Abstract
Introduction and importance
Primary central nervous system (CNS) lymphomas (PCNSLs) comprise a heterogeneous subset of intracranial disorders, predominantly of the intraparenchymal high-grade non-Hodgkin's lymphoma. Intracranial pseudolymphoma represents an exceedingly rare entity; as few as 3 reports in the English literature. We describe the first multiple large intracranial pseudolymphomata leading to increased intracranial pressure, visual loss, and recurrence during a short while. It also represents the first report of intracranial pseudolymphoma presented as a skull base tumor.
Case presentation
We describe a 67-year-old female suffering from left-sided loss of visual acuity, headache, nausea, vomiting, and improper balance. Axial brain computed tomography (CT) scan revealed an isodense anterior interhemispheric mass lesion with surrounding edema in both frontal lobes. T1 and T2 weighted magnetic resonance imaging (MRI) and T1 weighted with gadolinium injection revealed two extra-axial isointense dural-based mass lesions with homogenous enhancement compressing both frontal lobes. The morphologic findings favored B cell pseudolymphoma and meningeal B cell lymphoid hyperplasia. One year later, she developed headaches, disorientation, and progressive meaningless speech lasting 2 months. Subsequent MRI demonstrated the rapid growth of the lesion of the lesser sphenoid wing and recurrence of the lesion at the same site of surgery, thereby undergoing revision surgery in which both tumors were maximally resected using a pterional approach.
Clinical discussion
Intracranial pseudolymphoma remains exceedingly rare, and despite its benign cellular nature, it may proliferate and recur quickly.
Conclusion
Intracranial pseudolymphoma should always be considered a rare but potentially differential diagnosis leading to the intraventricular lesion.
Keywords: Intracranial pseudolymphoma, Meningioma, Primary CNS lymphomas
Highlights
-
•
Intracranial pseudolymphoma remains an exceedingly rare lesion with benign cellular nature, which may proliferate and recur quickly.
-
•
The lack of clonal lymphoid cells represents the pathognomonic features of pseudolymphoma
-
•
Watchful histopathological diagnosis and interpretation of flow cytometry may establish the diagnosis.
-
•
The first case of intracranial pseudolymphoma presented as a skull base tumor, and the first case of intracranial pseudolymphoma that promptly recurred quickly is described.
1. Introduction
Central nervous system (CNS) involvement in lymphoma is seen in primary and secondary forms. The secondary type occurs in the context of systemic lymphoma. Primary CNS lymphomas (PCNSLs) account for 2.4–3 % of all brain tumors [1] and predominantly manifest as intraparenchymal non-Hodgkin's lymphomas. PCNSLs were found to be located in the frontal lobe, temporal lobe, parietal lobe, occipital lobe basal ganglia, and periventricular brain parenchyma corpus callosum in 15 %, 8 %, 7 %, 3 %, 10 %, and 5 % of known cases, respectively [[2], [3], [4]]. Low-grade CNS lymphomas are much less common and can present as intraparenchymal lesions [5]. Low-grade B-cell and T-cell lymphomas, Burkitt lymphoma, high-grade T-cell, and NK/T-cell lymphomas are also reported primarily in the CNS [6,7]. Burkitt lymphoma represents a highly aggressive B-cell non-Hodgkin lymphoma highlighted by remarked tumor proliferation following the MYC oncogene translocation. All variants are shares the quickly disseminating tumor masses to the extranodal area, including CNS and the bone marrow [8].
Intracranial pseudolymphoma has been rarely reported, as only 3 cases have been previously reported in the English literature. Here, the authors describe a case of intracranial pseudolymphoma that mimics a large and relapsing dural-based anterior skull base tumor and reviews other reported cases. We believe that the current case is the first case of intracranial pseudolymphoma presented as a skull base tumor and also is the first case of intracranial pseudolymphoma that rapidly overgrew and recurred in a short while. This study has been provided in line with the SCARE 2020 guidelines to ensure quality reporting [9].
2. Case presentation
A 67-year-old female presented to our hospital in October 2019, suffering from visual loss in her left eye, headache lasting for 4 months, nausea, vomiting, and improper balance 12 months before admission. Except for hypothyroidism, she had no previous medical comorbidities. Systemic examination was unremarkable. Neurologic examination revealed no light perception in the left eye and normal visual acuity (at least 6-meter finger count) for the right side. On the other hand, she suffered left-sided 4th cranial nerve paresis resulting in concomitantly limited movement of the left eye to the inferior and medial aspects.
Computed Tomography (CT) scan revealed a large isodense frontal mass lesion with peripheral edema (Fig. 1A). Brain Magnetic resonance imaging (MRI) showed 2 extra-axial isointense masses in both T1 and T2 weighted images (Fig. 1B and C) with uniformly enhancing 2 masses during Gadolinium injection at the right frontal lobe and Planum part and tuberculum sella of sphenoid bone (Fig. 1D). Our probable preoperative diagnosis was a meningioma, so the patient underwent surgery regarding neurological findings via the right lateral subfrontal approach. The patient was scheduled to be operated by the senior author using a supine position. The head was fixed in 3 pins Mayfield holder and was contralaterally rotated 30 degrees. Following a pterional incision, a right frontotemporal craniotomy was done, and the patient's dura was subsequently opened. After retracting the right frontal lobe, two separated dural-based grayish sustainable masses were exposed. The origin of the first tumor was the dura mater of the anterior part of the frontal lobe, which had invaded the cortex, and the second one had originated from the diameter of the anterior cranial fossa from cribriform plate to diaphragm sella and had compressed both optic nerves and chiasma and right olfactory nerve. Tumors were dissected from optic nerves and were resected as much as possible. The dural base of the tumors was also coagulated. Post operation course remained uneventful, and the patient was discharged on postoperative day 5 without any newly developed neurological deficits.
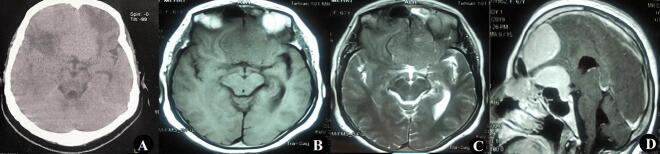
Fig. 1.
A; Axial Brain CT scan showed an isodense anterior interhemispheric mass lesion with surrounding edema in both frontal lobes. T1 (B) and T2 (C) weighted MRI and T1 weighted with gadolinium injection (D) demonstrated two extra-axial isointense dural-based mass lesions with the homogenous enhancement that compressed both frontal lobes.
Histopathologic examination showed dense lymphocytic infiltration of severely thickened meningeal nodules protruding to adjacent brain tissue with a pushing border (Fig. 2A–F). The infiltration also produced lymphoid follicles with prominent germinal centers containing scattered tingible body macrophages.
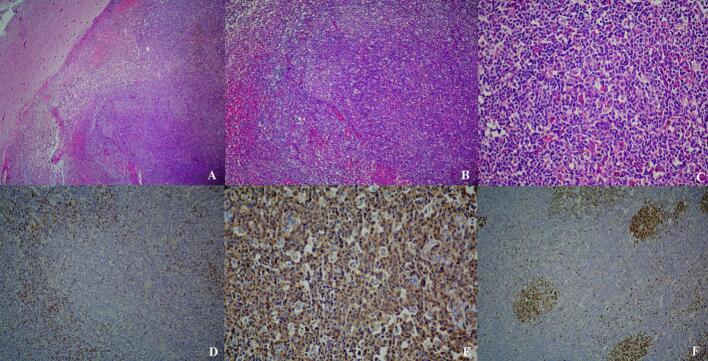
Fig. 2.
Histopathologic features, A) Dense meningeal lymphoid hyperplasia with pushing border on adjacent brain tissue, B) Lymphoid follicles with prominent germinal centers, C) Tingible body macrophages are present in germinal centers, D) CD3+ T cells mostly in interfollicular regions, E) CD20+ B-cells stain germinal centers, F) KI67 with high proliferative index in germinal centers.
Areas of vascular proliferation and fibrosis were noticed. Immunohistochemistry revealed CD20-positive B cells in lymphoid follicles and some CD3-positive T cells in interfollicular lymphoid cells. Ki-67 was mainly positive in germinal centers and 3 % in other areas. S100 and CD23 highlight follicular dendritic cells. Bcl2 was positive in some interfollicular lymphoid cells along with a few germinal centers lymphoid cells. CD138 was positive in a few scattered plasma cells. CD5 was positive in a few interfollicular T lymphocytes, and Bcl6 and CD10 were negative except for the few CD10-positive cells in germinal centers. GFAP and CK were negative. EMA was focally positive. These morphologic findings were compatible with B cell pseudolymphoma or meningeal B cell lymphoid hyperplasia.
After confirmation of histopathologic diagnosis, further evaluation, including whole-body CT scan, bone marrow biopsy, and flow cytometry, were performed, and there was no sign of malignancy in any of them. During the follow-up period, the patient's visual acuity in the left eye improved slightly, and she could detect light. Follow-up MRI 2 months after surgery revealed a new small lesion originating from a left lesser sphenoid wing (Fig. 3), requiring follow-up imaging and conservative management. About 1 year later, in October 2020, the patient presented again with headache, disorientation, and progressive meaningless speech during the last 2 months. Papillary edema on the right side and bilateral optic atrophy was detected on fundoscopic examination.
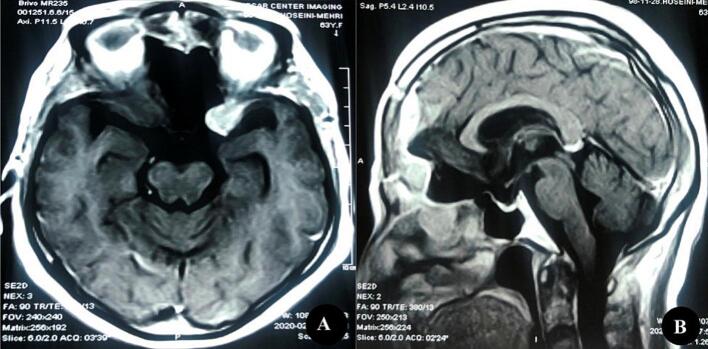
Fig. 3.
T1 Weighted MRI after gadolinium injection in axial (A) and Sagittal (B) planes revealed a new small lesion originating from the left lesser sphenoid wing 2 months after the operation.
MRI showed rapid growth of the lesion of the lesser sphenoid wing and recurrence of the lesion at the previous surgery site compared to the last imaging (Fig. 4B–D). After consulting with the hematology-oncology team, the patient was nominated for reoperation. She underwent surgery, and both tumors were removed using a pterional approach as much as possible. The tumor originated in the dura mater of the clinoid region and did not invade the vascular structure and bones. Histopathologic evaluation revealed the same microscopic features mentioned before (meningeal lymphoid follicle formation with prominent germinal centers (pseudolymphoma), compatible with pseudolymphoma recurrence). Early post-op MRI demonstrated gross total resection of the tumor (Fig. 3E, F).
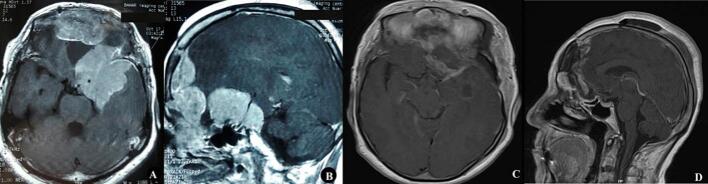
Fig. 4.
A and B; rapid growth and recurrence of tumor from the left lesser sphenoid wing and previous tumor sites are noticeable on T1 Weighted MRI after gadolinium injection one-year post-operation. C and D; early post-operation T1 with Gad MRI after the second surgery.
The patient was discharged on post-operation days 5 without any new morbidity nor evidence of regrowth or recurrence seen during the 6 months of follow-up due to the absence of any sign indicating malignancy in systemic re-examinations. No evidence of tumor recurrence nor neurological-related manifestations were noted during her 2 years of close follow-up.
3. Discussion
Non-Hodgkin's lymphoma is the most common type of PCNSL [6]. Although lymphoma occurs due to neoplastic proliferation, non-lymphomatous lymphoid disorders represent benign reactive polyclonal lymphoid proliferation involving a heterogeneous group of T, B, or mixed cells [5]. In pseudolymphomas, reactive lymphoid hyperplasia with mature lymphocytic infiltrates and germinal centers can mimic lymphoma [5,6]. Extranodal Pseudolymphomas were reported within sites such as the skin, lung, orbit, thyroid, pancreas, liver, and gastrointestinal tract [[10], [11], [12]].
We describe the first multiple large intracranial pseudolymphoma leading to increased intracranial pressure, visual loss, and recurrence during the first-year post-operation follow-up period. Numerous causes or mechanisms, such as foreign antigens, drugs [12], viruses (such as varicella-zoster infection, human immunodeficiency virus), and bacteria (Helicobacter pylori, Borrelia burgdorferi) have been variably implicated [11]. In cases where no causative factor is detected, continuous clinical evaluation should be considered for the patients [13].
Cerebrospinal fluid and radiography evaluations remained undiagnostic, and watchful histopathological diagnosis and interpretation of flow cytometry may establish the diagnosis. The lack of clonal lymphoid cells represents the pathognomonic features of pseudolymphoma [14].
Intracranial pseudolymphoma remains exceedingly rare, and we found only three reported cases in the literature listed in Table.1 [5,15,16]. Among the cases mentioned above, which were all middle-aged women, 2 lesions were dural-based and in 1 patient, located in the choroid plexus of the lateral ventricle. Headache [15,16], subcutaneous mass [15] mass, and grand mal seizures [5] have been the presentation in previous patients. A history of common variable immunodeficiency and Burkitt's lymphoma was present in only 1 patient [5], but others had no last underlying disease. All patients, like ours, underwent surgery and follow-up without adjuvant therapy.
Table 1-.
Summary of reported cases of intracranial pseudolymphoma.
| No | Author/year | Age(year)/gender(female/male) | Location | Presentation | Past medical history | Therapeutic choice | Follow up |
|---|---|---|---|---|---|---|---|
| 1 | Donnet [15], 2000 | 35/F | A left frontal dural-based lesion with extension to subcutaneous space | Headache and subcutaneous mass | None | Surgical resection | No recurrence was seen after 1-year follow-up without adjuvant therapy |
| 2 | Strowd [5], 2014 | 56/F | Two small dural-based lesions within the right temporal region | New-onset grand mal seizures | None | Surgical resection | The patient was seizure free during the 4-month follow-up without any medication |
| 3 | Rajah J. [16], 2020 | 44/F | Choroid plexus of the right lateral ventricle. | Headache | Common Variable Immunodeficiency (CVID) and Burkitt's lymphoma | Stereotactic biopsy | Follow-up without adjuvant therapy |
| 4 | Current case | 67/F | Multiple Large Anterior Skull Base | Headache and Visual Loss | None | Surgical resection | Recurrence and rapid growth of Lesions during 1-year follow-up without adjuvant therapy |
The dural tail sign, described as the adjacent enhancement of peripherally embedded extra-axial lesion, may raise concerns regarding meningioma [17].
Because of the well-circumscribed extra-axial mass with a dural tail and similar intensity on MRI, intracranial pseudolymphoma is radiographically indistinguishable from meningioma [5]. Pseudolymphoma, like most meningiomas, appears as well-circumscribed extra-axial masses isointense on T1-weighted imaging, with a variable appearance on T2-weighted imaging, and with marked homogenous enhancement following administration of gadolinium [5,18]. These characteristics are similar to primary dural lymphoma [19], and biopsy remains the only definitive means of differentiation [5].
It may broadly result from a spectrum of disorders hypervascularity, hyperemia, increased permeability, meningeal irritation, vascular congestion, and reactive/inflammatory lymphoid changes [5]. Fluid-attenuated inversion recovery imaging or surrounding edema visible on T2-weighted in approximately 67 % of symptomatic meningiomas could further cloud the issue. However, the abovementioned characteristics have also been remarked on in primary dural lymphomas [20,21].
4. Conclusions
Intracranial pseudolymphoma remains exceedingly rare and should always be considered a rare cause for a skull-based tumor in the differential diagnosis. Despite its benign cellular nature, this tumor can proliferate and recur quickly.
Declaration of competing interest
The authors have no conflicts of interest to declare. Also, there is no financial interest to declare.
Acknowledgments
Acknowledgments
We would like to extend our sincere gratitude to Prof. Dr. Farahnaz Bidari Zerehpoosh for providing the pathological insights for the current report.
Consent for publication
Written informed consent for the current work of clinical details and images was achieved from the patient.
Ethical approval
Ethical approval is not required since our patient's treatment was based on approved options, and it was not found to be controversial, according to the Ethics Committee of our institution.
Funding
Not applicable.
Author contribution
All authors contributed to drafting and final proofreading.
Guarantor
NA.
Data availability
NA.
References
- 1.Deckert M., Engert A., Brück W., Ferreri A.J., Finke J., Illerhaus G., et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011;25(12):1797–1807. doi: 10.1038/leu.2011.169. [DOI] [PubMed] [Google Scholar]
- 2.Deckert M., Engert A., Brück W., Ferreri A., Finke J., Illerhaus G., et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011;25(12):1797–1807. doi: 10.1038/leu.2011.169. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel U., Hochberg F.H. Neuro-Oncology of CNS Tumors. 2006. Primary CNS lymphoma; pp. 291–302. [Google Scholar]
- 4.Villano J., Koshy M., Shaikh H., Dolecek T., McCarthy B. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer. 2011;105(9):1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strowd R.E., Powers A.K., Beaty M.W., Ellis T.L. Intracranial pseudolymphoma presenting with grand mal seizures. J. Clin. Neurosci. 2014;21(5):874–876. doi: 10.1016/j.jocn.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Schlegel U. Primary CNS lymphoma. Ther. Adv. Neurol. Disord. 2009;2(2):93–104. doi: 10.1177/1756285608101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim T., Kim S.J., Kim K., Lee J.I., Lim D.H., Lee D.J., et al. Primary CNS lymphoma other than DLBCL: a descriptive analysis of clinical features and treatment outcomes. Ann. Hematol. 2011;90(12):1391–1398. doi: 10.1007/s00277-011-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum K.A., Lozanski G., Byrd J.C. Adult Burkitt leukemia and lymphoma. Blood. 2004;104(10):3009–3020. doi: 10.1182/blood-2004-02-0405. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Mitteldorf C., Kempf W. Cutaneous pseudolymphoma-a review on the spectrum and a proposal for a new classification. J. Cutan. Pathol. 2020;47(1):76–97. doi: 10.1111/cup.13532. [DOI] [PubMed] [Google Scholar]
- 11.Ploysangam T., Breneman D.L., Mutasim D.F. Cutaneous pseudolymphomas. J. Am. Acad. Dermatol. 1998;38(6 Pt 1):877–895. doi: 10.1016/s0190-9622(98)70154-9. quiz 96–7. [DOI] [PubMed] [Google Scholar]
- 12.Magro C.M., Daniels B.H., Crowson A.N. Drug induced pseudolymphoma. Semin. Diagn. Pathol. 2018;35(4):247–259. doi: 10.1053/j.semdp.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Donnet A., Horschowski N., Dufour H., Figarella-Branger D., Bryon P., Berger F., et al. Intracranial pseudolymphoma. J. Neuro-Oncol. 2000;47:175–179. doi: 10.1023/a:1006414717891. [DOI] [PubMed] [Google Scholar]
- 14.Rajah J., Cain S.A., Mews P. Intraventricular pseudolymphoma a case review. J. Clin. Neurosci. 2020;78:425–427. doi: 10.1016/j.jocn.2020.04.082. [DOI] [PubMed] [Google Scholar]
- 15.Donnet A., Horschowski N., Dufour H., Figarella-Branger D., Bryon P.A., Berger F., et al. Intracranial pseudolymphoma. J. Neuro-Oncol. 2000;47(2):175–179. doi: 10.1023/a:1006414717891. [DOI] [PubMed] [Google Scholar]
- 16.Rajah J., Cain S.A., Mews P. Intraventricular pseudolymphoma a case review. J. Clin. Neurosci. 2020;78:425–427. doi: 10.1016/j.jocn.2020.04.082. [DOI] [PubMed] [Google Scholar]
- 17.Rokni-Yazdi H., Sotoudeh H. Prevalence of “dural tail sign” in patients with different intracranial pathologies. Eur. J. Radiol. 2006;60(1):42–45. doi: 10.1016/j.ejrad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Engelhard H.H. Progress in the diagnosis and treatment of patients with meningiomas. Part I: diagnostic imaging, preoperative embolization. Surg. Neurol. 2001;55(2):89–101. doi: 10.1016/s0090-3019(01)00349-4. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M.D., Powell S.Z., Boyer P.J., Weil R.J., Moots P.L. Dural lesions mimicking meningiomas. Hum. Pathol. 2002;33(12):1211–1226. doi: 10.1053/hupa.2002.129200. [DOI] [PubMed] [Google Scholar]
- 20.Lobato R., Alday R., Gomez P., Rivas J., Dominguez J., Cabrera A., et al. Brain oedema in patients with intracranial meningioma: correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochir. 1996;138:485–494. doi: 10.1007/BF01411166. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M.D., Powell S.Z., Boyer P.J., Weil R.J., Moots P.L. Dural lesions mimicking meningiomas. Hum. Pathol. 2002;33(12):1211–1226. doi: 10.1053/hupa.2002.129200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.