HTR-8/svneo) trophoblast cells to Toxoplasma gondii infection is modulated by intracellular iron availability. Parasitol Res. 2019;118(5):1559–1572. doi: 10.1007/s00436-019-06257-2. [DOI] [PubMed] [Google Scholar]
- Al-Sandaqchi AT, Brignell C, Collingwood JF, Geraki K, Mirkes EM, et al. Metallome of cerebrovascular endothelial cells infected with Toxoplasma gondii using μ-XRF imaging and inductively coupled plasma mass spectrometry. METAJS. 2018;10(10):1401–1414. doi: 10.1039/c8mt00136g. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Franco A, Fernández-Novoa L, Cacabelos R. Blood levels of histamine, IL-1β, and TNF-α in patients with mild to moderate Alzheimer disease. Mol Chem Neuropathol. 1996;29(2):237–252. doi: 10.1007/BF02815005. [DOI] [PubMed] [Google Scholar]
- Arantes JM, Pedrosa ML, Martins HR, Veloso VM, de Lana M, et al. Trypanosoma cruzi: treatment with the iron chelator desferrioxamine reduces parasitemia and mortality in experimentally infected mice. Exp Parasitol. 2007;117(1):43–50. doi: 10.1016/j.exppara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 6. New York: Churchill Livingstone/Elsevier; 2008. pp. 726–744. [Google Scholar]
- Bergmann A, Floyd K, Key M, Dameron C, Rees KC, Thornton LB, Whitehead DC, Hamza I, Dou Z. Toxoplasma gondii requires its plant-like heme biosynthesis pathway for infection. PLoS Pathog. 2020;16(5):e1008499. doi: 10.1371/journal.ppat.1008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa TF, Cordeiro CA, Gonçalves RM, Young LH, Campos WR, et al. Increased serum levels of soluble tumor necrosis factor receptor-2 (sTNFR2) in patients with active toxoplasmic retinochoroiditis. Braz J Infect Dis. 2012;16:540–544. doi: 10.1016/j.bjid.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Blanchard N, Dunay IR, Schlüter D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. 2015;37:150–158. doi: 10.1111/pim.12173. [DOI] [PubMed] [Google Scholar]
- Bout D, Moretto M, Dimier-Poisson I, Gatel DB. Interaction between Toxoplasma gondii and Enterocyte. Immunobiol. 1999;201(2):225–228. doi: 10.1016/S0171-2985(99)80062-X. [DOI] [PubMed] [Google Scholar]
- Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematol Educ Progr Am Soc Hematol. 2013;1:1–8. doi: 10.1182/asheducation-2013.1.1. [DOI] [PubMed] [Google Scholar]
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260. doi: 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella D, Brenier-Pinchart MP, Braun L, van Rooyen JM, Bougdour A, Bastien O, et al. Mir-146a and mir155 delineate a microrna fingerprint associated with Toxoplasma persistence in the host brain. Cell Rep. 2014;6(5):928–937. doi: 10.1016/j.celrep.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Blanchard N, Besteiro S. The bradyzoite: a key developmental stage for the persistence and pathogenesis of toxoplasmosis. Pathogens. 2020;9(3):234. doi: 10.3390/pathogens9030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Konkel JE. TGF-beta and ‘‘adaptive’’ Foxp3(?) regulatory T cells. J Mol Cell Biol. 2010;2(1):30–36. doi: 10.1093/jmcb/mjp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R, Saha A, Chamani A, Schneider N, Shah R, et al. Iron pathways and iron chelation approaches in viral, microbial, and fungal infections. Pharmaceuticals. 2020;13(10):275. doi: 10.3390/ph13100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher D, Shaghlil A, Sobh E, Hamie M, Hassan ME, et al. Comprehensive overview of Toxoplasma gondii-induced and associated diseases. Pathogens. 2021;10(11):1351. doi: 10.3390/pathogens10111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Sun A, Zhang E, Ding L, et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κb signalling. Br J Nutr. 2013;110(4):599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. Treatment of toxoplasmosis: historical perspective. Animal Clin Microbiol Infect. 2018;31:1–33. doi: 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filisetti D, Candolfi E. Immune response to Toxoplasma gondii. Ann Ist Super Sanita. 2004;40(1):71–80. [PubMed] [Google Scholar]
- Fuentes-Castro BE, Reyes-García JG, Valenzuela-Vargas MT, Martínez-Gómez F. Histopathology of murine toxoplasmosis under treatment with dialyzable leukocyte extract. Inst Oswaldo Cruz. 2017;112:741–747. doi: 10.1590/0074-02760170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail M, Gross U, Bohne W. Transferrin receptor induction in Toxoplasma gondii-infected HFF is associated with increased iron-responsive protein 1 activity and is mediated by secreted factors. Parasitol Res. 2004;94(3):233–239. doi: 10.1007/s00436-004-1209-2. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev VS. Management of toxoplasmosis. Drugs. 1994;48:179–188. doi: 10.2165/00003495-199448020-00005. [DOI] [PubMed] [Google Scholar]
- Gunawardena S, Dunlap ME. Anemia and iron deficiency in heart failure. Curr Heart Failure Rep. 2012;9(4):319–327. doi: 10.1007/s11897-012-0112-x. [DOI] [PubMed] [Google Scholar]
- Gwamaka M, Kurtis JD, Sorensen BE, Holte S, Morrison R, et al. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis. 2012;54(8):1137–1144. doi: 10.1093/cid/cis010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashish H, Kamal R. Effect of curcumin on the expression of Caspase-3 and Bcl-2 in the spleen of diabetic rats. J Exp Clin Anat. 2015;14(1):18–23. doi: 10.1126/science.1104742. [DOI] [Google Scholar]
- Innes EA, Hamiliton C, Garcia JL, Chryssafidis A, Smith D. A one health approach to vaccines against Toxoplasma gondii. Food Waterborne Parasitol. 2019;15:e00053. doi: 10.1016/j.fawpar.2019.e00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz L, Jung G, Nemeth E, Ganz T. Erythroferrone contributes to recovery from anemia of inflammation. Blood, Am J Hematol. 2014;124(16):2569–2574. doi: 10.1182/blood-2014-06-584607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015;22(3):199. doi: 10.1097/MOH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo KL, Hung SC, Lee TS, Tarng DC. Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD. J Am Soc Nephrol. 2014;25(11):2596–2606. doi: 10.1681/ASN.2013080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gu X, Yi S. The regulatory effects of transforming growth factor-β on nerve regeneration. Cell Transplant. 2017;26(3):381–394. doi: 10.3727/096368916X693824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liline S, Rumahlatu D, Salmanu SI, Pattipeilohy M, Sangur K. Bioaccumulation of Chromium, Iron, and the Expression of TNF-α and Caspase-3 in Mudskipper (Periophthalmus spp.) from Ambon Island Waters, Indonesia. J Ecol Eng. 2022;23(7):104–190. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loreal O, Cavey T, Bardou-Jacquet E, Guggenbuhl P, Ropert M, et al. Iron, hepcidin, and the metal connection. Front Pharmacol. 2014;5:128. doi: 10.3389/fphar.2014.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykens JE, Terrell CE, Zoller EE, Divanovic S, Trompette A, et al. Mice with a selective impairment of IFN-γ signaling in macrophage lineage cells demonstrate the critical role of IFN-γ–Activated macrophages for the control of Protozoan parasitic infections in vivo. J Immunol. 2010;184(2):877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafaia G, MarconLde N, PereiraLde F, Pedrosa ML, Rezende SA. Leishmania chagasi: effect of the iron deficiency on the infection in BALB/c mice. Exp Parasitol. 2011;127:719–723. doi: 10.1016/j.exppara.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Marchioro AA, Colli CM, de Souza CZ, da Silva SS, Tiyo BT, et al. Analysis of cytokines IFN-γ, TNF-α, TGF-β and nitric oxide in amniotic fluid and serum of pregnant women with toxoplasmosis in southern Brazil. Cytokine. 2018;106:35–39. doi: 10.1016/j.cyto.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Meira CS, Pereira-Chioccola VL, Vidal JE, de Mattos CC, Motoie G, et al. Toxoplasma Groups. Cerebral and ocular toxoplasmosis related with IFN-γ, TNF-α, and IL-10 levels. Front Microbiol. 2014;13(5):492. doi: 10.3389/fmicb.2014.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi S, Kim TS, Jung CG, Masuda T, Urakawa S, et al. Enhanced neurogenesis from neural progenitor cells with G1/S-phase cell cycle arrest is mediated by transforming growth factor β1. Eur J Neurosci. 2008;28(6):1049–1059. doi: 10.1111/j.1460-9568.2008.06420.x. [DOI] [PubMed] [Google Scholar]
- Montazeri M, Mehrzadi S, Sharif M, Sarvi S, Tanzifi A, Aghayan SA, Daryani A. Drug resistance in Toxoplasma gondii. Front Microbiol. 2018;9:2587. doi: 10.3389/fmicb.2018.02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron—a metal at the host-pathogen interface. Cell Microbiol. 2010;12:1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Coutinho LB, Almeida MPO, Briceño MP, Araujo E, et al. The availability of iron is involved in the Murine experimental Toxoplasma gondii infection outcome. Microorganisms. 2020;8(4):560. doi: 10.3390/microorganisms8040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamukcu S, Cerutti A, Bordat Y, Hem S, Rofidal V, et al. Differential contribution of two organelles of endosymbiotic origin to iron-sulfur cluster synthesis and overall fitness in Toxoplasma. PLoS Pathog. 2021;17(11):e1010096. doi: 10.1371/journal.ppat.1010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117(7):1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Portes JA, Souza TG, Dos Santos TAT, Da Silva LLR, Ribeiro TP, et al. Reduction of Toxoplasma gondii development due to inhibition of parasite antioxidant enzymes by a dinuclear iron (III) compound. Antimicrob Agents Chemother. 2015;59(12):7374–7386. doi: 10.1128/AAC.00057-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Carret C, Recker M, Armitage AE, Gonçalves LA, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17(6):732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayoom A, Aneesha VA, Anagha S, Dar JA, Kumar P, et al. Lecithin-based deferoxamine nanoparticles accelerated cutaneous wound healing in diabetic rats. Euro J Pharm Sci. 2019;858:172478. doi: 10.1016/j.ejphar.2019.172478. [DOI] [PubMed] [Google Scholar]
- Raouf-Rahmati A, Ansar AR, Rezaee SA, Hosseini SM, Garweg JG, et al. Local and systemic gene expression levels of IL-10, IL-17 and TGF-β in active ocular toxoplasmosis in humans. Cytokine. 2021;146:155643. doi: 10.1016/j.cyto.2021.155643. [DOI] [PubMed] [Google Scholar]
- Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AE, Ashour DS, Dawood LM, El-Shorbagy SH. Age related changes in cerebral congenital toxoplasmosis: Histopathological and immunohistochemical evaluation. J Neuroimmunol. 2020;348:577384. doi: 10.1016/j.jneuroim.2020.577384. [DOI] [PubMed] [Google Scholar]
- Sagar P, Angmo S, Sandhir R, Rishi V, Yadav H, et al. Effect of hepcidin antagonists on anemia during inflammatory disorders. Pharmacol Ther. 2021;226:107877. doi: 10.1016/j.pharmthera.2021.107877. [DOI] [PubMed] [Google Scholar]
- Salama MA, Mostafa NE, El-Aal A, Fathy N, Moawad HS, et al. Efficacy of Zingiber officinale and Cinnamomum zeylanicum extracts against experimental Trichinella spiralis infection. J Parasit Dis. 2021;46(1):24–36. doi: 10.1007/s12639-021-01412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Codina N, Mancias JD. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals. 2018;11(4):114. doi: 10.3390/ph11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Silva LA, Fernandes MD, Machado AS, Reis-Cunha JL, Bartholomeu DC, Vitor RWA. Efficacy of sulfadiazine and pyrimetamine for treatment of experimental toxoplasmosis with strains obtained from human cases of congenital disease in Brazil. Exp Parasitol. 2019;202:7–14. doi: 10.1016/j.exppara.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Soares MP, Weiss G. The Iron age of host-microbe interactions. EMBO Rep. 2015;16(11):1482–1500. doi: 10.15252/embr.201540558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandara L, Salamunić I. Iron metabolism: current facts and future directions. Biochem Med. 2012;22(3):311–328. doi: 10.11613/bm.2012.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113(21):5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- Wang CY, Babitt JL. Liver iron sensing and body iron homeostasis. Blood. 2019;133(1):18–29. doi: 10.1182/blood-2018-06-815894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Iron availability and infection. Biochim Biophys Acta Gen Subj. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]