Abstract
Background
Early-life environmental exposures during critical windows (CWs) of development can impact life course health. Exposure to neuroactive metals such as manganese (Mn) during prenatal and early postnatal CWs may disrupt typical brain development, leading to persistent behavioral changes. Males and females may be differentially vulnerable to Mn, presenting distinctive CWs to Mn exposure.
Methods
We used magnetic resonance imaging to investigate sex-specific associations between early-life Mn uptake and intrinsic functional connectivity in adolescence. A total of 71 participants (15–23 years old; 53% female) from the Public Health Impact of Manganese Exposure study completed a resting-state functional magnetic resonance imaging scan. We estimated dentine Mn concentrations at prenatal, postnatal, and early childhood periods using laser ablation–inductively coupled plasma–mass spectrometry. We performed seed-based correlation analyses to investigate the moderating effect of sex on the associations between Mn and intrinsic functional connectivity adjusting for age and socioeconomic status.
Results
We identified significant sex-specific associations between dentine Mn at all time points and intrinsic functional connectivity in brain regions involved in cognitive and motor function: 1) prenatal: dorsal striatum, occipital/frontal lobes, and middle frontal gyrus; 2) postnatal: right putamen and cerebellum; and 3) early childhood: putamen and occipital, frontal, and temporal lobes. Network associations differed depending on exposure timing, suggesting that different brain networks may present distinctive CWs to Mn.
Conclusions
These findings suggest that the developing brain is vulnerable to Mn exposure, with effects lasting through late adolescence, and that females and males are not equally vulnerable to these effects. Future studies should investigate cognitive and motor outcomes related to these associations.
Keywords: Adolescence, Critical windows, Functional connectivity, Manganese, MRI, Sex-specific
The Developmental Origins of Health and Disease theory postulates that early-life environmental influences can result in long-lasting effects on health (1). Indeed, exposure to toxicants during critical windows (CWs) of development has been shown to alter tissue-specific plasticity by interfering with maturational processes, leading to functional changes in various organ systems throughout the life course (2). Such CWs result from gene-environment interactions, giving rise to sensitive periods during development in which the formation of a phenotype is responsive to external factors (3). These changes may increase susceptibility to disease and dysfunction later in life (4,5). This reprogramming process depends on 1) the sensitivity of a specific tissue to the chemical and 2) a temporal overlap between exposure and the tissue’s CW of development. In addition, the time window in which the phenotype/outcome is measured plays an important role in estimating the impact of exposure (1,6). The extended period of brain development, spanning the third gestational week through late adolescence, gives rise to many CWs of vulnerability to toxic chemicals across development (e.g., prenatal, early postnatal, and childhood periods) (7). Extensive evidence suggests that the effects of environmental exposure on neurodevelopment may depend as much on the exposure timing as on the concentration (8,9). A better understanding of CWs of vulnerability to developmental neurotoxicants and the biological mechanisms underlying these associations would inform our understanding of typically developmental trajectories as well as inform prevention and treatment strategies for reducing adverse environmentally associated brain outcomes.
Magnetic resonance imaging (MRI) provides insight into biological mechanisms underlying human cognition and behavior. Applied to studies of environmental epidemiology, MRI offers the possibility of linking exposure with changes in the brain. Specifically, resting-state functional MRI (rs-fMRI) can be used to map large-scale functional networks in the human brain by examining slow (<1 Hz) oscillatory intrinsic fluctuations in hemodynamics (10), with the assumption that regions with correlated activity form functional networks. Changes in brain functional connectivity have been reported in various neurodevelopmental disorders (11). rs-fMRI is particularly suited to benefit pediatric research because it is inherently noninvasive and does not require cooperation or motivation, minimizing many confounders related to task performance and increasing consistency across participants and datasets (12). Incorporating rs-fMRI into children’s environmental health studies allows us to examine the in vivo impacts of environmental exposures on the developing brain’s functional organization (13).
Throughout development, the brain undergoes a complex series of dynamic processes in which few cells develop into a highly interconnected brain (7,14). Environmental exposure to neuroactive metals such as manganese (Mn) during specific CWs of brain development may disrupt typical development and functioning, leading to persistent behavioral and cognitive changes (14,15). The general population can be exposed to Mn through inhalation, dietary intake, and drinking of contaminated water (16, 17, 18). Human activities such as ferroalloy industries may expose children and pregnant women residing in adjacent areas to higher levels of Mn (19). Mn can cross the placental and blood-brain barriers and lead to changes in the brain (20). Mn levels outside of the homeostatic range (i.e., deficiency or intoxication) alter neuron function (21,22), increase oxidative stress (23), accumulate in the basal ganglia (21, 22, 23), and adversely affect cognitive and behavioral outcomes including IQ (24,25), motor function (19,26,27), and hyperactivity (28,29). While CWs to Mn have been identified during gestation, infancy, early childhood, and adolescence (8,9,30,31), the brain mechanism underlying its neurotoxicity remains largely unknown. Further, animal and human studies demonstrate sex-specific associations between Mn exposure and behavioral outcomes (26,32, 33, 34), suggesting that males and females may present distinctive CWs to Mn exposure and may be differentially vulnerable to its effects. Sex-specific vulnerability may relate to the brain’s sexually dimorphic developmental trajectory, beginning in utero (35, 36, 37). Sex differences in developmental trajectories may emerge from endogenous developmental programming, developmental experience, or other environmental exposures (38, 39, 40). Sex-specific CWs may explain sex differences in the prevalence, severity, and progression of neurodevelopmental disorders. Understanding the impact of both timing and concentration of Mn exposure on brain functional connectivity may provide mechanistic insights into sex differences in Mn-associated neurotoxicity.
In this study, we used rs-fMRI to investigate sex-specific associations between prenatal, early postnatal, and childhood Mn exposure, measured in deciduous teeth, and brain intrinsic functional connectivity (iFC) in older adolescents. Notably, sex differences in associations between Mn and neurocognition have been previously reported in this cohort (32,41). Based on previous studies suggesting associations between early-life Mn exposure and changes in brain areas implicated in motor and cognitive control (42, 43, 44, 45, 46), we hypothesized that early-life exposure to Mn will have downstream impacts on brain functional connectivity in the basal ganglia, prefrontal cortex, parietal cortex, and motor cortex and that these effects will be different in females compared with males.
Methods and Materials
Participants
Participants were part of the ongoing Public Health Impact of Metal Exposure (PHIME) cohort based in the province of Brescia in Northern Italy. Details of the study recruitment and enrollment have been described previously (19). Briefly, participants were enrolled in the study using a community-based participatory approach from schools throughout the local public school system in Northern Italy. Schools are located in 3 geographically different but demographically similar communities in the province of Brescia, characterized by the presence of ferroalloy plants causing Mn exposure through airborne emission. All participants completed baseline questionnaires to evaluate inclusion and exclusion criteria. Inclusion criteria included birth in the study area of interest, residence in the study area since birth, and family residence in the study area since the 1970s. Exclusion criteria included known neurologic, hepatic, metabolic, endocrine, or major psychiatric disorder; medication usage with known neuropsychological side effects; clinically diagnosed motor deficits or cognitive impairment; and visual deficits that are not adequately corrected. A total of 720 adolescents were enrolled in the Public Health Impact of Metal Exposure cohort between 2007 and 2014. At the time of enrollment, participants were asked to provide a naturally shed deciduous tooth. Between 2015 and 2020, 207 subjects (age 16–23 years) participated in a multimodal MRI follow-up study and completed baseline questionnaires, including information on parental educational and occupational levels, and neuropsychological tests, including IQ using the Wechsler Intelligence Scale for Children-III assessment (47). Between 2015 and 2016, a convenience sample of teeth from 195 (27%) participants were analyzed for early-life Mn. From this sample, MRI scans were acquired for 73 subjects. Complete exposure data (i.e., dentine Mn), outcome (MRI data), and covariate data were available for the 73 adolescents (38 females) included in this analysis. After excluding 2 participants due to movement in the scanner, Mn uptake, MRI scans, and covariate data were available for 71 adolescents (38 females). This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai and the Public Health Agency of Brescia. Written informed consent was obtained from all participants.
Dentine Mn Biomarker
To identify early-life CWs of vulnerability to Mn, we used deciduous teeth as a biomarker of retrospective exposure. Because deciduous teeth accumulate metals in a pattern similar to the growth rings of a tree, they can provide estimates of both the timing and intensity of exposure, allowing us to detect when exposure is most hazardous (48). Only incisors, canines, and molars that were free of defects such as caries and extensive tooth wear, were analyzed. Detailed analytic methods have been described previously (48, 49, 50). Briefly, teeth were washed in an ultrasonic bath of ultrapure Milli-Q water (18.2 MΩ/cm) and sectioned on a vertical, labial-lingual, or buccal-lingual plane using a diamond-encrusted blade. The neonatal line, which histologically distinguishes pre- and postnatally formed regions of dentine, was identified using light microscopy. With the neonatal line as a reference point, the concentrations and spatial distribution of Mn in different developmental windows were determined using laser ablation–inductively coupled plasma–mass spectrometry. Dentine Mn concentrations were normalized to dentine calcium levels (55Mn:43Ca ratio) to account for variations in mineral density within and between teeth. A total of 30 sampling points were ablated parallel to the enamel-dentine junction and assigned to pre- or postnatal zones after identifying the neonatal line. Area under the curve was used to account for the different number of sampling points in each zone per tooth. Childhood cumulative Mn concentrations (1 to ∼6 years of age) were determined by averaging across 10 sampling locations within secondary dentine, the formation of which starts after the completion of the tooth root and proceeds at a slower rate. The limit of detection was 0.02 μg/g. Only one dentine measurement fell below the detection limit (n = 1) and was assigned half the lowest value among the samples above the detection limit.
Covariates
Given that age and socioeconomic status (SES) may impact iFC (51,52) and associations between Mn exposure and neurodevelopment (53), we included these variables as covariates in the analyses. SES index (low, medium, high) was determined using parental educational and occupational levels (54).
MRI Data Acquisition
The MRI scan was performed on a 3T MR unit (Skyra, Siemens) equipped with a 64-channel phased array head coil at the Neuroimaging Division at the ASST Spedali Civili Hospital of Brescia. The 10-minute rs-fMRI scans were acquired using a T2∗-weighted echo-planar imaging sequence (repetition time = 1000 ms, echo time = 27 ms, 70 axial slices, 2.1-mm thickness, matrix size 108 × 108, covering the brain from vertex to cerebellum). During this acquisition, lights were turned off and subjects were instructed to keep their eyes open and stare at a night skyline picture projected on the monitor, to not think of anything specific, and to not fall asleep. For registration purposes, we acquired a high-resolution anatomical T1-weighted scan using three-dimensional magnetization-prepared rapid gradient echo (repetition time = 2400 ms, echo time = 2.06 ms, 230 mm field of view, matrix size 256 × 256, 224 sagittal slices, 0.9 mm3 voxel size).
rs-fMRI Data Preprocessing
rs-fMRI data were preprocessed using the Functional Connectivity (CONN) toolbox (http://web.mit.edu/swg/software.htm) following the standard CONN preprocessing pipeline (55): functional realignment and unwarp (56), slice-timing correction (57), outlier identification (volumes exceeding >0.9 mm framewise displacement or global blood oxygen level–dependent signal changes above 5 SD), direct segmentation and normalization [images were normalized into standard Montreal Neurological Institute space and segmented into gray matter, white matter, and cerebral spinal fluid tissue classes using SPM12 unified segmentation and normalization procedure (58)], and functional smoothing (images were smoothed using spatial convolution with a Gaussian kernel of 8 mm full width at half maximum). After preprocessing, we applied CONN’s default denoising pipeline composed of linear regression of confounding effects and temporal bandpass filtering. White matter and cerebral spinal fluid noise were estimated and regressed out using an anatomical component-based noise correction procedure (59). To reduce motion artifacts, we included 12 noise parameters as nuisance regressors (3 translation and 3 rotation parameters and their associated first-order derivatives) at the single-subject level. Temporal frequencies were filtered to 0.01 to ∼0.09 Hz to focus on low-frequency fluctuations while minimizing the influence of physiological, head motion, and other noise sources. These noise and motion confounders were regressed out in the lower-level multiple regression analyses for each participant before any group-level analyses were carried out.
Seed-Based Correlation Analyses
Seed-based analyses were performed using the CONN toolbox by computing the temporal correlation between the blood oxygen level–dependent signals from regions of interest to all other voxels in the brain. Based on previous pilot results by our group (43), we selected 7 seeds from the probabilistic Harvard-Oxford Subcortical Structural Atlas (60): left and right putamen, left and right caudate, left and right pallidum, and bilateral middle frontal gyrus. Group-level analyses were performed using the general linear model implemented in the CONN toolbox with natural log-transformed Mn concentrations at each time point as predictors and iFC as the outcome. Given that Mn concentrations were moderately correlated across 2 of the 3 time points (r values = 0.03–0.42), we performed separate analyses for each of the 3 time points. To investigate the moderating effect of sex on the associations between dentine Mn and iFC, we examined interactions between sex and Mn adjusting for age and SES. Statistical images were thresholded using a cluster-corrected threshold of p < .05 false discovery rate correction (44,61, 62, 63). To control for the number of comparisons (n = 21; 7 seed regions × 3 Mn time points), we applied a secondary false discovery rate correction and only report findings surviving both false discovery rate corrections. Finally, in a sensitivity analysis, we investigated sex-stratified associations between dentine Mn and iFC in regions found to have a significant interaction term. Stratified models were implemented in R (version 3.5.1) with the glm package.
Results
Descriptive Statistics
Participant demographics are presented in Table 1. Among the 71 participants (38 [54%] female), the mean age of participants was 19.4 years (SD = 2.2 years). The average IQ score of participants was 104 (SD = 10.1). The majority of participants were from families reporting medium SES (66%). Dentine Mn was log-transformed to reduce skewness and approximate a normal distribution. There were no statistically significant differences found in dentine Mn, age at scan, IQ, and SES between male and female participants. Dentine Mn concentrations in each time point, stratified by sex, are presented in Figure 1.
Table 1.
Sex-Stratified Sociodemographic Characteristics of 71 Adolescents From Northern Italy
| Sociodemographic Characteristics | Female, n = 38 | Male, n = 33 | Total, N = 71 |
|---|---|---|---|
| Age, Years | |||
| Mean (SD) | 19.7 (2.08) | 19.1 (2.36) | 19.4 (2.22) |
| Median [min, max] | 19.7 [15.9, 23.1] | 19.1 [15.9, 23.4] | 19.4 [15.9, 23.4] |
| IQ | |||
| Mean (SD) | 103 (10.7) | 106 (9.25) | 104 (10.1) |
| Median [min, max] | 105 [78, 117] | 105 [77, 121] | 105 [77, 121] |
| SES, n (%) | |||
| Low | 5 (13.2%) | 2 (6.1%) | 7 (9.9%) |
| Medium | 25 (65.8%) | 23 (69.7%) | 48 (67.6%) |
| High | 8 (21.1%) | 8 (24.2%) | 16 (22.5%) |
| Site, n (%) | |||
| BM | 13 (34.2%) | 18 (54.5%) | 31 (43.7%) |
| GL | 13 (34.2%) | 6 (18.2%) | 19 (26.8%) |
| VC | 12 (31.6%) | 9 (27.3%) | 21 (29.6%) |
| Prenatal Mn | |||
| Mean (SD) | 0.433 (0.165) | 0.471 (0.192) | 0.451 (0.178) |
| Median [min, max] | 0.417 [0.168, 1.14] | 0.470 [0.148, 0.869] | 0.427 [0.148, 1.14] |
| Postnatal Mn | |||
| Mean (SD) | 0.137 (0.0477) | 0.119 (0.0555) | 0.129 (0.0519) |
| Median [min, max] | 0.125 [0.0486, 0.256] | 0.121 [0, 0.238] | 0.124 [0, 0.256] |
| Childhood Mn | |||
| Mean (SD) | 0.000821 (0.000422) | 0.000818 (0.000447) | 0.000820 (0.000431) |
| Median [min, max] | 0.000720 [0.000328, 0.00267] | 0.000757 [0, 0.00208] | 0.000727 [0, 0.00267] |
IQ was measured using the Wechsler Intelligence Scale for Children, 3rd edition (47). Sociodemographic characteristics did not differ between males and females (p > .05).
BM, Bagnolo Mella; GL, Garde Lake; max, maximum; min, minimum; Mn, manganese; SES, socioeconomic status; VC, Valcamonica.
Figure 1.
Prenatal, postnatal, and early childhood manganese (Mn) concentrations (log) measured in naturally shed deciduous teeth of 71 study participants by sex. The density curve represents the relative frequency of all observations, such that the area underneath it is exactly 1. Dentine Mn did not differ between males and females (logistic regression adjusted for socioeconomic status and age, p > .05). Ca, calcium.
Seed-Based Correlation Analyses of Sex-Specific Associations
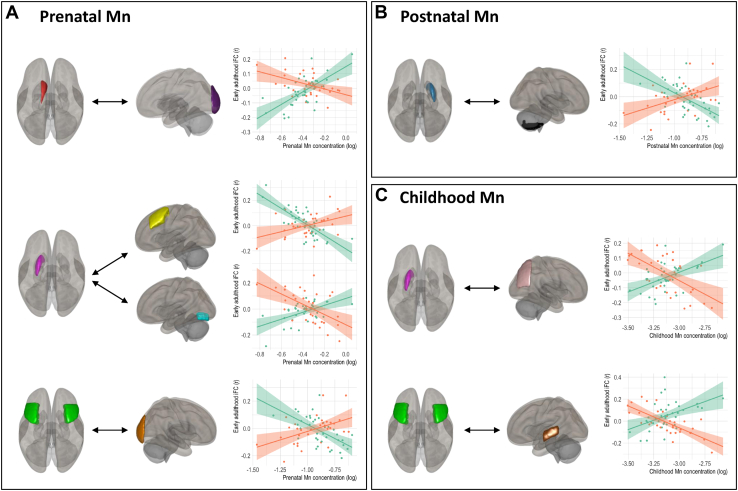
For all time points, the seed-based correlation analyses revealed sex-specific associations between dentine Mn and iFC (Table 2, Figure 2). Seed-to-region connectivity and peak coordinates in Montreal Neurological Institute space for models showing significant interactions between dentine Mn and sex are reported in Table 2. We observed sex-specific associations between prenatal dentine Mn and iFC in the following connections: 1) left caudate and left occipital pole, 2) left putamen and left middle frontal gyrus, 3) left putamen and left occipital fusiform gyrus, and 4) middle frontal gyrus and right occipital pole. Sex-stratified analyses showed that in connections 1 and 3, prenatal Mn exposure was associated with increased iFC in females and decreased iFC in males, whereas connections 2 and 4 show an inverse pattern (Table S1). Mn uptake during the early postnatal period (<1 year of age) showed sex-specific effects on iFC between the right putamen and the right cerebellum, with decreased iFC in females and increased iFC in males. Exposure to Mn during the early childhood period (1 to ∼6 years) showed sex-specific effects on iFC between 1) the left putamen and the right superior division of the lateral occipital cortex and 2) the middle frontal gyrus and the left posterior division of the middle temporal gyrus. In both connections, increased Mn exposure was associated with increased iFC in females and decreased iFC in males.
Table 2.
Results From Seed-Based Correlation Analysis Demonstrating Significant Sex-Specific Associations Between Dentine Mn at Three Time Points and Intrinsic Functional Connectivity in Adolescents
| Exposure Time Point | Seed-to-Region Connectivity | Cluster (x, y, z) | Cluster Size | β | p-FDR |
|---|---|---|---|---|---|
| Prenatal | Left caudate—left occipital pole | (−04, −90, +08) | 223 | 0.67 | <.001 |
| Left putamen—left middle frontal gyrus | (−30, +26, +42) | 64 | −0.71 | .014 | |
| Left putamen—left occipital fusiform gyrus | (−28, −78, −16) | 60 | 0.67 | .014 | |
| Middle frontal gyrus—right occipital pole | (+14, −98, +12) | 99 | −0.92 | .003 | |
| Postnatal | Right putamen—right cerebellum | (+42, −68, −54) | 74 | −0.70 | .009 |
| Early Childhood | Left putamen—lateral occipital cortex, right superior division | (+12, −52, +58) | 90 | 0.64 | .003 |
| Middle frontal gyrus—middle temporal gyrus, left posterior division | (−50, −22, −04) | 138 | 0.74 | .002 |
All models controlled for age (years) and SES. Voxel threshold p < .001 (uncorrected); cluster threshold p < .05 (p-FDR corrected). Coordinates presented are in MNI space. All coefficients refer to sex-Mn interaction term.
FDR, false discovery rate; Mn, manganese; MNI, Montreal Neurological Institute; SES, socioeconomic status.
Figure 2.
Sex-specific correlations between intrinsic functional connectivity (iFC) in adolescents and natural log-transformed dentine manganese (Mn) from 3 time points: (A) prenatal, (B) postnatal, and (C) childhood (N = 71). Brain regions are color-coded: left caudate, red; right putamen, blue; left putamen, pink; middle frontal gyrus, green; left occipital pole, purple; left middle frontal gyrus left, yellow; left occipital fusiform gyrus, teal; right occipital pole, orange; cerebellum, black; lateral occipital cortex, light pink; middle temporal gyrus, brown. Graphs plot regression lines and standard errors for females (green) and males (orange). Only significant interactions (p < .05) between dentine Mn and sex are shown. Regions are color-coded for visualization purposes. Exact cluster locations in Montreal Neurological Institute coordinates and cluster sizes are reported in Table 2.
Discussion
In this study, we sought to test our Developmental Origins of Health and Disease–based hypothesis that Mn uptake during early-life CWs of development is associated with changes in functional connectivity in adolescence, and that environmentally associated brain changes are sex specific. Using an objective, retrospective biomarker of Mn uptake during prenatal, early postnatal, and childhood periods and rs-fMRI in adolescence, we found significant sex-specific associations between dentine Mn at all time points and iFC in adolescents. Moreover, we report associations in different networks depending on the timing of exposure, suggesting that different brain networks may present distinctive CWs of vulnerability to Mn. During the prenatal period, we observed sex-specific associations between dentine Mn and functional connectivity between the dorsal striatum (caudate and putamen) and regions in the occipital and frontal lobes and between the middle frontal gyrus and the occipital lobe. During the early postnatal period (<1 year), dentine Mn was associated with sex-specific changes in the right putamen and the cerebellum. Finally, during the early childhood period (1 to ∼6 years), exposure to Mn showed sex-specific effects on iFC between the putamen and the occipital lobe and between the frontal and temporal lobes. Our results highlight that the effect of Mn on iFC differs in magnitude and directionality between females and males. Four connections showed increased iFC in females and decrease in males, whereas 3 connections showed a reverse pattern. These sex-specific effects may not be attributed to sex differences in exposure or absorption of Mn, because we report similar Mn uptake levels between males and females. Despite the proximity of study participants to active ferroalloy smelting activity, dentine Mn levels measured in this study are comparable to previously reported levels in other populations and are not suggestive of exceptionally heightened exposure (28,43,64,65). This study contributes to the growing literature suggesting that early-life Mn uptake impacts neurodevelopmental outcomes by providing mechanistic insights into the brain regions underlying these associations. Using our dentine biomarker to provide a retrospective marker of Mn uptake in 3 developmental stages, we identified 3 CWs of vulnerability to this neuroactive metal. Finally, our findings of sex-specific associations support our hypothesis that the effect of early-life exposure to Mn differs by sex.
MRI allows in vivo visualization of the brain. It is noninvasive and free of ionizing radiation, making it suitable for research in children. In the field of environmental epidemiology, MRI plays a critical role in elucidating biological mechanisms underlying Mn neurotoxicity. Until recently, the use of brain imaging in environmental health studies of Mn neurotoxicity has focused on structural brain abnormalities, primarily in the basal ganglia, and mostly targeted highly exposed workers (66,67). Numerous structural basal ganglia abnormalities have been reported in workers exposed to Mn, including T1 hyperintensities (68, 69, 70, 71), lower apparent diffusion coefficient (68), higher pallidal index (72,73), and lower fractional anisotropy (74, 75, 76). Only 2 occupational MRI studies have investigated functional brain changes related to Mn exposure (77,78). Chang et al. (77) showed Mn-induced alterations in brain activity during a working memory task, with higher activation in the basal ganglia (i.e., including the putamen) in exposed welders. Seo et al. (78) showed decreased activation of the frontal, parietal, and insular cortices in welders during an executive function task. Our findings are consistent with prior structural and functional MRI studies, because we detect effects in the caudate and putamen, which are central components of the basal ganglia. This study builds on the previous literature by adding 1) a focus on lower nonoccupational exposures in children, 2) longitudinal retrospective measures of Mn uptake allowing the detection CWs of susceptibility, 3) direct and objective measurement of Mn concentrations instead of self-reported exposure history, and 4) analyses of sex-specific effects.
Substantial research demonstrates associations between early-life Mn exposure and adverse neurodevelopmental outcomes (9,28,79,80). In a recent pilot study, de Water et al. (43) reported associations between postnatal Mn dentine concentrations and iFC in areas of the brain implicated in cognitive control and motor function. In this study, we built on this pilot work to explore sex-specific vulnerabilities to the effect of early-life Mn exposure on iFC. Our findings indicate that Mn exposure is differently associated with functional connectivity in males and females in brain regions involved in cognitive control and motor function. Mn concentrations were associated with sex-specific effects on iFC between regions of the basal ganglia (striatum or caudate–putamen) and cortical regions (occipital and frontal).
The cortical–subcortical connections impacted by Mn exposure in our study are known to be associated with neurologic health outcomes in adults. According to the current model of basal ganglia function in adults, the striatum is the main entry point of cortical information to the basal ganglia; it receives afferents from widespread areas of the cerebral cortex through the thalamus (i.e., corticobasal ganglia–thalamocortical loops) (81,82). These circuits are crucial for emotional, cognitive, and motor functions (83, 84, 85, 86, 87). The caudate and putamen are involved in the planning and execution of movement, learning, memory, and reward (88, 89, 90). Mn dyshomeostasis may disrupt the corticobasal ganglia circuitry, because Mn is known to accumulate in basal ganglia structures (42,91). Increased caudate connectivity (92), putamen dysfunction (93), and increased putamen–cerebellum connectivity (94) have been observed among adults diagnosed with Parkinsonism. Mn-exposed occupational workers demonstrate clinical symptoms mirroring Parkinson disease (95). Middle frontal gyrus and middle temporal gyrus connectivity have also been associated with cognitive and executive function, specifically literacy and numeracy abilities (96,97), with increased connectivity in the middle frontal gyrus within the occipital pole network reported among children with autism spectrum disorder (98). Little is known about the dynamic development of these circuits during childhood. Given the known vulnerability of these circuits to perturbation during development, our study adds to the literature by relating Mn exposure to alterations in typical neurodevelopment of subcortical–cortical connections.
Our novel approach combining neuroimaging in late adolescence with the use of deciduous teeth as a retrospective biomarker of early-life Mn uptake allows us to test our Developmental Origins of Health and Disease–based hypothesis. Traditional biomarkers of Mn exposure (e.g., urine, blood, hair) used in prior studies are unable to directly measure exposure in fetal life. Further, traditional biomarkers fail to provide longitudinal exposure spanning several potential CWs of development including the fetal, postnatal, and childhood periods. There is a lack of consensus regarding the appropriate biomarker to assess Mn-associated impacts on the developing brain because each biomarker reflects differences in pharmacokinetics, and therefore, associated health effects may be matrix dependent. Instead, dentine Mn is a validated biomarker providing a direct measure of Mn across prenatal and postnatal periods, which enabled us to detect CWs for effects of Mn exposure on iFC of the brain. Results from a recent study in the same cohort, using the dentine biomarker to study associations between Mn and neurocognition, suggest a subtle shift over time from a beneficial role of Mn during the prenatal period to a more detrimental role in childhood (41). Our unique study design is able to provide detailed insights into the neural mechanisms that may underpin these associations between early-life Mn exposure and cognitive and motor control reported in prior studies (26,28,32,41,99).
Very few previous investigations have explored sex-specific effects of early-life Mn on neurodevelopmental outcomes, and although most of them detect a difference in vulnerabilities by sex, the direction of reported associations is inconsistent. Early-life Mn has been positively associated with improved cognition and motor outcomes among females compared with males (26,100). Contrastingly, other groups have found a negative association between Mn concentrations and nonverbal performance, motor outcomes, visuospatial learning, and IQ scores among females (28,32,79,99). Discrepancies in direction of association may be due to differences in the task used to assess behavior, type of biomarker used to assess exposure, and timing of exposure and/or outcome assessment. Our results are in line with previous studies, suggesting that male and female neurodevelopment is not equally vulnerable to the effect of Mn exposure, and the direction of effect varies not only in magnitude but also in direction. Notably, unlike assessments of cognition and motor function, interpretation of directional changes in iFC is challenging. Increased functional connectivity does not necessarily indicate better performance, as it has been associated with memory and cognitive impairments (101,102), anxiety (103), and epilepsy (104), or may reflect a compensatory mechanism. Despite this limitation, our results add to the growing literature relating early-life Mn exposure with alterations in typical neurodevelopment and suggest that males and females are not equally vulnerable to Mn exposure, with effects persistent throughout extended adolescence.
To our knowledge, this is the first study to use a neuroimaging approach to examine sex-specific effects of early-life Mn exposure on neurodevelopmental outcomes in adolescence. We acknowledge several limitations to our research. The modest sample size of this study limits the generalization of our findings. Exclusion criteria such as diagnosis of neurologic or psychiatric disorders could potentially exclude participants with the highest levels of Mn exposure. The exact age at which teeth were shed was not documented in this study. However, because the age of shedding is not expected to be related to MRI data, any resulting bias would likely be toward the null. Pubertal stage data were not collected, which could potentially be considered as a covariate. Moreover, because the interpretation of the directionality of our observed associations is challenging, future studies with a larger sample size should include cognitive and motor outcomes to test whether Mn-associated changes in iFC are associated with increased or decreased performance. Finally, children and adolescents are rarely exposed to Mn alone and, in most cases, are exposed to low levels of several metals simultaneously. Coexposure to multiple metals may influence Mn toxicity (105,106). Thus, future studies should explore associations between early-life exposure to mixtures of metals and iFC later in life.
In conclusion, we identified sex-specific CWs of susceptibility to Mn exposure on iFC in areas of the brain implicated in cognitive and motor function. These findings suggest that the developing brain is especially vulnerable to Mn exposure, with effects lasting at least through late adolescence and possibly later. More research into identifying CWs of development that are sensitive to environmental insults will improve public health and risk management and may help identify especially susceptible subgroups for interventions and methods to optimize Mn exposure. Future research is needed to link sex-specific neural correlates with their behavioral and cognitive outcomes.
Acknowledgments and Disclosures
Funding was provided by the National Institute of Environmental Health Sciences (Grant Nos. R01 ES019222 and P30ES023515).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
ER and EN contributed equally to this work.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.03.016.
Supplementary Material
References
- 1.Barker D.J.P. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 2.Wells J.C.K. Adaptive variability in the duration of critical windows of plasticity: Implications for the programming of obesity. Evol Med Public Health 2014. 2014:109–121. doi: 10.1093/emph/eou019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burggren W.W., Mueller C.A. Developmental critical windows and sensitive periods as three-dimensional constructs in time and space. Physiol Biochem Zool. 2015;88:91–102. doi: 10.1086/679906. [DOI] [PubMed] [Google Scholar]
- 4.Harris A., Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker D.J. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 7.Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claus Henn B., Austin C., Coull B.A., Schnaas L., Gennings C., Horton M.K., et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res. 2018;161:588–598. doi: 10.1016/j.envres.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton M.K., Hsu L., Claus Henn B., Margolis A., Austin C., Svensson K., et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int. 2018;121:148–158. doi: 10.1016/j.envint.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner R.L., Krienen F.M., Yeo B.T.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D., Raichle M.E. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 12.Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton M.K., Margolis A.E., Tang C., Wright R. Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Curr Opin Pediatr. 2014;26:230–236. doi: 10.1097/MOP.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harischandra D.S., Ghaisas S., Zenitsky G., Jin H., Kanthasamy A., Anantharam V., Kanthasamy A.G. Manganese-induced neurotoxicity: New insights into the triad of protein misfolding, mitochondrial impairment, and neuroinflammation. Front Neurosci. 2019;13:654. doi: 10.3389/fnins.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dion L.A., Bouchard M.F., Sauvé S., Barbeau B., Tucholka A., Major P., et al. MRI pallidal signal in children exposed to manganese in drinking water. Neurotoxicology. 2016;53:124–131. doi: 10.1016/j.neuro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Wright R.O., Baccarelli A. Metals and neurotoxicology. J Nutr. 2007;137:2809–2813. doi: 10.1093/jn/137.12.2809. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger D.C. Prenatal exposures to environmental chemicals and children’s neurodevelopment: An update. Saf Health Work. 2013;4:1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucchini R.G., Guazzetti S., Zoni S., Donna F., Peter S., Zacco A., et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33:687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aschner M. Manganese: Brain transport and emerging research needs. Environ Health Perspect. 2000;108(suppl 3):429–432. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernández R.B., Farina M., Espósito B.P., Souza-Pinto N.C., Barbosa F., Jr., Suñol C. Mechanisms of manganese-induced neurotoxicity in primary neuronal cultures: The role of manganese speciation and cell type. Toxicol Sci. 2011;124:414–423. doi: 10.1093/toxsci/kfr234. [DOI] [PubMed] [Google Scholar]
- 22.Horning K.J., Caito S.W., Tipps K.G., Bowman A.B., Aschner M. Manganese is essential for neuronal health. Annu Rev Nutr. 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erikson K.M., Thompson K., Aschner J., Aschner M. Manganese neurotoxicity: A focus on the neonate. Pharmacol Ther. 2007;113:369–377. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menezes-Filho J.A., Novaes C. de O., Moreira J.C., Sarcinelli P.N., Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111:156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchard M.F., Sauvé S., Barbeau B., Legrand M., Brodeur M.È, Bouffard T., et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu Y.H.M., Claus Henn B., Hsu H.H.L., Pendo M.P., Coull B.A., Austin C., et al. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ Res. 2017;159:458–465. doi: 10.1016/j.envres.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-Bonilla D., Schilmann A., Montes S., Rodríguez-Agudelo Y., Rodríguez-Dozal S., Solís-Vivanco R., et al. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology. 2011;32:615–621. doi: 10.1016/j.neuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Mora A.M., Arora M., Harley K.G., Kogut K., Parra K., Hernández-Bonilla D., et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schullehner J., Thygesen M., Kristiansen S.M., Hansen B., Pedersen C.B., Dalsgaard S. Exposure to manganese in drinking water during childhood and association with attention-deficit hyperactivity disorder: A nationwide cohort study. Environ Health Perspect. 2020;128 doi: 10.1289/EHP6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu J., Wu C., Zheng T., Zhang B., Xia W., Peng Y., et al. Critical windows for associations between manganese exposure during pregnancy and size at birth: A longitudinal cohort study in Wuhan, China. Environ Health Perspect. 2018;126 doi: 10.1289/EHP3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rechtman E., Curtin P., Papazaharias D.M., Renzetti S., Cagna G., Peli M., et al. Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Transl Psychiatry. 2020;10:358. doi: 10.1038/s41398-020-01041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W., Xin Y., Li Q., Shang Y., Ping Z., Min J., et al. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ Health. 2020;19:104. doi: 10.1186/s12940-020-00659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamagata A.T., Guimarães N.C., Santana D.F., Gonçalves M.R., Souza V.C.O., Barbosa Júnior F., et al. Gender influence on manganese induced depression-like behavior and Mn and Fe deposition in different regions of CNS and excretory organs in intraperitoneally exposed rats. Toxicology. 2017;376:137–145. doi: 10.1016/j.tox.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Ingalhalikar M., Smith A., Parker D., Satterthwaite T.D., Elliott M.A., Ruparel K., et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111:823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruigrok A.N.V., Salimi-Khorshidi G., Lai M.C., Baron-Cohen S., Lombardo M.V., Tait R.J., Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheelock M.D., Hect J.L., Hernandez-Andrade E., Hassan S.S., Romero R., Eggebrecht A.T., Thomason M.E. Sex differences in functional connectivity during fetal brain development. Dev Cogn Neurosci. 2019;36 doi: 10.1016/j.dcn.2019.100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 39.Torres-Rojas C., Jones B.C. Sex differences in neurotoxicogenetics. Front Genet. 2018;9:196. doi: 10.3389/fgene.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozhemiako N., Nunes A.S., Vakorin V.A., Chau C.M.Y., Moiseev A., Ribary U., et al. Sex differences in brain connectivity and male vulnerability in very preterm children. Hum Brain Mapp. 2020;41:388–400. doi: 10.1002/hbm.24809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer J.A., White R.F., Coull B.A., Austin C., Oppini M., Zoni S., et al. Critical windows of susceptibility in the association between manganese and neurocognition in Italian adolescents living near ferro-manganese industry. Neurotoxicology. 2021;87:51–61. doi: 10.1016/j.neuro.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lao Y., Dion L.A., Gilbert G., Bouchard M.F., Rocha G., Wang Y., et al. Mapping the basal ganglia alterations in children chronically exposed to manganese. Sci Rep. 2017;7 doi: 10.1038/srep41804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Water E., Papazaharias D.M., Ambrosi C., Mascaro L., Iannilli E., Gasparotti R., et al. Early-life dentine manganese concentrations and intrinsic functional brain connectivity in adolescents: A pilot study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Water E., Proal E., Wang V., Medina S.M., Schnaas L., Téllez-Rojo M.M., et al. Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology. 2018;64:85–93. doi: 10.1016/j.neuro.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iannilli E., Gasparotti R., Hummel T., Zoni S., Benedetti C., Fedrighi C., et al. Effects of manganese exposure on olfactory functions in teenagers: A pilot study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0144783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aschner J.L., Anderson A., Slaughter J.C., Aschner M., Steele S., Beller A., et al. Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am J Clin Nutr. 2015;102:1482–1489. doi: 10.3945/ajcn.115.116285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolger C. In: Understanding Psychological Assessment. Perspectives on Individual Differences. Dorfman W.I., Hersen M., editors. Springer; Boston: 2001. Wechsler Intelligence Scale for Children-Third Edition (WISC-III) pp. 219–233. [Google Scholar]
- 48.Arora M., Austin C. Teeth as a biomarker of past chemical exposure. Curr Opin Pediatr. 2013;25:261–267. doi: 10.1097/MOP.0b013e32835e9084. [DOI] [PubMed] [Google Scholar]
- 49.Arora M., Hare D., Austin C., Smith D.R., Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–1319. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Arora M., Bradman A., Austin C., Vedar M., Holland N., Eskenazi B., Smith D.R. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46:5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solé-Padullés C., Castro-Fornieles J., de la Serna E., Calvo R., Baeza I., Moya J., et al. Intrinsic connectivity networks from childhood to late adolescence: Effects of age and sex. Dev Cogn Neurosci. 2016;17:35–44. doi: 10.1016/j.dcn.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tooley U.A., Mackey A.P., Ciric R., Ruparel K., Moore T.M., Gur R.C., et al. Associations between neighborhood SES and functional brain network development [published correction appears in Cereb Cortex 2021; 31:2307] Cereb Cortex. 2020;30:1–19. doi: 10.1093/cercor/bhz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lucchini R., Placidi D., Cagna G., Fedrighi C., Oppini M., Peli M., Zoni S. In: Neurotoxicity of Metals. Aschner M., Costa L.G., editors. Springer International Publishing; Cham, Switzerland: 2017. Manganese and developmental neurotoxicity; pp. 13–34. [Google Scholar]
- 54.Cesana G.C., Ferrario M., De Vito G., Sega R., Grieco A. [Evaluation of the socioeconomic status in epidemiological surveys: Hypotheses of research in the Brianza area MONICA project] Med Lav. 1995;86:16–26. [PubMed] [Google Scholar]
- 55.Nieto-Castanon A. Hilbert College Press; Hamburg, NY: 2020. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN. [Google Scholar]
- 56.Andersson J.L., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- 57.Henson R., Buechel C., Josephs O., Friston K.J. Presented at the 5th International Conference on Functional Mapping of the Human Brain. Düsseldorf; Germany: 1999. The slice-timing problem in event-related fMRI. June 22–26. [Google Scholar]
- 58.Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennedy D.N., Lange N., Makris N., Bates J., Meyer J., Caviness V.S., Jr. Gyri of the human neocortex: An MRI-based analysis of volume and variance. Cereb Cortex. 1998;8:372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- 61.Worsley K.J. In: Functional MRI: An Introduction to Methods. Jezzard P., Matthews P.M., Smith S.M., editors. Oxford University Press; Oxford, UK: 2002. Statistical analysis of activation images. [Google Scholar]
- 62.Shehzad Z., Kelly A.M.C., Reiss P.T., Gee D.G., Gotimer K., Uddin L.Q., et al. The resting brain: Unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Duijvenvoorde A.C.K., Achterberg M., Braams B.R., Peters S., Crone E.A. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage. 2016;124:409–420. doi: 10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 64.Gunier R.B., Arora M., Jerrett M., Bradman A., Harley K.G., Mora A.M., et al. Manganese in teeth and neurodevelopment in young Mexican-American children. Environ Res. 2015;142:688–695. doi: 10.1016/j.envres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassidy-Bushrow A.E., Wu K.H.H., Sitarik A.R., Park S.K., Bielak L.F., Austin C., et al. In utero metal exposures measured in deciduous teeth and birth outcomes in a racially-diverse urban cohort. Environ Res. 2019;171:444–451. doi: 10.1016/j.envres.2019.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin K.V., Edmondson D., Cecil K.M., Bezi C., Vance M.L., McBride D., Haynes E.N. Manganese exposure and neurologic outcomes in adult populations. Neurol Clin. 2020;38:913–936. doi: 10.1016/j.ncl.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li S.J., Jiang L., Fu X., Huang S., Huang Y.N., Li X.R., et al. Pallidal index as biomarker of manganese brain accumulation and associated with manganese levels in blood: A meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Criswell S.R., Perlmutter J.S., Huang J.L., Golchin N., Flores H.P., Hobson A., et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med. 2012;69:437–443. doi: 10.1136/oemed-2011-100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pesch B., Dydak U., Lotz A., Casjens S., Quetscher C., Lehnert M., et al. Association of exposure to manganese and iron with relaxation rates R1 and R2∗- Magnetic resonance imaging results from the WELDOX II study. Neurotoxicology. 2018;64:68–77. doi: 10.1016/j.neuro.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sen S., Flynn M.R., Du G., Tröster A.I., An H., Huang X. Manganese accumulation in the olfactory bulbs and other brain regions of “asymptomatic” welders. Toxicol Sci. 2011;121:160–167. doi: 10.1093/toxsci/kfr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long Z., Jiang Y.M., Li X.R., Fadel W., Xu J., Yeh C.L., et al. Vulnerability of welders to manganese exposure—A neuroimaging study. Neurotoxicology. 2014;45:285–292. doi: 10.1016/j.neuro.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Criswell S.R., Nielsen S.S., Warden M.N., Flores H.P., Lenox-Krug J., Racette S., et al. MRI signal intensity and Parkinsonism in manganese-exposed workers. J Occup Environ Med. 2019;61:641–645. doi: 10.1097/JOM.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucchini R., Albini E., Placidi D., Gasparotti R., Pigozzi M.G., Montani G., Alessio L. Brain magnetic resonance imaging and manganese exposure. Neurotoxicology. 2000;21:769–775. [PubMed] [Google Scholar]
- 74.Lee E.Y., Flynn M.R., Du G., Lewis M.M., Herring A.H., Van Buren E., et al. Editor’s highlight: Lower fractional anisotropy in the globus pallidus of asymptomatic welders, a marker for long-term welding exposure. Toxicol Sci. 2016;153:165–173. doi: 10.1093/toxsci/kfw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee E.Y., Flynn M.R., Lewis M.M., Mailman R.B., Huang X. Welding-related brain and functional changes in welders with chronic and low-level exposure. Neurotoxicology. 2018;64:50–59. doi: 10.1016/j.neuro.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis M.M., Lee E.Y., Jo H.J., Du G., Park J., Flynn M.R., et al. Synergy as a new and sensitive marker of basal ganglia dysfunction: A study of asymptomatic welders. Neurotoxicology. 2016;56:76–85. doi: 10.1016/j.neuro.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang Y., Lee J.J., Seo J.H., Song H.J., Kim J.H., Bae S.J., et al. Altered working memory process in the manganese-exposed brain. Neuroimage. 2010;53:1279–1285. doi: 10.1016/j.neuroimage.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Seo J., Chang Y., Jang K.E., Park J.W., Kim Y.T., Park S.J., et al. Altered executive function in the welders: A functional magnetic resonance imaging study. Neurotoxicol Teratol. 2016;56:26–34. doi: 10.1016/j.ntt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Gunier R.B., Mora A.M., Smith D., Arora M., Austin C., Eskenazi B., Bradman A. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Environ Sci Technol. 2014;48:14695–14702. doi: 10.1021/es503866a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claus Henn B., Ettinger A.S., Schwartz J., Téllez-Rojo M.M., Lamadrid-Figueroa H., Hernández-Avila M., et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leh S.E., Ptito A., Chakravarty M.M., Strafella A.P. Fronto-striatal connections in the human brain: A probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanciego J.L., Luquin N., Obeso J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aoki S., Smith J.B., Li H., Yan X., Igarashi M., Coulon P., et al. An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. Elife. 2019;8 doi: 10.7554/eLife.49995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 85.Jahanshahi M., Obeso I., Rothwell J.C., Obeso J.A. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16:719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- 86.Gunaydin L.A., Kreitzer A.C. Cortico-basal ganglia circuit function in psychiatric disease. Annu Rev Physiol. 2016;78:327–350. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- 87.Jin X., Costa R.M. Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol. 2015;33:188–196. doi: 10.1016/j.conb.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grahn J.A., Parkinson J.A., Owen A.M. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 89.Haber S.N., Calzavara R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Viñas-Guasch N., Wu Y.J. The role of the putamen in language: A meta-analytic connectivity modeling study. Brain Struct Funct. 2017;222:3991–4004. doi: 10.1007/s00429-017-1450-y. [DOI] [PubMed] [Google Scholar]
- 91.Balachandran R.C., Mukhopadhyay S., McBride D., Veevers J., Harrison F.E., Aschner M., et al. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem. 2020;295:6312–6329. doi: 10.1074/jbc.REV119.009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright N., Alhindi A., Millikin C., Modirrousta M., Udow S., Borys A., et al. Elevated caudate connectivity in cognitively normal Parkinson’s disease patients. Sci Rep. 2020;10 doi: 10.1038/s41598-020-75008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hensel L., Hoffstaedter F., Caspers J., Michely J., Mathys C., Heller J., et al. Functional connectivity changes of key regions for motor initiation in Parkinson’s disease. Cereb Cortex. 2019;29:383–396. doi: 10.1093/cercor/bhy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen B., Pan Y., Jiang X., Wu Z., Zhu J., Dong J., et al. Altered putamen and cerebellum connectivity among different subtypes of Parkinson’s disease. CNS Neurosci Ther. 2020;26:207–214. doi: 10.1111/cns.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Racette B.A., Searles Nielsen S., Criswell S.R., Sheppard L., Seixas N., Warden M.N., Checkoway H. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology. 2017;88:344–351. doi: 10.1212/WNL.0000000000003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koyama M.S., O’Connor D., Shehzad Z., Milham M.P. Differential contributions of the middle frontal gyrus functional connectivity to literacy and numeracy. Sci Rep. 2017;7 doi: 10.1038/s41598-017-17702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benischek A., Long X., Rohr C.S., Bray S., Dewey D., Lebel C. Pre-reading language abilities and the brain’s functional reading network in young children. Neuroimage. 2020;217 doi: 10.1016/j.neuroimage.2020.116903. [DOI] [PubMed] [Google Scholar]
- 98.Xu S., Li M., Yang C., Fang X., Ye M., Wei L., et al. Altered functional connectivity in children with low-function autism spectrum disorders. Front Neurosci. 2019;13:806. doi: 10.3389/fnins.2019.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Riojas-Rodríguez H., Solís-Vivanco R., Schilmann A., Montes S., Rodríguez S., Ríos C., Rodríguez-Agudelo Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118:1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Irizar A., Molinuevo A., Andiarena A., Jimeno-Romero A., San Román A., Broberg K., et al. Prenatal manganese serum levels and neurodevelopment at 4 years of age. Environ Res. 2021;197 doi: 10.1016/j.envres.2021.111172. [DOI] [PubMed] [Google Scholar]
- 101.Hafkemeijer A., Altmann-Schneider I., Oleksik A.M., van de Wiel L., Middelkoop H.A.M., van Buchem M.A., et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3:353–362. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hawellek D.J., Hipp J.F., Lewis C.M., Corbetta M., Engel A.K. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A. 2011;108:19066–19071. doi: 10.1073/pnas.1110024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin S., Young C.B., Duan X., Chen T., Supekar K., Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hsiao F.J., Yu H.Y., Chen W.T., Kwan S.Y., Chen C., Yen D.J., et al. Increased intrinsic connectivity of the default mode network in temporal lobe epilepsy: Evidence from resting-state MEG recordings. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanders A.P., Claus Henn B., Wright R.O. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: A review of recent literature. Curr Environ Health Rep. 2015;2:284–294. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merced-Nieves F.M., Arora M., Wright R.O., Curtin P. Metal mixtures and neurodevelopment: Recent findings and emerging principles. Curr Opin Toxicol. 2021;26:28–32. doi: 10.1016/j.cotox.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.