Introduction
Dyshidrotic eczema (DE), also known as dyshidrosis and acute palmoplantar eczema, is a recurrent vesicular eruption of the palms and soles that is typically associated with extreme pruritus.1 Although its etiology is unknown, DE is likely multifactorial and has an unclear association with atopic diseases.2 Treatment has classically involved topical medications, such as corticosteroids and calcineurin inhibitors, followed by immunomodulating agents for refractory cases, including dupilumab, cyclosporine, mycophenolate mofetil, azathioprine, or methotrexate.1 Disease-modifying antirheumatic drugs that inhibit Janus kinases (JAKs) have been employed to treat inflammatory conditions, such as atopic dermatitis and rheumatoid arthritis (RA); however, the use of such medications for the treatment of DE has not been reported. We present a case of a patient achieving remission of his DE on initiation of upadacitinib.
Case report
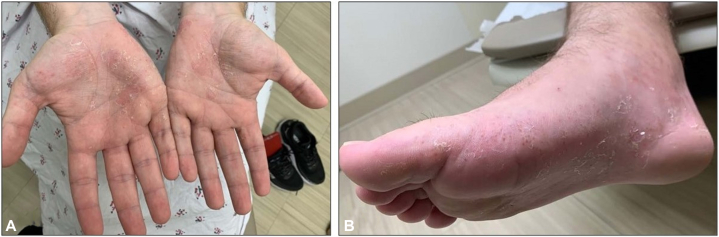
A 22-year-old man with a history of Crohn’s disease and RA presented to dermatology clinic with a 2-month history of a pruritic and painful rash mainly on his palms and soles that emerged shortly after completing a course of prednisone for a flare of RA. Over the previous 2 years, he had reported similar pruritic but milder rashes in the same distribution that were self-limited and resolved without any intervention. Physical examination was notable for deep-seated pustulovesicles on the bilateral palmar and plantar surfaces of the extremities that evolved into areas of desquamation with collarettes of scale. The rash also spread beyond the palmar and plantar surfaces to involve the medial and lateral surfaces of the hands and feet, as in a moccasin distribution (Fig 1). He denied a history of seasonal allergies or asthma. Topical corticosteroids had been yet ineffective; the patient was a longstanding user of methotrexate and had initiated ustekinumab 5 months prior. In addition to Crohn’s disease and RA, the patient’s medical history was significant for Gilles de la Tourette syndrome, for which topiramate was a longstanding therapy. Longstanding supplements included ferrous sulfate and folic acid.
Fig 1.
Fitzpatrick skin type II afflicted with probable dyshidrotic eczema. A, Pustules and vesicles with collarettes of scale on the palmar surfaces of the hands bilaterally. B, Few vesicles with collarettes of scale within a background of erythema extending from the plantar surface to the medial aspect of the foot.
The differential diagnosis included DE, pustular psoriasis, and tinea pedis et manuum. A potassium hydroxide scraping was negative for fungal elements, and the patient was started on topical 0.05% betamethasone dipropionate ointment twice daily to affected areas. Before returning to dermatology clinic, the patient’s rheumatologist transitioned his ustekinumab to upadacitinib, which was administered daily as 15 mg extended-release oral tablets. Due to improvement in his rash noted specifically after such change in medication, the patient was able to decrease his frequency of topical steroid use to once daily. After 3 months of taking upadacitinib, the patient reported a noticeable decrease in pain and pruritus. Physical examination revealed the absence of pustules and only sparse vesicles.
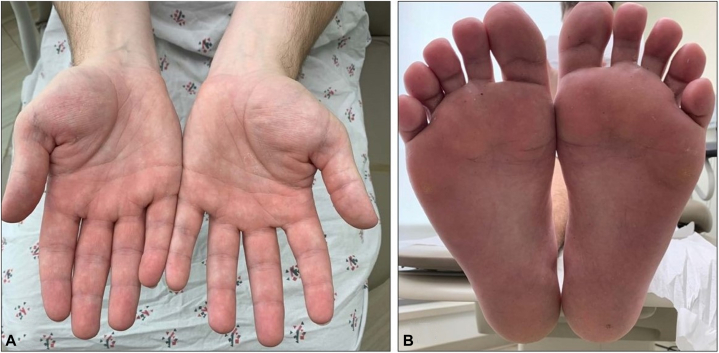
Six months later, the patient noted virtually complete resolution of his rash and was otherwise asymptomatic (Fig 2). The patient reported compliance with daily upadacitinib and only infrequently used the betamethasone ointment. Of note, rheumatology also discontinued methotrexate a week prior because of symptomatic control of his Crohn’s disease and RA. The patient denied any flares of his RA over the last 12 months and denied any adverse effects after starting upadacitinib.
Fig 2.
Excellent clinical response of dyshidrotic eczema 9 months after initiation of upadacitinib. A, Palmar aspects of hands with scant erythema and minimal scale but without pustules or vesicles. B, Plantar aspects of feet without any pustules, vesicles, erythema, or scale.
Discussion
Upadacitinib is a JAK inhibitor, or jakinib, that disrupts the JAK/signal transducer and activator of transcription signaling pathway. This highly conserved pathway is responsible for transducing proinflammatory, cytokine-mediated signals intracellularly, upregulating immune cell activity. As a second-generation jakinib, upadacitinib is selective for JAK1, interfering with the proinflammatory activity of a number of interleukins (ILs),3 including IL-4, IL-5, IL-13, IL-22, and IL-31, with relatively modest adverse effects.4 By comparison, the monoclonal antibody ustekinumab only has antagonist activity against IL-12 and IL-23, whose downstream effects are mediated by JAK2 and tyrosine kinase 2.5
To date, the US Food and Drug Administration has approved upadacitinib for the treatment of atopic dermatitis among other, rheumatologic indications.3 Although recent case reports have pointed toward the efficacy of upadacitinib in the treatment of other inflammatory dermatologic conditions, the authors, to their knowledge, believe that this represents the first reported case of upadacitinib’s clinical efficacy and tolerance in the treatment of DE.
Although the traditional immunosuppressive treatment for DE underscores its autoimmune etiology, its pathophysiology is not understood. Intraepidermal spongiosis in the setting of hyperkeratosis is a conserved histopathologic finding1 but the cellular and subcellular mechanisms driving these distortions are yet unknown. Thus, with a poorly understood pathogenesis and a reputation for being resistant to conventional treatment, DE represents an area for much further investigation. In the meantime, however, the efficacy of upadacitinib presented in this case represents an additional treatment option in the armamentarium for patients with severe, refractory DE or concomitant autoimmune inflammatory conditions.
Conflicts of interest
None disclosed.
Acknowledgments
We would like to thank the patient for his contributions to the advancement of medicine.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Lofgren S.M., Warshaw E.M. Dyshidrosis: epidemiology, clinical characteristics, and therapy. Dermatitis. 2006;17(4):165–181. doi: 10.2310/6620.2006.05021. [DOI] [PubMed] [Google Scholar]
- 2.Bryld L.E., Agner T., Menne T. Relation between vesicular eruptions on the hands and tinea pedis, atopic dermatitis and nickel allergy. Acta Derm Venereol. 2003;83(3):186–188. doi: 10.1080/00015550310007184. [DOI] [PubMed] [Google Scholar]
- 3.Samuel C., Cornman H., Kambala A., Kwatra S.G. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb) 2023;13(3):729–749. doi: 10.1007/s13555-023-00892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikhaylov D., Ungar B., Renert-Yuval Y., Guttman-Yassky E. Oral Janus kinase inhibitors for atopic dermatitis. Ann Allergy Asthma Immunol. 2023;130(5):577–592. doi: 10.1016/j.anai.2023.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Ye L., Wu Z., Li C., Zhao X., Wan M., Wang L. Off-label uses of ustekinumab. Dermatol Ther. 2022;35(12) doi: 10.1111/dth.15910. [DOI] [PubMed] [Google Scholar]