Abstract
Background
When brain networks deviate from typical development, this is thought to contribute to varying forms of psychopathology. However, research has been limited by the reliance on discrete diagnostic categories that overlook the potential for psychological comorbidity and the dimensional nature of symptoms.
Methods
This study examined the topology of functional networks in association with 4 bifactor-defined psychopathology dimensions—general psychopathology, internalizing symptoms, conduct problems, and attention-deficit/hyperactivity disorder symptoms—via the Child Behavior Checklist in a sample of 3568 children from the ABCD (Adolescent Brain Cognitive Development) Study. Local and global graph theory metrics were calculated at rest and during tasks of reward processing, inhibition, and working memory.
Results
Greater attention-deficit/hyperactivity disorder symptoms were associated with reduced modularity across rest and tasks as well as reduced local efficiency in motor networks at rest. Results survived sensitivity analyses for medication and socioeconomic status. Greater conduct problem symptoms were associated with reduced modularity on working memory and reward processing tasks; however, these results did not persist after sensitivity analyses. General psychopathology and internalizing symptoms showed no significant network associations.
Conclusions
Our findings suggest reduced efficiency in topology in those with greater attention-deficit/hyperactivity disorder symptoms across 4 critical cognitive states, with conduct problems also showing network deficits, although less consistently. This may suggest that modularity deficits are a neurobiological marker of externalizing behavior in children. Such specificity has not been demonstrated before using graph theory metrics and has the potential to redefine our understanding of network deficits in children with psychopathology symptoms.
Keywords: Adolescent Brain Cognitive Development Study, Attention-deficit/hyperactivity disorder, Child Behavior Checklist, Psychopathology symptoms
During childhood, the brain undergoes significant organization into functional networks that adapt and interact in response to incoming cognitive demands (1,2). Studies have demonstrated the modular organization of the human brain such that networks contain modules, or groups of densely interconnected nodes, that are thought to be efficient for information processing and specialized functions (3). In typical development, functional modules become more distinct across childhood and adolescence; within-module connectivity increases while connectivity between modules decreases (1,4,5). Deviations from typical development can result in large-scale network dysfunction, which is thought to contribute to a range of psychopathology symptoms (6, 7, 8, 9, 10).
Currently, the classification system for psychopathology relies heavily on traditional categorical diagnoses. However, several issues accompany this system. Traditional diagnoses are often marked by transdiagnostic symptoms, a high degree of disorder comorbidity, neurobiological nonspecificity, and inconsistent treatment response (11, 12, 13, 14, 15, 16, 17). Despite these concerns, traditional diagnoses often guide psychopathology research using case-control methods, which compare healthy control subjects to individuals who meet diagnostic criteria. This design overlooks the continuous nature of psychopathology symptoms, in which clinical symptomatology exists on a spectrum rather than finite groupings (8,18,19).
A growing body of literature indicates that psychopathology is better captured by a hierarchical dimensional model that identifies a common factor representing general symptoms across all disorders, also called the psychopathology or p factor, and factors defining specific psychological problems (12,20, 21, 22). Previous studies have revealed neurostructural associations with these general and specific psychopathology dimensions during development; reduced gray matter volume has been associated with both general and attention-deficit/hyperactivity disorder (ADHD) psychopathology dimensions, and white matter integrity has been linked to dimensions of ADHD and conduct problems (23, 24, 25). Research is just beginning to link functional network architecture with dimensions of psychopathology. Xia et al. (10) examined resting-state functional connectivity as it related to dimensions of mood, fear, psychosis, and externalizing behaviors, finding loss of network segregation common across all dimensions. This suggests that psychopathology dimensions may be associated with functional network–level deficits; however, this study was limited to a resting-state task. The analysis of varying cognitive states is crucial to understanding whether functional network properties emerge in association with psychopathology and whether these properties are consistent across varying cognitive demands. To build upon this work, this study sought to examine psychopathology dimensions and network properties beyond rest conditions.
We sought to characterize functional network properties across rest, reward processing, and executive functioning states to deepen our understanding of network-level deficits that are common across disorders or specific to varying forms of psychopathology. In a data-driven, exploratory analysis of a large sample of children ages 9–10 years from the ABCD (Adolescent Brain Cognitive Development) Study, we used a hierarchical model established in our prior work (26) to define a general factor of psychopathology and 3 specific factors of internalizing symptoms, conduct problems, and ADHD symptoms. We used structural equation modeling (SEM) to test the significance of individual path components between each dimension and functional network attributes. We characterized functional neural network topology with graph theory metrics—a mathematical framework for quantifying within- and between-network properties—during rest and the following 3 functional magnetic resonance imaging (fMRI) tasks: a monetary incentive delay task of reward processing, a stop signal task of inhibition, and an emotional n-back task of affective working memory (27).
Methods and Materials
Participants
We used data from the ABCD Study wave 1 (release 3.0), a study of youth brain development, for which consent was obtained from all participants. Vanderbilt University’s Institutional Review Board approved the use of this deidentified dataset. Participants included 11,875 children 9 and 10 years of age recruited from across the United States (for additional details on the representativeness of the sample, see the Supplement). We excluded participants based on missing data, failed quality assurance measures, and stringent motion parameters to ensure adequately clean data for the graph theory network metrics (Figure S1) (see Image Acquisition, Processing, and Quality Assurance for additional details). Final sample sizes for the 4 tasks were as follows: rest (n = 3568), monetary incentive delay (n = 1708), emotional n-back (n = 1652), and stop signal task (n = 1694). See Table 1 for demographics.
Table 1.
Demographics of the Sample (N = 3568)
| Characteristics | Mean (SD) or n (%) |
|---|---|
| Age, Years | 9.98 (0.63) |
| Sex | |
| Female | 1856 (52.0%) |
| Male | 1712 (48.0%) |
| Race/Ethnicity | |
| Black | 359 (10.0%) |
| Hispanic | 712 (20.0%) |
| Other | 418 (11.7%) |
| White | 2079 (58.3%) |
| Household Income, $ | |
| <5000 | 82 (2.3%) |
| 5000–11,999 | 82 (2.3%) |
| 12,000–15,999 | 76 (2.1%) |
| 16,000–24,999 | 135 (3.8%) |
| 25,000–34,999 | 174 (4.9%) |
| 35,000–49,999 | 283 (7.9%) |
| 50,000–74,999 | 468 (13.1%) |
| 75,000–99,999 | 547 (15.3%) |
| 100,000–199,999 | 1117 (31.3%) |
| ≥200,000 | 384 (10.8%) |
| Missing | 220 (6.2%) |
| Parent Education | |
| No degree | 125 (3.5%) |
| High school/GED | 371 (10.4%) |
| Some college | 554 (15.5%) |
| Associate degree | 460 (12.9%) |
| Bachelor’s degree | 1138 (31.9%) |
| Master’s degree | 694 (19.5%) |
| Professional/doctoral | 226 (6.3%) |
GED, general education development.
The “Other” Race/Ethnicity category includes study participants who were identified by their parent as American Indian/Native American, Alaska Native, Native Hawaiian, Guamanian, Samoan, Other Pacific Islander, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Other Asian, or Other Race.
Measures of Psychopathology
The Child Behavior Checklist was used to assess psychopathology through parent-reported emotional and behavioral problems (28). The Child Behavior Checklist is normed for children and adolescents ages 6–18 years and consists of 119 items related to various emotions and behaviors. Items are rated on a 3-point scale as follows: 0 = not true (as far as you know), 1 = somewhat or sometimes true, and 2 = very true or often true.
Hierarchical Models of Psychopathology
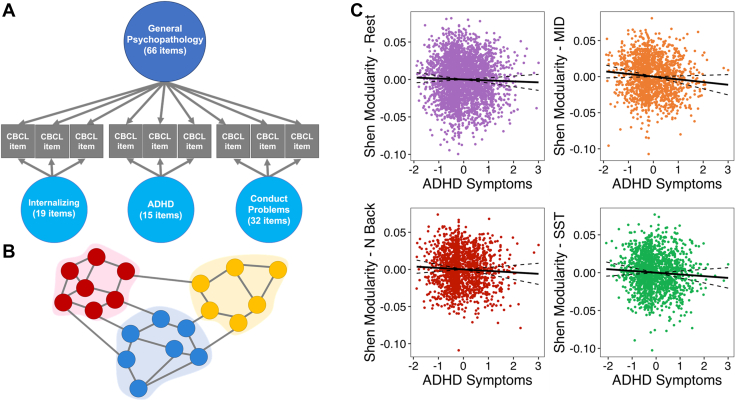
As outlined in our prior work, we defined latent factors of psychopathology derived from items from the Child Behavior Checklist (26). An exploratory factor analysis was used to identify 3 correlated dimensions of psychopathology—internalizing, ADHD, and conduct problems. Next, a general factor of psychopathology, which reflects the common symptoms across disorders, was identified using a confirmatory bifactor analysis (Figure 1A). The psychometric properties of the factors met all standards for construct reliability and factor determinacy recommended for bifactor models, and each factor demonstrated adequate criterion validity. Additional detail is provided in the Supplement.
Figure 1.
A hierarchical approach to examining modularity under varying cognitive demands. (A) Hierarchical model comprising a general factor, which represents the commonalities across all symptoms and 3 orthogonal, specific factors of internalizing, attention-deficit/hyperactivity disorder (ADHD), and conduct problems. (B) Visual depiction of the graph theory metric of atlas-derived modularity; circles indicate nodes, and their colors indicate various networks. Connecting lines indicate edges or “steps” between the nodes. Modularity quantifies the degree to which nodes form connections within or between modules (i.e., clusters of nodes shown by different colored areas). (C) Correlations between the ADHD dimension of psychopathology and its associated network modularity across tasks. Correlations display significance at the 30% threshold; solid line indicates line of best fit and dotted lines indicate a 95% confidence interval. Visualizations were created using factor scores, which are approximations of the latent factors estimated in Mplus [see Grice (60)]. CBCL, Child Behavior Checklist; MID, monetary incentive delay; SST, stop signal task.
fMRI Tasks
We examined 3 functional tasks. The stop signal task probes inhibition and impulse control. Performance is measured as reaction time, quantified as the mean stop signal delay subtracted from the mean reaction time on correct Go trials and the proportion correct on Go trials (29,30). Stop signal reaction times are reverse scored so that higher scores indicate better performance. The emotional n-back task probes working memory and emotion regulation processes. Performance is based on the rate of accuracy for 2-back trials. The monetary incentive delay task probes aspects of reward processing, including anticipation and motivation. Performance is based on total monetary earnings. Further task details are included in the Supplement.
Image Acquisition, Processing, and Quality Assurance
The imaging protocol was developed by the ABCD Data Analysis and Informatics Center and the ABCD Imaging Acquisition Workgroup (30). We downloaded minimally processed fMRI scans, which included head motion correction, B0 distortion correction, gradient nonlinearity distortion correction, resampling, and registration to T1 structural images (30). A detailed account of preprocessing can be found in the Supplement. Following preprocessing, all fMRI blood oxygen level–dependent time courses were spatially averaged within 268 previously defined functional regions (31,32). We used strict motion parameters for each task, excluding individual runs with greater than 0.2-mm mean and 2-mm maximum framewise displacement. In addition, data were lost because of a documented error with the Philips scanner data (see the Supplement for details). For each subject, when more than 1 run was retained, the parcellated time series were averaged over the 2 runs by spatially averaging within 268 defined functional regions. Additional details on the image acquisition, processing, and quality assurance procedures have been published elsewhere (29,30,33).
Deriving the Functional Networks
We analyzed the topology of networks derived from the Shen 268 atlas (32), which partitions the brain into 268 previously defined parcels based on group-level patterns of statistical similarities between brain region dynamics. This parcellation method has been used to study various disorders, development stages, and cognitive states and processes (34, 35, 36, 37, 38). These parcels were then grouped into established networks including subcortical-cerebellar, motor, medial frontal, frontoparietal, default mode, visual 1, visual 2, and visual association networks (31,32), henceforth referred to as canonical networks. In addition, we ran a Louvain community detection algorithm on each participant’s thresholded functional connectivity matrices, henceforth referred to as individualized networks. Separate from the Shen 268 atlas, the Louvain technique determines individualized partitions across the whole brain. Further details can be found in the Supplement.
Graph Theory Analyses
All measures derived from graph theory were computed using the Python package NetworkX (39). This analysis focused on graph theory metrics that are well suited to characterizing attributes on a network-wide scale, including modularity, path length, and varying measures of efficiency (27).
Connectivity Matrix Thresholding
To derive functional networks from correlation matrices of signals between brain regions, we applied 4 thresholds to evaluate only the strongest 10%, 16.67%, 23%, and 30% of connections between node pairs. The application of thresholds removes noisy edges. These thresholds were chosen to allow for sparse networks that were still largely connected. By the nature of this approach, thresholds affect the density of network connections included in the analysis (for discussion of alternative approaches, see Discussion). To combat potential bias, we only report results that were retained across at least 3 consecutive thresholds. This is similar to prior literature (40). We retained positive and negative connections that were stronger than each separate threshold within each network and within each task. Connections were binarized before all graph theory calculations.
Measures of Network Efficiency
This analysis examined the following graph theory metrics, as described and calculated in prior literature (27,41). Equations for metrics can be found in the Supplement. Modularity is a measure of a system’s balance of within-network connectivity and between-network connectivity via the extent to which a network can be subdivided into distinct and separate communities; it is defined by the strength of division of a network into modules. Average shortest path length is defined by the average number of edges along the shortest path for all possible node pairs. It is worth noting that graph theory mathematically conceptualizes efficiency, and while it highlights the most efficient path, signal may or may not traverse this path. The local efficiency of node i is defined by how well information is transferred by a node neighborhood when node i is removed. The diameter is a measure of the overall size of the graph and is calculated as the maximum eccentricity across all nodes; for a single node, the eccentricity is the maximum distance from that node to all other nodes in the graph. Small-world sigma metrics benchmark clustering and shortest path lengths against random reference graphs, whereas small-world omega metrics benchmark clustering against a reference lattice graph while shortest path length is compared against a random reference graph. Small-world omega metrics further allow the characterization of whether graphs are more random or more lattice-like in their deviations from small worldness.
Statistical Analysis
We examined associations between 4 orthogonal dimensions of psychopathology defined by our previous hierarchical model—general psychopathology, and specific internalizing, conduct problems, and ADHD—and functional network properties, including canonical and individualized modularity, average shortest path length, local efficiency, diameter, small-world sigma, and small-world omega (26). The data were weighted by the poststratification weights provided by the ABCD Study to make the sample more representative of the U.S. population, stratified based on site to control for site differences and clustered based on family membership to account for siblings and multiple births. We included age, sex, race/ethnicity, and MRI scanner model as covariates. For each of the 8 networks, 4 tasks, and 4 thresholds, we investigated associations between the dimensions of psychopathology and metrics through SEM as follows:
Of note, this equation exemplifies 1 network combination; however, all 8 networks were tested simultaneously in one structural equation model, and false discovery rate corrections were applied across the 8 networks. Owing to their orthogonality, the 4 psychopathology dimensions could be included together in the same model without concerns about multicollinearity. We controlled for the false discovery rate (q < .05) using the R Stats Package, version 3.6.1 (http://www.r-project.org/). Notably, we used SEM to assess individual paths while controlling for all other variables in the model; although we did not use SEM for model selection, model fit indices were adequate and are as follows: root mean square error of approximation = 0.020, 90% CI = 0.020–0.021; comparative fit index = 0.948; standardized root-mean-squared residual = 0.067.
In addition, we conducted analyses of behavioral measures to examine the associations between performance on working memory, reward processing, and inhibition tasks and the psychopathology dimensions while covarying for sex and race/ethnicity. We also performed sensitivity analyses with parental education, income, and medication (whether participants reported taking current medications or not) as additional covariates to determine whether associations between network properties and psychopathology would sustain when accounting for a proxy for socioeconomic status and medication status. Finally, we examined interactions with sex to test for sex differences in the relationship between network metrics and psychopathology.
Data and Code Availability
The data used in this study is from the ABCD Study and is available through the NDA (National Institute of Mental Health Data Archive) (https://nda.nih.gov/abcd). The Mplus and R code and a corresponding wiki for the analytic procedures can be found at https://github.com/VU-BRAINS-lab/Reimann_Network_Metrics.
Results
Graph theory metrics were considered reliable if they were significant across at least 3 consecutive thresholds; results will be referred to as significant if a significant association occurred across at least 3 consecutive thresholds for that network or task. Results will be referred to as inconsistent if significant associations occurred in individual networks and/or tasks but were not seen across at least 3 consecutive thresholds. The p values reflect results at the most liberal threshold. R2 reflects all predictors in each model, including covariates.
Link Between Whole-Brain Modularity and Psychopathology Dimensions
The specific ADHD dimension was associated with lower whole-brain canonical modularity. This was apparent during rest (pfdr = .01, R2 = 0.06), monetary incentive delay (pfdr = .001, R2 = 0.15), emotional n-back (pfdr = .008, R2 = 0.14), and stop signal task (pfdr < .001, R2 = 0.09) across all 4 thresholds (Figure 1C; Table 2). Lower modularity suggests that there are dense connections between modules but sparse connections within modules (Figure 1B). Lower modularity was also associated with poorer cognitive scores (4,42).
Table 2.
Results Examining the Relationship Between Psychopathology Dimensions and Whole-Brain Shen Modularity Across 10%, 16.67%, 23%, and 30% Thresholds
| Threshold | General |
Specific Conduct |
Specific Internalizing |
Specific ADHD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | β | pfdr | β | pfdr | β | pfdr | β | pfdr | R2 | |
| 10% | Rest | 0.007 | .982 | −0.051 | .064 | 0.024 | .356 | −0.081 | .010 | 0.07 |
| En-back | 0.036 | .286 | −0.086 | .048 | 0.019 | .619 | −0.130 | .009 | 0.14 | |
| MID | 0.001 | .983 | −0.106 | .014 | −0.020 | .754 | −0.140 | .001 | 0.15 | |
| SST | 0.072 | .079 | −0.081 | .057 | 0.000 | .995 | −0.170 | .001 | 0.09 | |
| 16.67% | Rest | 0.004 | .982 | −0.056 | .059 | 0.022 | .356 | −0.082 | .010 | 0.07 |
| En-back | 0.043 | .252 | −0.084 | .048 | 0.019 | .619 | −0.134 | .008 | 0.15 | |
| MID | 0.006 | .983 | −0.113 | .011 | −0.015 | .754 | −0.141 | .001 | 0.15 | |
| SST | 0.064 | .079 | −0.083 | .057 | 0.012 | .975 | −0.165 | .001 | 0.09 | |
| 23% | Rest | −0.001 | .982 | −0.057 | .059 | 0.024 | .356 | −0.081 | .010 | 0.07 |
| En-back | 0.047 | .252 | −0.085 | .048 | 0.017 | .619 | −0.135 | .008 | 0.15 | |
| MID | 0.007 | .983 | −0.114 | .011 | −0.013 | .754 | −0.142 | .001 | 0.15 | |
| SST | 0.062 | .079 | −0.080 | .057 | 0.020 | .975 | −0.161 | .001 | 0.09 | |
| 30% | Rest | −0.003 | .982 | −0.058 | .059 | 0.024 | .356 | −0.080 | .010 | 0.06 |
| En-back | 0.049 | .252 | −0.085 | .048 | 0.016 | .619 | −0.136 | .008 | 0.14 | |
| MID | 0.008 | .983 | −0.115 | .011 | −0.010 | .754 | −0.141 | .001 | 0.15 | |
| SST | 0.060 | .079 | −0.079 | .057 | 0.024 | .975 | −0.159 | .001 | 0.09 | |
R2 reflects all predictors in each model, including covariates.
ADHD, attention-deficit/hyperactivity disorder; En-back, emotional n-back; FDR, false discovery rate; MID, monetary incentive delay; SST, stop signal task.
Individuals with elevated levels on the specific conduct problems dimension displayed lower whole-brain canonical modularity, but only during tasks of reward processing (pfdr = .011, R2 = 0.15) and affective working memory (pfdr = .048, R2 = 0.14) (Table 2). This association was not seen at rest or during the inhibition task. The general psychopathology factor and the internalizing specific factor were not consistently associated with modularity across any task (Table 2).
Specific ADHD Is Associated With Deficits in the Motor Network at Rest
Greater ADHD symptoms were significantly associated with lower local efficiency in the motor network during rest (pfdr = .008, R2 = 0.06) (Table S1A–D), indicating reduced within-network efficiency and greater interruption if one of its nodes is removed. The specific factor of ADHD was not consistently associated with the average shortest path, diameter, small-world metrics (Tables S2–S5), or local efficiency in other networks. General psychopathology, conduct problems, and internalizing symptoms were not consistently associated with these graph theory metrics across networks (Tables S1–S5).
Task Performance and the Specific Dimensions
Given the association between network metrics and ADHD symptoms and conduct problems, we next examined the relationship between these psychopathology dimensions and behavioral measures derived from the various cognitive tasks. Findings showed that ADHD symptoms negatively predicted total earnings on the monetary incentive delay task (pfdr < .001), the proportion of correct responses across 2-back trials on the emotional n-back task (pfdr < .001), and the proportion of correct Go trials on the stop signal task (pfdr < .001). ADHD was not predictive of the mean response time for all correct Go trials during the stop signal task (pfdr = .071). Furthermore, the conduct problems specific factor negatively predicted the proportion of correct responses across 2-back trials on the emotional n-back (pfdr < .001) but not monetary incentive delay total earnings (pfdr = .071).
Sensitivity Analyses
Sensitivity analyses controlled for medication, parent education, and income (Table S12). Results showed that the specific ADHD factor retained significance for motor local efficiency at rest (pfdr = .005) and whole-brain canonical modularity during the monetary incentive delay task (pfdr = .01), the emotional n-back task (pfdr = .02), the stop signal task (pfdr = .008), and at rest (pfdr = .024). After controlling for medication, parent education, and income, the conduct problems factor did not retain significance for whole-brain canonical modularity during the emotional n-back task (pfdr = .10) and monetary incentive delay task (pfdr = .10). Finally, based on the known sex differences in the prevalence rates of ADHD (43), we examined sex differences in our ADHD results. As expected, boys endorsed greater ADHD symptoms than girls (pfdr < .001). However, there were no significant interactions between the specific factor of ADHD and sex after correction for multiple comparisons in motor local efficiency at rest or whole-brain modularity during any of the 4 tasks (psfdr ≥ .21).
Louvain-Derived Networks and the Psychopathology Dimensions
We examined the results from a Louvain community detection algorithm that defines individualized partitions across the whole brain. There were no significant associations between the Louvain-derived networks and psychopathology dimensions for the graph theory metrics across any task (Tables S6–S11).
Discussion
This study used a large subsample of children from the ABCD Study to examine associations between 4 orthogonal dimensions of psychopathology—general psychopathology, internalizing symptoms, ADHD symptoms, and conduct problems—and functional network efficiency at rest and during tasks of reward processing, inhibition, and affective working memory. Overall, findings provide evidence that altered network topology is consistent across rest and during various cognitive demands in those with greater externalizing symptomatology defined as ADHD symptoms and conduct problems. The ADHD factor was significantly associated with lower canonical modularity across all 4 tasks and reduced local efficiency in the motor network at rest. The conduct problems factor was associated with reduced canonical modularity during affective working memory and reward processing tasks; however, these results were less robust than the ADHD results. No consistent associations were found between canonical network metrics and general psychopathology or internalizing symptoms, nor were there associations between any psychopathology factor and individualized network metrics.
Studies in the past decade have placed great emphasis on the conceptualization of the brain as having a modular organization that develops early in life and continues across childhood (2,44,45). There has been considerable interest in how network properties can provide insight into psychopathology, with studies linking structural and functional network modularity to executive functioning and clinical symptoms (4,10). Of note, Xia et al. (10) found that lower network segregation was associated with externalizing behaviors, which was a composite of ADHD- and conduct-related behaviors. The findings from our study are consistent with the finding by Xia et al. (10) that modularity deficits are associated with externalizing behaviors, and we expanded upon this work to show a similar association across multiple tasks using a hierarchical model of psychopathology. Given the linkage between functional topology and clinical presentations, it is evident that network architecture can critically inform the ways in which atypical circuits give rise to psychiatric symptoms. This link may inform trajectories of psychiatric symptoms as well; although we did not see an association between network features and internalizing symptoms, this may emerge in adolescence when the incidence of anxiety and mood disorders increases (46). Furthermore, to our knowledge, this is the first study to examine the general psychopathology factor and network properties. Although we found no association between general psychopathology and the 8 networks, prior work has shown that the general factor tends to have a strong relationship with brain metrics; however, this is more apparent in certain brain modalities such as gray matter volume, but not cortical thickness (23,24). Current findings show the influence of other psychopathology dimensions, where functional topology differences were attributed more to the specific factor of ADHD rather than the general factor of psychopathology.
Modularity findings emerged as the most consistent association with ADHD, revealing significant associations across all 4 tasks and across all 4 thresholds. Lower canonical modularity indicates a bias toward global configuration (connections between modules) at the expense of local configuration (connections within modules), and this has been associated with poorer cognitive functioning (1,4,42). The lack of segregation found in this study may suggest that ADHD is associated with nonoptimal within-network topology and a lack of distinct hub formation. This is substantiated by our finding of reduced local efficiency in the motor cortex at rest, which also suggests local configuration deficits. It is worth noting that we did not find modularity deficits in the individualized networks. This suggests that individualized networks do not display psychopathology-network associations in subject-specific small, medium, and large communities detected across the whole brain. Our analysis of canonical networks may give us insight into brain areas linked in a large-scale network as they relate to cognitive abilities; findings showed that increased ADHD symptoms were associated with poorer cognitive performance. Taken together, these results suggest that ADHD is associated with deficits in the development of segregated network modules in the brain, which may negatively affect cognitive functioning.
The neural findings of this study align with and extend the research on network deficits in externalizing behaviors, especially for ADHD symptoms. Prior resting-state findings have indicated a link between greater ADHD symptoms and lower modularity (47). In addition to rest, we revealed reduced modularity present during reward processing, affective working memory, and inhibition tasks. Robust modularity findings across every task and threshold provide evidence for a broad canonical modularity deficit in children with greater ADHD symptoms. Overall, the significant association between modularity and ADHD could suggest that the neural systems of those with ADHD have not optimized topology on a local level. Given that prior findings suggest a developmental lag in ADHD functional networks, this local deficit may reflect a maturational delay (48). In addition, our findings revealed that in the absence of any cognitive demands, the ADHD motor network shows lower resilience to local failures in information exchange. Prior studies have shown that children with ADHD often display alterations in the functional connectivity strength of the motor network both within and between hemispheres and also show behavioral impairments during tasks of motor coordination (49,50). Considering our findings, motor network alterations may reflect reduced efficiency of within-network topology, which may contribute to motor disorganization.
In addition, our results revealed a significant association between conduct problems and whole-brain modularity under affective working memory and reward processing demands. Prior studies report working memory deficits and abnormal neural signatures during reward processing in individuals with conduct disorder and/or oppositional defiant disorder (51,52). Given the high comorbidity between ADHD and conduct disorder, the overlap in modularity deficits between conduct problems and ADHD may be expected (53). However, the lack of network segregation associated with conduct problems was not as robust across tasks compared to the ADHD results, and the findings for conduct problems did not persist when accounting for medication status, parental education, and income. This suggests that deficits in whole-brain modularity may confer greater risk for ADHD symptoms than for conduct problems.
There are several issues to consider in interpreting the results of our study. First, exclusions from analyses were largely based on the degree of in-scanner motion, given the impact of motion on efficiency metrics (5). Because hyperactivity is defined by greater motion, the results of this study may underestimate the actual effects for the upper end of the hyperactivity spectrum. In addition, no gold standard exists regarding thresholding. We applied global thresholds to connectivity matrices, which inherently affect the number of edges included for analysis. Prior research has discussed issues with thresholding including that thresholded network measures may be unstable, and modularity does not necessitate thresholding under certain models (54, 55, 56, 57, 58). We tested results at multiple thresholds; this is one potential solution but may be unfavorable compared with an approach that does not require arbitrary thresholds at all (57). In light of significant computational burden, we approached our work via a thresholding technique, and future work may consider exploring alternative models. Finally, the effect sizes of these findings are relatively small in magnitude, and significance may be affected by the large sample size. However, prior studies using large samples have consistently yielded brain-behavior associations that are small but reliable (59).
These findings lay the foundation for future work on network efficiency deficits in ADHD and conduct problems by demonstrating these associations in a large sample of children using multiple metrics and tasks. Overall, this work increases our understanding of network features of psychopathology, which may help to advance the classification of mental health disorders and aid in biologically driven interventions.
Acknowledgments and Disclosures
This research was supported by the National Institute on Drug Abuse (Grant Nos. UG3DA045251 and R01MH098098 [to BBL] and Grant No. R01MH117014 [to TMM]), National Institute of Mental Health (Grant No. R00MH117274 [to ANK]), National Center for Advancing Translational Sciences (Grants Nos. UL1TR000430 and UL1TR000445 [to BBL]), the National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to ANK), the Sloan Research Fellowship (to ANK), the Lifespan Brain Institute of the University of Pennsylvania and the Children’s Hospital of Philadelphia (to TMM), and National Institutes of Mental Health training (Grant No. T32-MH18921 [to ELD]).
The Adolescent Brain Cognitive Development (ABCD) Study is supported by the National Institutes of Health and additional federal partners (Grant Nos. U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147). A full list of supporters is available at https://abcdstudy.org/federal-partners.html.
This article reflects the views of the authors and may not reflect the opinions or views of the National Institute of Health or ABCD consortium investigators.
GER contributed to formal analysis, writing of the original draft of the manuscript, reviewing and editing the manuscript, and was responsible for visualization. AJS contributed to formal analysis, reviewing and editing of the manuscript, and conceptualization. TMM was responsible for methodology and validation and contributed to reviewing and editing of the manuscript. ELD contributed to writing of the original draft of the manuscript and reviewing and editing of the manuscript. HJJ contributed to writing of the original draft of the manuscript and reviewing and editing of the manuscript. CC-I contributed to reviewing and editing of the manuscript. RMD was responsible for data curation. JRP contributed to reviewing and editing of the manuscript. MGB contributed to conceptualization and reviewing and editing of the manuscript. BBL contributed to conceptualization and reviewing and editing of the manuscript. ANK contributed to conceptualization and reviewing and editing of the manuscript and was responsible for supervision.
Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org) held in the National Institutes of Mental Health Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. A listing of participating ABCD Study sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. The ABCD data repository grows and changes over time.
The ABCD data used in this report came from Research Resource Identifiers: SCR_015769, DOI 10.15154/1520591 (data release 3.0) and National Institutes of Mental Health Data Archive study DOI 10.15154.1520146.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.07.007.
Supplementary Material
References
- 1.Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5 doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporns O., Betzel R.F. Modular brain networks. Annu Rev Psychol. 2016;67:613–640. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum G.L., Ciric R., Roalf D.R., Betzel R.F., Moore T.M., Shinohara R.T., et al. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 2017;27:1561–1572.e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satterthwaite T.D., Wolf D.H., Ruparel K., Erus G., Elliott M.A., Eickhoff S.B., et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis F.C., Knodt A.R., Sporns O., Lahey B.B., Zald D.H., Brigidi B.D., et al. Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex. 2013;23:1444–1452. doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fekete T., Beacher F.D., Cha J., Rubin D., Mujica-Parodi L.R. Small-world network properties in prefrontal cortex correlate with predictors of psychopathology risk in young children: A NIRS study. Neuroimage. 2014;85:345–353. doi: 10.1016/j.neuroimage.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths K.R., Braund T.A., Kohn M.R., Clarke S., Williams L.M., Korgaonkar M.S. Structural brain network topology underpinning ADHD and response to methylphenidate treatment. Transl Psychiatry. 2021;11:150. doi: 10.1038/s41398-021-01278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R., Lin P., Wu Y. In: Advances in Cognitive Neurodynamics (IV). Advances in Cognitive Neurodynamics. Liljenström H., editor. Springer; Dordrecht: 2015. Exploring Dynamic Temporal-Topological Structure of Brain Network Within ADHD; pp. 93–98. [Google Scholar]
- 10.Xia C.H., Ma Z., Ciric R., Gu S., Betzel R.F., Kaczkurkin A.N., et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9:3003. doi: 10.1038/s41467-018-05317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalsgaard S., Thorsteinsson E., Trabjerg B.B., Schullehner J., Plana-Ripoll O., Brikell I., et al. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry. 2020;77:155–164. doi: 10.1001/jamapsychiatry.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotov R., Krueger R.F., Watson D., Achenbach T.M., Althoff R.R., Bagby R.M., et al. The hierarchical taxonomy of psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126:454–477. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 13.Monroe S.M., Anderson S.F., Harkness K.L. Life stress and major depression: The mysteries of recurrences. Psychol Rev. 2019;126:791–816. doi: 10.1037/rev0000157. [DOI] [PubMed] [Google Scholar]
- 14.Martin J.L., Sainz-Pardo M., Furukawa T.A., Martín-Sánchez E., Seoane T., Galán C. Benzodiazepines in generalized anxiety disorder: Heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol. 2007;21:774–782. doi: 10.1177/0269881107077355. [DOI] [PubMed] [Google Scholar]
- 15.Sinyor M., Schaffer A., Levitt A. The sequenced treatment alternatives to relieve depression (STAR∗D) trial: A review. Can J Psychiatry. 2010;55:126–135. doi: 10.1177/070674371005500303. [DOI] [PubMed] [Google Scholar]
- 16.Hermens D.F., Redoblado Hodge M.A., Naismith S.L., Kaur M., Scott E., Hickie I.B. Neuropsychological clustering highlights cognitive differences in young people presenting with depressive symptoms. J Int Neuropsychol Soc. 2011;17:267–276. doi: 10.1017/S1355617710001566. [DOI] [PubMed] [Google Scholar]
- 17.Barzilay R., Calkins M.E., Moore T.M., Boyd R.C., Jones J.D., Benton T.D., et al. Neurocognitive functioning in community youth with suicidal ideation: Gender and pubertal effects. Br J Psychiatry. 2019;215:552–558. doi: 10.1192/bjp.2019.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato J.R., Takahashi D.Y., Hoexter M.Q., Massirer K.B., Fujita A. Measuring network’s entropy in ADHD: A new approach to investigate neuropsychiatric disorders. Neuroimage. 2013;77:44–51. doi: 10.1016/j.neuroimage.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Lee W., Bindman J., Ford T., Glozier N., Moran P., Stewart R., et al. Bias in psychiatric case-control studies. Literature survey. Br J Psychiatry. 2007;190:204–209. doi: 10.1192/bjp.bp.106.027250. [DOI] [PubMed] [Google Scholar]
- 20.Lahey B.B., Krueger R.F., Rathouz P.J., Waldman I.D., Zald D.H. A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull. 2017;143:142–186. doi: 10.1037/bul0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahey B.B., Moore T.M., Kaczkurkin A.N., Zald D.H. Hierarchical models of psychopathology: Empirical support, implications, and remaining issues. World Psychiatry. 2021;20:57–63. doi: 10.1002/wps.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaczkurkin A.N., Moore T.M., Sotiras A., Xia C.H., Shinohara R.T., Satterthwaite T.D. Approaches to defining common and dissociable neurobiological deficits associated with psychopathology in youth. Biol Psychiatry. 2020;88:51–62. doi: 10.1016/j.biopsych.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczkurkin A.N., Park S.S., Sotiras A., Moore T.M., Calkins M.E., Cieslak M., et al. Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. Am J Psychiatry. 2019;176:1000–1009. doi: 10.1176/appi.ajp.2019.18070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durham E.L., Jeong H.J., Moore T.M., Dupont R.M., Cardenas-Iniguez C., Cui Z., et al. Association of gray matter volumes with general and specific dimensions of psychopathology in children. Neuropsychopharmacology. 2021;46:1333–1339. doi: 10.1038/s41386-020-00952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardenas-Iniguez C., Moore T.M., Kaczkurkin A.N., Meyer F.A.C., Satterthwaite T.D., Fair D.A., et al. Direct and indirect associations of widespread individual differences in brain white matter microstructure with executive functioning and general and specific dimensions of psychopathology in children. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:362–375. doi: 10.1016/j.bpsc.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore T.M., Kaczkurkin A.N., Durham E.L., Jeong H.J., McDowell M.G., Dupont R.M., et al. Criterion validity and relationships between alternative hierarchical dimensional models of general and specific psychopathology. J Abnorm Psychol. 2020;129:677–688. doi: 10.1037/abn0000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sporns O. Graph theory methods: Applications in brain networks. Dial Clin Neurosci. 2018;20:111–121. doi: 10.31887/DCNS.2018.20.2/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achenbach T. University of Vermont, Research Center of Children, Youth & Families; Vermont: 2009. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. [Google Scholar]
- 29.Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagler D.J., Jr., Hatton S., Cornejo M.D., Makowski C., Fair D., Dick A.S., et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., et al. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen X., Tokoglu F., Papademetris X., Constable R.T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stier A.J., Cardenas-Iniguez C., Kardan O., Moore T.M., Meyer F.A.C., Rosenberg M.D., et al. A scale-free gradient of cognitive resource disruptions in childhood psychopathology. bioRxiv. 2021 doi: 10.1101/2021.08.24.457554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y.L., Tu P.C., Huang T.H., Bai Y.M., Su T.P., Chen M.H., Wu Y.T. Identifying subtypes of bipolar disorder based on clinical and neurobiological characteristics. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghanbari M., Soussia M., Jiang W., Wei D., Yap P.T., Shen D., Zhang H. Alterations of dynamic redundancy of functional brain subnetworks in Alzheimer’s disease and major depression disorders. Neuroimage Clin. 2022;33 doi: 10.1016/j.nicl.2021.102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saberi M., Khosrowabadi R., Khatibi A., Misic B., Jafari G. Requirement to change of functional brain network across the lifespan. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon Y.H., Yoo K., Nguyen H., Jeong Y., Chun M.M. Predicting multilingual effects on executive function and individual connectomes in children: An ABCD study. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2110811118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg M.D., Finn E.S., Scheinost D., Papademetris X., Shen X., Constable R.T., Chun M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19:165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagberg A., Schult D., Swart P. Los Alamos National Lab; Los Alamos, NM: 2008. Exploring network structure, dynamics, and function using NetworkX. Proceedings of the 7th Python in Science Conference (SciPy 2008) pp. 11–16. [Google Scholar]
- 40.Fornito A., Zalesky A., Bullmore E.T. Network scaling effects in graph analytic studies of human resting-state fMRI data. Front Syst Neurosci. 2010;4:22. doi: 10.3389/fnsys.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Bertolero M.A., Yeo B.T.T., Bassett D.S., D’Esposito M. A mechanistic model of connector hubs, modularity and cognition. Nat Hum Behav. 2018;2:765–777. doi: 10.1038/s41562-018-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnett A.B., Pennington B.F., Willcutt E.G., DeFries J.C., Olson R.K. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry. 2015;56:632–639. doi: 10.1111/jcpp.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertolero M.A., Yeo B.T., D’Esposito M. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A. 2015;112:E6798–E6807. doi: 10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roza S.J., Hofstra M.B., Van Der Ende J., Verhulst F.C. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: A 14-year follow-up during childhood, adolescence, and young adulthood. Am J Psychiatry. 2003;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- 47.Qian X., Castellanos F.X., Uddin L.Q., Loo B.R.Y., Liu S., Koh H.L., et al. Large-scale brain functional network topology disruptions underlie symptom heterogeneity in children with attention-deficit/hyperactivity disorder. NeuroImage Clin. 2019;21 doi: 10.1016/j.nicl.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sripada C., Kessler D., Fang Y., Welsh R.C., Prem Kumar K., Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2014;35:4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLeod K.R., Langevin L.M., Dewey D., Goodyear B.G. Atypical within- and between-hemisphere motor network functional connections in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin. 2016;12:157–164. doi: 10.1016/j.nicl.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mokobane M., Pillay B.J., Meyer A. Fine motor deficits and attention deficit hyperactivity disorder in primary school children. S Afr J Psychiatry. 2019;25:1232. doi: 10.4102/sajpsychiatry.v25i0.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoorl J., van Rijn S., de Wied M., van Goozen S., Swaab H. Boys with oppositional defiant disorder/conduct disorder show impaired adaptation during stress: An executive functioning study. Child Psychiatry Hum Dev. 2018;49:298–307. doi: 10.1007/s10578-017-0749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawes S.W., Waller R., Byrd A.L., Bjork J.M., Dick A.S., Sutherland M.T., et al. Reward processing in children with disruptive behavior disorders and callous-unemotional traits in the ABCD study. Am J Psychiatry. 2021;178:333–342. doi: 10.1176/appi.ajp.2020.19101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angold A., Costello E.J., Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 54.Rubinov M., Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56:2068–2079. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 55.Alexander-Bloch A.F., Gogtay N., Meunier D., Birn R., Clasen L., Lalonde F., et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fornito A., Zalesky A., Breakspear M. Graph analysis of the human connectome: Promise, progress, and pitfalls. Neuroimage. 2013;80:426–444. doi: 10.1016/j.neuroimage.2013.04.087. [DOI] [PubMed] [Google Scholar]
- 57.Garrison K.A., Scheinost D., Finn E.S., Shen X., Constable R.T. The (in)stability of functional brain network measures across thresholds. Neuroimage. 2015;118:651–661. doi: 10.1016/j.neuroimage.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Heuvel M.P., de Lange S.C., Zalesky A., Seguin C., Yeo B.T.T., Schmidt R. Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: Issues and recommendations. Neuroimage. 2017;152:437–449. doi: 10.1016/j.neuroimage.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Paulus M.P., Thompson W.K. The challenges and opportunities of small effects: The new normal in academic psychiatry. JAMA Psychiatry. 2019;76:353–354. doi: 10.1001/jamapsychiatry.2018.4540. [DOI] [PubMed] [Google Scholar]
- 60.Grice J.W. Computing and evaluating factor scores. Psychol Methods. 2001;6:430–450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study is from the ABCD Study and is available through the NDA (National Institute of Mental Health Data Archive) (https://nda.nih.gov/abcd). The Mplus and R code and a corresponding wiki for the analytic procedures can be found at https://github.com/VU-BRAINS-lab/Reimann_Network_Metrics.