Abstract
Background
Convergent neuroimaging and neuromodulation studies implicate the right dorsolateral prefrontal cortex (dlPFC) as a key region involved in anxiety-cognition interactions. However, neuroimaging data are correlational, and neuromodulation studies often lack appropriate methodological controls. Accordingly, this work was designed to explore the role of right prefrontal cognitive control mechanisms in the expression/regulation of anxiety using continuous theta-burst transcranial magnetic stimulation (cTBS) and threat of unpredictable shock. Based on prior neuromodulation studies, we hypothesized that the right dlPFC contributed to anxiety expression, and that cTBS should downregulate this expression.
Methods
We measured potentiated startle and performance on the Sternberg working memory paradigm in 28 healthy participants before and after 4 sessions (600 pulses/session) of active or sham cTBS. Stimulation was individualized to the right dlPFC site of maximal working memory–related activity and optimized using electric-field modeling.
Results
Compared with sham cTBS, active cTBS, which is thought to induce long-term depression–like synaptic changes, increased startle during threat of shock, but the effect was similar for predictable and unpredictable threat. As a measure of target (dis)engagement, we also showed that active but not sham cTBS decreased accuracy on the Sternberg task.
Conclusions
Counter to our initial hypothesis, cTBS to the right dlPFC made individuals more anxious, rather than less anxious. Although preliminary, these results are unlikely to be due to transient effects of the stimulation, because anxiety was measured 24 hours after cTBS. In addition, these results are unlikely to be due to off-target effects, because target disengagement was evident from the Sternberg performance data.
Keywords: Anxiety-potentiated startle, Continuous theta-burst stimulation, Dorsolateral prefrontal cortex, Electric-field modeling, Transcranial magnetic stimulation, Working memory
Anxiety disorders are one of the most commonly diagnosed classes of psychiatric disorders, with 1 in 5 individuals meeting the criteria for an anxiety disorder within a given year (1). Individuals having anxiety disorders often find it difficult to focus, concentrate, and control their attention (2,3). These attention control difficulties can critically impact daily functioning (4). Despite the prevalence and severity of cognitive deficits in anxiety disorders, we still do not understand the mechanisms mediating these deficits. Without a mechanistic understanding of symptoms, treatment development pathways have relied on costly trial and error approaches with limited success. Key to understanding anxiety/cognition interactions is understanding how prefrontal cognitive control regions contribute to the expression and regulation of anxiety. The right dorsolateral prefrontal cortex (dlPFC) is known to be important for anxiety/cognition interactions (5, 6, 7, 8, 9), playing an active role in working memory (WM) (10, 11, 12, 13, 14), anxiety (9,15, 16, 17, 18), and emotional learning (19). However, it is currently unclear whether these contributions facilitate expression or regulation of anxiety.
Neuroimaging data suggest that the right dlPFC may regulate anxiety. We have shown that the right dlPFC is activated during unpredictable threat, and this activation is negatively correlated with startle recorded outside of the scanner (20). We have also shown that difficult cognitive tasks activate the right dlPFC during unpredictable threat, and this activation is positively correlated with cognitive task performance (21). In addition, tasks that engage the right dlPFC reduce state anxiety (22, 23, 24). In patients with anxiety (mixed generalized/social anxiety disorder samples), we have shown that the right dlPFC is either overactivated (25) or underactivated (26) compared with control subjects, depending on the task. These results are consistent with previous work linking abnormal right dlPFC activity with attentional control deficits in patients with anxiety (3,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39) and high anxious healthy participants alike (3,6,8,18,40,41). Although this convergent evidence seems to link right dlPFC activity with anxiety regulation, these results are correlational.
While there are neuromodulation data targeting the right dlPFC in anxiety, these results are much less clear about whether this region positively or negatively affects anxiety expression. Repetitive transcranial magnetic stimulation (rTMS) is one common noninvasive neuromodulatory technique to causally manipulate neural activity (42). Different patterns of rTMS can be used to up- or downregulate cortical excitability and induce lasting changes in synaptic plasticity (43, 44, 45, 46). rTMS tends to increase excitability at high frequencies (i.e., >5 Hz) and decrease excitability at low frequencies (47), although this is likely an oversimplification with exceptions depending on site, context, and other stimulation parameters. Similarly, patterned theta-burst stimulation (TBS) can induce long-term potentiation (LTP) (46) or long-term depression (LTD) (48) if delivered in an intermittent (iTBS) (LTP-like changes) or continuous (cTBS) (LTD-like changes) pattern. Accordingly, if the right dlPFC contributes to the expression of anxiety, one might expect inhibitory stimulation patterns (i.e., low-frequency rTMS and cTBS) to reduce anxiety. In contrast, if the right dlPFC contributes to the regulation of anxiety, one might expect inhibitory stimulation patterns to increase anxiety. For simplicity, we define inhibitory as neuromodulation techniques that interfere with or downregulate ongoing processes in a region. However, we understand that this is an outdated and reductionistic conceptualization that equates long-term therapeutic effects to the induced transient states of neuronal excitability and suppression, which is almost certainly false. Accordingly, it is critical for studies to establish a behavioral measure of target engagement and to distinguish between acute and long-term effects.
As mentioned above, the results are mixed. Results in patients with depression with comorbid anxiety suggest that high-frequency stimulation to the left dlPFC followed by low-frequency stimulation to the right dlPFC can reduce anxiety symptoms (49), consistent with the expression hypothesis. In contrast, data in patients with posttraumatic stress disorder suggest that either 5 Hz (50) or iTBS (51) to the right dlPFC can reduce posttraumatic stress disorder symptoms, consistent with the regulation hypothesis. As for patients with generalized anxiety disorder, there have actually been very few randomized controlled trials targeting the right dlPFC with rTMS, and across studies, the data are inconclusive (42,52, 53, 54). In a previous study, we delivered within-session 10-Hz stimulation and found that this increased anxiety-potentiated startle (APS), which is consistent with the expression hypothesis, potentially offering a (preliminary) mechanistic explanation for the low-frequency results in patients with anxious depression (55). It is important to note as well that targeting approaches vary across studies, and many of these trials were based on trial and error–like modifications of depression protocols rather than mechanistic work in patients with anxiety or model systems.
To address these gaps in the literature, this work is designed to explore the role of right prefrontal cognitive control mechanisms in the expression/regulation of anxiety using TBS and threat of unpredictable shock (20,55,56). In this study, we measured anxiety before and after either active or sham cTBS to the right dlPFC. We chose cTBS rather than standard rTMS (e.g., 1-Hz stimulation) because cTBS has been previously shown to induce long-term changes in synaptic plasticity (48) that should be observable across sessions. We targeted right dlPFC control circuits using the Sternberg WM paradigm (22,25,57,58). In addition, we measured performance on this task before and after stimulation as an index of target engagement. According to the expression hypothesis and the assumption that cTBS induces LTD-like changes in synaptic plasticity, we expected to see reductions in APS following active but not sham stimulation.
Methods and Materials
Participants
A total of 34 right-handed participants between the ages of 18 and 50 were recruited from the Philadelphia metropolitan area to take part in this study. Exclusion criteria included current or past Axis I psychiatric disorder(s) as identified with the Structured Clinical Interview for DSM-IV, nonpatient edition (2), use of psychoactive medications, any significant medical or neurologic problems (e.g., cardiovascular illness, respiratory illness, neurologic illness, seizure), and any magnetic resonance imaging (MRI)/TMS contraindications (e.g., implanted metal, history of epilepsy or seizure). For a complete list, see: http://www.clinicaltrial.gov (Identifier: NCT03993509).
A total of 28 participants completed the study (21 females, 7 males, mean age = 26.61 years, SD = 7.04). Six consented subjects were excluded from the final sample (2 screen failures, 1 pilot subject, 3 subjects withdrew [2 due to scheduling, 1 withdrew during consent]). All participants signed an informed consent form, and the protocol was approved by the Institutional Review Board for human subject research at the University of Pennsylvania. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
General Procedure
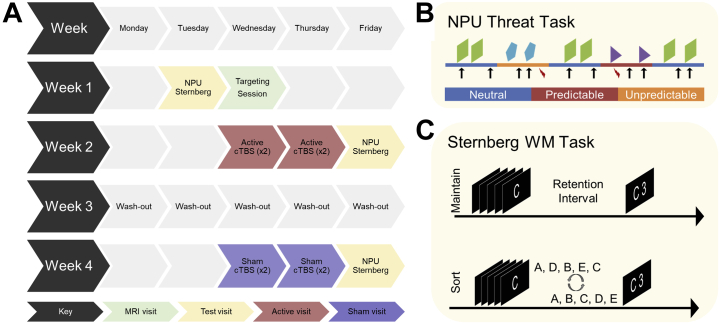
The basic procedure can be seen in Figure 1A. Subjects completed 8 study visits over the course of 4 weeks. During week 1, subjects completed an intake/pretest visit that included the consent, screening questionnaires, the no-shock, predictable-shock, unpredictable-shock (NPU) task, and the Sternberg task. They also completed a targeting session in the MRI scanner that included structural, resting-state, and task functional MRI runs. During weeks 2 and 4, subjects completed 2 days (2 sessions per day) of either active or sham cTBS. The order of the visits was counterbalanced across subjects. They also completed a post-cTBS testing session 24 hours after the final cTBS session that included the NPU and Sternberg WM tasks.
Figure 1.
Schematic of the study and task designs. (A) Diagram showing participant flow through the protocol. Performance on the no-shock, predictable-shock, unpredictable-shock (NPU) and Sternberg tasks was measured before and after 4 sessions of either active or sham continuous theta-burst transcranial magnetic stimulation (cTBS). (B) Diagram showing design of the NPU task. Blocks of neutral, predictable, and unpredictable conditions were presented. During the neutral periods, subjects could not receive a shock (red lightning bolt). During the predictable periods, subjects could receive a shock only during the cue (shapes). During the unpredictable periods, subjects could receive a shock at any time. Eyeblink responses to white noise probes (arrows) were measured throughout. (C) Diagram showing design of the Sternberg working memory (WM) task. Subjects viewed a series of letters and either 1) maintained them in the order presented or 2) sorted them in alphabetical order. MRI, magnetic resonance imaging.
Consent Visit
Subjects began by completing the informed consent form. They then completed the MRI safety form, the TMS adult safety screen (59), a medical history questionnaire, a demographics questionnaire, the State-Trait Anxiety Inventory (60), the Beck Anxiety Inventory (61), the Montgomery–Åsberg Depression Rating Scale (62), and an eligibility checklist. Afterward, the study coordinator administered the Structured Clinical Interview for DSM-IV (2). Participants that met screening criteria then completed the prestimulation test visit procedure.
Test Visits
Test Visit Procedure
The coordinator began the test visit by cleaning and preparing the skin for electrode placement. Then, electrodes for the blink recording, electrodermal activity recording, and shock delivery were attached and tested. Next a startle habituation task was completed, followed by a shock workup procedure. Once this initial setup was complete, the subjects completed 2 runs of the NPU threat task and 2 runs of the Sternberg+threat WM task (additional methods in the Supplement).
NPU Task
During each test visit, subjects had 2 runs of the NPU task (Figure 1B). Each run consisted of alternating blocks of neutral (no shock), predictable (at risk for shock only during cue), and unpredictable (at risk for shock throughout) conditions (20,55,56). Predictable and unpredictable blocks were always separated by a neutral block to yield the following 2 block orders: NPNUNUNP and NUNPNPNU. Subjects were informed of the contingencies before the task, and the block type was displayed at the top of the screen. Each block contained cue and intertrial interval (ITI) trials where a white noise probe was presented during the presence or absence of a visual cue. Cues were (8 s) simple colored (orange, teal, and purple) shapes (triangle, square, and pentagon), and the color and shape were varied across conditions. Each of the 4 neutral blocks had 2 trials per condition, while predictable (×2) and unpredictable (×2) blocks had 4 trials per condition, for a total of 8 trials per condition per run. Three shocks were presented during each run at random points during either the cue (predictable condition) or the ITI (unpredictable condition). Subjects rated their anxiety from 0 (not anxious) to 10 (extremely anxious) throughout the task using an onscreen numerical scale.
Sternberg+Threat WM Task
Following the NPU task, subjects completed 2 runs of the Sternberg+threat WM task (Figure 1C). The task consisted of a series of WM trials presented during safe (no shock) and threat (shock at any time) conditions. Each trial started with an instruction keyword to indicate the trial type. Next, subjects viewed a series of 5 letters presented sequentially. They retained them in WM for a brief interval and then gave a forced choice response during a subsequent response prompt. In maintain trials, subjects rehearsed the letters in the order that they were presented. In sort trials, subjects rearranged the letters in alphabetical order. When prompted with a letter/number combination, subjects indicated with a button press whether the position of the letter in the series matched the number.
Targeting Visit
Targeting Visit Procedure
Subjects arrived at the scanner and were cleared by the scanning technician or principal investigator to enter the scan room. They were given ear plugs, a button box, an emergency squeeze ball, and padding to minimize head movement. A pulse oximeter and respiration belt were also attached. Once setup was complete, structural scanning was completed from start to finish without intervention. Subjects then completed 1 run of the Sternberg WM task, followed by 2 resting-state runs (additional methods in the Supplement).
TMS Visits
TMS Visit Procedure
Subjects began the TMS visit by affirming their previous answers to the TMS adult safety screen and acknowledging any potential changes. The coordinator then secured the neuronavigation sensors using a swimcap and attached the e-stim electrodes. The subject was then registered to their MRI in Brainsight. On the first TMS visit, the subject’s resting motor threshold was obtained (specifications below). Next, the subject completed the remaining TMS visit procedures in the following order: Sternberg WM task (before stim run), cTBS (specifications below), and Sternberg WM task (after stim run). They were given a 30-minute break and the TMS visit procedures were repeated. We chose to administer 2 sessions per day for 2 days based on evidence that multiple spaced cTBS applications lead to more robust changes in plasticity that are less susceptible to de-depression (63). We also chose not to deliver a longer cTBS session to avoid potential excitatory effects (64). We gave participants a 30-minute break between the sessions to yield a net ∼50- to 60-minute temporal gap (gap = break + setup + 2× Sternberg runs) between the cTBS trains, which has been shown to be optimal for inducing metaplasticity (65) (additional methods in the Supplement).
Testing Session: NPU Task Anxiety Ratings and Startle
Anxiety ratings at the time of each white noise presentation were extracted and averaged across trials. Likewise, electromyography data were processed, and startle magnitude was averaged across trials. For both ratings and startle, difference scores were calculated to correspond to fear (fear-potentiated startle [FPS]: predictable cue − predictable ITI), anxiety during the ITI (APS_ITI: unpredictable ITI − neutral ITI), and anxiety during the cue (APS_cue: unpredictable cue − neutral cue). A 2 (coil: active vs. sham) × 3 (trial type: FPS vs. APS_ITI vs. APS_cue) repeated-measures analysis of variance was conducted on these values.
Testing Session: Sternberg Threat WM Performance
Percent correct and reaction time were calculated for the sort and maintain trials during safe and threat blocks. WM-related effects were calculated by creating WM-related difference scores (sort − maintain). A 2 (coil: active vs. sham) × 2 (condition: safe vs. threat) repeated-measures analysis of variance was conducted on these difference scores.
For all measures, outliers (i.e., values greater than 2× SD) were truncated to 2 standard deviations from the mean (i.e., x(|x > M ± 2 × SD|) = M ± 2 × SD). Significant two-way interactions and multilevel one-way main effects were probed using post hoc paired-sample t tests.
Results
Targeting Session: Whole-Brain Blood Oxygen Level–Dependent Data
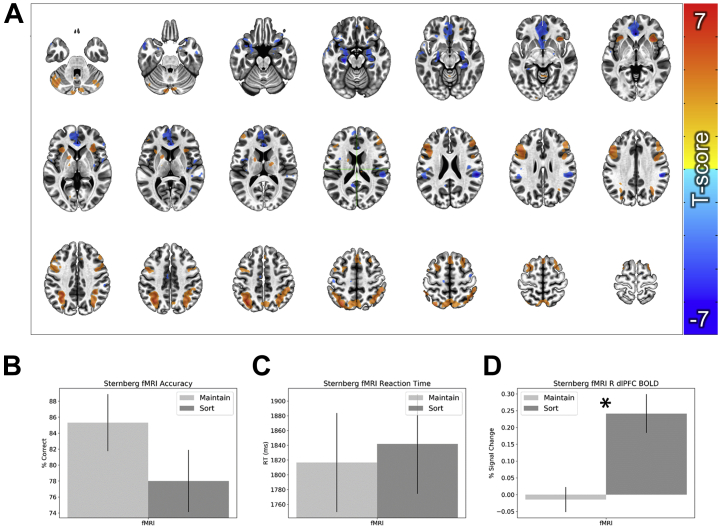
Figure 2 shows the data used to individualize targeting across subjects. The sort > maintain contrast yielded activations in previously identified task-positive regions of dorsal attention and cognitive control regions including the dorsomedial prefrontal cortex, bilateral dlPFC, anterior insula, and posterior parietal cortex/intraparietal sulcus (Figure 3A, Table S1; see Table S2 for dlPFC target coordinates). It also yielded deactivations in previously identified task-negative regions of the default mode network including the ventromedial prefrontal cortex, hippocampus, and posterior cingulate cortex. These results are largely replications of established WM manipulation findings.
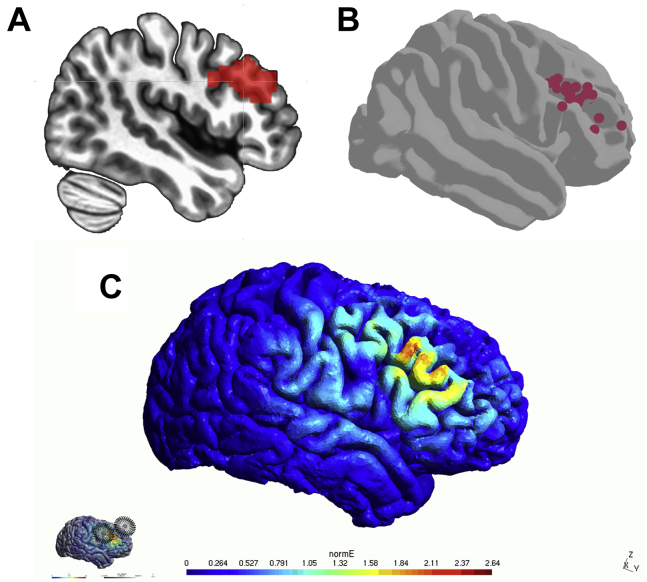
Figure 2.
Data used to individualize targeting across subjects. (A) Group-level region of interest for the right dorsolateral prefrontal cortex that was used to mask the single-subject blood oxygen level–dependent data from the Sternberg working memory task. (B) Single-subject peaks for working memory–related activity during the Sternberg working memory task. (C) Map showing the electric field at the site and orientation of stimulation for an example subject.
Figure 3.
Functional magnetic resonance imaging (fMRI) and performance data from the Sternberg targeting run. (A) Whole-brain blood oxygen level–dependent (BOLD) responses for the sort > maintain contrast. (B) Accuracy during the sort and maintain trials. (C) Reaction time (RT) during the sort and maintain trials. (D) BOLD data extracted from the group-level mask shown in Figure 2A. Bars represent the mean ± SEM. ∗p < .05. dlPFC, dorsolateral prefrontal cortex; R, right.
Targeting Session: Performance and dlPFC Blood Oxygen Level–Dependent Data
Results from the targeting session showed no significant difference in accuracy (t24 = 1.89; p = .071; d = 0.38) (Figure 3B) or reaction time (t24 = 0.48; p = .635; d = 0.1) (Figure 3C). However, consistent with our previous studies, accuracy was marginally better on maintenance trials than on sort trials. In contrast, sort trials evoked significantly greater blood oxygen level–dependent responses in the dlPFC target mask (t27 = 5.04; p < .001; d = 0.96) (Figure 3D), validating the current targeting approach.
Testing Session: NPU Anxiety Ratings and Startle
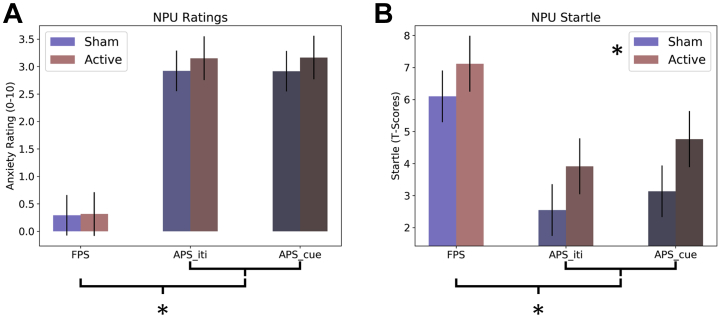
For ratings during the NPU task (Figure 4A; Table S3), there was a significant main effect for trial type (F2,54 = 64.35; p < .001; η2 = 0.7), but no main effect for coil (sham vs. active; F1,27 = 1.75; p = .2; η2 = 0.06) and no coil × trial type interaction (F2,54 = 1.47; p = .24; η2 = 0.05). These effects are comparable when order of stimulation is included as a fixed factor. To characterize the significant main effect, we conducted pairwise post hoc t tests for the levels of trial type. FPS was significantly reduced compared with APS_cue (t27 = −7.86; p < .001; Cohen’s d = −1.49) and APS_ITI (t27 = 8.32; p < .001; Cohen’s d = 1.57), but APS_cue and APS_ITI were not significantly different from one another (t27 = −0.07; p = .94; Cohen’s d = −0.01).
Figure 4.
Anxiety ratings and startle during the no-shock, predictable-shock, unpredictable-shock (NPU) threat task. (A) Anxiety ratings reported on a scale from 0 to 10. (B) Potentiated startle represented as T scores. For both measures, difference scores were calculated to correspond to fear (fear-potentiated startle [FPS]: predictable cue − predictable intertrial interval [iti]), anxiety during the ITI (APS_ITI: unpredictable ITI − neutral ITI), and anxiety during the cue (APS_cue: unpredictable cue − neutral cue). Bars represent the mean ± SEM. ∗p < .05.
In contrast, for startle during the NPU task (Figure 4B and Table S3), there was a significant main effect for coil (active > sham; F1,27 = 7.08; p = .01; η2 = 0.21) and trial type (F2,54 = 18.49; p = 0; η2 = 0.41), but no coil × trial type interaction (F2,54 = 0.1; p = .9; η2 = 0). These effects are comparable when order of stimulation is included as a fixed factor. To characterize the significant main effect of trial type, we conducted pairwise post hoc t tests for the levels of trial type. FPS was significantly larger compared with APS_cue (t27 = 4.17; p < .001; Cohen’s d = 0.79) and APS_ITI (t27 = −5.67; p < .001; Cohen’s d = −1.07), but APS_cue and APS_ITI were not significantly different from one another (t27 = −1.39; p = .18; Cohen’s d = −0.26).
It should also be noted that when counterbalance was included in the model, there was a counterbalance × stimulation interaction (F1,26 = 5.14; p = .032). When probed further with paired-sample t tests, we found that the group that received active stimulation first showed a significantly larger startle response for the active than the sham condition (t14 = 3.89; p = .002; Cohen’s d = 1.00). However, this effect was not significant for the group that received sham stimulation first (t12 = 0.269; p = .793; Cohen’s d = 0.07). This pattern seems to be inconsistent with carryover effects, which would likely lead to a larger active versus sham effect for the group receiving sham stimulation first. The most likely explanation for these findings is that there was a tendency for startle to habituate over time. In the active-first counterbalance, this effect was additive with the stimulation effect. However, in the sham-first counterbalance, the stimulation and habituation effects were counter to each other.
Testing Session: Sternberg Threat WM Performance
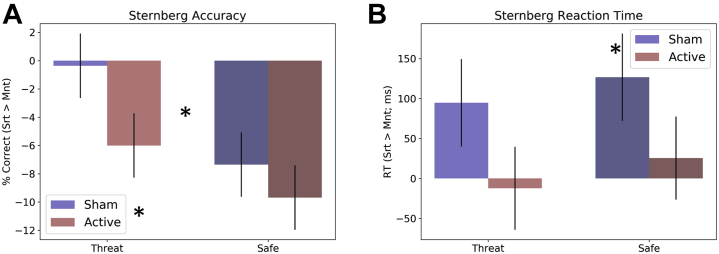
For accuracy during the Sternberg threat WM sessions (Figure 5A), there was a significant main effect for threat (F1,27 = 4.53; p = .04; η2 = 0.14), with larger WM-related differences (i.e., sort > maintain) in safe compared with threat conditions. There was also a significant main effect for coil (F1,27 = 4.22; p = .05; η2 = 0.14), with larger WM-related differences following active compared with sham stimulation, suggesting that active stimulation reduced accuracy on sort trials (Table S4). However, there was no threat × coil interaction (F1,27 = 0.73; p = .4; η2 = 0.03). These effects are comparable when order of stimulation is included as a fixed factor.
Figure 5.
Accuracy and reaction time (RT) during the Sternberg+threat working memory task. (A) Percent correct during the threat periods of the Sternberg+threat working memory task. (B) RT during the threat periods of the Sternberg+threat working memory task. Bars represent the mean ± SEM. ∗p < .05. Mnt, maintain; Srt, sort.
For reaction time (Figure 5B), there was a significant main effect for coil (F1,27 = 5.37; p = .03; η2 = 0.17), with greater WM-related differences following sham compared with active stimulation. However, there was no significant threat main effect (F1,27 = 0.68; p = .42; η2 = 0.02) or threat × coil interaction (F1,27 = 0.01; p = .93; η2 = 0). These effects are comparable when order of stimulation is included as a fixed factor.
Discussion
Here, we examined anxiety after 4 sessions (600 pulses/session) of active or sham cTBS to the right dlPFC. We used functional MRI during the Sternberg WM task to identify subject-specific stimulation sites (55), and electric-field modeling to optimize stimulation at those individualized sites (66). We found that active cTBS increased potentiated startle during both predictable and unpredictable threat compared with sham cTBS. However, there was no effect of stimulation type on anxiety ratings during the task, suggesting that these results were not driven by explicit expectations for how TMS should affect anxiety (i.e., placebo effects) (55). In addition, we have concurrent evidence from the Sternberg WM paradigm that suggests that performance during the sort trials of this task (i.e., WM manipulation trials) was reduced following active cTBS compared with sham cTBS, consistent with the hypothesis that cTBS induces LTD-like effects at the stimulation site (46). These results suggest that applying cTBS to the right dlPFC can lead to increases in anxiety expression, which was counter to our initial hypothesis.
These results leave open two questions. The first question pertains to the role of the right dlPFC in the expression/regulation of anxiety. While it is clear from neuroimaging data that both the left and right dlPFC are activated during the manipulation of items in WM (11,25,67), it is unclear how this executive control relates to emotion regulation. In a recent study, we showed that left dlPFC deficits in WM manipulation exhibited by patients with anxiety could be rescued by recruiting the right dlPFC (25). Accordingly, this led to the hypothesis that both the left and right dlPFC were specialized executive control centers, but that the domain of function differed across the hemispheres. While the left dlPFC was specialized for verbal information (11,25,67), the right dlPFC may be specialized for emotional content (9,15, 16, 17, 18, 19). In other words, our hypothesis is that the primary domain of function of the right dlPFC is the flexible manipulation of emotional content in WM.
The second question pertains to the short- and long-term effects of different TMS protocols on right dlPFC cognitive control circuits. It is hypothesized that cTBS induces LTD-like metaplastic effects at the site of stimulation (46) [see (68) for within-session effects]. Consistent with this hypothesis, we observed a performance deficit following active cTBS compared with sham cTBS on our Sternberg WM task. Accordingly, our results suggest that inducing LTD-like processes in right dlPFC control circuits that are important for WM manipulation (10, 11, 12, 13, 14), which can make people more anxious.
Assuming that low-frequency rTMS should have a similar effect (47), we might expect low-frequency rTMS to increase rather than decrease anxiety. Instead, there is a common understanding among clinical TMS practitioners that excitatory stimulation to the right dlPFC can increase anxiety symptoms, while inhibitory stimulation to the right dlPFC can decrease anxiety symptoms, supported by early research into the effects of high (assumed to be excitatory) and low (assumed to be inhibitory) frequency rTMS on mood/anxiety (69). While there have been some preliminary studies in both patients with primary generalized anxiety disorder (70, 71, 72, 73) and patients with generalized anxiety disorder/major depressive disorder (49) to support the low-frequency hypothesis, these studies have small sample sizes and lack of adequate control conditions (74,75). In addition, there are counterexamples suggesting that high-frequency right dlPFC stimulation can reduce anxiety symptoms as well (76), and there is no mechanistic explanation that can sufficiently explain this pattern of results. While we are not questioning the efficacy of 1-Hz stimulation, we instead suggest that the mechanism of action is unlikely due to downregulation of plasticity at the stimulation site. Indeed, there is some evidence to suggest that low-frequency stimulation is not sufficient to induce observable metaplastic effects outside the window for transient effects on excitability (77). While it may be tempting to equate cTBS with low-frequency stimulation and begin using right dlPFC cTBS in place of the longer 1-Hz protocol to treat anxiety, current results suggest that such an approach might not yield favorable clinical outcomes. However, because this study was an exploratory, preclinical study on a small group of healthy volunteers, it would be premature to use the findings to anticipate clinical outcomes in patients with anxiety.
These results are also seemingly inconsistent with our previous work showing that 10 Hz to the stimulation site increases anxiety within session. Aside from the stimulation pattern, the primary difference between these studies is the interval between the stimulation and the poststimulation anxiety test. In the 10-Hz study, we measured anxiety immediately following a single course of 10-Hz stimulation (55). In contrast, in this cTBS study, we measured anxiety 24 hours following four 600-pulse sessions of stimulation. Critically, this difference means that in the cTBS study, our results are unlikely to be due to acute, transient fluctuations in excitability. Instead, these results are likely driven by long-term changes in synaptic plasticity (46). Likewise, our Sternberg results showing decreased WM manipulation performance following active compared with sham stimulation confirm the predicted cTBS target (dis)engagement, potentially ruling out the possibility of paradoxical excitatory cTBS effects.
While most therapeutic clinical neuromodulation trials measure their effects at longer intervals after repeated sessions (offline stimulation), many mechanistic studies measure their effects at shorter intervals during a single session (online stimulation). Accordingly, it may be possible to explain both the 1-Hz and the 10-Hz findings based on distinct effects of online and offline stimulation. In the case of the 1-Hz stimulation, we have already shown that patients with anxiety have deficits in WM manipulation processing in the left dlPFC (25). Although pure speculation, perhaps temporarily decreasing excitability in the right dlPFC forces the left dlPFC to compensate, leading to increased processing efficiency over time. Consistent with this hypothesis, 1-Hz stimulation to the right dlPFC seems to be most effective as an add-on to 10-Hz stimulation to the left dlPFC (49,70). Based on this hypothesis, one might also expect improved WM performance and increased WM manipulation–related left dlPFC activity following a therapeutic course of 1-Hz stimulation to the right dlPFC. In the case of the 10-Hz stimulation (55), it is possible that the increase in excitability at the stimulation site is nonspecific. According to our right dlPFC emotional WM hypothesis, this nonspecific increase in cortical excitability may actually interfere with the pattern-specific activity needed to flexibly manipulate emotional content in WM (10, 11, 12, 13, 14). If this is the case, one might expect different effects for online and offline stimulation. Specifically, one might hypothesize offline 10-Hz stimulation to strengthen right dlPFC cognitive control circuits, which would lead to better regulation of, and thus decreases in, anxiety. In contrast, one might expect nonspecific transient increases in excitability induced by online 10-Hz stimulation to potentially interfere with the specific patterns of right dlPFC activity needed for manipulation of emotional content in WM, which would lead to transient impairments in regulation and transient increases in anxiety similar to the ones we observed in our previous work.
Broader Implications
Although it is common practice in the neuromodulation literature to base a study’s rationale, hypotheses, and design on hypotheses about the excitatory and inhibitory properties of rTMS/TBS, much of the data supporting these assumptions were derived from motor cortical conditioning studies using motor-evoked potentials as a stand-in for excitability (78, 79, 80, 81, 82). There have been few neuromodulatory studies showing a clear generalization of these properties specifically to prefrontal areas. Accordingly, the generalization of assumptions from motor cortex to prefrontal cortex could be questionable and should be critically evaluated and discussed if it forms of the basis of a study. In addition, studies should be designed with reliable behavioral indicators of target engagement whenever possible.
Likewise, many of these cortical conditioning studies were measured within session (78), with little data to suggest that these transient states of neuronal excitability and suppression can account for the longer-term changes in synaptic plasticity driving the therapeutic effects of most neuromodulatory treatments. We believe that synaptic plasticity is the key to understanding the long-term effects, and thus the therapeutic impact, of neuromodulatory treatments. There is an extensive literature showing that changes at the synaptic level undergo an active consolidation process that includes the synthesis of new proteins (83), degradation of old proteins (84), and remodeling of the synapse (85), a collection of processes that can last several hours (86). Accordingly, we believe that it is critical to evaluate the performance of a potential neuromodulatory treatment at intervals outside the window for transient increases in excitability.
Limitations
Despite the strengths of the study (see Supplemental Discussion), the following limitations should be noted. First, the results were counter to our hypotheses. Although not technically a limitation, these data need to be replicated in an independent sample. Another limitation is that we included a single baseline visit rather than a within-week baseline visit for the NPU paradigm, which would have provided a more flexible baseline that could have potentially accounted for any plasticity effects related to order of administration. Although counterbalancing should control for this, it could be argued that a baseline closer in temporal proximity to the cTBS/sham would have been preferable.
Conclusions
Here, we measured fear and anxiety following active or sham cTBS to the right dlPFC and found that active cTBS increases both fear and anxiety. Results are consistent with a role for the right dlPFC in anxiety regulation but require replication. This is important because it is a potential first step toward understanding the mechanism of action of neuromodulatory treatments for anxiety aimed at the prefrontal cortex. Future research should examine how other types of stimulation paradigms (high-frequency rTMS, low-frequency rTMS, iTBS, sequential bilateral cTBS + iTBS, etc.) affect fear and anxiety. In addition, despite the translational nature of the threat paradigm used (87, 88, 89, 90, 91, 92, 93), these stimulation paradigms should be tested in patients with clinical anxiety. Finally, our results highlight the importance of studying TMS-related effects outside of the acute administration window.
Acknowledgments and Disclosures
This project was supported in part by two NARSAD Young Investigator Grants from the Brain & Behavior Research Foundation (2018, 2021 [to NLB]) and by a K01 award (Grant No. K01MH121777 [to NLB]).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conceptualization: DJO, YS, NLB; Formal Analysis: MT, NLB; Funding Acquisition: NLB; Investigation: MT, WM, NLB; Methodology: NLB; Project Administration: NLB; Software: MT, NLB; Supervision: DJO, YS, NLB; Visualization: MT, NLB; Writing - Original Draft: MT, NLB; Writing - Review and Editing: MT, WM, Z-DD, DJO, YS, NLB.
The study team would like to thank the following individuals who contributed to Dr. Balderston’s K01 project: Dr. Kerry Ressler, Dr. Michael Thase, and Dr. Kristin Linn. The study team would like to also thank the Data and Safety Monitoring Board members who oversaw the project: Dr. Lindsay Oberman (Chair), Dr. Alex Shackman, and Dr. Gang Chen. This study used the high-performance computational capabilities of the CUBIC computing cluster at the University of Pennsylvania (https://www.med.upenn.edu/cbica/cubic.html). We thank Maria Prociuk for her expertise and assistance in submitting the paper. We would also like to thank the participants for their time and effort.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.04.001.
Supplementary Material
References
- 1.Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication [published correction appears in Arch Gen Psychiatry 2005; 62:709. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. American Psychiatric Publishing; Washington, DC: 2012. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. [Google Scholar]
- 3.Eysenck M.W., Derakshan N., Santos R., Calvo M.G. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 4.Wang P.S., Lane M., Olfson M., Pincus H.A., Wells K.B., Kessler R.C. Twelve-month use of mental health services in the United States: Results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- 5.Cieslik E.C., Zilles K., Caspers S., Roski C., Kellermann T.S., Jakobs O., et al. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23:2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basten U., Stelzel C., Fiebach C.J. Trait anxiety and the neural efficiency of manipulation in working memory. Cogn Affect Behav Neurosci. 2012;12:571–588. doi: 10.3758/s13415-012-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding I.H., Yücel M., Harrison B.J., Pantelis C., Breakspear M. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. Neuroimage. 2015;106:144–153. doi: 10.1016/j.neuroimage.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Fales C.L., Barch D.M., Burgess G.C., Schaefer A., Mennin D.S., Gray J.R., Braver T.S. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- 9.Peers P.V., Simons J.S., Lawrence A.D. Prefrontal control of attention to threat. Front Hum Neurosci. 2013;7:24. doi: 10.3389/fnhum.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbey A.K., Koenigs M., Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altamura M., Elvevåg B., Blasi G., Bertolino A., Callicott J.H., Weinberger D.R., et al. Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res. 2007;154:103–114. doi: 10.1016/j.pscychresns.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Curtis C.E., D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 13.Geier C.F., Garver K.E., Luna B. Circuitry underlying temporally extended spatial working memory. Neuroimage. 2007;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feredoes E., Heinen K., Weiskopf N., Ruff C., Driver J. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc Natl Acad Sci U S A. 2011;108:17510–17515. doi: 10.1073/pnas.1106439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitschke J.B., Sarinopoulos I., Mackiewicz K.L., Schaefer H.S., Davidson R.J. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Shang J., Fu Y., Ren Z., Zhang T., Du M., Gong Q., et al. The common traits of the ACC and PFC in anxiety disorders in the DSM-5: Meta-analysis of voxel-based morphometry studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster S., Nunez Elizalde A.O., Castle E., Bishop S.J. Unraveling the anxious mind: Anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb Cortex. 2015;25:609–618. doi: 10.1093/cercor/bht248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop S.J. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 19.Carter R.M., O’Doherty J.P., Seymour B., Koch C., Dolan R.J. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Balderston N.L., Liu J., Roberson-Nay R., Ernst M., Grillon C. The relationship between dlPFC activity during unpredictable threat and CO2-induced panic symptoms. Transl Psychiatry. 2017;7:1266. doi: 10.1038/s41398-017-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balderston N.L., Hsiung A., Ernst M., Grillon C. Effect of threat on right dlPFC activity during behavioral pattern separation. J Neurosci. 2017;37:9160–9171. doi: 10.1523/JNEUROSCI.0717-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balderston N.L., Quispe-Escudero D., Hale E., Davis A., O’Connell K., Ernst M., Grillon C. Working memory maintenance is sufficient to reduce state anxiety. Psychophysiology. 2016;53:1660–1668. doi: 10.1111/psyp.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vytal K.E., Cornwell B.R., Letkiewicz A.M., Arkin N.E., Grillon C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Front Hum Neurosci. 2013;7:93. doi: 10.3389/fnhum.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vytal K., Cornwell B., Arkin N., Grillon C. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balderston N.L., Flook E., Hsiung A., Liu J., Thongarong A., Stahl S., et al. Patients with anxiety disorders rely on bilateral dlPFC activation during verbal working memory. Soc Cogn Affect Neurosci. 2020;15:1288–1298. doi: 10.1093/scan/nsaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balderston N.L., Vytal K.E., O’Connell K., Torrisi S., Letkiewicz A., Ernst M., Grillon C. Anxiety patients show reduced working memory related dlPFC activation during safety and threat. Depress Anxiety. 2017;34:25–36. doi: 10.1002/da.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinholdt-Dunne M.L., Mogg K., Bradley B.P. Attention control: Relationships between self-report and behavioural measures, and symptoms of anxiety and depression. Cogn Emot. 2013;27:430–440. doi: 10.1080/02699931.2012.715081. [DOI] [PubMed] [Google Scholar]
- 28.Reinholdt-Dunne M.L., Mogg K., Bradley B.P. Effects of anxiety and attention control on processing pictorial and linguistic emotional information. Behav Res Ther. 2009;47:410–417. doi: 10.1016/j.brat.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Grillon C., Robinson O.J., Mathur A., Ernst M. Effect of attention control on sustained attention during induced anxiety. Cogn Emot. 2016;30:700–712. doi: 10.1080/02699931.2015.1024614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braver T.S., Cole M.W., Yarkoni T. Vive les differences! Individual variation in neural mechanisms of executive control. Curr Opin Neurobiol. 2010;20:242–250. doi: 10.1016/j.conb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derryberry D., Reed M.A. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- 32.Berggren N., Derakshan N. Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biol Psychol. 2013;92:440–446. doi: 10.1016/j.biopsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Berggren N., Derakshan N. The role of consciousness in attentional control differences in trait anxiety. Cogn Emot. 2013;27:923–931. doi: 10.1080/02699931.2012.750235. [DOI] [PubMed] [Google Scholar]
- 34.Coombes S.A., Higgins T., Gamble K.M., Cauraugh J.H., Janelle C.M. Attentional control theory: Anxiety, emotion, and motor planning. J Anxiety Disord. 2009;23:1072–1079. doi: 10.1016/j.janxdis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong T., Zald D.H., Olatunji B.O. Attentional control in OCD and GAD: Specificity and associations with core cognitive symptoms. Behav Res Ther. 2011;49:756–762. doi: 10.1016/j.brat.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price R.B., Eldreth D.A., Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: An fMRI investigation. Transl Psychiatry. 2011;1:e46. doi: 10.1038/tp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Najmi S., Amir N., Frosio K.E., Ayers C. The effects of cognitive load on attention control in subclinical anxiety and generalised anxiety disorder. Cogn Emot. 2015;29:1210–1223. doi: 10.1080/02699931.2014.975188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najmi S., Kuckertz J.M., Amir N. Attentional impairment in anxiety: Inefficiency in expanding the scope of attention. Depress Anxiety. 2012;29:243–249. doi: 10.1002/da.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison A.S., Heimberg R.G. Attentional control mediates the effect of social anxiety on positive affect. J Anxiety Disord. 2013;27:56–67. doi: 10.1016/j.janxdis.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basten U., Stelzel C., Fiebach C.J. Trait anxiety modulates the neural efficiency of inhibitory control. J Cogn Neurosci. 2011;23:3132–3145. doi: 10.1162/jocn_a_00003. [DOI] [PubMed] [Google Scholar]
- 41.Telzer E.H., Mogg K., Bradley B.P., Mai X., Ernst M., Pine D.S., Monk C.S. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79:216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefaucheur J.P., André-Obadia N., Antal A., Ayache S.S., Baeken C., Benninger D.H., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Neves G., Cooke S.F., Bliss T.V.P. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality [published correction appears in Nat Rev Neurosci 2012; 13:878. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 44.Dudek S.M., Bear M.F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudek S.M., Bear M.F. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y.Z., Rothwell J.C., Chen R.S., Lu C.S., Chuang W.L. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1011–1018. doi: 10.1016/j.clinph.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Lazzaro V., Dileone M., Pilato F., Capone F., Musumeci G., Ranieri F., et al. Modulation of motor cortex neuronal networks by rTMS: Comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011;105:2150–2156. doi: 10.1152/jn.00781.2010. [DOI] [PubMed] [Google Scholar]
- 48.Barr D.S., Lambert N.A., Hoyt K.L., Moore S.D., Wilson W.A. Induction and reversal of long-term potentiation by low- and high-intensity theta pattern stimulation. J Neurosci. 1995;15:5402–5410. doi: 10.1523/JNEUROSCI.15-07-05402.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White D., Tavakoli S. Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Ann Clin Psychiatry. 2015;27:192–196. [PubMed] [Google Scholar]
- 50.Carpenter L.L., Conelea C., Tyrka A.R., Welch E.S., Greenberg B.D., Price L.H., et al. 5 Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J Affect Disord. 2018;235:414–420. doi: 10.1016/j.jad.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Philip N.S., Barredo J., Aiken E., Larson V., Jones R.N., Shea M.T., et al. Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am J Psychiatry. 2019;176:939–948. doi: 10.1176/appi.ajp.2019.18101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pallanti S., Bernardi S. Neurobiology of repeated transcranial magnetic stimulation in the treatment of anxiety: A critical review. Int Clin Psychopharmacol. 2009;24:163–173. doi: 10.1097/YIC.0b013e32832c2639. [DOI] [PubMed] [Google Scholar]
- 53.Zwanzger P., Fallgatter A.J., Zavorotnyy M., Padberg F. Anxiolytic effects of transcranial magnetic stimulation-an alternative treatment option in anxiety disorders? J Neural Transm (Vienna) 2009;116:767–775. doi: 10.1007/s00702-008-0162-0. [DOI] [PubMed] [Google Scholar]
- 54.Pigot M., Loo C., Sachdev P. Repetitive transcranial magnetic stimulation as treatment for anxiety disorders. Expert Rev Neurother. 2008;8:1449–1455. doi: 10.1586/14737175.8.10.1449. [DOI] [PubMed] [Google Scholar]
- 55.Balderston N.L., Beydler E.M., Roberts C., Deng Z.D., Radman T., Lago T., et al. Mechanistic link between right prefrontal cortical activity and anxious arousal revealed using transcranial magnetic stimulation in healthy subjects. Neuropsychopharmacology. 2020;45:694–702. doi: 10.1038/s41386-019-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz A., Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat Protoc. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balderston N.L., Hsiung A., Liu J., Ernst M., Grillon C. Reducing state anxiety using working memory maintenance. J Vis Exp. 2017;125 doi: 10.3791/55727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 59.Keel J.C., Smith M.J., Wassermann E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 60.Spielgberger C.D., Gorsuch R., Lushene R.E., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 61.Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 62.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 63.Goldsworthy M.R., Müller-Dahlhaus F., Ridding M.C., Ziemann U. Resistant against de-depression: LTD-like plasticity in the human motor cortex induced by spaced cTBS. Cereb Cortex. 2015;25:1724–1734. doi: 10.1093/cercor/bht353. [DOI] [PubMed] [Google Scholar]
- 64.Gamboa O.L., Antal A., Moliadze V., Paulus W. Simply longer is not better: Reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. 2010;204:181–187. doi: 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomson A.C., Sack A.T. How to design optimal accelerated rTMS protocols capable of promoting therapeutically beneficial metaplasticity. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.599918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balderston N.L., Roberts C., Beydler E.M., Deng Z.D., Radman T., Luber B., et al. A generalized workflow for conducting electric field–optimized, fMRI-guided, transcranial magnetic stimulation. Nat Protoc. 2020;15:3595–3614. doi: 10.1038/s41596-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altamura M., Goldberg T.E., Elvevåg B., Holroyd T., Carver F.W., Weinberger D.R., Coppola R. Prefrontal cortex modulation during anticipation of working memory demands as revealed by magnetoencephalography. Int J Biomed Imaging. 2010;2010 doi: 10.1155/2010/840416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCalley D.M., Lench D.H., Doolittle J.D., Imperatore J.P., Hoffman M., Hanlon C.A. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci Rep. 2021;11:8726. doi: 10.1038/s41598-021-87916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.George M.S., Avery D., Nahas Z., Molloy M., Oliver N.C., Risch S.C., Arana G.W. rTMS studies of mood and emotion. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:304–314. [PubMed] [Google Scholar]
- 70.Lu R., Zhang C., Liu Y., Wang L., Chen X., Zhou X. The effect of bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum brain-derived neurotropic factor and serotonin in patients with generalized anxiety disorder. Neurosci Lett. 2018;684:67–71. doi: 10.1016/j.neulet.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 71.Diefenbach G.J., Bragdon L.B., Zertuche L., Hyatt C.J., Hallion L.S., Tolin D.F., et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: A pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 2016;209:222–228. doi: 10.1192/bjp.bp.115.168203. [DOI] [PubMed] [Google Scholar]
- 72.Diefenbach G.J., Assaf M., Goethe J.W., Gueorguieva R., Tolin D.F. Improvements in emotion regulation following repetitive transcranial magnetic stimulation for generalized anxiety disorder. J Anxiety Disord. 2016;43:1–7. doi: 10.1016/j.janxdis.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Bystritsky A., Kaplan J.T., Feusner J.D., Kerwin L.E., Wadekar M., Burock M., et al. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder. J Clin Psychiatry. 2008;69:1092–1098. doi: 10.4088/jcp.v69n0708. [DOI] [PubMed] [Google Scholar]
- 74.Sagliano L., Atripaldi D., De Vita D., D’Olimpio F., Trojano L. Non-invasive brain stimulation in generalized anxiety disorder: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:31–38. doi: 10.1016/j.pnpbp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Parikh T.K., Strawn J.R., Walkup J.T., Croarkin P.E. Repetitive transcranial magnetic stimulation for generalized anxiety disorder: A systematic literature review and meta-analysis. Int J Neuropsychopharmacol. 2022;25:144–146. doi: 10.1093/ijnp/pyab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dilkov D., Hawken E.R., Kaludiev E., Milev R. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: A randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61–65. doi: 10.1016/j.pnpbp.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 77.Gersner R., Kravetz E., Feil J., Pell G., Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Y.Z., Rothwell J.C., Lu C.S., Wang J., Weng Y.H., Lai S.C., et al. The effect of continuous theta burst stimulation over premotor cortex on circuits in primary motor cortex and spinal cord. Clin Neurophysiol. 2009;120:796–801. doi: 10.1016/j.clinph.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 80.Suppa A., Huang Y.Z., Funke K., Ridding M.C., Cheeran B., Di Lazzaro V., et al. Ten years of theta burst stimulation in humans: Established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 81.Wischnewski M., Schutter D.J.L.G. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 2015;8:685–692. doi: 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Goldsworthy M.R., Müller-Dahlhaus F., Ridding M.C., Ziemann U. Inter-subject variability of LTD-like plasticity in human motor cortex: A matter of preceding motor activation. Brain Stimul. 2014;7:864–870. doi: 10.1016/j.brs.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Baltaci S.B., Mogulkoc R., Baltaci A.K. Molecular mechanisms of early and late LTP. Neurochem Res. 2019;44:281–296. doi: 10.1007/s11064-018-2695-4. [DOI] [PubMed] [Google Scholar]
- 84.Jarome T.J., Devulapalli R.K. The ubiquitin-proteasome system and memory: Moving beyond protein degradation. Neuroscientist. 2018;24:639–651. doi: 10.1177/1073858418762317. [DOI] [PubMed] [Google Scholar]
- 85.Asok A., Leroy F., Rayman J.B., Kandel E.R. Molecular mechanisms of the memory trace. Trends Neurosci. 2019;42:14–22. doi: 10.1016/j.tins.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellfy L., Kwapis J.L. Molecular mechanisms of reconsolidation-dependent memory updating. Int J Mol Sci. 2020;21:6580. doi: 10.3390/ijms21186580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grillon C., Ameli R., Woods S.W., Merikangas K., Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 88.Grillon C., Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 89.Gorka S.M., Liu H., Sarapas C., Shankman S.A. Time course of threat responding in panic disorder and depression. Int J Psychophysiol. 2015;98:87–94. doi: 10.1016/j.ijpsycho.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lieberman L., Stevens E.S., Funkhouser C.J., Weinberg A., Sarapas C., Huggins A.A., Shankman S.A. How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. Int J Psychophysiol. 2017;114:24–30. doi: 10.1016/j.ijpsycho.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaye J.T., Bradford D.E., Curtin J.J. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks [published correction appears in Psychophysiology 2017; 54:158] Psychophysiology. 2016;53:1241–1255. doi: 10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bradford D.E., Starr M.J., Shackman A.J., Curtin J.J. Empirically based comparisons of the reliability and validity of common quantification approaches for eyeblink startle potentiation in humans. Psychophysiology. 2015;52:1669–1681. doi: 10.1111/psyp.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grillon C., Baas J.M.P., Pine D.S., Lissek S., Lawley M., Ellis V., Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.