Abstract
Simian virus 40 large-T antigen transactivates the ribosomal genes which are transcribed by RNA polymerase (pol I), as well as genes that are dependent on either pol II or pol III. This report identifies regions and activities of T antigen that are required to transactivate a pol I-dependent rat ribosomal gene promoter. By using the rat ribosomal gene (rDNA) promoter linked to a chloramphenicol acetyltransferase gene, we show that at least three separable T-antigen regions are necessary to achieve wild-type levels of transactivation of rDNA in transiently transfected monkey cells. One activity depends on the region of T antigen shared with small-t antigen (T/t common region). A second activity maps to amino acids 109 to 626 and is highly sensitive to mutational inactivation. Complementation analyses suggest that at least one activity in this region is independent of and must be in cis with the activity within the T/t common region. In addition, a functional nuclear localization signal is required for maximal T-antigen-mediated transactivation of rat rDNA. The three activities work in concert to override cellular species-specific controls and transactivate the rat ribosomal gene promoter. Finally, we provide evidence that although the tumor suppressor protein Rb has been shown to repress a pol I-dependent promoter, transactivation of the rat rDNA promoter does not depend on T antigen’s ability to bind the tumor suppressor product Rb.
Expression of the simian virus 40 (SV40) large-T antigen (or, for simplicity, T antigen) is sufficient to initiate and maintain transformation of cells in culture and tumorigenesis in experimental animals (see references 21 and 40 for recent reviews). Regions and activities of the multifunctional protein that are involved in specific growth property changes that accompany transformation have been defined (40). The aggressive growth that characterizes transformed and tumor cells places increased demands on protein synthesis. In support of this contention, current evidence indicates that increased protein synthesis, brought about by overexpression of translation initiation factors, can result in transformation (for a review, see reference 30). Increased expression of ribosomal genes might provide a second mode of increasing protein synthesis. One consequence of T-antigen expression is transactivation of ribosomal genes (36, 53, 55, 68). The relationship of this activity to transformation remains to be determined. Genetic analysis to identify the regions of T antigen required for transactivation of the ribosomal genes in vivo is a first step in correlating this capability with the oncogenic activities of the protein.
The ribosomal genes are transcribed by RNA polymerase I (pol I) in a species-specific manner with the aid of at least two transcription factors, the upstream binding factor (UBF) and the species selectivity factor SL1. The rat ribosomal gene (rDNA) contains a core promoter element (CPE) located between nucleotides (nt) −31 and +6 (6, 20, 27, 36, 69, 70), an upstream promoter element (UPE), whose exact location varies slightly between species (nt −50 to −186) (27, 50), an enhancer (nt −2357 to −2183) (16), and terminator sequences (29). UBF binds to specific sequences in both the UPE and the CPE (2, 42) and stimulates pol I-mediated transcription. SL1 has low DNA-binding affinity unless it is accompanied by UBF (2). SL1 is needed for efficient transcription of the ribosomal genes and is responsible for conferring the species-specific nature of the transcription (3, 25, 35).
Human SL1 is a complex composed of the TATA-binding protein (TBP; 38 kDa) and three TATA-associated factors, TAFI48 (48 kDa), TAFI63 (63 kDa), and TAFI110 (110 kDa) (12, 18). TAFI48 and TBP efficiently bind to UBF, whereas TAFI110 and TAFI63 contact the promoter directly (1). Thus, during transcription, UBF and pol I interact with cis elements in the promoter, whereas SL1 associates with these proteins to form the active initiation complex (1, 12).
T antigen is a promiscuous transcriptional transactivator. It transactivates the ribosomal genes, which are transcribed by pol I (36, 53, 54), as well as genes that are dependent on either pol II or pol III. T antigen’s ability to transactivate pol II- and III-dependent promoters (38, 47, 63) and the regions of the protein involved have been investigated in detail (4, 26, 31, 56, 72). However, less is known concerning the T-antigen activities required for transactivation of pol I-dependent promoters. In HeLa cell extracts, purified T antigen increases in vitro transcription from a human ribosomal promoter (36). Recently, Zhai et al. (71) showed that T antigen binds to the SL1 complex through interactions with TAFI48, TAFI110, and TBP and that the T antigen-SL1 association is crucial to activation of the ribosomal gene promoter. They showed further that T-antigen amino acids 1 to 436 were sufficient to bind SL1 and that amino acids 1 to 538 were sufficient to stimulate pol I-mediated transcription of the human ribosomal gene in HeLa cell extracts.
Early investigations into T antigen’s ability to override the species specific nature of ribosomal transcription in vivo assessed reactivation silent rDNA promoters within mouse-human hybrid cell lines (53, 55). These assays defined the T-antigen region necessary for the reactivation of a heterologous rRNA gene as amino acids 1 to 509 (54).
The role of specific T-antigen activities in pol I-dependent transactivation remains to be determined. It was shown recently that the retinoblastoma gene product, Rb, plays a role in the regulation of ribosomal transcription (7, 67). In vitro Rb binds to UBF (7) and inhibits its ability to bind to the UPE. This interaction represses transcription from the ribosomal promoter (67). It is not known whether T-antigen binding to Rb is involved in activation of ribosomal genes.
The study reported here focused on the ability of T antigen to transcriptionally activate the rat ribosomal gene promoter when transiently transfected into monkey cells and investigated the specific regions and activities of T antigen necessary for this heterologous ribosomal gene transactivation. The results indicate that at least three activities cooperate to transactivate the rat ribosomal gene and that Rb binding is not essential.
MATERIALS AND METHODS
Plasmids.
The rat ribosomal promoter was cloned into plasmid pU3RIIICAT (51) as follows. Plasmid pDJ3 (16), used as the source of the rat ribosomal promoter, contains the rat ribosomal promoter within 2.16 kb of external transcribed sequence. The promoter was released from pDJ3 by first digesting the plasmid with KpnI. The KpnI end was made blunt by excessive treatment with the Klenow fragment of DNA polymerase, and an XhoI linker was added. The resulting DNA was digested with XhoI and HindIII, and the released fragment containing nt −527 to +124 was gel purified. Plasmid pU3RIIICAT was digested with SalI and HindIII to release the human immunodeficiency virus long terminal repeat (LTR), and the rDNA promoter was inserted in its place to generate the reporter plasmid prDCAT. Loss of the SalI site provided evidence of insertion. The rat promoter cloned upstream of the chloramphenicol acetyltransferase (CAT) gene contains the CPE and UPE (−167 to +124), encompassing the region protected by rat UBF binding (69), and 360 bp of far-upstream sequence.
Plasmid pPVU0 (33) contains the SV40 enhancer, promoter, origin, and early region coding sequences from the PvuII (nt 272) to BamHI (nt 2533) sites cloned into pBR328 at the corresponding sites. pPVU0 produces functional large-T and small-t antigens. Plasmid Wt-2 was generated by cloning the entire SV40 genome into the EcoRI site of pBR322. Plasmid pdl2005 contains the genome of the mutant dl2005, which has a 230-bp deletion within the large-T intron and does not produce detectable quantities of small-t antigen (49).
Plasmid pdl536 was generously provided by L. Sompayrac. The pdl536 (52) construct contains the genome of the deletion mutant dl536 cloned into the BamHI site of pBR322. The dl536 deletion removes the splice acceptor site for the large-T-antigen and small-t-antigen mRNAs. Thus, pdl536 encodes an authentic small-t antigen but no large-T antigen.
Plasmids dl1265, dl1066, dl2465, dl1061, dl2433, and dl1263 contain the deletion mutant genomes cloned into pBR322. The mutants and their properties have been described previously (62, 65). They encode T antigens containing amino acids 1 to 699, 1 to 670, 1 to 626, and 1 to 590 and T antigen missing amino acids 586 to 589 and 663 to 674, respectively. Plasmid K1 (33) encodes a T antigen (T-Glu107Lys) with the amino acid substitution Glu107Lys. Plasmid D10 (33) encodes a T antigen (T-Lys128Thr) with the amino acid substitution Lys128Thr.
Plasmids dl105-108 (dl2441) (73), dl127-250 (S11-S24) (33), dl252-300, dl301-350, dl336-484, dl347-370, dl357-370, dl351-400, dl382-400, dl400, dl401-436, dl401-450, dl434-444, dl451-465, dl451-532, dl501, and dl501-550 (34) have been described previously. They contain the early coding regions of the mutants in a pBR328 plasmid backbone. They express T antigens missing the amino acids indicated by the plasmid names.
The SV40 nuclear localization signal (NLS; amino acids 126 to 132) (32) was inserted into D10, and also into dl127-250, between amino acids 650 and 651 in the following steps. Initially, annealed complementary oligonucleotides containing T-antigen codons 126 to 132 bounded by EcoRI-compatible ends of appropriate length to conserve the reading frame were inserted at the EcoRI site between codons 650 and 651 of plasmid pPVU0RI650 (34). Then the small DNA fragment generated by digesting the resulting plasmid, pPVU0RI650NLS, with PvuII and BamHI was purified from an agarose gel following electrophoresis and was ligated to the large PvuII-plus-BamHI digest fragment of dl127-250 to produce dl127-250NLS650. The NLS was inserted into plasmid D10 by the equivalent fragment exchange between dl127-250NLS650 and plasmid D10. In a separate construction, the SV40 NLS was inserted between codons 126 and 251 of dl127-250 by using annealed oligonucleotides as described for the generation of pPVU0RI650NLS.
Plasmid CAV83-708 was constructed as follows. First, the SV40 early coding region and promoter/enhancer contained between the KpnI and BamHI sites was cloned into the vector p-Select (Promega). Then the EcoRI site in the vector was converted to a blunt end to remove the enzyme recognition sequence. Oligonucleotide-directed mutagenesis was performed on the resulting plasmid to insert an EcoRI linker between codons 82 and 83. The resulting plasmid was digested with EcoRI and SalI. The fragment released from the vector contained the early-region sequences encoding T-antigen amino acids 83 to 708 and the poly(A) site. The released fragment was cloned between the corresponding sites of the modified (8) vector pBluescript SK+ (Stratagene) and subsequently was transferred into the modified expression vector as described previously (8). The final construct encodes a fusion protein containing the first seven amino acids of the β-galactosidase α-peptide followed by 31 amino acids encoded by the synthetic polylinker in pBluescript SK, two amino acids encoded by the EcoRI linker, and T-antigen amino acids 83 to 708 under control of the cytomegalovirus transcriptional promoter/enhancer.
CAT assays.
Two and a half micrograms of prDCAT reporter, 5 μg of salmon sperm (carrier) DNA, and 5 μg of wild-type or mutant T-antigen plasmid or an additional 5 μg of carrier DNA were transfected into TC7 cells by the DEAE-dextran-chloroquine procedure essentially as described elsewhere (8). For complementation assays, the carrier DNA was replaced by 5 μg of DNA of the complementing mutant. Specifically, 6 × 105 cells were seeded into 60-cm2 tissue culture dishes 1 day prior to transfection. For each DNA sample, two to four replicate plates were seeded in Dulbecco’s modified Eagle’s medium supplemented with 100 μg of streptomycin per ml, 100 μg of kanamycin per ml, 100 U of penicillin per ml, 0.03% glutamine, 0.15% Na2HCO3, 25 mM HEPES, and 10% fetal bovine serum (DMEM10×2+HEPES). For each transfection, DNA was added to 750 μl of Tris-buffered saline (TBS; pH 7.4). Then 250 μl of a 2-mg/ml stock solution of DEAE-dextran was added to give a final DEAE-dextran concentration of 500 μg/ml. Cell monolayers were rinsed once with TBS. One-half of each DNA mixture then was placed onto each of two cultures, and the dishes were rocked for 20 min at room temperature. TBS (5 ml) was added to each plate, aspirated, and replaced with 4.5 ml DMEM10×2+HEPES containing 100 μM chloroquine phosphate. The cultures were incubated at 37°C for precisely 3.5 h. The medium then was replaced with DMEM10×2+HEPES, and the cells were incubated at 37°C for an additional 48 h.
Proteins were extracted from the transfected cells, and the extracts were processed for CAT assays essentially as described previously (48). Briefly, medium was removed from the cells, and the monolayers were washed once with 4 ml of TBS. Monolayers were incubated in an additional 4 ml of TBS for 5 min. Following removal of the TBS, 0.5 ml of 0.25 M Tris-Cl (pH 8.0) was added, and the monolayers were incubated at room temperature for an additional 5 min. Cell monolayers were scraped into the buffer, and the cell suspensions were placed into microcentrifuge tubes. Cells were lysed by three cycles of freezing and thawing, and endogenous acetylase activity was inactivated by heating at 65°C for 10 min. Lysates were cleared by microcentrifugation at 14,000 rpm for 10 min at 4°C. Then 70 μl of each extract was assayed for CAT activity after addition of n-butyryl coenzyme A (0.2 mg/ml), 100 μCi of [14C]chloramphenicol, and 0.25 M Tris-Cl (pH 8.0) to a final volume of 125 μl. The samples were incubated at 37°C for 16 to 20 h. The butyrated product was isolated by xylene extraction. Specifically, 350 μl of mixed xylenes was added to each reaction; the tubes were vortexed for 40 s and centrifuged for 5 min at 14,000 rpm. Next, 300 μl of the upper layer containing the butyrated product was transferred to a microcentrifuge tube containing 125 μl of 0.25 M Tris-Cl (pH 8.0), vortexed for 20 s, and centrifuged for 5 min at 14,000 rpm. Two hundred microliters of the butyrated product was transferred to a glass scintillation vial, and 5 ml of aqueous fluor was added. The samples were counted for 1 min in a Beckman scintillation counter. The means of the counts per minute of replicate samples were calculated. The level of transactivation is expressed as the mean percentage of wild-type activity in the same experiment; the percent standard error of the mean was calculated.
Antibodies.
To detect T antigen and various mutant T antigens, monoclonal antibodies PAb901 and PAb419 were used. PAb901 recognizes a denaturation-resistant epitope located between amino acids 684 and 698 (57). PAb419 is directed towards the N-terminal 82 amino acids of large-T and small-t antigens, and it recognizes a denaturation-resistant epitope (28).
Immunoblot analysis.
Immunoblot analysis was performed essentially as described by Cavender et al. (8). TC7 cells were seeded into T150 tissue culture flasks. Cells were transfected by the DEAE-dextran-chloroquine method with 40 μg of plasmid DNA. Proteins were extracted 48 h posttransfection. Equal protein amounts were used in immunoprecipitation reactions. Detection of the specific proteins was accomplished by using protein A-Sepharose conjugated with horseradish peroxidase obtained from Amersham.
RESULTS
Transactivation of the rat ribosomal promoter by wild-type large-T antigen.
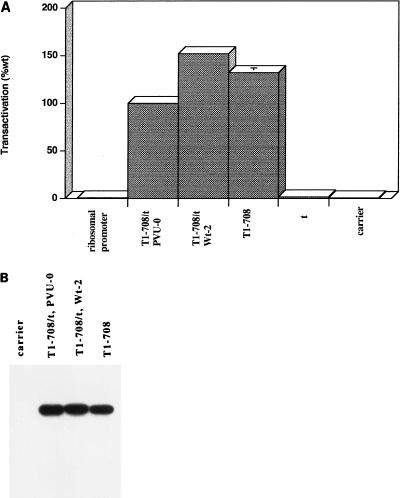
Figure 1A shows the results of representative experiments assessing the ability of large-T and small-t antigens to transactivate the rat ribosomal promoter in transiently transfected monkey cells. The basal level of CAT activity produced from the rat ribosomal promoter reporter plasmid was not significantly different from that in extracts prepared from cells transfected with carrier DNA only, confirming the species-specific nature of the rDNA promoter. Since there was no measurable activity from the rat ribosomal promoter in the monkey cells, fold activation by T antigen could not be measured. Therefore, transactivation is expressed as percentage of wild-type activity in the same experiment.
FIG. 1.
Transactivation of the rat ribosomal promoter by wild-type large-T antigens and the accumulated levels of T antigens in transiently transfected cells. (A) Transactivation of the rat ribosomal promoter-CAT construct was assayed by determining the amount of butyrated chloramphenicol produced by extracts prepared from cells transfected with the reporter construct only or in conjunction with plasmids that produce wild-type large-T antigen only, small-t antigen only, or both large-T and small-t antigens (pPVU0 or Wt-2), as described in Materials and Methods. Each construct or construct combination was transfected in duplicate or quadruplicate. Replicate assays were performed on extracts from each transfection. Means were determined from the four or eight replicate samples. The level of transactivation is expressed as the mean percentage of wild-type (wt) activity in the same experiment. Each error bar represents the percent standard error of the mean; the absence of an error bar indicates that the error was less than 2%. The entire experiment was performed four times. The pattern of results was consistent; results of one representative experiment are shown. (B) Accumulated levels of T antigen produced from cells transiently transfected with pPVU0, Wt-2, or a T-antigen-only (T1-708) construct. Immunoblot analysis of transfected cell protein extracts was performed as described in Materials and Methods with monoclonal antibody PAb901, which recognizes an epitope in the C terminus of T antigen.
Initially, three large-T-antigen expression plasmids were tested. In the experiments described below, the majority of mutant T antigens used were cloned in or derived from two plasmid sources. Wt-2 and dl2005 contain SV40 genetic information in a pBR322 background. In pPVU0, the SV40 information is contained in the pBR328 background. All three plasmids showed a high level of transactivation. As shown in Fig. 1A, 3A, and 4, cells transfected with prDCAT and the large-T antigen-expressing plasmid dl2005 or plasmid Wt-2, which expresses large-T and small-t antigens, produced a higher level of CAT activity than cells cotransfected with plasmid pPVU0. pPVU0, like Wt-2, produces both large-T and small-t antigens. Immunoblot analysis of cells transfected with the plasmids is shown in Fig. 1B. In all cases, T antigen accumulated to approximately the same level. Therefore, the reason for the differences in levels of transactivation is not clear. The majority of mutant T-antigen constructs examined in subsequent transactivation experiments were derived from pPVU0. Therefore, in all experiments the levels of transactivation were compared with results for pPVU0. In addition, in those cases involving mutant T-antigen constructs in a pBR322 background, the level of transactivation achieved by Wt-2 was included. Small-t antigen did not contribute to the transactivation by wild-type large-T antigen, as the percentages of transactivation conferred by Wt-2 and dl2005 were similar. Thus, as shown previously by microinjection (53, 55) and in vitro transcription assays (36), large-T antigen alone was sufficient to transactivate the rat rDNA promoter in transient transfection assays.
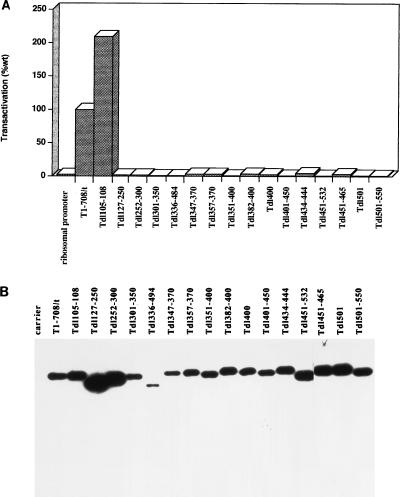
FIG. 3.
Transactivation by T antigens containing internal or N-terminal deletions and the accumulated levels of mutant T antigens in transiently transfected cells. (A) The ability of each mutant T antigen to transactivate the rat ribosomal promoter was determined as described for Fig. 1A. Each mutant was tested two to six times. The pattern of results was consistent; the results of one representative experiment are shown. Each error bar represents the percent standard error of the mean; the absence of an error bar indicates that the error was less than 2%. (B) Accumulated levels of T antigen produced from cells transiently transfected with each mutant T-antigen-producing construct. Immunoblot analysis of transfected cell protein extracts was performed as described in Materials and Methods with monoclonal antibody PAb901, which recognizes an epitope in the C terminus of T antigen.
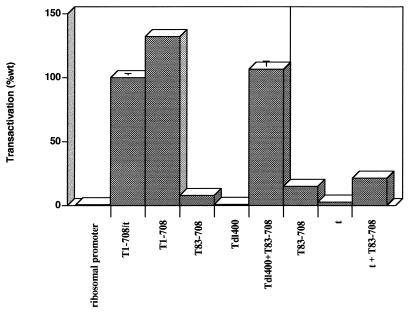
FIG. 4.
Complementation of an N-terminally truncated T antigen. The ability of the N-terminally truncated T antigen T83-708 was tested for its ability to transactivate the rat ribosomal promoter either alone and in combination with Tdl400 or small-t antigen. Each T antigen or combination was tested at least six times. The pattern of results was consistent; results of a representative experiment for each complementation are shown. Each error bar represents the percent standard error of the mean; the absence of an error bar indicates that the error was less than 2%.
Transactivation capacity of C-terminally truncated T antigens.
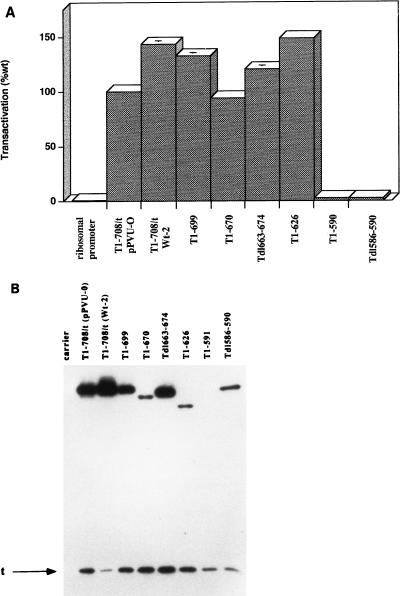
The C-terminal region of T antigen is dispensable for multiple functions of the protein (10, 45, 62, 65, 66). To determine the extent to which the C-terminal sequences of T antigen are necessary for transactivation of the rat ribosomal promoter in monkey cells, each of a series of deletion mutants was cotransfected with the reporter plasmid and tested three to six times. The pattern of transactivation by the mutants was consistent. Figure 2A shows the results of a representative experiment. T antigen missing amino acids 700 to 708 (T1-699), 671 to 708 (T1-670), 663 to 674 (Tdl663-674), or 627-708 (T1-626) transactivated the rat ribosomal promoter to wild-type levels. However, transactivation was abrogated by deleting amino acids 591 to 708 (T1-590) or 587 to 589 (Tdl586-590). Immunoblot analysis of cells transiently transfected with the mutant T-antigen constructs is shown in Fig. 2B. T1-699 and Tdl663-674 accumulated approximately to the level of T1-708. The levels of the T1-670, T1-626, and Tdl586-590 were readily detected but reduced; the T1-591 protein did not accumulate to a detectable level. Therefore, a T antigen consisting of amino acids 1 to 626 was sufficient to transactivate the rat ribosomal promoter to wild-type levels, and wild-type levels of protein are not required to achieve full transactivation potential. In addition, removal of amino acids 586 to 590 abrogated transactivation, suggesting a requirement for integrity of that portion of T antigen. The results obtained with T1-590 also confirmed that small-t antigen was not sufficient for transactivation of the ribosomal promoter.
FIG. 2.
Transactivation by C-terminally truncated T antigens and the accumulated levels of mutant T antigens in transiently transfected cells. (A) The ability of each mutant T antigen to transactivate the rat ribosomal promoter was tested three to six times as described for Fig. 1A. The pattern of results was consistent; results of a representative experiment are shown. Each error bar represents the percent standard error of the mean; the absence of an error bar indicates that the error was less than 2%. (B) Accumulated levels of T antigen produced from cells transiently transfected with Wt-2, dl1265 (T1-699), dl1066 (T1-670), dl1263 (Tdl663-674), dl2465 (T1-626), dl1061 (T1-590), and dl2433 (Tdl586-590) constructs. Immunoblot analysis of transfected cell protein extracts was performed as described in Materials and Methods with monoclonal antibodies PAb901, which recognizes an epitope in the C terminus of T antigen, and PAb419, which recognizes an epitope in the T/t common region.
Transactivation by T antigens containing internal or N-terminal deletions.
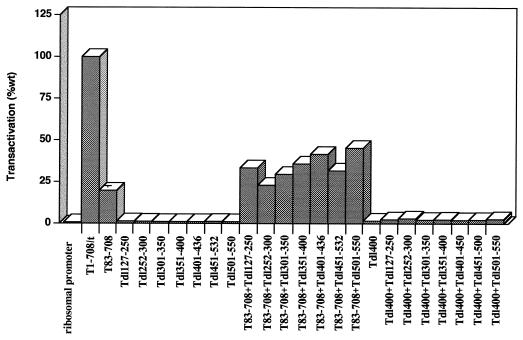
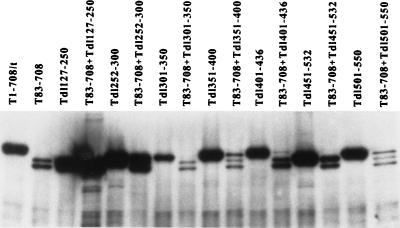
To further define the T-antigen segments that are sufficient to transactivate the rat ribosomal promoter in vivo, mutants with deletions spanning amino acids 127 to 550 were examined. Figure 3A shows that the T antigen missing amino acids 105 to 108 transactivated to a wild-type level. However, T antigens bearing internal deletions of amino acids 127 to 250, 252 to 300, 301 to 350, 336 to 484, 347 to 370, 357 to 370, 351 to 400, 382 to 400, 400, 401 to 436, 401 to 450, 434 to 444, 451 to 532, 451 to 465, 501, or 501 to 550 failed to transactivate the rat ribosomal promoter. Even deletion of the single amino acid at position 400 or 501 yielded no transactivational activity. Figure 3B shows that the mutant T antigens accumulated to approximately wild-type levels or greater with the exception of Tdl336-494. However, T antigens with smaller deletions within the region encompassed by amino acids 336 to 494 accumulated to the wild-type level. These results suggest that transactivation of the rat ribosomal gene promoter by T antigen requires integrity of the T-antigen region between amino acids 108 and 550.
To determine whether N-terminal amino acids were needed, mutant T83-708, which is missing amino acids 1 to 82, the region shared by large-T and small-t antigens (the T/t common region), was examined. The results presented in Fig. 4 showed that removal of this T/t common region substantially reduced transactivation. Immunoblot analysis (Fig. 5) indicated that T83-708 accumulated to a level lower than the wild-type level but greater than those for other mutants that retained full transactivation potential (Fig. 2B). These results suggested that alterations in the N terminus as well as in the region from amino acids 108 to 550 of T antigen abrogate transactivation.
FIG. 5.
Immunoblot analysis of mutant T antigens used in complementation experiments and the effect of cotransfection on protein accumulation. Immunoblot analysis of transfected cell protein extracts was performed as described in Materials and Methods with monoclonal antibody PAb901, which recognizes an epitope in the C terminus of T antigen.
Complementation assays.
The observation that interrupting multiple regions of T antigen disrupted transactivation suggested two possibilities. Multiple independent activities may cooperate to provide full transactivating potential, or transactivation may depend on a specific conformation of T antigen dictated by several regions of the protein. Complementation assays were performed to distinguish between these alternatives. Initially, complementation between the small internal deletion (dl400) and T83-708 was examined. The results in Fig. 4 showed that neither mutant T antigen alone transactivated the rat ribosomal promoter substantially; however, wild-type levels of transactivation were achieved in complementation assays between Tdl400 and T83-708. Figure 5 shows the relative levels of the mutant T antigens when transfected singly or in combination. In cells expressing both T83-708 and Tdl400, the level of T83-708 was unchanged. However, the level of Tdl400 was lower than the level in cells transfected with Tdl400 alone. This reduction may result from promoter competition between the strong cytomegalovirus promoter driving expression of T83-708 and the SV40 promoter driving expression of Tdl400. Nonetheless, the level of each protein was sufficient for complementation to the wild-type level. This complementation suggested that at least two T-antigen activities were required for transactivation and that one or both could operate in trans. One of these activities requires integrity of the T/t common region. However, cotransfection of the N-terminally deleted T antigen, T83-708, and the small-t-antigen-expressing plasmid did not substantially increase the level of transactivation (Fig. 4). Thus, the T/t common region could not act in trans to restore wild-type levels of transactivation, suggesting that the N-terminal activity either required large T-antigen-specific sequences beyond amino acid 82 or required another region of T antigen to be supplied in cis.
If the N-terminal activity extended beyond amino acid 82 and could be supplied in trans, then the C-terminal limit of the region might be determined by examining the ability of T83-708 to complement T antigens containing additional internal deletions. The results of such an analysis appear in Fig. 6. In no case was complementation to a wild-type level achieved. In this and a second experiment (data not shown), slight apparent increases in transactivation were observed when T83-708 was cotransfected with Tdl127-250, Tdl351-400, Tdl401-436, or Tdl501-550 and the reporter plasmid; nonetheless, in contrast to complementation between T83-708 and Tdl400, they did not approach the wild-type level. Because coexpression of T83-708 and Tdl400 resulted in reduced levels of Tdl400, it was important to examine the protein levels of the complementation pairs (Fig. 7). The protein levels of mutant T antigens Tdl301-350, Tdl351-400, Tdl401-436, Tdl451-532, and Tdl501-550 were reduced substantially in cotransfected cells (Fig. 7); lighter exposure of the immunoblot (not shown) revealed a decreased level of Tdl252-300. Since the minimal amount of T antigen needed for complementation is not known, it was not possible to define the limit of the activity marked by the deletion of amino acids 1 to 82. The protein level of Tdl127-250, however, was not diminished in cells cotransfected with the T83-708 plasmid. Thus, it was possible to conclude that removal of amino acids 127 to 250 prevented complementation. These results suggested three possibilities. The deletion could be part of the N-terminal activity compromised in T83-708. Alternatively, the deletion could impinge on the activity marked by dl400 or could identify a third activity which must be in cis with the N terminus.
FIG. 6.
Complementation between internally deleted T antigens and T83-708 or Tdl400. The abilities of the T antigens with internal deletions to transactivate the rat ribosomal promoter either alone and in combination with T83-708 or Tdl400 were examined. Each T antigen or combination was tested twice. The pattern of results was consistent; results of a representative experiment for each complementation are shown. Each error bar represents the percent standard error of the mean; the absence of an error bar indicates that the error was less than 2%.
FIG. 7.
Accumulated T-antigen levels in cells coexpressing T83-708 and other mutant T antigens. Immunoblot analysis of transfected cell protein extracts was performed as described in Materials and Methods with monoclonal antibody PAb901, which recognizes an epitope in the C terminus of T antigen.
To further explore whether the T83-708 T-antigen segment contains two complementing activities, one or both of which operate in cis with the N terminus of T antigen, we tested mutants with internal deletions for the ability to complement mutant dl400. Mutant dl400 would contain the putative N-terminal activity in cis with the region containing amino acids 127 to 250 or 252 to 300, for instance. Similarly, T antigens with internal deletions (except Tdl401-450) would contain the N-terminal activity in cis with the activity marked by dl400. The results in Fig. 6 showed that dl400 could not cooperate with any of the internal deletion mutants tested to transactivate the rat ribosomal promoter. Immunoblot analysis (not shown) showed no evidence of substantial reduction in T-antigen accumulation in cells coexpressing mutant T antigens under transcriptional control of the SV40 promoter. Therefore, although T83-708 complements dl400, trans-complementing activities between amino acids 127 and 550 of T antigen were not identified. The results did not distinguish between the possibilities that transactivation requires the structural integrity of that large region of T antigen or that more than two activities of the protein are required in cis in order to complement T83-708.
Involvement of specific T-antigen functions in transactivation.
Two activities located in the N terminus of T antigen, Rb binding and nuclear localization, were examined for involvement in transactivation of the rat ribosomal gene promoter. The amino acid deletion or substitution in the conserved LXCXE (amino acids 103 to 107) motif (43) of T antigen disables binding of the Rb family (Rb/p107/p130) of proteins (14). The mutant T antigens T-Glu107Lys and Tdl105-108 were examined for protein accumulation and the ability to transactivate the ribosomal gene promoter. Both proteins accumulated to wild-type protein levels (Fig. 8 and 3B) and transactivated (Fig. 9 and 3A) to wild-type levels, indicating that Rb/p107/p130 binding was not required.
FIG. 8.
Immunoblot analysis of mutant T antigens with or without an NLS or Rb-binding capability in single transfections and cotransfections with T83-708. Immunoblot analysis of transfected cell protein extracts was performed as described in Materials and Methods with monoclonal antibody PAb901, which recognizes an epitope in the C terminus of T antigen.
FIG. 9.
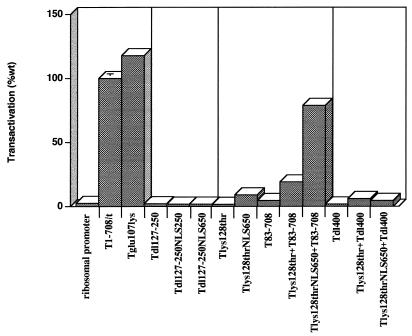
Involvement of specific T-antigen functions in transactivation of the ribosomal promoter. The impacts of Rb binding and nuclear localization were investigated. Each mutant was tested at least twice for the ability to transactivate the rat ribosomal promoter; results of representative experiments are shown. The graph is divided into four units for ease of comparing related functions. T-Glu107Lys does not bind the Rb family (Rb/p107/p130) of proteins. Tdl127-250NLS250 and Tdl127-250NLS650 are T antigens missing amino acids 127 to 250 and containing the SV40 NLS immediately preceding amino acid 250 and between amino acids 650 and 651, respectively. T-Lys128ThrNLS contains the SV40 NLS between amino acids 650 and 651. Each error bar represents the percent standard error of the mean; the absence of an error bar indicates that the error was less than 2%.
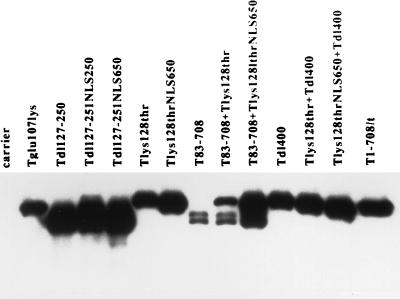
The mutant T antigen Tdl127-250 accumulates in the cytoplasm. Its inability to transactivate suggested three possibilities: that nuclear localization is essential; that the amino acids comprising the NLS (amino acids 126 to 132) are part of a larger region containing a required activity; and that nuclear localization is inconsequential, but amino acids 133 to 250 are required. To distinguish among these possibilities, an NLS was introduced into Tdl127-250 between amino acids 126 and 250 or between amino acids 650 and 651. The location adjacent to amino acid 126 was used in order to place the NLS close to its natural position in the protein. The location between amino acids 650 and 651 was chosen on the basis of two observations. We showed previously that extraneous sequences could be inserted at that position without compromising protein accumulation or any of the T-antigen functions tested (22, 61). In addition, we show here that removal of amino acids 626 to 708 did not compromise transactivation of the rat ribosomal promoter (Fig. 2A). Both mutant T antigens containing the NLS accumulated in the nucleus in immunofluorescence assays (data not shown) and accumulated to greater than wild-type levels in transiently transfected cells (Fig. 8). The results in Fig. 9 showed that neither T antigen transactivated, suggesting that disruption of the region from amino acids 127 to 250 abrogates transactivation even when the protein accumulates in the nucleus.
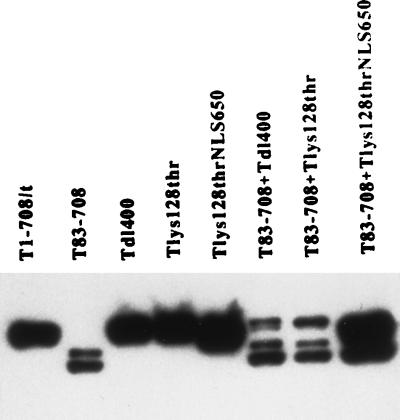
To investigate whether amino acids that constitute the NLS are required only to direct the protein to the nucleus or are part of a region directly associated with transactivation, we examined the ability of the mutant T-Lys128Thr to transactivate the rat ribosomal gene promoter. The Lys128Thr mutation inactivates transport to the nucleus, resulting in cytoplasmic accumulation of the protein. As shown in Fig. 9, T-Lys128Thr did not transactivate the rat ribosomal promoter. To determine whether nuclear localization was the only activity disrupted by the Lys128Thr mutation, the NLS was introduced between amino acids 650 and 651; the resulting T antigen was shown to accumulate in the nucleus (data not shown) and to wild-type levels (Fig. 5 and 8) in transiently transfected cells. Introduction of the NLS into T-Lys128Thr did not restore a wild-type level of transactivation in transiently transfected (Fig. 9). It appeared, therefore, that nuclear localization affected transactivation of the rat ribosomal gene promoter. However, the amino acid substitution at position 128 significantly diminished the ability of T antigen to transactivate.
We next determined whether the Lys128Thr mutation could be complemented by T83-708 and the impact of nuclear location on that complementation. The results are shown in Fig. 9; corresponding T-antigen levels are shown in Fig. 5 and 8. T-Lys128ThrNLS650 and T83-708 complemented to produce a nearly wild-type level of transactivation, whereas coexpression of T-Lys128Thr and T83-708 did not. Coexpression of T-Lys128ThrNLS650 and T83-708 resulted in an increased level of T83-708 relative to the amount of protein that accumulates in cells transfected with the T83-708-expressing plasmid (Fig. 5 and 8). The reason for this increase is not known. Nonetheless, it is unlikely that this increase in the level of T83-708 is sufficient to explain the near wild-levels of transactivation. A lighter exposure of the immunoblot in Fig. 7 (not shown) revealed a similar increase in T83-708 when cells were coexpressing Tdl252-300, yet complementation was not observed. These results indicated a requirement for nuclear accumulation to achieve maximal transactivation of the rat ribosomal gene promoter in complementation assays.
Since mutation of amino acid 128 abrogated transactivation independent of its effect on nuclear localization, it was appropriate to determine whether the activities marked by the alterations at amino acids 400 and 128 were separable and operated in trans. Therefore, we performed complementation assays between the two mutants. The results appear in Fig. 9; corresponding protein levels appear in Fig. 5 and 8. T-Lys128-Thr and Tdl400 did not complement. Introduction of an NLS into T-Lys128Thr did not alter the result. Thus, the activities marked by the dl400 and Lys128Thr mutations either constitute distal elements of a compound function or represent independent activities that operate only in cis.
DISCUSSION
The genetic analysis presented here showed that a T antigen containing amino acids 1 to 626 was sufficient to transactivate the rat ribosomal promoter linked to a CAT reporter gene in transiently transfected monkey cells. These results are consistent with other reports of T antigen’s capacity to transactivate pol I-dependent promoters. Previously, Soprano et al. (54) demonstrated reactivation of silent human ribosomal genes by microinjecting cloned T-antigen segments into mouse-human hybrid cell lines. They found that a mutant T antigen containing amino acids 1 to 509 (T1-509) was sufficient for reactivation. More recently, using in vitro transcription assays, Zhai et al. (71) showed that a T-antigen segment consisting of amino acids 1 to 538 (T1-538) was sufficient to transactivate a human ribosomal gene promoter in HeLa cell extracts. Each of these investigations indicated that C-terminal regions of T antigen are not needed to transactivate pol I-dependent promoters. The reasons for the difference observed in the T-antigen segment required may relate to the methodologies used (microinjection, transfection, in vitro transcription) or the steady-state level of the mutant proteins maintained in the various systems. Finally, these studies may indicate that different activities of T antigen are required to transactivate a rat ribosomal gene in monkey cells compared to the human genes in a mouse-human hybrid cell line or in vitro.
The genetic analysis presented here indicated that at least three T-antigen regions or activities were needed to transactivate the rat ribosomal promoter in vivo. One of these relied on the integrity of amino acids spanning the length of the T polypeptide from amino acids 109 to 626. The requirement for an extensive region of T antigen in vivo is consistent with results obtained by others using in vitro transcription assays. Zhai et al. (71) showed that a T antigen containing amino acids 1 to 538 was sufficient to stimulate transcription of a human rDNA gene in HeLa cell extracts and that deletion of amino acids 436 to 538 from T antigen sharply diminished transcriptional stimulation. They showed further that T1-538 contained two regions essential for transactivation. An N-terminal T antigen segment extending to amino acid 436 was sufficient to bind the SL1 components TBP and the polymerase-specific TAFs that are required for efficient transcription of pol I-dependent promoters and that confer the species-specific nature of transcription. However, the additional amino acids between positions 436 and 538 were needed for transactivation. The data presented here examined the consequence in vivo of deletions within the T1-538 region and indicated that the T-antigen region extending from amino acids 109 to 550 is highly sensitive to mutational inactivation of its ability to transactivate the rat ribosomal gene promoter. Even small deletions within that region such as deletion of amino acid 400 or 501 abolished T antigen’s capacity to transactivate. These findings suggested that additional functions in the region that is sufficient for SL1 binding are needed to transactivate pol I-dependent promoters, that SL1 binding requires integrity of the entire region, or that even small alterations in amino acid sequence distort this region of T antigen globally or lead to rapid degradation of the protein. Immunoblot analyses of the mutant T antigens (Fig. 2B, 3B, and 8) revealed that less than wild-type protein levels were sufficient to transactivate, and rarely could the lack of transactivation be directly related to a reduced level of the mutant T antigen. It is unlikely, therefore, that differences in protein accumulation can account for failure of mutant T antigens with deletions between amino acids 109 and 550 to transactivate the ribosomal gene promoter.
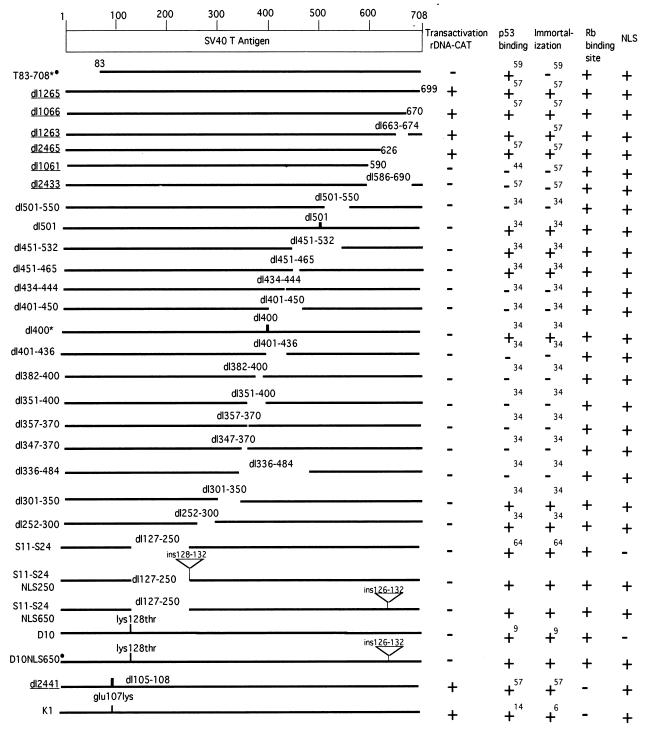
As summarized in Fig. 10, each of the T antigens with internal deletions of amino acids 127 to 350, 451 to 532, 400, or 501, as well as T-Lys128Thr, retained the ability to bind p53 and to immortalize primary cells. Integrity of activities in both the N and C termini of T antigen are required for immortalization (13, 58), and separate regions within the C-terminal half of the protein are required for binding the tumor suppressor p53 in vivo (34). Thus, retention of these activities indicates that the proteins are not distorted globally. Nonetheless, they all failed to transactivate the ribosomal promoter. It seems likely, therefore, that maintaining integrity of at least the regions containing amino acids 127 to 350, 451 to 532, 400, 501, and Lys128Thr is essential for transactivation. The T antigens containing deletions within the regions defined by amino acids 351 to 450 and 532 to 626 lose simultaneously the abilities to bind the tumor suppressor p53 and to immortalize primary mouse cells (34). The loss of multiple T-antigen activities may indicate substantial distortion of at least the C-terminal half of the protein. Thus, on the basis of deletion mutant analysis, the involvement of the regions 351 to 450 and 532 to 626 in transactivation of pol I-dependent promoters in vivo is less certain.
FIG. 10.
Diagrammatic representation of mutant T antigens used in this study. The names of the plasmids encoding the mutant T antigens are given on the left. The corresponding T antigens are represented by lines in which the deleted amino acids are represented by gaps. Single amino acid substitutions and deletions of three or fewer amino acids are represented by vertical lines at the positions of their occurrence. Insertions of amino acids are represented by an inverted triangle at the position of the insertion. The deleted amino acids, substitutions, and insertions are indicated above the sites of alteration. Mutant T antigen sequences cloned in a pBR322 background are underlined; mutant T-antigen sequences cloned in a pBR328 background are not underlined; mutant T antigens that complement one another are designated by an asterisk or dot. Numbers in superscript correspond to literature citations.
The finding that T83-708 could not transactivate the rat ribosomal gene promoter indicated that a second region, the extreme N-terminal portion of T antigen, was required. Complementation analyses were performed to determine whether the activity marked by the deletion of amino acids 1 to 82 was separable from that marked by internal deletions. T83-708 complemented Tdl400. Thus, deletion of amino acids 1 to 82 and deletion of amino acid 400 alter different T-antigen activities that can operate in trans to increase transcription of a pol I-dependent promoter. To determine if the C-terminal limit of the activity marked by deletion of amino acids 1 to 82 extends further into the protein, attempts were made to complement T83-708 with additional T antigens containing internal deletions. This analysis was compromised by decreased levels of mutant T antigens in cells coexpressing T83-708. Nonetheless, the finding that deletion of amino acids 105 to 108 did not diminish transactivation of the rat ribosomal gene promoter indicated that amino acids 1 to 82 is not part of a contiguous transactivation function of T antigen extending beyond amino acid 104.
The ability of T antigen to localize to the nucleus also affected activation of the rat ribosomal gene promoter. Mutant T antigens with alterations that prevent nuclear localization failed to transactivate alone or in combination with T83-708. Addition of an NLS to T-Lys128Thr at an alternate site did not result in transactivation to wild-type levels, indicating that this mutation marks a required activity independent of nuclear localization. The observation that T-Lys128ThrNLS complemented the N-terminally truncated protein T83-708 indicated that the activity marked by the substitution at residue 128 is independent of the activity lost when the first 82 amino acids of T antigen are deleted. In addition, the results showed that accumulation of T antigen in the nucleus was essential for wild-type levels of transactivation.
The finding that T-Lys128ThrNLS would not complement dl400 suggested two possibilities. Either T-Lys128ThrNLS and Tdl400 must be present on the same molecule to cooperate functionally or they mark a single activity that depends on integrity of a substantial portion of T antigen. The finding that T antigens with internal deletions other than dl400 were devoid of transactivating function supports the latter contention.
The capacity of T83-708 and dl400 and of T83-708 and T-Lys128ThrNLS to complement constitutes intracistronic complementation. Intracistronic complementation could occur if transactivation operated through the independent action of mutant T-antigen monomers. Alternatively, oligomerization of T antigens with alterations in separate regions might be required to form an active protein structure. Although oligomerization is not needed for transactivation of pol II-dependent promoters (31), it is not known whether transactivation of pol I-dependent promoters depends on oligomerization of T antigen. T-antigen regions between amino acids 114 and 152 and the C-terminal region up to 669 are necessary and sufficient for oligomerization (41), yet a T-antigen fragment extending only to amino acid 538 transactivates the human ribosomal gene promoter in vitro (71). The close correspondence between the T-antigen regions required for in vitro and in vivo transcription of pol I-dependent promoters suggests that oligomerization would not be needed. If oligomerization were required, it clearly would not be sufficient. T83-708 has been shown to oligomerize in a fashion identical to that of wild-type T antigen (37), indicating that it should be able to form oligomers with mutant T antigen that retain oligomerization domains. With the exception of T125-250 and its derivatives containing a translocated NLS, and T-Lys128Thr and its NLS-containing derivative, all of the mutant T antigens tested contain the T-antigen regions that are sufficient for oligomerization, yet complementation to wild-type levels was observed only in the two cases noted.
It was shown recently that Rb represses transcription of ribosomal genes by binding to UBF and disrupting initiation complexes (7, 67). Thus, it seemed likely that T antigen might transactivate ribosomal genes by binding to Rb and preventing its association with UBF. This, however, does not appear to be the case. T-Glu107Lys, in which T-Rb binding is disrupted, and Tdl105-108, which bears a deletion within the Rb-binding site, both transactivated to wild-type levels. Thus, T-antigen binding to Rb is not the mechanism used to transactivate the rat ribosomal gene.
The relationship between activities of the SV40 early proteins that participate in transactivation of pol I-dependent genes and those reported to contribute to transactivation of pol II-dependent genes is difficult to decipher. Large-T antigen transactivates a variety of pol II-dependent promoters containing different upstream activation sequences (23, 46). The requirement for specific activities of T antigen in transcriptional activation of pol II-dependent genes varies with the specific promoters utilized. For instance, transactivation of the Rous sarcoma virus (RSV) LTR and SV40 late promoters by T antigen does not require integrity of the Rb-binding site (72), whereas Rb binding appears to participate in transactivation of the E2A promoter (39). Nonetheless, some comparisons can be made. Like transactivation of the RSV and SV40 late promoters, transactivation of the rat rDNA did not require an intact T-antigen Rb-binding region. In addition, efficient nuclear localization was not essential for transactivation of the RSV or SV40 late promoter (72). However, nuclear localization substantially affected transactivation of the rat ribosomal gene promoter. The N-terminal 121-amino-acid segment of T antigen is sufficient to transactivate certain pol II-dependent promoters in transient transfection assays (56), and multiple insertions and internal deletions in T antigen are tolerated with little or no substantial loss of transactivating activity (72). In contrast, we show here that with the exception of amino acids 105 to 108, internal deletions within T antigen abrogated transactivation of the rat ribosomal gene promoter. Similarly, the role of small-t antigen in transactivation depends on the specific promoter utilized. Small-t antigen transactivates the pol II-dependent adenovirus E2A and SV40 early promoters but not the RSV or SV40 late promoter. Small-t antigen did not independently transactivate the rat ribosomal gene promoter to a measurable extent (Fig. 1A and 2A), nor could it supply the transactivating activity missing from T83-708. Thus, the activities of large-T antigen that are reported to be sufficient to transactivate a pol II-dependent promoter are not sufficient to transactivate a rat pol I-dependent promoter in a transient transfection assay in monkey cells.
Four conclusions can be drawn from the results presented here. First, activation of the rat ribosomal gene in monkey cells depends on an N-terminal activity of T antigen that can be supplied in trans by large-T but not small-t antigen. Second, accumulation of T antigen in the nucleus is required for maximal transactivation of the rat rDNA promoter. Third, one or more activities encompassed by amino acids 109 to 626 are required. The results do not distinguish between the possibilities that multiple activities within the region from amino acids 109 to 626 must be in cis, as is the case for activities involved in replication of the viral genome (11, 19), or that a single activity determined by a specific conformation of the region is involved. Finally, the capacity to transactivate ribosomal genes did not correlate with the capacity of T antigen to immortalize primary cells or to bind either the Rb or p53 tumor suppressor.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant CA67303 (J.F.C.) and (in part) CA24694 (M.J.T.) from the National Cancer Institute of the National Institutes of Health.
REFERENCES
- 1.Beckmann H, Chen J-L, O’Brien T, Tjian R. Coactivator and promoter-selective properties of RNA polymerase I TAFs. Science. 1995;270:1506–1509. doi: 10.1126/science.270.5241.1506. [DOI] [PubMed] [Google Scholar]
- 2.Bell S P, Jantzen H-M, Tjian R. Assembly of alternative multiprotein complexes directs rRNA promoter selectivity. Genes Dev. 1990;4:943–954. doi: 10.1101/gad.4.6.943. [DOI] [PubMed] [Google Scholar]
- 3.Bell S P, Learned R M, Jantzen H-M, Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988;241:1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- 4.Berger, L. C., D. B. Smith, I. Davidson, J.-J. Hwang, E. Fanning, and A. G. Wildeman. Interaction between T antigen and TEA domain of the factor TEF-1 derepresses simian virus 40 late promoter I vitro: identification of T-antigen domains important to transcription control. J. Virol. 70:1203–1212. [DOI] [PMC free article] [PubMed]
- 5.Bikel I, Loeken M R. Involvement of simian virus 40 (SV40) small t antigen in trans activation of SV40 early and late promoters. J Virol. 1992;66:1489–1494. doi: 10.1128/jvi.66.3.1489-1494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassidy B, Haglund R, Rothblum L. Regions upstream from the core promoter of the rat ribosomal gene are required for the formation of a stable transcription initiation complex by RNA polymerase I in vitro. Biochim Biophys Acta. 1987;909:113–144. doi: 10.1016/0167-4781(87)90035-2. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh A H, Hempel W M, Taylor L J, Rogalsky V, Todorov G, Rothblum L I. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995;374:177–180. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- 8.Cavender J F, Conn A, Epler M, Lacko H, Tevethia M J. Simian virus 40 contains two independent activities that cooperate with an ras oncogene to transform rat embryo fibroblasts. J Virol. 1995;69:923–934. doi: 10.1128/jvi.69.2.923-934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Paucha E. Identification of a region of simian virus 40 large T antigen required for cell transformation. J Virol. 1990;64:3350–3357. doi: 10.1128/jvi.64.7.3350-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark R, Peden K, Pipas J M, Nathans D, Tjian R. Biochemical activities of T antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1883;3:220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole C N, Tornow J, Clark R, Tjian R. Properties of the simian virus 40 (SV40) large T antigen encoded by SV40 mutants with deletions in gene A. J Virol. 1986;57:539–546. doi: 10.1128/jvi.57.2.539-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comai L, Tanese N, Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 13.Conzen S D, Cole C N. Three transforming regions of SV40 T antigen are required for immortalization of primary mouse embryo fibroblasts. Oncogene. 1995;11:2295–2302. [PubMed] [Google Scholar]
- 14.DeCaprio J A, Ludlow J W, Figge J, Shew J-Y, Huang C-M, Lee W-H, Marsilio E, Paucha E, Livingston D M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 15.Dickmanns A, Zeitvogen A, Simmersbach F, Weber R, Arthur A K, Dehde S, Wildeman A G, Fanning E. The kinetics of simian virus 40-induced progression of quiescent cells into S phase depends on four independent functions of large T antigen. J Virol. 1994;68:5496–5508. doi: 10.1128/jvi.68.9.5496-5508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixit A, Garg L C, Chao W, Jacob S T. An enhancer element in the far upstream spacer region of rat ribosomal RNA gene. J Biol Chem. 1987;262:11616–11622. [PubMed] [Google Scholar]
- 17.Dobbelstein M, Aurthur A K, Dehde S, van Zee K, Dickmanns A, Fanning E. Intracistronic complementation reveals a new function of SV40 T antigen that co-operates with Rb and p53 binding to stimulate DNA synthesis in quiescent cells. Oncogene. 1991;7:837–847. [PubMed] [Google Scholar]
- 18.Eberhard C, Tora L, Egly J M, Grummt I. A TBP-containing multiprotein complex (TIF-IB) mediates transcription specificity of murine RNA polymerase I. Nucleic Acids Res. 1993;21:4180–4186. doi: 10.1093/nar/21.18.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanning E, Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- 20.Financsek I, Mizumoto K, Mishima Y, Muramatsu M. Human ribosomal RNA gene: nucleotide sequence of the transcription initiation region and comparison of three mammalian genes. Proc Natl Acad Sci USA. 1982;79:3092–3096. doi: 10.1073/pnas.79.10.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowlis D J, Balmain A. Oncogenes and tumor suppressor genes in transgenic mouse models of neoplasia. Eur J Cancer. 1993;29A:638–645. doi: 10.1016/s0959-8049(05)80170-4. [DOI] [PubMed] [Google Scholar]
- 22.Fu T-M, Bonneau R H, Tevethia M J, Tevethia S S. Simian virus 40 T antigen as a carrier for the expression of cytotoxic T-lymphocyte recognition epitopes. J Virol. 1993;67:6866–6871. doi: 10.1128/jvi.67.11.6866-6871.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilinger G, Alwine J C. Transcriptional activation by simian virus 40 large T antigen: requirements for simple promoter structures containing either TATA or initiator elements with variable upstream factor binding sites. J Virol. 1993;67:6682–6688. doi: 10.1128/jvi.67.11.6682-6688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grummt I. Nucleotide sequence requirements for specific initiation of transcription by RNA polymerase I. Proc Natl Acad Sci USA. 1982;79:6908–6911. doi: 10.1073/pnas.79.22.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grummt I, Roth E, Paule M. Ribosomal RNA transcription in vitro is species specific. Nature. 1982;296:173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- 26.Grunda M C, Zabolotny M M, Xiao J H, Davidson I, Alwine J S. Transcriptional activation by simian virus 40 large T antigen: interactions with multiple components of the transcription complex. Mol Cell Biol. 1993;13:961–969. doi: 10.1128/mcb.13.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haltiner M M, Smale S T, Tjian R. Two distinct promoter elements in the human rRNA gene identified by linker scanning mutagenesis. Mol Cell Biol. 1986;6:227–235. doi: 10.1128/mcb.6.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson S, Sollner-Webb B. A transcriptional terminator is a novel element of the promoter of the mouse ribosomal RNA gene. Cell. 1986;47:891–900. doi: 10.1016/0092-8674(86)90804-4. [DOI] [PubMed] [Google Scholar]
- 30.Hunter T. Oncoprotein networks. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 31.Johnson S D, Yu X-M, Mertz J E. The major transcriptional transactivation domain of simian virus 40 large T antigen associates nonconcurrently with multiple components of the transcription preinitiation complex. J Virol. 1996;70:1191–1202. doi: 10.1128/jvi.70.2.1191-1202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalderon D, Roberts B L, Richardson W D, Smith A E. A short amino acid sequence able to specify nuclear localization. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 33.Kalderon D, Smith A E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984;139:109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- 34.Kierstead T D, Tevethia M J. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J Virol. 1993;67:1817–1828. doi: 10.1128/jvi.67.4.1817-1829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Learned R M, Cordes S, Tjian R. Purification and characterization of a transcription factor that confers promoter specificity to human RNA polymerase I. Mol Cell Biol. 1985;5:1358–1369. doi: 10.1128/mcb.5.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Learned R M, Smale S T, Haltiner M H, Tjian R. Regulation of human ribosomal RNA transcription. Proc Natl Acad Sci USA. 1983;80:3558–3562. doi: 10.1073/pnas.80.12.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin H J L, Upson R H, Simmons D T. Nonspecific DNA binding activity of simian virus 40 large T antigen: evidence for cooperation of two regions for full activity. J Virol. 1992;66:5443–5452. doi: 10.1128/jvi.66.9.5443-5452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeken M, Bikel I, Livingston D M, Brady J. Trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988;55:1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- 39.Loeken M R. Multiple, distinct trans-activation functions are encoded by the simian virus 40 large T and small t antigens, only some of which require the 82-residue amino-terminal common domain. J Virol. 1993;67:7684–6789. doi: 10.1128/jvi.67.12.7684-7689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manfredi J J, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198:65–83. doi: 10.1016/0304-419x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 41.Montenarh M, Vesco C, Kemmerling G, Mueller D, Henning R. Regions of SV40 large T antigen necessary for oligomerization and complex formation with the cellular oncoprotein p53. FEBS Lett. 1986;204:51–55. doi: 10.1016/0014-5793(86)81386-2. [DOI] [PubMed] [Google Scholar]
- 42.Pikaard C S, McStay B, Schultz M C, Bell S P, Reeder R H. The Xenopus ribosomal gene enhancers bind an essential polymerase I transcription factor, xUBF. Genes Dev. 1989;3:1779–1788. doi: 10.1101/gad.3.11.1779. [DOI] [PubMed] [Google Scholar]
- 43.Pipas J M. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pipas, J. M. Personal communication.
- 45.Pipas J M, Peden K W C, Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983;3:202–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice P W, Cole C N. Efficient transcriptional activation of many simple modular promoters by simian virus 40 large T antigen. J Virol. 1993;67:6689–6697. doi: 10.1128/jvi.67.11.6689-6697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott M R D, Westphal K-H, Rigby P W J. Activation of mouse genes in transformed cells. Cell. 1983;34:557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 48.Seed B, Sheen J-Q. A simple phase-extraction assay for chloramphenicol acetyl transferase. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 49.Sleigh J J, Topp W C, Harich R, Sambrook J F. Mutants of SV40 with altered small t protein are reduced in their ability to transform cells. Cell. 1978;14:79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- 50.Smale S, Tjian R. Transcription of herpes simplex tk sequences under the control of wild-type and mutant human RNA polymerase I promoter. Mol Cell Biol. 1985;5:352–362. doi: 10.1128/mcb.5.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sodrosky J, Patarca R, Rosen C, Wong-Staal F, Hazeltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985;229:74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- 52.Sompayrac L, Danna K J. Simian virus 40 deletion mutants that transform with reduced efficiency. Mol Cell Biol. 1983;3:484–489. doi: 10.1128/mcb.3.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soprano K J, Dev V G, Croce C M, Beserga R. Reactivation of silent rRNA genes by simian virus 40 in human-mouse hybrid cells. Proc Natl Acad Sci USA. 1979;76:3885–3889. doi: 10.1073/pnas.76.8.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soprano K J, Galanti N, Jonak G J, McKercher S, Pipas J M, Peden K W C, Beserga R. Mutational analysis of simian virus 40 T antigen: stimulation of cellular DNA synthesis and activation of rRNA genes by mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983;3:214–219. doi: 10.1128/mcb.3.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soprano K J, Rossini M, Croce C, Beserga R. The role of large T antigen in simian virus 40-induced reactivation of silent rRNA genes in human-mouse hybrid cells. Virology. 1980;102:317–326. doi: 10.1016/0042-6822(80)90099-9. [DOI] [PubMed] [Google Scholar]
- 56.Srinivasan A, Peden K W C, Pipas J M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989;63:5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tevethia M J, Bonneau R H, Griffith J W, Mylin L. A simian virus 40 large T-antigen segment containing amino acids 1 to 127 and expressed under the control of the rat elastase-1 promoter produces pancreatic acinar carcinomas in transgenic mice. J Virol. 1997;71:8157–8166. doi: 10.1128/jvi.71.11.8157-8166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tevethia M J, Lacko H A, Conn A. Two regions of simian virus 40 large T-antigen independently extend the life-span of primary C57BL/6 mouse embryo fibroblasts and cooperate in immortalization. Virology. 1998;243:303–312. doi: 10.1006/viro.1998.9056. [DOI] [PubMed] [Google Scholar]
- 59.Tevethia, M. J., and H. A. Lacko. Unpublished data.
- 60.Tevethia M J, Pipas J M, Kierstead T, Cole C. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3′ end of SV40 gene A. Virology. 1988;162:76–89. doi: 10.1016/0042-6822(88)90396-0. [DOI] [PubMed] [Google Scholar]
- 61.Tevethia M J, Lacko H A, Kierstead T D, Thompson D L. Adding an Rb-binding site to an N-terminally truncated simian virus 40 large T antigen restores growth to high cell density, and the T common region in trans provides anchorage-independent growth and rapid growth in low serum concentrations. J Virol. 1997;71:1888–1896. doi: 10.1128/jvi.71.3.1888-1896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tevethia M J, Pipas J M, Kierstead T, Cole C. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3′ end of SV40 gene A. Virology. 1988;162:76–89. doi: 10.1016/0042-6822(88)90396-0. [DOI] [PubMed] [Google Scholar]
- 63.Tevethia M J, Spector D J. Heterologous transactivation among viruses. Prog Med Virol. 1989;36:120–190. [PubMed] [Google Scholar]
- 64.Thompson D L, Kalderon D, Smith A E, Tevethia M J. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology. 1990;178:15–34. doi: 10.1016/0042-6822(90)90375-2. [DOI] [PubMed] [Google Scholar]
- 65.Tornow J, Cole C N. Nonviable mutants of simian virus 40 with deletions near the 3′ end of the gene A define a function for large T antigen required after onset of viral DNA replication. J Virol. 1983;47:487–494. doi: 10.1128/jvi.47.3.487-494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tornow J, Polvino-Bodmar M, Santangelo G, Cole C N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985;53:415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voit R, Schaffer K, Grummt I. Mechanism of repression of RNA polymerase I transcription by the retinoblastoma protein. Mol Cell Biol. 1997;17:4230–4237. doi: 10.1128/mcb.17.8.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whelly S, Ide T, Beserga R. Stimulation of RNA in isolated nucleoli by preparations of simian virus 40 T antigen. Virology. 1978;88:82–91. doi: 10.1016/0042-6822(78)90112-5. [DOI] [PubMed] [Google Scholar]
- 69.Xie W Q, O’Mahony D J, Smith S D, Rothblum L. Complementary in vivo and in vitro analyses of the interactions between cis-acting elements of the rat rDNA promoter. Mol Cell Biochem. 1991;104:127–135. doi: 10.1007/BF00229812. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto O, Takakusa N, Mishima Y, Kominami R, Murammatsu M. Determination of the promoter region of the mouse ribosomal RNA gene by an in vitro transcription system. Proc Natl Acad Sci USA. 1984;81:299–303. doi: 10.1073/pnas.81.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhai W, Tuan J, Comai L. SV40 large T antigen binds to TBP-TAF1 complex SL1 and coactivates ribosomal RNA transcription. Genes Dev. 1998;11:1603–1617. doi: 10.1101/gad.11.12.1605. [DOI] [PubMed] [Google Scholar]
- 72.Zhu J, Rice P W, Chamberlain M, Cole C N. Mapping the transcriptional transactivation function of simian virus 40 large T antigen. J Virol. 1991;65:2778–2790. doi: 10.1128/jvi.65.6.2778-2790.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu J, Rice P W, Gorsch L, Abate M, Cole C N. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992;66:2780–2791. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]