Abstract
Background
Early-life stressors can adversely affect the developing brain. While hierarchical modeling has established the existence of a general factor of psychopathology, no studies have modeled a general factor of environmental stress and related this factor to brain development. Using a large sample of children from the ABCD (Adolescent Brain Cognitive Development) Study, the current study aimed to identify general and specific factors of environmental stress and test their associations with brain structure and psychopathology.
Methods
In a sample of 11,878 children, bifactor modeling and higher-order (second-order) modeling identified general and specific factors of environmental stress: family dynamics, interpersonal support, neighborhood socioeconomic status deprivation, and urbanicity. Structural equation modeling was performed to examine associations between these factors and regional gray matter volume (GMV) and cortical thickness as well as general and specific factors of psychopathology.
Results
The general environmental stress factor was associated with globally smaller cortical and subcortical GMV as well as thinner cortices across widespread regions. Family dynamics and neighborhood socioeconomic status deprivation were associated with smaller GMV in focal regions. Urbanicity was associated with larger cortical and subcortical GMV and thicker cortices in frontotemporal regions. The environmental factors were associated with psychopathology in the expected directions. The general factors of environmental stress and psychopathology were both predictors of smaller GMV in children, while remaining distinct from each other.
Conclusions
This study reveals a unifying model of environmental influences that illustrates the inherent organization of environmental stressors and their relationship to brain structure and psychopathology.
Keywords: Brain development, Environmental risk factors, Hierarchical modeling, Neuroimaging, Psychopathology, Stress
The developing brain is susceptible to stress such as neglect, abuse, and unsupportive interpersonal relationships (1), as well as environmental influences including resource availability, urban living, and pollution (2). Features of the child’s environment interact in complex ways to impact the developing brain (3, 4, 5, 6, 7) and have important implications for the onset of psychopathology (8), adverse physical health (9), and poor psychosocial functioning (10) later in life.
A unifying model that captures environmental influences on the developing brain is needed. Traditionally, researchers have taken either a specificity approach or a cumulative-risk approach (11). Using a specificity approach, different types of adversities such as poverty or abuse are considered distinct categories, which fails to account for the high co-occurrence among multiple forms of adversity. Conversely, the cumulative-risk approach aggregates the number of occurrences of adversity into a count variable, which assumes that all events have equal weights and can be additive. To overcome the limitations presented by the specificity and cumulative-risk approaches, a dimensional approach has been suggested as an alternative. A dimensional approach assumes that there are core underlying dimensions across different types of adversities with shared features (11).
A comprehensive dimensional model of the child’s environment needs to account for both the common and the specific influences of environmental stressors. Hierarchical models, in particular, have transformed the way we conceptualize psychopathology by revealing a general psychopathology factor (p factor) that represents the shared variance across symptoms and subfactors representing specific symptom domains (12,13). These psychopathology dimensions are associated with abnormal development of brain structure (3,14), suggesting that hierarchical models can be useful for identifying transdiagnostic brain features underlying mental health disorders. Likewise, a hierarchical model of environmental stressors could uncover the dimensional structure underlying attributes of the child’s environment and how these factors are associated with the developing brain and psychopathology. While prior work has shown a relationship between socioeconomic/environmental variables and the brain using dimensional approaches (15, 16, 17), these studies used canonical correlation analysis and moderated nonlinear factor analysis, which are methods that address different questions than the current study. Our approach is distinct because it tells us what is common across all environmental stressors and parses the unique variance left over after accounting for a general factor of environmental stress.
The current study used hierarchical modeling to delineate common and specific factors of environmental stress and related these dimensions to brain structure (gray matter volume [GMV] and cortical thickness) in a large sample of children from the ABCD (Adolescent Brain Cognitive Development) Study. In addition, we examined the association between the environmental stress factors and dimensions of psychopathology: internalizing, attention-deficit/hyperactivity disorder (ADHD) symptoms, and conduct problems. Based on prior work showing that environmental factors such as low socioeconomic status (SES) and childhood abuse are associated with abnormalities in brain structure and psychopathology (18,19), we hypothesized that a general factor of environmental stress would be related to globally smaller cortical and subcortical brain volumes, thinner cortices, and greater psychopathology. As the discovery of the specific environmental stress factors was exploratory, we had no a priori predictions about the relationships between the specific environmental factors and brain or psychopathology.
Methods and Materials
Participants
Participants were 11,878 children between 9 and 10 years old from wave 1 (release 3.0) of the ABCD Study (20). The ABCD Study group obtained parental consent and child assent. The Vanderbilt University Institutional Review Board approved the use of this deidentified dataset. Data collection was performed at 21 sites across the United States (21).
Measures of the Environment
A broad range of environmental stressors were examined, including early-life stress (e.g., neglect and abuse), family characteristics (e.g., family history of mental illness, family conflict), community characteristics (e.g., poverty levels, residential density, crime rates, availability of substances), attributes of the physical environment (e.g., pollution exposure, lead exposure, population density, proximity to major roads), and interpersonal factors (e.g., supportive relationships in the child’s immediate social environment). For additional details on the stressor measures, see the Supplement.
Psychopathology Measures
Psychopathology factors were derived based on a previous study using the Child Behavior Checklist items from the baseline data from the ABCD Study (22). This model identified a general factor of psychopathology symptoms (also called the psychopathology [p] factor) and 3 specific factors of psychopathology: internalizing problems, ADHD symptoms, and conduct problems. The validity and reliability of this model have been published elsewhere (22). For additional details on the psychopathology factors, see the Supplement.
Image Acquisition, Processing, and Quality Assurance
A description of the brain image acquisition, processing, and quality assurance procedures for the ABCD Study is given elsewhere (23). A brief summary on the procedures developed and performed by the ABCD Study group is provided in the Supplement.
Statistical Analyses
All analyses were performed with Mplus Version 8.4 (https://www.statmodel.com/) using structural equation modeling (SEM). In analyzing this complex survey data, the data were 1) clustered based on family membership to account for siblings and multiple births, 2) stratified based on site to account for site differences, 3) weighted by the poststratification weights provided by the ABCD Study to make the sample more representative of the U.S. population, 4) weighted by nonparticipation weights to adjust for differences between the included and excluded samples, and 5) covaried by scanner model to control for differences between scanners. For additional details on the handling of dependencies and weighting, see the Supplement. Of the total sample of 11,878 youths, about three fourths of participants (9000 youths) were randomly selected for an exploratory structural equation modeling (ESEM) analysis (24) to determine what underlying factors may be present, and the remaining participants (2878 youths) were reserved for a confirmatory bifactor analysis (25) and a higher-order model analysis (26) to model general and specific factors. There were 1555 participants from the ESEM sample and 505 participants from the holdout confirmatory sample that were missing nonparticipation weights, leaving 7445 participants for the ESEM analysis and 2373 participants for the confirmatory analyses.
To examine the associations between the general and specific environmental stress factors and measures of brain structure, SEM was used while controlling for age, sex, race/ethnicity, and magnetic resonance imaging (MRI) scanner model as in equation 1.
| (1) |
where i = 1 … 68 (i.e., the number of brain regions) for cortical thickness and i = 1 … 87 for GMV analyses.
Participants with missing data and participants failing to pass quality assurance measures for MRI data were excluded, leaving 9818 participants for these analyses (Figure S1). Sensitivity analyses were performed to control for global brain measures to test whether the main findings were regionally specific. Intracranial volume and average cortical thickness were added as additional covariates in analyses of GMV and cortical thickness, respectively. SEM was also used to examine the associations between our general and specific environmental stress factors and the general and specific factors of psychopathology defined by Moore et al. (22): general psychopathology, internalizing symptoms, ADHD symptoms, and conduct problems. The demographic characteristics based on each sample are presented in Table 1. The false discovery rate (q < .05) was controlled to account for multiple tests. For additional details on the analyses, see the Supplement.
Table 1.
Demographic Characteristics of the Sample
| ESEM (n = 7445) | Bifactor Modeling (n = 2373) | SEM (n = 9818) | |
|---|---|---|---|
| Age, Months | 119.15 (7.51) | 119.23 (7.52) | 119.17 (7.51) |
| Sex | |||
| Female | 3604 (48.41%) | 1185 (49.94%) | 4789 (48.78%) |
| Male | 3841 (51.59%) | 1188 (50.06%) | 5029 (51.22%) |
| Race/Ethnicity | |||
| Black | 1052 (14.13%) | 343 (14.45%) | 1395 (14.21%) |
| Hispanic | 1503 (20.19%) | 508 (21.41%) | 2011 (20.48%) |
| Other | 910 (12.22%) | 270 (11.38%) | 1180 (12.02%) |
| White | 3980 (53.46%) | 1252 (52.76%) | 5232 (53.29%) |
| Household Annual Income | |||
| <$5000 | 251 (3.37%) | 83 (3.50%) | 334 (3.40%) |
| $5000–$11,999 | 258 (3.47%) | 71 (2.99%) | 329 (3.35%) |
| $12,000–$15,999 | 168 (2.26%) | 58 (2.44%) | 226 (2.30%) |
| $16,000–$24,999 | 286 (3.84%) | 133 (5.60%) | 419 (4.27%) |
| $25,000–$34,999 | 399 (5.36%) | 126 (5.31%) | 525 (5.35%) |
| $35,000–$49,999 | 570 (7.66%) | 182 (7.67%) | 752 (7.66%) |
| $50,000–$74,999 | 939 (12.61%) | 302 (12.73%) | 1241 (12.64%) |
| $75,000–$99,999 | 992 (13.32%) | 338 (14.24%) | 1330 (13.55%) |
| $100,000–$199,999 | 2176 (29.23%) | 643 (27.10%) | 2819 (28.71%) |
| ≥$200,000 | 793 (10.65%) | 238 (10.03%) | 1031 (10.50%) |
| Missing | 613 (8.23%) | 199 (8.39%) | 812 (8.27%) |
| Parental Education | |||
| No degree | 372 (5.00%) | 119 (5.01%) | 491 (5.00%) |
| High school degree/GED | 888 (11.93%) | 288 (12.14%) | 1176 (11.98%) |
| Some college | 1196 (16.06%) | 393 (16.56%) | 1589 (16.18%) |
| Associate’s degree | 925 (12.42%) | 326 (13.74%) | 1251 (12.74%) |
| Bachelor’s degree | 2121 (28.49%) | 670 (28.23%) | 2791 (28.43%) |
| Master’s degree | 1470 (19.74%) | 444 (18.71%) | 1914 (19.49%) |
| Professional/doctoral degree | 473 (6.35%) | 133 (5.60%) | 606 (6.17%) |
Values are mean (SD) or n (%).
ESEM, exploratory structural equation modeling; SEM, structural equation modeling.
Data and Code Availability
The ABCD Study data used in the current study are available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The code and a corresponding wiki for the analytic procedures can be found at https://github.com/VU-BRAINS-lab/Jeong_Environmental_Bifactor.
Results
ESEM Identifies 4 Environmental Stress Factors
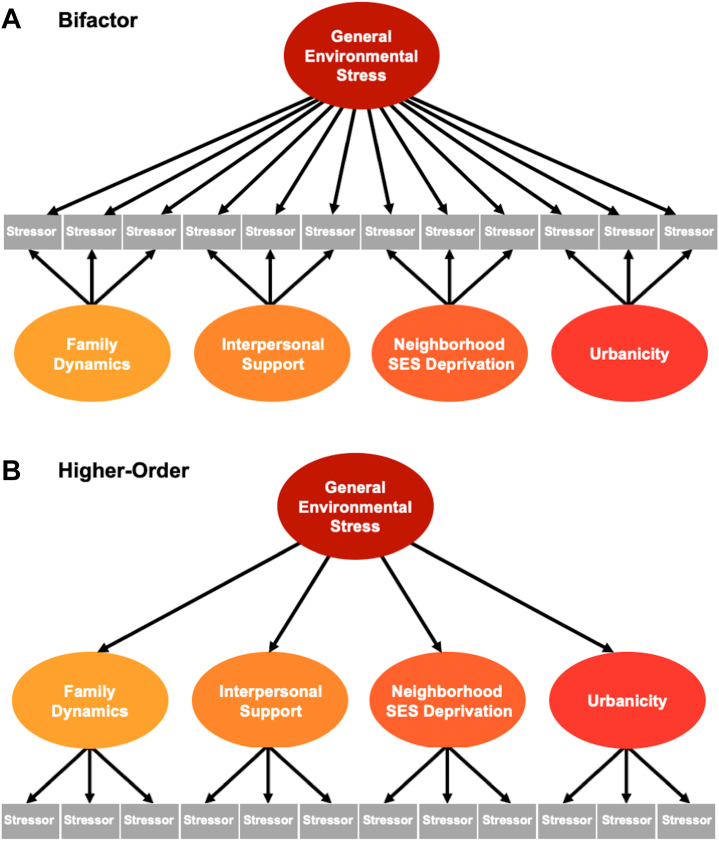
Using ESEM, a scree plot indicated that 4 factors can be extracted from items reflecting the environmental stressors (Figure S2; Table S2). Factor 1 was predominantly made up of items related to the dynamics of the child’s family environment. Items included those indicating a history of mental illness in any blood relative of the child, traumatic events experienced by the child (e.g., physical/sexual abuse, witnessing violence at home), the presence of conflict within the family, and financial difficulty experienced by the immediate family (e.g., inability to pay for food, rent, hospital services). Factor 1 was labeled “family dynamics.”
Factor 2 was composed of items reflecting interpersonal support at school and home. This factor included items related to the child’s perception of his or her connectedness in school, such as the child’s relationship with teachers, perception of the school environment, involvement in school, and feelings of alienation from academic goals. Additionally, the child’s perception of the primary caregiver’s warmth, acceptance, and responsiveness loaded onto factor 2, as well as items reflecting parental involvement in monitoring the child. Factor 2 was labeled “interpersonal support.”
Factor 3 primarily contained items related to the availability of resources in the child’s neighborhood. Items indicating the SES disadvantage of the neighborhood in which the child resides clustered together, such as the median rent and home value, the percentage of families living below or close to the poverty level, income disparity, median family income, and the percentage of the population with at least a high school diploma. Factor 3 was labeled “neighborhood SES deprivation.”
Finally, factor 4 comprised items indicating the quality of the child’s physical environment, many of which are related to urban living. Items measuring features of the physical environment included pollution levels, population density, walkability, and lead exposure risk. Additionally, items related to safety loaded onto factor 4, including crime rates as well as parents’ perceptions of neighborhood safety. Factor 4 was labeled “urbanicity.”
Hierarchical Modeling Defines a General Environmental Stress Factor
Following the ESEM, we then performed bifactor and higher-order modeling to model these 4 factors as well as extract a general factor to account for commonalities across stressors. A schematic representation of the bifactor model and the higher-order model with the identified factors is shown in Figure 1. Results from the bifactor model are presented in Table S3, and results from the higher-order model are presented in Table S4. Two items measuring parents’ perception of neighborhood safety (“I feel safe walking in my neighborhood, day or night,” “My neighborhood is safe from crime”) had highly correlated residuals; therefore, we allowed the residuals of these 2 items to correlate, which resulted in fit indices as follows: root mean square error of approximation = 0.029, 90% CI = 0.028–0.030, comparative fit index = 0.902, and standardized root mean square residual = 0.080 for the bifactor model and root mean square error of approximation = 0.038, 90% CI = 0.037–0.039, comparative fit index = 0.829, and standardized root mean square residual = 0.102 for the higher-order model. Psychometric indices (27) for each latent factor defined in the bifactor model and higher-order model are provided in Table S5.
Figure 1.
Bifactor and higher-order models delineate general factors and subfactors of environmental stress. Exploratory analyses identified 4 factors from the environmental stress items: family dynamics, interpersonal support, neighborhood socioeconomic status (SES) deprivation, and urbanicity. (A) We then used a bifactor model to model these 4 factors plus a general factor representing what is common across all the items. Each item loads onto both the general factor and only one of the specific subfactors. All factors are orthogonal to each other in the bifactor model. (B) In a higher-order model, the same 4 factors were modeled, plus a general factor that represents the common variance across the subfactors. The subfactors in a higher-order model are allowed to correlate.
Environmental Stress Factors Identified by Bifactor Modeling Show Dissociable Relationships With Brain Structure
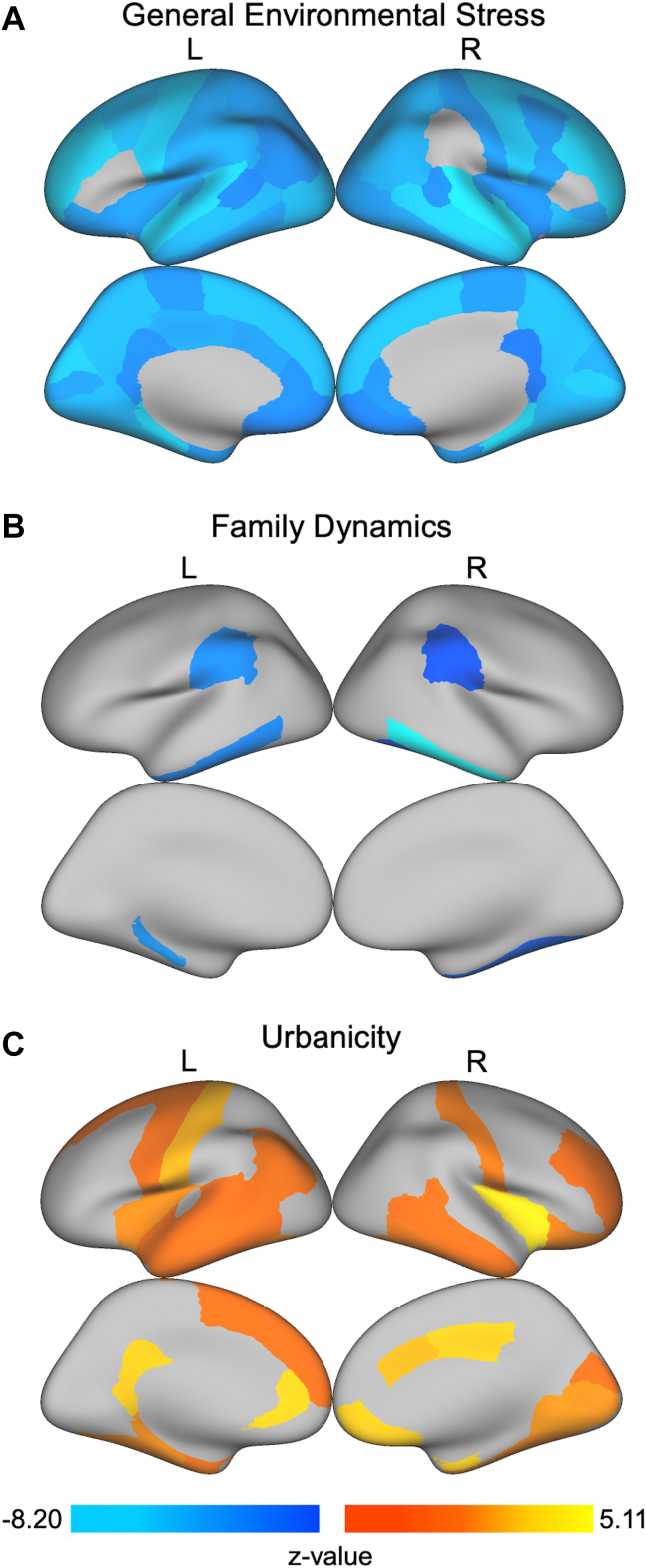
After false discovery rate correction for multiple comparisons and regressing out age, sex, race/ethnicity, and MRI scanner model, a global pattern of associations between the general environmental stress factor obtained from bifactor modeling and GMV was found (Figure 2; Table S6). Of the 68 cortical regions and 19 subcortical regions tested, the general environmental stress factor was negatively associated with 62 cortical regions and all subcortical GMV regions where higher general environmental stress was related to smaller GMV. Greater scores on family dynamics were associated with smaller GMV in bilateral inferior temporal gyri, bilateral supramarginal gyri, left parahippocampal gyrus, right fusiform gyrus, left putamen, right pallidum, and right amygdala (Figure 2; Table S6). Greater neighborhood SES deprivation was associated with smaller GMV in left putamen and right amygdala (Table S6). Conversely, for the specific factor of urbanicity, 28 cortical GMV regions and 15 subcortical GMV regions were positively associated with urbanicity (Figure 2; Table S6). No significant associations were found between interpersonal support and regional GMV.
Figure 2.
Regions with significant associations between regional gray matter volume (GMV) and environmental stress factors obtained from bifactor modeling. After controlling for age, sex, race/ethnicity, and magnetic resonance imaging scanner model, we found that (A) general environmental stress was associated with smaller GMV in almost all regions of the brain (see Table S6 for a complete list); (B) family dynamics was associated with smaller GMV in the bilateral inferior temporal gyri, bilateral supramarginal gyri, left parahippocampal gyrus, right fusiform gyrus, left putamen, right pallidum, and right amygdala; and (C) urbanicity was associated with greater GMV in 28 cortical and 15 subcortical regions (Table S6). Although not shown on these cortical surface projections, greater neighborhood socioeconomic status deprivation was associated with smaller GMV in left putamen and right amygdala. All analyses account for multiple testing using the false discovery rate (q < .05). L, left; R, right.
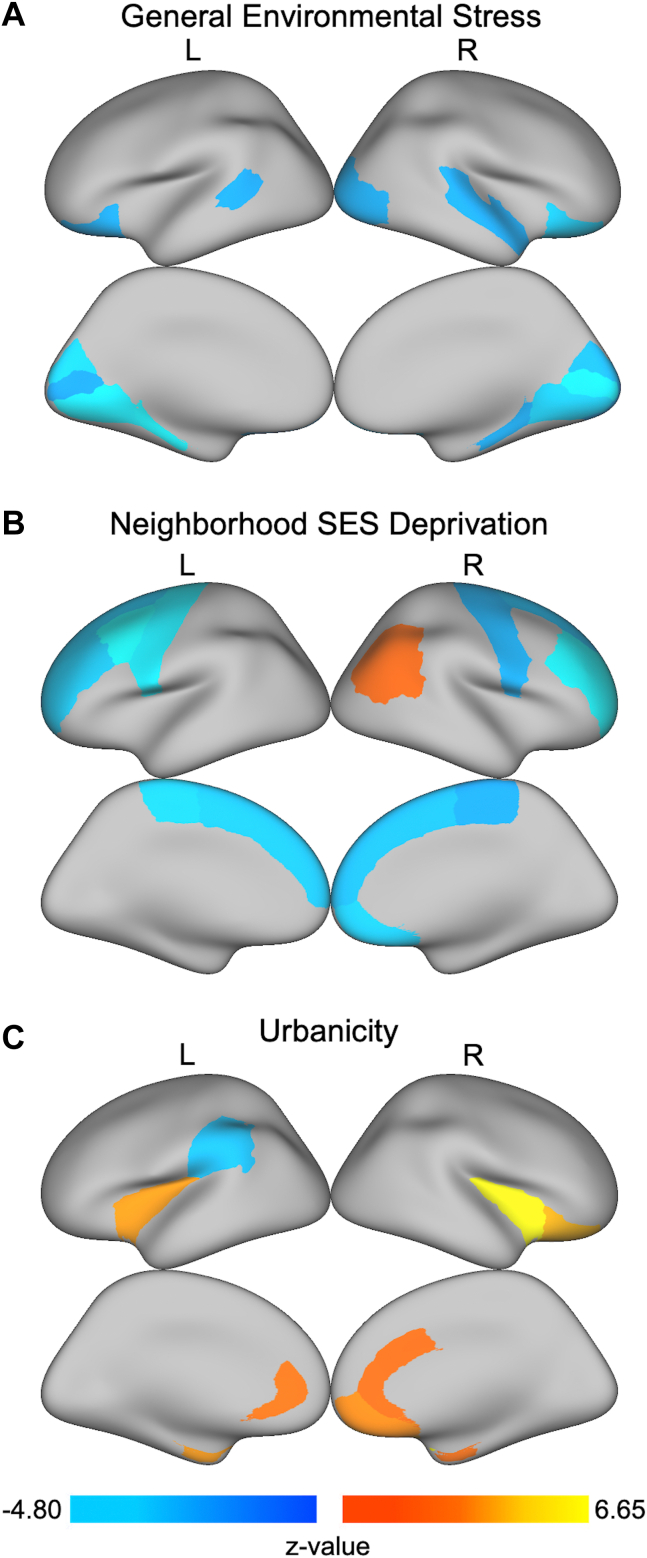
In terms of cortical thickness, the general environmental stress factor obtained from bifactor modeling was associated with thinner cortices in bilateral cuneus, bilateral lateral orbitofrontal gyri, bilateral lingual gyri, bilateral parahippocampal gyri, bilateral pericalcarine, left banks of superior temporal sulcus, right lateral occipital gyrus, and right superior temporal gyrus (Figure 3; Table S7). The specific factor of neighborhood SES deprivation was associated with thinner cortices in bilateral rostral middle frontal gyri, bilateral superior frontal gyri, bilateral paracentral gyri, bilateral precentral gyri, left caudal middle frontal gyrus, and right medial orbitofrontal gyrus as well as thicker cortices in right inferior parietal cortex (Figure 3; Table S7). Urbanicity was associated with thicker cortices in bilateral rostral anterior cingulate gyri, bilateral insulae, bilateral entorhinal cortices, right caudal anterior cingulate gyrus, right lateral orbitofrontal cortex, and right medial orbitofrontal cortex as well as thinner cortices in left supramarginal gyrus (Figure 3; Table S7). No significant associations were found between cortical thickness and the specific factors of family dynamics and interpersonal support in any regions.
Figure 3.
Regions with significant associations between regional cortical thickness and environmental stress factors obtained from bifactor modeling. After controlling for age, sex, race/ethnicity, and magnetic resonance imaging scanner model, we found that (A) general environmental stress was associated with thinner cortices in bilateral cuneus, bilateral lateral orbitofrontal gyri, bilateral lingual gyri, bilateral parahippocampal gyri, bilateral pericalcarine, left banks of superior temporal sulcus, right lateral occipital gyrus, and right superior temporal gyrus; (B) neighborhood socioeconomic status (SES) deprivation was associated with thinner cortices in bilateral rostral middle frontal gyri, bilateral superior frontal gyri, bilateral paracentral gyri, bilateral precentral gyri, left caudal middle frontal gyrus, and right medial orbitofrontal gyrus as well as thicker cortices in right inferior parietal cortex; and (C) urbanicity was associated with thicker cortices in bilateral rostral anterior cingulate gyri, bilateral insulae, bilateral entorhinal cortices, right caudal anterior cingulate gyrus, right lateral orbitofrontal cortex, and right medial orbitofrontal cortex as well as thinner cortices in left supramarginal gyrus. All analyses account for multiple testing using the false discovery rate (q < .05). L, left; R, right.
Environmental Stress Factors Identified by Higher-Order Modeling Show Dissociable Relationships With Brain Structure
A global pattern of associations between the general environmental stress factor obtained from higher-order modeling and GMV was found (Figure S3; Table S8). The general environmental stress factor was negatively associated with 65 cortical regions and all subcortical GMV regions, indicating that higher general environmental stress was associated with smaller GMV at the global level. Greater scores on family dynamics were associated with smaller GMV in 23 cortical regions as well as 12 subcortical regions (Figure S3; Table S8). Greater neighborhood SES deprivation was associated with smaller GMV in 65 cortical regions and all subcortical regions (Figure S3; Table S8). Greater urbanicity was associated with larger GMV in 19 cortical regions and 8 subcortical regions (Figure S3; Table S8). No significant associations were found between interpersonal support and regional GMV.
In terms of cortical thickness, the general environmental stress factor obtained from higher-order modeling was associated with thinner cortices in 23 of 68 cortical regions tested (Figure S4; Table S9). Neighborhood SES deprivation was associated with thinner cortices in 26 regions (Figure S4; Table S9). In contrast, urbanicity was associated with thicker cortices in the right medial orbitofrontal cortex and right insula (Figure S4; Table S9). No significant associations were found for family dynamics or interpersonal support and regional cortical thickness.
Sensitivity Analyses
Sensitivity analyses were conducted controlling for intracranial volume or average cortical thickness to test for regional specificity. After controlling for intracranial volume, the volume results for both the bifactor model and the higher-order model showed a similar direction as the effects found in the main analyses (for details on the specific regions that remained significant, see Supplement and Tables S10 and S11). After controlling for average cortical thickness, the general environmental stress factor obtained from bifactor and higher-order modeling was associated with thinner cortices in a number of regions (Supplement and Tables S12 and S13). In contrast, neighborhood SES deprivation and urbanicity in the bifactor and higher-order models showed both thicker and thinner cortices in several regions (Supplement and Tables S12 and S13).
Environmental Stress Factors Are Highly Related to Psychopathology Dimensions
Next, we examined the relationship between each of the general and specific psychopathology factors (internalizing symptoms, ADHD symptoms, and conduct problems) and the general and specific environmental stress factors (family dynamics, interpersonal support, neighborhood SES deprivation, and urbanicity). As shown in Table 2, the general environmental stress factor was positively associated with ADHD symptoms and conduct problems. The family dynamics factor was positively associated with all psychopathology factors. Greater interpersonal support was associated with lower general psychopathology, ADHD symptoms, and conduct problems. Greater scores on neighborhood SES deprivation were associated with greater scores on the ADHD and conduct problem factors. Finally, urbanicity was associated with lower scores on ADHD symptoms.
Table 2.
Results Examining the Relationship Between Psychopathology Dimensions and Environmental Stress Factors
| Predictor | General Environmental Stress |
Family Dynamics |
Interpersonal Support |
Neighborhood SES Deprivation |
Urbanicity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | pFDR | R2 | β | pFDR | R2 | β | pFDR | R2 | β | pFDR | R2 | β | pFDR | R2 | |
| General Psychopathology | 0.02 | .256 | 0.000 | 0.47 | <.0001 | 0.220 | −0.15 | <.0001 | 0.021 | 0.00 | .837 | 0.000 | −0.02 | .256 | 0.000 |
| Internalizing | −0.02 | .355 | 0.001 | 0.17 | <.0001 | 0.029 | 0.01 | .713 | 0.000 | 0.00 | .936 | 0.000 | −0.02 | .510 | 0.000 |
| ADHD | 0.16 | <.0001 | 0.027 | 0.10 | <.0001 | 0.009 | −0.17 | <.0001 | 0.028 | 0.11 | <.0001 | 0.013 | −0.06 | .005 | 0.003 |
| Conduct Problems | 0.26 | <.0001 | 0.069 | 0.17 | <.0001 | 0.030 | −0.16 | <.0001 | 0.026 | 0.14 | <.0001 | 0.019 | −0.03 | .163 | 0.001 |
ADHD, attention-deficit/hyperactivity disorder; pFDR, false discovery rate–corrected p value; SES, socioeconomic status.
Discussion
The current study used hierarchical modeling to delineate general and specific factors of environmental stress in a large sample of children. An initial exploratory analysis revealed 4 factors of environmental stress: family dynamics, interpersonal support, neighborhood SES deprivation, and urbanicity. A confirmatory bifactor analysis modeled these 4 factors and identified a general factor of environmental stress, which represents the common variance across all environmental measures. The results showed that the general environmental stress factor was associated with globally smaller brain volumes, suggesting that a wide range of influences in the child’s environment can adversely affect the developing brain. The results also showed focal differences in volume and cortical thickness associated with family dynamics, neighborhood SES deprivation, and urbanicity. The general and specific environmental stress factors were also associated with psychopathology dimensions, indicating that these environmental influences may be associated with risk for broad and specific psychopathology symptoms as well.
The specific environmental stress factors identified in the current study are consistent with prior theories of child development, such as Bronfenbrenner’s ecological systems theory (28). Bronfenbrenner (28) posited that child development occurs within a complex, multilevel system of influences that span from the immediate family/school environments to the broadest influences of cultural values, customs, and laws. The hierarchical model derived in the current study supports aspects of Bronfenbrenner’s theory while revealing important differences. Our specific factor of interpersonal support maps onto Bronfenbrenner’s microsystem, where the child’s relationships in the immediate environment (family, peers, school) are thought to have a direct impact on the child (28). In contrast to Bronfenbrenner’s theory, our specific factor of family dynamics, which would likely also fall under the microsystem, separated itself out from the other factors, suggesting that there are important distinctions within the child’s immediate environment. Likewise, while both neighborhood SES deprivation and urbanicity could theoretically fall under Bronfenbrenner’s exosystem (28), they diverged into separate specific factors in our model. This demonstrates the utility of using hierarchical models to test existing theories and to reveal the underlying latent structure of environmental stressors using a data-driven approach.
While the breadth of environmental measures included in the current study was comprehensive, there may be other variables not captured in the ABCD Study that would be important to include in a model of environmental influences. Based on prior work (2,4,6,18,19,29), we used a broad array of measures, including measures of early-life stress such as abuse/neglect, family dynamics, interpersonal relationships, and community characteristics and resources, and attributes of the physical environment such as pollution and density. Other important environmental factors may include media influences, political climate, green space exposure, and pandemics. With additional environmental measures in future work, it is possible that more diverse factors will become apparent. Additionally, we controlled for race/ethnicity based on previous work showing an association between racial/ethnic background, SES, and the brain (30). As race/ethnicity and SES are highly confounded with each other (31), it is possible that by controlling for race/ethnicity, we are also controlling for some variance associated with SES. Furthermore, stress exposure differs by race/ethnicity and SES, including stressors such as racism, discrimination, and class prejudice (32). While structural and social factors and their interaction with race/ethnicity (i.e., structural racism) were not considered in the present study, these effects are highly collinear with SES effects in the United States and could be examined in future work.
In terms of brain-behavior associations, we found that the general environmental stress factor was associated with smaller GMV across the brain, consistent with prior work showing smaller volumes associated with a broad range of measures of environmental stress, including lower SES (18), abuse/neglect (29), and urban living (4). This is also analogous to prior studies showing globally smaller brain volumes associated with a general factor of psychopathology (3,14). The general factor of environmental stress was also associated with thinner cortices in orbitofrontal, occipital/lingual, superior temporal, and parietal regions, which are involved in goal-directed behavior (33), visual processing (34), social cognition (35), and motor functioning (36). This is consistent with prior work relating SES and maltreatment to thinner cortices in similar regions (6,37,38). The specific factors of family dynamics and neighborhood SES deprivation were associated with smaller GMV and thinner cortices in distinctive regions. Notably, both specific stressors were associated with smaller GMV in the putamen and right hippocampus, which are known to be susceptible to early-life stress (39).
In terms of normative brain development, we would expect to see accelerated growth in utero and following birth with the brain reaching about 80% of its adult size by 2 years of age (40). Early childhood demonstrates continued gains in cortical GMV, followed by a slow, protracted period of decline that is likely due to synaptic pruning (41). At the same time, white matter increases throughout development as a result of increased myelination (40). While the general environmental stress factor in the current study was associated with globally smaller brain volume, we cannot tell from this cross-sectional analysis whether children with higher scores on the general environmental stress factor had smaller brains to begin with or whether these children underwent accelerated synaptic pruning and/or reduced myelination throughout early life. Both scenarios are plausible given prior work showing that environmental variables such as lower SES and childhood maltreatment are associated with smaller brains at birth (42) and faster reductions in global brain volume (29,42). Given that the first wave of data begins at ages 9 to 10 years, it remains a limitation of the current dataset that we cannot know what the developmental trajectories of these children were from in utero.
In contrast, urbanicity was associated with larger GMV and thicker cortices in several regions. In a bifactor model, urbanicity is orthogonal or uncorrelated with the general environmental stress factor. Thus, the urbanicity factor reflects the residual variance left over when the common variance associated with the general factor is partitioned, leaving the unique variance that urbanicity explains above and beyond the general factor. Our results would suggest that some aspects of urbanicity are detrimental, which could be absorbed into the general factor, and some aspects of urbanicity are beneficial, which is revealed by the residual variance represented by the urbanicity factor. The positive and negative implications of urban living for brain development have been demonstrated in prior work. On one hand, urban living is associated with risk factors such as air pollution and community violence, which are associated with smaller brain volumes and thinner cortices (4,43). On the other hand, living in an urban area may have a number of benefits such as more opportunities for social support, greater access to health care systems, more job opportunities, inexpensive transportation (44), and lower rates of depression (45). Lower levels of depression in larger urban areas are hypothesized to be driven by exponential increases in social interactions (45). In support of this, we found that urbanicity was associated with lower general psychopathology and specific symptoms in children. While we initially thought that the orthogonal nature of the general and specific factors would explain the negative association between urbanicity and brain volume, we found the same effect when using a higher-order (second-order) model that does not impose orthogonality on the subfactors after defining a general factor. In the higher-order model, greater urbanicity continued to be associated with larger brain volumes and thicker cortices. This suggests that the urbanicity findings are not an artifact of orthogonality; instead, these results suggest that urban living has some protective effects for brain development, likely for the reasons noted above (more social support, better access, more opportunities, etc.). Future work that can dissociate the positive and negative effects of urban living on brain development is needed.
Conclusions
The current study reveals the hierarchical structure of measures of environmental stress and broadens our understanding of the associations between environmental stress, psychopathology, and brain structure. The convergent findings of globally smaller brain volumes associated with both the general environmental stress factor and the general psychopathology factor (14) in the ABCD Study sample suggest the possibility of an important link between environmental stressors, psychopathology, and brain structure. Our findings on the effects of environmental stressors on the developing brain call for early intervention strategies at systemic levels.
Acknowledgments and Disclosures
This work was supported by the National Institute on Drug Abuse (Grant No. UG3DA045251 [to BBL]), National Institute of Mental Health (Grant Nos. R01MH098098 [to BBL], R01MH117014 [to TMM], R00MH117274 [to ANK], and T32-MH18921 [ELD is supported as a trainee on this grant]), National Center for Advancing Translational Sciences (Grant Nos. UL1TR000430 and UL1TR000445 [to BBL]), NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (to ANK), Sloan Research Fellowship (to ANK), and Lifespan Brain Institute of the University of Pennsylvania and the Children's Hospital of Philadelphia (to TMM).
Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org), held in the National Institute of Mental Health Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children 9 to 10 years of age and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners (Grant Nos. U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147). A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A list of participating sites and a complete list of the study investigators are available at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data, but did not necessarily participate in the analysis or writing of this report. This article reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from ABCD Study data release 3.0 (RRID: SCR_015769, DOI 10.15154/1520591) and National Institute of Mental Health Data Archive study DOI 10.15154/1520063. DOIs can be found at https://nda.nih.gov/abcd/.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
CC-I is currently at the Department of Population and Public Health Sciences, Keck School of Medicine of University of Southern California, Los Angeles, California.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.04.004.
Supplementary Material
References
- 1.Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 2.Olden K., Lin Y.S., Gruber D., Sonawane B. Epigenome: Biosensor of cumulative exposure to chemical and nonchemical stressors related to environmental justice. Am J Public Health. 2014;104:1816–1821. doi: 10.2105/AJPH.2014.302130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczkurkin A.N., Park S.S., Sotiras A., Moore T.M., Calkins M.E., Cieslak M., et al. Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. Am J Psychiatry. 2019;17:1000–1009. doi: 10.1176/appi.ajp.2019.18070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderón-Garcidueñas L., Torres-Jardón R., Kulesza R.J., Park S.B., D’Angiulli A. Air pollution and detrimental effects on children’s brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front Hum Neurosci. 2014;8:613. doi: 10.3389/fnhum.2014.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman L., Sivaratnam C., Komiti A. Attachment and early brain development—neuroprotective interventions in infant-caregiver therapy. Transl Dev Psychiatry. 2015;3 [Google Scholar]
- 6.Vargas T., Damme K.S.F., Mittal V.A. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. Neuroimage. 2020;220 doi: 10.1016/j.neuroimage.2020.117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman M.G., Kardan O., Kotabe H.P., Nusbaum H.C., London S.E. The promise of environmental neuroscience. Nat Hum Behav. 2019;3:414–417. doi: 10.1038/s41562-019-0577-7. [DOI] [PubMed] [Google Scholar]
- 8.Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich K.B., Miller G.E., Chen E. In: Developmental Psychopathology: Risk, Resilience, and Intervention. Cicchetti D., editor. John Wiley & Sons; Hoboken, NJ: 2016. Childhood adversity and adult physical health; pp. 1–42. [Google Scholar]
- 10.Beal S.J., Wingrove T., Mara C.A., Lutz N., Noll J.G., Greiner M.V. Childhood adversity and associated psychosocial function in adolescents with complex trauma. Child Youth Care Forum. 2019;48:305–322. doi: 10.1007/s10566-018-9479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin K.A., Sheridan M.A., Humphreys K.L., Belsky J., Ellis B.J. The value of dimensional models of early experience: Thinking clearly about concepts and categories. Perspect Psychol Sci. 2021;16:1463–1472. doi: 10.1177/1745691621992346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahey B.B., Applegate B., Hakes J.K., Zald D.H., Hariri A.R., Rathouz P.J. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121:971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotov R., Waszczuk M.A., Krueger R.F., Forbes M.K., Watson D., Clark L.A., et al. The hierarchical taxonomy of psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126:454–477. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 14.Durham E.L., Jeong H.J., Moore T.M., Dupont R.M., Cardenas-Iniguez C., Cui Z., et al. Association of gray matter volumes with general and specific dimensions of psychopathology in children. Neuropsychopharmacology. 2021;46:1333–1339. doi: 10.1038/s41386-020-00952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alnæs D., Kaufmann T., Marquand A.F., Smith S.M., Westlye L.T. Patterns of sociocognitive stratification and perinatal risk in the child brain. Proc Natl Acad Sci U S A. 2020;117:12419–12427. doi: 10.1073/pnas.2001517117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modabbernia A., Janiri D., Doucet G.E., Reichenberg A., Frangou S. Multivariate patterns of brain-behavior-environment associations in the Adolescent Brain and Cognitive Development Study. Biol Psychiatry. 2021;89:510–520. doi: 10.1016/j.biopsych.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeJoseph M.L., Herzberg M.P., Sifre R.D., Berry D., Thomas K.M. Measurement matters: An individual differences examination of family socioeconomic factors, latent dimensions of children’s experiences, and resting state functional brain connectivity in the ABCD sample. Dev Cogn Neurosci. 2022;53 doi: 10.1016/j.dcn.2021.101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gur R.E., Moore T.M., Rosen A.F.G., Barzilay R., Roalf D.R., Calkins M.E., et al. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry. 2019;76:966–975. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong H.J., Durham E.L., Moore T.M., Dupont R.M., McDowell M., Cardenas-Iniguez C., et al. The association between latent trauma and brain structure in children. Transl Psychiatry. 2021;11:240. doi: 10.1038/s41398-021-01357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., et al. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore T.M., Kaczkurkin A.N., Durham E.L., Jeong H.J., McDowell M.G., Dupont R.M., et al. Criterion validity and relationships between alternative hierarchical dimensional models of general and specific psychopathology. J Abnorm Psychol. 2020;129:677–688. doi: 10.1037/abn0000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagler D.J., Hatton S., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asparouhov T., Muthén B. Exploratory structural equation modeling. Structural Equation Modeling: A Multidisciplinary Journal. 2009;16:397–438. [Google Scholar]
- 25.Reise S.P. The rediscovery of bifactor measurement models. Multivariate Behav Res. 2012;47:667–696. doi: 10.1080/00273171.2012.715555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carragher N., Teesson M., Sunderland M., Newton N.C., Krueger R.F., Conrod P.J., et al. The structure of adolescent psychopathology: A symptom-level analysis. Psychol Med. 2016;46:981–994. doi: 10.1017/S0033291715002470. [DOI] [PubMed] [Google Scholar]
- 27.Hancock G.R., Mueller R.O. In: Structural Equation Modeling: Present and Future—A Festschrift in Honor of Karl Jöreskog. Cudeck S.T.R., Sörbom D., editors. Scientific Software International; Lincolnwood, IL: 2001. Rethinking construct reliability within latent variable systems; pp. 195–216. [Google Scholar]
- 28.Bronfenbrenner U. Harvard University Press; Cambridge, MA: 1979. The Ecology of Human Development: Experiments by Nature and Design. [Google Scholar]
- 29.Belsky J., De Haan M. Annual research review: Parenting and children’s brain development: The end of the beginning. J Child Psychol Psychiatry Allied Discip. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 30.Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan G.J., Magnuson K.A. Can family socioeconomic resources account for racial and ethnic test score gaps? Future Child. 2005;15:35–54. doi: 10.1353/foc.2005.0004. [DOI] [PubMed] [Google Scholar]
- 32.Myers H.F. Ethnicity- and socio-economic status-related stresses in context: An integrative review and conceptual model. J Behav Med. 2009;32:9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 33.Hollerman J.R., Tremblay L., Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- 34.Arroyo S., Lesser R.P., Poon W.T., Robert W., Webber S., Gordon B. Neuronal generators of visual evoked potentials in humans: Visual processing in the human cortex. Epilepsia. 1997;38:600–610. doi: 10.1111/j.1528-1157.1997.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 35.Bigler E.D., Mortensen S., Neeley E.S., Ozonoff S., Krasny L., Johnson M., et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- 36.Leisman G., Moustafa A.A., Shafir T. Thinking, walking, talking: Integratory motor and cognitive brain function. Front Public Heal. 2016;4:1–19. doi: 10.3389/fpubh.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whittle S., Dennison M., Vijayakumar N., Simmons J.G., Yücel M., Lubman D.I., et al. Childhood maltreatment and psychopathology affect brain development during adolescence. J Am Acad Child Adolesc Psychiatry. 2013;52:940–952.e1. doi: 10.1016/j.jaac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Kelly P.A., Viding E., Wallace G.L., Schaer M., De Brito S.A., Robustelli B., Mccrory E.J. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: Neural markers of vulnerability? Biol Psychiatry. 2013;74:845–852. doi: 10.1016/j.biopsych.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Fan C.C., Marshall A., Smolker H., Gonzalez M.R., Tapert S.F., Barch D.M., et al. Adolescent Brain Cognitive Development (ABCD) study Linked External Data (LED): Protocol and practices for geocoding and assignment of environment data. Dev Cogn Neurosci. 2021;52 doi: 10.1016/j.dcn.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaczkurkin A.N., Raznahan A., Satterthwaite T.D. Sex differences in the developing brain: Insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44:71–85. doi: 10.1038/s41386-018-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bethlehem R.A.I., Seidlitz J., White S.R., Vogel J.W., Anderson K.M., Adamson C., Alexopoulos G.S. Brain charts for the human lifespan. Nature. 2022;604:525–533. doi: 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth [published correction appears in PLoS One 2015; 10:e0146434] PLoS One. 2013;8 doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Besteher B., Gaser C., Spalthoff R., Nenadić I. Associations between urban upbringing and cortical thickness and gyrification. J Psychiatr Res. 2017;95:114–120. doi: 10.1016/j.jpsychires.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Dye C. Health and urban living. Science. 2008;319:766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- 45.Stier A.J., Schertz K.E., Rim N.W., Cardenas-Iniguez C., Lahey B.B., Bettencourt L.M.A., Berman M.G. Evidence and theory for lower rates of depression in larger US urban areas. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2022472118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ABCD Study data used in the current study are available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd). The code and a corresponding wiki for the analytic procedures can be found at https://github.com/VU-BRAINS-lab/Jeong_Environmental_Bifactor.