Abstract
An acute exacerbation of COPD (AECOPD) is associated with increased risk of cardiovascular (CV) events. The elevated risk during an AECOPD may be related to changes in vascular function, arterial stiffness, and systemic inflammation; the time course of these measures and their corresponding recovery are poorly understood. Further, physical activity is reduced during an AECOPD, and physical activity may influence the cardiovascular responses to an AECOPD. The purpose of the study was to examine the acute impact of an AECOPD requiring hospitalization on vascular function, arterial stiffness, and systemic inflammation and examine whether physical activity modulates these variables during recovery. Patients hospitalized for an AECOPD were prospectively recruited and compared to control patients with stable COPD. Vascular function, arterial stiffness, and systemic inflammation (CRP, IL-6) were measured at hospital admission, hospital discharge and within 14 days of discharge. Physical activity was electronically tracked daily while in hospital and for 7 days following discharge using a Fitbit. One hundred and twenty-one patients with an AECOPD requiring hospitalization and 33 control patients with stable COPD were enrolled in the study. Vascular function was significantly lower, and systemic inflammation higher at hospital admission in patients with an AECOPD compared to stable COPD. Significant improvements in vascular function and inflammation were observed within 14 days of hospital discharge; however, vascular function remained lower than stable COPD. Physical activity was low at admission and increased following discharge; however, physical activity was unrelated to measures of vascular function or inflammation at any time point. An AECOPD requiring hospitalization is associated with impaired vascular function that persists during recovery. These findings provide a mechanistic link to help explain the enduring increase in CV risk and mortality following a severe AECOPD event.
Clinical trial registration: ClinicalTrials.gov #NCT01949727; Registered: 09/20/2013.
Subject terms: Vascular diseases, Vascular diseases, Outcomes research
Introduction
Patients who experience an acute exacerbation of COPD (AECOPD) often seek medical assessment in emergency departments (ED) and many require hospital admission. Moreover, these patients experience a four-fold increase in cardiovascular (CV) events with an AECOPD, and a greater than two-fold increase in myocardial infarction risk within the first 5 days following an exacerbation1. The potential mechanism(s) for the increased CV and mortality risk during a severe AECOPD are unclear and requires further investigation.
Arterial stiffness and vascular function are both independent predictors of CV risk in healthy populations2–7 even when adjusted for traditional risk factors of CV disease such as age, sex, systolic and diastolic blood pressure, diabetes mellitus, total cholesterol, and smoking status6. In patients with stable COPD, arterial stiffness is elevated compared to age-matched controls2–5 and arterial stiffness increases during an AECOPD8. Vascular dysfunction, as evaluated by flow-mediated dilatation (FMD), is widely regarded as an early manifestation of CV disease9, and is impaired in patients with stable COPD10. An acute increase in inflammation appears to impair vascular function11, and a recent small study demonstrated that vascular function was lower during a severe AECOPD relative to healthy non-smokers12.
An AECOPD has a detrimental affect on multiple facets of everyday life including sleep, nutrition and physical activity13,14. Physical inactivity, an independent risk factor of CV disease2,3,5, is common in patients with stable COPD15–18 and physical activity is reduced even further during an AECOPD19. Patients hospitalized with an AECOPD undergo extended periods of bedrest. Prolonged bedrest in healthy participants has been shown to reduce CV function20,21 and increase inflammation22,23, while regular mobilization during prolonged bedrest has been shown to prevent the rise in inflammation22. The combined inflammatory response from a severe AECOPD requiring hospitalization as well as the hospitalization-associated bedrest may have synergistic effects and potentiate CV risk22 secondary to impairments in vascular function and arterial stiffness.
Several studies have examined connections between physical activity, arterial stiffness and systemic inflammation24–29; however, to our knowledge, no study has elucidated the pattern of inflammation during a severe AECOPD requiring hospitalization, and subsequently how physical activity may modulate inflammation, arterial stiffness, and vascular function. Thus, the purpose of the present study was to (1) examine the impact and time course of a severe AECOPD requiring hospitalization on vascular function, arterial stiffness, and systemic inflammation, and (2) examine whether physical activity was associated with vascular function, arterial stiffness, and systemic inflammation during an AECOPD.
Methods
Study design
The present study was a single-centre observational cohort study (ClinicalTrials.gov #NCT01949727) approved by the University of Alberta Health Research Ethics Board (Biomedical Panel, Pro00038838). Participants provided written informed consent prior to any research procedures and all methods were carried out in accordance with relevant guidelines and regulations. This study was performed in line with the principles of the Declaration of Helsinki.
Measurement assessments
For each AECOPD inpatient, data were obtained over three periods; (1) within 24 h of admission to hospital (admission); (2) 24 h prior to discharge from hospital (discharge); and (3) within 14 days following discharge from hospital (14 Day Follow-up). Each testing day consisted of an assessment of vascular function, arterial stiffness, blood biomarkers and health related quality of life. Measurements were obtained at a consistent time to minimize diurnal variation and participants refrained from smoking a minimum of 12 h prior to assessments. Physical activity was assessed daily while in hospital and for a 7-day period following discharge. Control patients with stable COPD were assessed for primary and secondary outcomes at a single time point.
Participants
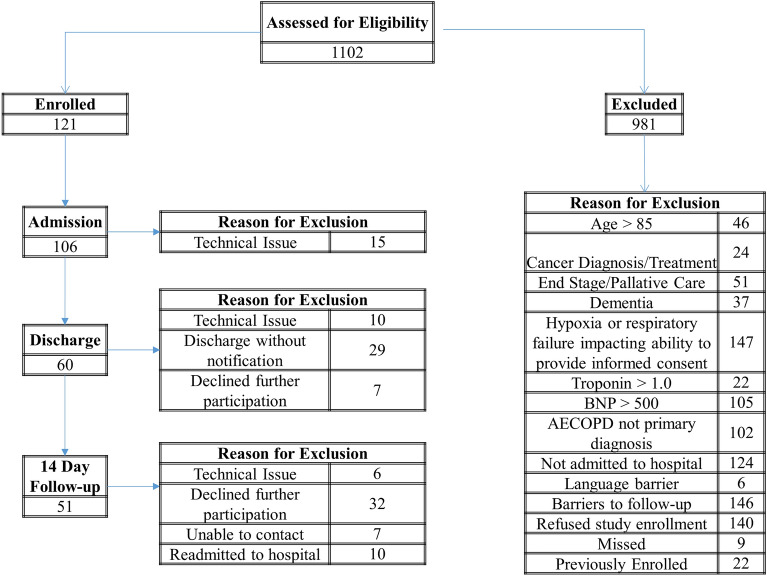
Adult patients (between 40–85 years of age) were enrolled if the primary diagnosis was an AECOPD and they were admitted to the University of Alberta Hospital (UAH) in Edmonton, AB (AECOPD inpatient). The UAH is a quaternary care, referral, academic teaching hospital with a large catchment area. Patients with respiratory failure impacting consent, conventional troponin-I (TnI) > 1.0 ng/ml, brain natriuretic peptide (BNP) > 500 pg/ml, or a diagnosis of cancer, dementia, palliative and/or end-stage disease were excluded (Fig. 1). A consort diagram indicating the number of patients assessed at Admission, Discharge and 14 Day Follow-up is available in Fig. 1. Patients that were not assessed at discharge were still contacted for the 14 day follow-up assessment. To confirm COPD, in-hospital spirometry (two flow-volume loops) was performed once on each patient with an AECOPD when they were sufficiently stable (typically within 2–3 days following admission) to provide reproducible maximal flow-volume loops.
Figure 1.
Patient eligibility, inclusion and exclusion. Trop: troponin; BNP: brain natriuretic peptide.
For comparison, control patients with stable COPD were recruited from the community. All participants had COPD as defined by the American Thoracic Society criteria of irreversible post-bronchodilator airflow obstruction (i.e. FEV1/FVC below the lower limit of normal predicted from height, age, sex)30 and smoking history (> 10 pack-years). Stable COPD controls were classified as Gold Stage 1 and 2 or Gold Stage 3 and 4, had no history of an AECOPD within the previous 6 months, did not have cancer or dementia, and were not receiving end-stage care.
Biomarkers
Clinical blood biomarkers of heart failure and cardiovascular disease (BNP and TnI)) were assessed while in the ED prior to hospital admission.
Arterial stiffness
Following instrumentation, participants rested for at least 10 min in the supine position. Blood pressure and heart rate were recorded to confirm that the participant was stable before resting carotid-radial pulse wave data were collected. Pulse waveforms were simultaneously acquired by non-invasive pressure sensors (Complior, Alam Medical, Vincennes, France) from the carotid and radial arteries and used to calculate pulse wave velocity (PWV). Pulse wave velocity was calculated as PWV = D∙Δt−1, where D was the distance (m) between sites and Δt was the time difference (s) between pulse waves using the foot-to-foot method31. Distance between the recording sites at the carotid and radial arteries was measured on the surface of the body using a tape measure. Mean PWV was calculated as the average of at least five consecutive beats in order to cover a full respiratory cycle31.
Vascular function
Vascular function was then assessed as outlined by Faizi et al.32 using the Endo-PAT® (Endo-PAT®, Itamar Medical Ltd., Caesarea, Israel), which examines the vasodilatory response (measured at the finger) to 5 min of circulatory occlusion. Similar to other flow-mediated techniques, the vascular response assessed by the Endo-PAT® has been shown to be related to coronary endothelial dysfunction33. Forearm occlusion was chosen to allow for consistency as the majority of patients had at least one intravenous site in the antecubital space which might be moved during hospitalization. Pulse amplitude response to hyperemia (RHI) was automatically calculated using the Endo-PAT® proprietary algorithm34,35. Previous research has shown an RHI score of < 1.67 represents endothelial dysfunction33,35.
Systemic inflammation
A venous puncture was performed by an experienced phlebotomist after arterial stiffness and RHI data were obtained. Blood samples were collected in plain vacutainer tubes and centrifuged for 10 min at 1200 rpm; serum was aliquoted (100 µl) into 0.6 mL cryovials. Samples were then stored at − 80 °C until analyzed. Serum analysis of human C-reactive protein (CRP) and interlukin-6 (IL-6) was performed using commercial ELISA assays (ELISA Human CRP assay and ELISA Human IL-6 assay R&D Systems, Minneapolis, MN), consistent with previous work36,37. CRP has previously been shown to be significantly related to flow mediated dilation10.
Physical activity
Physical activity monitors (Fitbit, Fitbit Inc, San Francisco, CA) were worn by AECOPD inpatients at admission and at 14 day follow-up to assess number of steps taken during their hospital stay and while in the community38. The physical activity monitors selected for the study have been validated in patients with COPD both in the community and in a hospital setting39,40. Minute by minute data were extracted using the Fitbit developer application programming interface (Fitbit, Fitbit Inc, San Francisco, CA). Admission and discharge data represent the daily step count collected over that day for a patient, and 14 day follow-up represents the average step count measured over 7 days. Step counts were averaged over seven days in the control patients with stable COPD.
Health related quality of life: CAT and mMRC
The impact of the AECOPD on the patient’s quality of life was evaluated over time using the COPD Assessment Test (CAT) and scores were totaled out of 4041. The modified Medical Research Council dyspnea scale (mMRC) questionnaire was used to assess perceived breathlessness41.
Statistical analysis
Data are presented as mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables and as counts and proportions for dichotomous variables. Differences in baseline clinical characteristics, including smoking history and lung function, were evaluated using a one-way analysis of variance (ANOVA) followed by a Bonferroni-adjustment for multiple comparisons where statistical significance (p < 0.05) was found for continuous variables. A chi-square (χ2) test for was used for categorical variables.
Change in vascular function, arterial stiffness and systemic inflammation (primary outcomes) were analyzed using mixed effects linear regression models. The model had a fixed effect for timing discharge and 14 day follow-up, with admission as the reference level. The model allowed for easy reporting of the relevant comparisons of interest and had a random effect for subjects to capture within-subject dependence. Separate models also adjusted for the following covariates one at a time: age, sex, BMI, FEV1% predicted, mean arterial blood pressure (MAP), systolic blood pressure, diastolic blood pressure, length of stay (LOS) and estimated arterial O2 saturation (SPO2). Bonferroni correction was applied to the p-values to account for the three contrasts and a p-value of 0.05 was selected to indicate statistical significance. A Kruskal–Wallis test was used to determine statistical significance in step count between admission, discharge and 14 day follow up followed by a Bonferroni adjustment for multiple comparisons where statistical significance was set at p < 0.05.
In addition, Pearson correlations were used to determine if a relationship was present between primary outcomes and step count and partial correlations were used to determine the relationship between primary outcomes while controlling for step count.
All statistical analyses were performed in SPSS Statistical software version 26.0.0.0 IBM Corp™, Armonk, NY, USA, and Studio Team 2020, RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, USA.
Sample size
A convenience sample of COPD patients was recruited in an attempt to provide sufficient data for multiple regression analysis. Preliminary analysis indicated 100 COPD patients would provide reasonable confidence intervals on important variables and outcomes.
Results
The study screened 1102 patients who presented at the ED for an AECOPD, and 121 patients were recruited prior to admission to hospital (Fig. 1, CONSORT diagram). Out of these, 111 were included in the analysis (AECOPD Inpatients). Baseline patient characteristics are outlined in Table 1. The groups were well matched for age, height, weight and smoking history. Patients with an AECOPD had similar airflow limitation (FEV1% predicted = 50 vs. 38), more likely to be on supplemental oxygen (49 vs. 23%), and had higher levels of perceived breathlessness as compared to stable patients with GOLD 3 and 4 COPD (see Table 1).
Table 1.
Participant characteristics.
| Stable COPD controls GOLD 1 & 2 | Stable COPD controls GOLD 3 & 4 | AECOPD inpatients | |
|---|---|---|---|
| n | 11 | 22 | 111 |
| Males/females, n | 6/5 | 16/6 | 51/60 |
| Age (years) | 72 (9) | 64 (9)* | 69 (10) |
| Height (cm) | 164 (10) | 171 (9) | 167 (11) |
| Mass (kg) | 77 (20) | 86 (23) | 78 (24) |
| BMI (kg/m2) | 28 (8) | 29 (8) | 28 (9) |
| Smoking history (pack years; median (IQR)) | 40 (32) | 49 (15) | 41 (25) |
| Length of hospitalization (days) | – | – | 4.7 (3.1) |
| O2 supplementation (%) | 0 | 23 | 49* |
| Anitbiotics (%) | 79 | ||
| Systemic corticosteroids (%) | 82 | ||
| Pulmonary function (% predicted) | |||
| FEV1 | 87 (13) | 38 (12)* | 50 (22)* |
| FVC | 97 (13) | 72 (18)* | 76 (26)* |
| FEV1/FVC % | 86 (9) | 53 (15)* | 67 (24)*¥ |
| Troponin (ng/ml) | – | – | 0.1 (0.1) |
| Brain natriuretic peptide (pg/ml; median (IQR)) | – | – | 84 (137) |
| mMRC | 1.2 (0.9) | 2.4 (1.1)* | 2.7 (1.2)* |
| COPD assessment tool | 12 (8) | 17 (7) | 27 (8)*¥ |
Values are mean (SD) unless otherwise indicated.
Antibiotics and Systemic Corticosteroids prescribed during AECOPD.
FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, mMRC modified Medical Research Council Questionnaire.
Summaries are based on completed data.
*p < 0.05 vs. Stable COPD control Gold 1 & 2.
¥p < 0.05 vs. Stable COPD control Gold 3 & 4.
The length of hospitalization for patients with an AECOPD ranged from 1 to 19 days with a median of 4 (IQR = 1, 7). There were no between-group differences in age, BMI, airway obstruction, smoking history or perceived dyspnea between inpatients with AECOPD and control patients with stable COPD (GOLD 3 and 4, Table 1). Health related QoL was significantly decreased at admission compared to control patients (Table 1) and improved significantly at 14-day follow up (see Table 2).
Table 2.
Clinical characteristics of patients with an AECOPD from admission, discharge and 14 day follow-up (mean (SD)).
| Admission | Discharge | 14 day follow up | |
|---|---|---|---|
| Heart rate (bpm) | 88 (17) | 82 (13)* | 80 (10)* |
| Systolic blood pressure (mmHg) | 131 (24) | 121 (17)* | 122 (16)* |
| Diastolic blood pressure (mmHg) | 76 (16) | 70 (11)* | 73 (9) |
| Mean arterial blood pressure (mmHg) | 94 (15) | 87 (11)* | 89 (10) |
| Pulse oximetry (SpO2) | 92 (5) | 93 (3) | 94 (3) |
| IL-6 (pg/ml) | 7.2 (12.9) | 9.9 (15.1) | 8.6 (7.0) |
| mMRC | 2.8 (1.2) | 2.4 (1.0) | 2.0 (1.0)* |
| COPD assessment tool | 27 (8) | 20 (7)* | 18 (7)* |
| Step count (steps/day; median (IQR)) | 830 (1051) | 964 (1844) | 1740 (2830)*¥ |
Values are mean (SD).
Summaries are based on completed data.
*p < 0.05 vs. Admission.
¥p < 0.05 vs. Discharge.
Vascular function, arterial stiffness, systemic inflammation
Measurements of RHI, PWV and CRP in stable control patients and patients with AECOPD at admission are shown in Fig. 2. The RHI was significantly lower in patients with an AECOPD compared to both GOLD 1 and 2 and GOLD 3 and 4 control COPD groups. A greater proportion of patients with an AECOPD had an impaired RHI at admission compared to control patients with stable COPD (AECOPD: 59% (31/53), GOLD 1 and 2 Stable COPD controls: 18% (2/11), GOLD 3 and 4 Stable COPD controls 33% (7/21); chi-square = 8.05; p = 0.018). Arterial stiffness was not significantly different between patients with stable COPD and patients with AECOPD at admission. Admitted patients with an AECOPD had significantly higher CRP values compared to control patients. IL-6 was not significantly different between patients with AECOPD and control patients (AECOPD inpatients: 7.2 ± 12.9; GOLD 1 and 2 Stable COPD controls: 5.8 ± 8.2; GOLD 3 and 4 Stable COPD controls: 3.2 ± 2.4; p = 0.787). A partial correlation was used to determine the relationship between RHI and CRP within the AECOPD patients while controlling for step count at admission (see Fig. 3). There was a statistically significant, moderate, negative partial correlation (− 0.512, p = 0.008) between RHI and CRP whilst controlling for step count. However, zero-order correlations showed that step count had little influence on the relationship between RHI and CRP. No relationship was present between arterial stiffness and CRP (− 0.115; p = 0.594) at admission.
Figure 2.
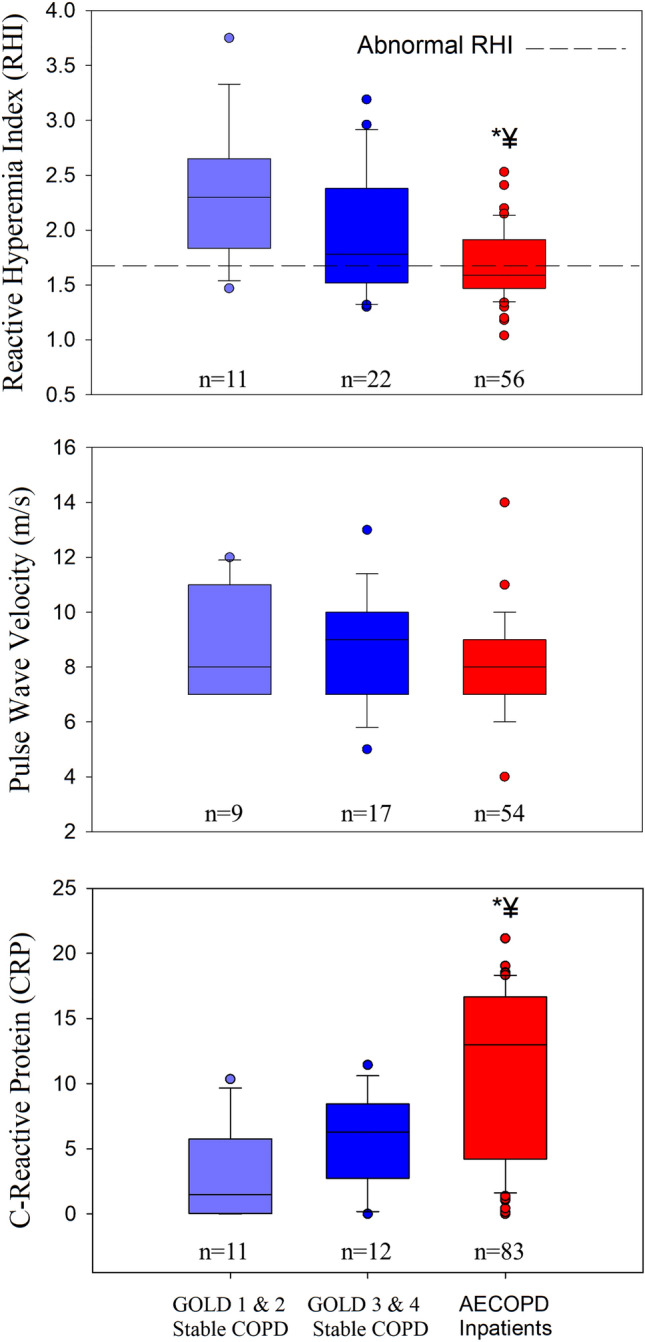
Vascular function, pulse wave velocity and systemic inflammation at admission. *p < 0.05 vs. GOLD 1 and 2 Stable COPD controls; ¥p < 0.05 vs. GOLD 3 and 4 Stable COPD control.
Figure 3.

Correlation between Reactive hyperemia index (RHI) and C-Reactive Protein (CRP) at admission. (− 0.512; p = 0.008).
The time course of change in RHI, PWV and CRP are shown in Fig. 4. RHI significantly improved (estimated increase of 0.215; p = 0.031) from admission to 14 day follow up, however, regression analysis showed that age, sex, BMI and LOS did not contribute to this increase. CRP significantly decreased from admission to 14 day follow up and age, sex, FEV1, MAP and LOS were shown to not contribute to this difference. IL-6 and arterial stiffness remained unchanged from admission to discharge to 14 day follow-up.
Figure 4.
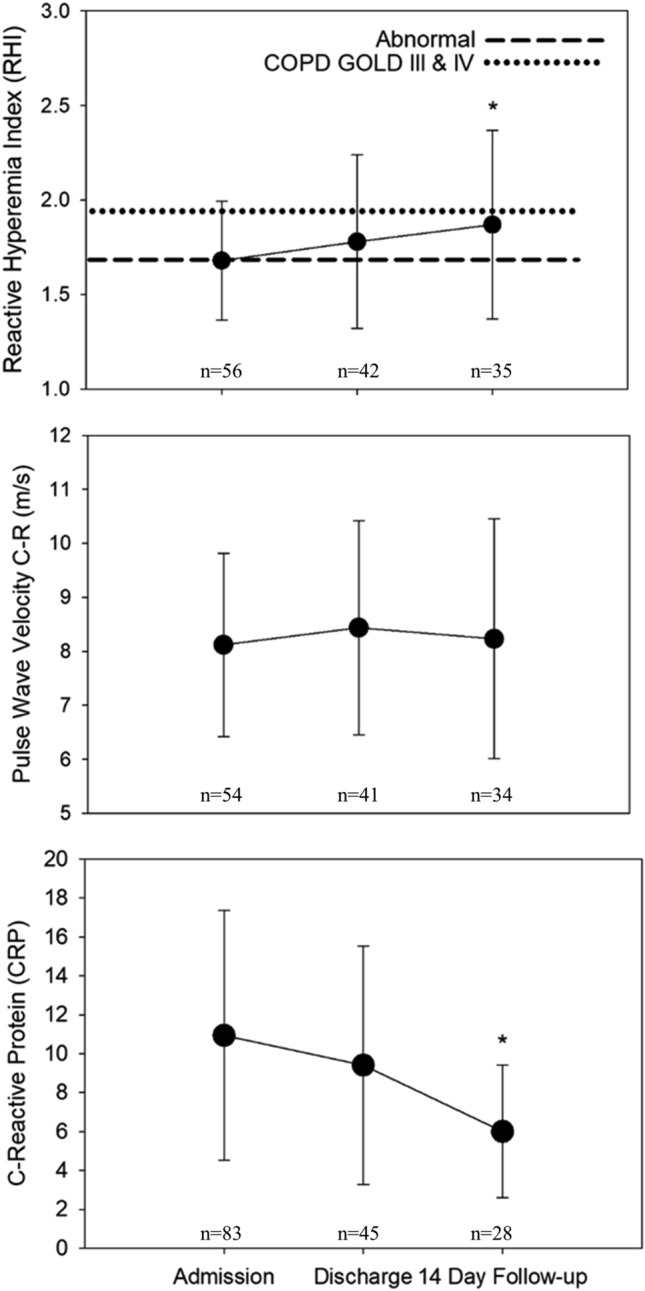
Vascular function, pulse wave velocity and systemic inflammation for patients with AECOPD across admission, discharge and 14-day follow-up. *p < 0.05 vs. admission. RHI score of < 1.67 represents endothelial dysfunction.
Relationship between physical activity and vascular function, pulse wave velocity, inflammation
At admission, patients with an AECOPD had significantly lower physical activity compared to controls (AECOPD: median = 830 (IQR = 1051) steps/day; GOLD 1 and 2 Stable COPD controls: 5130 (3336) steps/day; GOLD 3 and 4 Stable COPD controls: 4202 (4311) steps/day; p < 0.001). Within the AECOPD group, step count increased significantly by an estimated 1865 steps/day from admission to 14 day post discharge (p = 0.025). Age, sex, BMI and SPO2 were not statistically significant predictors, and the step count increased even after adjustment for these predictors. Step count at 14-days in patients with an AECOPD remained below the average step count in stable GOLD 1 and 2 and GOLD 3 and 4 (AECOPD 14 Day: median = 1740 (IQR = 2831) steps/day vs. GOLD 1 and 2 Stable COPD controls 5130 (3336) steps/day; GOLD 3 and 4 Stable COPD controls 4202 (4311), p = 0.02).
There was no association between step count and RHI data obtained at admission (− 0.287; p = 0.106), discharge (− 0.190; p = 0.332) and 14 day follow up (0.182; p = 0.431). Arterial Stiffness was significantly related to step count at admission (− 0.428; p = 0.012) and discharge (− 0.447; p = 0.019); however, there was no relationship at 14 day follow-up (0.071; p = 0.747). Step count and CRP showed no relationship at admission (0.111; p = 0.511), discharge (0.328; p = 0.354) or 14 day follow up (− 0.252; p = 0.454). When examining patients who experienced the greatest improvements in RHI (> 0.215), step count was not significantly different between the two RHI groups (> 0.215 units: 2450 (2302) steps/day; < 0.215 units: 4472 (4534) steps/day; p = 0.315).
Discussion
Findings from the current study indicate that vascular function was impaired during an acute exacerbation of COPD, and this impairment was accompanied by an acute increase in systemic inflammation. Importantly, the reduction in vascular function persisted for at least 2 weeks following discharge from hospital. Surprisingly, our findings indicate that arterial stiffness was unaffected by an AECOPD. Physical activity was very low during the AECOPD hospitalization, and improved following discharge; however, physical activity did not appear to influence vascular function or inflammation in-hospital or within the first 2 weeks of discharge. The impairment in vascular function observed during an AECOPD may be an important factor contributing to the increased CV and mortality risk reported during a severe AECOPD.
Vascular function during an AECOPD hospitalization has been previously examined;12,42 however, our study is the first to demonstrate that vascular function is impaired when matched to patients with stable COPD. By examining vascular function at discharge and again at 2 weeks post discharge, we were able to demonstrate that while there is a relative improvement in vascular function from hospital admission, vascular dysfunction persists during recovery from an AECOPD. This provides insight into potential changes in vascular function during and following hospitalization for an AECOPD, which was previously unknown, and builds on earlier research which examined vascular function during recovery at approximately 1 month42 and 3 months12 following an AECOPD. The slight improvement in vascular function observed in the present study is consistent with previous work where FMD increased during recovery from an AECOPD hospitalization12,42; however and importantly, our data indicate that vascular function remains well below values observed in control patients with stable COPD. These findings suggest that persistent vascular impairment after an AECOPD hospitalization may contribute to the increased CV risk and mortality following a severe AECOPD. Recovery from an AECOPD is complex and further research targeting interventions to improve vascular health and reduce CV risk in COPD post-hospitalization is warranted.
The underlying mechanisms for impaired vascular function during an AECOPD hospitalization are not well understood. Systemic inflammation is associated with reduced vascular function in stable COPD patients2,10, and both Marchetti et al.12 and Ozben et al. (2010) postulated that the acute increase in systemic inflammation with an AECOPD likely contributed to impaired vascular function in hospitalized patients with COPD. Supporting the hypothesis of a link between systemic inflammation and impaired vascular function2,10,12,42, we found high circulating CRP and reduced vascular function in the AECOPD group in comparison to patients with stable COPD (see Fig. 2). Further, within the AECOPD group, a high CRP was associated with lower vascular function (see Fig. 3). Interestingly, IL-6, another marker of systemic inflammation, remained unchanged throughout the study; however, previous work has also found that CRP is elevated independently of IL-643. CRP has been shown to induce vascular dysfunction by suppressing endothelial nitric oxide synthase expression and activity44, and our findings suggest that the acute increase in systemic inflammation observed with an AECOPD may contribute to impaired vascular function in hospitalized patients with COPD.
Surprisingly, no difference in arterial stiffness was observed between patients experiencing an exacerbation and those with stable COPD. These findings suggest that the elevated arterial stiffness often observed in patients with COPD may be the result of long-standing/chronic changes, and not impacted by a AECOPD event45. However these findings are contrary to previous work demonstrating an acute increase in PWV of 1.2 m/s above baseline in patients experiencing an AECOPD8. The divergent findings may be explained by differences in PWV assessment between the two studies. Patel et al.8 examined central PWV (carotid-femoral) which is predominately dependent on the elastic properties of the artery, whereas the current study assessed peripheral PWV (carotid-radial) which is predominantly dependent on the muscular properties of the artery8. It may be that other aspects of an AECOPD (e.g., hypoxemia, hypercapnia, medication) may influence peripheral PWV differently than central PWV and suggests that peripheral PWV may not change during a COPD hospitalization46–48.
As expected, physical activity was extremely low during the AECOPD hospitalization, and while step count increased following discharge, physical activity within 14 days following hospitalization was still significantly below values obtained in patients with stable COPD. Physical activity was not associated with RHI, PWV or inflammation during and following hospitalization, suggesting that physical activity/mobilization had no short-term effect on modulating CV risk during an AECOPD. One explanation for the lack of association between physical activity and CV risk parameters may be the substantially reduced physical activity observed in patients with an AECOPD throughout recovery. Average step count at 14 day follow-up in the AECOPD group remained well below the average step count observed in stable GOLD 3 and 4 patients (1740 (2831) steps/day vs. 5130 (3336) steps/day). Previous research in healthy populations has shown that increases in physical activity can reduce CV risk by as much as 21%, although this finding is based on an increase in step count above baseline physical activity values. In patients with stable COPD participating in pulmonary rehabilitation, the minimal clinically important difference (MCID) in step count was calculated to be between 599 to 1131 steps per day49. Patients who exceeded the MCID in steps per day demonstrated a significant reduction in hospital admission in the first 2 years following rehabilitation49. Importantly, this MCID was determined from a mean baseline of 3839 steps per day, which is well above the mean of 2730 steps per day observed 14-days following an AECOPD in the current study. It may be that comprehensive interventions such as pulmonary rehabilitation are needed to increase physical activity sufficiently to reduce CV risk following an AECOPD50.
As a project conducted within an acute care hospital, this study has several limitations. We were unable to assess vascular function and PWV while patients were in isolation in hospital resulting in missing data at admission and discharge (n = 12), and 22 patients were discharged before being assessed at admission. It was not possible to characterize the exacerbation into causative pathology, and medications were not standardized throughout the study. Nonetheless, most of the patients hospitalized with AECOPD received evidence-based care with systemic corticosteroids (82%) and antibiotics (79%). It was not feasible for patients to fast prior to vascular assessments, which may have impacted vascular outcomes; however, to control for this and diurnal variations, patients were assessed at the same time of day throughout the study. Because of the complexity of obtaining data within the ED, peripheral (carotid-radial) PWV data were obtained to assess arterial stiffness, which differs from central (carotid-femoral) arterial stiffness. Additionally, arterial blood gas data were not available, limiting our ability to investigate the influence of hypercapnia on PWV. At the bedside, it was not possible to perform full lung function testing, and only two flow-volume loops were conducted at admission, and therefore we were are unable to examine the association between hyperinflation and vascular function. Biomarkers of inflammation (e.g., CRP and IL-6) were selected a-priori because of their link to vascular function and PWV. We acknowledge that other inflammatory biomarkers may be altered during an AECOPD and may impact the results. Finally, control patients were recruited from our local pulmonary clinics and may not be representative of all patients with stable COPD.
An AECOPD hospitalization was associated with impaired vascular function, which improved during recovery, but remained reduced 14 days post- hospitalization relative to patients with stable COPD. Physical activity was low throughout the hospitalization, and improved following discharge; however, physical activity did not influence vascular function or inflammation. The persistent vascular dysfunction after an AECOPD hospitalization provide a mechanistic link to help explain the persistent increase in CV risk and mortality following a severe AECOPD event. In addition to focusing on respiratory recovery, clinicians would be encouraged to support the CV health of patients recovering from an AECOPD. Additional studies are needed to determine whether targeted mobilization/pulmonary rehabilitation strategies in hospital and during recovery may help normalize vascular dysfunction and CV risk in patients recovering from a COPD hospitalization.
Acknowledgements
The authors would like to thank the Emergency Medicine Research Group (EMeRG) in the Department of Emergency Medicine at the University of Alberta for their invaluable assistance with recruitment and data collection for this study.
Abbreviations
- AECOPD
Acute exacerbation of COPD
- BNP
Brain natriuretic peptide
- COPD
Chronic obstructive pulmonary disease
- CAT
COPD assessment test
- CRP
C-reactive protein
- CV
Cardiovascular function
- ED
Emergency department
- FMD
Flow mediated dialatation
- IL-6
Interlukin-6
- IQR
Interquartile range
- MAP
Mean arterial blood pressure
- mMRC
Modified medical research council
- PWV
Pulse wave velocity
- RHI
Reactive hyperemia index
- SD
Standard deviation
- TnI
Troponin
Author contributions
Conception and design of the work: D.P.F., B.H.R., M.B., R.J.R., M.K.S. Acquisition and analysis: D.P.F., A.R.B., M.B., R.J.R., M.K.S. Interpretation of data: D.P.F., A.R.B., B.H.R., M.B., R.J.R., M.K.S. Drafted and/or revised manuscript: D.P.F., A.R.B., B.H.R., M.B., R.J.R., M.K.S. Approved submission: D.P.F., A.R.B., B.H.R., M.B., R.J.R., M.K.S.
Funding
This study was funded by Alberta Innovates Health Solutions (AIHS: #201200821) through the Collaborative Research and Innovation Opportunities (CRIO) program. Dr. Rowe’s research is supported by a Scientific Director’s Grant (SOP 168483) from the Canadian Institutes of Health Research (CIHR) through the Government of Canada (Ottawa, ON).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 2.Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175(12):1259–1265. doi: 10.1164/rccm.200701-067OC. [DOI] [PubMed] [Google Scholar]
- 3.McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;176(12):1208–1214. doi: 10.1164/rccm.200707-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maclay J, McAllister DA, Mills NL, Paterson F, Ludlam CA, Drost EM, et al. Vascular dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009 doi: 10.1164/rccm.200903-0414OC. [DOI] [PubMed] [Google Scholar]
- 5.Mills NL, Miller JJ, Anand A, Robinson SD, Frazer GA, Anderson D, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: A mechanism for increased cardiovascular risk. Thorax. 2008;63(4):306–311. doi: 10.1136/thx.2007.083493. [DOI] [PubMed] [Google Scholar]
- 6.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 7.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel AR, Kowlessar BS, Donaldson GC, Mackay AJ, Singh R, George SN, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188(9):1091–1099. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;178(12):1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 11.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102(9):994–999. doi: 10.1161/01.CIR.102.9.994. [DOI] [PubMed] [Google Scholar]
- 12.Marchetti N, Ciccolella DE, Jacobs MR, Crookshank A, Gaughan JP, Kashem MA, et al. Hospitalized acute exacerbation of COPD impairs flow and nitroglycerin-mediated peripheral vascular dilation. COPD. 2011;8(2):60–65. doi: 10.3109/15412555.2011.558541. [DOI] [PubMed] [Google Scholar]
- 13.McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: The forgotten dimension. Eur. Respir. Rev. 2013;22(129):365–375. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur. Respir. J. 1997;10(10):2264–2269. doi: 10.1183/09031936.97.10102264. [DOI] [PubMed] [Google Scholar]
- 15.Albarrati AM, Gale NS, Munnery MM, Cockcroft JR, Shale DJ. Daily physical activity and related risk factors in COPD. BMC Pulm. Med. 2020;20(1):60. doi: 10.1186/s12890-020-1097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir. Med. 2012;106(7):962–969. doi: 10.1016/j.rmed.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troosters T, Sciurba F, Battaglia S, Langer D, Valluri SR, Martino L, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir. Med. 2010;104(7):1005–1011. doi: 10.1016/j.rmed.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waschki B, Spruit MA, Watz H, Albert PS, Shrikrishna D, Groenen M, et al. Physical activity monitoring in COPD: Compliance and associations with clinical characteristics in a multicenter study. Respir. Med. 2012;106(4):522–530. doi: 10.1016/j.rmed.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax. 2006;61(9):772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor HL, Henschel A, et al. Effects of bed rest on cardiovascular function and work performance. J. Appl. Physiol. 1949;2(5):223–239. doi: 10.1152/jappl.1949.2.5.223. [DOI] [PubMed] [Google Scholar]
- 21.Convertino VA. Cardiovascular consequences of bed rest: Effect on maximal oxygen uptake. Med. Sci. Sports Exerc. 1997;29(2):191–196. doi: 10.1097/00005768-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Arinell K, Christensen K, Blanc S, Larsson A, Frobert O. Effect of prolonged standardized bed rest on cystatin C and other markers of cardiovascular risk. BMC Physiol. 2011;11:17. doi: 10.1186/1472-6793-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurdana M, Jenko-Praznikar Z, Mohorko N, Petelin A, Jakus T, Simunic B, et al. Impact of 14-day bed rest on serum adipokines and low-grade inflammation in younger and older adults. Age (Dordr.) 2015;37(6):116. doi: 10.1007/s11357-015-9848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes JN, Nualnim N, Sugawara J, Sommerlad SM, Renzi CP, Tanaka H. Arterial stiffening, wave reflection, and inflammation in habitually exercising systemic lupus erythematosus patients. Am. J. Hypertens. 2011 doi: 10.1038/ajh.2011.143. [DOI] [PubMed] [Google Scholar]
- 25.Provan SA, Angel K, Semb AG, Mowinckel P, Agewall S, Atar D, et al. Early prediction of increased arterial stiffness in patients with chronic inflammation: A 15-year followup study of 108 patients with rheumatoid arthritis. J. Rheumatol. 2011;38(4):606–612. doi: 10.3899/jrheum.100689. [DOI] [PubMed] [Google Scholar]
- 26.Yiu KH, Yeung CK, Chan HT, Wong RM, Tam S, Lam KF, et al. Increased arterial stiffness in patients with psoriasis is associated with active systemic inflammation. Br. J. Dermatol. 2011;164(3):514–520. doi: 10.1111/j.1365-2133.2010.10107.x. [DOI] [PubMed] [Google Scholar]
- 27.van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, et al. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011;58(4):588–595. doi: 10.1161/HYPERTENSIONAHA.111.174557. [DOI] [PubMed] [Google Scholar]
- 28.Yasmin McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25(2):372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 29.Yasmin McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler. Thromb. Vasc. Biol. 2004;24(5):969–74. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell DE, Sanii R, Anthonisen NR, Younes M. Effect of dynamic airway compression on breathing pattern and respiratory sensation in severe chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1987;135(4):912–918. doi: 10.1164/arrd.1987.135.4.912. [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 32.Faizi AK, Kornmo DW, Agewall S. Evaluation of endothelial function using finger plethysmography. Clin. Physiol. Funct. Imaging. 2009;29(5):372–375. doi: 10.1111/j.1475-097X.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 33.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 34.Meeme A, Buga GA, Mammen M, Namugowa A. Endothelial dysfunction and arterial stiffness in pre-eclampsia demonstrated by the EndoPAT method. Cardiovasc. J. Afr. 2017;28(1):23–29. doi: 10.5830/CVJA-2016-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syvanen K, Korhonen P, Partanen A, Aarnio P. Endothelial function in a cardiovascular risk population with borderline ankle-brachial index. Vasc. Health Risk Manag. 2011;7:97–101. doi: 10.2147/VHRM.S17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subrahmanyam RM, Srikantaiah C, Krishna P, Delphine Silvia CR, Thirunavukkarasu S, Devi K, et al. Can bronchial asthma be classified based on the immunological status? Lung India. 2011;28(2):110–113. doi: 10.4103/0970-2113.80323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, et al. High sensitivity C-reactive protein in asthma. Eur. Respir. J. 2006;27(5):908–912. doi: 10.1183/09031936.06.00114405. [DOI] [PubMed] [Google Scholar]
- 38.Storm FA, Heller BW, Mazza C. Step detection and activity recognition accuracy of seven physical activity monitors. PLoS ONE. 2015;10(3):e0118723. doi: 10.1371/journal.pone.0118723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vooijs M, Alpay LL, Snoeck-Stroband JB, Beerthuizen T, Siemonsma PC, Abbink JJ, et al. Validity and usability of low-cost accelerometers for internet-based self-monitoring of physical activity in patients with chronic obstructive pulmonary disease. Interact. J. Med. Res. 2014;3(4):e14. doi: 10.2196/ijmr.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prieto-Centurion V, Bracken N, Norwick L, Zaidi F, Mutso AA, Morken V, et al. Can commercially available pedometers be used for physical activity monitoring in patients with COPD following exacerbations? Chronic Obstr. Pulm. Dis. 2016;3(3):636–642. doi: 10.15326/jcopdf.3.3.2015.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng SL, Lin CH, Wang CC, Chan MC, Hsu JY, Hang LW, et al. Comparison between COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) dyspnea scores for evaluation of clinical symptoms, comorbidities and medical resources utilization in COPD patients. J. Formos Med. Assoc. 2019;118(1 Pt 3):429–435. doi: 10.1016/j.jfma.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Ozben B, Eryuksel E, Tanrikulu AM, Papila-Topal N, Celikel T, Basaran Y. Acute exacerbation impairs endothelial function in patients with chronic obstructive pulmonary disease. Turk. Kardiyol. Dern. Ars. 2010;38(1):1–7. [PubMed] [Google Scholar]
- 43.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439–1441. doi: 10.1161/01.CIR.0000033116.22237.F9. [DOI] [PubMed] [Google Scholar]
- 45.Vivodtzev I, Tamisier R, Baguet JP, Borel JC, Levy P, Pepin JL. Arterial stiffness in COPD. Chest. 2014;145(4):861–875. doi: 10.1378/chest.13-1809. [DOI] [PubMed] [Google Scholar]
- 46.Stickland MK, Fuhr DP, Edgell H, Byers BW, Bhutani M, Wong EY, et al. Chemosensitivity, cardiovascular risk, and the ventilatory response to exercise in COPD. PLoS ONE. 2016;11(6):e0158341. doi: 10.1371/journal.pone.0158341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edgell H, Moore LE, Chung C, Byers BW, Stickland MK. Short-term cardiovascular and autonomic effects of inhaled salbutamol. Respir. Physiol. Neurobiol. 2016;231:14–20. doi: 10.1016/j.resp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Moore LE, Kapoor K, Byers BW, Brotto AR, Ghods-Esfahani D, Henry SL, et al. Acute effects of salbutamol on systemic vascular function in people with asthma. Respir. Med. 2019;155:133–140. doi: 10.1016/j.rmed.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 49.Demeyer H, Burtin C, Hornikx M, Camillo CA, Van Remoortel H, Langer D, et al. The minimal important difference in physical activity in patients with COPD. PLoS ONE. 2016;11(4):e0154587. doi: 10.1371/journal.pone.0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore LE, Byers BW, Fuhr DP, Wong E, Bhutani M, Stickland MK. Cardiovascular benefits from standard pulmonary rehabilitation are related to baseline exercise tolerance levels in chronic obstructive pulmonary disease. Respir. Med. 2017;132:56–61. doi: 10.1016/j.rmed.2017.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.