Abstract
Thoracic outlet syndrome (TOS) is caused by entrapment of the neurovascular bundle in the interscalene, costoclavicular, or subpectoral minor space. Compression in the interscalene or costoclavicular space with the first rib and scalene muscle leads to vascular and neurogenic TOS, whereas compression in the subpectoral minor space leads to pectoralis minor syndrome. Various surgical approaches exist for the treatment of TOS. The introduction and development of surgical approaches have minimized surgical invasiveness and complications. The reported approaches include transaxillary, supraclavicular, infraclavicular, posterior, combined transaxillary and supraclavicular, combined supraclavicular and infraclavicular (paraclavicular), endoscopic-assisted transaxillary, and video-assisted thoracoscopic approaches. In this review, we summarize the reported surgical approaches for TOS treatment, in terms of the history of the approach, surgical procedure, advantages and disadvantages, clinical outcomes, and complications. An adequate excision of compression structures, including the first rib and scalene muscles, provides satisfactory outcomes regardless of the approach selected, whereas an inadequate release of compression structures leads to failed or recurrent outcomes. Reducing the risk of complications is the most important aspect of TOS management. Surgery should be performed safely, with sufficient resection of compression structures. Additionally, the approach should be selected based on the surgeon’s skill, surgeon’s preferences, surgical invasiveness, cosmetic appearance, and the presence of special equipment, as well as other advantages and disadvantages of each approach.
Key words: Approach, Incision, Surgical approach, Thoracic outlet compression syndrome, Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is caused by entrapment of the neurovascular bundle in the thoracic outlet. Anatomically, the thoracic outlet is divided into 3 sections: the interscalene, costoclavicular, and subpectoral minor spaces. Compression of the interscalene or costoclavicular space by the anterior and middle scalene muscles or the first rib leads to arterial TOS (ATOS) or neurogenic TOS (NTOS). In patients with venous TOS (VTOS), compression occurs at the anterior costoclavicular space, which includes the anterior scalene muscle, first rib, costocoracoid ligament, and subclavius tendon. Infraclavicular compression in the subpectoral minor space leads to pectoralis minor syndrome. Various surgical approaches have been reported for the treatment of TOS (Fig 1).1, 2, 3 The transaxillary and supraclavicular approaches are commonly used for the resection of the first rib and scalene muscles.4 Additionally, the introduction of advanced technology with endoscopic- or robotic-assisted first-rib resection, or arthroscopic pectoralis minor release, addresses the limitations of traditional approaches of TOS treatment.5, 6, 7, 8, 9, 10 No firm evidence exists for justifying which approach is better, despite debates on the subject. In this review, we summarize and update information on the reported surgical approaches for TOS treatment, including the history of the approach, surgical procedure, advantages and disadvantages, clinical outcomes, and complications (Table 1, Table 2, Table 3).4, 6, 8, 11, 12, 13, 14
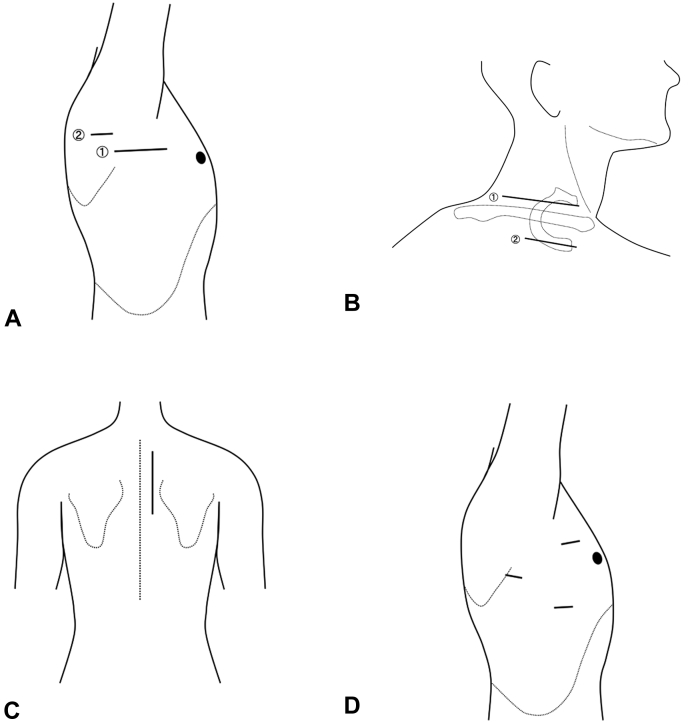
Figure 1.
Incisions in each approach of thoracic outlet syndrome treatment. A Transaxillary approach (①) and endoscopic-assisted transaxillary approach (①+②, ②: endoscopic portal). B Supraclavicular approach (①), infraclavicular approach (②), and combined supra- and infraclavicular approach (①+②). C Posterior approach. D Video-assisted thoracoscopic approach with a few portals.
Table 1.
Advantages and Disadvantages of Each Surgical Approach
| Approach | Advantages | Disadvantages |
|---|---|---|
| Transaxillary | Good cosmetic appearance Easy access to the first rib without muscle dissection |
Manipulation in a deep and narrow surgical field Difficulty of vascular reconstruction |
| Supraclavicular | Easy scalenectomy Manipulation in a shallow field |
Difficulty in approaching anterior structures Poor cosmetic appearance |
| Infraclavicular | Easy access to anterior structures Manipulation in a shallow field |
Difficulty in approaching the posterior aspect of the thoracic outlet |
| Posterior | Might be indicated for reoperation or radiation-induced tissue fibrosis of the anterior chest wall | Difficulty in approaching anterior structures and vascular reconstruction Rarely performed for the initial operation |
| Combined transaxillary and supraclavicular | Secure resection of the scalene muscles and first rib | Prolonged operative time with repositioning Greater invasiveness with 2 incisions |
| Combined supraclavicular and infraclavicular (paraclavicular) | Secure resection of the scalene muscles and first rib Well indicated for vascular reconstruction |
Greater invasiveness with 2 incisions |
| Endoscopic-assisted transaxillary | Good cosmetic appearance Easy access to the first rib without muscle dissection Clear visualization in a deeper and narrower field |
Technically demanding Requires special equipment |
| Video-assisted thoracoscopic (with or without robot) | Requires only a few small portals Clear visualization of the intrathoracic operative field |
Technically demanding Necessity of a pleural drain due to opened pleura Requires special equipment |
Table 2.
Clinical Outcomes of Each Surgical Approach
| Approach | Clinical Outcomes |
|---|---|
| Transaxillary (n = 2,326; systematic review)4 | Success rate: 76% Complete relief rate: 53% |
| Supraclavicular scalenectomy with first-rib excision (n = 683; systematic review)4 | Success rate: 77%, Complete relief rate: 57% |
| Supraclavicular scalenectomy without first-rib excision (n = 674; systematic review)4 | Success rate: 85% Complete relief rate: 61% |
| Infraclavicular (n = 55; case-control study)11 | Patency rate of subclavian vein: 95% |
| Posterior (n = 2,305; case series)12 | Good, 75%; fair, 16%; poor, 9% |
| Combined transaxillary and supraclavicular (n = 94; case-control study)13 | Excellent, 95%: good, 4%; poor, 1% |
| Combined supraclavicular and infraclavicular (n = 100; case series)14 | Derkash score: excellent, 41%; good, 35%; fair, 19%; poor, 5% |
| Endoscopic-assisted transaxillary (n = 131; case series)6 | Roos and Derkash score: excellent, 40%; good, 41%; fair, 14%; poor, 6% |
| Video-assisted thoracoscopic (n = 30; case-control study)8 | Excellent, 37%; good, 30%; partial, 20%; no significant improvement, 13% |
Table 3.
Complication Rate of Each Surgical Approach
| Approach | Complication Rate |
|---|---|
| Transaxillary (n = 2,326; systematic review)4 | Pleural opened or pneumothorax: 14% Neurological injury: 5% Vascular injury: 0.1% (3 veins) Death: 0.04% (1 case) |
| Supraclavicular scalenectomy with first rib excision (n = 683; systematic review)4 | Pleural opened or pneumothorax: 19% Neurological injury: 3% Vascular injury: 0.3% (3 veins, 1 artery) Death: 0.07% (1 case) |
| Supraclavicular scalenectomy without first rib excision (n = 674; systematic review)4 | Pleural opened or pneumothorax: 5% Neurological injury: 7% Vascular injury: 0.2% (1 vein) Death: 0% |
| Infraclavicular (n = 55; case-control study)11 | Pneumothorax: 7% Neurological injury: 0% |
| Posterior (n = 2,305; case series)12 | Pleural opened: most cases (unknown rate) Neurological injury: unknown rate |
| Combined transaxillary and supraclavicular (n = 94; case-control study)13 | Pneumothorax: 4% Neurological (phrenic nerve) injury: 1% |
| Combined supraclavicular and infraclavicular (n = 100; case series)14 | Pneumothorax: 0% Neurological injury: 6% Pleural effusion and hemothorax: 6% |
| Endoscopic-assisted transaxillary (n = 131; case series)6 | Pneumothorax: 3% Neurological (long thoracic and axially nerve) injury: 2% |
| Video-assisted thoracoscopic (n = 30; case-control study)8 | Pleural opened:100% Neurological (brachial plexus) injury: 3% |
Principle of the Surgical Treatment of TOS
The main principle of TOS surgical treatment is to relieve compression of the neurovascular structures in the thoracic outlet. The treatment procedure for NTOS includes the excision of anomalous anatomical structures, excision of the anterior and middle scalene muscles, neurolysis of the brachial plexus, and resection of the first rib if it is compressing the brachial plexus or exerting a traction force on the plexus.2 In addition to the excision of anomalous anatomical structures, the first rib, and scalene muscles, vascular reconstruction is sometimes necessary for the treatment of ATOS with an aneurysm or mural thrombus. Venous TOS treatment requires resection of the subclavian muscle, anterior aspect of the first rib, anterior scalene muscle, and costoclavicular ligament, as well as anticoagulation, if necessary. Pectoralis minor muscle or coracocostal ligament release is performed for pectoralis minor syndrome. Scalenectomy directly decompresses the nerves or prevents elevation of the first rib up to the clavicle. A previous review reported that scalenectomy alone, without first-rib resection, tends to fail or results in a greater recurrence rate compared with scalenectomy with first-rib resection (57% vs 99% patient satisfaction, respectively).2,15 However, another review reported that isolated supraclavicular scalenectomy showed better clinical outcomes and fewer complications than scalenectomy with first-rib resection through the transaxillary or supraclavicular approach (success rates, 85% vs 76% vs 77%, respectively; complication rates, 13% vs 23% vs 26%, respectively).4 Although the necessity of first-rib resection is controversial, resections of both the scalene muscle and first rib are widely performed.2, 3, 4
Transaxillary Approach
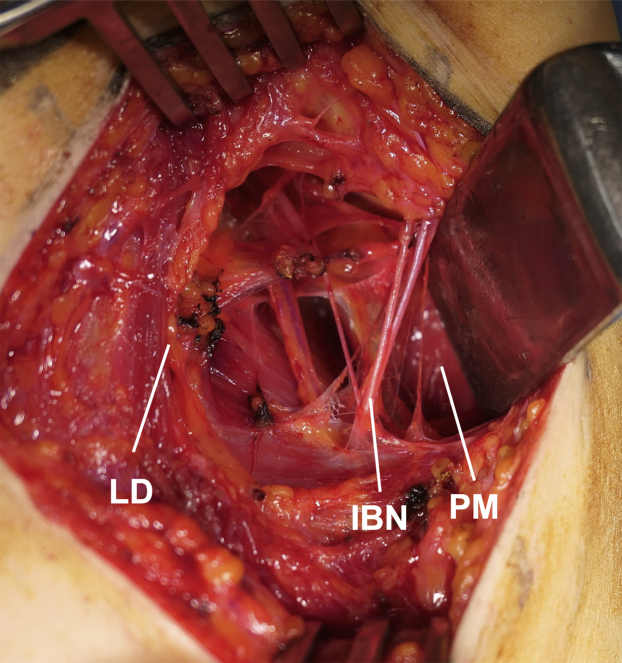
Roos16 introduced the transaxillary approach for first-rib resection, which is the most widely used approach, in 1966. The incision for this approach is made between the pectoralis major and latissimus dorsi muscles, and thus a rapid and easy exposure of the first rib and anterior scalene muscle is possible without any muscle dissection (Figs 1A and 2). The anterior and middle scalene muscles are dissected free of their origins at the first rib using electrocautery. The first rib is elevated from the pleura and resected with a rongeur, microsaw, or rib cutter to avoid damaging the pleura. The use of lighted retractors and a headlight are helpful to obtain good visualization. This approach provides a cosmetically desirable incision. A systematic review reported that the pooled success rate of transaxillary first-rib resection was 76%, and the probability of greater than 70% improvement was 90%; moreover, complete relief was observed in 53% of 2,326 cases.4 The most difficult aspect of this approach is performing manipulations in a deep and narrow surgical field, especially when excising the posterior aspect of the first rib. The reported complication rate of the transaxillary approach is approximately 23%, and the incidence of pneumothorax is approximately 14%.4 Surgery is performed with the patient in the lateral position, and the arm is stabilized using a specialized arm holder or via manual traction by an assistant. Furthermore, the neurovascular bundle is retracted to obtain good visualization. Neurological complications, including nerve traction injury, are common with the transaxillary approach, and occur in approximately 5% of patients.2,4 The reported rate of permanent brachial plexus injury is 0.1%.4 Although most of the symptoms disappear within a short period, the occurrence of dysesthesia due to intercostobrachial nerve injury is a disadvantage of the transaxillary approach.2 Another disadvantage is difficulty in the management of the subclavian artery and vein. When vascular reconstruction is required in the presence of an arterial aneurysm or mural thrombus, other approaches are used.
Figure 2.
Transaxillary approach. The incision for this approach is made between the PM and LD muscles. IBN, intercostobrachial nerve; LD, latissimus dorsi; PM, pectoralis major.
Supraclavicular Approach
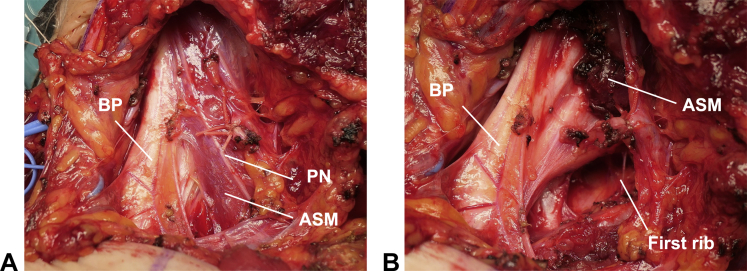
In 1927, Adson and Coffey17 published a report on scalenotomy without first-rib resection through the supraclavicular approach (Fig 1B). In the 1960s, scalenectomy with first-rib resection through the supraclavicular approach was introduced.1 A transverse incision is made 1 to 2 cm above the clavicle, and the platysma is dissected. The lateral head of the sternocleidomastoid muscle and the scalene fat pad are carefully dissected to preserve the supraclavicular nerve; thereafter, the scalene muscles and brachial plexus are explored. Anterior and middle scalenectomies are performed using electrocautery, followed by first-rib resection. The supraclavicular approach facilitates neurolysis and the resection of the scalene muscles, abnormal bands, first rib, and cervical rib, if present (Fig 3). Compared to the transaxillary approach, the advantages of the supraclavicular approach are good visualization and direct manipulation of the thoracic outlet structures in a shallow field, which increases the safety and feasibility of the scalenectomy procedure. A systematic review revealed that the pooled success rate of supraclavicular scalenectomy with first-rib resection was 77%, and the probability of greater than 70% improvement was 87%; additionally, complete relief was observed in 57% of 683 cases, almost the same as the rate with the transaxillary approach.4 The greatest disadvantage of the supraclavicular approach is the difficulty in approaching the anterior aspect of the first rib via the costoclavicular space. Since dissection and retraction of the supraclavicular nerve, phrenic nerve, long thoracic nerve, and brachial plexus are required, these structures are at a high risk of injury.2,4 The rates of neurological injuries are approximately 3% and 7% for scalenectomy with and without first-rib resection, respectively.4 The reported incidences of pneumothorax are approximately 19% and 5% for scalenectomy with and without first-rib resection, respectively, suggesting that first-rib resection increases the risk of pneumothorax.4 Additionally, the incision for this approach has less cosmetic appeal.
Figure 3.
Supraclavicular approach. A Macroscopic appearance of supraclavicular structures. B Exposure of the first rib after scalenectomy. ASM, anterior scalene muscle; BP, brachial plexus; PN, phrenic nerve.
Infraclavicular Approach
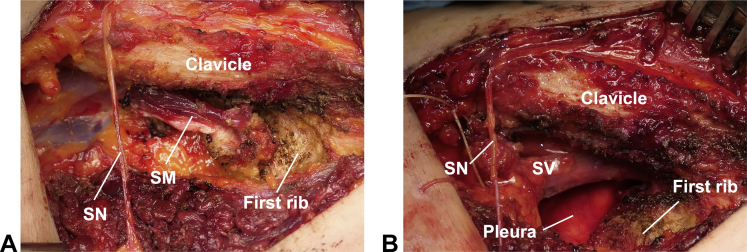
Gol et al18 originally introduced the infraclavicular approach for the removal of the first rib in 1968 (Fig 1B). This approach is used for the treatment of VTOS, which is usually diagnosed using venography, computed tomography, or ultrasonography to examine the presence of stenosis or thrombosis of the axillary-subclavian vein (Paget-Schroetter syndrome). A transverse incision is made 1 to 2 cm below the clavicle (Fig 1B). The pectoralis major muscle is spared or released from the clavicle to expose the subclavian muscle, which is excised; subsequently, the subclavian vein and first rib are explored. The infraclavicular approach allows for the resection of the subclavian muscle, anterior aspect of the first rib, costoclavicular ligament, and anterior scalene muscle, which compress the subclavian vein (Fig 4). The approach provides excellent exposure of the subclavian vein in a shallow field, and allows for subclavian venolysis and reconstruction, when required. When the infraclavicular incision is extended, a pectoralis minor tenotomy can be performed to treat pectoralis minor syndrome. Infraclavicular thoracic outlet decompression for VTOS showed good axillosubclavian vein patency (in 95% of 55 patients), which was comparable to that with supraclavicular decompression (good patency in 94% of 54 patients).11 Moreover, the infraclavicular approach does not require retraction of the nerve around the thoracic outlet; this reduces the risk of brachial plexus, phrenic nerve, and supraclavicular nerve injuries compared to risks of these injuries from the supraclavicular approach. The reported incidences of neurological injuries and pneumothorax are approximately 0% and 7% in infraclavicular approach, and 2% and 11% in supraclavicular approach, respectively.11 The complication rate after VTOS surgery is significantly lower with the infraclavicular approach than with the supraclavicular approach (P < 0.05).11 Although the infraclavicular approach provides easy access to the anterior aspect of the costoclavicular space, it is difficult to access the posterior aspect of the first rib and middle scalene muscle using this approach. Furthermore, the neurolysis procedure is difficult with this approach; thus, it is not easily applicable to NTOS treatment. The use of the isolated infraclavicular approach is limited to VTOS or pectoralis minor syndrome treatment. Cosmetically, the infraclavicular incision is better than the supraclavicular incision, albeit not as satisfactory as the transaxillary incision.
Figure 4.
Infraclavicular approach. A Macroscopic appearance of infraclavicular structures. B Exposure of the SV after resection of the SM, anterior scalene muscle, and first rib. SM, subclavian muscle; SN, supraclavicular nerve; SV, subclavian vein.
Posterior Approach
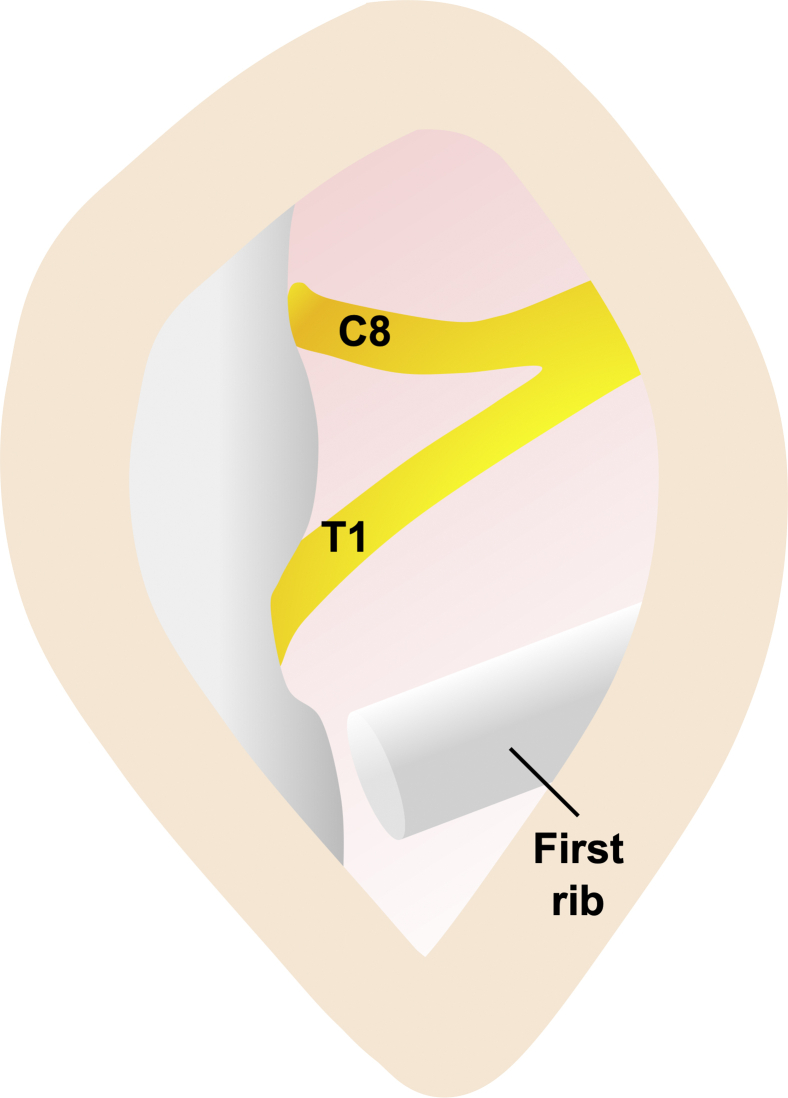
Clagett19 introduced first-rib resection through the posterior approach in 1962. A longitudinal periscapular incision is made between the spinous processes and the medial border of the scapula (Fig 1C). The trapezius and rhomboid minor muscles are separated, after which the first rib is explored and resected. An elongated C7 transverse process is removed, if necessary. The C7, C8, and T1 nerve roots are released, and dorsal sympathectomy is performed, if necessary (Fig 5).12 The posterior approach may be useful for patients with a history of surgery through the anterior or transaxillary approach who require reoperation, or for those who have radiation-induced tissue fibrosis of the anterior chest wall. Reoperation through the posterior approach in 2,305 patients with recurrent TOS showed satisfactory outcomes (good, 75%; fair, 16%; and poor, 9%).12 However, visualization of the anterior structures and arterial reconstruction is difficult using this approach. Potential complications include scapular winging, cervical spine instability (when more than 2 facets are removed), pleural tears, pneumothorax, hemothorax, and phrenic nerve palsy. Based on the advantages of other approaches, the posterior approach is rarely used for the initial operation; it is limited to reoperations only.
Figure 5.
Posterior approach. The C8 and T1 nerve roots and the first rib are explored.
Combined Transaxillary and Supraclavicular Approach
During the 1980s, a combined transaxillary and supraclavicular approach was introduced to compensate for the disadvantages of each approach.1,13 The same incisions and procedures described above are performed through both approaches to explore the scalene muscle and first rib (Fig 1A and B). The first rib is resected through the transaxillary approach, followed by anterior and middle scalene muscle resection through the supraclavicular approach.1 An advantage of this approach is the secure resection of the structures around the neurovascular bundle through the double incision. This combined approach showed superior outcomes (excellent, 95%; good, 4%; and poor, 1%) in 94 extremities compared to those of the isolated transaxillary approach (excellent, 72%; good, 7%; and poor, 21%) in 97 extremities.13 The reported incidence of pneumothorax is lower with the combined approach (4%) than with the isolated transaxillary approach (11%), probably because scalenectomy is not necessarily performed in a deep surgical field during the combined approach.13 However, the double incision potentially increases the risk of nerve injury associated with each approach, such as an intercostobrachial or supraclavicular nerve injury. Additionally, the prolonged operative time due to repositioning the patient after the first procedure and the greater invasiveness are disadvantages of the combined procedure. This procedur is not indicated for an initial surgery; moreover, this technique may be used for a “failed” initial surgery or recurrent TOS.2
Combined Supra- and Infraclavicular Approach (Paraclavicular Approach)
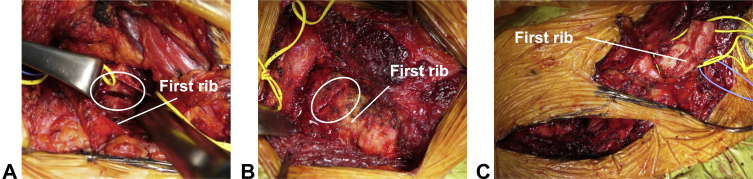
The combined supra- and infraclavicular (paraclavicular) approach was introduced for VTOS treatment in 1992 (Fig 1B).20 The efficacy of this procedure has been reported in patients with NTOS or ATOS.3 Two transverse incisions are made above and below the clavicle (Fig 1B). The ensuing procedures are performed as described above, to explore the scalene muscle and first rib. An anterior and middle scalenectomy and resection of the posterior aspect of the first rib are performed through the supraclavicular approach, followed by resection of the anterior aspect of the first rib through the infraclavicular approach (Fig 6A and B). The rib is released from the pleura and intercostal muscle through both incisions, after which en bloc resection of the first rib is performed (Fig 6C). The paraclavicular approach provides sufficient exposure for the effective decompression of all neurovascular structures and the performance of potential interventions. It enables en bloc resection of the first rib, from the anterior to posterior aspects, and avoids insufficient resection, leading to satisfactory postoperative outcomes (Fig 5). Approximately 85% to 94% of patients with vascular TOS who underwent paraclavicular decompression demonstrated ‘‘excellent’’ or ‘‘good’’ functional outcomes based on the Derkash classification.3,14 Since the first rib can be accessed and released through both incisions, the risk of pneumothorax is low. The reported incidences of neurological injuries and pneumothorax are approximately 6% and 0%, respectively.14 In contrast, the disadvantages of this approach include greater invasiveness and less cosmetic appeal due to the double incision around the clavicle. In total, 3% to 19% of patients who undergo this approach require postoperative management, such as drainage, or reoperation due to hematoma or bleeding.3,14 If necessary, vascular reconstruction can be performed via this approach, which tends to be selected for patients with VTOS or ATOS. 3,14
Figure 6.
Combined supra- and infraclavicular approach. A First rib resection through the supraclavicular approach. B First rib resection through the infraclavicular approach. C En bloc resection of the first rib through both approaches.
Endoscopic-Assisted Transaxillary Approach
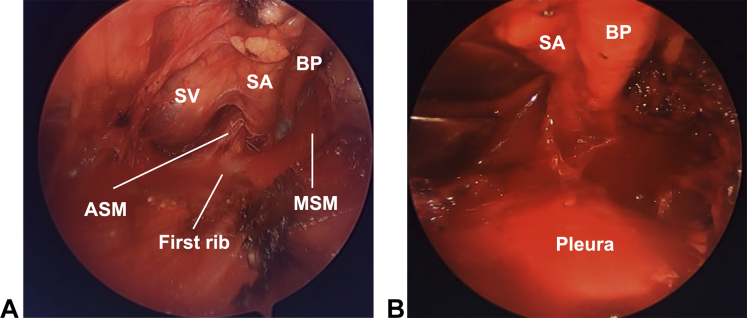
In 2005, endoscopic-assisted transaxillary first-rib resection was introduced to overcome the limitations of the transaxillary approach.5 After making an incision according to the traditional transaxillary approach, an arthroscope is introduced with an additional small port placed anterior to the latissimus dorsi (Fig 1A).6 Under endoscopic assistance, the anterior and middle scalene muscles are dissected free of their origins at the first rib using electrocautery. The first rib is elevated from the pleura and resected in a piece-by-piece fashion with a rongeur (Video). Endoscopic-assisted surgery provides excellent visualization of the thoracic outlet, especially in the posterior aspect of the first rib and middle scalene muscle (Fig 7). This advantage potentially minimizes complications that occur during surgery in a deeper and narrower field. This approach demonstrated good clinical outcomes (Roos and Derkash score: excellent, 40%; good, 41%; fair, 14%; and poor, 6%) in 131 cases.6 The reported incidence of pneumothorax ranges from 0% to 3%.5,6 Although endoscopic-assisted transaxillary first-rib resection is an effective procedure for the management of TOS, it is technically demanding; additionally, special instruments, such as an endoscopic device or a specialized arm traction holder, are required.6
Figure 7.
Endoscopic-assisted transaxillary approach. A Endoscopic appearance of the thoracic outlet. B After resection of the scalene muscles and first rib. ASM, anterior scalene muscle; BP, brachial plexus; MSM, middle scalene muscle; SA, subclavian artery; SV, subclavian vein.
Video-Assisted Thoracoscopic Surgery
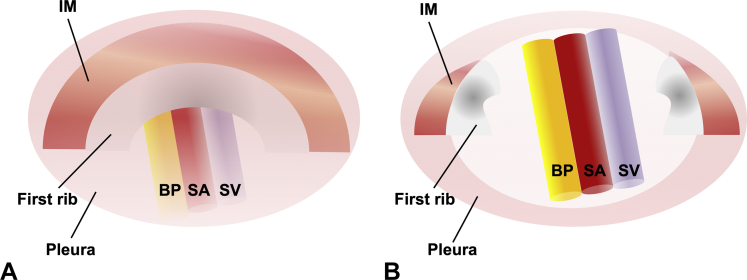
Video-assisted thoracoscopic surgery (VATS) was first applied as a minimally invasive approach for first-rib resection in patients with TOS in 1999; subsequently, several reports have demonstrated the feasibility and reproducibility of this approach.7Video-assisted thoracoscopic surgery is performed with the patient in the lateral position, and several portals are used for the scope and working instruments (Fig 1D).7,8 A thoracoscope is introduced into the fifth intercostal space at the midaxillary line, and the first rib is visible through the pleura. The parietal pleura of the first rib is opened, and intercostal muscles are dissected from the first rib using diathermy. The first rib is resected using a rongeur or rib cutter, and the attachments of the anterior and middle scalene muscles are divided (Fig 8). The advantages of VATS include its small incisions and the clear intrathoracic visualization of the operative field. A retrospective case-control study of patients that underwent thoracoscopic (n = 30) versus traditional transaxillary (n = 30) approaches for first-rib resection showed similar recovery rates for the 2 approaches (excellent or good recovery, 67% vs 63%, respectively).8 Difficulty in the full observation of neurovascular bundles before dissection and resection of the rib might result in a potential risk of neurovascular injury or insufficient decompression. The reported complication rate of VATS ranges from 3% to 25%; complications include wound infections, pneumothorax, hemothorax, transient arm weakness, bleeding, pulmonary embolism, pneumonia, and brachial plexus injury.8,9 The VATS approach has been further developed by robotic assistance. The robotic platform offers magnified, high-resolution, 3-dimensional images of the target structure anatomy, and shows fewer complications.10 Since VATS with or without robotic assistance is performed by opening the pleura, it is necessary to place a pleural drain, which is a disadvantage of this approach.8 Furthermore, VATS is technically highly demanding and requires special equipment; accordingly, it can only be performed by thoracic surgeons who are well trained to perform the procedure.
Figure 8.
Video-assisted thoracoscopic surgery. A Intrathoracic appearance of the first rib on thoracoscopy. B After opening the pleura and resection of the first rib. BP, brachial plexus; IM, intercostal muscle; SA, subclavian artery; SV, subclavian vein.
Conclusions
Each approach has advantages and disadvantages for the surgical treatment of TOS. When compression structures around the neurovascular bundle in the thoracic outlet are adequately released, surgical outcomes are generally satisfactory, regardless of the approach used. An inadequate release of compressed structures leads to failed or recurrent outcomes. The total complication rate for the surgical treatment of TOS ranges from 13% to 26%.4 Reported complications include death, major bleeding requiring vascular reconstruction, pneumothorax, hemothorax, increased pain, plexus or peripheral nerve injury, stellate ganglion injury, and peri-incisional numbness.2, 3, 4 Reducing the risk of complications is the most important aspect of TOS management. The surgical procedure should be safe, with sufficient resection. Furthermore, the surgical approach should be selected based on the surgeon’s skill, surgeon’s preferences, surgical invasiveness, cosmetic appearance, and the presence of special equipment, as well as other advantages and disadvantages of each approach. Technically safe and feasible, minimally invasive approaches that allow for adequate resection should be used. Most studies on surgical approaches for TOS treatment are case series or retrospective case-control studies; therefore, no well-established evidence exists for selecting a particular approach for the treatment of TOS. Prospective randomized controlled trials should be conducted to clarify the choice of treatment for patients with TOS.
Acknowledgments
We thank Professor M. Nakamura, Professor M. Matsumoto, and M. Hosotani in the Department of Orthopaedic Surgery, Keio University School of Medicine, for their helpful discussion and technical support.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
Supplementary Data
Surgical techniques of endoscopic-assisted transaxillary first-rib resection and scalenectomy.
References
- 1.Atasoy E. A hand surgeon’s further experience with thoracic outlet compression syndrome. J Hand Surg Am. 2010;35(9):1528–1538. doi: 10.1016/j.jhsa.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Meyer R. In: Green’s Operative Hand Surgery. 7th ed. Scott W.W., William C.P., Scott H.K., Mark S.C., editors. Churchill Livingstone; 2017. Thoracic outlet compression syndrome; pp. 959–978. [Google Scholar]
- 3.Peek J., Vos C.G., Ünlü Ç., van de Pavoordt H.D.W.M., van den Akker P.J., de Vries J.P.M. Outcome of surgical treatment for thoracic outlet syndrome: systematic review and meta-analysis. Ann Vasc Surg. 2017;40:303–326. doi: 10.1016/j.avsg.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 4.Yin Z.G., Gong K.T., Zhang J.B. Outcomes of surgical management of neurogenic thoracic outlet syndrome: a systematic review and Bayesian perspective. J Hand Surg Am. 2019;44(5):416.e1–416.e17. doi: 10.1016/j.jhsa.2018.06.120. [DOI] [PubMed] [Google Scholar]
- 5.Martinez B.D., Wiegand C.S., Evans P., Gerhardinger A., Mendez J. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular. 2005;13(6):327–335. doi: 10.1258/rsmvasc.13.6.327. [DOI] [PubMed] [Google Scholar]
- 6.Furushima K., Funakoshi T., Kusano H., et al. Endoscopic-assisted transaxillary approach for first rib resection in thoracic outlet syndrome. Arthrosc Sports Med Rehabil. 2021;3(1):e155–e162. doi: 10.1016/j.asmr.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtsuka T., Wolf R.K., Dunsker S.B. Port-access first-rib resection. Surg Endosc. 1999;13(9):940–942. doi: 10.1007/s004649901141. [DOI] [PubMed] [Google Scholar]
- 8.Nuutinen H., Riekkinen T., Aittola V., Mäkinen K., Kärkkäinen J.M. Thoracoscopic versus transaxillary approach to first rib resection in thoracic outlet syndrome. Ann Thorac Surg. 2018;105(3):937–942. doi: 10.1016/j.athoracsur.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Buero A., Chimondeguy D.J., Auvieux R., et al. Resection of the first rib by video thoracoscopy in Paget-Schroetter syndrome. Med (B Aires) 2021;81(1):31–36. [PubMed] [Google Scholar]
- 10.Pupovac S.S., Lee P.C., Zeltsman D., Jurado J., Hyman K., Singh V. Robotic-assisted first rib resection: our experience and review of the literature. Semin Thorac Cardiovasc Surg. 2020;32(4):1115–1120. doi: 10.1053/j.semtcvs.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Dua A., Rothenberg K.A., Gologorsky R.C., Deslarzes-Dubuis C., Lee J.T. Long-term quality of life comparison between supraclavicular and infraclavicular rib resection in patients with vTOS. Ann Vasc Surg. 2020;62:128–132. doi: 10.1016/j.avsg.2019.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Urschel H.C., Kourlis H. Thoracic outlet syndrome: a 50-year experience at Baylor University Medical Center. Proc (Bayl Univ Med Cent) 2007;20(2):125–135. doi: 10.1080/08998280.2007.11928267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qvarfordt P.G., Ehrenfeld W.K., Stoney R.J. Supraclavicular radical scalenectomy and transaxillary first rib resection for the thoracic outlet syndrome. A combined approach. Am J Surg. 1984;148(1):111–116. doi: 10.1016/0002-9610(84)90297-6. [DOI] [PubMed] [Google Scholar]
- 14.Al Rstum Z., Tanaka A., Sandhu H.K., et al. Differences in quality of life outcomes after paraclavicular decompression for thoracic outlet syndrome. J Vasc Surg. 2020;72(4):1421–1426. doi: 10.1016/j.jvs.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Sanders R.J. Results of the surgical treatment for thoracic outlet syndrome. Semin Thorac Cardiovasc Surg. 1996;8(2):221–228. [PubMed] [Google Scholar]
- 16.Roos D.B. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg. 1966;163(3):354–358. doi: 10.1097/00000658-196603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adson A.W., Coffey J.R. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839–857. doi: 10.1097/00000658-192785060-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gol A., Patrick D.W., McNeel D.P. Relief of costoclavicular syndrome by infraclavicular removal of first rib. Technical note. J Neurosurg. 1968;28(1):81–84. doi: 10.3171/jns.1968.28.1.0081. [DOI] [PubMed] [Google Scholar]
- 19.Clagett O.T. Research and prosearch. J Thorac Cardiovasc Surg. 1962;44:153–166. [PubMed] [Google Scholar]
- 20.Thompson R.W., Schneider P.A., Nelken N.A., Skioldebrand C.G., Stoney R.J. Circumferential venolysis and paraclavicular thoracic outlet decompression for “effort thrombosis” of the subclavian vein. J Vasc Surg. 1992;16(5):723–732. doi: 10.1067/mva.1992.41523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surgical techniques of endoscopic-assisted transaxillary first-rib resection and scalenectomy.